Abstract
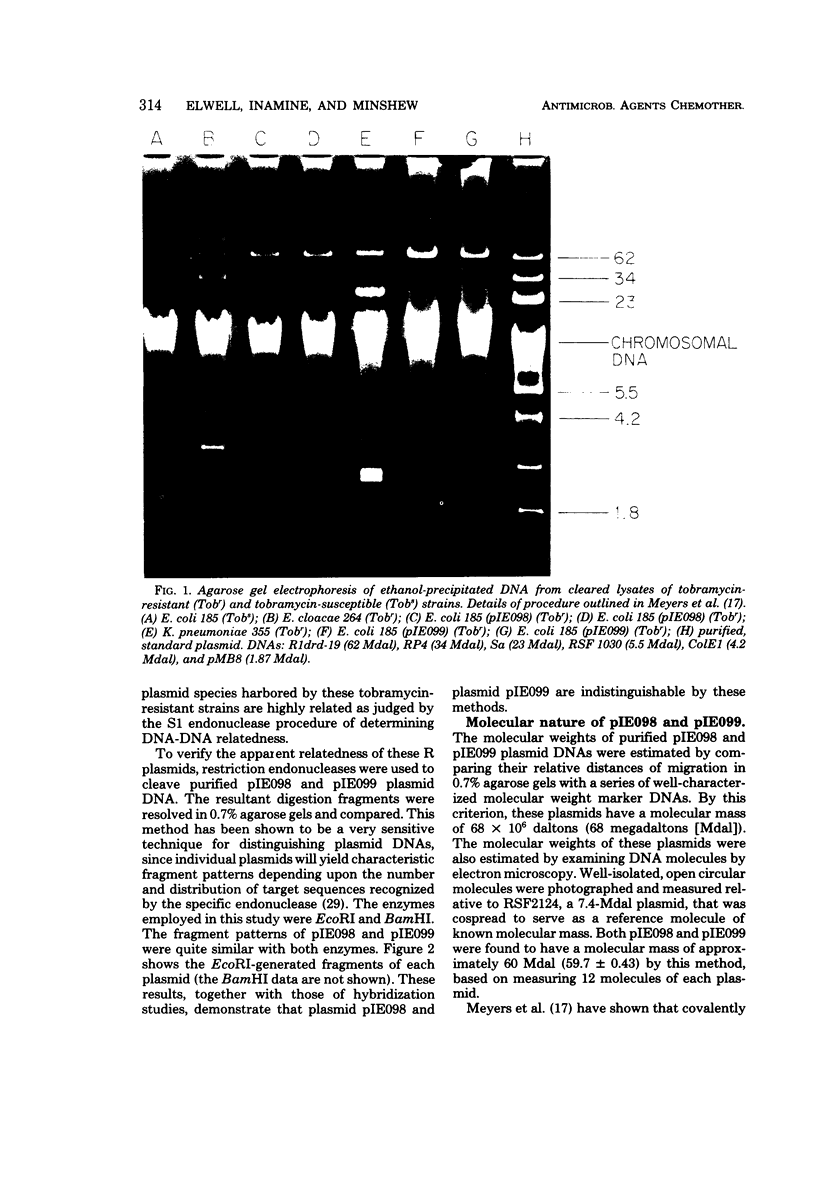
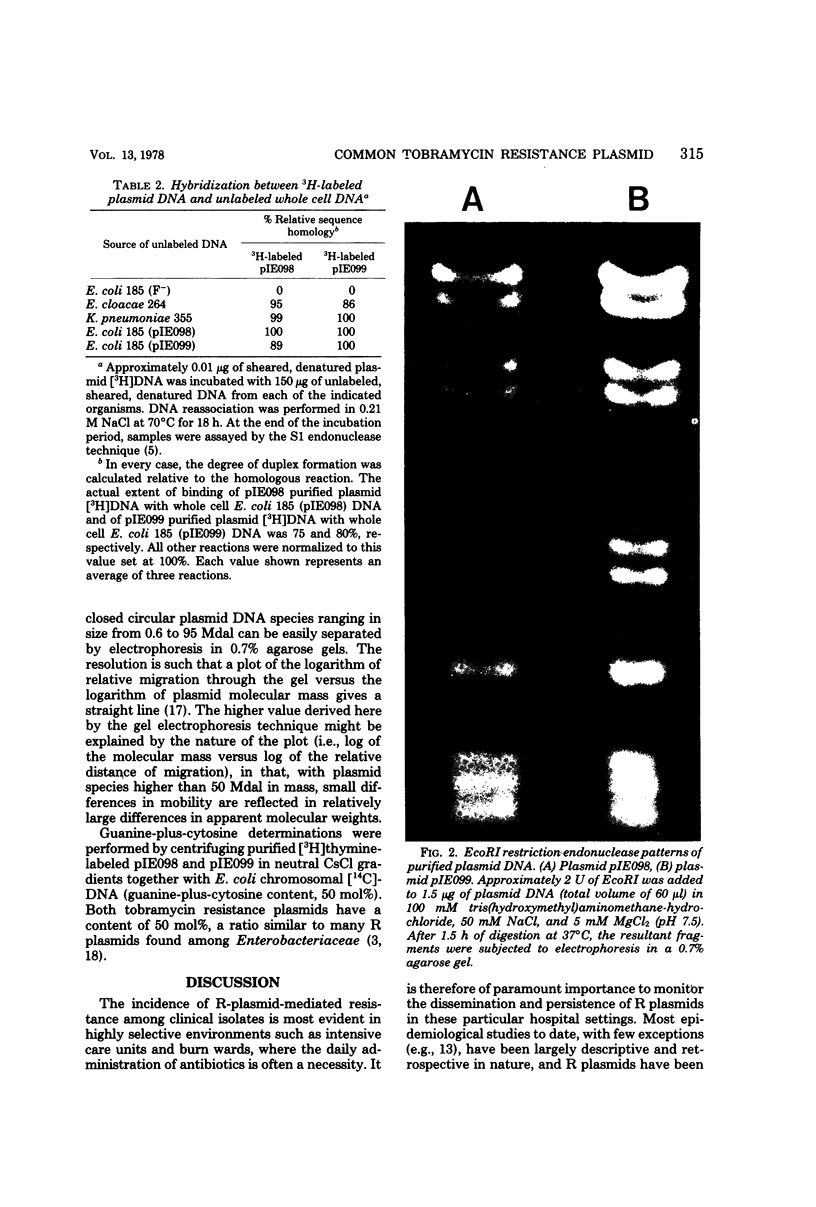
Tobramycin-resistant burn wound isolates of Klebsiella pneumoniae and Enterobacter cloacae, together with Escherichia coli K-12 transconjugants from these two strains, were examined for plasmid deoxyribonucleic acid (DNA). All the resistant strains contained a common, high-molecular-weight, covalently closed circular DNA plasmid that was absent in the tobramycin-susceptible E. coli recipient strain. The common plasmid residing in E. cloacae was designated pIE098, and that residing in K. pneumoniae was designated pIE099. Both plasmid species were found to have a molecular mass of approximately 60 × 106 daltons and a guanine-plus-cytosine content of 50 mol%. The DNA that was extracted from all of the tobramycin-resistant strains tested was able to hybridize to 86 to 100% with pIE098 and pIE099 [3H]DNA generated by EcoRI to produce fragments of a size similar to those generated by BamHI. This study illustrates the usefulness of simple screening methods for antibiotic resistance plasmids in a hospital epidemiological situation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R., Davies J. Enzymatic acetylation of aminoglycoside antibiotics by Escherichia coli carrying an R factor. Biochemistry. 1971 May 11;10(10):1787–1796. doi: 10.1021/bi00786a009. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Kopecko D. J. Structural evolution of bacterial plasmids: role of translocating genetic elements and DNA sequence insertions. Fed Proc. 1976 Jul;35(9):2031–2036. [PubMed] [Google Scholar]
- Crosa J. H., Brenner D. J., Falkow S. Use of a single-strand specific nuclease for analysis of bacterial and plasmid deoxyribonucleic acid homo- and heteroduplexes. J Bacteriol. 1973 Sep;115(3):904–911. doi: 10.1128/jb.115.3.904-911.