Abstract
Chronic alcohol abuse is an established risk factor for osteoporosis. However, the precise mechanisms for the bone loss are largely unknown. Alcohol decreases skeletal expression of insulin-like growth factor-I (IGF-I), an important growth hormone (GH)-regulated skeletal growth factor. Therefore, we investigated the effects of alcohol on the skeletal response to GH in male Sprague Dawley rats made GH-deficient by hypophysectomy (HYPOX). Four groups of sexually mature (3-month-old) rats were studied: pituitary-intact (control), HYPOX, HYPOX + GH, and HYPOX + alcohol + GH. All animals were transferred to a liquid diet 6 days following surgery. The alcohol-fed group was adapted to a graded increase in alcohol beginning 11 days following surgery. GH or vehicle was administered during the final 8 days of study and all animals were sacrificed 25 days following surgery. HYPOX resulted in cessation of body weight gain and tibial growth. Compared to controls, longitudinal bone growth and cancellous bone formation were lower following HYPOX. The latter was associated with lower mineralizing perimeter/bone perimeter. Bone marrow adiposity was higher following HYPOX. Compared to HYPOX, GH treatment increased body weight gain and bone formation rate, and decreased bone marrow adiposity. In contrast to the effects of GH treatment without alcohol, bone marrow adiposity did not differ between HYPOX and alcohol-fed GH-treated HYPOX rats. Alcohol did not alter GH-induced weight gain or increases in serum IGF-I levels but significantly impaired the effects of GH on tibial growth and cancellous bone formation. We conclude that the detrimental skeletal effects of alcohol abuse observed in this experiment are mediated, at least in part, by skeletal resistance to GH.
Keywords: hypophysectomy, IGF-I, bone histomorphometry, osteoporosis, osteoblast
Introduction
Chronic alcohol abuse is an established risk factor for osteoporosis [1, 2]. At the cellular level, heavy alcohol consumption reduces biochemical markers of bone formation in humans [3] and histomorphometric measures of bone formation in rats [2]. However, the precise mechanisms mediating the inhibitory effect of alcohol on bone formation have yet to be elucidated.
We and others have shown that ethanol or its metabolites have direct inhibitory effects on cultured osteoblasts. Specifically, alcohol decreases osteoblast proliferation and inhibits the synthesis of bone matrix proteins [4–6]. However, the concentration of ethanol necessary to achieve inhibitory effects on cultured cells is generally very high (>100 mM), suggesting that osteoblasts are resistant to the direct toxic effects of alcohol [7]. In contrast, ingestion of alcohol that contributed as little as 3% of caloric intake (estimated peak blood levels of <5 mM) suppressed bone formation by >50% in adult rats [7], implying that the inhibitory actions of alcohol on bone formation may involve indirect mechanisms.
Disruption of endocrine signaling is a potential molecular mechanism by which alcohol can indirectly suppress bone formation. Chronic alcohol consumption has been reported to alter levels of insulin-like growth factor-I (IGF-I), estrogen, and parathyroid hormone (PTH) [8–12]. However, the changes in the levels of these hormones are generally modest in magnitude and are not detected in all studies [13, 14]. Alternatively, alcohol may disrupt endocrine signaling by inducing resistance to one or more hormones. We have shown that alcohol down-regulates mRNA levels for IGF-I in skeletal tissue prior to decreasing bone formation [15]. Because IGF-I gene expression generally reflects GH status, alcohol may reduce IGF-I gene expression by antagonizing GH signaling.
The hypothesis that decreased GH/IGF-I signaling contributes to the detrimental effects of alcohol abuse is supported by similarities between GH-deficient patients and chronic alcohol abusers. For example, in addition to decreased bone formation, both conditions are associated with increased fat accumulation [16, 17]. Reductions in bone formation and increased bone marrow adiposity are also observed in rat models for GH deficiency and for chronic alcohol abuse [2, 18]. The numerous ways that alcohol could disrupt GH/IGF-I signaling fall into two non-mutually exclusive classes of action: 1) decreased hormone bioavailability and 2) end-organ resistance. The purpose of the present study was to evaluate the potential role of GH resistance as a mechanism for the detrimental effects of alcohol abuse on bone metabolism by determining the skeletal response to GH in alcohol-fed, growth hormone-deficient hypophysectomized (HYPOX) rats.
Methods
Animal Procedures
The animal procedures were approved by the Institutional Animal Use and Care Committee at Oregon State University. Three-month-old HYPOX (n=18) or pituitary intact (controls; n=7) male Sprague Dawley rats weighing approximately 250 g were obtained from Harlan (Indianapolis, IN). The rats were housed individually in plastic shoebox cages and maintained on a 14:10 hour light-dark cycle at an ambient temperature of 21–23°C.
Rat chow and water were provided ad libitum to all animals until they were switched on post-op day 6 to a liquid diet (Lieber-DeCarli liquid diet, BioServ, #F1258SP). The HYPOX rats were randomized by weight into two treatment groups: HYPOX (n=6) and HYPOX + alcohol (ethanol, EtOH) (n=12). Alcohol was introduced to the alcohol-fed group on post-op day 11 at a concentration that contributed 12% of total caloric intake. Alcohol was increased on post-op day 18 to 23% caloric intake and further increased, on post-op day 21, to 35% caloric intake. Alcohol-free controls and HYPOX rats consumed an isocaloric liquid diet with alcohol replaced with maltose-dextran (BioServ, #F1259SP). The food was provided ad libitum to all animals during the 14-day alcohol intervention. We did not pair-feed the rats to the HYPOX groups because one of the goals of the study was to determine whether alcohol antagonizes the orexigenic effects of GH.
On post-op day 18, the alcohol-fed HYPOX rats were randomized into two groups (n=6/group): GH- and vehicle-treated. The GH-treated group received recombinant human GH (a gift from Genentech; 800 µg/kg/day via 2× daily sc injections spaced 12 h apart). Treatment of HYPOX rats with vehicle (normal saline solution) or GH continued until the experiment was completed 8 days later (post-op day 25).
Double fluorochrome labeling was used to measure bone formation. Rats were injected sc with declomycin (15 mg/kg; Sigma Chemical Co., St. Louis, MO) and calcein (15 mg/kg; Sigma Chemical Co.) 8 and 2 days prior to necropsy, respectively. For tissue collection, all rats were anesthetized with isoflurane and death was induced by exsanguination from the heart, followed by cardiac excision. Tibiae were excised and placed in 70% ethanol for histological processing. White adipose tissue (WAT) was dissected out from the peritoneal cavity and weighed.
Serum Analysis
Serum IGF-I was measured with a radioimmunoassay using a polyclonal antibody to IGF-I after separation of IGF-binding proteins by acid ethanol extraction. The inter-assay coefficient of variation is 4.6% and the lower limit of detection is 0.1 ng/ml. Serum leptin was measured by ELISA as recommended by the manufacturer (Diagnostic Systems Laboratories, Webster, TX).
