Abstract
Objective
Inflammation promotes epidermal wound healing but is considered detrimental to recovery from CNS injury. Sick infants have increased levels of cytokines in their CSF that correlate with poor neurological outcome. In this study we investigated the role of neuroinflammation and more specifically, IL-6, in the amplification of subventricular zone (SVZ) and subgranular zone (SGZ) neural precursors after neonatal brain injury.
Methods
Neonatal hypoxia/ischemia (H/I) was induced in P6 rat pups and IL-6 was quantified with or without Indomethacin administration. Neural precursor responses were evaluated by neurosphere assays as well as by stereological analyses. Studies were performed to determine how IL-6 and LIF affect SVZ cell expansion, proliferation and self-renewal.
Results
Consistent with earlier studies, SVZ cells expanded after H/I. Contrary to our expectations, Indomethacin significantly decreased both the initial reactive increase in these precursors as well as their ability to self-renew. By contrast, Indomethacin increased proliferation in the SGZ and lateral SVZ. Indomethacin diminished the accumulation of microglia/macrophages and IL-6 production after H/I. In vitro IL-6 enhanced neurosphere growth, self-renewal and tripotentiality and was more effective than LIF in promoting self-renewal. Enhanced precursor self-renewal also was obtained using PGE2, which is downstream of cyclooxygenase-2 and a target of Indomethacin.
Interpretation
These data implicate neuroinflammation and in particular IL-6 as a positive effector of primitive neural precursor expansion after neonatal brain injury. These findings have important clinical implications, as Indomethacin and other anti-inflammatory agents are administered to premature infants for a variety of reasons.
Keywords: Neurogenesis, subventricular zone, stem cells, regenerative medicine, inflammation, rats, brain damage
Introduction
Inflammatory processes occur almost immediately after tissue damage to peripheral organs and the central nervous system. While inflammation is ubiquitous its effect will differ depending upon the location and type of injury. In wound healing inflammation is regarded as necessary for repair1, 2 with IL-6 regarded as especially important3, 4. Thus, knocking out IL-6 delays healing whereas increased levels accelerate wound repair5. By contrast, inflammation is viewed as detrimental to recovery from neurological injury. Neuroinflammation has been implicated in Alzheimer’s disease, Parkinson’s disease, ALS, Multiple Sclerosis, H/IV dementia, spinal cord injury and stroke with anti-inflammatory drugs showing improved outcome6–11.
In the infant increased levels of cytokines in the brain and CSF correlate with poor neurological outcomes12–16 and reducing inflammation and levels of pro-inflammatory cytokines is known to decrease the severity of injury17–20. IL-6 is consistently and strongly elevated in the neonatal CNS after injury15, 21–24. For instance, Ellison et al., 2005 found that IL-6 was elevated 8 fold in the CSF of preterm infants who exhibited abnormalities on their MRIs, whereas TNFα was increased 3 fold 22. Savman et al. (1998) found that IL-6 was significantly elevated to 250 pg/mL in the CSF of asphyxiated infants23. Elevated levels of IL-6 have also been reported in animal models of developmental brain damage 25. Recent evidence has implicated a functional polymorphism in the IL-6 gene as a risk factor in cerebral palsy, which is associated with perinatal brain injury26, 27.
Studies on the specific role of IL-6 in CNS pathogenesis have produced mixed results, supporting the view that the effects of IL-6 are dose and context dependent. Mice overexpressing IL-6 develop severe neurologic syndromes that included ataxia, seizure and tremor, and as well as profound astrogliosis and microgliosis. Hippocampal neurons in these mice possess abnormal dendrites and the animals develop a progressive learning impairment28. On the other hand, adult IL-6 null mice sustain more severe traumatic and excitotoxic injuries29, 30.
Only a few studies have evaluated the role of IL-6 in CNS regeneration. Monje et al. (2003) and Hoehn et al (2005) demonstrated that LPS induced inflammation decreased the proliferation of precursors in the hippocampal SGZ, depressing hippocampal neurogenesis 31, 32. They correlated this decrease with increased activated microglia and IL-6 and found that injecting Indomethacin, a non-specific anti-inflammatory drug, reversed the effects on the precursors in the hippocampus and the cerebral cortex. Co-culturing hippocampal progenitors with microglial cells activated by LPS, or exposing them to microglial-conditioned medium (CM) altered survival and differentiation31, 33, 34. Using transgenic techniques Vallieres et al showed that long term exposure to IL-6 decreased overall neurogenesis by 63% in the hippocampal dentate gyrus35. These studies have strengthened the prevailing view that depressing inflammation will decrease the severity of damage and promote repair.
The SVZ is the largest reservoir of somatic stem cells in the brain and it expands in response to CNS injuries36–39. This regenerative response persists after injury37, 40, 41. There is a doubling of tripotential stem/progenitor cells within the SVZ 36 that precedes the production of new neocortical and striatal neurons37, 39, 42. While the SVZ expands we do not fully understand the mechanisms that mediate this increase. Previously we found that LIF, a member of the IL-6 family of cytokines, increases in the SVZ after H/I and promotes the expansion and proliferation of neural stem/progenitors (NSPs)43 but the role of IL-6 itself has not been evaluated. Therefore, the aim of the studies reported here was to examine the effect of neuroinflammation, and more specifically, the role of IL-6, in the expansion of neural precursors in the SVZ and SGZ after a neonatal brain injury.
Methods and Materials
Neonatal Hypoxia/Ischemia and drug administration
All experiments were performed in accordance with research guidelines set forth by the IACUC committee of the New Jersey Medical School. Cerebral H/I was produced in 6-day-old rats (day of birth = P0) by cauterizing the right common carotid artery followed by systemic hypoxia (pups were exposed to 80 minutes of humidified 8% O2/92% N2) as described previously44. Hypoxic-ischemic or control pups were injected with either 2.5mg/kg Indomethacin (Sigma, St. Louis, MO) or vehicle (1% EtOH/PBS) intraperitoneally immediately following H/I and then once every 12 hours for 2 days. Intraperitoneal injections of 5′-bromo-2′-deoxyuridine (BrdU) (50mg/kg, Sigma), were administered once at 3 days of recovery from H/I. Rats were perfused 4 h later. This short interval is sufficient for BrdU to incorporate into cells in S-phase but too brief for migration to occur 45, 46.
Immunohistochemistry
Animals were deeply anesthetized, perfused with 4% paraformaldehyde, and the brains cryoprotected. Immunofluorescent staining was performed on 20μm cryostat sections. The following antibodies were used: anti-BrdU (rat monoclonal, 1:30, Accurate, Westbury, NY); anti-GFAP (rabbit polyclonal, 1:500, Dako, Carpinteria, CA); anti-IBA-1 (1:200 Wako, Tx). Secondary antibodies against the appropriate species were incubated for 2 h at RT (Jackson, West Grove, PA). DAPI (Sigma, 1 μg/ml) was used for 15 min. to counterstain nuclei.
Microscopy
Images of fluorescently immunolabeled sections were acquired using an Olympus AX70 microscope and imaged using a Photometrics cooled charged coupled device camera (Tucson, AZ) interfaced with IP Lab scientific imaging software (Scanalytics Fairfax, VA). Cell counts for BrdU+ cells in the medial SVZ, the mediolateral SVZ and the SGZ were obtained using the Cavalieri and optical dissector methods. We defined the medial zone as the highly dense region that extends approximately 20μm from the lateral ventricle. The mediolateral region extends from approximately 20μm to 200 μm from the lateral ventricle. The SGZ was defined as the region between the granular cell layer and the hilus (CA4 layer) of the hippocampus. The Cavalieri method was used to measure the total reference volume (Vref) and the number of BrdU+ cells per unit volume of the regions of interest. Neuronal density was measured using the optical dissector method.
