Abstract
Given the crucial roles for mitochondria in ATP energy supply, Ca2+ handling and cell death, mitochondrial dysfunction has long been suspected to be an important pathogenic feature in Duchenne muscular dystrophy (DMD). Despite this foresight, mitochondrial function in dystrophin-deficient muscles has remained poorly defined and unknown in vivo. Here, we used the mdx mouse model of DMD and non-invasive spectroscopy to determine the impact of dystrophin-deficiency on skeletal muscle mitochondrial localization and oxidative phosphorylation function in vivo. Mdx mitochondria exhibited significant uncoupling of oxidative phosphorylation (reduced P/O) and a reduction in maximal ATP synthesis capacity that together decreased intramuscular ATP levels. Uncoupling was not driven by increased UCP3 or ANT1 expression. Dystrophin was required to maintain subsarcolemmal mitochondria (SSM) pool density, implicating it in the spatial control of mitochondrial localization. Given that nitric oxide-cGMP pathways regulate mitochondria and that sildenafil-mediated phosphodiesterase 5 inhibition ameliorates dystrophic pathology, we tested whether sildenafil's benefits result from decreased mitochondrial dysfunction in mdx mice. Unexpectedly, sildenafil treatment did not affect mitochondrial content or oxidative phosphorylation defects in mdx mice. Rather, PDE5 inhibition decreased resting levels of ATP, phosphocreatine and myoglobin, suggesting that sildenafil improves dystrophic pathology through other mechanisms. Overall, these data indicate that dystrophin-deficiency disrupts SSM localization, promotes mitochondrial inefficiency and restricts maximal mitochondrial ATP-generating capacity. Together these defects decrease intramuscular ATP and the ability of mdx muscle mitochondria to meet ATP demand. These findings further understanding of how mitochondrial bioenergetic dysfunction contributes to disease pathogenesis in dystrophin-deficient skeletal muscle in vivo.
INTRODUCTION
Duchenne muscular dystrophy (DMD) is the most common muscular dystrophy caused by mutations in the dystrophin gene (1). DMD is characterized by progressive muscle wasting and weakness, culminating in respiratory or cardiac failure. At the cellular level, the loss of dystrophin initiates a complex series of pathophysiological changes that drive skeletal muscle cell weakness, atrophy and death. Most prominent is abnormal Ca2+ influx and handling that is thought to activate proteases and cause mitochondrial Ca2+ overload and dysfunction (2–4). Despite a potentially important role for mitochondria in this pathogenic cascade, the impact of dystrophin deficiency on fundamental aspects of mitochondrial biology remains incompletely understood.
Mitochondria are pivotal players in skeletal muscle energetics due to their well-known ability to synthesize ATP by oxidative phosphorylation. However, they are also crucial players in muscle cell signaling due to their regulation of intramuscular Ca2+ and cell death. Skeletal muscle contains two spatially and functionally distinct mitochondrial populations: the subsarcolemmal mitochondria (SSM) and the intermyofibrillar mitochondria (IFM). SSM reside beneath the sarcolemma and comprise ∼10–15% of total muscle mitochondria (5). IFM localize to the I band and intermyofibrillar space, make up the vast majority of mitochondria and provide most of the ATP required for muscle contraction (6). The impact of dystrophin deficiency on SSM and IFM is unknown.
Recently, insights into aberrant mitochondrial function in muscular dystrophy have come from studies of their role in Ca2+ handling and necrotic cell death. In dystrophic muscle, mitochondrial dysfunction is thought to result from Ca2+ overload that can lead to: (i) prolonged mitochondrial permeability transition pore (PTP) opening and (ii) inhibition of mitochondrial ATP synthesis, which together drive cell death. Excessive PTP opening leading to mitochondria-dependent cell necrosis was observed in dystrophin-deficient muscle and was thought to be an important cause of muscle cell death (3,7,8). Also, there is limited evidence supporting defective mitochondrial oxidative phosphorylation function. In vitro studies of permeabilized quadricep fibers from one (9) and three (10) DMD patient biopsies showed an ∼2-fold reduction in maximal mitochondrial respiration. A similar reduction in maximal mitochondrial respiration was also reported by Kuznetsov et al. (9) in the quadriceps muscles from the mdx mouse model of DMD. These two studies (with the caveat of small sample size) suggest a reduction in mitochondrial oxidative phosphorylation capacity in DMD consistent with the Ca2+ overload.
Although these studies suggest a reduced maximal ATP synthesis capacity of the mitochondria in DMD, they were performed in vitro under optimal conditions permitting full mitochondrial enzyme activation. However, it remains unclear whether these in vitro results translate to significant effects on mitochondrial and muscle energetics under the suboptimal metabolic conditions present in vivo. In addition, mitochondrial function reflects the totality of interactions of the organelle with its intracellular signaling (‘regulatory’) environment. These mitochondria–environment interactions are lost in in vitro studies by permeabilization. Thus, in vitro studies largely reflect inherent (‘structural’) changes to the mitochondria themselves and do not necessarily reflect alterations in the cell signaling (‘regulatory’) environment impacting mitochondrion function. An advantage of in vivo measurements of mitochondrial function, such as those used in the present study, is that they reflect both structural and regulatory influences on the mitochondrial ATP synthesis. This is particularly advantageous to analyses of the impact of therapeutics that may indirectly modulate mitochondria function. Thus, in vivo approaches provide additional unique and potentially complimentary insights into mitochondrial dysfunction.
Targeting mitochondrial dysfunction shows promise for alleviating disease burden in muscular dystrophy. Increased mitochondrial biogenesis, or the generation of new mitochondria from existing ones, has been shown to reduce dystrophic muscle pathology (11,12). In addition, mdx mice treated with the cyclophilin inhibitor Debio 025 exhibited normal mPTP opening, enhanced mitochondrial resistance to Ca2+ overload and decreased muscle necrosis (3,7). Phase II clinical studies demonstrated that idebenone, an antioxidant thought to enhance mitochondrial electron transport, significantly reduced respiratory muscle dysfunction in DMD patients, the primary cause of mortality in DMD (13). These data provide compelling evidence that mitochondria represent an important drug target for muscular dystrophy. Better defining mitochondrial dysfunction will assist in the identification of new therapeutic targets for the treatment of DMD.
One pathway that has emerged as an important therapeutic target in DMD is the neuronal nitric oxide synthase-cyclic guanosine monophosphate (nNOS-cGMP) signaling axis, which is disrupted by the absence of dystrophin. Dystrophin deficiency prevents normal expression and localization of nNOSμ, a source of nitric oxide (NO) in skeletal muscle (14,15). An important function of nNOS-derived NO is to stimulate soluble guanylyl cyclase to synthesize cGMP, which in turn activates downstream effectors including protein kinase G (PKG). Defective nNOSμ signaling and concomitant decreases in cGMP promote skeletal muscle weakness, vascular dysfunction and impaired exercise performance in mdx mice (16–19). In preclinical studies, the use of phosphodiesterase 5 (PDE5) inhibitors sildenafil (Viagra®) and tadalafil (Cialis®) to inhibit cGMP breakdown, and thus amplify NO-cGMP signaling, substantially reduces dystrophic pathology in mdx mice (16,17,19,20–22). Thus, PDE5 inhibitor-mediated amplification of NO-cGMP signaling shows promise as a novel treatment option for muscular dystrophy.
While PDE5 inhibition reduces dystrophin-deficient muscle dysfunction, the molecular mechanisms responsible remain unclear. Elucidating these mechanisms is important for understanding and refining the potential therapeutic utility of PDE5 inhibitors. Although PDE5 inhibitors are potent vasodilators, such that their benefits likely result in part from improved muscle perfusion, they may also impact mitochondrial pathways (8,21,23). Indeed, NO-cGMP signaling pathways can drive mitochondrial biogenesis and regulate both mitochondrial fission and respiratory function (24–26). Therefore, sildenafil-mediated increases in cGMP may increase mdx skeletal muscle mitochondrial content. An additional ‘mitoprotective’ activity of sildenafil may be increased PKG-mediated inactivation of glycogen synthase kinase 3β. GSK3β inactivation promotes normal PTP function (23,27). Similarly, sildenafil-mediated cardioprotection in dystrophin-deficient mdx hearts is also accompanied by increased GSK3β inactivation, attenuation of mitochondrial Ca2+ uptake and normalized PTP opening which may promote mitochondrial ATP synthesis (8,21). Taken together, these data suggest the possibility that sildenafil may benefit dystrophin-deficient muscle by increasing mitochondrial biogenesis and ATP synthesis.