Tissue Processing
For histomorphometric evaluation of cancellous bone and marrow adiposity, proximal tibiae were dehydrated in a graded series of ethanol and xylene, and embedded undecalcified in modified methyl methacrylate as described [19]. Longitudinal sections (4 µm thick) were cut with a vertical bed microtome (Leica 2065) and affixed to slides. One section/animal was stained according to the Von Kossa method with a tetrachrome counter stain (Polysciences, Warrington, PA) and used for assessing bone area and bone marrow adiposity. A second section was left unstained and used for assessing fluorochrome-based measurements of bone mineralization.
Histomorphometry
Histomorphometric data were collected within the proximal tibial epiphysis using the OsteoMeasure System (OsteoMetrics, Inc., Atlanta, GA). We have previously demonstrated that the epiphysis responds to regulatory factors such as estrogen and to conditions such as mechanical loading [20]. The epiphyseal area of interest included cancellous bone and bone marrow but excluded cortical bone. Static cancellous bone endpoints included: 1) cancellous bone area/tissue area (area of total tissue occupied by cancellous bone (%), 2) trabecular thickness (µm), 3) trabecular number (1/mm), and 4) trabecular separation (µm). Measurements of bone marrow adiposity included: 1) adipocyte area/tissue area (%), 2) adipocyte number/tissue area (#/mm2), and 3) adipocyte size (µm2). Adipocytes were identified morphologically as large circular or oval shaped cells, bordered by a prominent cell membrane and deficient in cytoplasmic staining due to alcohol extraction of intracellular lipids during processing.
Fluorochrome-based indices of bone formation were determined in the unstained sections illuminated with UV light. Mineralizing perimeter was determined as percentage of cancellous bone perimeter covered with double fluorochrome labels + ½ single labels. Mineral apposition rate was calculated as the mean distance between two fluorochrome markers that comprise a double label divided by the number of days between label administrations. Bone formation rate was calculated using a tissue area referent (%/year). Longitudinal bone growth rate was determined as the mean distance between the declomycin labeling front located in the primary spongiosa and the calcein label in the mineralizing growth plate cartilage divided by the labeling interval of 6 days. All bone histomorphometric data are reported using standard nomenclature [21].
Statistical Analysis
The effects of HYPOX were analyzed using a t-test or Mann-Whitney test. Because treatment with GH was short-term (final 8 days of study), a one-way ANOVA followed by a Bonferroni post-hoc multiple comparison test was used to analyze treatment effects in HYPOX animals (SPSS 13.0, SPSS Inc., Chicago, IL). Differences were considered significant at P < 0.05. All data are reported as mean ± SE.
Results
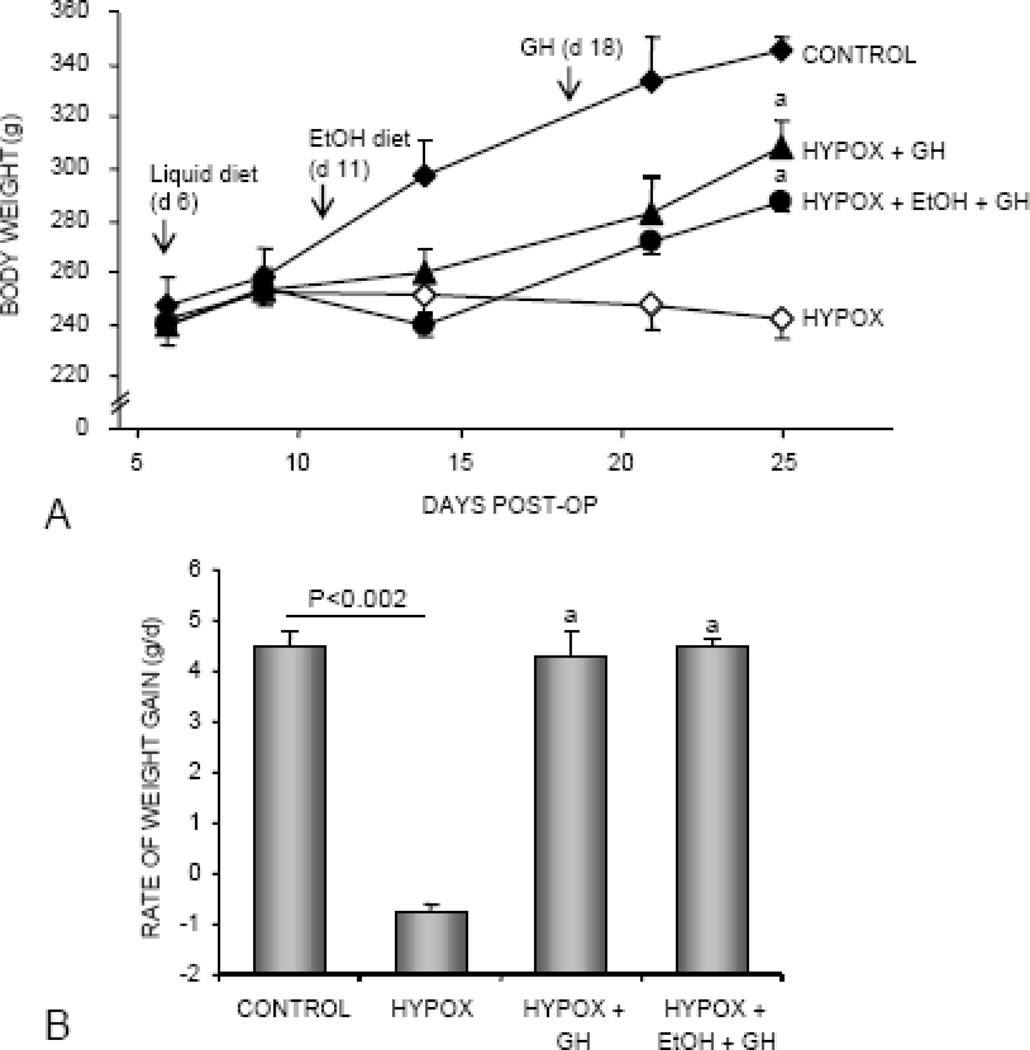
Changes in body weight during the 25 day duration of study are shown in Figure 1A. Body weight did not increase in HYPOX rats. In contrast, pituitary-intact control rats gained 98 g (increased weight to 140 ± 7% of starting weight). GH treatment during the final 8 days of study increased the rate of weight gain in HYPOX rats, irrespective of alcohol consumption (Figure 1B).
Figure 1.
The effects of HYPOX and treatment of HYPOX rats with GH ± alcohol on body weight (A) and rate of body weight gain during the last 4 days of GH treatment (B). Surgery was performed on day 0, alcohol feeding commenced on post-op day 11 and GH treatment commenced on post-op day 18. Values are mean ± SE; aDifferent from HYPOX, P<0.05.