Protein Isolation and IL-6 ELISA
SVZs were micro-dissected from control, H/I, Indomethacin treated H/I and Vehicle treated H/I rat pups and placed into ice cold PBS containing protease inhibitor cocktail, PSMF, sodium orthovanadate and sodium fluoride (Sigma). Samples were homogenized, sonicated and then centrifuged at 15,000 rpm at 4°C for 15 minutes. The protein concentration of each lysate was measured using a BCA assay (Pierce, Rockford, IL) prior to storage at −80°C.
Flat bottom 96 well plates (Nunc, Maxisorp immunoplates) were coated overnight with anti-IL-6 antibody at 4° C (R & D Systems, Minneapolis, MN). Wells were washed, blocked and incubated with either dilutions of rmIL-6 or samples overnight at 4° C. The next day wells were washed and biotinylated anti-IL-6 antibody (R & D Systems) added for 2 hours at 37°C followed by washes and then incubation with AP-conjugated Streptavidin for 1 hour at 37°C. Wells were incubated with p-nitrophenyl phosphate (Pierce) in substrate buffer for 90 minutes at 37°C. The plate was read at 405 nm on a plate reader (Thermo, Waltham, MA) and the data analyzed.
Neurosphere Experiments
Neurospheres were prepared from normal Wistar pups (P2-7) as previously described36. After 6–7 days primary neurospheres were collected and passaged to form secondary neurospheres. Secondary neurospheres were cultured in a chemically defined medium (ProN) supplemented to 20 ng/mL EGF and 10 ng/ml FGF-2. rmIL-6 and rrLIF were used at 5 ng/mL (R & D Systems). Indomethacin (Sigma) was dissolved in ethanol and used at 2 μg/mL. NS398 (Sigma) was used at 3 μg/mL. Dimethyl PGE2 (Cayman Chemicals) was used at 5 μg/mL. Cells were propagated for 6 days prior to analysis.
A neurosphere was defined as a free-floating, cohesive cluster that was at least 30 μm in diameter, although the vast majority of neurospheres (>98%) were substantially larger than this. Plates were gently shaken before counting each well to ensure an even distribution of spheres. 5 random 4X fields were counted per well and at least 3 wells evaluated per group. The frequency of sphere-forming cells was calculated from the average number of spheres per field, the area of the field and the area of the well.
Quantitative real-time PCR analysis
Total RNA was isolated from the SVZs after 1, 2, 3, and 4 days of recovery from H/I, as described previously36. Primers for quantitative real-time polymerase chain reaction (Q-PCR) were purchased from Invitrogen (La Jolla, CA) or Applied Biosystems (Foster City, CA). Analysis was performed using an ABI Prism 7700 Sequence Detection System (Applied Biosystems). The relative amount of message was normalized to the level of 18S ribosomal RNA. Fold-changes in gene expression relative to a housekeeping gene were obtained using the Relative Expression Software Tool (REST) for groupwise comparison and statistical analysis of relative expression results in real-time PCR47.
Statistical analysis
The results obtained from the qPCR analysis were analyzed using the REST program47 as noted above. The REST program utilizes pair-wise fixed reallocation randomized testing to determine if there is a significant change in the expression of the target gene. This test is considered more flexible than non-parametric tests and does not suffer from the same reduction of power as parametric tests. Results from the other experiments were analyzed for statistical significance using a t test or by ANOVA, and all error bars represented SEMs. Post-hoc analysis was applied to evaluate inter-group differences. Comparisons were interpreted as significant when associated with p<0.05.
Results
IL-6 but not CNTF mRNA increases after neonatal H/I
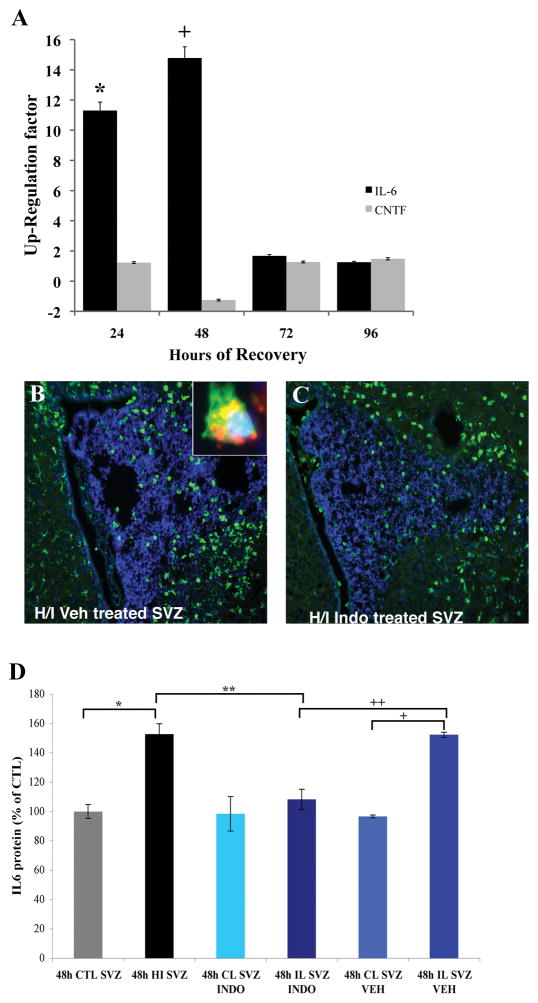
It has been shown that the LIFR/gp130 receptor heterodimer maintains embryonic and adult neural stem cells in vitro48, supporting the hypothesis that ligands for this complex might stimulate the increase in NSPs observed during recovery from H/I. To assess levels of IL-6 after H/I we microdissected SVZs from the ipsilateral (ILH) and contralateral hemispheres (CLH) of H/I animals at intervals of recovery spanning from 24 hours to 4 days. Using qPCR we observed a significant increase in IL-6 mRNA relative to 18S in the ILH compared to the CLH (Figure 1A). At 24 and 48 hours of recovery IL-6 was induced 11.5 and 15 fold respectively (Figure 1A, n= 6, *, + p < 0.05). At 72 hours IL-6 expression returned to control levels and remained unchanged at 4 days of recovery (Figure 1A, p > 0.05).
Figure 1.
IL-6 production increases in the SVZ after injury but is decreased by treatment with Indomethacin. Panel A shows the change in IL-6 mRNA expression over 4 days of recovery after neonatal H/I. IL-6 mRNA in the ipsilateral SVZ was compared to the contralateral SVZ by qPCR. There is a peak in expression 48h after H/I (n = 6 at all time points) (*, p < 0.05). There is no significant increase in the expression of CNTF mRNA. Statistical significance was determined using the REST program 47. Panels B and C depict IBA-1 staining for microglia in the SVZ at 72 hours of recovery in the H/I Vehicle compared to H/I Indomethacin treated animals. There was a decrease in IBA-1 staining in the H/I Indomethacin treated SVZ. The inset in panel B depicts IL-6 (green) in an IBA-1+ (red) microglial cell. Panel E shows the change in IL-6 protein level after 2 days of treatment with either Vehicle or Indomethacin. IL-6 protein was significantly elevated in the SVZ after H/I compared to Control (* p < 0.05) and in the IL H/I Vehicle SVZ compared to CL H/I Vehicle SVZ (+ p < 0.05). Indomethacin significantly decreased IL-6 levels in the H/I Indomethacin SVZ compared to H/I SVZ and H/I Vehicle SVZ (**, ++ p < 0.05).
