Abstract
Purpose.
To present a method to analyze circadian intraocular pressure (IOP) patterns in glaucoma patients and suspects undergoing repeated continuous 24-hour IOP monitoring.
Methods.
Forty patients with established (n = 19) or suspected glaucoma (n = 21) underwent ambulatory 24-hour IOP monitoring on two sessions 1 week apart using a contact lens sensor (CLS). The CLS provides its output in arbitrary units (a.u.). A modified cosinor rhythmometry method was adapted to the CLS output to analyze 24-hour IOP patterns and their reproducibility. Nonparametric tests were used to study differences between sessions 1 and 2 (S1 and S2). Patients pursued their routine daily activities and their sleep was uncontrolled. CLS data were used to assess sleep times.
Results.
Complete 24-hour data from both sessions were available for 35 patients. Mean (SD) age of the patients was 55.8 ± 15.5 years. The correlation of the cosinor fitting and measured CLS values was r = 0.38 (Spearman r; P < 0.001) for S1, r = 0.50 (P < 0.001) for S2, whereas the correlation between S1 and S2 cosinor fittings was r = 0.76 (P < 0.001). Repeated nocturnal acrophase was seen in 62.9% of patients; 17.1% of patients had no repeatable acrophase. The average amplitude of the 24-hour curve was 143.6 ± 108.1 a.u. (S1) and 130.8 ± 68.2 a.u. (S2) (P = 0.936).
Conclusions.
Adapting the cosinor method to CLS data is a useful way for modeling the rhythmic nature of 24-hour IOP patterns and evaluating their reproducibility. Repeatable nocturnal acrophase was seen in 62.9% of patients. (ClinicalTrials.gov number, NCT01319617.)
The recent availability of devices for continuous monitoring of 24-hour IOP patterns creates new challenges for data analysis. The current article applies a modified cosinor method to data from repeated 24-hour IOP monitoring and demonstrates its usefulness for modeling the rhythmic nature of IOP patterns.
Introduction
Elevated intraocular pressure (IOP) is a major risk factor for glaucoma onset1 and progression.2 Current treatment for glaucoma is based on lowering of IOP to reduce the rate of progression.3 Treatment success is frequently expressed as reaching a target range of IOP on subsequent clinic visits when IOP is generally assessed using Goldmann applanation tonometry (GAT). Despite its limitations,4 the Goldmann technique is widely accessible, easy to perform, and remains the worldwide standard procedure for measuring IOP. Although it generates reliable results, it can provide only a single temporal value that does not reflect the dynamic nature of IOP and its circadian rhythm.5,6
As a consequence, variations in IOP are commonly noticed, but not well recognized, and often underappreciated in the management of glaucoma patients. These variations are the result of complex interactions between external environmental stimuli and the biologic IOP rhythm. Although previous studies have implicated peak IOP as a major contributor to glaucoma progression,7,8 data on the time course of IOP rhythms and their reproducibility in glaucoma patients remain scarce. Evidence suggests that reproducibility of several daytime IOP measurements with GAT is only moderate at best in glaucoma patients.9–11 Important shortcomings of these studies, next to the limited number of available IOP measurements, are the nonphysiological conditions (including interrupted sleep) in which IOP data are obtained.
The recent development of a contact lens sensor (CLS) for noninvasive continuous 24-hour IOP monitoring has enabled the assessment of 24-hour IOP rhythms in an ambulatory environment including undisturbed sleep.12–14 The device is based on the assumption that circumferential changes at the corneoscleral junction correspond to changes in IOP.15,16 The interpretation of the results of this new technology, however, poses challenges. These include processing large amounts of data on continuous measurements that are collected over time compared with a single measurement obtained when using GAT. Another complicating factor is that the output signal is not displayed in millimeters of mercury but in an arbitrary unit (a.u.) proportional to the electric signal (in mV) generated by the contact lens–embedded strain gauge. At present, no calibration to mm Hg is available for the CLS.
Cosinor rhythmometry is a commonly used method to study periodic functions associated with circadian (24-hour) biological rhythms.17,18 Previous investigators have used this approach to assess static IOP readings obtained from hospital-based diurnal tension curves or from sleep laboratories.5,19,20 The present study applies a modified cosinor rhythmometry model21 to two full cycles of continuous 24-hour data from the CLS to test the hypothesis that the chronobiology of IOP rhythms in glaucoma patients is reproducible in the short term.9,10 This approach may also serve to facilitate visualization and interpretation of CLS data. To this end, we evaluated circadian IOP patterns and their time course in patients with established and suspected glaucoma undergoing repeated 24-hour IOP monitoring with the CLS at a 1-week interval.
Methods
This study followed the tenets of the Declaration of Helsinki and was approved by the University of California at San Diego, Human Research Protections Program/Institutional Review Board. Informed written consent was obtained from all patients.
