Abstract
Rett syndrome (RTT) is an X-linked neurological disorder caused by mutations in the gene encoding the transcriptional modulator methyl-CpG-binding protein 2 (MeCP2). Typical RTT primarily affects girls and is characterized by a brief period of apparently normal development followed by the loss of purposeful hand skills and language, the onset of anxiety, hand stereotypies, autistic features, seizures and autonomic dysfunction. Mecp2 mouse models have extensively been studied to demonstrate the functional link between MeCP2 dysfunction and RTT pathogenesis. However, the majority of studies have focused primarily on the molecular and behavioral consequences of the complete absence of MeCP2 in male mice. Studies of female Mecp2+/− mice have been limited because of potential phenotypic variability due to X chromosome inactivation effects. To determine whether reproducible and reliable phenotypes can be detected Mecp2+/− mice, we analyzed Mecp2+/− mice of two different F1 hybrid isogenic backgrounds and at young and old ages using several neurobehavioral and physiological assays. Here, we report a multitude of phenotypes in female Mecp2+/− mice, some presenting as early as 5 weeks of life. We demonstrate that Mecp2+/− mice recapitulate several aspects of typical RTT and show that mosaic expression of MeCP2 does not preclude the use of female mice in behavioral and molecular studies. Importantly, we uncover several behavioral abnormalities that are present in two genetic backgrounds and report on phenotypes that are unique to one background. These findings provide a framework for pre-clinical studies aimed at improving the constellation of phenotypes in a mouse model of RTT.
INTRODUCTION
Rett syndrome (RTT), an X-linked neurological disorder that affects girls, is characterized by a period of normal development followed by regression and the manifestation of behavioral and physiological problems (1,2). RTT is caused by mutations in the gene encoding the transcriptional modulator Methyl-CpG-Binding Protein 2 (MECP2) (3). MeCP2 binds to methylated-CpG dinucleotides, influencing gene expression both positively and negatively (4–6). Several mouse models have been generated to understand how MeCP2 dysfunction leads to neurological deficits in RTT (7–16).
The majority of work involving Mecp2 mouse models has focused on the loss of MeCP2 in male mice (Mecp2−/y). Studying Mecp2−/y mice has revealed the consequences of a total loss of MeCP2 function without the potential confounding effects of variable X chromosome inactivation (XCI) in female mice. Pathogenesis studies in Mecp2−/y mice aim to reveal mechanisms that might be therapeutically manipulated, and have revealed potential avenues for pre-clinical studies aimed at improving RTT-like phenotypes in the mouse (17,18). The severe phenotype and short lifespan of Mecp2−/y mice, however, render interventional studies challenging. Female Mecp2+/− mice (9) may serve as a better model for pre-clinical studies, but it is unclear whether these animals display the spectrum of phenotypic abnormalities typical of RTT. The few studies of neurobehavioral and physiological defects in Mecp2+/− mice reported general health evaluations (9,19), motor defects (9,19–21), breathing abnormalities (9,22–25) and abnormal early neurological reflexes (20), but apparently normal autonomic cardiovascular function and anxiety-like behavior (20,24). In most cases, these phenotypes were described in older mice.
We therefore reasoned that the detailed characterization of Mecp2+/− mice to identify as many RTT-like phenotypes as possible and to establish their onset and reproducibility is an important step for pre-clinical trials in an RTT model. Accordingly, we characterized Mecp2+/− mice of two different F1 hybrid isogenic backgrounds using several neurobehavioral assays, starting from 5 weeks of life through 22 weeks of life. We chose F1 hybrid animals that receive an equal genetic contribution from two pure inbred strains to minimize the strain-specific issues typical of inbred strains (26–28). We also evaluated physiological parameters such as weight and breathing. Our data detail the onset and nature of the various abnormalities in Mecp2+/− mice and highlight the ideal F1 hybrid background for specific phenotypes, providing a framework for future studies aimed at testing in vivo interventions.
RESULTS
Mecp2+/− mice display reduced anxiety-like behavior
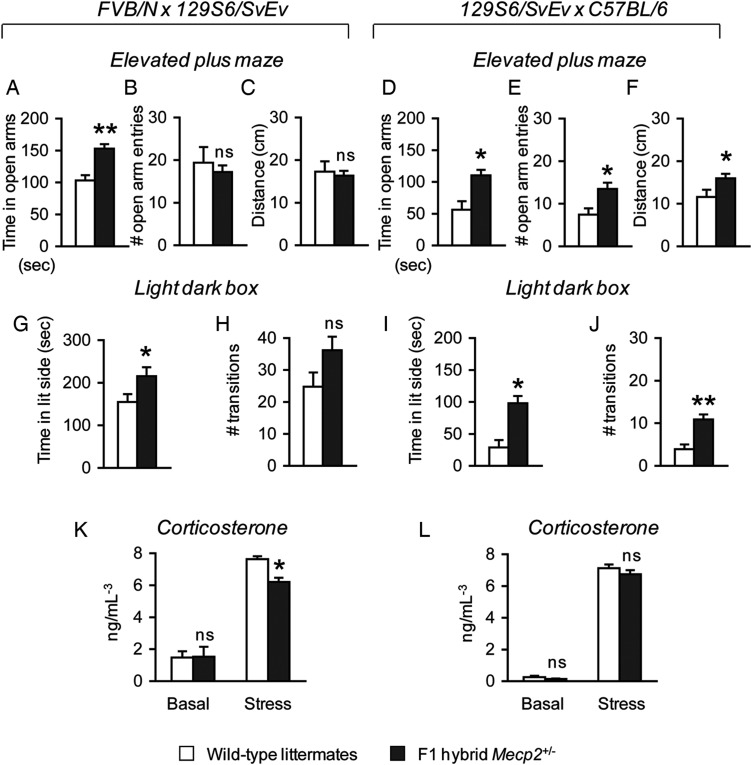
Previous studies of female mice harboring either an Mecp2-mutant allele (8,29,30) or an Mecp2-null allele (9,20) have shown conflicting results regarding the presence of either elevated or decreased anxiety-like behavior in these animals, despite the fact that anxiety is a prominent component of typical RTT. Therefore, to resolve the issue of whether anxiety is indeed present in Mecp2+/− mice, we tested 5-week-old animals using two conventional assays that assess elevated anxiety-like behavior in rodents, the elevated plus maze and the light-dark box (31). To minimize the strain-specific issues associated with the study of pure inbred strains in anxiety-related behavioral tasks, we used F1 hybrid animals that receive an equal genetic contribution from two inbred strains (26,27,32). The two F1 hybrid strains used in this study are (FVB/N × 129S6/SvEv)F1, indicated as FVB.129F1, and (129S6/SvEv × C57BL/6)F1, indicated as 129.B6F1. We discovered that both FVB.129F1 and 129.B6F1 Mecp2+/− mice spent significantly more time in the open arms of the elevated plus maze compared with their respective wild-type littermates [FVB.129F1, F(1, 28) = 15.90, P < 0.001; 129.B6F1, F(1,17) = 11.90, P < 0.05] (Fig. 1A and D). Although FVB.129F1 Mecp2+/− mice compared with wild-type littermates entered the open arm and traveled in the maze similarly (Fig. 1B and C), 129.B6F1 Mecp2+/− mice made a greater number of open-arm entries in the elevated plus maze [F(1, 17) = 8.67, P < 0.05] (Fig. 1E), and traveled more in the maze [F(1,17) = 5.03, P < 0.05] (Fig. 1F). An analysis of the first 5min of activity during this task also revealed similar results (Supplementary Material, Fig. S1A–F). In the light–dark box exploration task, both FVB.129F1 and 129.B6F1 Mecp2+/− mice spent more time in the lit compartment [FVB.129F1, F(1,26) = 4.68, P < 0.05; 129.B6F1, F(1,17) = 17.83, P < 0.05] (Fig. 1G and I). 129.B6F1 but not FVB.129F1 Mecp2+/− mice made more transitions to the lit compartment of the light–dark box [F(1,17) = 19.05, P < 0.001] (Fig. 1H and J). Because previous studies indicated that decreased anxiety-like behavior correlates with an impaired response to stress (33), we measured basal serum corticosterone levels before and after stress exposure. FVB.129F1 and 129.B6F1 Mecp2+/− mice had normal basal serum corticosterone levels; however, only FVB.129F1 Mecp2+/− mice showed reduced corticosterone levels in response to stress [F(1, 8) = 21.09, P < 0.05] (Fig. 1K and L).
Figure 1.
Mecp2+/− are less anxious and have an abnormal corticosterone response to stress. FVB.129F1 Mecp2+/− mice spend more time in the open arms of the elevated plus maze compared with wild-type littermates (A), but do not show differences in the number of open arm entries (B) or in the distance traveled in the maze (C). 129.B6F1 Mecp2+/− mice show significant differences in all three test parameters, spending more time in the open arms (D), making more open arm entries (E) and traveling further in the elevated plus maze (F) compared with wild-type littermates. This anxiolytic effect in female mice was confirmed in the light–dark box exploration task. FVB.129F1 Mecp2+/− mice compared with wild-type littermates spend more time in the lit compartment (G) and show a non-significant increase in the number of transitions between the light and dark compartments (H), whereas 129.B6F1 Mecp2+/− mice show significant increases in both time spent in the lit compartment (I) and the number of transitions between the two compartments (J). Animals from both genetic backgrounds show normal basal corticosterone levels (K and L). However, FVB.129F1, but not 129.B6F1 Mecp2+/−, mice show a decreased corticosterone response to stress (K and L). *P < 0.05; **P < 0.001; ns, not significant.
