Abstract
Objectives
To define the impact of coinfection with hepatitis B virus (HBV) or hepatitis C virus (HCV) on viroimmunological response to raltegravir-based salvage regimens that also include new HIV inhibitors such as maraviroc, darunavir and etravirine.
Methods
We used data from a national observational study of patients starting raltegravir-based regimens to compare virological suppression and CD4 cell change from baseline in patients with and without concomitant HBV or HCV infection.
Results
Overall, 275 patients (107 coinfected and 168 non-coinfected) were evaluated. Coinfected patients were more commonly former intravenous drug users and had a longer history of HIV infection and higher baseline aminotransferase levels. Both HIV-RNA and CD4 response were similar in the two groups. Mean time to first HIV-RNA copy number <50 copies/mL was 4.1 months (95% CI 3.5–4.6) in non-coinfected patients and 3.9 months (95% CI 3.3–4.5) in coinfected patients (hazard ratio 1.039, 95% CI 0.761–1.418, P = 0.766, log-rank test). The risk of developing new grade 3–4 hepatic adverse events was significantly higher in coinfected patients (hazard ratio 1.779, 95% CI 1.123–2.817, P = 0.009). The two groups of coinfected and non-coinfected patients had similar rates of interruption of any baseline drug (hazard ratio 1.075, 95% CI 0.649–1.781, P = 0.776) and of raltegravir (hazard ratio 1.520, 95% CI 0.671–3.447, P = 0.311). Few AIDS-defining events and deaths occurred.
Conclusions
Viroimmunological response to regimens based on raltegravir and other recent anti-HIV inhibitors is not negatively affected by coinfection with HBV or HCV. Liver toxicity, either pre-existing or new, is more common in coinfected patients, but with no increased risk of treatment interruption.
Keywords: antiretroviral therapy, HIV-1, HBV, HCV, integrase inhibitors, viral hepatitis, viral load, CD4 response, darunavir, maraviroc, etravirine, HIV resistance, liver disease
Introduction
Most of the available data suggest that coinfection with hepatitis B virus (HBV) or hepatitis C virus (HCV) has no major effect on HIV disease progression and HIV-related mortality, and that virological and immunological response to initial HIV combination regimens is substantially similar compared with non-coinfected patients.1–3 However, some studies have reported among coinfected patients a blunted CD4 cell response,4,5 a more frequent occurrence of AIDS-defining illnesses6 and an increased mortality attributable to liver disease.1,5
Most of the above studies have evaluated regimens based on HIV reverse transcriptase inhibitors and protease inhibitors (PIs), and there are more limited data on second- and third-line regimens, which frequently include the integrase inhibitor raltegravir, the C-C chemokine receptor type 5 (CCR5) antagonist maraviroc and other recently introduced drugs, such as darunavir and etravirine.
Most of the currently available information on raltegravir-based regimens in HIV-infected patients coinfected with HCV or HBV is also limited to safety data from controlled clinical trials and observational studies. Such studies overall suggest an increased risk of liver-related toxicities in the presence of HCV or HBV coinfection,7–9 but more data are still needed.
In order to provide more information on this issue, with particular reference to viroimmunological response and toxicity in a context of common use of maraviroc, darunavir and etravirine, we further explored the response to raltegravir-based salvage regimens in coinfected patients participating in a cohort study of triple-class [nucleoside reverse transcriptase inhibitor (NRTI) + non-nucleoside reverse transcriptase inhibitor (NNRTI) + PI] drug-experienced patients followed in a setting of common clinical practice.
Methods
We used data from the ISS-NIA (Istituto Superiore di Sanità—New Inhibitors Against HIV) study, an ongoing national cohort study designed to evaluate in a clinical practice setting the effects of starting anti-HIV regimens based on raltegravir or maraviroc. Only triple-class (NRTI + NNRTI + PI) drug-experienced patients with treatment failure, resistance or intolerance are eligible for this observational study, and no indication is given for the selection of regimens, which are decided by the treating physician. The study received ethics approval and all patients gave written informed consent. Enrolment started in 2008 and was closed in late 2010. Patients are followed for at least 3 years.