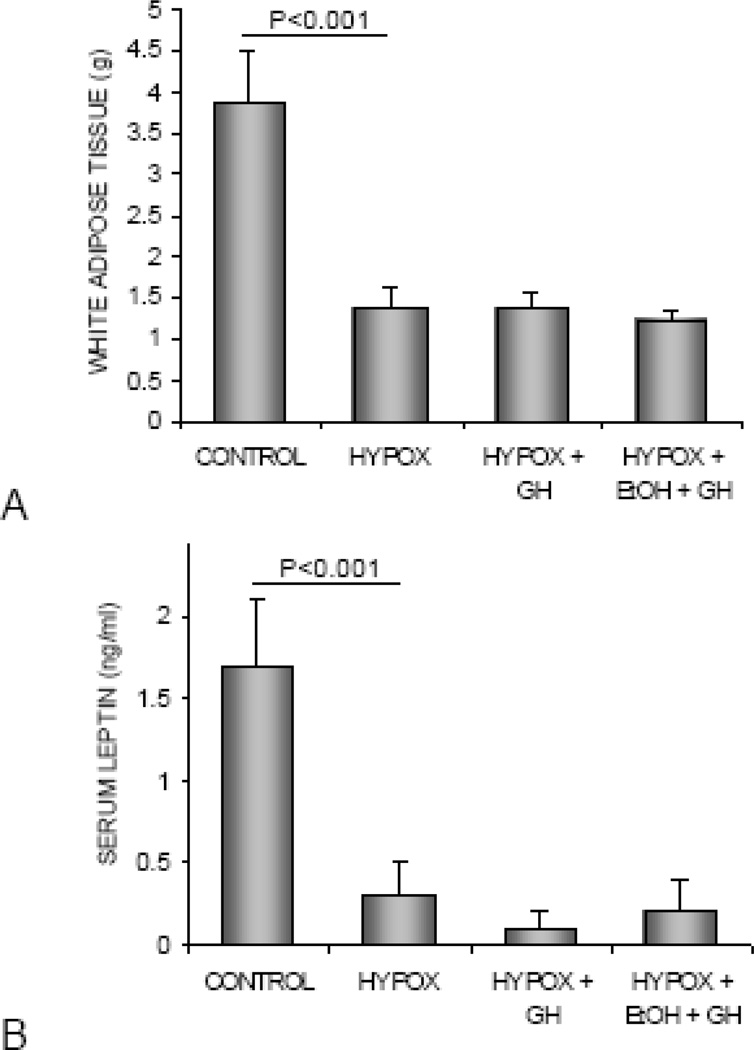
The effects of HYPOX and treatment with GH or alcohol + GH on abdominal WAT weight and serum leptin levels are shown in Figure 2. Compared to control rats, HYPOX rats had lower abdominal WAT weight (Figure 2A) and lower serum leptin levels (Figure 2B). GH treatment during the final 8 days of study had no effect on abdominal WAT or serum leptin in rats fed either alcohol-free or alcohol-containing diets.
Figure 2.
The effects of HYPOX and treatment of HYPOX rats with GH ± alcohol on abdominal white adipose tissue weight (A) and serum leptin levels (B). Values are mean ± SE.
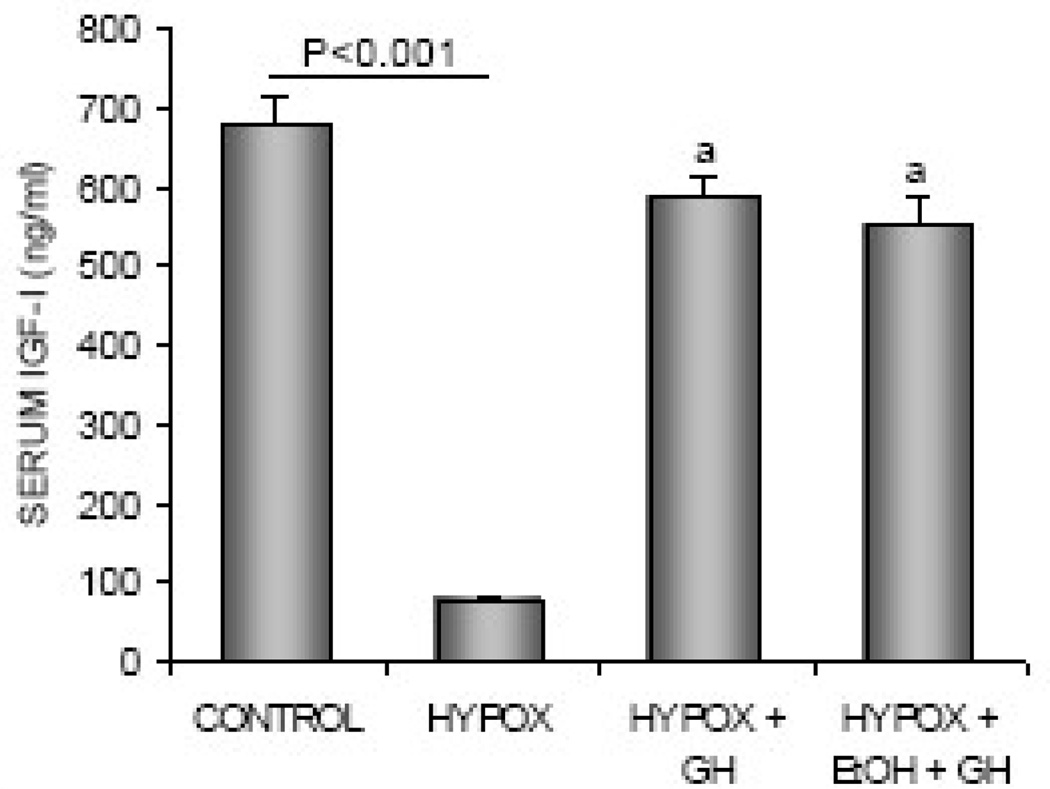
The effects of HYPOX and treatments with GH or alcohol + GH on serum IGF-I levels are shown in Figure 3. HYPOX rats had lower serum IGF-I levels than control rats. Administration of GH to HYPOX rats resulted in higher IGF-I levels, irrespective of alcohol treatment.
Figure 3.
The effects of HYPOX and treatment of HYPOX rats with GH ± alcohol on serum IGF-I levels. Values are mean ± SE; aDifferent from HYPOX, P<0.05.