Mecp2+/− mice have progressive motor impairments
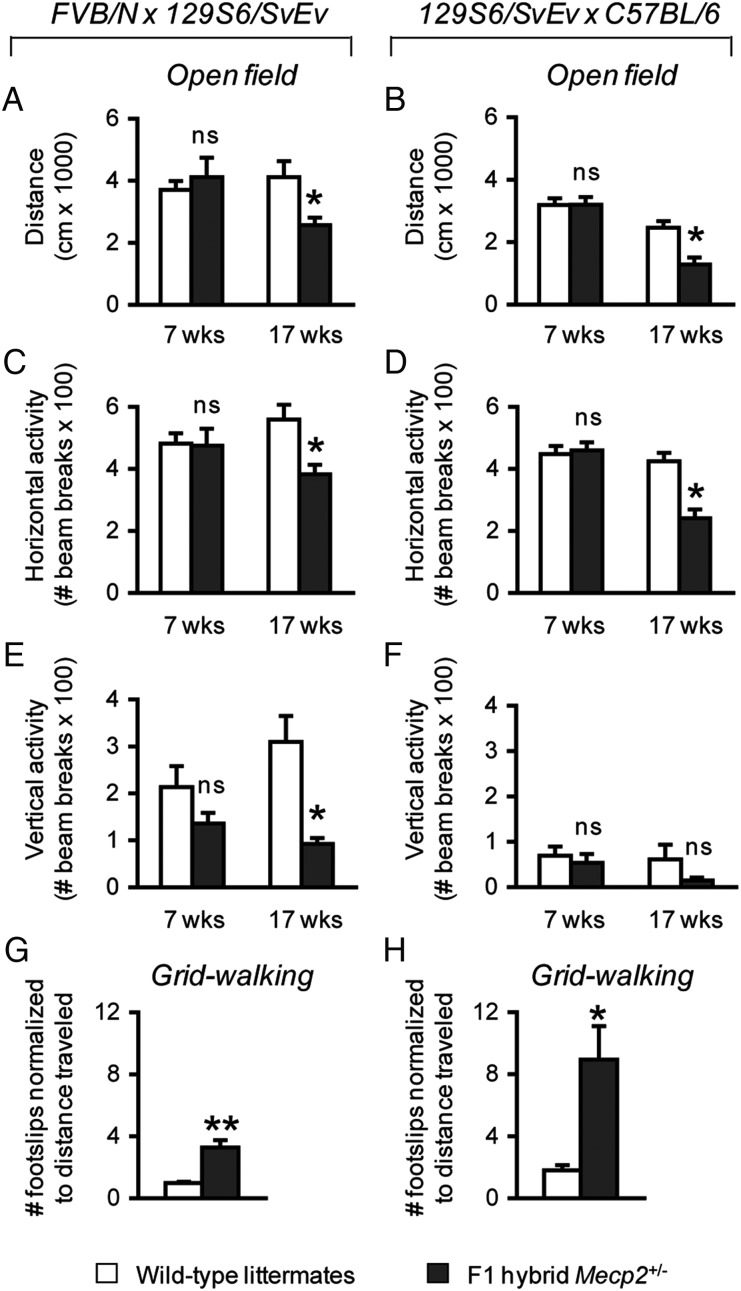
The loss of motor function is an important clinical feature of typical RTT that manifests early during the course of disease. Male mice lacking MeCP2 display a deficit in motor function that begins at 3–4 weeks of life and that dramatically worsens over time (9,20). In contrast, the data on the onset and course of motor deficits in female Mecp2+/− mice are sparse and often limited to the later stages of life (9,19–21). Decreased motor function has also been reported in a few studies of female Mecp2-mutant mice (29). Therefore, we assessed motor function of Mecp2+/− mice in an open field and in the grid-walking test to establish the earliest age of onset in these genetic backgrounds and to determine whether this deficit worsened with age. In the open field, the total distance traveled as well as horizontal and vertical activities of 7-week-old Mecp2+/− mice compared with wild-type littermates were not significantly different (Fig. 2A–F). At 17 weeks of age, however, both FVB.129F1 and 129.B6F1 Mecp2+/− mice traveled less in the open field [FVB.129F1, F(1,21) = 7.28, P < 0.05; 129.B6F1, F(1,17) = 14.61, P < 0.05] and showed reduced horizontal activity [FVB.129F1, F(1,21) = 10.24, P < 0.05; 129.B6F1, F(1,17) = 21.72, P < 0.001] (Fig. 2A–D). FVB.129F1 Mecp2+/− mice also showed reduced vertical activity [F(1,21) = 14.88, P < 0.05]; however, 129.B6F1 Mecp2+/− mice did not show a significant difference in vertical activity compared with their wild-type littermates (Fig. 2E and F). To assess motor coordination at this age, we tested the animals in the grid-walking test. Unlike the accelerating rotating rod used in previous studies (20,21), this assay quantifies the number of footslips through an elevated wire grid and is not influenced by the weight of the animals (34). We found that both FVB.129F1 and 129.B6F1 Mecp2+/− mice made a greater number of footslips per centimeter traveled compared with their wild-type littermates [FVB.129F1, F(1,24) = 27.28, P < 0.001; 129.B6F1, F(1,17) = 9.64, P < 0.05] (Fig. 2G–H).
Figure 2.
Mecp2+/− mice display motor impairments at a later age. At 7 weeks of life, F1 hybrid Mecp2+/− mice of both backgrounds travel a similar distance compared with their respective wild-type littermates (A and B), and display normal horizontal (C and D) and vertical (E and F) activities in an open field. At 17 weeks of life, however, Mecp2+/− mice of both backgrounds travel less than their respective wild-type littermates (A and B) and show a reduction in horizontal (C and D). Only FVB.129F1 Mecp2+/− mice show reduced vertical activity (E and F). Mecp2+/− mice of both backgrounds display a greater number of footslips in the gridwalking test compared with their respective wild-type littermates (G and H), further confirming motor dysfunction as the number of footslips is normalized to the total distance traveled. *P < 0.05; **P < 0.001; ns, not significant.
Mecp2+/− mice have reduced startle, enhanced prepulse inhibition and reduced pain recognition and normal olfaction
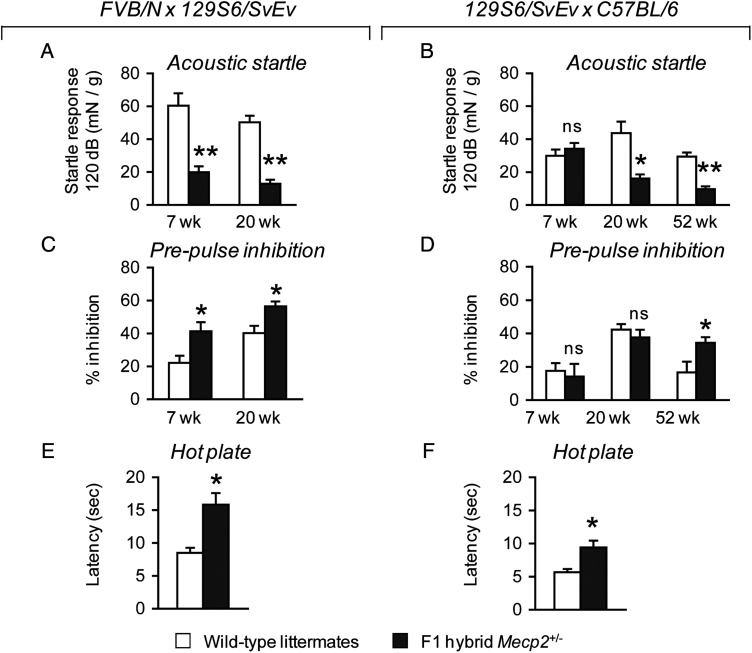
To investigate abnormalities in sensory function, we examined sensorimotor gating, pain sensitivity and olfaction. Alterations in sensorimotor gating are prominent in neurological disorders such as Fragile X syndrome (35,36), autism (37) and schizophrenia (38,39). This phenomenon can be evaluated in mice by measuring the inhibition of an acoustic startle response (ASR) after animals are presented with a weaker prepulse sound. We tested the ASR threshold of Mecp2+/− mice and their wild-type littermates by exposing the animals to a series of sounds ranging from 70 to 118 dB presented in random order, then evaluated the inhibition of a maximum startle response (120 dB) when animals were presented with a prepulse sound of lower intensity. Our analysis of the ASR threshold indicated no difference in the ASR of 7-week-old FVB.129F1 Mecp2+/− mice compared with their wild-type littermates presented with sound in the range of 70–102 dB; in the range of 106–118 dB, however, FVB.129F1 Mecp2+/− mice displayed a lower ASR compared with their wild-type littermates (Supplementary Material, Fig. S1G). In contrast, 129.B6F1 Mecp2+/− mice showed no differences in their startle responses across the presented range of sounds at this age (Supplementary Material, Fig. S1H). An analysis of ASR at 20 weeks of life revealed that both FVB.129F1 and 129.B6F1 Mecp2+/− mice showed a reduction in the ASR when presented with sound ranging from 102 to 118 dB (Supplementary Material, Fig. S1I and J). In a separate test incorporating prepulse sounds of varied intensity prior to a maximum of 120 dB sound, we confirmed the ASR deficit of FVB.129F1 Mecp2+/− mice at 7 and 20 weeks of life [7 weeks, F(1,24) = 16.70, P < 0.001; 20 weeks, F(1,24) = 59.05, P < 0.001] (Fig. 3A), and the ASR deficit of 129.B6F1 Mecp2+/− mice at 20 weeks of life [F(1,17) = 15.38, P < 0.05] (Fig. 3B). Prepulse inhibition (PPI) of the ASR was significantly enhanced in both 7- and 20-week-old FVB.129F1 Mecp2+/− mice [7 weeks, F(1,24) = 59.05, P < 0.001; 20 weeks, F(1,24) = 8.72, P < 0.05] (Fig. 3C), but normal in 129.B6F1 Mecp2+/− mice at these ages (Fig. 3D). To determine whether the PPI phenotype manifests later in 129.B6F1 Mecp2+/− mice, we tested a separate cohort of naïve 1-year-old 129.B6F1 Mecp2+/− mice. We confirmed a deficit in the ASR at this age [F(1,21) = 36.53, P < 0.001] (Fig. 3B), and discovered that 129.B6F1 Mecp2+/− mice indeed showed enhanced PPI [F(1,21) = 4.78, P < 0.05], indicating a delay in the onset of this specific phenotype when the Mecp2-null allele is on this particular genetic background (Fig. 3D). To test gross olfaction ability, we measured the time that animals spent sniffing a cotton swab impregnated with either a familiar odor (water) or a novel odor (vanilla extract). Mecp2+/− mice of both genetic backgrounds spent the same amount of time sniffing the cotton swabs with both familiar and novel odors as their respective wild-type littermates (Supplementary Material, Fig. S2). This indicates that Mecp2+/− mice likely have normal olfaction, similar to observations made in other MeCP2 mouse models (40,41). Previous work showed that male animals harboring a hypomorphic allele of Mecp2 displayed a decrease in pain recognition (42); therefore, we investigated whether female Mecp2+/− mice also showed a similar defect using the hot plate test. Mecp2+/− mice of both genetic backgrounds compared with wild-type littermates displayed a slower reactivity to a conductive heat stimulus, indicated by the increase in latency to respond when placed on a heated metal surface [FVB.129F1, F(1,24) = 16.51, P < 0.001; 129.B6F1, F(1,17) = 10.17, P < 0.05] (Fig. 3E and F).