All patients on raltegravir-based regimens (including concomitant maraviroc) with known hepatitis virus coinfection status and at least one follow-up visit were eligible for the present analysis, which refers to data available up to January 2012. The following study timepoints were used: baseline; month 3 (2–4); month 6 (5–7); month 9 (8–10); month 12 (11–13); month 15 (14–16); and month 18 (17–19). For patients with >18 months of follow-up, follow-up was censored at month 18.
The main efficacy measures were represented by time to virological suppression (defined by <50 copies/mL of HIV-1 RNA in plasma during follow-up) and by mean CD4 cell change from baseline. The main toxicity measures were represented by new grade 3–4 toxicities according to the definition of the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events.10 For the purpose of this analysis HCV coinfection was defined by positive commercial enzyme-linked immunosorbent tests for anti-HCV antibodies, and HBV coinfection by a positive hepatitis B surface antigen (HBsAg) test. Patients who in addition to HIV infection had also HCV and/or HBV coinfection were compared with patients with HIV infection only. In patients with HBV or HCV coinfection, liver fibrosis was assessed using the stepwise algorithm described by Pineda et al.11
Descriptive statistics and parametric and non-parametric tests were used to summarize and compare baseline characteristics in the two groups (non-coinfected and coinfected patients). Time to virological failure, to discontinuation of the baseline regimen and to first occurrence of severe hepatic adverse events (aminotransferase or bilirubin levels >5× upper limit of normal, or terminal liver disease) were analysed using the Kaplan–Meier method and the log-rank test. The Cox proportional hazards model was used to estimate hazard ratios of events.
The CD4 response was analysed at each follow-up timepoint, evaluating 95% CIs of mean CD4 change from baseline (cells/mm3) for the two groups, with any overlap in CI indicating no statistically significant difference between groups. All analyses were performed using SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Overall, 326 patients were enrolled in the cohort study. All had available information on HBV and HCV coinfection status. Following exclusion of 30 patients on other new inhibitor-based regimens that did not include raltegravir (essentially maraviroc-based) and of 21 patients with only baseline visit data, 275 patients (107 coinfected, 38.9%; and 168 non-coinfected, 61.1%) were considered for all subsequent analyses. Their baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics
| Non-coinfected (n = 168) | Coinfected (n = 107) | P value | |
|---|---|---|---|
| HIV + HCV, n (%)a | — | 88 (82.2) | ND |
| HIV + HBV, n (%)b | — | 12 (11.2) | |
| HIV + HBV + HCV, n (%) | — | 7 (6.5) | |
| Female, n (%) | 52 (31.0) | 27 (25.2) | 0.307* |
| Age (years), mean ± SD (n, range) | 47 ± 10.0 (168, 21–80) | 46 ± 6.2 (107, 24–65) | 0.156† |
| HIV transmission, n (%) | <0.001* | ||
| men who have sex with men | 47 (28.0) | 9 (8.4) | |
| intravenous drug use | 19 (11.3) | 66 (61.7) | |
| heterosexual | 95 (56.5) | 28 (26.2) | |