To better understand the role of mitochondria in dystrophic pathogenesis and the mechanisms by which sildenafil enhances dystrophin-deficient muscle function, we used electron microscopy and non-invasive 31P magnetic resonance spectroscopy (MRS)/optical spectroscopy (OS) to answer two questions. First, does the absence of dystrophin negatively impact mitochondrial localization, content and ATP synthesis function in vivo. Second, do the protective effects of sildenafil result from enhanced mitochondrial biogenesis and oxidative phosphorylation function in vivo.
RESULTS
Dystrophin is required to maintain normal SSM pool density
Initially, we focused on the impact of dystrophin deficiency on the SSM and IFM pools. We hypothesized that the absence of dystrophin may preferentially disrupt the SSM pool, since dystrophin is necessary for organelle localization to the subsarcolemmal space (28). Transmission electron microscopy was used to analyze SSM and IFM localization in the tibialis anterior muscles of wild-type, untreated and sildenafil-treated mdx mice. Ultrastructural analysis of wild-type skeletal muscle revealed high SSM concentrations beneath the sarcolemma (Fig. 1A and D). To gain a quantitative understanding of SSM density, we determined the number of mitochondria per micron length of sarcolemma (Fig. 1G). Wild-type muscles averaged ∼3 SSM per micron of sarcolemma (Fig. 1G). In contrast, the numbers of SSM were substantially decreased in mdx skeletal muscle (Fig. 1B and E). The absence of dystrophin significantly reduced SSM density by 39% (P < 0.01) compared with wild-type controls (Fig. 1G). Note also that while SSM from dystrophin-deficient muscle appeared slightly smaller than controls, the difference in size was not statistically significant (compare Fig. 1D and E; wild-type: 0.45 ± 0.05 μm2; mdx: 0.38 ± 0.05 μm2, n = 4 for both groups). Fourteen weeks of chronic sildenafil-mediated PDE5 inhibition had no overt impact on SSM localization or morphology in mdx muscle (Fig. 1C and F compared with B and E, respectively). Sildenafil-treated mdx muscles also had a similar SSM density as mdx controls, suggesting that PDE5-regulated cGMP does not regulate SSM localization or number (Fig. 1G). Taken together, these data suggest that dystrophin is necessary for the strict spatial control of SSM localization.
Figure 1.
Dystrophin-deficiency reduces SSM density. Transmission electron microscopy micrographs of the SSM pool in tibialis anterior skeletal muscles from wild-type (A), dystrophin-deficient mdx (B) and sildenafil treated mdx mice (C). (D), (E) and (F) represent magnified regions within (A), (B) and (C), respectively. SSM densities (number of mitochondria per micron length of sarcolemma) were determined from transmission electron micrographs (G). In wild-type skeletal muscle, SSM were heavily concentrated along the subsarcolemmal space and averaged ∼3 SSM per micron of sarcolemma (A, D, G). SSM density was significantly reduced (P < 0.01) in both untreated mdx (B, E, G) and sildenafil-treated mdx (C, F, G) skeletal muscle compared with wild-type controls. Chronic PDE5 inhibition had no impact on SSM density in mdx muscle (C, F, G). In (A), N indicates a nucleus. S marks the sarcolemma. White asterisks (*) mark individual mitochondria. Number of mice per group: wild-type (6), mdx (5), mdx + sildenafil (5). Scale bars, 2 μm.
The absence of dystrophin did not have the same impact on IFM (Fig. 2). Ultrastructural analyses of wild-type muscle showed that IFM exhibited a stereotypical localization to the I band (Fig. 2A, arrowheads) and formed long trains in the intermyofibrillar space (Fig. 2B, arrowheads). However, IFM were not localized as frequently to the I band in mdx muscle, even in regions where sarcomere integrity was largely preserved (Fig. 2C). IFM often accumulated near sites of necrotic muscle breakdown, which were easily distinguished by sarcomere disruption (Fig. 2D, arrowhead) or myofibril loss (Fig. 2E, arrowhead). In addition, large numbers of IFM accumulated between the centrally localized nuclei of regenerating mdx fibers (Fig. 3A and B) which parallel the accumulation of the Golgi complex organelle at this site (28). To quantitate changes in IFM content in mdx skeletal muscle, we used stereological methodologies to determine IFM volume density (Vv, volume of mitochondria per volume of muscle fiber). In contrast to the SSM pool, mdx IFM volume density was increased, but not significantly, relative to wild-type controls (Fig. 2H). Taken together, these findings highlight a redistribution of IFM in necrotic and regenerating mdx muscle cells. Ultrastructural analyses also showed that chronic sildenafil treatment did not prevent aberrant IFM localization either in regions with reasonably preserved (Fig. 2F) or poor (Fig. 2G) sarcomere organization. As with the SSM, sildenafil treatment had no significant impact on IFM densities in mdx muscle (Fig. 2H). Therefore, increases in the pool of cGMP regulated by PDE5 are not sufficient to increase mitochondrial density in dystrophin-deficient skeletal muscle.
Figure 2.
Aberrant IFM localization in dystrophin-deficient skeletal muscle. Transmission electron microscopy micrographs of the IFM pool in tibialis anterior skeletal muscles from wild-type (A and B), dystrophin-deficient mdx (C–E) and sildenafil-treated mdx (F and G). IFM volume density (the volume of mitochondria per volume of muscle fiber) was determined stereologically using the grid point counting method (H). In wild-type skeletal muscle, IFM localize to the I band on either side of the z disk (A, arrowheads) and can be found in long trains between myofibrils (B, arrowheads). In contrast, in dystrophin-deficient skeletal muscle, IFM do not show as tight localization to the I band, even in regions where sarcomere organization is largely preserved (C). IFM also accumulate at sites of focal muscle necrosis where sarcomere filament organization is lost (D, arrowhead) or at large intermyofibrillar gaps that presumably result from myofibril loss (E, arrowhead). Chronic sildenafil treatment did not impact IFM distribution in regions of preserved sarcomere organization (F) or muscle breakdown (G). IFM volume density in dystrophin-deficient muscle did not differ significantly from controls and was unaffected by sildenafil treatment (H). Number of mice per group: wild-type (6), mdx (5), mdx + sildenafil (5). Scale bars represent 1 μm.
Figure 3.
High densities of mitochondria between centrally localized nuclei or regenerating mdx muscle cells. A representative transmission electron microscopy micrograph of a regenerating dystrophin-deficient tibialis anterior muscle cell with centrally localized nuclei (A). Higher magnification image of panel A (B). Large numbers of mitochondria localize to the intermyonuclear space between centrally localized nuclei of regenerating mdx muscle cells. N indicates a centrally localized nucleus. Scale bars in (A) and (B) represent 2 and 1 μm, respectively.
Stereological analysis of mitochondrial volume density was supported by biochemical analysis of mitochondrial marker voltage-dependent anion channel 1 (VDAC1) protein expression (Fig. 4A). In agreement with IFM volume density, analysis of VDAC1 expression revealed that total mitochondrial content in untreated mdx skeletal muscle, although increased, was not significantly different from wild-type controls (Fig. 4A). Note that the changes in VDAC1 expression largely reflect changes in the IFM pool since IFM account for ∼90% of total mitochondrial content; therefore, the changes in IFM number masked the decrease in the smaller SSM pool. Similarly, sildenafil treatment had no impact on VDAC1 expression (Fig. 4A). In summary, these ultrastructural and biochemical analyses suggest a significant decrease in the SSM pool without significant compensatory IFM mitochondrial biogenesis. Importantly, these findings suggest that dystrophin is required for normal mitochondrial localization and is necessary for maintaining the density of mitochondria in the subsarcolemmal space.
Figure 4.
Mitochondrial content and respiratory chain complex composition in untreated and sildenafil-treated mdx skeletal muscle. Relative levels of the mitochondrial marker voltage-dependent anion channel (VDAC1) and the five respiratory chain complexes were determined by western immunoblotting. VDAC1 protein levels were not significantly different between wild-type, untreated mdx and sildenafil-treated mdx skeletal muscles suggesting that total mitochondrial content was unaffected by dystrophin deficiency or treatment (A). Expression of complex I subunit NDUFB8 (B), complex II subunit 30 kDa (C), complex IV subunit I (E) and complex V ATPase synthase α subunit (F) were not significantly affected by dystrophin deficiency or sildenafil treatment. In contrast, the ubiquinol cytochrome C reductase subunit II of complex III was significantly increased in mdx muscle suggesting an increase in mitochondrial complex III respiratory complex content or altered CIII subunit composition (D). Number of mice per group: wild-type (8), mdx (6), and mdx+ sildenafil (8).