Figure 3.
Mecp2+/− mice show deficits in the ASR and enhanced PPI of the startle response, and have abnormal thermal nociception. The ASR is reduced in FVB.129F1 Mecp2+/− mice at 7 and 20 weeks of life (A) and in 129.B6F1 Mecp2+/− mice at 20 weeks of life (B). At these ages, FVB.129F1 Mecp2+/− mice show enhanced PPI of the ASR compared with wild-type littermates (C), whereas 129.B6F1 Mecp2+/− mice have normal PPI compared with wild-type littermates (D). At 52 weeks of life, 129.B6F1 Mecp2+/− mice have an ASR deficit and show enhanced PPI compared with wild-type littermates (B and D). In the hot plate test, Mecp2+/− mice of both backgrounds respond slower when placed on a 50°C heated metal surface compared with wild-type littermates, indicating reduced pain sensitivity (E and F). *P < 0.05; **P < 0.001; ns, not significant.
Mecp2+/− mice display altered social approach behavior
In typical RTT, autistic behavior manifests during the regression phase of the disorder and some features persist throughout the course of the disease (43); however, social behavior has yet to be studied in Mecp2+/− mice. To evaluate social behavior, we tested two separate cohorts of Mecp2+/− mice either at 12 weeks of life or at 22 weeks of life using the three-chamber assay for sociability. This test is a validated assay for social approach behavior in mice and has been useful in uncovering alterations in social behavior in mouse models of autism (44–46).
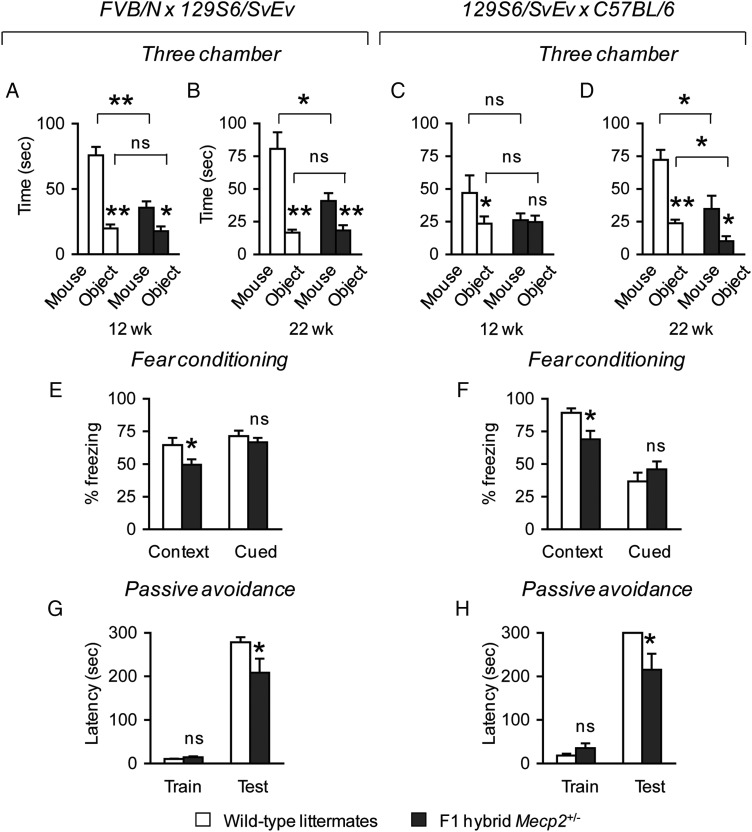
When tested for social approach behavior toward a novel mouse versus a novel object, we first compared the amount of time mice spent investigating a novel mouse versus a novel object within a genotype. We then compared whether there was a difference across genotypes in the time spent investigating a novel mouse, and in the time spent investigating a novel object. Both FVB.129F1 Mecp2+/− and wild-type littermate mice at 12 weeks of life spent more time investigating a novel mouse compared with a novel object [Mecp2+/−, F(1,19) = 8.80, P < 0.05; wild-type, F(1,19) = 62.52, P < 0.001]. Compared with wild-type littermate mice, FVB.129F1 Mecp2+/− mice spent less time investigating a novel mouse [F(1,18) = 24.77, P < 0.001] (Fig. 4A), but a similar amount of time investigating a novel object. These results were confirmed in a separate cohort of animals at 22 weeks of life [time spent with mouse versus object, Mecp2+/−, F(1,23) = 8.17, P < 0.05; wild-type, F(1,19) = 24.90, P < 0.001; time spent with mouse across genotypes, F(1,21) = 8.65, P < 0.05] (Fig. 4B). In contrast, 129.B6F1 Mecp2+/− mice at 12 weeks of life were different from wild-type littermates in that they spent an equal amount of time investigating either a novel mouse or a novel object (Fig. 4C), and showed a significant decrease in social approach behavior toward novel mice only at 22 weeks of life [time spent with mouse across genotypes, F(1,17) = 8.56, P < 0.05] (Fig. 4D). It is noteworthy that 129.B6F1 Mecp2+/− mice at 22 weeks of life also showed a decrease in the amount of time spent investigating a novel object [F(1,17) = 8.15, P < 0.05]. The time mice spent in each chamber (either mouse, center or object chamber), in addition to the control measurements performed during the habituation and test phases at both time points, is described in Supplementary Material, Figures S3 and S4.
Figure 4.
F1 hybrid Mecp2+/− mice have altered social behavior, and impaired contextual fear memory and passive avoidance learning. At 12 weeks of life, FVB.129F1 Mecp2+/− and wild-type littermate mice spend more time investigating a novel mouse compared with a novel object. However, FVB.129F1 Mecp2+/− spend less time investigating a novel mouse, and investigate a novel object similarly compared with wild-type animals (A). In contrast, 129.B6F1 Mecp2+/− mice show no preference for a novel object or mouse, whereas wild-type littermates spend more time investigating a novel mouse compared with a novel object. Across genotypes, no difference is observed in the time spent investigating a novel mouse (C). Similar to the findings at 12 weeks of life, at 22 weeks of life FVB.129F1 Mecp2+/− and wild-type littermate mice spend more time investigating a novel mouse compared with a novel object, and FVB.129F1 Mecp2+/− mice spend less time investigating a novel mouse. No difference was observed in the time spent investigating a novel object (B). Similarly, 129.B6F1 Mecp2+/− and wild-type littermate mice spend more time investigating a novel mouse compared with a novel object. However, 129.B6F1 Mecp2+/− mice spend less time investigating both the novel mouse and the novel object (D), due to reduced motor activity in the three-chamber apparatus (Supplementary Material, Fig. S4). Mecp2+/− mice of both F1 hybrid backgrounds compared with wild-type littermates freeze less in a test for contextual fear memory but not cued fear memory (E and F). In the passive avoidance learning task, no differences among genotypes were observed for the latency to enter the dark compartment during the training phase (G and H). However, during the test phase, Mecp2+/− mice of both F1 hybrid backgrounds compared with their respective littermates showed a decrease in the latency to enter the dark compartment (G and H). *P < 0.05; **P < 0.001; ns, not significant.
Mecp2+/− mice show deficits in contextual fear memory and passive avoidance learning
One hallmark of MeCP2 dysfunction is impaired cognition. For example, intellectual disability is a prominent clinical feature of typical RTT, and women with MECP2 mutations that do not meet the diagnostic criteria for RTT also display learning disabilities that range from mild to severe (47–50). Impaired learning and memory is also apparent in male mice harboring either a Mecp2-null (12) or Mecp2-mutant allele (51). We therefore evaluated Mecp2+/− mice and their wild-type littermates for fear conditioning and passive avoidance learning. We found that Mecp2+/− mice of both genetic backgrounds displayed a deficit in contextual fear memory [FVB.129F1, F(1,22) = 4.78, P < 0.05; 129.B6F1, F(1,17) = 7.20, P < 0.05], but normal cued fear memory (Fig. 4E and F). This deficit is not due to differences in response to shock because Mecp2+/− mice and wild-type littermates respond equally to different shock intensities (Supplementary Material, Fig. S5A and B). In the passive avoidance task, no differences were observed among genotypes for both strain backgrounds during the training day. Mecp2+/− mice of both genetic backgrounds, however, showed a decreased latency to enter the dark compartment [FVB.129F1, U(1) = 49.5, Z = −2.03, P < 0.05; 129.B6F1, U(1) = 31.5, Z = −1.96, P = 0.05] (Fig. 4G and H). Differences were also observed for the number of animals that successfully learned to associate the shock with the dark compartment. Fewer FVB.129F1 Mecp2+/− mice (∼42%, 5/12) compared with wild-type littermates (∼79%, 11/14) remained in the lit side [X2 = 3.72, P = 0.05]. Similarly, fewer 129.B6F1 Mecp2+/− mice (∼64%, 7/11) compared with wild-type littermates (100%, 9/9) remained in the lit side [X2 = 4.09, P < 0.05]. Because we were interested in the behavior of animals that remained in the lit side during the test, we measured freezing behavior and the number of approaches toward the dark compartment. Of the two genetic backgrounds, FVB.129F1 Mecp2+/− mice were more active in the lit side during the duration of the test compared with wild-type littermates [U(1) = 8.50, Z = −2.17, P < 0.05], and made a greater number of approaches toward the dark compartment [U(1) = 6.00, Z = −2.57, P < 0.05] (Supplementary Material, Fig. S5C–F).
Mecp2+/− mice of a reciprocal genetic strain background show similar phenotypic defects
Because of the possibility that there may be an interaction between genetic strain background and maternal genotype on the neurobehavioral phenotypes studied, we sought to control for a possible maternal effect and generated a separate cohort of animals of a 129.FVBF1 background by breeding 129S6/SvEv Mecp2+/− females to FVB/N wild-type males. These mice were tested in select assays for anxiety-like behavior, motor dysfunction, social behavior, sensorimotor gating and learning and memory (see Supplementary Material, Methods). We found that 129.FVBF1 Mecp2+/− mice had exactly the same phenotypes as FVB.129F1 Mecp2+/− mice (Supplementary Material, Fig. S6).