| maternal–fetal/other | 7 (4.2) | 4 (3.7) | |
| Ethnicity, n (%) | 0.920* | ||
| Caucasian | 157 (93.5) | 101 (94.4) | |
| African | 8 (4.8) | 4 (3.7) | |
| other | 3 (1.8) | 2 (1.9) | |
| CD4 + /mm3 | |||
| mean ± SD (n, range) | 378 ± 280 (168, 1–1442) | 340 ± 235 (107, 2–1127) | 0.253† |
| ≤200, n (%) | 45 (26.8) | 32 (29.9) | 0.574* |
| HIV-RNA | |||
| copies/mL (log10), mean ± SD (n, range) | 3.2 ± 1.5 (168, 1.3–6.5) | 3.1 ± 1.4 (107, 1.3–6.2) | 0.884† |
| <50 copies/mL, n (%) | 62 (37) | 35 (33) | 0.478* |
| CDC HIV disease stage, n (%) | 0.361* | ||
| A | 70 (41.7) | 37 (34.6) | |
| B | 36 (21.4) | 30 (28.0) | |
| C | 62 (37.0) | 40 (37.4) | |
| Time from HIV diagnosis (years), mean ± SD (n, range) | 13 ± 6.3 (168, 1–29) | 18 ± 5.8 (71, 1–25) | <0.001† |
| Time on antiretroviral therapy (years), mean ± SD (n, range) | 11 ± 5.4 (167, 1–22) | 12 ± 4.8 (107, 1–21) | 0.039† |
| AST (IU/mL), mean ± SD (n, range) | 33.3 ± 28.5 (153, 9–200) | 49.3 ± 39.3 (100, 11–256) | <0.001† |
| ALT (IU/mL), mean ± SD (n, range) | 34.2 ± 27.6 (168, 10–164) | 47.4 ± 35.9 (107, 11–256) | 0.001† |
| Baseline AST or ALT >5× ULN, n (%) | 1 (0.6) | 3 (2.8) | ND |
| Total bilirubin (mg/dL), mean ± SD (n, range) | 0.78 ± 0.82 (167, 0.1–5.5) | 0.98 ± 1.1 (106, 0.1–5.9) | 0.075† |
| Baseline total bilirubin >5× ULN, n (%) | 7 (4.2) | 6 (5.6) | ND |
| Triglycerides (mg/dL), mean ± SD (n, range) | 199 ± 139 (167, 43–867) | 168 ± 100 (107, 42–565) | <0.001† |
| Total cholesterol (mg/dL), mean ± SD (n, range) | 190 ± 48 (168, 68–354) | 170 ± 44 (107, 81–354) | 0.045† |
| Concomitant drugs in the regimen, n (%) | |||
| maraviroc | 28 (16.7) | 18 (16.8) | 0.973* |
| etravirine | 22 (13.1) | 13 (12.1) | 0.819* |
| darunavir | 75 (44.6) | 55 (51.4) | 0.274* |
| Drug classes in the regimen, n (%) | |||
| NRTIs | 122 (72.6) | 77 (72.0) | 0.906* |
| NNRTIs | 28 (16.7) | 20 (18.7) | 0.666* |
| PIs | 122 (72.6) | 82 (76.6) | 0.583* |
| CCR5 inhibitors | 28 (16.7) | 18 (16.8) | 0.973* |
| fusion inhibitors | 17 (10.1) | 11 (10.3) | 0.966* |
ND, not done; ULN, upper limit of normal.
aSix patients in this group received HCV treatment (pegylated interferon and ribavirin) during follow-up.
bHBV-active nucleosides in patients of this group: tenofovir (n = 8), emtricitabine (n = 7) and lamivudine (n = 2).
*χ2 test.
†t-test.
The two groups were well balanced for gender, ethnicity, CD4 cell count, HIV-RNA copy number, CDC HIV disease stage and concomitant drugs in the regimen, including maraviroc, etravirine and darunavir (Table 1).
The main differences between the two groups were represented by a higher proportion of former intravenous drug users among coinfected patients (a likely route of transmission of hepatitis viruses as well as HIV), a longer history of HIV infection and antiretroviral treatment in the same group (consistent with the earlier spreading of HIV among drug users) and higher baseline aminotransferase [aspartate transaminase (AST) and alanine transaminase (ALT)] levels, consistent with liver damage due to coinfection with hepatitis viruses. Mean follow-up was similar in the two groups (14.5 months in the non-coinfected group and 13.8 months in the coinfected group, P = 0.182).