Microglial activation is decreased by Indomethacin
Several studies have shown that H/I injury activates microglia and we observed microglial accumulation in the SVZ after H/I. To assess whether inhibiting neuroinflammation would reduce the number of microglia in the SVZ we administered Indomethacin immediately after H/I and for the first 2 days of recovery. The abundance of microglia in either vehicle or 2.5mg/kg Indomethacin (H/I Indo) was compared to controls. We found strong microgliosis in the H/I Vehicle both within the infarct and SVZ compared to controls, but there were fewer microglia in the H/I Indo group (Figure 1B, C, Supplemental Figure 1A, *, **, + p < 0.05). Staining for IBA-1 and IL-6 showed IL-6 production by IBA-1 positive microglia (Figure 1B, inset). We also observed a significant increase in GFAP staining intensity in the H/I groups that was decreased by Indomethacin. (Supplemental Figure 1B, *, **p < 0.05). To determine whether H/I was sufficient to activate microglia, we exposed cultured microglia to hypoxic/hypoglycemic conditions and found that this stimulus significantly induce IL-6 mRNA expression (Supplemental Figure 1C, *, **p < 0.05).
Indomethacin decreases the levels of IL-6 protein in the SVZ after H/I
To determine whether reducing inflammation with Indomethacin would reduce IL-6 protein levels we microdissected SVZs from experimental and control animals at 3 days of recovery and analyzed IL-6 levels by ELISA. In H/I and H/I Vehicle animals IL-6 protein increased compared to CTL and administering Indomethacin returned IL-6 levels to control (Figure 1D, ** p < 0.05).
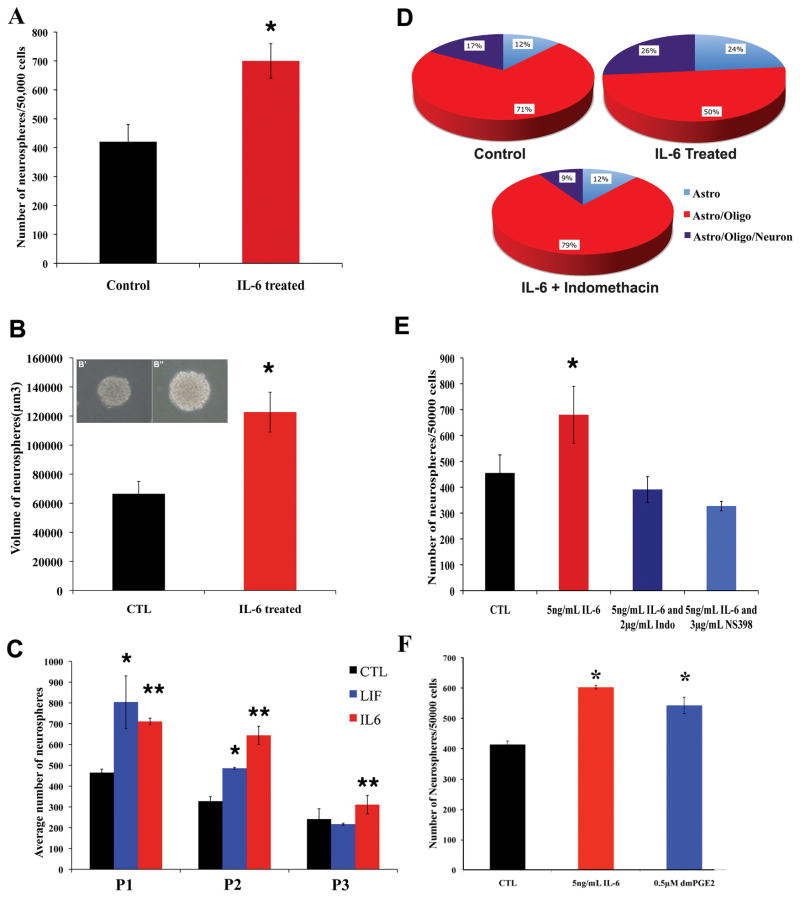
A greater number of neurospheres are formed after the addition of IL-6
The clonal neurosphere assay is an important method for quantifying numbers of NSPs in the brain. Adding IL-6 to the media increased the number of secondary neurospheres by ~2 fold (Figure 2A, p < 0.05). These neurospheres were also significantly larger than control (Figure 2B, p < 0.05). When continuously maintained in IL-6 over passages there were significantly more tertiary and quaternary neurospheres than control (Figure 2C, p < 0.05). Furthermore, IL-6 more effectively maintained NSPs than LIF. When secondary neurospheres were differentiated to measure potentiality, NSPs, maintained in IL-6 produced more tri-lineage colonies (Figure 2D, p < 0.05). These results show that IL-6 can serve as a signal to expand NSPs.
Figure 2.
IL-6 enhances neural stem cell expansion/maintenance, which can be inhibited by COX inhibitors. Primary neurospheres were dissociated and replated into either control media or media supplemented with 5ng/mL of rmIL-6. The number (A) and size (B) of spheres was quantified after 6 days. IL-6 increased the number of secondary neurospheres (*, p < 0.05) and the spheres were also larger (*, p < 0.05). Panels B′ and B″ show representative neurospheres cultured in standard medium or medium supplemented with IL-6. Panel C depicts the average number of neurospheres obtained over multiple passages in medium supplemented with either 5 ng/mL IL-6 or 5 ng/mL LIF. Only IL-6 increased the number of neurospheres produced at each passage compared to control media (*, **, ***, p < 0.05). Panel D summarizes results of cell differentiation assays after neurosphere growth in IL-6, IL-6 + 2 μg/mL Indomethacin or control medium. A greater percentage of the spheres grown in 5ng/mL of IL-6 were tripotential compared to Control, which was completely antagonized by Indomethacin. In Panel E, primary neurospheres were dissociated and replated into either control media, media supplemented with 5ng/mL of IL-6, media supplemented with 5ng/mL of IL-6 and 2 μg/mL Indomethacin (non specific COX inhibitor), or media supplemented with 5 ng/mL of IL-6 and 3 μg/mL NS398 (Cox 2 inhibitor) for 6 days. Both Indomethacin and NS398 antagonized the IL-6 stimulated increase in neurosphere expansion/maintenance (* p < 0.05). Panel F depicts effects of 5ng/mL of IL-6, or medium supplemented with 0.5 μM of dmPGE2 for 6 days. Both IL-6 and dmPGE2 increased the number of secondary neurospheres.