Breathing abnormalities are detectable in young Mecp2+/− mice
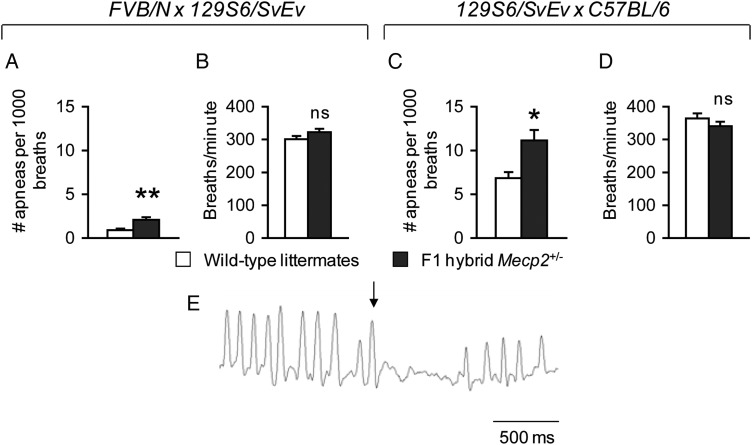
Breathing irregularities are a key feature of typical RTT (52,53), and several studies have demonstrated breathing abnormalities in male mice lacking MeCP2 (22,23,54–56). Four reports of formal breathing measurements have documented abnormalities in female Mecp2+/− mice that were older than 2.6 months of life (9,22,23,25). To determine whether breathing problems were present in younger mice, we examined the number of apneas and the respiratory rate in Mecp2+/− mice of both F1 hybrid genetic backgrounds at ∼7 weeks of life. Classification of apneas as breaths which had an expiratory phase greater than 1 s (25) did not identify a substantial number of apneas in either wild-type or Mecp2+/− mice (data not shown). In contrast, using a method in which apneic breaths were defined by the degree to which they exceeded both global and local average exhalation time, we found that Mecp2+/− mice compared with wild-type littermates had a greater number of apneas per 1000 breaths [FVB.129F1, F(1,49) = 12.91, P < 0.001; 129.B6F1, F(1,34) = 7.42, P < 0.05] (Fig. 5A and C) but showed normal respiration (Fig. 5B and D). A breathing trace of a representative apnea is shown in Fig. 5E. These findings demonstrate that disordered breathing in Mecp2+/− mice is apparent at younger ages than previously reported.
Figure 5.
Mecp2+/− mice show breathing abnormalities at 7 weeks of life. FVB.129F1 Mecp2+/− mice of both F1 hybrid backgrounds show an increase in the number of apneas per breath (A and C), but a normal breathing rate (B and D). (E) A representative breathing trace displaying an apnea is shown, with the arrow indicating the breath preceding the occurrence of an apnea. *P < 0.05; **P < 0.001; ns, not significant.
Increased weight gain in female Mecp2+/− mice is influenced by strain background and correlates with MeCP2 levels
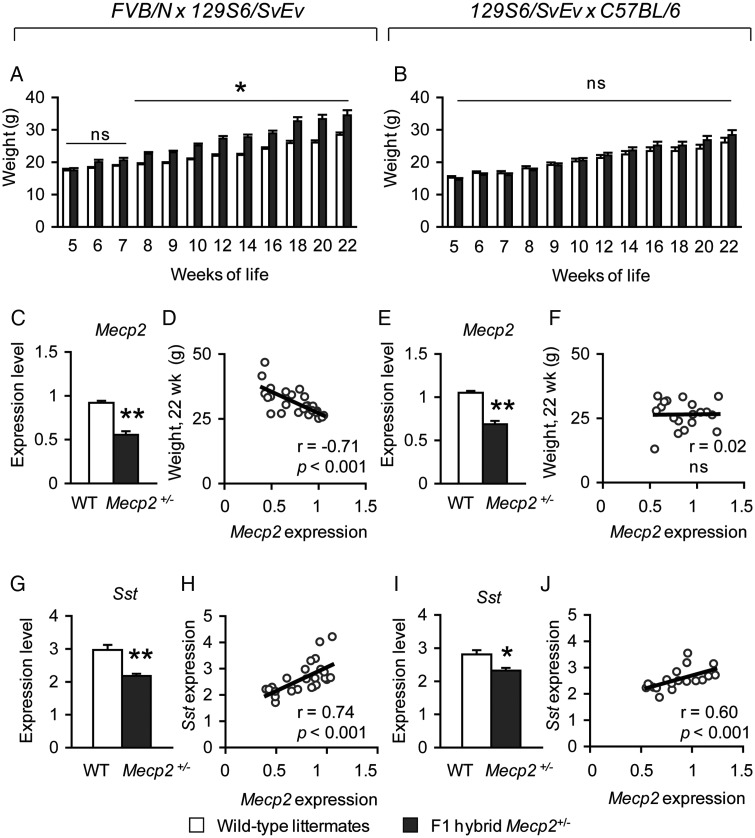
Although typical RTT individuals have reduced weight gain, some disease-causing MECP2 mutations have been reported in females with increased weight (48,57,58). Work in MeCP2 conditional knock-out mouse models have demonstrated that the loss of MeCP2 in hypothalamic Sim1-positive neurons causes abnormal weight gain (59). Abnormal weight in MeCP2 mouse models also appears to be strongly influenced by genetic strain background. Specifically, male Mecp2-null (9) and -mutant animals (29) of a pure C57BL/6 are lighter, whereas male animals harboring the same Mecp2 alleles on other genetic strain backgrounds are overweight (56). Because work in MeCP2 mouse models have underscored the role of MeCP2 in the homeostatic regulation of this particular phenotype, we monitored the weight of FVB.129F1 and 129.B6F1 Mecp2+/− animals from 5 weeks of life through 22 weeks of life. We found that FVB.129F1 Mecp2+/− animals are heavier starting at 8 weeks of life through 22 weeks of life [F(8,192) = 2.86, P < 0.001] (Fig. 6A); in contrast, 129.B6F1 Mecp2+/− animals do not show abnormal weight throughout this period (Fig. 6B). However, 129.B6F1 Mecp2+/− mice at 52 weeks of life are indeed overweight [Mecp2+/− = 49.9 g ± 2.8 g, wild-type = 35.0 g ± 2.0; F(1,22) = 17.35, P < 0.001].
Figure 6.
Early weight gain is observed in FVB.129F1 Mecp2+/− mice and negatively correlates with the level of MeCP2 expression. FVB.129F1 Mecp2+/− mice are heavier compared with wild-type littermates starting at 8 weeks and through 22 weeks of life (A), whereas weight is normal in F1 hybrid 129.B6 Mecp2+/− mice compared with wild-type littermates during this period (B). Hypothalamic Mecp2 levels of both wild-type and Mecp2+/− mice for each F1 hybrid genetic background is shown; although significantly decreased in female Mecp2+/− mice, some variation in the levels is observed (C and E). The levels of hypothalamic Mecp2 are negatively correlated with weight in FVB.129F1 (D), but not 129.B6F1 Mecp2+/− mice (F). Sst expression is down-regulated in Mecp2+/− mice (G and I). The levels of Sst positively correlate with the levels of Mecp2 in the hypothalamus from both F1 hybrid lines (H and J). *P < 0.05; **P < 0.001; ns, not significant.
Previous work demonstrated a correlation between non-random XCI and abnormal phenotypes in female mice harboring a Mecp2-mutant allele (60), raising the question of whether a similar correlation exists in females harboring an Mecp2-null allele. Among the phenotypes tested in this study, weight gain is one physiological parameter that is clearly sensitive to MeCP2 dysfunction in the hypothalamus (59). Therefore, we asked whether hypothalamic Mecp2 expression levels influenced weight. Mecp2 levels in the hypothalami of F1 hybrid Mecp2+/− animals compared with their respective wild-type littermates were reduced as expected (Fig. 6C and E). Due to variation in XCI patterns, the distribution of Mecp2 levels ranged from ∼40 to ∼85% of wild-type levels in FVB.129F1 Mecp2+/− mice (Fig. 6C) and ∼55 to ∼85% of wild-type levels in 129.B6F1 Mecp2+/− mice (Fig. 6E). Examination of Mecp2 levels and weight together revealed that increased weight gain negatively correlated with Mecp2 levels in FVB.129F1 mice (Fig. 6D), but not in 129.B6F1 mice that are not overweight at this time point (Fig. 6F). We also examined the expression levels of Somatostatin, a gene previously identified as being sensitive to MeCP2 in the hypothalamus (5,61), and known to play a role in body weight homeostasis (62). Similar to the observation in male Mecp2-null mice, we found that Sst was reduced in Mecp2+/− mice (Fig. 6G and I). In addition, Sst levels positively correlated with Mecp2 levels in both genetic backgrounds (Fig. 6H and J). Together, these data suggest that MeCP2 influences only weight in female mice when the mutant allele is present on an FVB.129F1 background.
DISCUSSION
Women and girls with typical RTT suffer from several neurological and physiological problems that occur early in life and persist throughout adulthood. Studies using MeCP2 mouse models have focused on the absence of normal MeCP2 function in male mice, avoiding the confounding effects of variable XCI in female mice, which is also documented as a cause of phenotypic variation in RTT (63,64). Although we did indeed find variable MeCP2 expression in Mecp2+/− mice, our data suggest that while variation in XCI patterns modulates phenotypes and gene expression changes, they are not sufficient to compromise their penetrance. In this study, we uncovered multiple robust and reproducible phenotypes in female FVB.129F1 and 129.B6F1 Mecp2+/− mice (Supplementary Material, Table S1). It is noteworthy that many of these findings were observed at young ages, and occurred much earlier than previously reported in some cases, such as breathing abnormalities (9,22,23,25).
Animal models of neurological disorders serve as one of the most valuable tools for the discovery of potential therapies. An important step toward the end goal of ameliorating disease phenotypes in affected individuals is the design and successful implementation of pre-clinical trials in such animal models. Surprisingly, very little is known about the onset and progression of several neurobehavioral deficits in female Mecp2+/− mice. The existing data on female Mecp2tm1.1Bird (Mecp2+/−) mice are sparse, with only eight studies documenting phenotypic defects such as poor health, breathing abnormalities, motor dysfunction and defects in early postnatal neurological reflexes (Supplementary Material, Table S2). By showing that female Mecp2tm1.1Bird mice (Mecp2+/−) have excellent face validity for several neurobehavioral features present in typical RTT, we demonstrate a number of quantifiable, independent phenotypes that can be used as outcome measures in pre-clinical trials. We also show that some phenotypes are not reproducible across the two genetic backgrounds studied, or are only reproducible at certain time points, suggesting that the phenotypes used as readouts and the genetic background of animals studied in future pre-clinical trials should be carefully selected. It is noteworthy that, for a select number of assays, we observed similar results in both FVB.129F1 and 129.FVBF1 mice, indicating that maternal genotype does not affect these particular phenotypes for this Mecp2 allele.