Most coinfected patients (77/107) were evaluable for fibrosis according to the algorithm described: 40 of them (51.9%) had fibrosis of grade 2 or higher. Among patients with HCV coinfection and available information on HCV-RNA (82/95, 86.3%), 66 (80.5%) had detectable HCV-RNA in plasma.
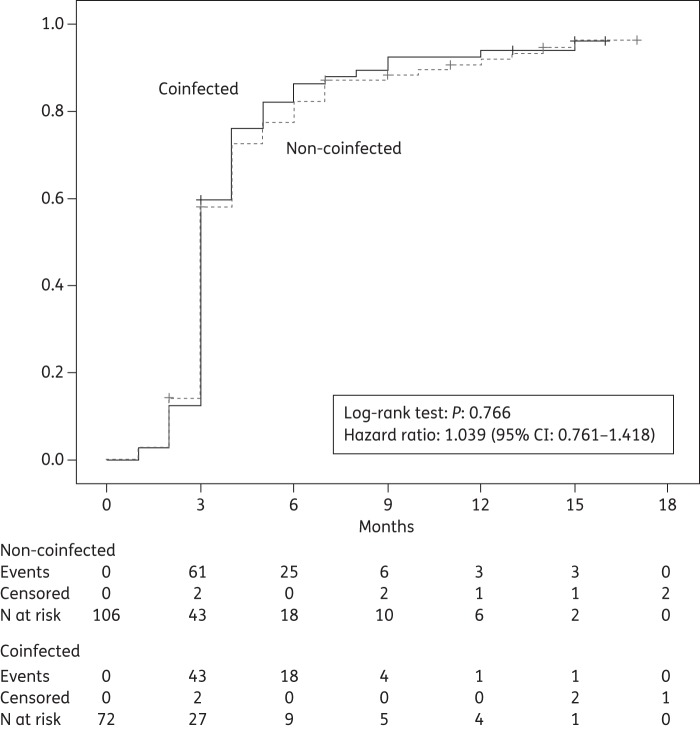
Both HIV-RNA and CD4 response during follow-up were similar in the two groups: mean time to first plasma HIV-RNA copy number <50 copies/mL was 4.1 months (95% CI 3.5–4.6) in non-coinfected patients and 3.9 months (95% CI 3.3–4.5) in coinfected patients (hazard ratio 1.039, 95% CI 0.761–1.418, P = 0.766, log-rank test) (Figure 1).
Figure 1.
Cumulative probability of reaching HIV-RNA suppression (plasma HIV-RNA <50 copies/mL) during follow-up (months 0–18).
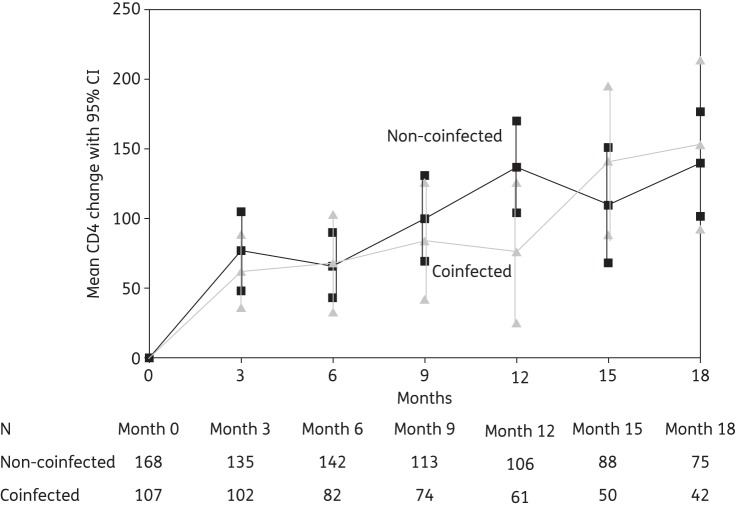
During 18 months of follow-up, CD4 cell count increased steadily in both groups with respect to baseline values, with no significant differences by coinfection status (Figure 2). Subgroup analyses of viroimmunological response that considered as coinfected patients those with HCV coinfection only (excluding patients with HBV or HBV/HCV coinfection) gave substantially identical results (hazard ratio for plasma HIV-RNA copy number <50 copies/mL 1.212, 95% CI 0.866–1.696, P = 0.156). No differences in viroimmunological response were observed in this subgroup between patients with and without detectable plasma HCV-RNA (data not shown).