Dystrophin deficiency promotes uncoupling of mitochondrial oxidative phosphorylation
We then focused on determining the functional properties of mitochondria in untreated and sildenafil-treated dystrophin-deficient skeletal muscle in vivo. It is well established that mitochondrial ATP synthesis depends on normal respiratory chain function (29). The respiratory chain, also known as the oxidative phosphorylation system, comprises five multimeric enzyme complexes (complexes I–IV) and the ATP synthase complex (complex V) that together coordinate electron transfer with the establishment of proton gradients to generate ATP. Mitochondrial respiratory chain defects can cause skeletal muscle weakness and exercise intolerance, two hallmark pathological features of DMD and mdx mice; suggesting the possibility of respiratory chain abnormalities in mdx muscle (29).
We determined the relative expression levels of complex I through V using antibodies to specific protein subunits of each complex (Fig. 4B–F). Complex I, II, IV and V expression levels were not significantly changed in untreated or sildenafil-treated mdx mice (Fig. 4B, C, E, F). In contrast, the ubquinol cytochrome C reductase subunit 2 (UQCRC2) of complex III was increased nearly 2-fold (P < 0.01) in mdx muscle compared with wild-type, but unaffected by sildenafil treatment (Fig. 4D). These data suggest either an increase in complex III content or a change in complex III subunit composition in mitochondria from mdx muscle and mark a previously unrecognized abnormality in mitochondrial respiratory chain organization in dystrophin-deficient skeletal muscle.
The disruption of mitochondrial subcellular distribution and perturbation of respiratory chain complex expression suggested that mitochondrial function may be impaired in mdx skeletal muscle in vivo. To determine mitochondrial oxidative phosphorylation function in vivo, we used a unique combination of non-invasive 31P MRS and OS (30,31). We measured two key functional properties of mitochondria in vivo: (i) the degree of coupling of ATP synthesis to O2 consumption (P/O ratio) and (ii) the maximal rate of ATP synthesis (ATPmax) (31). Moderate uncoupling (low P/O ratio) indicates mitochondrial inefficiency where increased oxygen consumption is required to make ATP. Uncoupling is a characteristic of aged or oxidatively stressed skeletal muscle (31–33). ATPmax is a measure of maximal mitochondrial ATP production and reflects the ability of skeletal muscle mitochondria to meet ATP demand.
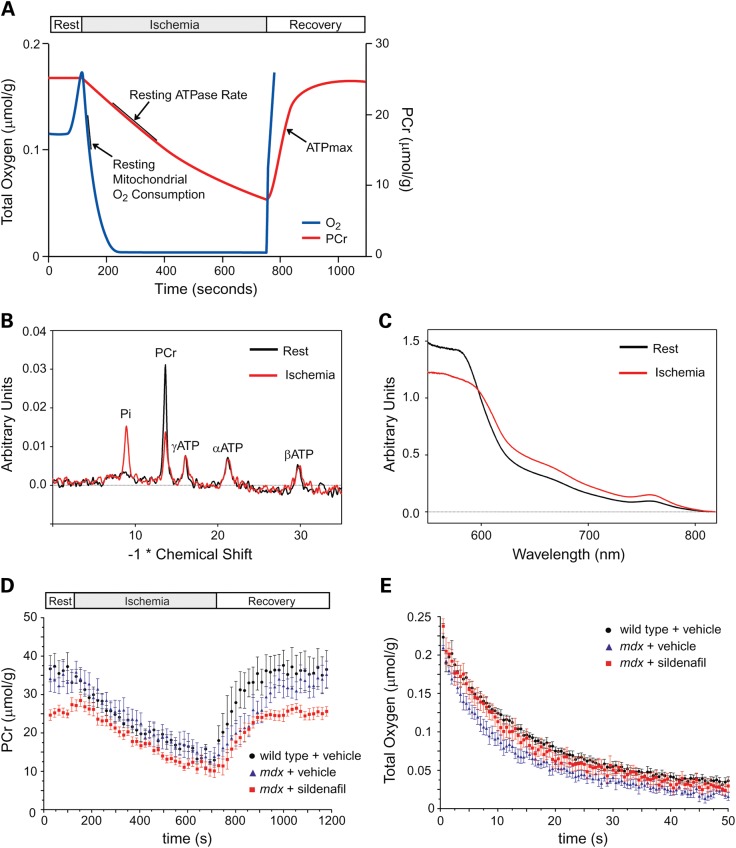
The degree of in vivo mitochondrial uncoupling and maximal ATP production capacity was determined from changes in hindlimb phosphocreatine (PCr) and oxygen concentrations in anesthetized mice during a mild ischemia-recovery protocol. The method is outlined schematically in Figure 5A. PCr buffers cellular ATP levels through the creatine kinase reaction, so the decline in PCr during early ischemia can be used to determine the rate of mitochondrial ATP production under steady state normoxic conditions (30,34, Fig. 5A). ATPmax was determined from the maximal rate of PCr restoration during the recovery phase. A series of 31P MRS spectra were sequentially captured during ischemia and recovery periods and used to measure PCr concentration (Fig. 5B and D). Rates of mitochondrial oxygen consumption were calculated from the initial rates of hemoglobin (Hb) and myoglobin (Mb) oxygen desaturation determined from optical spectra collected at the same time as 31P MRS spectra during ischemia (Fig. 5C and E) (35). The rate of oxygen consumption was calculated during the initial period of ischemia (where Mb oxygen saturation >50%) before oxygen becomes limiting to respiration (36).
Figure 5.
Phosphocreatine and oxygen fluxes in wild-type, untreated and sildenafil-treated mdx skeletal muscle. Schematic representation of the mild ischemia-recovery protocol used to measure mitochondrial oxidative phosphorylation function in vivo (A). Decreases in phosphocreatine (PCr) and O2 concentration were determined during mild hindlimb ischemia and used to calculate resting ATPase rates (P), a measure of resting mitochondria ATP synthesis, resting mitochondrial O2 consumption (O) and P/O coupling ratio. During muscle recovery and the resupply of O2 for oxidative phosphorylation, the rate of PCr recovery was used to calculate the maximal ATP synthesis rate (ATPmax) (B). Representative 31P spectra showing a decrease in PCr and proportional increase in inorganic phosphate (Pi) at the end of ischemia. ATP levels remain essentially constant since they are buffered by PCr (C). Representative optical spectra at rest of oxygenated hemoglobin (Hb) and myoglobin (Mb) and after ischemia of oxygen-desaturated Hb and Mb. These spectra were used to calculate muscle O2 concentration. Mean changes in PCr levels during ischemia–reperfusion in wild-type, mdx and sildenafil-treated mdx mice (D). Mean changes in O2 concentration during ischemia in wild-type, mdx and sildenafil-treated mdx mice (E). Number of mice per group: wild-type (7), mdx (6) and mdx + sildenafil (9).
Mean PCr concentrations during the rest, ischemia and recovery periods in wild-type, untreated mdx mice and sildenafil-treated mdx mice are shown in Figure 5D. Resting PCr levels were not significantly different between wild-type and mdx skeletal muscle; however, sildenafil treatment significantly decreased resting PCr compared with wild-type and untreated mdx (both P < 0.05) (Fig. 5D). PCr concentrations in wild-type, mdx and sildenafil-treated mdx muscle were: 33.3 ± 1.6, 31.6 ± 1.5 and 27.1 ± 1.3 mm, respectively. However, the resting rate of mitochondrial ATP synthesis (resting ATPase or P) was not affected in untreated or sildenafil-treated mdx mice relative to wild-type controls (Fig. 6A).
Figure 6.
Dystrophin deficiency leads to uncoupling of mitochondrial oxidative phosphorylation. Resting ATPase activity (P, which reflects resting mitochondria ATP synthesis) was unaffected by the loss of dystrophin (A). Sildenafil had no significant impact on resting ATPase activity (A). Resting mitochondrial O2 consumption in mdx skeletal muscle was not statistically different from controls and was unaffected by PDE5 inhibition (B). The mitochondrial oxidative phosphorylation coupling ratio (P/O) was significantly decreased in mdx skeletal muscle (C). These data suggest marked mitochondrial ATP synthesis inefficiency in mdx muscle. Sildenafil had no impact on the coupling efficiency of mdx mitochondria. Number of mice per group: wild-type (7), mdx (6) and mdx + sildenafil (7).