Indomethacin directly inhibits the stimulating effects of IL-6 on self-renewal
To determine whether Indomethacin attenuates the stimulating effects of IL-6 directly or indirectly, neurospheres were cultured in the presence of IL-6 and their differentiation potential was measured. The addition of Indomethacin completely antagonized the expansion of tripotential NSPs and even reduced their number to below control (Fig 2D). To determine whether these effects were due to activation of Cox 1 or Cox 2 NSPs were stimulated with IL-6 in the presence of either Indomethacin or NS398 (Cox 2 inhibitor). Quantitative analyses revealed that the addition of either Indomethacin or NS398 completely inhibited the ability of IL-6 to promote NSPs expansion and reduced the percentage of tripotential colonies (Figure 2E, p < 0.05). Culturing neurospheres in 5 μg/mL dimethyl prostaglandin E2 (dmPGE2) increased neurosphere number similar to that observed with IL-6 (Figure 2F).
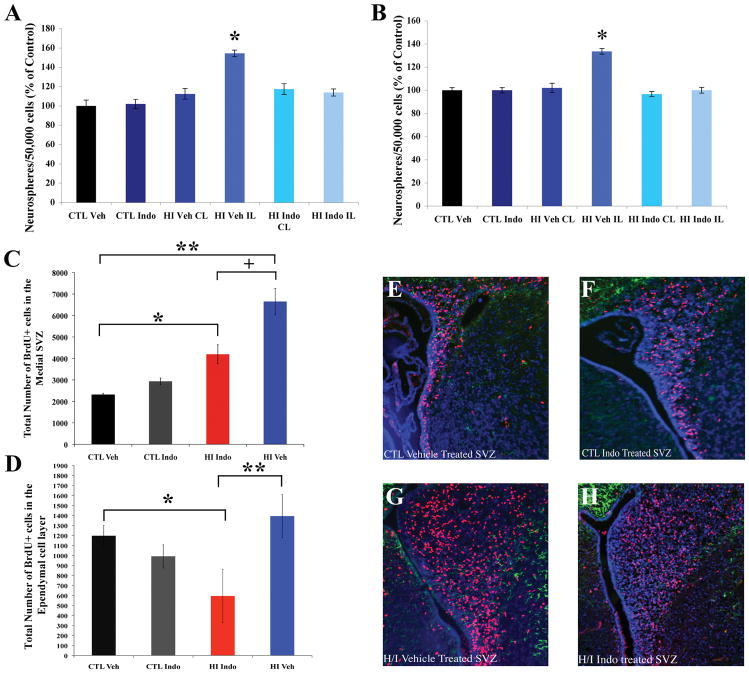
Indomethacin inhibits neural stem/progenitor cell expansion after hypoxia/ischemia
To assess whether treating neonatal pups with Indomethacin would inhibit NSP expansion after H/I we isolated SVZs from CTL Vehicle, CTL Indomethacin, H/I Vehicle and H/I Indomethacin treated animals and assayed the number of primary neurospheres formed. Quantitative analyses revealed that administering Indomethacin for the first two days of recovery after H/I significantly decreased the number of neurospheres formed (Figure 3A, p < 0.05). To determine whether this difference was due to a change in the number of NSPs or their responsiveness to growth factors we passaged the primary neurospheres to create secondary neurospheres. The number of secondary neurospheres was increased in the H/I Vehicle group when compared to the other groups (Figure 3B, p < 0.05).
Figure 3.
Indomethacin antagonizes NSP amplification of after H/I. Animals were separated into treatment groups and the neurosphere assay was performed at 3 days of recovery. Panel A depicts the number of primary neurospheres obtained. Panel B depicts the number of secondary spheres obtained. Cumulatively, these data indicate that Indomethacin decreases the number of NSPs rather than changing their responsiveness to growth factors (* p < 0.05). Another cohort of animals received BrdU at 3 days of recovery, 4 hours prior to sacrifice. Panel C depicts the number of BrdU+ cells in the medial SVZ across treatment groups. There was 3-fold increase in BrdU labeled cells in H/I Vehicle treatment when compared to Controls (** p < 0.05) and this increase was reduced with Indomethacin treatment. However, there were more proliferating cells in the Indomethacin treated animals compared to Controls (* p < 0.05). Panel D depicts the number of BrdU+ cells in the ependymal cell layer of SVZ across treatment groups. There was 2-fold decrease in BrdU labeled cells in H/I Indo treatment when compared to Controls (* p < 0.05) and to H/I Vehicle treatment (** p < 0.05). Panels E–H show representative images of BrdU+/GFAP+ staining in the SVZ of Vehicle, Indomethacin, H/I Vehicle, and H/I Indomethacin treated animals. Scale bar represents 20 μm.
As the neurosphere assay is a retrospective analysis we injected a single pulse of BrdU on day 3 of recovery, 4 h prior to sacrifice, to analyze proliferation of precursors in the medial aspect of the SVZ and progenitors in the more lateral aspect. There was a significant decrease in the number of BrdU+ cells in the medial aspect of the SVZ of H/I Indomethacin group compared to H/I Vehicle animals (Figure 3C; 3E–H, p < 0.05). Studies suggest that the ependymal cell layer contains a subset of neural stem cells, and previously we had documented proliferation in both the ependymal cell; however, we had not quantified the relative numbers of proliferating ependymal cells 36, 49. At 3 days of recovery, the number of BrdU+ cells in the ependymal cell layer did not change after H/I and actually decreased in the H/I Indomethacin group compared to CTL and H/I Vehicle animals (Figure 3D, p < 0.05).
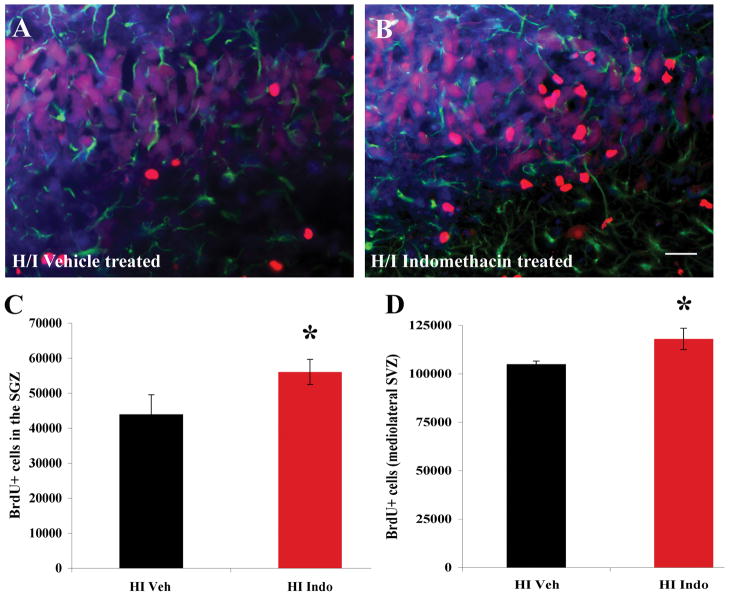
Indomethacin increases the number of BrdU+ cells in the SGZ of the hippocampus and in the mediolateral SVZ but does not confer neuroprotection
Previous studies have reported that Indomethacin increases BrdU+ cells in the SGZ of the hippocampus during recovery from H/I and irradiation induced damage in adults. As our studies were performed on neonates the differences in the results we observed could be due to either age or regional differences. To rule out age related differences we quantified the total number of BrdU+ cells in the SGZ of the hippocampus of H/I Vehicle vs. H/I Indomethacin treated animals. We found more BrdU+ cells in the SGZ of the H/I Indomethacin group compared to the H/I Vehicle group consistent with previous reports (Figure 4A – C) (* p < 0.05). Quantifying the total number of BrdU+ cells in the mediolateral SVZ revealed a similar result where the H/I Indomethacin treated animals had more BrdU+ cells compared to the H/I Vehicle group (Figure 4D * p < 0.05). To evaluate whether the decrease in NSPs observed was due to a decrease in the severity of H/I we examined the brains of CTL Vehicle, Indo, H/I Vehicle and Indo 30 days after the last injection of Indomethacin. A quantitative analysis of neocortical volume failed to reveal any significant difference in the area of the H/I Indo cortices compared to the H/I Vehicle cortices. Indomethacin had no effect on the neocortical volume of uninjured animals (Supplemental figure 2).