Figure 2.
Mean CD4 cell count change with 95% CIs during follow-up (months 0–18). Any overlap in CI indicates no significant difference between the two groups.
The two main groups were also similar in terms of interruption of any baseline drug and of raltegravir: mean time to any drug interruption within initial regimen was 7.3 months (95% CI 5.7–9.0) in non-coinfected patients and 7.1 months (95% CI 5.1–9.1) in coinfected patients (hazard ratio 1.075, 95% CI 0.649–1.781, P = 0.776, log-rank test) and mean time to interruption of raltegravir was 8.0 months (95% CI 5.4–10.8) in non-coinfected patients and 9.3 months (95% CI 6.3–12.3) in coinfected patients (hazard ratio 1.520, 95% CI 0.671–3.447, P = 0.311, log-rank test). The reasons for interruption of raltegravir were: virological failure (non-coinfected = 2 and coinfected = 3), terminal liver disease (coinfected = 2), adverse event (non-coinfected = 4 and coinfected = 1), patient request or physician decision for other reasons (non-coinfected = 5 and coinfected = 3) and death due to other reasons (non-coinfected = 1 and coinfected = 2).
Overall, AIDS-defining events and deaths were relatively uncommon during follow-up: only five new AIDS-defining events were observed, two among non-coinfected patients (recurrent pneumonia and visceral leishmaniasis) and three among coinfected patients (non-Hodgkin lymphoma, diffuse herpes zoster and cerebral toxoplasmosis). Five patients died within 18 months: four were coinfected patients (two with terminal liver disease, defined by cirrhosis or hepatocellular carcinoma, one with complications due to non-Hodgkin lymphoma and one with body wasting possibly due to lymphoma) and one was a non-coinfected patient (sudden death during sleep, limited information available).
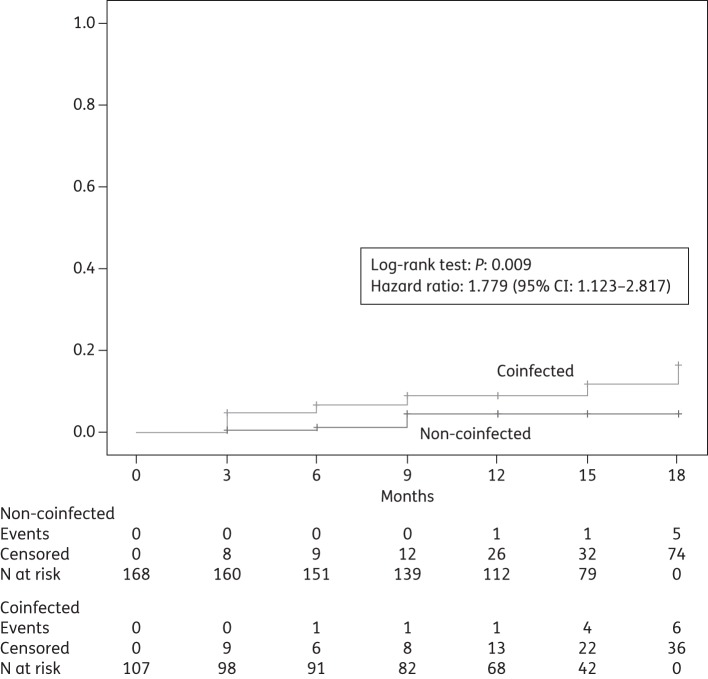
New occurrences of severe hepatic grade 3–4 adverse events during follow-up were relatively infrequent: overall, 23 such events occurred in the first 18 months, 7 among non-coinfected patients and 16 among coinfected patients, with a significantly higher risk in coinfected patients (hazard ratio 1.779, 95% CI 1.123–2.817, P = 0.009, Figure 3). Among coinfected patients, none of the above events occurred as a consequence of reactivation of HBV disease, because no HBV-active NRTI was interrupted in HBV-coinfected patients. Other adverse events of grade 3–4 were even less frequent, and overall they were balanced between coinfected and non-coinfected patients (data not shown).