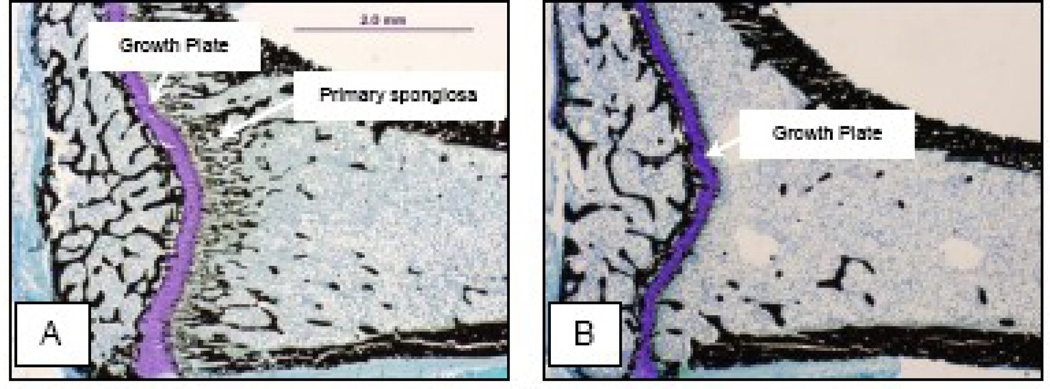
Routine nondestructive screening of tibia by µCT [22] revealed very low cancellous bone volume/tissue volume (< 2%) in the proximal tibial metaphysis of HYPOX animals. This finding of severe osteopenia was confirmed by histomorphometry (Figure 4) and precluded the use of this site for subsequent analyses of static and dynamic bone histomorphometric measurements [22]. As a result, analysis of the proximal tibia utilized the epiphysis, a skeletal compartment that is rich in cancellous bone [20].
Figure 4.
Representative photomicrographs of the proximal tibia of a control rat (A) and a HYPOX rat (B). Please note that the cancellous bone area is much lower in the metaphysis as opposed to the epiphysis in both the control and the HYPOX rat. Please also note the dramatic decrease in primary spongiosa in the HYPOX compared to the control rat.
Static bone histomorphometry results are summarized in Table 1. Compared to control rats, HYPOX rats had lower epiphyseal cancellous bone area/tissue area, lower trabecular number, and greater trabecular separation. Significant differences in trabecular thickness were not detected with HYPOX. Differences with treatment (vehicle, GH or alcohol + GH) in the HYPOX animals were not detected for any of the endpoints evaluated.
Table 1.
Effect of HYPOX and treatment of HYPOX rats with GH ± alcohol on static bone histomorphometry in the proximal tibial epiphysis.
| Endpoint | Control | HYPOX | HYPOX+ GH | HYPOX + EtOH + GH |
|---|---|---|---|---|
| Bone area/Tissue area (%) | 27.1 ± 1.0 | 18.8 ± 1.1* | 20.1 ± 0.6 | 23.0 ± 2.0 |
| Trabecular number (1/mm) | 5.5 ± 0.3 | 4.0 ± 0.2* | 4.2 ± 0.4 | 4.0 ± 0.5 |
| Trabecular thickness (µm) | 51 ± 2 | 48 ± 3 | 50 ± 5 | 58 ± 5 |
| Trabecular separation (µm) | 136 ± 9 | 208 ± 11* | 194 ± 20 | 201 ± 30 |
Data are mean ± SE
Different from pituitary-intact Control, P<0.001
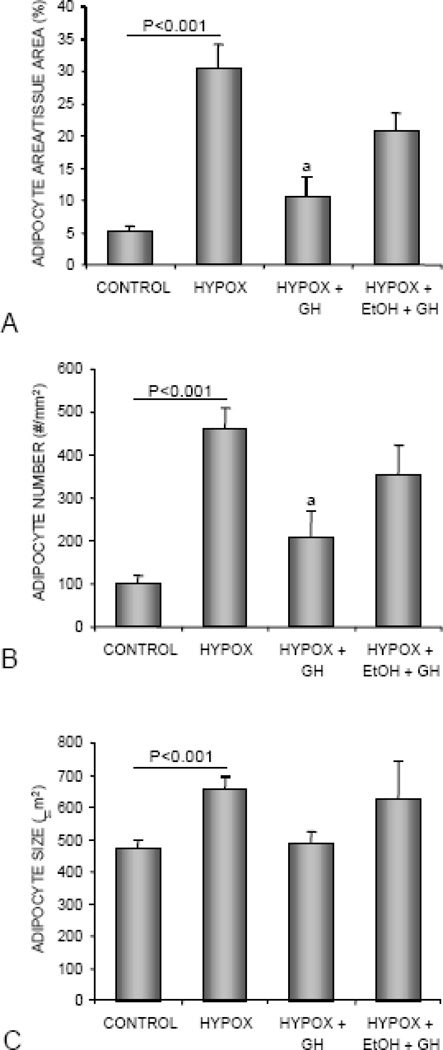
The effects of HYPOX and treatments on bone marrow adiposity are shown in Figure 5. Compared to control rats, HYPOX rats had higher levels of bone marrow adiposity (Figure 5A). This was due to greater adipocyte number (Figure 5B) and size (Figure 5C). Compared to HYPOX rats, GH-treated HYPOX rats had lower bone marrow adiposity, due to lower adipocyte number. Significant differences between HYPOX rats and alcohol-fed GH-treated HYPOX rats were not detected for any of the adiposity endpoints evaluated. Representative histological specimens from the proximal tibia of control, HYPOX, HYPOX + GH, and HYPOX + alcohol + GH rats are shown in Figure 6.
Figure 5.
The effects of HYPOX and treatment of HYPOX rats with GH ± alcohol on adipocyte area/tissue area (A), adipocyte number (B), and adipocyte size (C). Values are mean ± SE; aDifferent from HYPOX, P<0.05.