Among the robust and reproducible phenotypes we identified, we found that F1 hybrid Mecp2+/− mice displayed normal motor function at a young age, and motor deficits in adulthood, which is one of the most consistently reported phenotypes in Mecp2 loss-of-function models (20,21). The progressive decline in motor function is a hallmark of typical RTT and is faithfully reproduced in the mouse; thus, it can likely be used as one primary outcome measure in pre-clinical trials. In contrast, decreased ASR and enhanced PPI are two examples of outcome measures that may be less ideal for pre-clinical studies. FVB.129F1 Mecp2+/− mice displayed these phenotypic abnormalities during early and later stages of life, whereas 129.B6F1 Mecp2+/− mice showed these defects at only a late-stage time point. Although decreased acoustic startle and enhanced PPI are present across the two genetic background strains studied, more work is needed to determine whether these readouts are sufficiently robust for use in pre-clinical work.
In regards to social behavior abnormalities in RTT, some studies suggest that the social behavior deficits identified in some RTT cases improve with age (65), yet recent evidence shows that some impairments persist throughout adulthood (43). Our work demonstrates altered social behavior in female Mecp2+/− mice, but the interpretation of social behavior data is complex and warrants some discussion. The three-chamber data suggest that only 129.B6F1 Mecp2+/− mice display an impairment at 12 weeks of life, as these animals showed no difference in the time spent with either the novel mouse or object, and that 12- and 22-week-old FVB.129F1, and 12-week-old 129.FVBF1 Mecp2+/− mice are social because they spend more time investigating a novel mouse compared with a novel object. An additional interpretation of the three-chamber data, however, suggests a relative reduction in social approach behavior in Mecp2+/− mice of these two reciprocal F1 isogenic strains, given that the time mutants spent sniffing/investigating novel mice is reduced compared with wild-type mice. This interpretation is reliable for 12-week-old FVB.129F1 and 129.FVBF1 F1 Mecp2+/− mice, but less so for 129.B6F1 Mecp2+/− mice. 129.B6F1 Mecp2+/− mice at 12 weeks do not show a relative reduction in the time spent investigating novel mice compared with wild-type littermates, and at 22 weeks these animals have reduced motor activity in the apparatus which makes the three-chamber data for these mice at this later time point difficult to interpret. Therefore, we caution investigators to take into consideration the disease progression of Mecp2+/− female mice of different strain backgrounds in the analysis of social behavior phenotypes. Of note, unlike some studies that have reported a correlation between the time spent in the chamber, and the time spent investigating/sniffing either a novel mouse or object (66), we did not observe such a positive correlation in any of our F1 hybrid lines.
Other phenotypes uncovered in our work that were not previously reported in female Mecp2+/− mice include decreased reaction to conductive heat, impaired contextual fear memory and a deficit in passive avoidance learning. Decreased reaction to conductive heat in Mecp2+/− mice is consistent with the findings in male mice with a 50% reduction in MeCP2 (42). Although the relationship between MeCP2 function and pain sensitivity is unclear, it is known that RTT individuals have an abnormal pain response (67). Further work on the regulation of pain sensitivity in animals that lack MeCP2 is needed to understand the etiology of nociceptive defects in RTT.
The deficit observed in contextual fear memory is consistent with a previous study of male mice lacking MeCP2 (12), and male Mecp2tm1Hzo mice; however, passive avoidance learning has not been previously performed in any MeCP2 mouse model. These data suggest a defect in hippocampal-dependent memory, consistent with the finding of synaptic plasticity defects in Mecp2+/− mice (68). Together, these new findings in Mecp2+/− mice contribute to the list of phenotypes that may be used as outcome measures when implementing pre-clinical studies.
Finally, to complement our neurobehavioral studies, we monitored physiological parameters such as breathing and weight. Abnormal respiration is a hallmark of typical RTT, and several studies have shown an increase in apneas in male Mecp2−/y and female Mecp2+/− mice (69). Consistent with previous reports of increased apneas in older Mecp2+/− mice (22,25), we now show that young F1 hybrid Mecp2+/− mice display a similar breathing abnormality. These findings demonstrate the reproducibility of this specific phenotype at a younger age and in different genetic backgrounds, highlighting the reliability of increased apneas in Mecp2+/− mice as a useful outcome measure. In terms of weight, we found increased weight in FVB.129F1, but not 129.B6F1 Mecp2+/− mice at 22 weeks of life, suggesting that weight gain at this time point may not be an appropriate outcome measure in pre-clinical studies. Obesity has been reported in the preserved speech variant of RTT and other individuals that carry a disease-causing MECP2 mutation but do not meet the criteria for typical RTT (48,57). In the MeCP2 loss-of-function mouse model, abnormal weight gain is heavily influenced by genetic background (9), and our work demonstrates that the C57BL/6 background likely modifies weight gain in young female Mecp2+/− mice. To determine whether XCI patterns vary in adult female Mecp2+/− mice, we examined the expression of Mecp2 transcript in a specific brain region known to control weight gain (59). Instead of the expected 50% of wild-type levels, we found Mecp2 levels to vary from ∼40 to 85% in Mecp2+/− mice. Interestingly, of the two genetic backgrounds, we found a negative correlation between Mecp2 expression and increased weight gain in FVB.129F1 animals, and a positive correlation between Mecp2 expression and the expression of the neuropetide Sst in the hypothalamus. Additional correlation analyses of Mecp2 expression in brain regions related to specific phenotypic abnormalities in Mecp2+/− mice will provide further insight into how variable Mecp2 expression influenced by XCI patterns affects disease severity.
Genetic background effects were observed in both the onset of phenotypes, such as impaired social behavior, enhanced PPI and increased weight gain, and the manifestation of phenotypes, such as reduced corticosterone levels in response to stress. Pinpointing such genetic background differences will aid investigators in choosing the proper genetic background when evaluating new therapies that may impact specific phenotypes. In addition, the finding that female Mecp2+/− mice of two F1 hybrid backgrounds are less anxious, which is in contrast to the heightened anxiety in RTT individuals, underscores the notion that ablation of normal MeCP2 function in the mouse may reproduce many, but not necessarily all, of the features present in the human disorder. Decreased anxiety-like behavior was documented in male mice expressing either a constitutive Mecp2-null allele (12) or a hypomorphic Mecp2Flox allele (42), and in male and female mice that harbor the Mecp2tm1.1Jae allele (29). The fact that we found reduced anxiety-like behavior in Mecp2+/− mice of two F1 hybrid backgrounds at 5 weeks of life is in agreement with these studies and could be attributed to reduced signaling of components in the hypothalamic-pituitary axis, as Corticosterone receptor hormone, the neuropeptide gene involved in anxiety, is reduced in Mecp2null/y animals (5).
In conclusion, Mecp2+/− mice recapitulate multiple features observed in typical RTT. Our study is the first to provide a comprehensive overview of neurobehavioral and physiological phenotypes of female Mecp2tm1.1Bird mice of two different genetic backgrounds. It is also the first report to examine some of these phenotypes over time. Our findings suggest the phenotypic defects consistently observed across these two different genetic backgrounds may serve as the strongest readouts for pre-clinical studies. Previous studies that used specific compounds to improve phenotypes in Mecp2tm1.1Bird mice relied on a limited number of phenotypes (25,70). As we plan to translate studies from mouse models to human patients, it is important to identify compounds that affect as many of the RTT-like phenotypes as possible in pre-clinical studies. Furthermore, establishing that such phenotypes can be improved on more than one genetic background will increase the likelihood of success in the highly heterogeneous clinical population.
MATERIALS AND METHODS
Animal husbandry
Mice were maintained on a 12 h light:12 h dark cycle with standard mouse chow and water ad libitum. F1 hybrid FVB/N × 129S6/SvEv (FVB.129F1) animals were generated by mating female Mecp2+/− mice of a pure FVB/N background to wild-type male mice of a pure 129SvEv background (Taconic Farms, Inc., Germantown, NY, USA), and F1 hybrid 129S6/SvEv × C57BL/6 (129.B6F1) animals were generated by mating female Mecp2+/− mice of a pure 129S6/SvEv background to wild-type male mice of a pure C57BL/6 background (Jackson Laboratories, Bar Harbor, ME, USA). The Mecp2 allele used in this study was the Mecp2tm1.1Bird allele, which lacks exons 3 and 4, and generates a true Mecp2-null allele (9). Animals were housed four to five animals per cage, and were weighed weekly starting at 5 weeks of life through 22 weeks of life. All research and animal care procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Test cohorts
A total of 232 animals separated into five independent test cohorts were used for neurobehavioral and physiological studies. The first test cohort consisted of 46 animals (n = 13 FVB.129F1 Mecp2+/−, n = 14 wild-type littermates, n = 10 129.B6F1 Mecp2+/− and n = 9 wild-type littermates) that were tested with the following assays at the indicated time points: elevated plus maze, 5 weeks; light–dark box exploration task, 6 weeks; open field, 7 weeks; ASR and PPI of ASR, 7 weeks; fear conditioning, 8 weeks; open field, 17 weeks; grid-walking/footslip, 17 weeks; ASR and PPI, 20 weeks; three chamber, 22 weeks; and hot plate, 22 weeks. One FVB.129F1 Mecp2+/− mouse died unexpectedly at 8 weeks of life. The second test cohort consisted of 46 animals (n = 12 FVB.129F1 Mecp2+/−, n = 14 wild-type littermates and n = 10 129.B6 Mecp2+/− and n = 10 wild-type littermates) that were tested with the following assays at the indicated time points: ASR, 7 weeks; three chamber, 12 weeks; olfaction, 12 weeks; and passive avoidance learning, 14 weeks. A subset of these animals (n = 5 per genotype per strain background) were used in foot shock control tests at the end of 14 weeks of life. The third test cohort consisted of 85 animals that were tested for breathing abnormalities at 7 weeks (n = 25 FVB.129F1 Mecp2+/− and n = 25 wild-type littermates, and n = 21 129.B6 Mecp2+/− and n = 14 wild-type littermates). The fourth test cohort consisted of 23 129.B6 animals tested for ASR and weight at 52 weeks of life (n = 13 129.B6 Mecp2+/− and n = 10 wild-type littermates). The fifth test cohort consisted of 12 animals used for baseline serum corticosterone measurements (n = 3 per genotype per strain background), and 20 animals used for stress-induced serum corticosterone measurements (n = 5 per genotype per strain background).
Neurobehavioral assays
The majority of neurobehavioral assays were performed as previously described (28,41,42,59,71,72), with slight modifications in some cases.