Participants
Forty eyes of 40 patients were included in the study. Nineteen eyes (48%) had a diagnosis of primary open-angle glaucoma (POAG) and 21 eyes (52%) were suspected of having glaucoma. For inclusion in the study, patients were required to have a best-corrected visual acuity (BCVA) of 20/80 or better in the study eye, spherical refraction between −5 diopters (D) and +3 D, cylinder correction ≤ 2 D, open angles on gonioscopy, and be between 18 and 80 years of age. Patients were excluded if they had a history of intraocular surgery or intraocular laser treatment within the past 3 months, and contraindications for contact lens wear, such as known intolerance to silicone, severe dry eye disease, keratoconus, or other corneal abnormalities. Eyes with a corneal radius outside the manufacturer's recommended range of 40–48 D were also excluded from the study. When both eyes of a patient were eligible, one eye was chosen at the discretion of the investigators. To be classified as glaucomatous, eyes had to have at least two consecutive, reliable, and repeatable standard automated perimetry examinations with either a pattern SD outside the 95% normal limits or a glaucoma hemifield test result outside the 99% normal limits. Suspect glaucoma was defined as eyes with abnormal-appearing optic discs (presence of neuroretinal rim thinning or localized or diffuse retinal nerve fiber layer defects characteristic for glaucoma) by masked stereophotograph assessment without repeatable abnormal perimetry results. Suspect glaucoma also included eyes with IOP ≥ 22 mm Hg but with healthy-appearing optic discs and without repeatable abnormal perimetry results.22
All patients underwent a screening visit, followed by two study sessions (S1 and S2) each 6–8 days apart. At the screening visit, patients underwent a comprehensive ophthalmologic examination, including review of medical history, BCVA, automated refraction, keratometry, ultrasound pachymetry, slit-lamp biomicroscopy, gonioscopy, dilated fundus examination with a 78-D lens, and standard automated perimetry with the 24-2 Swedish interactive threshold algorithm (Carl Zeiss Meditec, Inc., Dublin, CA). On day 1, patients underwent an ophthalmologic examination including BCVA, automated refraction, keratometry, ultrasound pachymetry, and slit-lamp biomicroscopy. The CLS was then placed on the study eye (one eye per patient) and 24-hour IOP monitoring was started. The CLS exists in three different base curves (8.4 mm [steep], 8.7 mm [medium], 9.0 mm [flat]). According to the manufacturer's manual, patients with a central corneal radius in the flatter meridian between 7.54 and 8.44 mm were fitted with a medium-sized CLS, those with lower values with a steep CLS, and those with higher values with a flat CLS. At 5 and 30 minutes after CLS placement, the fitting of the CLS was evaluated with regard to centering and its mobility on blinking and during push-up maneuver were assessed on the slit lamp. Patients were provided with a standardized activity diary for half-hourly recording of information on sleep and wakefulness times, intake of medications and meals, physical activity, emotional status, and other events. After 24 hours (day 2), patients returned, the CLS was removed, the activity diary was collected, and an ophthalmologic examination was performed. At the end of the monitoring, data from the portable recorder were transferred to a computer (via a Bluetooth device). The same procedures were repeated a week later.
Continuous 24-h IOP Monitoring
Twenty-four–hour IOP monitoring was conducted with a CLS (Triggerfish; Sensimed AG, Lausanne, Switzerland). Leonardi et al.15 have previously reported that the technology provides good correlation to manometric pressure measurements in cannulated porcine eyes. However, because the CLS measurements are provided in an arbitrary unit (a.u.) corresponding to mV, direct comparisons to tonometry measurements could not be obtained. The sensor consists of microfabricated strain gauges that record circumferential changes in the area of the corneoscleral junction and is embedded in a soft silicone contact lens. The CLS is powered by inductive coupling through an external antenna patched around the eye. The microprocessor reads the strain gauges at a frequency of 10 Hz and transmits data to the external antenna, which is connected to a portable recorder unit. The device can record IOP patterns in an ambulatory setting for up to 24 hours and remains active during sleep. Three hundred data points are acquired during a 30-second measurement period, repeated every 5 minutes, providing a total of 288 recording periods over a 24-hour period. Recorded profiles are visualized graphically on a computer interface. The device is described in more detail elsewhere.12,13
Modeling of Circadian IOP Patterns
Mathematical estimation of the circadian IOP rhythms was done using the cosinor rhythmometry method, which uses sine and cosine terms. This method assumes that the circadian IOP rhythm resembles a cosine profile and different formulations of it have previously been used to express 24-hour IOP variations.5 The model can be written as follows: y(t) = b0 + b1 × cos[(2π/24) × t] + b2 × sin[(2π/24) × t], where y is the observed signal in a.u. at time t and b0, b1, and b2 are regression coefficients, estimated from the data. The periodicity of the 24-hour IOP pattern is represented by the constant (2π/24). Unbiased estimates and confidence limits of amplitude, mesor (mean), and acrophase (time of peak value) were obtained from the individual waveforms. The amplitude was defined as half the distance between the cosine-fit maximum and minimum (A = [max(predicted value) − min(predicted value)]/2 ∼ squareroot[b cos2 + b sin2]). It represented the parameter estimate of the variation for the 24-hour period. The clock time of the acrophase represented the phase timing of the rhythm.
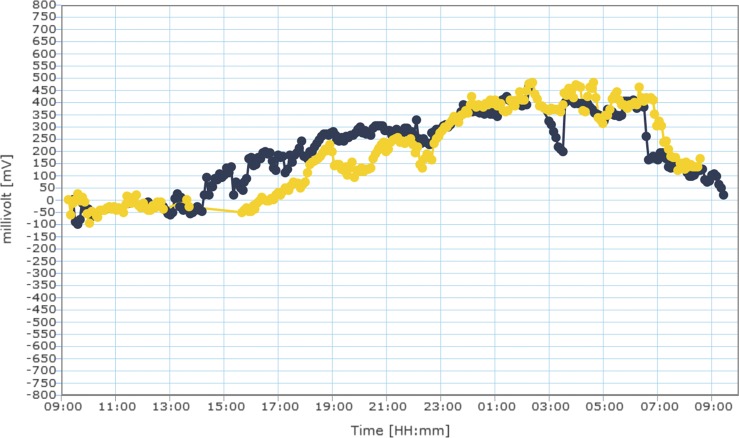
The Spearman correlation (r) between the measured CLS values and the values predicted by the cosinor fitting was then calculated for each patient. Figure 1 presents an example of a patient's overlapped CLS monitoring results as well as the results of cosinor modeling. The null hypothesis of random distribution of all circadian acrophases in the S1 and S2 sessions was evaluated by using the Rayleigh test.23 Lack of a statistical significance indicated no synchronized circadian rhythm, whereas the alternative conclusion demonstrated synchronized rhythm in the group.