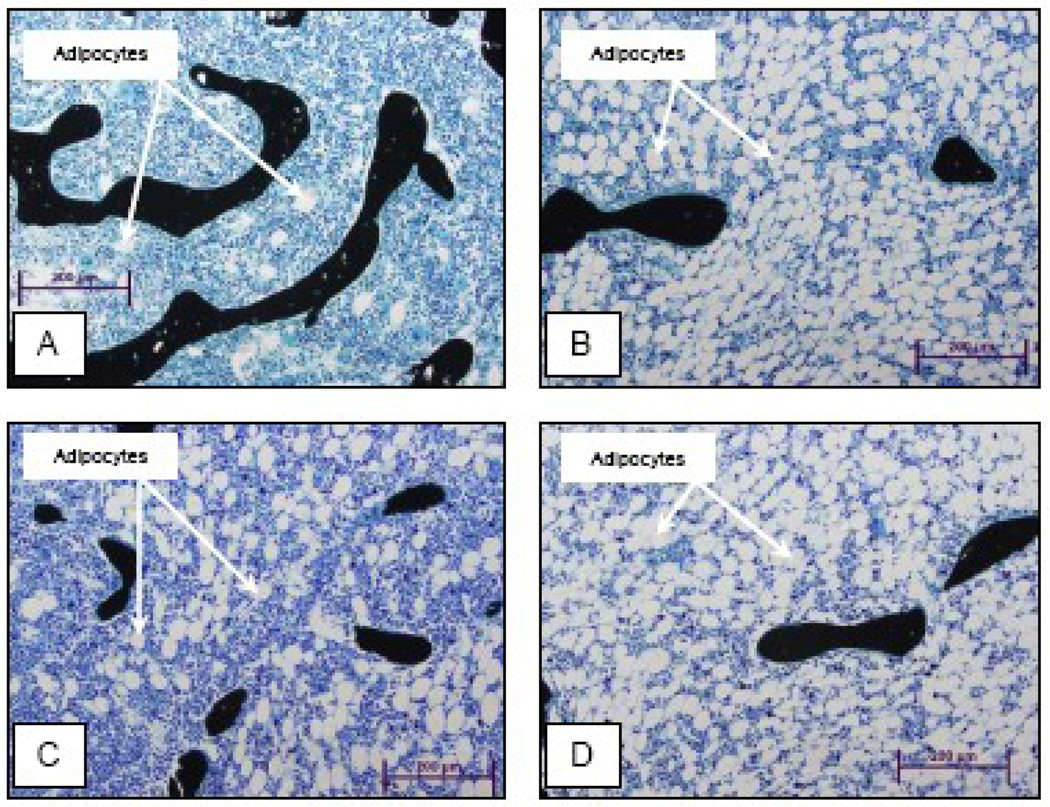
Figure 6.
Representative photomicrographs of the proximal tibial metaphysis showing adipocytes in a control rat (A), HYPOX rat (B), HYPOX + GH rat (C) and HYPOX + alcohol + GH rat (D).
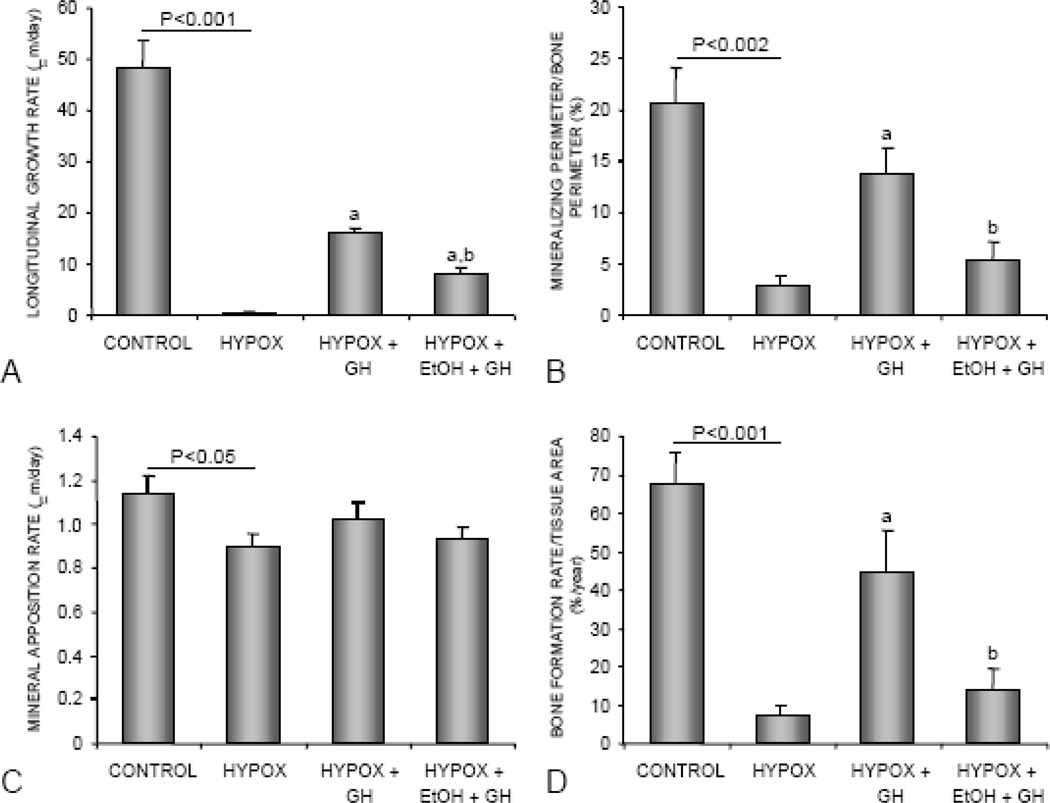
The effects of HYPOX and treatments on longitudinal bone growth and indices of cancellous bone formation are shown in Figure 7. Compared to control rats, HYPOX rats had lower longitudinal growth rate (Figure 7A), cancellous bone mineralizing perimeter/bone perimeter (Figure 7B), mineral apposition rate (Figure 7C), and bone formation rate/tissue area (Figure 7D). GH treatment resulted in greater longitudinal bone growth and greater cancellous bone formation rates. The latter was due predominantly to higher mineralizing perimeter/bone perimeter. Alcohol intake antagonized the effects of GH on indices of bone growth and formation. Longitudinal bone growth rate, mineralizing perimeter/bone perimeter, and bone formation rate/tissue area were lower in alcohol-fed GH-treated rats compared to GH-treated rats consuming the alcohol-free control diet.
Figure 7.
The effects of HYPOX and treatment of HYPOX rats with GH ± alcohol on tibial longitudinal growth rate (A), mineralizing perimeter/bone perimeter (B), mineral apposition rate (C), and bone formation rate/tissue area (D). Values are mean ± SE; aDifferent from HYPOX, P<0.05.
Discussion
This study investigated the effects of alcohol on the skeletal response to GH in sexually mature, GH-deficient, male HYPOX rats. HYPOX prevented body weight gain and this effect was reversed by GH treatment regardless of dietary alcohol. Compared to control rats, HYPOX rats had less cancellous bone and lower rates of longitudinal bone growth and cancellous bone formation, but higher bone marrow adiposity. Short duration (8 days) treatment of HYPOX rats with GH increased cancellous bone formation and longitudinal growth rates, and decreased bone marrow adiposity. Alcohol consumption blunted the effects of GH on bone elongation and cancellous bone formation.
GH couples energy expenditure to growth, including the growth of bone. GH deficiency in humans and other animals is associated with decreased bone growth and osteoporosis [23, 24]. Osteoblasts, chondrocytes, and adipocytes have receptors for GH, as do their stromal cell precursors, and the hormone elicits rapid effects on these cells in culture [25]. GH increases stromal cell number and differentiation of these cells to osteoblasts. In contrast, GH suppresses adipocyte differentiation. As a consequence, alcohol-induced changes in GH signaling could change the balance between osteoblast and adipocyte differentiation [18].