Elevated plus maze
Animals were habituated to the test room (150 lux, 60 dB white noise) for 30 min. After the habituation period, animals were placed in the center of a maze consisting of two arms (each arm 25 × 7.5 cm) enclosed by approximately 15 cm high walls, and two open arms (each arm 25 × 7.5 cm, with a raised 0.5 cm lip around the edges) elevated 50 cm above ground level; the arms of the maze were equidistant from the center platform. The amount of time animals spent in the open arms, the number of arm entries and the total distance traveled were recorded for 10 min using a camera and detection software (ANY-maze video tracking system, Stoelting Co., Wood Dale, IL, USA).
Light–dark box exploration task
Animals were habituated to the test room (150 lux, 60 dB white noise) for 30 min. After the habituation period, animals were placed in the lit side (36 × 20 × 26 cm) of a chamber separated by dark side, which was a covered black plastic chamber (15.5 × 20 × 26 cm) containing a 10.5 × 5 cm opening. The amount of time animals spent in the lit side and the number of transitions were recorded for 10 min using a handheld computer (Psion Workabout mx, Psion Teklogix, Inc., Hebron, KY, USA); data acquisition was performed using the Observer program (Noldus Information Technologies, Leesburg, VA, USA).
Open field
Animals were habituated to the test room (150 lux, 60 dB white noise) for 30 min. After the habituation period, animals were placed in the center of a 40 × 40 × 30-cm chamber equipped with photobeams (Accuscan, Columbus, OH, USA) to record activity during a 30 min test period.
Three-chamber test for sociability
Novel partner mice were placed within a small wire cage as previously described (28,41) to habituate them to their test environment, these animals were placed randomly in either the left or right test chamber for ∼30 min to 1 h per day for at least 2 consecutive days prior to the actual test day. On the day of testing, test animals were first habituated to the test room for 30 min (150 lux, 60 dB). A test for side preference was then performed by placing the test animals in the center of the three-chamber apparatus. The entries to either the left or right chambers were unobstructed during the side preference test, allowing animals to freely explore and spend time in all three chambers. The time spent in each chamber was measured for 10 min. Next, during the social approach test (novel mouse versus object), the amount of time test animals spent in all three chambers and interacting with either a novel mouse or object was measured. Novel partner mice were randomly assigned in either the left or right chamber to avoid the potential of a side bias; this was designed in a manner to expose half of each test subject genotype (Mecp2+/− mice or wild-type littermates) to a novel mouse in the left chamber, and the remaining half of each test subject genotype to a novel mouse in the right chamber.
Olfaction
A test for olfaction was performed as previously described (41). Animals were habituated to the test room (ambient lighting) for 30 min. After the habituation period, animals were individually placed into standard housing cages. A cotton swab was presented to the animals through the cage top; animals were allowed to habituate to this environment for at least 20 min. The cotton swab was then replaced with a new cotton swab with either 60 µl of vanilla or water placed on the tip. The amount of time animals spent sniffing the cotton swab was measured for 2 min. Following an inter-trial interval (5 min), the swab was replaced with another cotton swab with the opposite scent used in the first trial. The amount of time animals spent sniffing the cotton swab during both trials was recorded for 2 min using a handheld computer (Psion Workabout mx, Psion Teklogix Inc., Hebron, KY, USA); data acquisition was performed using the Observer program (Noldus Information Technologies, Leesburg, VA, USA). Odors were presented in random order (vanilla then water, or water then vanilla) to ensure that the olfaction testing was specific to the vanilla-scented swab.
Grid-walking/footslip test
Animals were habituated to the test room (ambient lighting) for 30 min. After the habituation period, animals were individually placed into the center of a wire grid laid within an open-field chamber (Accuscan, Columbus, OH, USA). The number of both fore- and hindpaw slips through the wire grid and the total distance traveled were recorded for 5 min. The data shown are the number of slips normalized to the total distance traveled.
ASR and PPI of ASR
The ASR and PPI of the ASR were measured as previously described (41,42). To determine ASR to sound ranging from 78 to 118 dB, a total of 13 sounds (0 and 78–118 dB in 4 dB increments) were randomly presented, and activity was recorded as previously described using a startle chamber for mice (SR-Lab, San Diego Instruments, San Diego, CA, USA) (41,42).
Hot plate
Animals were placed on a metal surface heated to 55°C (Hot-Plate Analgesia Meter, Columbus Instruments, Columbus, OH, USA). The latency to respond was recorded as previously described (42).
Pavlovian fear conditioning
Contextual and cued fear conditioning was performed as previously described with a shock intensity level of 0.7 mA (41,42). Animals are first exposed to two successive rounds of a conditioned stimulus-unconditioned stimulus pair (80 dB sound, followed by a mild foot shock), then are tested 24 h later for both contextual memory, which is hippocampal dependent, and cued memory, which is hippocampal and amygdalar dependent. Footshock control tests were performed as previously described (73).
Passive avoidance learning
Passive avoidance learning, a similar learning and memory task that involves contributions from both the hippocampus and amygdala, was performed using a two-day assay as previously described (74). In this task, animals placed in a brightly lit side of a two compartment chamber receive a mild foot shock upon entering the dark compartment (74). Animals are returned to the chamber 24 h later and tested to determine whether they successfully remembered to associate the shock with the dark compartment. For animals that remained in the lit side on the second day of testing, activity was manually recorded. The number of approaches toward the dark compartment was scored, and freezing behavior indicated by the complete lack of movement was scored every 30 s for the duration of the test.
Corticosterone studies
Serum corticosterone levels were determined as described previously (71). Briefly, basal corticosterone levels were obtained from animals that were undisturbed for at least 12 h. Stress-induced corticosterone levels were obtained from animals restrained in 50 ml conical tubes for 30 min. For both test measurements, animals were rapidly decapitated after the indicated time period. Trunk blood was collected and placed in 1.5 ml conical tubes on ice for at least 30 min. Blood was centrifuged at a maximum speed for 10 min. Serum was collected and analyzed using an enzyme-linked immunoassay (IDS, Inc., Scottsdale, AZ, USA).
Unrestrained whole-body plethysmography and data analysis
Mice were allowed to acclimate for 20 min in unrestrained whole-body plethysmography chambers (Buxco, Wilmington, NC, USA) with a continuous flow of fresh air as described previously (56), and baseline breathing was then recorded for 30 min. Breath waveforms were captured using Biosystem XA software (Buxco, USA) and processed in Matlab (Mathworks, Natick, MA, USA). Apneic breaths were defined for each mouse as breaths whose TE (exhalation time) exceeded both the global average TE for the recording period by 3-fold and the local average TE (5-breath window, centered on the breath) by 2-fold.
Statistical analysis of behavioral and physiological data
Statistical analysis of data was performed using SPSS (version 19). Data from elevated plus maze, light–dark box exploration, open field, gridwalking/footslip, acoustic startle, PPI, hot plate, fear conditioning and breathing were analyzed using a one-way analysis of variance (ANOVA) with genotype as a factor. Weight was analyzed using a one-way ANOVA with repeated measures. Data from passive avoidance learning were analyzed using Mann–Whitney U non-parametric tests. Data related to the three-chamber test were analyzed using a one-way ANOVA with genotype as a factor to first determine within-genotype differences, then to determine between-genotype differences.
RNA, cDNA synthesis and quantitative real-time reverse transcriptase (QRT-PCR)
Total RNA using Trizol (Invitrogen, Carlsbad, CA, USA) was extracted from the hypothalami of FVB.129F1 and 129.B6F1 Mecp2+/− mice and wild-type littermates of both genetic backgrounds (Test cohort 1, at the end of 22 weeks of life: FVB.129F1 Mecp2+/− mice, n = 12 and wild-type littermates, n = 14; 129.B6F1 Mecp2+/− mice, n = 10 and wild-type littermates, n = 9), and column purified using RNeasy mini kits (Qiagen, Valencia, CA, USA). Three micrograms of RNA was used to synthesize cDNA according to the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA). QRT-PCR was performed as previously described using PerfeCTa qPCR FastMix (Quanta Biosciences, Inc., Gaithersburg, MD, USA) (72). Mecp2 expression levels were quantified using previously published primers (42). Sst levels were quantified using the following primers: forward, 5′-CCCAGACTCCGTCAGTTTCT-3′, reverse, 5′-GAAGTTCTTGCAGCCAGCTT-3′. Expression levels were normalized to S16. Significant differences were determined using paired t-tests. Correlation analyses were performed using Matlab (Mathworks, Natick, MA, USA).
AUTHORS’ CONTRIBUTION
R.C.S. and H.Y.Z. designed research; R.C.S. performed majority of research; R.C.S. and C.M.M. analyzed data; C.M.M., and C.S.W. performed the breathing studies and analyzed breathing data; Y.S. provided technical expertise; J.L.N. provided intellectual contribution; R.C.S. and H.Y.Z. wrote the manuscript. All authors reviewed the data and edited the manuscript.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by the U.S. National Institutes of Health [grants NS043124 (R.C.S.), NS073317 (C.M.M.), NS066601 (C.S.W.), NS052240 (J.L.N.), HD062553 (J.L.N.), NS057819 (H.Y.Z.), HD024064 (H.Y.Z.)], Baylor College of Medicine Intellectual and Developmental Disabilities Research Center, IDDRC), the International Rett Syndrome Foundation (J.L.N.), the Rett Syndrome Research Trust and the Carl C. Anderson, Sr. and Marie Jo Anderson Charitable Foundation (H.Y.Z.). J.L.N. is the Cynthia and Anthony G. Petrello Endowed Scholar of the Jan and Dan Duncan Neurological Research Institute. H.Y.Z. is a Howard Hughes Medical Institute investiagtor. Funding to pay the Open Access publication charges for this article was provided by The Howard Hughes Medical Institute.