Although resting mitochondrial ATP synthesis was not significantly different between the three groups, resting mitochondrial O2 consumption trended higher in both untreated and sildenafil-treated mdx muscle relative to wild-type muscle (Fig. 6B). Importantly, the amount of ATP produced per O2 consumed (P/O = 0.5× ATPase/O2 consumption) was decreased 39% (P < 0.05) in mdx skeletal muscle compared with wild type skeletal muscle (Fig. 6C). The reduction in the P/O ratio demonstrated a significant uncoupling of mitochondrial oxidative phosphorylation. This meant that mdx mitochondria were consuming more O2 to make the same amount of ATP as wild-type mitochondria. In addition, PDE5 inhibition by sildenafil did not significantly reduce mdx mitochondria uncoupling in vivo (Fig. 6C). These data indicate that mitochondria from dystrophin-deficient muscles are functionally inefficient and that mdx mitochondrial inefficiency is not ameliorated by PDE5 inhibition.
We then sought to elucidate the molecular mechanism(s) responsible for mitochondrial uncoupling in mdx skeletal muscle. Uncoupling of O2 consumption from ATP production results from proton leak across the mitochondrial inner membrane (37). In skeletal muscle, proton leak is thought to be regulated by the mitochondrial inner membrane proteins adenine nucleotide translocase 1 (ANT1) and uncoupling protein 3 (UCP3) (37–39). Therefore, we determined ANT1 and UCP3 protein expression by western immunoblotting (Fig. 7). Interestingly, UCP3 and ANT1 protein expression in mdx muscle were not significantly different from wild-type controls and were also unaffected by PDE5 inhibition (Fig. 7A and B, respectively). These results suggested that uncoupling was not driven by increases in ANT1 and UCP3 protein expression in mdx skeletal muscle and that other mechanisms are responsible for uncoupling. In summary, mdx mitochondria uncoupling occurred independently of changes in ANT1 or UCP3 protein expression.
Figure 7.
UCP3 and ANT1 uncoupling protein expression unaffected in mdx skeletal muscle. Relative levels of uncoupling protein 3 (UCP3) and adenine nucleotide translocase type 1 (ANT1) were determined by western immunoblotting. Neither UCP3 (A) nor ANT1 (B) protein levels differed significantly between wild-type, mdx and sildenafil-treated mdx skeletal muscles. Uncoupling of mdx mitochondria occurred independently of changes in UCP3 and ANT1 protein expression. Number of mice per group: wild-type (8), mdx (6) and mdx + sildenafil (8).
Mitochondria exhibit reduced maximal ATP synthesis capacity in mdx skeletal muscle
Further evidence of mitochondrial dysfunction in vivo caused by dystrophin-deficiency emerged from analyses of maximal mitochondrial ATP synthesis rates (ATPmax) during the recovery period (Fig. 8A). ATPmax was normalized to mitochondrial volume density (Vv) content to provide a measure of maximal ATP synthesis rate per mitochondrion (Fig. 8A). In the absence of dystrophin, the ATPmax per mitochondrion was significantly decreased by 43% (P < 0.01) relative to wild-type controls. Sildenafil-mediated PDE5 inhibition did not alleviate this attenuated oxidative phosphorylation capacity (Fig. 8A). ATPmax normalized to VDAC1 also showed a similar decrease (data not shown).
Figure 8.
Dystrophin deficiency reduces maximal mitochondrial ATP synthesis capacity. The maximum mitochondrial oxidative phosphorylation rate (ATPmax) was determined during muscle recovery and normalized to IFM volume density (A). Normalized ATPmax was significantly decreased in dystrophin-deficient skeletal muscle; however, ATPmax was not significantly affected by PDE5 inhibition in mdx mice (A). Levels of the muscle oxygen carrier myoglobin in wild-type, mdx and sildenafil-treated mdx skeletal muscle (B). Myoglobin levels were significantly lower in mdx skeletal muscle and were further decreased by chronic sildenafil treatment (B). Mean time (in seconds) for the reoxygenation of myoglobin (C). Although myoglobin reoxygenation times trended longer in mdx muscle, they did not reach statistical significance (C). Sildenafil did not impact myoglobin reoxygenation times in mdx muscle (C). Number of mice per group: wild-type (6), mdx (6) and mdx + sildenafil (5).
Reductions in ATPmax may have resulted from restricted O2 availability during blood reperfusion after ischemia. Indeed, expression of the muscle oxygen storage protein myoglobin was significantly lower in mdx muscle compared with controls and were further decreased by sildenafil treatment, consistent with impaired intramuscular O2 supply (Fig. 8B). To evaluate this possibility, we determined the rate of myoglobin reoxygenation during recovery where longer reoxygenation times would indicate a limit on O2 availability. Although myoglobin reoxygenation times were longer in mdx skeletal muscle, they were not statistically different from wild-type controls (Fig. 8C). Surprisingly, given the potent vasodilatation resulting from PDE5 inhibition, sildenafil did not significantly impact myoglobin reoxygenation times in mdx muscle (Fig. 8C). Thus, the decrease in ATPmax was unlikely to be a consequence of impaired blood reperfusion and O2 availability. This finding is also consistent with the observation that sildenafil did not impact ATPmax in mdx skeletal muscle. Together, these data indicate that the absence of dystrophin leads to a reduction in the maximal ATP synthesis capacity of skeletal muscle mitochondria in mdx mice.
One possible consequence of the restricted ATP generating capacity resulting from decreased mitochondrial coupling and maximal oxidative phosphorylation capacity is a reduction in intramuscular ATP content. As anticipated, ATP concentrations were significantly decreased in dystrophin-deficient muscle (Fig. 9A). Sildenafil treatment also significantly decreased ATP in mdx hindlimb, indicating a potential concern for the long-term use of sildenafil (Fig. 9A). Thus, the restriction in mitochondrial ATP synthesizing capacity manifests as a decrease in intramuscular ATP. Importantly, these data suggest that it is highly unlikely that mdx mitochondria are able to effectively meet the ATP energy demands of skeletal muscle thereby promoting muscle cell dysfunction and death.
Figure 9.
Mitochondrial dysfunction is associated with decreased intramuscular ATP levels. ATP concentrations were determined in wild-type, untreated and sildenafil-treated mdx skeletal muscle (A). ATP levels were significantly reduced in mdx skeletal muscle and further decreased by sildenafil treatment. Number of mice per group: wild-type (7), mdx (6) and mdx + sildenafil (9).
DISCUSSION
This study provides novel insights into the defects in mitochondrial bioenergetics and their contribution to disease pathogenesis in dystrophin-deficient skeletal muscle. Here, we present the first in vivo evidence of mitochondrial mislocalization and oxidative phosphorylation dysfunction in the skeletal muscles of the mdx mouse model of the DMD. The loss of dystrophin specifically decreased the SSM pool, led to an uncoupling of oxidative phosphorylation and reduced the maximal rate at which mitochondria synthesize ATP. These mitochondrial defects culminated in decreased intramuscular ATP concentrations. These data demonstrate that dystrophin-deficiency impairs the strict spatial control of mitochondria localization and restricts global mitochondrial ATP generating capacity that together serve to diminish ATP availability and promote skeletal muscle cell death, a hallmark pathological feature of the dystrophic phenotype.
These findings are consistent with recent reports that mitochondria may be useful targets for ameliorating muscular dystrophy (3,7,11–13). Given that NO-cGMP pathways regulate mitochondria biogenesis and function and that sildenafil may reduce dystrophic mdx muscle pathology in mice by improving mitochondrial Ca2+ handling and mPTP function, we tested whether sildenafil could also increase mitochondrial content and/or improve ATP synthesis (8,16,17,19–21). Surprisingly, after chronically increasing the PDE5-regulated cGMP pool, we found neither an increase in mitochondrial content nor a significant change in ATP synthesis. This contrasts with recent reports that cGMP drives functional mitochondrial biogenesis in normal cells (24,25). At present, the reasons for this are unclear; however, there are at least two possible explanations. First, the PDE5-regulated cGMP pool plays no role in mitochondrial biogenesis. Second, the cellular machinery for cGMP-stimulated biogenesis in dystrophin-deficient skeletal muscle is defective or insensitive to increased cGMP. It is also worth noting that changes in PTP function may not necessarily accompany improved mitochondrial ATP synthesis capacity (8). However, chronic sildenafil treatment did lead to a small but significant decrease in intramuscular ATP levels by mechanisms that are presently unclear. This change may not necessarily be deleterious. For example, sildenafil-treated wild-type tibialis anterior muscles exhibit normal specific force output (19). Overall, our data suggest that the impact of chronic sildenafil treatment on dystrophic skeletal muscle pathology does not result from alleviation of defective mitochondrial distribution or oxidative phosphorylation dysfunction.