Figure 3.
Cumulative probability of developing new severe hepatic adverse events (aminotransferase or bilirubin levels >5× upper limit of normal, or terminal liver disease) during follow-up (months 0–18).
Discussion
Our data show that viroimmunological response to raltegravir-based salvage regimens is similar in patients with and without coinfection with hepatitis viruses, even in a context of combinations including etravirine, darunavir and maraviroc that adequately reflects current clinical practice. Interestingly, our data did not show for coinfected patients the blunted CD4 response reported by others.4,5 It is possible that this difference may be attributable to the inclusion of newer and more active drugs in currently prescribed regimens or to differences in patient characteristics. In extensively pretreated patients the CD4 response may be lower compared with naive patients, making the detection of a significant difference between groups less likely. Clinicians currently caring for triple-class (NRTI + NNRTI + PI) drug-experienced patients with HIV may therefore expect similar HIV-RNA and CD4 cell count response for both coinfected and non-coinfected patients assigned raltegravir. This information is consistent with recently published data from clinical9 and observational7,8 studies, and adds confidence to using raltegravir and other new inhibitors in patients with HBV or HCV coinfection.
Several studies have shown that patients with hepatitis virus coinfection have a higher risk of liver toxicity when taking highly active antiretroviral therapy (‘HAART’) regimens based on different anti-HIV inhibitors, including raltegravir.7–9 Our data indicate that coinfected patients usually enter new raltegravir-based regimens with higher mean plasma levels of hepatic enzymes and that they have a significantly higher risk of developing new grade 3–4 liver-related adverse events during follow-up. Although the above findings indicate a higher risk of hepatotoxicity with currently prescribed raltegravir-based regimens in the presence of HBV or HCV coinfection, it is important to note that this increased occurrence of liver-related adverse events was not associated in our study with a higher risk of discontinuing treatment, because the two groups had similar probabilities of treatment interruption, with no evidence of a higher risk of discontinuation due to adverse events among coinfected patients. These findings are reassuring and indicate that the possibly more common occurrence of liver-related adverse events in coinfected patients can be managed without discontinuing the regimen.
Our findings have been limited by the small sample size and the relatively short duration of follow-up: we could not provide comparative data on HIV disease progression or of mortality between groups, because very few AIDS-defining events occurred during follow-up and only five patients died (four coinfected patients and one non-coinfected patient). Overall, most of these deaths were not attributable to HIV disease, reinforcing the observation of large cohort studies that have shown that non-HIV-related events currently represent a major cause of death among persons with HIV and advanced disease.12 In our observational study, two coinfected patients died from worsening of clinically advanced liver disease already present at baseline, reinforcing the assumption that in a context of prolonged survival among people with HIV, liver disease in coinfected patients may become a relevant cause of death.13,14
In evaluating the results, the definition of HBV and HCV infection that we used should be considered, because it might differ from that used in other studies and may have influenced the results. Even though most of the HCV-positive patients in our study had detectable HCV-RNA, indicating active disease, we also included a minority of patients with missing information on HCV-RNA and a few patients with undetectable HCV-RNA who might have better outcomes compared with patients with evidence of active HCV replication. Conversely, we did not exclude patients with double coinfection (HBV/HCV), who may have worse outcomes compared with patients with HBV or HCV coinfection only. Finally, we were not able to evaluate HBV-DNA and resistance data in patients with HBV coinfection and therefore could not evaluate possible HBV reactivation as a result of developing resistance.