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., Saunders J. R., Richmond M. H., Falkow S. Relationships among some R plasmids found in Haemophilus influenzae. J Bacteriol. 1977 Jul;131(1):356–362. doi: 10.1128/jb.131.1.356-362.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. E., Jr, Eidson M., Guerry P., Falkow S., Drusin L. M., Roberts R. B. Interbacterial transfer of R factor in the human intestine: in-vivo acquisition of R-factor-mediated kanamycin resistance by a multiresistant strain of Shigella sonnei. J Infect Dis. 1972 Jul;126(1):27–33. doi: 10.1093/infdis/126.1.27. [DOI] [PubMed] [Google Scholar]
- Haas M., Biddlecome S., Davies J., Luce C. E., Daniels P. J. Enzymatic modification of aminoglycoside antibiotics: a new 6'-N-acetylating enzyme from a Pseudomonas aeruginosa isolate. Antimicrob Agents Chemother. 1976 Jun;9(6):945–950. doi: 10.1128/aac.9.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Translocation of a plasmid DNA sequence which mediates ampicillin resistance: molecular nature and specificity of insertion. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3623–3627. doi: 10.1073/pnas.72.9.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. C., Anderson J. D., Arrand J. E., Richmond M. H. A probable example of R-factor recombination in the human gastro-intestinal tract. J Med Microbiol. 1974 May;7(2):251–257. doi: 10.1099/00222615-7-2-251. [DOI] [PubMed] [Google Scholar]
- Ingram L. C., Richmond M. H., Sykes R. B. Molecular characterization of the R factors implicated in the carbenicillin resistance of a sequence of Pseudomonas aeruginosa strains isolated from burns. Antimicrob Agents Chemother. 1973 Feb;3(2):279–288. doi: 10.1128/aac.3.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. Translocatable elements in procaryotes. Cell. 1977 May;11(1):11–23. doi: 10.1016/0092-8674(77)90313-0. [DOI] [PubMed] [Google Scholar]