Figure 1.
(A) Example of the raw data obtained from 24-hour IOP curves from the same eye during sessions 1 (yellow) and 2 (blue). (B) Cosinor rhythmometry fitting of the same curves for session 1 and session 2. The predictive value of the cosinor fitting on CLS data was r = 0.87 (P < 0.001) and r = 0.90 (P < 0.001) for sessions 1 and 2, respectively.
Nocturnal/sleep periods were defined through the observation of blink cessation (identified as short and high-amplitude spikes that are displayed by the software) on the CLS signal using the software zoom function and were confirmed using individual diary-reported sleep times. These were within ±15 minutes of CLS-derived sleep times in 33 out of 35 patients (94%). Therefore, CLS-derived sleep times were used for the analysis in all cases. Patients were then classified into pattern groups based on the following definitions:
Diurnal acrophase: Peak occurring during the diurnal/wakefulness period.
Nocturnal acrophase: Peak occurring during the nocturnal/sleep period.
No significant acrophase: If at least one parameter from the model equation (i.e., one of the sine or cosine parameters or the intercept) did not have a significant influence on the dependent variable. The rational for this definition was that models that do not contain any significant coefficients indicate that the cosine model does not sufficiently fit the data. Therefore, the acrophase provided by such models was not included in the analyses.
Low amplitude: If the amplitude was lower than the lower bound amplitude threshold of 75.4 a.u. This cutoff value was determined from analysis of the whole population and was calculated as the mean of the following three values: 10% quantile amplitude; 25% quantile amplitude; mean − (minus) standard deviation (SD). The rationale for calculating a cutoff for low amplitude was to be able to determine which CLS plots should be considered “flat.” Since “flat” is a relative term, the described formula was adopted, taking into account relative parameters: mean, SD, and quantiles. By doing this, population characteristics were taken into account to determine what should be considered relatively “flat” for an individual in the population.
Patients who were classified in different categories in the two sessions were classified as “nonmatching phases.”
In continuous IOP monitoring with the CLS, many more data points (288 continuous 30-second periods) are obtained than in standard sleep laboratory monitoring (approximately 12 single values). All CLS data points were used for cosinor modeling. Another difference of the CLS from tonometry is that the measurement units are given in arbitrary units, corresponding to mV changes of the strain gauges.
Statistical Analysis
Data are expressed as mean ± SD or interquartile range (IQR), where appropriate. Categorical variables are described in terms of frequencies and percentages. The distribution of all variables was examined using the Shapiro–Wilk test of normality. Variables were analyzed for correlation using Pearson for parametric and Spearman correlation for nonparametric data. Missing data were not imputed and output values indicated as invalid by the device were excluded from the analysis. Statistical significance was defined at P < 0.05. All analyses were conducted using commercial analytic software (SAS software; SAS Institute, Cary, NC).