While sildenafil had no impact on mitochondria content and ATP synthesis in mdx hindlimb muscle, novel insights were gained into the spatial control of mitochondrial organelle localization in skeletal muscle. The absence of dystrophin had a profound impact on mitochondrial distribution, particularly the SSM. Ultrastructural analyses of mdx skeletal muscle revealed a 39% decrease in SSM density, indicating that dystrophin was required to maintain the localization of the SSM pool. Despite the decrease in SSM pool, total mitochondria content was not significantly altered in dystrophin-deficient skeletal muscle. These data are consistent with reports that cytochrome c oxidase (COX) activity, a biochemical measure of mitochondrial content, was unaffected in both DMD patients and mdx mice (10,40). However, Kuznetsov et al. (9) reported that COX activity was substantially reduced in mdx quadriceps muscle homogenates. The reasons for this discrepancy remain unclear.
The decrease in SSM density raises the question as to how the absence of dystrophin leads to the loss of mitochondria from the subsarcolemmal space. It is well established that microtubules play a crucial role in the spatial control of mitochondria in mammalian cells. In skeletal muscle cells, dystrophin is required for the alignment and stabilization of the subsarcolemmal microtubule cytoskeleton, which we have shown is required for normal subsarcolemmal organelle localization (28,41,42). Thus, it is highly likely that the disruption of the subsarcolemmal microtubule network impairs the normal scaffolding of mitochondria in the subsarcolemmal space. Taken together, these data suggest an intriguing link between dystrophin-mediated cytoskeletal integrity and cellular energy supply pathways, where dystrophin-deficiency disrupts subsarcolemmal microtubule integrity and the local spatial control of mitochondria.
Since it is well accepted that mitochondria must be positioned properly to perform their cellular roles, SSM pool function may be disproportionately impacted by the absence of dystrophin (43). Not only do SSM regulate ATP production and Ca2+ handling, they also participate in the adaptation of muscle to exercise, glucose homeostasis and fat metabolism (5,44,45). In mdx mice, fewer SSM may impair local Ca2+ handling by reducing dystrophic muscle's ability to buffer excessive Ca2+ influx. This would lead to further elevations in intracellular Ca2+, forming a deleterious feed forward loop or death spiral that promotes further mitochondrial Ca2+ overload, cellular dysfunction and death (2). Indeed, aberrant Ca2+ handling, particularly in the subsarcolemmal space, is thought to be a significant contributor to dystrophic disease pathogenesis and the decrease in SSM suggests a possible contributory mechanism (4,46). A functionally defective SSM pool may decrease local ATP availability and impair the activity of sarcolemmal ATP-dependent proteins that regulate Ca2+ and other ion influx/efflux (2). This would promote further Ca2+ accumulation, thereby amplifying the death spiral and leading to progressive muscle wasting and weakness. Future studies are required to address the consequences of the disruption of the SSM pool in mdx muscle.
In addition to being aberrantly localized, mdx mitochondria were also functionally impaired. Their ability to produce ATP was highly inefficient due to uncoupling, which drove them to consume larger amounts of O2 to make the same amount of ATP as wild-type mitochondria. Sustained mitochondrial uncoupling is deleterious to muscle, since it decreases ATP and ATP reserve, which in turn compromises skeletal muscle integrity (47). Our demonstration of uncoupling is consistent with findings from in vitro experiments on two DMD patient biopsies showing a lower respiratory control index (10). Together, these data support the idea that mitochondrial uncoupling may be a general and important pathogenic feature of human and murine dystrophin-deficient skeletal muscle.
The uncoupling of oxygen consumption from ATP synthesis is caused by proton leak across the inner mitochondrial membrane that can be regulated by UCP2, UCP3 and ANT1 (37–39). Our investigation of mechanisms governing the uncoupling of mdx mitochondria suggests that it was not driven by increased ANT1, UCP2 or UCP3 protein expression. These findings are in partial agreement with gene expression studies of DMD biopsies that reported no change in UCP3 mRNA expression, but a small decrease in ANT1 expression (48,49). Also, although gene expression meta-analyses predict a significant role for UCP2 in dystrophic pathogenesis, we could not detect UCP2 expression in wild-type or mdx skeletal muscle homogenates (50). This is not unusual since UCP2 protein is not typically expressed in skeletal muscle (37). Many factors could account for these discrepancies in ANT1 and UCP2 expression including the well-established imperfect correlation of mRNA and protein expression as well as disease stage and profound differences in disease severity between human and mouse.
One potential alternative mechanism to explain the decreased mitochondria coupling in vivo is the increase in oxidative stress in skeletal muscles of mdx mice (51,52). Mild oxidative stress can reduce mitochondrial coupling P/O in mouse skeletal muscle in vivo independently of causing damage to mitochondria (31). This oxidative stress-induced uncoupling is also independent of UCP3 expression. Therefore, in mdx skeletal muscle, oxidative stress alone may be sufficient to activate an increase in proton leak and concomitant uncoupling.
While the mechanisms causing uncoupling remain to be elucidated, the reprogramming of skeletal muscle mitochondrial coupling has important whole body metabolic consequences. At high coupling efficiencies, ATP is made and calories are stored as fat. At low coupling efficiency, less ATP is produced per O2, fat is burned and calories are dissipated as heat. This metabolic inefficiency increases energy expenditure (increased calories and O2 consumed per ATP synthesized) and metabolic rate, thereby decreasing fat stores (37). Since skeletal muscle accounts for ∼40% of body mass, muscle mitochondria uncoupling can significantly increase whole body energy expenditure and is being intensively studied for application to the treatment of obesity. Our data predict that skeletal muscle mitochondria uncoupling would make mdx mice resistant to weight gain induced by high fat diets. Indeed, mdx mice fed a moderately high fat diet were recently shown to be resistant to weight gain (53). While mitochondrial uncoupling represents an important feature of the metabolic reprogramming leading to energetic dysfunction in mdx mice, it is consistent with an important role for uncoupling in preventing obesity and provides a plausible molecular mechanism for the resistance of mdx mice to obesogenic diets.
Not only were the mdx mitochondria significantly uncoupled, they exhibited a decreased maximal rate of ATP synthesis (ATPmax). As expected from this degree of mitochondrial dysfunction, ATP levels were significantly reduced. The reduction in ATPmax in vivo described here is consistent with previous in vitro studies of DMD patient biopsies and mdx mice showing a significant reduction in maximal state 3 mitochondrial respiration (9,10). The decrease in ATPmax in vivo may have resulted in part from mitochondrial uncoupling, since mitochondrial inefficiency can reduce the maximal rate of ATP synthesis. Additional potential mechanisms include mitochondrial Ca2+ overload which can decrease mitochondrial inner membrane enzyme content (9,10). We also find evidence of abnormalities in mdx mitochondria protein expression, specifically the UQCRC2 subunit of complex III of the respiratory chain. The significance of increased UQCRC2 expression remains to be determined. Together, these data provide strong evidence of mitochondrial dysfunction in mdx mice and suggest that the reduction in maximal rate of ATP synthesis is a pathogenic characteristic of both human and murine dystrophin deficient skeletal muscle.
In conclusion, given its important roles in ATP energy supply, Ca2+ handling and necrotic cell death, mitochondria dysfunction has been suspected for decades to be an important pathogenic feature of muscular dystrophy (2). Despite this foresight, mitochondrial function in dystrophin-deficient muscles has remained relatively poorly defined and unknown in vivo. In the present study, we demonstrate that dystrophin is necessary to maintain the normal localization and oxidative phosphorylation function of mitochondria in vivo. Furthermore, we found that the cytoprotective effects of sildenafil on dystrophin-deficient muscle do not result from improved mitochondrial function. Thus, while sildenafil may promote normal mitochondria Ca2+ handling and PTP function, these effects appear separable from an impact on ATP synthesis (8).
MATERIALS AND METHODS
Animal models
All experimental procedures performed on mice were approved by the Institutional Animal Care and Use Committee of the University of Washington. Dystrophin-deficient C57BL/10ScSn-Dmdmdx/J (mdx) mice and C57BL/10ScSn/J strain controls were purchased from The Jackson Laboratory. All comparisons are made between age-matched (17-week-old) male mice.