- Lowbury E. J., Lilly H. A., Kidson A., Ayliffe G. A., Jones R. J. Sensitivity of Pseudomonas aeruginosa to antibiotics: emergence of strains highly resistant to carbenicillin. Lancet. 1969 Aug 30;2(7618):448–452. doi: 10.1016/s0140-6736(69)90163-9. [DOI] [PubMed] [Google Scholar]
- Mayer L. W., Holmes K. K., Falkow S. Characterization of plasmid deoxyribonucleic acid from Neisseria gonorrhoeae. Infect Immun. 1974 Oct;10(4):712–717. doi: 10.1128/iai.10.4.712-717.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew B. H., Holmes R. K., Sanford J. P., Baxter C. R. Transferrable resistance to tobramycin in Klebsiella pneumoniae and Enterobacter cloacae associated with enzymatic acetylation of tobramycin. Antimicrob Agents Chemother. 1974 Oct;6(4):492–497. doi: 10.1128/aac.6.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan P., Holder I. A., MacMillan B. G. Burn wounds: microbiology, local host defenses, and current therapy. CRC Crit Rev Clin Lab Sci. 1973 Jul;4(1):61–100. doi: 10.3109/10408367309151684. [DOI] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Schaberg D. R., Highsmith A. K., Wachsmuth I. K. Resistance plasmid transfer by Serratia marcescens in urine. Antimicrob Agents Chemother. 1977 Mar;11(3):449–450. doi: 10.1128/aac.11.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So M., Crosa J. H., Falkow S. Polynucleotide sequence relationships among Ent plasmids and the relationship between Ent and other plasmids. J Bacteriol. 1975 Jan;121(1):234–238. doi: 10.1128/jb.121.1.234-238.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens P., Young L. S., Hewitt W. L. Improved acetylating radioenzymatic assay of amikacin, tobramycin, and sisomicin in serum. Antimicrob Agents Chemother. 1975 Mar;7(3):374–376. doi: 10.1128/aac.7.3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R., Hughes S. G., Broda P. Plasmid identification using specific endonucleases. Mol Gen Genet. 1974;133(2):141–149. doi: 10.1007/BF00264835. [DOI] [PubMed] [Google Scholar]