Results
Complete data from both monitoring sessions were available from 35 of 40 patients (88%). Of these, 6 patients had previously been treated with selective laser trabeculoplasty and 23 patients (65.7%) were on IOP-lowering eye drops during the study period. Thirty-seven of 40 patients (92.5%) received a medium CLS. Thirty minutes after placement, all CLS had minimal or no movement on blinking, indicating a stable positioning on the eye. Demographic and clinical data are summarized in Table 1. Reasons for incomplete or unusable data were: battery insufficiency (n = 2) and unknown or presumed device manipulation leading to signal loss (n = 3).
Table 1. .
Demographic and Ophthalmic Characteristics (n = 35)
|
Factor |
Value |
| Age, y | 55.8 ± 15.5 |
| Sex, n (male) | 22 (62.9%) |
| Ancestry, n | |
| Caucasian | 26 (74.3%) |
| Asian | 5 (14.3%) |
| African American | 1 (2.9%) |
| Hispanic | 3 (8.8%) |
| Pseudophakia | 3 (8.8%) |
| Visual field MD, dB | −1.5 ± 2.6 |
| Visual field PST, dB | 2.6 ± 1.9 |
| Use of IOP-lowering drops, n | 23 (65.7%) |
| PGA monotherapy | 13 (37.1%) |
| CAI monotherapy | 1 (2.9%) |
| Combination PGA–CAI | 2 (5.7%) |
| Combination PGA + CAI–β-blocker | 2 (5.7%) |
| Combination PGA + α-agonist–β-blocker | 2 (5.7%) |
| Combination PGA–β-blocker | 1 (2.9%) |
| Combination α-agonist–β-blocker | 1 (2.9%) |
| Combination PGA + α-agonist–CAI | 1 (2.9%) |
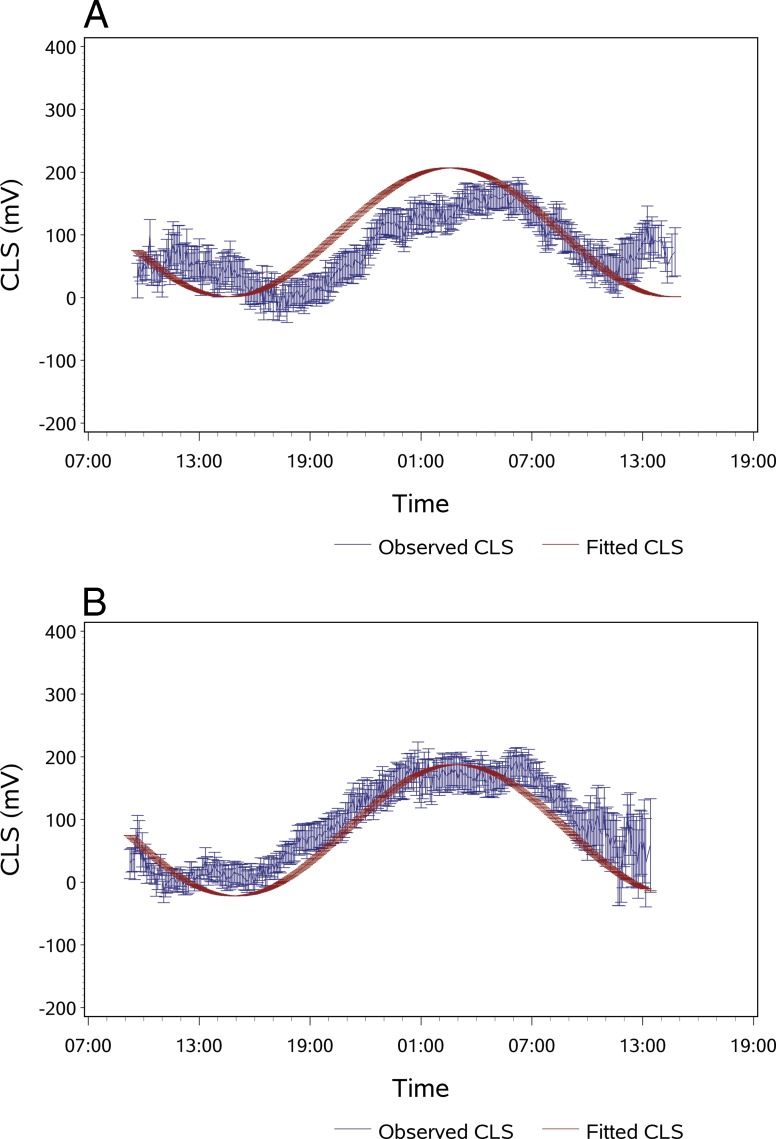
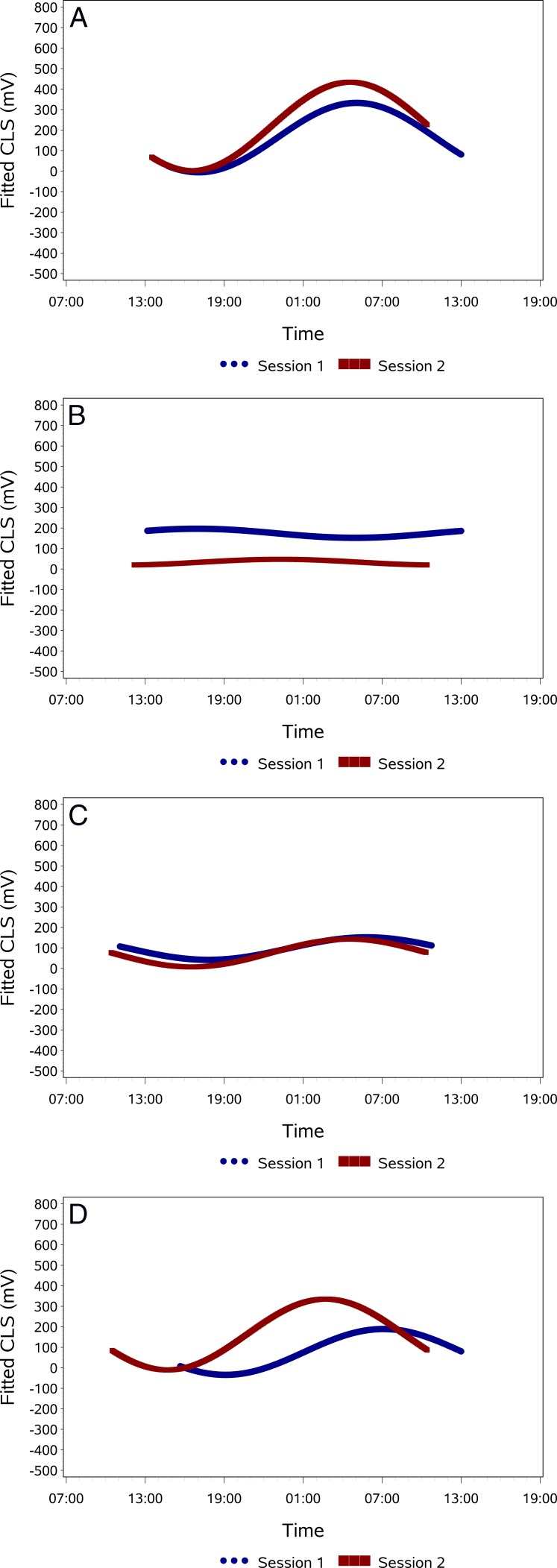
Overall, the correlation between CLS measurements and values predicted from cosinor rhythmometry fitting was r = 0.38 (P < 0.001) for session S1 and r = 0.50 (P < 0.001) for session S2. Figure 1 provides an example of a subject's superimposed CLS curves for both sessions and cosinor fitting of the CLS data. Applying cosinor rhythmometry increased correlation between both sessions from r = 0.58 (P < 0.001) for the raw data to r = 0.76 (P < 0.001) for the fitted data. After cosinor fitting, 25 patients (71.4%) had an r > 0.6 for the correlation between sessions S1 and S2 and 24 patients (68.6%) had an r > 0.7. Cosinor rhythmometry modeling of the 24-hour curves for the entire group indicated a nocturnal/sleep acrophase, with the peak occurring at approximately 1:45 (S1) and 2:00 (S2) (Fig. 2). In session 1, 23 patients (65.7%) were classified as having a nocturnal acrophase, 3 patients (8.6%) as diurnal acrophase, 5 patients as (14.3%) low amplitude, and 4 patients (11.4%) as no significant acrophase. Corresponding values for session 2 were 26 (74.3%), 2 (5.7%), 5 (14.3%), and 2 (5.7%), respectively. Based on the repeatability of the patterns in both sessions, the patients were classified into the following pattern types: repeatable nocturnal acrophase (n = 22, 62.9%), repeatable diurnal acrophase (0), no significant repeatable acrophase (n = 2, 5.7%), repeatable low amplitude (n = 5, 14.3%), and nonmatching acrophases (n = 6, 17.1%). Figure 3 provides examples for each of the four pattern groups present in this study.