Supplementary Material
ACKNOWLEDGEMENTS
We thank R. James Cotton for helpful discussion of breathing data analysis, Drs Corinne Spencer and Richard Paylor for advice on neurobehavioral experiments and the IDDRC for the use of the Neurobehavioral Core Facility.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Rett A. [On a unusual brain atrophy syndrome in hyperammonemia in childhood] Wien Med Wochenschr. 1966;116:723–726. [PubMed] [Google Scholar]
- 2.Hagberg B. Rett's syndrome: prevalence and impact on progressive severe mental retardation in girls. Acta Paediatr. Scand. 1985;74:405–408. doi: 10.1111/j.1651-2227.1985.tb10993.x. doi:10.1111/j.1651-2227.1985.tb10993.x. [DOI] [PubMed] [Google Scholar]
- 3.Amir R.E., Van den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. doi:10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 4.Yasui D.H., Peddada S., Bieda M.C., Vallero R.O., Hogart A., Nagarajan R.P., Thatcher K.N., Farnham P.J., Lasalle J.M. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. Proc. Natl Acad. Sci. USA. 2007;104:19416–19421. doi: 10.1073/pnas.0707442104. doi:10.1073/pnas.0707442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chahrour M., Jung S.Y., Shaw C., Zhou X., Wong S.T.C., Qin J., Zoghbi H.Y. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. doi:10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skene P.J., Illingworth R.S., Webb S., Kerr A.R.W., James K.D., Turner D.J., Andrews R., Bird A.P. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol. Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. doi:10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tate P., Skarnes W., Bird A. The methyl-CpG binding protein MeCP2 is essential for embryonic development in the mouse. Nat. Genet. 1996;12:205–208. doi: 10.1038/ng0296-205. doi:10.1038/ng0296-205. [DOI] [PubMed] [Google Scholar]
- 8.Chen R.Z., Akbarian S., Tudor M., Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 2001;27:327–331. doi: 10.1038/85906. doi:10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 9.Guy J., Hendrich B., Holmes M., Martin J.E., Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 2001;27:322–326. doi: 10.1038/85899. doi:10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 10.Shahbazian M., Young J., Yuva-Paylor L., Spencer C., Antalffy B., Noebels J., Armstrong D., Paylor R., Zoghbi H. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. doi:10.1016/S0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 11.Collins A.L., Levenson J.M., Vilaythong A.P., Richman R., Armstrong D.L., Noebels J.L., David Sweatt J., Zoghbi H.Y. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum. Mol. Genet. 2004;13:2679–2689. doi: 10.1093/hmg/ddh282. doi:10.1093/hmg/ddh282. [DOI] [PubMed] [Google Scholar]
- 12.Pelka G.J., Watson C.M., Radziewic T., Hayward M., Lahooti H., Christodoulou J., Tam P.P.L. Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain. 2006;129:887–898. doi: 10.1093/brain/awl022. doi:10.1093/brain/awl022. [DOI] [PubMed] [Google Scholar]
- 13.Lawson-Yuen A., Liu D., Han L., Jiang Z.I., Tsai G.E., Basu A.C., Picker J., Feng J., Coyle J.T. Ube3a mRNA and protein expression are not decreased in Mecp2R168X mutant mice. Brain Res. 2007;1180:1–6. doi: 10.1016/j.brainres.2007.08.039. doi:10.1016/j.brainres.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao J., Hu K., Chang Q., Wu H., Sherman N.E., Martinowich K., Klose R.J., Schanen C., Jaenisch R., Wang W., et al. Phosphorylation of MeCP2 at Serine 80 regulates its chromatin association and neurological function. Proc. Natl Acad. Sci. USA. 2009;106:4882–4887. doi: 10.1073/pnas.0811648106. doi:10.1073/pnas.0811648106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jentarra G.M., Olfers S.L., Rice S.G., Srivastava N., Homanics G.E., Blue M., Naidu S., Narayanan V. Abnormalities of cell packing density and dendritic complexity in the MeCP2 A140V mouse model of Rett syndrome/X-linked mental retardation. BMC Neurosci. 2010;11:19. doi: 10.1186/1471-2202-11-19. doi:10.1186/1471-2202-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brendel C., Belakhov V., Werner H., Wegener E., Gärtner J., Nudelman I., Baasov T., Huppke P. Readthrough of nonsense mutations in Rett syndrome: evaluation of novel aminoglycosides and generation of a new mouse model. J. Mol. Med. 2011;89:389–398. doi: 10.1007/s00109-010-0704-4. doi:10.1007/s00109-010-0704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadalla K.K.E., Bailey M.E.S., Cobb S.R. MeCP2 and Rett syndrome: reversibility and potential avenues for therapy. Biochem. J. 2011;439:1–14. doi: 10.1042/BJ20110648. doi:10.1042/BJ20110648. [DOI] [PubMed] [Google Scholar]
- 18.Samaco R.C., Neul J.L. Complexities of Rett syndrome and MeCP2. J. Neurosci. 2011;31:7951–7959. doi: 10.1523/JNEUROSCI.0169-11.2011. doi:10.1523/JNEUROSCI.0169-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guy J., Gan J., Selfridge J., Cobb S., Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. doi:10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos M., Silva-Fernandes A., Oliveira P., Sousa N., Maciel P. Evidence for abnormal early development in a mouse model of Rett syndrome. Genes Brain Behav. 2007;6:277–286. doi: 10.1111/j.1601-183X.2006.00258.x. doi:10.1111/j.1601-183X.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- 21.Jugloff D.G.M., Vandamme K., Logan R., Visanji N.P., Brotchie J.M., Eubanks J.H. Targeted delivery of an Mecp2 transgene to forebrain neurons improves the behavior of female Mecp2-deficient mice. Hum. Mol. Genet. 2008;17:1386–1396. doi: 10.1093/hmg/ddn026. doi:10.1093/hmg/ddn026. [DOI] [PubMed] [Google Scholar]
- 22.Bissonnette J.M., Knopp S.J. Separate respiratory phenotypes in methyl-CpG-binding protein 2 (Mecp2) deficient mice. Pediatr. Res. 2006;59:513–518. doi: 10.1203/01.pdr.0000203157.31924.4a. doi:10.1203/01.pdr.0000203157.31924.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bissonnette J.M., Knopp S.J. Effect of inspired oxygen on periodic breathing in methy-CpG-binding protein 2 (Mecp2) deficient mice. J. Appl. Physiol. 2008;104:198–204. doi: 10.1152/japplphysiol.00843.2007. doi:10.1152/japplphysiol.00843.2007. [DOI] [PubMed] [Google Scholar]
- 24.Bissonnette J.M., Knopp S.J., Maylie J., Thong T. Autonomic cardiovascular control in methyl-CpG-binding protein 2 (Mecp2) deficient mice. Auton. Neurosci. 2007;136:82–89. doi: 10.1016/j.autneu.2007.04.007. doi:10.1016/j.autneu.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdala A.P.L., Dutschmann M., Bissonnette J.M., Paton J.F.R. Correction of respiratory disorders in a mouse model of Rett syndrome. Proc. Natl Acad. Sci. USA. 2010;107:18208–18213. doi: 10.1073/pnas.1012104107. doi:10.1073/pnas.1012104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mutant mice and neuroscience: recommendations concerning genetic background. Banbury conference on genetic background in mice. Neuron. 1997;19:755–759. doi: 10.1016/s0896-6273(00)80958-7. doi:10.1016/S0896-6273(00)80958-7. [DOI] [PubMed] [Google Scholar]
- 27.Wolfer D.P., Lipp H.P. Dissecting the behaviour of transgenic mice: is it the mutation, the genetic background, or the environment? Exp. Physiol. 2000;85:627–634. [PubMed] [Google Scholar]
- 28.Samaco R.C., Mandel-Brehm C., McGraw C.M., Shaw C.A., McGill B.E., Zoghbi H.Y. Crh and Oprm1 mediate anxiety-related behavior and social approach in a mouse model of MECP2 duplication syndrome. Nat. Genet. 2012;44:206–211. doi: 10.1038/ng.1066. doi:10.1038/ng.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stearns N.A., Schaevitz L.R., Bowling H., Nag N., Berger U.V., Berger-Sweeney J. Behavioral and anatomical abnormalities in Mecp2 mutant mice: a model for Rett syndrome. Neuroscience. 2007;146:907–921. doi: 10.1016/j.neuroscience.2007.02.009. doi:10.1016/j.neuroscience.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Lonetti G., Angelucci A., Morando L., Boggio E.M., Giustetto M., Pizzorusso T. Early environmental enrichment moderates the behavioral and synaptic phenotype of MeCP2 null mice. Biol. Psychiatry. 2010;67:657–665. doi: 10.1016/j.biopsych.2009.12.022. doi:10.1016/j.biopsych.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 31.Crawley J.N., Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm. Behav. 1997;31:197–211. doi: 10.1006/hbeh.1997.1382. doi:10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- 32.Cook M.N., Williams R.W., Flaherty L. Anxiety-related behaviors in the elevated zero-maze are affected by genetic factors and retinal degeneration. Behav. Neurosci. 2001;115:468–476. doi:10.1037/0735-7044.115.2.468. [PubMed] [Google Scholar]
- 33.Smith G.W., Aubry J.M., Dellu F., Contarino A., Bilezikjian L.M., Gold L.H., Chen R., Marchuk Y., Hauser C., Bentley C.A., et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. doi:10.1016/S0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 34.Brooks S.P., Dunnett S.B. Tests to assess motor phenotype in mice: a user's guide. Nat. Rev. Neurosci. 2009;10:519–529. doi: 10.1038/nrn2652. doi:10.1038/nrn2652. [DOI] [PubMed] [Google Scholar]
- 35.Frankland P.W., Wang Y., Rosner B., Shimizu T., Balleine B.W., Dykens E.M., Ornitz E.M., Silva A.J. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol. Psychiatry. 2004;9:417–425. doi: 10.1038/sj.mp.4001432. doi:10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- 36.Hessl D., Berry-Kravis E., Cordeiro L., Yuhas J., Ornitz E.M., Campbell A., Chruscinski E., Hervey C., Long J.M., Hagerman R.J. Prepulse inhibition in fragile X syndrome: feasibility, reliability, and implications for treatment. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009;150B:545–553. doi: 10.1002/ajmg.b.30858. doi:10.1002/ajmg.b.30858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perry W., Minassian A., Lopez B., Maron L., Lincoln A. Sensorimotor gating deficits in adults with autism. Biol. Psychiatry. 2007;61:482–486. doi: 10.1016/j.biopsych.2005.09.025. doi:10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 38.Braff D.L., Grillon C., Geyer M.A. Gating and habituation of the startle reflex in schizophrenic patients. Arch. Gen. Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. doi:10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- 39.Quednow B.B., Frommann I., Berning J., Kühn K.-U., Maier W., Wagner M. Impaired sensorimotor gating of the acoustic startle response in the prodrome of schizophrenia. Biol. Psychiatry. 2008;64:766–773. doi: 10.1016/j.biopsych.2008.04.019. doi:10.1016/j.biopsych.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 40.Moretti P., Bouwknecht J.A., Teague R., Paylor R., Zoghbi H.Y. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum. Mol. Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. doi:10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- 41.Chao H.-T., Chen H., Samaco R.C., Xue M., Chahrour M., Yoo J., Neul J.L., Gong S., Lu H.-C., Heintz N., et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. doi:10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samaco R.C., Fryer J.D., Ren J., Fyffe S., Chao H.-T., Sun Y., Greer J.J., Zoghbi H.Y., Neul J.L. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum. Mol. Genet. 2008;17:1718–1727. doi: 10.1093/hmg/ddn062. doi:10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaufmann W.E., Tierney E., Rohde C.A., Suarez-Pedraza M.C., Clarke M.A., Salorio C.F., Bibat G., Bukelis I., Naram D., Lanham D.C., et al. Social impairments in Rett syndrome: characteristics and relationship with clinical severity. J. Intellect. Disabil. Res. 2012;56:233–247. doi: 10.1111/j.1365-2788.2011.01404.x. doi:10.1111/j.1365-2788.2011.01404.x. [DOI] [PubMed] [Google Scholar]