HYPOX animals are deficient in several hormones known to influence bone metabolism, including GH, leptin, sex steroids, and adrenal and thyroid hormones. Thus, the profound skeletal changes resulting from HYPOX need not be due exclusively to GH deficiency. However, the present study found that GH replacement increased bone formation and decreased bone marrow adiposity in HYPOX rats. In contrast, administration of thyroxine, cortisol or 17β-estradiol to HYPOX rats was ineffective [26, 27]. Also in the present study, short duration treatment with GH did not restore WAT or serum leptin levels to normal. These findings suggest that the profound changes in leptin levels following HYPOX do not mediate the immediate actions of GH on bone growth, turnover, or adiposity. Thus, GH appears to be sufficient to increase bone formation and reduce bone marrow adiposity in HYPOX rats.
The present histomorphometric analyses were performed in the proximal tibial epiphysis rather than the more commonly studied proximal tibial metaphysis. This was done because HYPOX resulted in a virtual obliteration of the metaphyseal primary spongiosa which normally provides a template for bone formation and subsequent remodeling. Although a decrease in cancellous bone was also detected in the epiphysis, it was much less severe. Originating as a secondary ossification center, the epiphysis is skeletally more mature than the corresponding metaphysis in growing animals. In the age range used for the present investigation, the cancellous bone in the epiphysis is composed entirely of secondary spongiosa whereas the metaphysis contains similar amounts of primary and secondary spongiosa. Although a smaller compartment than the metaphysis, the greater isotropy of the trabecular network in the epiphysis can be an advantage, making this is a highly reproducible sampling site. In addition, the epiphysis, like the metaphysis, is responsive to physiological regulators of bone turnover such as parathyroid hormone, estrogen and mechanical loading [20, 28] and, as shown in this study, GH.
GH treatment did not alter bone area in HYPOX rats. This was likely due to the short duration of treatment (8 days) compared to the duration of GH deficiency (25 days). The 8 day treatment interval did, however, reduce bone marrow adiposity. This finding is in agreement with earlier studies showing a dramatic increase in bone marrow adiposity 10 days following HYPOX that was completely restored to normal following GH treatment for 17 days [18]. Also, dynamic histomorphometric measurements obtained after 8 days of GH treatment are likely to underestimate the effects of treatment because the initial fluorochrome label was given on the day GH treatment was initiated. Nevertheless, GH was effective in increasing cancellous bone mineralizing perimeter/bone perimeter and longitudinal bone growth rate in HYPOX rats. The observed rapid effects of GH on bone formation in HYPOX rats are in agreement with other reports [27]. Taken together, these findings suggest that bone marrow fat stores as well as bone formation are tightly regulated by GH.
IGF-I is necessary for most, if not all, of the actions of GH on bone and multiple lines of evidence indicate that IGF-I is essential for normal bone growth and remodeling. As shown in this and previous studies, HYPOX dramatically decreases serum IGF-I levels [29]. Alcohol consumption, in addition to reducing serum IGF-I levels, alters hepatic synthesis of IGF binding proteins [30]. Locally, alcohol decreases IGF-I gene expression in bone [15]. Alcohol may also reduce IGF receptor numbers in target cells [30]. In contrast, alcohol did not reduce the levels of serum IGF-I in GH-treated HYPOX rats, a finding which suggests GH bioavailability was not impaired by ethanol. The present study therefore implicates skeletal resistance to GH as a mechanism for alcohol impaired GH/IGF-I signaling.
IGF-I, in addition to stimulating osteoblast differentiation in vitro, acts as an autocrine growth and survival factor for osteoblasts and may be essential for these cells to maintain their fully differentiated phenotype. As with GH, the bone anabolic actions of parathyroid hormone (PTH) require IGF-I [31–36]. PTH stimulates bone formation, apparently by activating bone lining cells, in HYPOX rats [28]. Illustrating the important role of locally produced IGF-I, increased bone formation is accompanied by an increase in skeletal IGF-I mRNA levels with no rise in circulating IGF-I [37]. This finding also indicates that the bone anabolic actions of PTH do not require GH receptor-mediated actions. PTH and alcohol were shown to have independent effects on bone formation [38], findings which suggest that alcohol does not interfere with IGF-I signaling. In the present study, alcohol consumption significantly impaired skeletal responses of HYPOX rats to GH. Thus, the putative alcohol-induced defect in GH signaling is likely due to impaired GH receptor function.
There is precedence for end organ resistance to GH in rats; skeletal resistance to the hormone was reported in rat models for disuse [29, 39–41] and senescence [42]. In regard to the former, alcohol has been shown to accentuate the detrimental skeletal effects of disuse [43]. Further studies are required to determine whether the putative resistance to GH is due to reduced receptor number or impaired signaling.
The bone loss associated with alcohol abuse or with GH deficiency is primarily due to decreased bone formation [27]. Similar to GH deficiency, the decrease in bone formation in alcohol-fed rats is largely due to decreased mineralizing perimeter [10]. Although the skeletal changes in alcohol-fed rats are remarkably similar to those observed in GH-deficient rats, GH resistance may not be the only indirect mechanism by which alcohol impacts the skeleton. Recent studies suggest that alcohol disrupts estrogen receptor signaling in skeletal tissue in rats and in vitro in cultured osteosarcoma cells [44, 45]. Estrogen receptor signaling plays an important role in bone metabolism, and as recently reviewed, the skeletal effects of estrogen are mediated, in part, through IGF-I signaling [46]. Interestingly, the skeletal effects of estrogen appear to require GH signaling [27]. Alcohol-induced skeletal resistance to GH may, therefore, negatively impact estrogen signaling. However, it is not clear whether the much lower levels of alcohol exposure experienced by moderate drinkers would impact estrogen action.
The pituitary is the site of synthesis of gonadotrophin releasing hormone and as a consequence HYPOX rats are severely hypogonadal [26, 27]. HYPOX female rats are unresponsive to the actions of estrogen on bone, indicating a critical role for pituitary hormones as permissive factors in the skeletal actions of estrogen [27]. Also, in contrast to females, estrogen receptor blockade using the potent antiestrogen ICI 182,780 had no effect on bone growth and turnover in young male rats [47, 48]. Therefore, it is unlikely that alcohol-induced alterations in estrogen receptor signaling contributed to the results observed in the present study.
By evaluating HYPOX animals, the present study focused on the role of skeletal resistance to GH in the etiology of alcohol-induced changes in bone metabolism. Alcohol consumption was shown to reduce the ability of GH to stimulate longitudinal bone growth and cancellous bone formation in HYPOX rats without reducing the ability of the hormone to increase serum IGF-I levels. These findings support the hypotheses that the detrimental effects of alcohol abuse on the skeleton result, at least in part, from acquired skeletal resistance to GH.
Acknowledgements
This work was supported by NIH grant AA011140, DOD grant PRO43181, and Oregon State University.
