Abstract
Background
This study was conducted to compare oral contraceptive (OC) pharmacokinetics (PK) in normal weight (BMI 19.0-24.9) and obese (BMI 30.0-39.9) women.
Study Design
During the third week of the third cycle of OC use, we admitted 15 normal weight and 15 obese women for collection of 12 venous specimens over 24 h. Using RIA techniques, we measured levels of ethinyl estradiol (EE) and levonorgestrel (LNG). During the same cycle, women underwent twice-weekly sonography to assess ovarian follicular development and blood draws to measure endogenous estradiol (E2) and progesterone levels.
Results
Obese women had a lower area under the curve (AUC; 1077.2 pg*h/mL vs 1413.7 pg*h/mL) and lower maximum values (85.7 pg/mL vs 129.5 pg/mL) for EE than normal weight women (p = 0.04 and 0.01, respectively); EE trough levels were similar between BMI groups. The similar, but smaller, differences in their LNG levels for AUC and maximum values (Cmax) were not statistically significant. While peak values differed somewhat, the LNG trough levels were similar for obese and normal weight women (2.6 ng/mL and 2.5 ng/mL, respectively). Women with greater EE AUC had smaller follicular diameters (p = 0.05) and lower E2 levels (p = 0.04). While follicular diameters tended to be larger among obese women, these differences were not statistically significant.
Conclusion
OC hormone peak levels are lower among obese women compared to normal weight women, but their trough levels are similar. In this small study, the observed PK differences did not translate into more ovarian follicular activity among obese OC users.
1. Introduction
Several studies have reported higher oral contraceptive (OC) failure rates in overweight and obese women than in normal weight women, while other studies have not found this association [1-3]. These previous studies largely relied on self-reported patterns of OC use, pregnancy, and body weight, sometimes long after these events took place. Thus, the studies published so far are not able to distinguish method failure from user error, and also may be limited by misclassification of body weight.
A plausible mechanism for increased OC failure among obese women would be more frequent ovulation during OC use due to incomplete ovarian suppression. Among obese women, incomplete ovarian suppression leading to ovulation could be a consequence if they achieve lower serum levels of the OC hormones compared to normal weight women. The effective availability of drugs may differ by body weight for many reasons including variations in absorption, distribution, binding, metabolism, or clearance [4, 5]. Steroids are lipophilic and will have a larger volume of distribution in obese compared to normal weight individuals. This finding has been confirmed in studies of contraceptive injections and implants that find lower serum levels among obese women [6-8]. Differences in circulating levels of contraceptive hormones may not, however, predict failure rates. In studies of contraceptive implants and injections, the lower serum levels found in the obese women were not associated with contraceptive failure. To permit ovulation and OC failure, pharmacokinetic (PK) differences must be associated with less ovarian suppression.
Most PK studies of OC hormones have been small and limited to normal weight women; thus, we found little data regarding PK differences between normal weight and obese OC users. A recent study compared 10 normal weight and 10 obese OC users, and found that the obese women had more hypothalamic-pituitary-ovarian axis activity during OC use [9]. The purpose of the present study was to assess oral contraceptive PK among obese and normal weight women and to explore whether PK results are associated with differential ovarian suppression. Because of the sustained popularity of the OC and the increasing prevalence of obesity in the U.S., clarifying any differential effectiveness by body weight has enormous clinical relevance.
2. Materials and methods
The 30 women who participated in this PK study were a volunteer sub-group recruited from 226 women enrolled in a randomized, double-blind clinical trial that assessed ovarian function among normal weight and obese women using one of two OC formulations. The Columbia University Institutional Review Board approved these studies and all participants gave informed consent.
2.1. Study participants
Eligible women were aged 18-35 years with a recent history of regular, spontaneous menstrual cycles. We excluded women with any medical contraindications to the use of combined hormonal contraception based on the WHO Medical Eligibility Criteria [10], only enrolling women in WHO Category 1. We also specifically excluded women recently pregnant or breastfeeding, those with preexisting renal disease, diabetes, those with thyroid, pituitary, adrenal or ovarian disorders, and those smoking ≥10 cigarettes per day. Women using medications known to affect the cytochrome P450 system including antiseizure medicines, rifampin, griseofulvin or fluconazole, were ineligible as were women regularly consuming grapefruit juice, which affects the CYP450 system. All participants had normal-appearing ovaries on a baseline sonogram using a TITAN (SonoSite, Inc., Bothell, WA) with a 7.5 MHz transvaginal probe, and were either normal weight (body mass index, kg/m2, BMI 19.0-24.9) or obese (BMI 30.0-39.9) based on standardized height and body weight measurement on the day of enrollment. We excluded overweight women to create a clear differentiation between BMI groups. We measured standing height (cm) using a fixed stadiometer. We measured body weight (kg), and composition (total body water (kg), percent body fat, fat mass (kg), and fat free mass (kg)) by bioelectrical impedance analysis using the BC-418 (Tanita Corp., Tokyo, Japan), an 8-contact electrode single frequency 50 kHz body composition analyzer. We used the same machine for all body weight and composition measurements.