Figure 2 .
Cosinor rhythmometry modeling of average circadian IOP patterns of the entire study group (n = 35), by session. (A) Session 1 (S1): Bars indicate SEM. The equations' parameters were calculated as: y(t) = 104.11 + 79.90 × cos[(2π/24) × time] + 64.71 × sin[(2π/24) × time]. (B) Session 2 (S2): The equations' parameters were calculated as: y(t) = 82.79 + 74.39 × cos[(2π/24) × time] + 73.71 × sin[(2π/24) × time]. Units on the y-axis correspond to mV. The increased variability at the end of the monitoring is assumed to be due to the increase in manipulation of the globe to remove a tightly fitting CLS in a few patients, which may have produced an artifactual signal rise. Also, the error bars increase because there are fewer data points available because not all patients' sessions lasted exactly the same time.
Figure 3. .
Examples of pattern types of 24-hour curves. (A) Nocturnal acrophase. (B) No significant acrophase. (C) Low amplitude. (D) Nonmatching acrophases. There was no case of repeated diurnal acrophase in this series.
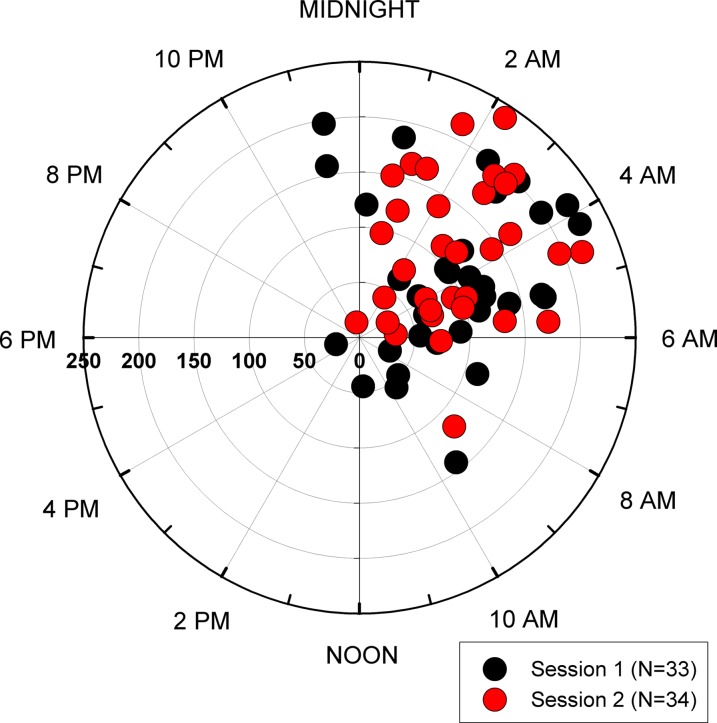
The absolute intersession time difference of peaks was 140 ± 108 (IQR, 170) minutes. For 22 patients with repeatable nocturnal acrophase this difference was 87 ± 54 minutes. Table 2 provides 10% quantiles for the time difference of peaks. The Rayleigh test detected synchronized 24-hour rhythms of habitual IOP for both sessions (P < 0.001). Figure 4 summarizes all the individual phase timings. The mean amplitudes of the 24-hour curves were 143.6 ± 108.1 a.u. and 130.8 ± 68.2 a.u. for sessions 1 and 2, respectively (P = 0.936). The mean intersession difference between individual amplitudes was 12.8 ± 99.1 a.u. The intersession correlation between amplitudes was moderate for the entire group (Spearman r = 0.60, P < 0.001).
Table 2. .
10% Quantiles of Intersession Difference for Time Course of Acrophases
|
Percentile |
Time Difference (minutes) |
| 0% | 14 |
| 10% | 40 |
| 20% | 49 |
| 30% | 57 |
| 40% | 81 |
| 50% | 105 |
| 60% | 127 |
| 70% | 168 |
| 80% | 235 |
| 90% | 281 |
| 100% | 427 |
Figure 4. .
Estimated 24-hour IOP rhythms in the habitual body positions. The clock time of the cosinor rhythmometry-derived acrophase (phase timing) is shown with the amplitude in the radial scale (in arbitrary units [a.u.]). Three individuals' measurements are not included due to high outlier a.u. values. Their acrophases and amplitudes were 0:22, 579 a.u.; 0:43, 427 a.u.; and 22:28, 328 a.u., respectively.
Discussion
Peak IOP has been shown to be a risk factor for glaucoma progression.7,8 However, the pathophysiologic consequences of the timing of peaks and other IOP parameters are presently unknown. Given a possible altered circadian timing system in glaucoma,24 the time course of IOP rhythms may have additional implications for disease management. In the present study, we applied cosinor rhythmometry modeling to investigate characteristics of 24-hour IOP monitoring obtained using a recently introduced telemetric CLS in a mixed group of glaucoma patients and suspects. We defined and evaluated two parameters of the 24-hour IOP curve (acrophase and amplitude) that may be helpful in the conceptualization and interpretation of basic features of continuous 24-hour IOP curves. Using this method, our results show that categorization of circadian IOP rhythms and patterns based on acrophase and amplitudes showed relatively good reproducibility in the short term.