In conclusion, our study showed that viroimmunological response to current regimens based on raltegravir and other recently introduced anti-HIV inhibitors (as salvage therapy) is not negatively affected by coinfection with HBV or HCV. Liver toxicity, either pre-existing or new, is more common in coinfected patients, but with no increased risk of treatment interruption. Further research and data from large cohort studies will have to explore the extent to which coinfection with hepatitis virus is responsible for an increased mortality in patients with HIV, identify the causes and determinants of liver-related mortality, and define strategies to prevent its occurrence.
Members of the ISS-NIA Study Group
G. Angarano, N. Ladisa, A. Volpe, Clinic of Infectious Diseases, University of Bari; V. Vullo, G. D'Ettorre, G. Ceccarelli, Department of Infectious Diseases, La Sapienza University, Rome; M. Andreoni, L. Sarmati, D. Delle Rose, Infectious Diseases Unit, Tor Vergata University, Rome; V. Tozzi, P. Narciso, N. Petrosillo, V. Pucillo, R. Bellagamba, R. Libertone, S. Cicalini, C. Tommasi, INMI ‘Lazzaro Spallanzani’, Rome; L. Sighinolfi, D. Segala, Infectious Diseases Unit, Arcispedale S. Anna, Ferrara; O. Armignacco, R. Preziosi, ASL Viterbo, Belcolle Hospital, Viterbo; C. Ferrari, A. Degli Antoni, A. Cavalli, Ospedale Maggiore, Parma; G. Parruti, F. Sozio, L. Cosentino, Infectious Diseases Unit, Presidio Ospedaliero Santo Spirito, Pescara; A. Vivarelli, Infectious Diseases Unit, Ospedale Civile, Pistoia; P. E. Manconi, F. Ortu, University Policlinic, Cagliari; P. Viale, G. Verucchi, S. Tedeschi, R. Manfredi, Infectious Diseases Section, University and Policlinico S. Orsola, Bologna; M. S. Mura, M. Mannazzu, G. Cattari, Clinic of Infectious Diseases, University of Sassari; M. Tavio, R. Del Gobbo, A. Mataloni Paggi, Infectious Diseases Unit, Ospedali Riuniti, Torrette, Ancona; A. Giacometti, O. Cirioni, E. Marchionni, C. Silvestri, L. Brescini, S. Sebastianelli, Clinic of Infectious Diseases, Ospedali Riuniti, Marche Polytechnic University; F. Baldelli, D. Francisci, A. Mercuri, S. Bastianelli, Ospedale S. Maria della Misericordia and University of Perugia; G. Guaraldi, G. Nardini, Department of Internal Medicine, Clinic of Infectious Diseases, University of Modena and Reggio Emilia, Modena; R. Bucciardini, M. Floridia, L. E. Weimer, V. Fragola, M. Massella, S. Baroncelli, C. M. Galluzzo, M. F. Pirillo, M. G. Mancini, R. Amici, A. Cara, R. Bona, P. Leone, P. Filati, M. Franco, S. Donnini, Department of Therapeutic Research and Medicines Evaluation, Istituto Superiore di Sanità, Rome.
Funding
This work was supported by a grant from the National Program on Research on AIDS 2009–2010 (project grant ID: I85J08000040005, to M. F.). No funding was received for this work from any of the following organizations: National Institutes of Health (NIH); Wellcome Trust; and the Howard Hughes Medical Institute (HHMI).
Transparency declarations
None to declare.
Acknowledgements
We thank Ms Stefania Donnini and Ms Alessandra Mattei for secretarial help.