Figure 4.
Indomethacin increases the number of proliferating precursors in the SGZ of the hippocampus and the lateral aspect of the SVZ. Stereological counting methods were used to quantify the number of BrdU+ cells in the SGZs and SVZs of control and injured brains. Panels A and B show representative images of the BrdU+ cells (red) in the SGZ of H/I Vehicle and H/I Indomethacin, respectively. Sections were also stained for GFAP (green) and for DAPI (blue). Scale bar represent 20 μm. Panel C depicts the average # of BrdU+ cells/SGZ. As illustrated, Indomethacin increased the number of BrdU+ cells in the SGZ after H/I compared to H/I Vehicle (* p < 0.05). Panel D depicts the average # of BrdU+ cells/lateral SVZ. Indomethacin increased the number of BrdU+ cells in the lateral SVZ after H/I compared to H/I Vehicle (* p < 0.05).
Discussion
There is an unavoidably large cohort of infants who sustain developmental brain injuries where intrauterine infection and hypoxemia are contributing factors. These injuries contribute to a range of neurodevelopmental disorders that include cerebral palsy, attention deficit and hyperactivity disorder, epilepsy and more subtle cognitive, emotional and motor disorders 50,51. As reviewed in the Introduction, elevated levels of cytokines in the CSF correlate with poor outcome and in animal models of developmental brain injuries suppressing neuroinflammation is generally neuroprotective. Clinically the prophylactic use of Indomethacin decreases intraventricular and periventricular hemorrhage52–57. Ment and colleagues have reported favorable neurological outcomes subsequent to Indomethacin treatment, predominantly in males, but a number of other studies have reported no significant long-term improvement56–59. In light of the clinical use of Indomethacin and the continued interest in anti-inflammatory therapies to prevent brain injury to infants, we were interested in evaluating how suppressing neuroinflammation after neonatal hypoxic/ischemic brain damage will affect the regenerative responses of the subventricular and subgranular zones.
Here we report that IL-6 mRNA and protein increase significantly during the acute recovery interval from H/I and by immunofluorescence, IL-6 co-localized to microglia/macrophages within the SVZ. Goings et al. (2006) reported that while IL-6 was produced in the SVZ by microglia, aspiration lesions of the cortex didn’t induce an increase in SVZ IL-6 levels60. However, those studies were performed in mice, whereas ours were performed in rats; and in parallel studies that we have performed in neonatal mice, IL-6 is more rapidly increased and decreased in mice vs. rats. Consistent with earlier studies, neural stem/progenitors proliferated in the SVZ after H/I. But, contrary to our expectations, administering Indomethacin significantly decreased both the initial reactive increase in these precursors as well as their ability to self-renew. Furthermore, there were reduced numbers of proliferating cells in the neural stem cell niche of the SVZ after Indomethacin treatment following H/I. By contrast, Indomethacin increased proliferation in the SGZ during recovery from injury. Supporting an important role for microglial produced IL-6, Indomethacin diminished the accumulation of microglia/macrophages and IL-6 production in the SVZ after H/I, and IL-6 enhanced neurosphere growth, self-renewal and tripotentiality in vitro. Consistent with a direct effect of IL-6 on SVZ precursors, Indomethacin and NS398 blocked the IL-6 induced increase in neurospheres in vitro. Altogether, our results indicate that some inflammatory modulators, like IL-6, may be necessary for neural stem/progenitor cell amplification.
Injuries to the brain stimulate a reactive increase in the size of the SVZ, number of tripotential NSPs and neuron production36, 37, 39, 61. We previously showed that LIF is induced within the SVZ with the same time course as we show here for IL-6 and that in vitro, LIF promotes NSC self-renewal. Similar in vitro effects have been reported for the related cytokine CNTF62. Both CNTF and LIF are members of the IL-6 superfamily and they have been shown to regulate NSC homeostasis via the LIFR/gp130 receptor heterodimer through the induction of Notch48. Compared to LIF, IL-6, which stimulates cells through a gp130 homodimer, more effectively promoted NSC self-renewal (data not shown). The related cytokine CT-1 is neuroprotective63, however, we have previously shown that CT-1 is not elevated after neonatal H/I43.
There is evidence to suggest that inflammation creates an inhospitable environment for precursor proliferation and neurogenesis in the adult brain64. A number of studies have evaluated the effect of Minocycline on adult neural precursor responses to inflammation and to stroke 65, 66 while other studies evaluated the effects of Indomethacin 31, 32. They all reported that anti-inflammatory compounds enhanced the proliferation of precursors in both the hippocampal SGZ and the forebrain SVZ, thus they concluded that neuroinflammation suppresses CNS regeneration. One study however has reported a decrease in BrdU+ cells in the SVZ following Minocycline administration after focal cerebral ischemia67. This, coupled with our results reveals a more complex response by neural precursors to neuroinflammation. One might conclude that neuroinflammation differentially affects neonatal vs. adult neural stem/progenitors, but this explanation is not tenable as we also found that Indomethacin increased the proliferation of precursors in the neonatal SGZ and in lateral aspects of the SVZ. Thus, it is not likely that the differences in our conclusions are due to differential responses of neonatal vs. adult neural precursors to neuroinflammation. Rather it is more likely that neuroinflammation, and more specifically, IL-6, exerts different effects on stem cells vs. progenitors.
Seaburg and Van der Kooy (2002) and Bull and Bartlett (2005) reported that the precursors in the hippocampus are lineage restricted progenitors and not stem cells68, 69. Furthermore, their data indicated that there are two discrete populations of progenitors in the SGZ, one giving rise to neurons while the other gives rise to glial cells. Monje et al 31 reported that neuroinflammation inhibited hippocampal neurogenesis and that IL-6 reduced neurogenesis in vitro. We observed a similar effect on the neonatal SGZ precursor responses to H/I. However, we report that Indomethacin reduces the proliferation of cells in the most medial aspect of the SVZ after injury, which numerous studies have shown is where the NSCs reside. We also report that IL-6 promotes neural stem cell self-renewal more effectively than LIF, and that in vivo Indomethacin directly abrogates NSP expansion, as measured by the neurosphere self-renewal and differentiation. Supporting the conclusion that neuroinflammation inhibits the proliferation of progenitors, we observed increased proliferation of precursors in the more lateral regions of the SVZ with Indomethacin administration after neonatal H/I, and we also observed increased proliferation in the neonatal SGZ with Indomethacin treatment. We cannot however, discount another interpretation of our data which is that the neural stem cells of the SVZ respond positively to neuroinflammation whereas the neural stem cells of the hippocampal SGZ respond negatively.