Cosinor rhythmometry modeling of 24-hour CLS data is an objective method of assessing circadian IOP rhythms that may have advantages over other methods using infrequent IOP measurements. Previously, Wilensky et al.9 assessed reproducibility of IOP patterns by home tonometry over 4 to 8 consecutive days. In their study, subjective assessment of curve shapes showed that only 28% of patients with ocular hypertension and 34% of patients with open-angle glaucoma had reproducible curve shapes. However, in addition to device-related limitations of self-tonometry,25 subjective curve assessment can be further affected by interpreter bias. More recently, Realini et al.10 revisited this issue. They studied the repeatability of diurnal IOP (measured with GAT every 2 hours) in treated glaucoma patients. They found intraclass correlation coefficients ranging from 0.45 to 0.71 for IOP values at each time point between the two visits and concluded that short-term IOP patterns were not repeatable in glaucoma patients. Rotchford et al.11 investigated repeatability of diurnal GAT-IOP measurements (8:00 AM, 11:00 AM, 4:00 PM) in 30 patients with glaucoma or ocular hypertension at three weekly visits. The measurements were then repeated after introduction of topical prostaglandin analog therapy. Although the authors reported intraclass correlation coefficients of as high as 0.89, indicating “excellent” agreement, they cautioned that repeatability was lower when other measures of agreement that were less dependent on the range of IOP (such as coefficients of variation and coefficients of repeatability) were applied. These measures indicated high levels of IOP variability for pre- and posttreatment visits with a limit of precision in excess of ±20% for diurnal IOP measurements. This level of uncertainty is of clinical significance given the fact that the IOP-lowering effect of glaucoma medications is generally around 30%. The findings reported by Rotchford et al.11 further highlight the inadequacy of current IOP assessment techniques. It is doubtful whether results obtained from a few diurnal IOP measurements over 8- to 12-hour periods can be extrapolated to the full circadian cycle. In addition, comparisons of single temporal IOP readings may be of limited adequacy. That is because, given the highly dynamic nature of IOP, the reliance on a few seconds of IOP measurements increases the effect of single deviating data points on the overall analysis.
The current study avoids some of these pitfalls by using a methodology based on 288 continuous 30-second measurements over 24 hours, thus significantly minimizing the impact of measurement error of single measurements. Previously, variations of cosinor rhythmometry have been used to evaluate 24-hour IOP rhythms using either simple cosine or sine models or more complex nonlinear least-squares regression analysis involving multiple harmonics.20,26–28 In the latter, when two sinusoids are fit, the first is usually constrained to 24 hours with a large amplitude, whereas the second has a relatively low amplitude but can alter the general shape. Since cosine and sine terms are a shift of π/2 away from one another, using only one of them would produce a “shifted” model, with the subsequent need to search for a “shift” term between 0 to π/2 to optimally fit the curve. In our approach of modified cosinor rhythmometry, by using both cosine and sine terms together, the model is obtained exactly in the same location without the need to shift one term to compensate for the other.
In the current study, a majority of patients (62.9%) repeatedly had their acrophase during the nocturnal/sleep period. Although alterations in hormonal and neural activity influence the 24-hour IOP rhythm, the most significant contributor to a nocturnal acrophase is believed to be the recumbent position assumed during sleep.6,29 In our study, all patients spent the nocturnal/sleep period in the supine (“habitual”) body position. Earlier studies conducted in sleep laboratories also found that a majority of glaucoma patients had circadian IOP rhythms with a nocturnal acrophase.27,30 In addition to the 62.9% of patients with repeatable acrophase, 14.3% had repeatable low amplitude. In these eyes, no discernible acrophases were identified and their IOP profiles would be best described as “flat.” Such a pattern could potentially occur as the result of less IOP elevation in recumbent position, absence of disease-related IOP spikes, or as the result of ocular hypotensive treatment. In total, 77.2% of patients had repeatable patterns (repeatable acrophase or repeatable low amplitude) as measured by the CLS. However, in 17.1% of patients the acrophases did not match between sessions. It is unclear whether the pattern differences between sessions as observed in the current study are a consequence of actual IOP changes or of unidentified device-related issues. These may include any of the following: signal drift, inadvertent manipulation of CLS instrumentation during sleep, changes in corneal astigmatism and biomechanical properties, and CLS fit and motion on the globe. There is currently no objective method to evaluate the presence of a signal drift with the CLS. When we qualitatively defined “drift” as an absence of signal drop after awakening to presleep levels, this was observed in 7 (9%) of cases. More research, however, is needed to study this question. During sleep, patients may inadvertently manipulate parts of the CLS instrumentation and produce artifactual readings. For instance, when strong pressure is applied on the periocular soft patch containing the antenna (e.g., by a pillow), signal transmission can be interrupted temporarily. This is demonstrated in Figure 1A, where a signal loss lasting 30 minutes occurred at 2:00. Circadian changes in corneal biomechanical properties are repeatable in the short term31,32 and the effect on IOP measurements has been shown to be insignificant.33 Therefore, changes in corneal biomechanical properties are unlikely to result in pattern differences. In this series, all patients were fitted with the CLS according to the manufacturer's recommendation and were controlled 30 minutes after CLS installation to verify good CLS fit, defined as no spontaneous CLS movement. After 24-hour wear, there was a tight fit of CLS on the globe in all eyes and the absence of CLS movement during the push-up maneuver.
Figure 1 .
Continued.