Sildenafil administration
The dosing regimen has been previously described (19). Briefly, the PDE5 inhibitor sildenafil citrate (Pfizer Inc.) was administered in the drinking water ad libitum (400 mg/l) to wild-type and mdx mice for 14 weeks from 3 weeks of age. Using this approach, the average concentration of circulating sildenafil is 70 ± 0.05 nm over a 24 h period (21).
Quantitation of mitochondrial densities by transmission electron microscopy
Transmission electron microscopy was performed as described previously (14). Briefly, tibialis anterior skeletal muscle tissue from wild-type, mdx and sildenafil-treated mdx mice was fixed in half-strength Karnovsky's fixative (2.0% paraformaldehyde, 2.5% glutaraldehyde, 0.1 m cacodylate buffer, 3 mm CaCl2, pH 7.3), post-fixed for 1 h in 1.0% OsO4, and then stained with 2% uranyl acetate. Samples were then rinsed and dehydrated through a graded series of alcohol and propylene oxide. Samples were embedded with Eponate resin and 90–100 nm sections were cut and mounted on 150 mesh rhodium/copper grids and stained with uranyl acetate and lead citrate. Samples were analyzed with a JEM 1200EX II transmission electron microscope and images were acquired with an Olympus-SIS Morada digital camera. SSM densities (the number of mitochondria per micron length of sarcolemma) were determined between 20 and 36 electron micrographs (×10 000 magnification) of randomly selected fields per animal (≥10 images per grid and ≥2 grids per mouse). A subsarcolemmal mitochondrion was defined as one which did not have a myofibril between it and the sarcolemma. All other mitochondria were considered IFM. IFM volume density (the volume of mitochondria per volume of muscle fiber) was determined stereologically using the point counting grid (144 point grid) method (54). This stereological approach is a well-established quantitative method for determining skeletal muscle mitochondrial content that avoids the potentially confounding contribution of infiltrating cell mitochondria in dystrophic muscle (54). IFM volume densities were determined between 20 and 26 electron micrographs (×25 000 magnification) of randomly selected fields per animal (≥10 images per grid and ≥2 grids per mouse). For IFM measurements, electron micrographs containing central nuclei were excluded from counts. SSM areas (≥100 per mouse, four mice per group) were measured manually using Image J v1.38 from randomly selected electron micrographs.
Multi-mode MRS and OS
These methods have been described previously (31). Briefly, mice were anesthetized with an intraperitoneal injection of 0.01 ml/g of 2.5% tribromoethanol (avertin) and the left distal hindlimb was then shaved. Mice were then secured with velcro straps in a custom-built multi-mode probe (for MRS and OS) developed for a 7T vertical bore spectrometer (Varian). The distal hindlimb was centered within a horizontal, solenoid magnetic resonance coil tunable to both 1H and 31P. In order to deliver light to the lateral surface of the hindlimb, fiber optic bundles were positioned on either side of the hindlimb and coil. The fiber optic bundles collected light transmitted through the hindlimb, and then delivered this light to a spectrograph coupled to a CCD camera (Princeton Instruments).
After positioning the mouse, the magnetic resonance signal was optimized by shimming the 1H peak using endogenous tissue water. The optical signal was optimized by adjusting acquisition time (typically 30–60 ms). A high signal to noise 31P spectrum was acquired under fully relaxed conditions, and then dynamic optical (0.5 s delay) and 31P spectra (1.5 s interpulse delay) were acquired continuously through periods of rest (∼2 min), ischemia (∼11 min) and recovery (∼7 min). After the first minute of rest, mice breathed 100% O2 for the remainder of each experiment. Experimental methodology is outlined schematically in Figure 5A.
Multi-mode magnetic resonance and optical spectroscopy data analysis
31P MR spectra were exponentially multiplied, Fourier transformed and then phase-corrected manually using the Varian VNMR software. Spectra were analyzed with custom-written algorithms in MATLAB (Mathworks, Natick, MA, USA). The methodology used to analyze MR spectra has been described previously (30). Briefly, during the ischemia reperfusion protocol, PCr and Pi peak magnitudes relative to rest were calculated from three summed consecutive dynamic spectra (to improve the signal-to-noise ratio) using the fit-to-standard algorithm. After correcting for variable relaxation, skeletal muscle ATP concentration determined by HPLC analysis was used as an internal reference to calculate absolute PCr concentrations over time.
The methodology used for analyzing optical spectra has been described in detail (30). Briefly, after taking the second derivative of optical spectra to minimize the influence of tissue scattering, we used a partial-least squares routine to determine the O2 saturations of Hb and Mb throughout dynamic spectral acquisition. The concentrations and well-described O2 binding kinetics of Hb and Mb were then used to calculate net O2 flux in the ischemic hindlimb.
The resting rates of mitochondrial ATP production (proportional to endogenous ATPase consumption of ATP) and O2 consumption were calculated during ischemia from least-squares linear approximations of the decrease in PCr and O2, respectively, during the initial phase of ischemia. Using the initial phase of ischemia allows the ATPase and O2 consumption rates to be determined before the onset of significant glycolytic contribution to PCr synthesis and before O2 tension becomes rate-limiting to oxidative phosphorylation (36,55). The maximum rate of oxidative phosphorylation (ATPmax) was calculated using a least-squares monoexponential approximation of PCr recovery during recovery from ischemia (34).
Skeletal muscle metabolite quantification
Immediately following in vivo multi-mode spectroscopy, mice were injected with a supplemental and non-lethal dose of avertin. The skeletal muscles of the left distal hindlimb were rapidly dissected and flash frozen in liquid nitrogen. Extensor digitorum longus, gastrocnemius, soleus and tibialis anterior muscles were pooled and pulverized under liquid nitrogen for measurement of high energy phosphate metabolite (ATP) levels as well as hemoglobin (Hb), and myoglobin (Mb) concentrations. Tissue concentrations of ATP and phosphocreatine (PCr), and creatine were determined by HPLC (Waters, Milford, MA, USA) as previously described (31).
Absolute hemoglobin and myoglobin concentrations were determined from Coomassie-stained gels using standards of known concentration. Pulverized skeletal muscles were combined 1:25 with Cellytic MT lysis buffer containing 0.1% protease inhibitor and homogenized at 4° using a Bullet Blender 24 (Next Advance, Averill Park, NY, USA). The resulting lysate was combined 1:1 with tricine sample buffer containing 350 mm dithiothreitol and incubated for 8 min at 95°C. Proteins were separated on 10–20% gradient gels at 150 V for 2.5 h and then stained overnight in Coomassie Brilliant Blue. After destaining, gels were imaged with the Bio-Rad ChemiDoc imaging system and band intensities were analyzed using the QuantityOne software (Bio-Rad).
Western immunoblotting
The gastrocnemius muscle from right hindlimb right leg was also pulverized in liquid nitrogen and used for western immunoblotting as previously described (31). VDAC1 was used as a marker of mitochondrial content. VDAC1 and ANT1 rabbit polyclonal antibodies were purchased from Sigma-Aldrich. UCP2 polyclonal antibody was purchased from Millipore. The UCP3 rabbit polyclonal antibody was purchased from Abcam. The MitoProfile® Total OXPHOS Rodent WB Antibody Cocktail was ALOS purchased from Abcam and was used to determine the relative levels of the five mitochondrial respiratory chain complexes. Sample protein concentrations were determined using BCA assay (Pierce) and equal protein loadings were normalized to Ponceau S intensity on nitrocellulose membranes post-transfer.
Statistics
All values are reported as mean ± SEM. The numbers of mice analyzed per group are shown in the figure legends. Statistically significant differences between wild-type, mdx and sildenafil-treated mdx groups were determined by one-way ANOVA, followed by the Tukey–Kramer multiple comparison post hoc tests between paired groups. Statistical calculations were performed using the Prism version 4 software (Graphpad Software Inc.). P-values of >0.05 were considered significant.
FUNDING
This work was supported by the National Institutes of Health grants AG036606 and AG028455 (D.J.M.), NS59514 and AR056221 (S.C.F.) and the Muscular Dystrophy Association (69075 to J.M.P.).