It has been suggested that Indomethacin, by releasing the inhibitory effects of cytokines on neuronogenesis will promote regeneration. However, preventing neural stem cell expansion may negate the beneficial outcomes to be expected from Indomethacin as pertains to cell replacement. Progenitors, by definition, possess limited self-renewal capacity, and it has been shown that most neural progenitors can only divide 8 times70. If a progenitor divides symmetrically 8 times, then 1 progenitor will make 256 cells. By contrast, if a stem cell divides asymmetrically every 14 days, with each division producing one stem cell and one progenitor, then 1 stem cell will produce 25 progenitors each year71. If each of those progenitors produces 256 cells, then for each additional stem cell produced there will be 6500 new cells generated per year. Clearly conditions that permit or promote the expansion of neural stem cells will promote regeneration more effectively than those that enhance progenitor cell expansion.
North et al (2007) showed that hematopoietic stem cell maintenance required Cox-2 and that dmPGE2 increased the numbers of hematopoietic stem cells in zebrafish and mice72. IL-6 has been shown to induce Cox-2 in microglia and Cox-2 will increase production of prostaglandins73. In this study we show that Indomethacin, a non-specific Cox inhibitor, and NS398, a specific Cox-2 inhibitor, prevented IL-6 from increasing the number of NSPs. Furthermore, dmPGE2 increased the number of self-renewing neural precursors. These results suggest that IL-6 is activating Cox-2 in neural precursors to catalyze the production of a number of prostanoids including prostaglandin E2. But, IL-6 is likely activating several pathways to induce NSP expansion, which are areas of investigation that we are actively pursuing.
The findings from this study, that inflammation and more specifically IL-6 promote the expansion of SVZ NSPs to increase the production of multipotent progenitors has important implications for managing sick infants. Using anti-inflammatory compounds or other techniques to suppress inflammation may alter the response of microglia and rob the damaged brain of factors that are necessary to launch a robust endogenous repair response 74, 75. Recently, Iosif et al reported that another pro-inflammatory cytokine, TNFα, decreased neural precursor proliferation, and SVZ proliferation after stroke was increased in mice lacking the TNF type 1 receptor76. Katakowski et al implicated tumor necrosis factor-alpha-converting enzyme proteolysis77. Clearly further studies are needed to more fully understand when neuroinflammation might be beneficial during recovery from ischemic injuries and which cytokines (at specific concentrations) are promoting or inhibiting regenerative processes.
Supplementary Material
Supplemental Figure 1: Indomethacin decreases both IBA-1 and GFAP florescent intensity. Panel A shows there was an increase in IBA-1 florescent intensity in the H/I Veh treated SVZ when compared to Controls (*, p < 0.05) and H/I Indomethacin treated (**, p < 0.05). However, there was more IBA-1 florescent intensity in the Indomethacin treated animals compared to Controls (+ p < 0.05). Panel B shows IBA-1 staining (red) and IL-6 staining (green) of the striatum and SVZ at 72 hours of recovery. Virtually all of the IL-6+ cells were IBA-1 positive. Panel C depicts an increase in GFAP florescent intensity in the H/I Veh treated SVZ when compared to Controls (*, p < 0.05) and H/I Indomethacin treated (**, p < 0.05). However, there was more GFAP florescent intensity in the Indomethacin treated animals compared to Controls (+ p < 0.05). Panel D shows the change in IL-6 mRNA expression in cultured astrocytes, microglia or mixed brain cells exposed to in vitro hypoxia (2% O2) and glucopenia (3 mM glucose). There is a significant increase in IL-6 mRNA expression in the microglia and mixed brain cells compared to controls.
Supplemental Figure 2: Indomethacin does not provide neuroprotection from Hypoxia/Ischemia. Panel A shows Control Vehicle brain, Panel B shows Control Indo brain, Panel C shows HI Vehicle, and Panel D shows HI Indomethacin. Panel E shows that there is a significant decrease in the area of the cortex of both HI Vehicle (*, p < 0.05) and HI Indomethacin treated (**, p < 0.05) compared to Control Vehicle. There is no significant difference between HI Vehicle and HI Indo.
Acknowledgments
This work was presented in part at the 2007 Society for Neuroscience meeting78 and was supported by a grant from the National Institute of Mental Health (5R01MH059950) and a grant from the Leducq Foundation awarded to SWL.
Abbreviations
- ANOVA
Analysis of Variance
- BrdU
5-bromo-2-deoxyuridine
- CCA
Common Carotid Artery
- CLH
Contralateral Hemisphere
- CM
Conditioned Media
- CNTF
Ciliary Neurotrophic Factor
- dmPGE2
dimethyl Prostaglandin E2
- EGF
Epidermal Growth Factor
- FGF
Fibroblast Growth Factor
- H/I
Hypoxia/Ischemia
- ILH
Ipsilateral Hemisphere
- IL-6
Interleukin-6
- LPS
Lipopolysaccharide
- NSP
Neural Stem/Progenitor Cell
- NV
Neuronal density
- PGM
Phosphate Buffered Saline with Glucose and Magnesium
- qPCR
Quantitative Real-time PCR
- REST
Relative Expression Software Tool
- SGZ
Subgranular Zone
- SVZ
Subventricular Zone
- TNFα
Tumor Necrosis Factor alpha
- Vref
Total reference volume
References
- 1.Simpson DM, Ross R. The neutrophilic leukocyte in wound repair a study with antineutrophil serum. J Clin Invest. 1972;51:2009–2023. doi: 10.1172/JCI107007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- 3.Myers SR, Leigh IM, Navsaria H. Epidermal repair results from activation of follicular and epidermal progenitor keratinocytes mediated by a growth factor cascade. Wound Repair Regen. 2007;15:693–701. doi: 10.1111/j.1524-475X.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- 4.Loo WT, Sasano H, Chow LW. Pro-inflammatory cytokine, matrix metalloproteinases and TIMP-1 are involved in wound healing after mastectomy in invasive breast cancer patients. Biomed Pharmacother. 2007;61:548–552. doi: 10.1016/j.biopha.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Swartz KR, Liu F, Sewell D, et al. Interleukin-6 promotes post-traumatic healing in the central nervous system. Brain Res. 2001;896:86–95. doi: 10.1016/s0006-8993(01)02013-3. [DOI] [PubMed] [Google Scholar]
- 6.D’Amelio FE, Smith ME, Eng LF. Sequence of tissue responses in the early stages of experimental allergic encephalomyelitis (EAE): immunohistochemical, light microscopic, and ultrastructural observations in the spinal cord. Glia. 1990;3:229–240. doi: 10.1002/glia.440030402. [DOI] [PubMed] [Google Scholar]
- 7.Griffin WS, Sheng JG, Royston MC, et al. Glial-neuronal interactions in Alzheimer’s disease: the potential role of a ‘cytokine cycle’ in disease progression. Brain Pathol. 1998;8:65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolson DL, Lavi E, Gonzalez-Scarano F. The effects of human immunodeficiency virus in the central nervous system. Adv Virus Res. 1998;50:1–47. doi: 10.1016/s0065-3527(08)60804-0. [DOI] [PubMed] [Google Scholar]
- 9.Prineas JW, Kwon EE, Sternberger NH, Lennon VA. The distribution of myelin-associated glycoprotein and myelin basic protein in actively demyelinating multiple sclerosis lesions. J Neuroimmunol. 1984;6:251–264. doi: 10.1016/0165-5728(84)90012-2. [DOI] [PubMed] [Google Scholar]