The present study was designed to address the issue of short-term reproducibility of circadian IOP rhythms in a group of patients seen at a tertiary glaucoma clinic in an ambulatory setting. As such it has some strengths. First, whereas previous studies evaluating diurnal or circadian IOP behaviors were conducted within the limited confines of research institutions, the CLS used in our study allowed us to monitor patients in their natural habitat with unrestricted activities and undisturbed sleep. It is likely that higher reproducibility of IOP rhythms would have been found if this study was conducted in a controlled environment. However, being able to obtain IOP data in a real-life scenario could prove to be an important advancement in glaucoma research. Second, we did not only rely on patient diaries to determine the clock times of activity and sleep but were able to determine sleep times through characteristic signs on the CLS output. In fact, we found good correlation between patient-reported and CLS-based sleep times, with differences not greater than 15 minutes for 33 of 35 patients.
There are several limitations to the current study including device-related ones. Measurements obtained from the CLS are reported in arbitrary units and the absolute values in a.u. are not directly comparable among different eyes. This is due to the internal recalibration process of the sensors. For this reason, it is not possible to apply a single calibration factor that would allow conversion to mm Hg and directly compare absolute pressure values from different sessions. This is an important drawback of the device, which limits its ability to evaluate absolute IOP reductions following interventions. It also does not allow direct evaluation of the agreement in absolute IOP measurements between different sessions. In fact, due to this limitation, we were able to evaluate reproducibility of patterns using only a correlational approach. Although our proposed method overcomes some of the device limitations by relying on the analysis of the shape of a 24-hour IOP pattern and analysis of relative measures (amplitudes), there is currently no evidence relating continuous 24-hour IOP patterns or amplitudes to the risk of glaucoma development or progression. Prospective longitudinal studies should be conducted to investigate this issue. One drawback of cosinor modeling is that due to its simplifying nature, data on individual time points are lost. Therefore, this method should be used in a complementary fashion to the analysis of the raw CLS data. Since the CLS signal is dependent on changes occurring at the corneoscleral junction, it could potentially be affected by non–IOP-related changes in corneal thickness, particularly during nocturnal sleep.34 Freiberg et al.35 have evaluated the effect on central corneal thickness (CCT) of 9-hour overnight CLS wear in 20 glaucoma patients. Although they found a statistically significant increase in CCT from baseline in the study eye, the magnitude of the increase was small (14.0 μm). In addition, there is evidence that most of the overnight corneal swelling is directed inward into the anterior chamber.36 For these reasons we do not expect the nocturnal CCT increase to have had a significant effect on CLS readings.
An important question is whether the use of the CLS could influence absorption of ocular hypotensive medications and potentially affect comparison of IOP patterns. In our study, 65.7% of patients were on IOP-lowering medications and continued to use them after the CLS was inserted. Manufacturer's data (Matteo Leonardi, written communication, 2012) suggest minimal absorption by the CLS of the three most commonly used classes of ocular hypotensive medications. Therefore, it seems unlikely that the analysis of IOP patterns would be affected by changes in drug absorption. However, this is an issue that requires further investigation.
In conclusion, the analytical approach presented in the current study provides guidance to the interpretation of 24-hour IOP monitoring results from the CLS. Due to the recent emergence of 24-hour IOP monitoring, this is a rapidly evolving field and the proposed method may be one among several approaches. Using this method, we found that a majority of glaucoma patients and suspects had a reproducible nocturnal/sleep acrophase in the short term. Future studies should evaluate the prognostic significance of the parameters extracted from 24-hour IOP data on the clinical prognosis of glaucoma patients.
Footnotes
Supported in part by National Eye Institute Grant R01021818 (FAM), Sensimed AG (Lausanne, Switzerland), an unrestricted grant from Research to Prevent Blindness (New York, New York) to the Department of Ophthalmology at the University of California, San Diego, and Velux Foundation, Zurich, Switzerland (KM).
Disclosure: K. Mansouri, Sensimed AG (C); J.H.K. Liu, Sensimed AG (F); R.N. Weinreb, Sensimed AG (C); A. Tafreshi, None; F.A. Medeiros, Sensimed AG (F)
References
- 1.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713; discussion, 829–830 [DOI] [PubMed] [Google Scholar]
- 2.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972 [DOI] [PubMed] [Google Scholar]
- 3.Mansouri K, Leite MT, Medeiros FA, et al. Assessment of rates of structural change in glaucoma using imaging technologies. Eye. 2011;25:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitacre MM, Stein R. Sources of error with use of Goldmann-type tonometers. Surv Ophthalmol. 1993;38:1–30 [DOI] [PubMed] [Google Scholar]
- 5.Liu JH, Kripke DF, Hoffman RE, et al. Nocturnal elevation of intraocular pressure in young adults. Invest Ophthalmol Vis Sci. 1998;39:2707–2712 [PubMed] [Google Scholar]