ACKNOWLEDGEMENTS
The authors would like to thank Dr Kevin Conley for insightful discussions and Dr Kimberley Craven for critical editing of the manuscript.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Hoffman E.P., Brown R.H., Jr, Kunkel L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. doi:10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Wrogemann K., Pena S.D. Mitochondrial calcium overload: a general mechanism for cell-necrosis in muscle diseases. Lancet. 1976;1:672–674. doi: 10.1016/s0140-6736(76)92781-1. doi:10.1016/S0140-6736(76)92781-1. [DOI] [PubMed] [Google Scholar]
- 3.Millay D.P., Sargent M.A., Osinska H., Baines C.P., Barton E.R., Vuagniaux G., Sweeney H.L., Robbins J., Molkentin J.D. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat. Med. 2008;14:442–447. doi: 10.1038/nm1736. doi:10.1038/nm1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millay D.P., Goonasekera S.A., Sargent M.A., Maillet M., Aronow B.J., Molkentin J.D. Calcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanism. Proc. Natl Acad. Sci. USA. 2009;106:19023–19028. doi: 10.1073/pnas.0906591106. doi:10.1073/pnas.0906591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoppeler H., Fluck M. Plasticity of skeletal muscle mitochondria: structure and function. Med. Sci. Sports Exerc. 2003;35:95–104. doi: 10.1249/01.MSS.0000043292.99104.12. doi:10.1097/00005768-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Ogata T., Yamasaki Y. Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat. Rec. 1997;248:214–223. doi: 10.1002/(SICI)1097-0185(199706)248:2<214::AID-AR8>3.0.CO;2-S. doi:10.1002/(SICI)1097-0185(199706)248:2<214::AID-AR8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 7.Reutenauer J., Dorchies O.M., Patthey-Vuadens O., Vuagniaux G., Ruegg U.T. Investigation of Debio 025, a cyclophilin inhibitor, in the dystrophic mdx mouse, a model for Duchenne muscular dystrophy. Br. J. Pharmacol. 2008;155:574–584. doi: 10.1038/bjp.2008.285. doi:10.1038/bjp.2008.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ascah A., Khairallah M., Daussin F., Bourcier-Lucas C., Godin R., Allen B.G., Petrof B.J., Des Rosiers C., Burelle Y. Stress-induced opening of the permeability transition pore in the dystrophin-deficient heart is attenuated by acute treatment with sildenafil. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H144–H153. doi: 10.1152/ajpheart.00522.2010. doi:10.1152/ajpheart.00522.2010. [DOI] [PubMed] [Google Scholar]
- 9.Kuznetsov A.V., Winkler K., Wiedemann F.R., von Bossanyi P., Dietzmann K., Kunz W.S. Impaired mitochondrial oxidative phosphorylation in skeletal muscle of the dystrophin-deficient mdx mouse. Mol. Cell Biochem. 1998;183:87–96. doi: 10.1023/a:1006868130002. doi:10.1023/A:1006868130002. [DOI] [PubMed] [Google Scholar]
- 10.Sperl W., Skladal D., Gnaiger E., Wyss M., Mayr U., Hager J., Gellerich F.N. High resolution respirometry of permeabilized skeletal muscle fibers in the diagnosis of neuromuscular disorders. Mol. Cell Biochem. 1997;174:71–78. doi:10.1023/A:1006880529195. [PubMed] [Google Scholar]
- 11.Handschin C., Kobayashi Y.M., Chin S., Seale P., Campbell K.P., Spiegelman B.M. PGC-1α regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev. 2007;21:770–783. doi: 10.1101/gad.1525107. doi:10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selsby J.T., Morine K.J., Pendrak K., Barton E.R., Sweeney H.L. Rescue of dystrophic skeletal muscle by PGC-1α involves a fast to slow fiber type shift in the mdx mouse. PLoS ONE. 2012;7:e30063. doi: 10.1371/journal.pone.0030063. doi:10.1371/journal.pone.0030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buyse G.M., Goemans N., van den Hauwe M., Thijs D., de Groot I.J., Schara U., Ceulemans B., Meier T., Mertens L. Idebenone as a novel, therapeutic approach for Duchenne muscular dystrophy: results from a 12 month, double-blind, randomized placebo-controlled trial. Neuromuscul. Disord. 2011;21:396–405. doi: 10.1016/j.nmd.2011.02.016. doi:10.1016/j.nmd.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Percival J.M., Anderson K.N., Huang P., Adams M.E., Froehner S.C. Golgi and sarcolemmal neuronal NOS differentially regulate contraction-induced fatigue and vasoconstriction in exercising mouse skeletal muscle. J. Clin. Invest. 2010;120:816–826. doi: 10.1172/JCI40736. doi:10.1172/JCI40736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Percival J.M. nNOS regulation of skeletal muscle fatigue and exercise performance. Biophys. Rev. 2011;3:1–9. doi: 10.1007/s12551-011-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asai A., Sahani N., Kaneki M., Ouchi Y., Martyn J.A., Yasuhara S.E. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS ONE. 2007;2:e806. doi: 10.1371/journal.pone.0000806. doi:10.1371/journal.pone.0000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi Y.M., Rader E.P., Crawford R.W., Iyengar N.K., Thedens D.R., Faulkner J.A., Parikh S.V., Weiss R.M., Chamberlain J.S., Moore S.A., Campbell K.P. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. doi:10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai Y., Thomas G.D., Yue Y., Yang H.T., Li D., Long C., Judge L., Bostick B., Chamberlain J.S., Terjung R.L., Duan D. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J. Clin. Invest. 2009;119:624–635. doi: 10.1172/JCI36612. doi:10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Percival J.M., Whitehead N.P., Adams M.E., Adamo C.M., Beavo J.A., Froehner S.C. Sildenafil reduces respiratory muscle weakness and fibrosis in the mdx mouse model of Duchenne muscular dystrophy. J. Pathol. 2012;228:77–87. doi: 10.1002/path.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khairallah M., Khairallah R.J., Young M.E., Allen B.G., Gillis M.A., Danialou G., Deschepper C.F., Petrof B.J., Des Rosiers C. Sildenafil and cardiomyocyte-specific cGMP signaling prevent cardiomyopathic changes associated with dystrophin deficiency. Proc. Natl Acad. Sci. USA. 2008;105:7028–7033. doi: 10.1073/pnas.0710595105. doi:10.1073/pnas.0710595105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adamo C.M., Dai D.F., Percival J.M., Minami E., Willis M.S., Patrucco E., Froehner S.C., Beavo J.A. Sildenafil reverses cardiac dysfunction in the mdx mouse model of Duchenne muscular dystrophy. Proc. Natl Acad. Sci. USA. 2010;107:19079–19083. doi: 10.1073/pnas.1013077107. doi:10.1073/pnas.1013077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Percival J.M., Adamo C.M., Beavo J.A., Froehner S.C. Evaluation of the therapeutic utility of phosphodiesterase 5A inhibition in the mdx mouse model of duchenne muscular dystrophy. Handb. Exp. Pharmacol. 2011;204:323–344. doi: 10.1007/978-3-642-17969-3_14. doi:10.1007/978-3-642-17969-3_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das A., Xi L., Kukreja R.C. Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J. Biol. Chem. 2008;283:29572–29585. doi: 10.1074/jbc.M801547200. doi:10.1074/jbc.M801547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nisoli E., Clementi E., Paolucci C., Cozzi V., Tonello C., Sciorati C., Bracale R., Valerio A., Francolini M., Moncada S., et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. doi:10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 25.Nisoli E., Falcone S., Tonello C., Cozzi V., Palomba L., Fiorani M., Pisconti A., Brunelli S., Cardile A., Francolini M., et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc. Natl Acad. Sci. USA. 2004;101:16507–16512. doi: 10.1073/pnas.0405432101. doi:10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knott A.B., Bossy-Wetzel E. Impact of nitric oxide on metabolism in health and age-related disease. Diabetes Obes. Metab. 2010;12:126–133. doi: 10.1111/j.1463-1326.2010.01267.x. doi:10.1111/j.1463-1326.2010.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burwell L.S., Brookes P.S. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid. Redox Signal. 2008;10:579–599. doi: 10.1089/ars.2007.1845. doi:10.1089/ars.2007.1845. [DOI] [PubMed] [Google Scholar]