References
- 1.Rockstroh JK, Mocroft A, Soriano V, et al. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis. 2005;192:992–1002. doi: 10.1086/432762. [DOI] [PubMed] [Google Scholar]
- 2.Yacisin K, Maida I, Ríos MJ, et al. Hepatitis C virus coinfection does not affect CD4 restoration in HIV-infected patients after initiation of antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:935–40. doi: 10.1089/aid.2008.0069. [DOI] [PubMed] [Google Scholar]
- 3.Fuping G, Wei L, Yang H, et al. Impact of hepatitis C virus coinfection on HAART in HIV-infected individuals: multicentric observation cohort. J Acquir Immune Defic Syndr. 2010;54:137–42. doi: 10.1097/QAI.0b013e3181cc5964. [DOI] [PubMed] [Google Scholar]
- 4.Carmo RA, Guimarães MD, Moura AS, et al. The influence of HCV coinfection on clinical, immunological and virological responses to HAART in HIV-patients. Braz J Infect Dis. 2008;12:173–9. doi: 10.1590/s1413-86702008000300003. [DOI] [PubMed] [Google Scholar]
- 5.Weis N, Lindhardt BO, Kronborg G, et al. Impact of hepatitis C virus coinfection on response to highly active antiretroviral therapy and outcome in HIV-infected individuals: a nationwide cohort study. Clin Infect Dis. 2006;42:1481–7. doi: 10.1086/503569. [DOI] [PubMed] [Google Scholar]
- 6.D'Arminio Monforte A, Cozzi-Lepri A, Castagna A, et al. Risk of developing specific AIDS-defining illnesses in patients coinfected with HIV and hepatitis C virus with or without liver cirrhosis. Clin Infect Dis. 2009;49:612–22. doi: 10.1086/603557. [DOI] [PubMed] [Google Scholar]
- 7.Vispo E, Mena A, Maida I, et al. Hepatic safety profile of raltegravir in HIV-infected patients with chronic hepatitis C. J Antimicrob Chemother. 2010;65:543–7. doi: 10.1093/jac/dkp446. [DOI] [PubMed] [Google Scholar]
- 8.Macías J, Neukam K, Portilla J, et al. Liver tolerance of raltegravir-containing antiretroviral therapy in HIV-infected patients with chronic hepatitis C. J Antimicrob Chemother. 2011;66:1346–50. doi: 10.1093/jac/dkr083. [DOI] [PubMed] [Google Scholar]
- 9.Rockstroh J, Teppler H, Zhao J, et al. Safety and efficacy of raltegravir in patients with HIV-1 and hepatitis B and/or C virus coinfection. HIV Med. 2012;13:127–31. doi: 10.1111/j.1468-1293.2011.00933.x. [DOI] [PubMed] [Google Scholar]
- 10.AIDS Clinical Trials Group. Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events. http://www.hptn.org/web%20documents/hptn046/ssp/appendices/appendixe-toxicitytables_daids_ae_gradingtable_finaldec2004.pdf. (27 August 2012, date last accessed) [Google Scholar]
- 11.Pineda JA, Santos J, Rivero A, et al. Liver toxicity of antiretroviral combinations including atazanavir/ritonavir in patients co-infected with HIV and hepatitis viruses: impact of pre-existing liver fibrosis. J Antimicrob Chemother. 2008;61:925–32. doi: 10.1093/jac/dkn045. [DOI] [PubMed] [Google Scholar]
- 12.Smith CJ, Sabin CA, Lundgren JD, et al. Data Collection on Adverse Events of Anti-HIV drugs (D:A:D) Study Group. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 2010;24:1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann CJ, Seaberg EC, Young S, et al. Hepatitis B and long-term HIV outcomes in coinfected HAART recipients. AIDS. 2009;23:1881–9. doi: 10.1097/QAD.0b013e32832e463a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas S. Causes of death in the HAART era. Curr Opin Infect Dis. 2012;25:36–41. doi: 10.1097/QCO.0b013e32834ef5c4. [DOI] [PubMed] [Google Scholar]