- 6.Mansouri K, Weinreb RN, Liu JH. Effects of aging on 24-hour intraocular pressure measurements in sitting and supine body positions. Invest Ophthalmol Vis Sci. 2012;53:112–116 [DOI] [PubMed] [Google Scholar]
- 7.Zeimer RC, Wilensky JT, Gieser DK, Viana MA. Association between intraocular pressure peaks and progression of visual field loss. Ophthalmology. 1991;98:64–69 [DOI] [PubMed] [Google Scholar]
- 8.Konstas AG, Quaranta L, Mikropoulos DG, et al. Peak intraocular pressure and glaucomatous progression in primary open-angle glaucoma. J Ocul Pharmacol Ther. 2012;28:26–32 [DOI] [PubMed] [Google Scholar]
- 9.Wilensky JT, Gieser DK, Dietsche ML, et al. Individual variability in the diurnal intraocular pressure curve. Ophthalmology. 1993;100:940–944 [DOI] [PubMed] [Google Scholar]
- 10.Realini T, Weinreb RN, Wisniewski S. Short-term repeatability of diurnal intraocular pressure patterns in glaucomatous individuals. Ophthalmology. 2011;118:47–51 [DOI] [PubMed] [Google Scholar]
- 11.Rotchford AP, Uppal S, Lakshmanan A, King AJ. Day-to-day variability in intraocular pressure in glaucoma and ocular hypertension. Br J Ophthalmol. 2012;96:967–970 [DOI] [PubMed] [Google Scholar]
- 12.Mansouri K, Shaarawy T. Continuous intraocular pressure monitoring with a wireless ocular telemetry sensor: initial clinical experience in patients with open angle glaucoma. Br J Ophthalmol. 2011;95:627–629 [DOI] [PubMed] [Google Scholar]
- 13.Mansouri K, Weinreb R. Continuous 24-hour intraocular pressure monitoring for glaucoma—time for a paradigm change. Swiss Med Wkly. 2012;142:w13545 [DOI] [PubMed] [Google Scholar]
- 14.Mansouri K, Weinreb RN. Meeting an unmet need in glaucoma: continuous 24-h monitoring of intraocular pressure. Expert Rev Med Devices. 2012;9:225–231 [DOI] [PubMed] [Google Scholar]
- 15.Leonardi M, Leuenberger P, Bertrand D, et al. First steps toward noninvasive intraocular pressure monitoring with a sensing contact lens. Invest Ophthalmol Vis Sci. 2004;45:3113–3117 [DOI] [PubMed] [Google Scholar]
- 16.Hjortdal JO, Jensen PK. In vitro measurement of corneal strain, thickness, and curvature using digital image processing. Acta Ophthalmol Scand. 1995;73:5–11 [DOI] [PubMed] [Google Scholar]
- 17.Tong YL. Parameter estimation in studying circadian rhythms. Biometrics. 1976;32:85–94 [PubMed] [Google Scholar]
- 18.Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6:305–323 [PubMed] [Google Scholar]
- 19.Yamagami J, Araie M, Shirato S. A comparative study of optic nerve head in low- and high-tension glaucomas. Graefes Arch Clin Exp Ophthalmol. 1992;230:446–450 [DOI] [PubMed] [Google Scholar]
- 20.Renard E, Palombi K, Gronfier C, et al. Twenty-four hour (Nyctohemeral) rhythm of intraocular pressure and ocular perfusion pressure in normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2010;51:882–889 [DOI] [PubMed] [Google Scholar]
- 21.Lee YR, Kook MS, Joe SG, et al. Circadian (24-hour) pattern of intraocular pressure and visual field damage in eyes with normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2012;53:881–887 [DOI] [PubMed] [Google Scholar]
- 22.Mansouri K, Leite MT, Weinreb RN, et al. Association between corneal biomechanical properties and glaucoma severity. Am J Ophthalmol. 2012;153:419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zar J. Biostatistical Analysis. 5th ed. Upper Saddle River, NJ: Prentice-Hall; 1999 [Google Scholar]
- 24.Drouyer E, Dkhissi-Benyahya O, Chiquet C, et al. Glaucoma alters the circadian timing system. PLoS One. 2008;3:e3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang SY, Lee GA, Shields D. Self-tonometry in glaucoma management—past, present and future. Surv Ophthalmol. 2009;54:450–462 [DOI] [PubMed] [Google Scholar]
- 26.Brown EN, Czeisler CA. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. J Biol Rhythms. 1992;7:177–202 [DOI] [PubMed] [Google Scholar]
- 27.Buguet A, Py P, Romanet JP. 24-hour (nyctohemeral) and sleep-related variations of intraocular pressure in healthy white individuals. Am J Ophthalmol. 1994;117:342–347 [DOI] [PubMed] [Google Scholar]
- 28.Yamagami J, Araie M, Aihara M, Yamamoto S. Diurnal variation in intraocular pressure of normal-tension glaucoma eyes. Ophthalmology. 1993;100:643–650 [DOI] [PubMed] [Google Scholar]
- 29.Friberg TR, Sanborn G, Weinreb RN. Intraocular and episcleral venous pressure increase during inverted posture. Am J Ophthalmol. 1987;103:523–526 [DOI] [PubMed] [Google Scholar]
- 30.Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44:1586–1590 [DOI] [PubMed] [Google Scholar]
- 31.Lam AK, Chen D. Pentacam pachometry: comparison with non-contact specular microscopy on the central cornea and inter-session repeatability on the peripheral cornea. Clin Exp Optom. 2007;90:108–114 [DOI] [PubMed] [Google Scholar]
- 32.Oncel B, Dinc UA, Gorgun E, Yalvac BI. Diurnal variation of corneal biomechanics and intraocular pressure in normal subjects. Eur J Ophthalmol. 2009;19:798–803 [DOI] [PubMed] [Google Scholar]
- 33.Kida T, Liu JH, Weinreb RN. Effect of 24-hour corneal biomechanical changes on intraocular pressure measurement. Invest Ophthalmol Vis Sci. 2006;47:4422–4426 [DOI] [PubMed] [Google Scholar]
- 34.Fan S, Hejkal JJ, Gulati V, et al. Aqueous humor dynamics during the day and night in volunteers with ocular hypertension. Arch Ophthalmol. 2011;129:1162–1166 [DOI] [PubMed] [Google Scholar]
- 35.Freiberg FJ, Lindell J, Thederan LA, Leippi S, Shen Y, Klink T. Corneal thickness after overnight wear of an intraocular pressure fluctuation contact lens sensor. Acta Ophthalmol. 2012;90:e534–e539 [DOI] [PubMed] [Google Scholar]
- 36.Erickson P, Comstock TL, Doughty MJ, Cullen AP. The cornea swells in the posterior direction under hydrogel contact lenses. Ophthalmic Physiol Opt. 1999;19:475–480 [DOI] [PubMed] [Google Scholar]