- 28.Percival J.M., Gregorevic P., Odom G.L., Banks G.B., Chamberlain J.S., Froehner S.C. rAAV6-microdystrophin rescues aberrant Golgi complex organization in mdx skeletal muscles. Traffic. 2007;8:1424–1439. doi: 10.1111/j.1600-0854.2007.00622.x. doi:10.1111/j.1600-0854.2007.00622.x. [DOI] [PubMed] [Google Scholar]
- 29.DiMauro S. Mitochondrial myopathies. Curr. Opin. Rheumatol. 2006;18:636–641. doi: 10.1097/01.bor.0000245729.17759.f2. doi:10.1097/01.bor.0000245729.17759.f2. [DOI] [PubMed] [Google Scholar]
- 30.Marcinek D.J., Schenkman K.A., Ciesielski W.A., Conley K.E. Mitochondrial coupling in vivo in mouse skeletal muscle. Am. J. Physiol. Cell Physiol. 2004;286:C457–C463. doi: 10.1152/ajpcell.00237.2003. doi:10.1152/ajpcell.00237.2003. [DOI] [PubMed] [Google Scholar]
- 31.Siegel M.P., Kruse S.E., Knowels G., Salmon A., Beyer R., Xie H., Van Remmen H., Smith S.R., Marcinek D.J. Reduced coupling of oxidative phosphorylation in vivo precedes electron transport chain defects due to mild oxidative stress in mice. PLoS ONE. 2011;6:e26963. doi: 10.1371/journal.pone.0026963. doi:10.1371/journal.pone.0026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcinek D.J., Schenkman K.A., Ciesielski W.A., Lee D., Conley K.E. Reduced mitochondrial coupling in vivo alters cellular energetics in aged mouse skeletal muscle. J Physiol. 2005;569:467–473. doi: 10.1113/jphysiol.2005.097782. doi:10.1113/jphysiol.2005.097782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conley K.E., Marcinek D.J., Villarin J. Mitochondrial dysfunction and age. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:688–692. doi: 10.1097/MCO.0b013e3282f0dbfb. doi:10.1097/MCO.0b013e3282f0dbfb. [DOI] [PubMed] [Google Scholar]
- 34.Blei M.L., Conley K.E., Kushmerick M.J. Separate measures of ATP utilization and recovery in human skeletal muscle. J. Physiol. 1993;465:203–222. doi: 10.1113/jphysiol.1993.sp019673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel M.P., Wilbur T., Mathis M., Shankland E., Trieu A., Harper M-E., Marcinek D.J. Impaired adaptability of in vivo mitochondrial energetics to acute oxidative insult in aged skeletal muscle. Mech. Ageing Dev. 2012;133:620–628. doi: 10.1016/j.mad.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcinek D.J., Ciesielski W.A., Conley K.E., Schenkman K.A. Oxygen regulation and limitation to cellular respiration in mouse skeletal muscle in vivo. Am. J. Physiol. Heart. Circ. Physiol. 2003;285:H1900–H1908. doi: 10.1152/ajpheart.00192.2003. [DOI] [PubMed] [Google Scholar]
- 37.Harper M.E., Green K., Brand M.D. The efficiency of cellular energy transduction and its implications for obesity. Annu. Rev. Nutr. 2008;28:13–33. doi: 10.1146/annurev.nutr.28.061807.155357. doi:10.1146/annurev.nutr.28.061807.155357. [DOI] [PubMed] [Google Scholar]
- 38.Nadtochiy S.M., Tompkins A.J., Brookes P.S. Different mechanisms of mitochondrial proton leak in ischaemia/reperfusion injury and preconditioning: implications for pathology and cardioprotection. Biochem. J. 2006;395:611–618. doi: 10.1042/BJ20051927. doi:10.1042/BJ20051927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arvier M., Lagoutte L., Johnson G., Dumas J., Sion B., Grizard G., Malthièry Y., Simard G., Ritz P. Adenine nucleotide translocator promotes oxidative phosphorylation and mild uncoupling in mitochondria after dexamethasone treatment. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1320–E1324. doi: 10.1152/ajpendo.00138.2007. doi:10.1152/ajpendo.00138.2007. [DOI] [PubMed] [Google Scholar]
- 40.Rezvani M., Cafarelli E., Hood D.A. Performance and excitability of mdx mouse muscle at 2, 5, and 13 wk of age. J. Appl. Physiol. 1995;78:961–967. doi: 10.1152/jappl.1995.78.3.961. [DOI] [PubMed] [Google Scholar]
- 41.Percival J.M., Froehner S.C. Golgi complex organization in skeletal muscle: a role for Golgi-mediated glycosylation in muscular dystrophies? Traffic. 2007;8:184–194. doi: 10.1111/j.1600-0854.2006.00523.x. doi:10.1111/j.1600-0854.2006.00523.x. [DOI] [PubMed] [Google Scholar]
- 42.Prins K.W., Humston J.L., Mehta A., Tate V., Ralston E., Ervasti J.M. Dystrophin is a microtubule-associated protein. J. Cell Biol. 2009;186:363–369. doi: 10.1083/jcb.200905048. doi:10.1083/jcb.200905048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romanello V., Sandri M. Mitochondrial biogenesis and fragmentation as regulators of muscle protein degradation. Curr. Hypertens. Rep. 2010;12:433–439. doi: 10.1007/s11906-010-0157-8. doi:10.1007/s11906-010-0157-8. [DOI] [PubMed] [Google Scholar]
- 44.Koves T.R., Noland R.C., Bates A.L., Henes S.T., Muoio D.M., Cortright R.N. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am. J. Physiol. Cell Physiol. 2005;288:C1074–C1782. doi: 10.1152/ajpcell.00391.2004. doi:10.1152/ajpcell.00391.2004. [DOI] [PubMed] [Google Scholar]
- 45.Ritov V.B., Menshikova E.V., He J., Ferrell R.E., Goodpaster B.H., Kelley D.E. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. doi:10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 46.Hopf F.W., Turner P.R., Steinhardt R.A. Calcium misregulation and the pathogenesis of muscular dystrophy. Subcell. Biochem. 2007;45:429–464. doi: 10.1007/978-1-4020-6191-2_16. doi:10.1007/978-1-4020-6191-2_16. [DOI] [PubMed] [Google Scholar]
- 47.Maglara A.A., Vasilaki A., Jackson M.J., McArdle A. Damage to developing mouse skeletal muscle myotubes in culture: protective effect of heat shock proteins. J. Physiol. 2003;548:837–846. doi: 10.1113/jphysiol.2002.034520. doi:10.1113/jphysiol.2002.034520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y.W., Zhao P., Borup R., Hoffman E.P. Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J. Cell Biol. 2000;151:1321–1336. doi: 10.1083/jcb.151.6.1321. doi:10.1083/jcb.151.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y.W., Nagaraju K., Bakay M., McIntyre O., Rawat R., Shi R., Hoffman E.P. Early onset of inflammation and later involvement of TGFβ in Duchenne muscular dystrophy. Neurology. 2005;65:826–834. doi: 10.1212/01.wnl.0000173836.09176.c4. doi:10.1212/01.wnl.0000173836.09176.c4. [DOI] [PubMed] [Google Scholar]
- 50.Kotelnikova E., Shkrob M.A., Pyatnitskiy M.A., Ferlini A., Daraselia N. Novel approach to meta-analysis of microarray datasets reveals muscle remodeling-related drug targets and biomarkers in Duchenne muscular dystrophy. PLoS Comput. Biol. 2012;8:e1002365. doi: 10.1371/journal.pcbi.1002365. doi:10.1371/journal.pcbi.1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haycock J.W., MacNeil S., Jones P., Harris J.B., Mantle D. Oxidative damage to muscle protein in Duchenne muscular dystrophy. Neuroreport. 1996;8:357–361. doi: 10.1097/00001756-199612200-00070. doi:10.1097/00001756-199612200-00070. [DOI] [PubMed] [Google Scholar]
- 52.Ragusa R.J., Chow C.K., Porter J.D. Oxidative stress as a potential pathogenic mechanism in an animal model of Duchenne muscular dystrophy. Neuromuscul. Disord. 1997;7:379–386. doi: 10.1016/s0960-8966(97)00096-5. doi:10.1016/S0960-8966(97)00096-5. [DOI] [PubMed] [Google Scholar]
- 53.Crabb H., Fiorotto M.L., Grounds M.D. The different impact of a high fat diet on dystrophic mdx and control C57Bl/10 mice. PLoS Curr. 2011;3:RRN1276. doi: 10.1371/currents.RRN1276. doi:10.1371/currents.RRN1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Medeiros D.M. Assessing mitochondria biogenesis. Methods. 2008;46:288–294. doi: 10.1016/j.ymeth.2008.09.026. doi:10.1016/j.ymeth.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 55.Marcinek D.J., Kushmerick M.J., Conley K.E. Lactic acidosis in vivo: testing the link between lactate generation and H+ accumulation in ischemic mouse muscle. J. Appl. Physiol. 2010;108:1479–1486. doi: 10.1152/japplphysiol.01189.2009. doi:10.1152/japplphysiol.01189.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]