Funding: This work was supported by NIH grant AA011140, DOD grant PRO43181, and Oregon State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Sampson HW. Alcohol and other factors affecting osteoporosis risk in women. Alcohol Res Health. 2002;26:292–298. [PMC free article] [PubMed] [Google Scholar]
- 2.Turner RT. Skeletal response to alcohol. Alcohol Clin Exp Res. 2000;24:1693–1701. [PubMed] [Google Scholar]
- 3.Rapuri PB, Gallagher JC, Balhorn KE, Ryschon KL. Alcohol intake and bone metabolism in elderly women. Am J Clin Nutr. 2000;72:1206–1213. doi: 10.1093/ajcn/72.5.1206. [DOI] [PubMed] [Google Scholar]
- 4.Giuliani N, Girasole G, Vescovi PP, Passeri G, Pedrazzoni M. Ethanol and acetaldehyde inhibit the formation of early osteoblast progenitors in murine and human bone marrow cultures. Alcohol Clin Exp Res. 1999;23:381–385. [PubMed] [Google Scholar]
- 5.Maran A, Zhang M, Spelsberg TC, Turner RT. The dose-response effects of ethanol on the human fetal osteoblastic cell line. J Bone Miner Res. 2001;16:270–276. doi: 10.1359/jbmr.2001.16.2.270. [DOI] [PubMed] [Google Scholar]
- 6.Vignesh RC, Sitta Djody S, Jayasudha E, Gopalakrishnan V, Ilangovan R, Balaganesh M, Veni S, Sridhar M, Srinivasan N. Effect of ethanol on human osteosarcoma cell proliferation, differentiation and mineralization. Toxicology. 2006;220:63–70. doi: 10.1016/j.tox.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Turner RT, Kidder LS, Kennedy A, Evans GL, Sibonga JD. Moderate alcohol consumption suppresses bone turnover in adult female rats. J Bone Miner Res. 2001;16:589–594. doi: 10.1359/jbmr.2001.16.3.589. [DOI] [PubMed] [Google Scholar]
- 8.Dumitrescu RG, Shields PG. The etiology of alcohol-induced breast cancer. Alcohol. 2005;35:213–225. doi: 10.1016/j.alcohol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Iwaniec UT, Trevisiol CH, Maddalozzo GF, Rosen CJ, Turner RT. Effects of low-dose parathyroid hormone on bone mass, turnover, and ectopic osteoinduction in a rat model for chronic alcohol abuse. Bone. 2008;42:695–701. doi: 10.1016/j.bone.2007.12.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maddalozzo GF, Turner RT, Edwards CH, Howe KS, Widrick JJ, Rosen CJ, Iwaniec UT. Alcohol alters whole body composition, inhibits bone formation, and increases bone marrow adiposity in rats. Osteoporos Int. 2009 doi: 10.1007/s00198-009-0836-y. [DOI] [PubMed] [Google Scholar]
- 11.Ronis MJ, Wands JR, Badger TM, de la Monte SM, Lang CH, Calissendorff J. Alcohol-induced disruption of endocrine signaling. Alcohol Clin Exp Res. 2007;31:1269–1285. doi: 10.1111/j.1530-0277.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 12.Sibonga JD, Iwaniec UT, Shogren KL, Rosen CJ, Turner RT. Effects of parathyroid hormone (1–34) on tibia in an adult rat model for chronic alcohol abuse. Bone. 2007;40:1013–1020. doi: 10.1016/j.bone.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Sampson HW, Hebert VA, Booe HL, Champney TH. Effect of alcohol consumption on adult and aged bone: composition, morphology, and hormone levels of a rat animal model. Alcohol Clin Exp Res. 1998;22:1746–1753. [PubMed] [Google Scholar]
- 14.Sampson HW, Spears H. Osteopenia due to chronic alcohol consumption by young actively growing rats is not completely reversible. Alcohol Clin Exp Res. 1999;23:324–327. [PubMed] [Google Scholar]
- 15.Turner RT, Wronski TJ, Zhang M, Kidder LS, Bloomfield SA, Sibonga JD. Effects of ethanol on gene expression in rat bone: transient dose-dependent changes in mRNA levels for matrix proteins, skeletal growth factors, and cytokines are followed by reductions in bone formation. Alcohol Clin Exp Res. 1998;22:1591–1599. doi: 10.1111/j.1530-0277.1998.tb03953.x. [DOI] [PubMed] [Google Scholar]
- 16.Morita Y, Iwamoto I, Mizuma N, Kuwahata T, Matsuo T, Yoshinaga M, Douchi T. Precedence of the shift of body-fat distribution over the change in body composition after menopause. J Obstet Gynaecol Res. 2006;32:513–516. doi: 10.1111/j.1447-0756.2006.00437.x. [DOI] [PubMed] [Google Scholar]
- 17.Pei L, Tontonoz P. Fat's loss is bone's gain. J Clin Invest. 2004;113:805–806. doi: 10.1172/JCI21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menagh PJ, Turner RT, Lowry MB, Wong C, Yakar S, Rosen CJ, Iwaniec UT. Growth hormone regulates the balance between bone formation and bone marrow adiposity. J Bone Miner Res. doi: 10.1359/jbmr.091015. lSubmitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwaniec UT, Wronski TJ, Turner RT. Histological analysis of bone. Methods Mol Biol. 2008;447:325–341. doi: 10.1007/978-1-59745-242-7_21. [DOI] [PubMed] [Google Scholar]
- 20.Westerlind KC, Wronski TJ, Ritman EL, Luo ZP, An KN, Bell NH, Turner RT. Estrogen regulates the rate of bone turnover but bone balance in ovariectomized rats is modulated by prevailing mechanical strain. Proc Natl Acad Sci U S A. 1997;94:4199–4204. doi: 10.1073/pnas.94.8.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 22.Iwaniec U, Turner R. Animal models for osteoporosis. In: Marcus RF D, Nelson DA, Rosen CJ, editors. Osteoporosis. 3 ed. New York: Elsevier Press; 2008. pp. 985–2009. [Google Scholar]
- 23.Kasukawa Y, Miyakoshi N, Mohan S. The anabolic effects of GH/IGF system on bone. Curr Pharm Des. 2004;10:2577–2592. doi: 10.2174/1381612043383764. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson A, Swolin D, Enerback S, Ohlsson C. Expression of functional growth hormone receptors in cultured human osteoblast-like cells. J Clin Endocrinol Metab. 1995;80:3483–3488. doi: 10.1210/jcem.80.12.8530587. [DOI] [PubMed] [Google Scholar]
- 25.Ohlsson C, Bengtsson BA, Isaksson OG, Andreassen TT, Slootweg MC. Growth hormone and bone. Endocr Rev. 1998;19:55–79. doi: 10.1210/edrv.19.1.0324. [DOI] [PubMed] [Google Scholar]