- 10.Spleiss O, Gourmala N, Boddeke HW, et al. Cloning of rat HIV-1-chemokine coreceptor CKR5 from microglia and upregulation of its mRNA in ischemic and endotoxinemic rat brain. J Neurosci Res. 1998;53:16–28. doi: 10.1002/(SICI)1097-4547(19980701)53:1<16::AID-JNR3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 11.Thornalley PJ. Cell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEs. Cell Mol Biol (Noisy-le-grand) 1998;44:1013–1023. [PubMed] [Google Scholar]
- 12.Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 13.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Kadhim H, Tabarki B, Verellen G, et al. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 2001;56:1278–1284. doi: 10.1212/wnl.56.10.1278. [DOI] [PubMed] [Google Scholar]
- 15.Hansen-Pupp I, Hallin AL, Hellstrom-Westas L, et al. Inflammation at birth is associated with subnormal development in very preterm infants. Pediatr Res. 2008;64:183–188. doi: 10.1203/PDR.0b013e318176144d. [DOI] [PubMed] [Google Scholar]
- 16.Chau V, Poskitt KJ, McFadden DE, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol. 2009;66:155–164. doi: 10.1002/ana.21713. [DOI] [PubMed] [Google Scholar]
- 17.Liu XH, Kwon D, Schielke GP, et al. Mice deficient in interleukin-1 converting enzyme are resistant to neonatal hypoxic-ischemic brain damage. J Cereb Blood Flow Metab. 1999;19:1099–1108. doi: 10.1097/00004647-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Hedtjarn M, Leverin AL, Eriksson K, et al. Interleukin-18 involvement in hypoxic-ischemic brain injury. Journal of Neuroscience. 2002;22:5910–5919. doi: 10.1523/JNEUROSCI.22-14-05910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballabh P, Xu H, Hu F, et al. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med. 2007;13:477–485. doi: 10.1038/nm1558. [DOI] [PubMed] [Google Scholar]
- 20.Wolfberg AJ, Dammann O, Gressens P. Anti-inflammatory and immunomodulatory strategies to protect the perinatal brain. Semin Fetal Neonatal Med. 2007;12:296–302. doi: 10.1016/j.siny.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Silveira RC, Procianoy RS. Interleukin-6 and tumor necrosis factor-alpha levels in plasma and cerebrospinal fluid of term newborn infants with hypoxic-ischemic encephalopathy. J Pediatr. 2003;143:625–629. doi: 10.1067/S0022-3476(03)00531-6. [DOI] [PubMed] [Google Scholar]
- 22.Ellison VJ, Mocatta TJ, Winterbourn CC, et al. The relationship of CSF and plasma cytokine levels to cerebral white matter injury in the premature newborn. Pediatr Res. 2005;57:282–286. doi: 10.1203/01.PDR.0000148286.53572.95. [DOI] [PubMed] [Google Scholar]
- 23.Savman K, Blennow M, Gustafson K, et al. Cytokine response in cerebrospinal fluid after birth asphyxia. Pediatr Res. 1998;43:746–751. doi: 10.1203/00006450-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Aly H, Khashaba MT, El-Ayouty M, et al. IL-1beta, IL-6 and TNF-alpha and outcomes of neonatal hypoxic ischemic encephalopathy. Brain Dev. 2006;28:178–182. doi: 10.1016/j.braindev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Hagberg H, Gilland E, Bona E, et al. Enhanced expression of interleukin (IL)-1 and IL-6 messenger RNA and bioactive protein after hypoxia-ischemia in neonatal rats. Pediatric Research. 1996;40:603–609. doi: 10.1203/00006450-199610000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Wu YW, Croen LA, Torres AR, et al. Interleukin-6 genotype and risk for cerebral palsy in term and near-term infants. Ann Neurol. 2009;66:663–670. doi: 10.1002/ana.21766. [DOI] [PubMed] [Google Scholar]
- 27.Resch B, Radinger A, Mannhalter C, et al. Interleukin-6 G(--174)C polymorphism is associated with mental retardation in cystic periventricular leucomalacia in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2009;94:F304–306. doi: 10.1136/adc.2008.140244. [DOI] [PubMed] [Google Scholar]
- 28.Campbell IL, Stalder AK, Chiang CS, et al. Transgenic models to assess the pathogenic actions of cytokines in the central nervous system. Mol Psychiatry. 1997;2:125–129. doi: 10.1038/sj.mp.4000225. [DOI] [PubMed] [Google Scholar]
- 29.Penkowa M, Giralt M, Carrasco J, et al. Impaired inflammatory response and increased oxidative stress and neurodegeneration after brain injury in interleukin-6-deficient mice. Glia. 2000;32:271–285. doi: 10.1002/1098-1136(200012)32:3<271::aid-glia70>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Penkowa M, Espejo C, Martinez-Caceres EM, et al. Altered inflammatory response and increased neurodegeneration in metallothionein I+II deficient mice during experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001;119:248–260. doi: 10.1016/s0165-5728(01)00357-5. [DOI] [PubMed] [Google Scholar]
- 31.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 32.Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36:2718–2724. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- 33.Cacci E, Claasen JH, Kokaia Z. Microglia-derived tumor necrosis factor-alpha exaggerates death of newborn hippocampal progenitor cells in vitro. J Neurosci Res. 2005;80:789–797. doi: 10.1002/jnr.20531. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Imai H, Sadamatsu M, et al. Cytokines participate in neuronal death induced by trimethyltin in the rat hippocampus via type II glucocorticoid receptors. Neurosci Res. 2005;51:319–327. doi: 10.1016/j.neures.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. Journal of Neuroscience. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Felling RJ, Snyder MJ, Romanko MJ, et al. Neural stem/progenitor cells participate in the regenerative response to perinatal hypoxia/ischemia. Journal of Neuroscience. 2006;26:4359–4369. doi: 10.1523/JNEUROSCI.1898-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plane JM, Liu R, Wang T-W, et al. Neonatal hypoxic-ischemic injury increases forebrain subventricular zone neurogenesis in the mouse. Neurobiology of Disease. 2004;16:585–595. doi: 10.1016/j.nbd.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Szele FG. Pharmacology. Philadelphia: University of Pennsylvania; 1994. Plasticity in the striatum and subependymal layer of adult rats in response to cortical lesions; p. 137. [Google Scholar]
- 39.Yang Z, Levison SW. Hypoxia/ischemia expands the regenerative capacity of progenitors in the perinatal subventricular zone. Neuroscience. 2006;139:555–564. doi: 10.1016/j.neuroscience.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 40.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 41.Greenberg RA, Allsopp RC, Chin L, et al. Expression of mouse telomerase reverse transcriptase during development, differentiation and proliferation. Oncogene. 1998;16:1723–1730. doi: 10.1038/sj.onc.1201933. [DOI] [PubMed] [Google Scholar]
- 42.Yang Z, Covey MV, Bitel CL, et al. Sustained Neocortical Neurogenesis after Neonatal Hypoxic/Ischemic Injury. Annals of Neurology. 2007;61:199–208. doi: 10.1002/ana.21068. [DOI] [PubMed] [Google Scholar]