- 44.Moy S.S., Nadler J.J., Perez A., Barbaro R.P., Johns J.M., Magnuson T.R., Piven J., Crawley J.N. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. doi:10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 45.Jamain S., Radyushkin K., Hammerschmidt K., Granon S., Boretius S., Varoqueaux F., Ramanantsoa N., Gallego J., Ronnenberg A., Winter D., et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl Acad. Sci. USA. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. doi:10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peça J., Feliciano C., Ting J.T., Wang W., Wells M.F., Venkatraman T.N., Lascola C.D., Fu Z., Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. doi:10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lam C.-W. Spectrum of mutations in the MECP2 gene in patients with infantile autism and Rett syndrome. J. Med. Genet. 2000;37:41e–e41. doi: 10.1136/jmg.37.12.e41. doi:10.1136/jmg.37.12.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zappella M., Meloni I., Longo I., Hayek G., Renieri A. Preserved speech variants of the Rett syndrome: molecular and clinical analysis. Am. J. Med. Genet. 2001;104:14–22. doi: 10.1002/ajmg.10005. doi:10.1002/ajmg.10005. [DOI] [PubMed] [Google Scholar]
- 49.Carney R.M., Wolpert C.M., Ravan S.A., Shahbazian M., Ashley-Koch A., Cuccaro M.L., Vance J.M., Pericak-Vance M.A. Identification of MeCP2 mutations in a series of females with autistic disorder. Pediatr. Neurol. 2003;28:205–211. doi: 10.1016/s0887-8994(02)00624-0. doi:10.1016/S0887-8994(02)00624-0. [DOI] [PubMed] [Google Scholar]
- 50.Huppke P., Maier E.M., Warnke A., Brendel C., Laccone F., Gärtner J. Very mild cases of Rett syndrome with skewed X inactivation. J. Med. Genet. 2006;43:814–816. doi: 10.1136/jmg.2006.042077. doi:10.1136/jmg.2006.042077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaevitz L.R., Moriuchi J.M., Nag N., Mellot T.J., Berger-Sweeney J. Cognitive and social functions and growth factors in a mouse model of Rett syndrome. Physiol. Behav. 2010;100:255–263. doi: 10.1016/j.physbeh.2009.12.025. doi:10.1016/j.physbeh.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 52.Julu P.O., Kerr A.M., Apartopoulos F., Al-Rawas S., Engerström I.W., Engerström L., Jamal G.A., Hansen S. Characterisation of breathing and associated central autonomic dysfunction in the Rett disorder. Arch. Dis. Child. 2001;85:29–37. doi: 10.1136/adc.85.1.29. doi:10.1136/adc.85.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weese-Mayer D.E., Lieske S.P., Boothby C.M., Kenny A.S., Bennett H.L., Silvestri J.M., Ramirez J.-M. Autonomic nervous system dysregulation: breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr. Res. 2006;60:443–449. doi: 10.1203/01.pdr.0000238302.84552.d0. doi:10.1203/01.pdr.0000238302.84552.d0. [DOI] [PubMed] [Google Scholar]
- 54.Viemari J.-C., Roux J.-C., Tryba A.K., Saywell V., Burnet H., Peña F., Zanella S., Bévengut M., Barthelemy-Requin M., Herzing L.B.K., et al. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J. Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. doi:10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Voituron N., Zanella S., Menuet C., Dutschmann M., Hilaire G. Early breathing defects after moderate hypoxia or hypercapnia in a mouse model of Rett syndrome. Respir. Physiol. Neurobiol. 2009;168:109–118. doi: 10.1016/j.resp.2009.05.013. doi:10.1016/j.resp.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 56.Ward C.S., Arvide E.M., Huang T.-W., Yoo J., Noebels J.L., Neul J.L. MeCP2 is critical within HoxB1-derived tissues of mice for normal lifespan. J. Neurosci. 2011;31:10359–10370. doi: 10.1523/JNEUROSCI.0057-11.2011. doi:10.1523/JNEUROSCI.0057-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kleefstra T., Yntema H.G., Oudakker A.R., Romein T., Sistermans E., Nillessen W., van Bokhoven H., de Vries B.B.A., Hamel B.C.J. De novo MECP2 frameshift mutation in a boy with moderate mental retardation, obesity and gynaecomastia. Clin. Genet. 2002;61:359–362. doi: 10.1034/j.1399-0004.2002.610507.x. doi:10.1034/j.1399-0004.2002.610507.x. [DOI] [PubMed] [Google Scholar]
- 58.Adegbola A.A., Gonzales M.L., Chess A., LaSalle J.M., Cox G.F. A novel hypomorphic MECP2 point mutation is associated with a neuropsychiatric phenotype. Hum. Genet. 2009;124:615–623. doi: 10.1007/s00439-008-0585-6. doi:10.1007/s00439-008-0585-6. [DOI] [PubMed] [Google Scholar]
- 59.Fyffe S.L., Neul J.L., Samaco R.C., Chao H.-T., Ben-Shachar S., Moretti P., McGill B.E., Goulding E.H., Sullivan E., Tecott L.H., et al. Deletion of Mecp2 in Sim1-expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress. Neuron. 2008;59:947–958. doi: 10.1016/j.neuron.2008.07.030. doi:10.1016/j.neuron.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young J.I., Zoghbi H.Y. X-chromosome inactivation patterns are unbalanced and affect the phenotypic outcome in a mouse model of rett syndrome. Am. J. Hum. Genet. 2004;74:511–520. doi: 10.1086/382228. doi:10.1086/382228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGraw C.M., Samaco R.C., Zoghbi H.Y. Adult neural function requires MeCP2. Science. 2011;333:186. doi: 10.1126/science.1206593. doi:10.1126/science.1206593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luque R.M., Park S., Kineman R.D. Role of endogenous somatostatin in regulating GH output under basal conditions and in response to metabolic extremes. Mol. Cell. Endocrinol. 2008;286:155–168. doi: 10.1016/j.mce.2007.12.005. doi:10.1016/j.mce.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Knudsen G.P.S., Neilson T.C.S., Pedersen J., Kerr A., Schwartz M., Hulten M., Bailey M.E.S., Orstavik K.H. Increased skewing of X chromosome inactivation in Rett syndrome patients and their mothers. Eur. J. Hum. Genet. 2006;14:1189–1194. doi: 10.1038/sj.ejhg.5201682. doi:10.1038/sj.ejhg.5201682. [DOI] [PubMed] [Google Scholar]
- 64.Archer H., Evans J., Leonard H., Colvin L., Ravine D., Christodoulou J., Williamson S., Charman T., Bailey M.E.S., Sampson J., et al. Correlation between clinical severity in patients with Rett syndrome with a p.R168X or p.T158M MECP2 mutation, and the direction and degree of skewing of X-chromosome inactivation. J. Med. Genet. 2007;44:148–152. doi: 10.1136/jmg.2006.045260. doi:10.1136/jmg.2006.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zappella M., Meloni I., Longo I., Canitano R., Hayek G., Rosaia L., Mari F., Renieri A. Study of MECP2 gene in Rett syndrome variants and autistic girls. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003;119B:102–107. doi: 10.1002/ajmg.b.10070. doi:10.1002/ajmg.b.10070. [DOI] [PubMed] [Google Scholar]
- 66.Silverman J.L., Turner S.M., Barkan C.L., Tolu S.S., Saxena R., Hung A.Y., Sheng M., Crawley J.N. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. doi:10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Downs J., Géranton S.M., Bebbington A., Jacoby P., Bahi-Buisson N., Ravine D., Leonard H. Linking MECP2 and pain sensitivity: the example of Rett syndrome. Am. J. Med. Genet. A. 2010;152A:1197–1205. doi: 10.1002/ajmg.a.33314. doi:10.1002/ajmg.a.33314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Asaka Y., Jugloff D.G.M., Zhang L., Eubanks J.H., Fitzsimonds R.M. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol. Dis. 2006;21:217–227. doi: 10.1016/j.nbd.2005.07.005. doi:10.1016/j.nbd.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Lioy D.T., Wu W.W., Bissonnette J.M. Autonomic dysfunction with mutations in the gene that encodes methyl-CpG-binding protein 2: insights into Rett syndrome. Auton. Neurosci. 2011;161:55–62. doi: 10.1016/j.autneu.2011.01.006. doi:10.1016/j.autneu.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 70.Tropea D., Giacometti E., Wilson N.R., Beard C., McCurry C., Fu D.D., Flannery R., Jaenisch R., Sur M. Partial reversal of Rett syndrome-like symptoms in MeCP2 mutant mice. Proc. Natl Acad. Sci. USA. 2009;106:2029–2034. doi: 10.1073/pnas.0812394106. doi:10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McGill B.E., Bundle S.F., Yaylaoglu M.B., Carson J.P., Thaller C., Zoghbi H.Y. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc. Natl Acad. Sci. USA. 2006;103:18267–18272. doi: 10.1073/pnas.0608702103. doi:10.1073/pnas.0608702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samaco R.C., Mandel-Brehm C., Chao H.-T., Ward C.S., Fyffe-Maricich S.L., Ren J., Hyland K., Thaller C., Maricich S.M., Humphreys P., et al. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc. Natl Acad. Sci. USA. 2009;106:21966–21971. doi: 10.1073/pnas.0912257106. doi:10.1073/pnas.0912257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moretti P., Levenson J.M., Battaglia F., Atkinson R., Teague R., Antalffy B., Armstrong D., Arancio O., Sweatt J.D., Zoghbi H.Y. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J. Neurosci. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. doi:10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marubio L.M., Paylor R. Impaired passive avoidance learning in mice lacking central neuronal nicotinic acetylcholine receptors. Neuroscience. 2004;129:575–582. doi: 10.1016/j.neuroscience.2004.09.003. doi:10.1016/j.neuroscience.2004.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.