- 26.Yeh JK, Chen MM, Aloia JF. Effects of estrogen and growth hormone on skeleton in the ovariectomized rat with hypophysectomy. Am J Physiol. 1997;273:E734–E742. doi: 10.1152/ajpendo.1997.273.4.E734. [DOI] [PubMed] [Google Scholar]
- 27.Kidder LS, Schmidt IU, Evans GL, Turner RT. Effects of growth hormone and low dose estrogen on bone growth and turnover in long bones of hypophysectomized rats. Calcif Tissue Int. 1997;61:327–335. doi: 10.1007/s002239900343. [DOI] [PubMed] [Google Scholar]
- 28.Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995;136:3632–3638. doi: 10.1210/endo.136.8.7628403. [DOI] [PubMed] [Google Scholar]
- 29.Kostenuik PJ, Harris J, Halloran BP, Turner RT, Morey-Holton ER, Bikle DD. Skeletal unloading causes resistance of osteoprogenitor cells to parathyroid hormone and to insulin-like growth factor-I. J Bone Miner Res. 1999;14:21–31. doi: 10.1359/jbmr.1999.14.1.21. [DOI] [PubMed] [Google Scholar]
- 30.Lang CH, Liu X, Nystrom G, Wu D, Cooney RN, Frost RA. Acute effects of growth hormone in alcohol-fed rats. Alcohol Alcohol. 2000;35:148–158. doi: 10.1093/alcalc/35.2.148. [DOI] [PubMed] [Google Scholar]
- 31.Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res. 2002;17:1570–1578. doi: 10.1359/jbmr.2002.17.9.1570. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Nishida S, Boudignon BM, Burghardt A, Elalieh HZ, Hamilton MM, Majumdar S, Halloran BP, Clemens TL, Bikle DD. IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J Bone Miner Res. 2007;22:1329–1337. doi: 10.1359/jbmr.070517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohan S, Baylink DJ. Impaired skeletal growth in mice with haploinsufficiency of IGF-I: genetic evidence that differences in IGF-I expression could contribute to peak bone mineral density differences. J Endocrinol. 2005;185:415–420. doi: 10.1677/joe.1.06141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohan S, Richman C, Guo R, Amaar Y, Donahue LR, Wergedal J, Baylink DJ. Insulin-like growth factor regulates peak bone mineral density in mice by both growth hormone-dependent and -independent mechanisms. Endocrinology. 2003;144:929–936. doi: 10.1210/en.2002-220948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Zhou J, Cheng CM, Kopchick JJ, Bondy CA. Evidence supporting dual, IGF-I-independent and IGF-I-dependent, roles for GH in promoting longitudinal bone growth. J Endocrinol. 2004;180:247–255. doi: 10.1677/joe.0.1800247. [DOI] [PubMed] [Google Scholar]
- 36.Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt IU, Dobnig H, Turner RT. Intermittent parathyroid hormone treatment increases osteoblast number, steady state messenger ribonucleic acid levels for osteocalcin, and bone formation in tibial metaphysis of hypophysectomized female rats. Endocrinology. 1995;136:5127–5134. doi: 10.1210/endo.136.11.7588250. [DOI] [PubMed] [Google Scholar]
- 38.Turner RT, Evans GL, Zhang M, Sibonga JD. Effects of parathyroid hormone on bone formation in a rat model for chronic alcohol abuse. Alcohol Clin Exp Res. 2001;25:667–671. [PubMed] [Google Scholar]
- 39.Bikle DD, Harris J, Halloran BP, Currier PA, Tanner S, Morey-Holton E. The molecular response of bone to growth hormone during skeletal unloading: regional differences. Endocrinology. 1995;136:2099–2109. doi: 10.1210/endo.136.5.7720659. [DOI] [PubMed] [Google Scholar]
- 40.Sakata T, Halloran BP, Elalieh HZ, Munson SJ, Rudner L, Venton L, Ginzinger D, Rosen CJ, Bikle DD. Skeletal unloading induces resistance to insulin-like growth factor I on bone formation. Bone. 2003;32:669–680. doi: 10.1016/s8756-3282(03)00088-7. [DOI] [PubMed] [Google Scholar]
- 41.Sakata T, Wang Y, Halloran BP, Elalieh HZ, Cao J, Bikle DD. Skeletal unloading induces resistance to insulin-like growth factor-I (IGF-I) by inhibiting activation of the IGF-I signaling pathways. J Bone Miner Res. 2004;19:436–446. doi: 10.1359/JBMR.0301241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren J, Jefferson L, Sowers JR, Brown RA. Influence of age on contractile response to insulin-like growth factor 1 in ventricular myocytes from spontaneously hypertensive rats. Hypertension. 1999;34:1215–1222. doi: 10.1161/01.hyp.34.6.1215. [DOI] [PubMed] [Google Scholar]
- 43.Hefferan TE, Kennedy AM, Evans GL, Turner RT. Disuse exaggerates the detrimental effects of alcohol on cortical bone. Alcohol Clin Exp Res. 2003;27:111–117. doi: 10.1097/01.ALC.0000047348.23797.6E. [DOI] [PubMed] [Google Scholar]
- 44.Chen JR, Haley RL, Hidestrand M, Shankar K, Liu X, Lumpkin CK, Simpson PM, Badger TM, Ronis MJ. Estradiol protects against ethanol-induced bone loss by inhibiting up-regulation of receptor activator of nuclear factor-kappaB ligand in osteoblasts. J Pharmacol Exp Ther. 2006;319:1182–1190. doi: 10.1124/jpet.106.109454. [DOI] [PubMed] [Google Scholar]
- 45.Chen JR, Lazarenko OP, Haley RL, Blackburn ML, Badger TM, Ronis MJ. Ethanol impairs estrogen receptor signaling resulting in accelerated activation of senescence pathways, whereas estradiol attenuates the effects of ethanol in osteoblasts. J Bone Miner Res. 2009;24:221–230. doi: 10.1359/jbmr.081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner RT, Rickard DJ, Iwaniec UT, Speleberg TC. Estrogens and progestins. In: Bilezikian JP, Raisz LG, Martin J, editors. Principles of Bone Biology. Academic Press; 2008. pp. 847–877. [Google Scholar]
- 47.Sibonga JD, Dobnig H, Harden RM, Turner RT. Effect of the high-affinity estrogen receptor ligand ICI 182,780 on the rat tibia. Endocrinology. 1998;139:3736–3742. doi: 10.1210/endo.139.9.6172. [DOI] [PubMed] [Google Scholar]
- 48.Turner RT, Evans GL, Dobnig H. The high-affinity estrogen receptor antagonist ICI 182,780 has no effect on bone growth in young male rats. Calcif Tissue Int. 2000;66:461–464. doi: 10.1007/s002230010092. [DOI] [PubMed] [Google Scholar]