- 43.Covey MV, Levison SW. Leukemia inhibitory factor participates in the expansion of neural stem/progenitors after perinatal hypoxia/ischemia. Neuroscience. 2007;148:501–509. doi: 10.1016/J.NEUROSCIENCE.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alagappan D, Lazzarino DA, Felling RJ, et al. ASN Neuro. 2009. Brain injury expands the numbers of neural stem cells and progenitors in the SVZ by enhancing their responsiveness to EGF; p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 46.Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemistry determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. Journal of Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- 47.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chojnacki A, Shimazaki T, Gregg C, et al. Glycoprotein 130 signaling regulates Notch1 expression and activation in the self-renewal of mammalian forebrain neural stem cells. Journal of Neuroscience. 2003;23:1730–1741. doi: 10.1523/JNEUROSCI.23-05-01730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carlen M, Meletis K, Goritz C, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- 50.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 51.Rezaie P, Dean A. Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology. 2002;22:106–132. doi: 10.1046/j.1440-1789.2002.00438.x. [DOI] [PubMed] [Google Scholar]
- 52.Bada HS, Green RS, Pourcyrous M, et al. Indomethacin reduces the risks of severe intraventricular hemorrhage. J Pediatr. 1989;115:631–637. doi: 10.1016/s0022-3476(89)80300-2. [DOI] [PubMed] [Google Scholar]
- 53.Bandstra ES, Montalvo BM, Goldberg RN, et al. Prophylactic indomethacin for prevention of intraventricular hemorrhage in premature infants. Pediatrics. 1988;82:533–542. [PubMed] [Google Scholar]
- 54.Ment LR, Oh W, Ehrenkranz RA, et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics. 1994;93:543–550. [PubMed] [Google Scholar]
- 55.Ment LR, Oh W, Ehrenkranz RA, et al. Low-dose indomethacin therapy and extension of intraventricular hemorrhage: a multicenter randomized trial. J Pediatr. 1994;124:951–955. doi: 10.1016/s0022-3476(05)83191-9. [DOI] [PubMed] [Google Scholar]
- 56.Ment LR, Vohr BR, Makuch RW, et al. Prevention of intraventricular hemorrhage by indomethacin in male preterm infants. J Pediatr. 2004;145:832–834. doi: 10.1016/j.jpeds.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt B, Davis P, Moddemann D, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. New England Journal of Medicine. 2001;344:1966–1972. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]
- 58.Fowlie PW, Davis PG. Prophylactic indomethacin for preterm infants: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2003;88:F464–466. doi: 10.1136/fn.88.6.F464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vohr BR, Allan WC, Westerveld M, et al. School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2003;111:e340–346. doi: 10.1542/peds.111.4.e340. [DOI] [PubMed] [Google Scholar]
- 60.Goings GE, Kozlowski DA, Szele FG. Differential activation of microglia in neurogenic versus non-neurogenic regions of the forebrain. Glia. 2006;54:329–342. doi: 10.1002/glia.20381. [DOI] [PubMed] [Google Scholar]
- 61.Ong J, Plane JM, Parent JM, Silverstein FS. Hypoxic-ischemic injury stimulates subventricular zone proliferation and neurogenesis in the neonatal rat. Pediatr Res. 2005;58:600–606. doi: 10.1203/01.PDR.0000179381.86809.02. [DOI] [PubMed] [Google Scholar]
- 62.Bauer S. Cytokine control of adult neural stem cells. Ann N Y Acad Sci. 2009;1153:48–56. doi: 10.1111/j.1749-6632.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- 63.Wen TC, Rogido MR, Moore JE, et al. Cardiotrophin-1 protects cortical neuronal cells against free radical-induced injuries in vitro. Neurosci Lett. 2005;387:38–42. doi: 10.1016/j.neulet.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 64.Pluchino S, Muzio L, Imitola J, et al. Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain. 2008;131:2564–2578. doi: 10.1093/brain/awn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ekdahl CT, Claasen JH, Bonde S, et al. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Z, Fan Y, Won SJ, et al. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- 67.Kim BJ, Kim MJ, Park JM, et al. Reduced neurogenesis after suppressed inflammation by minocycline in transient cerebral ischemia in rat. J Neurol Sci. 2009;279:70–75. doi: 10.1016/j.jns.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 68.Bull ND, Bartlett PF. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J Neurosci. 2005;25:10815–10821. doi: 10.1523/JNEUROSCI.3249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. Journal of Neuroscience. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raff MC, Lillien LE, Richardson WD, et al. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988;333:562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- 71.Morshead CM, Reynolds BA, Craig CG, et al. Neural stem cells in the adult mammalian forebrain: A relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 72.North TE, Goessling W, Walkley CR, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krady JK, Lin HW, Liberto CM, et al. Ciliary neurotrophic factor and interleukin-6 differentially activate microglia. Journal of Neuroscience Research. 2008;86:1538–1547. doi: 10.1002/jnr.21620. [DOI] [PubMed] [Google Scholar]
- 74.Vexler ZS, Yenari MA. Does inflammation after stroke affect the developing brain differently than adult brain? Dev Neurosci. 2009;31:378–393. doi: 10.1159/000232556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thored P, Heldmann U, Gomes-Leal W, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- 76.Iosif RE, Ahlenius H, Ekdahl CT, et al. Suppression of stroke-induced progenitor proliferation in adult subventricular zone by tumor necrosis factor receptor 1. J Cereb Blood Flow Metab. 2008;28:1574–1587. doi: 10.1038/jcbfm.2008.47. [DOI] [PubMed] [Google Scholar]
- 77.Katakowski M, Chen J, Zhang ZG, et al. Stroke-induced subventricular zone proliferation is promoted by tumor necrosis factor-alpha-converting enzyme protease activity. J Cereb Blood Flow Metab. 2007;27:669–678. doi: 10.1038/sj.jcbfm.9600390. [DOI] [PubMed] [Google Scholar]
- 78.Covey M, Levison SW. Interleukin 6 (IL-6) stimulates the expansion of neural stem/progenitors and is highly expressed in the subventricular zone after perinatal hypoxia/ischemia. Society for Neuroscience; San Diego, CA: 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Indomethacin decreases both IBA-1 and GFAP florescent intensity. Panel A shows there was an increase in IBA-1 florescent intensity in the H/I Veh treated SVZ when compared to Controls (*, p < 0.05) and H/I Indomethacin treated (**, p < 0.05). However, there was more IBA-1 florescent intensity in the Indomethacin treated animals compared to Controls (+ p < 0.05). Panel B shows IBA-1 staining (red) and IL-6 staining (green) of the striatum and SVZ at 72 hours of recovery. Virtually all of the IL-6+ cells were IBA-1 positive. Panel C depicts an increase in GFAP florescent intensity in the H/I Veh treated SVZ when compared to Controls (*, p < 0.05) and H/I Indomethacin treated (**, p < 0.05). However, there was more GFAP florescent intensity in the Indomethacin treated animals compared to Controls (+ p < 0.05). Panel D shows the change in IL-6 mRNA expression in cultured astrocytes, microglia or mixed brain cells exposed to in vitro hypoxia (2% O2) and glucopenia (3 mM glucose). There is a significant increase in IL-6 mRNA expression in the microglia and mixed brain cells compared to controls.
Supplemental Figure 2: Indomethacin does not provide neuroprotection from Hypoxia/Ischemia. Panel A shows Control Vehicle brain, Panel B shows Control Indo brain, Panel C shows HI Vehicle, and Panel D shows HI Indomethacin. Panel E shows that there is a significant decrease in the area of the cortex of both HI Vehicle (*, p < 0.05) and HI Indomethacin treated (**, p < 0.05) compared to Control Vehicle. There is no significant difference between HI Vehicle and HI Indo.