2. Treatment and measurements
For the PK study, the study drug contained 30 mcg ethinyl estradiol (EE) and 150 mcg levonorgestrel (LNG) packaged with 21 active and 7 placebo tablets (Portia®, Barr Laboratories, Inc.). Participants completed two to three OC cycles prior to a study cycle. During the study cycle, participants underwent sonography twice weekly approximately 3-5 days apart to measure ovarian follicles using a SonoSite TITAN (Bothell, WA) with a 7.5 MHz transvaginal probe; we measured follicles in two perpendicular diameters and recorded the dimensions of all follicles with a mean diameter of 8 mm or greater. Participants underwent eight venipunctures on the same days as the sonograms to obtain samples to measure endogenous estradiol (E2) and progesterone (P). We also measured LNG in the blood specimens collected from OC cycle Day 2-21 as a compliance check. These venipunctures were not linked to the time the participants took their pills. We considered the two normal weight participants with LNG values consistently below 0.16 ng/mL (the sensitivity limit of the assay) to be non-compliant with study drug (OC non-user). Therefore, we excluded these two participants from all analyses because they would not have steady-state levels for the PK study.
On one day between Days 15-21 of the study cycle, the PK study participants were admitted for 24 h to the Irving Institute of Clinical and Translational Research at Columbia University. Using an indwelling intravenous catheter, 8.5-mL samples were collected at 0, ½, 1, 1½, 2, 3, 4, 6, 8, 12, 16 and 24 h (noted below as t0, t1/2, t1,…, t24). We measured all of these specimens for EE and LNG, and measured the t0 specimens for sex hormone binding globulin (SHBG). Oral administration of the OC tablet was scheduled to occur immediately after the t0 blood draw. Specimens were allowed to clot for at least 60 min at room temperature and then separated by centrifugation at 2500 rpm. The serum was transferred to a clean tube and stored in aliquots at −80°C until analysis.
2.3. Laboratory methods
Assays were performed in the Reproductive Endocrine Research Laboratory at University of Southern California under the direction of one of the authors (FZS). LNG and EE levels in serum samples were quantified by specific and sensitive radioimmunoassays (RIAs) as described in detail elsewhere [11, 12]. Briefly, prior to RIA, each analyte is extracted with ethyl acetate:hexane (3:2) to remove interfering steroids. Procedural losses are estimated by adding approximately 600 d.p.m. of titrated internal standard (3H-LNG or 3H-EE) to the serum prior to the extraction step. The losses range from 15-20% and the values are used to correct the RIA results. A highly specific antiserum is used in conjunction with an iodinated radioligand in each RIA. Separation of free from antiserum-bound LNG or EE is achieved by use of a second antibody. Intra-assay and inter-assay coefficients of variation are 4.4% and 8.9% for LNG RIAs and 6.9% and 11.0% for EE RIAs, respectively. SHBG was quantified by a solid-phase, two-site chemoluminescent immunoassay using the Immulite Analyzer (Siemens Medical Solution Diagnostics, Los Angeles, CA)
The Core Laboratory of the Irving Institute of Clinical and Translational Research at Columbia University Medical Center performed the E2 and P assays using the RIA kits, “Double Antibody Estradiol” and “Coat – A – Count Progesterone”, respectively (Siemens Medical Solution Diagnostics; Malvern, PA).
3.4. Statistical analysis
For the PK analyses, serum concentration values of LNG and EE for each participant were fitted using a noncompartmental approach. We recorded individual values of maximum concentration (Cmax), the time of maximum concentration (Tmax), and the trough concentration at t24 (Cmin). We calculated the area under the curve (AUC) from t0 to t24 using linear trapezoidal approximation. We calculated elimination half-life for each hormone using values from t12, t16, and t24. We also calculated corrected values of AUC, Cmax,, Tmax, and Cmin to correct for the unknown time that the participant took the previous day’s tablet; the corrected values adjust the parameters to a standard 24 h interval since the previous OC was taken. The correction used the elimination half-life of the hormone (LNG or EE) using mixed models, to calculate the values at t½, t1, t1½,…, t16 and t24 eliminating the contribution of the prior OC use. These values were then used repeatedly – together with the estimated elimination half-life after each OC – to calculate steady-state PK values from which the corrected parameters were estimated. The differences between the normal weight and obese women were similar using the original observations and the corrected values; only results using the original observations are presented here.
Data analyses focused on comparing AUC (t0-t24), Cmax, the terminal elimination half-life, and Cmin for EE and LNG in normal weight versus obese participants. A priori power calculations indicated that a sample size of 15 participants in each group was needed in order to have 80% power to detect a 20% difference in AUC. We used the Wilcoxon rank sum test to assess the relationships between obesity and each of the PK estimates. We also used linear regression to assess the relationships between obesity and each of the PK estimates, using both original and log-transformed values, as appropriate, while adjusting for covariates.
We used rank correlation and linear regression to evaluate the relationships between AUC, Cmax, Cmin, and maximum follicular diameter and maximum E2 during the study cycle. In addition to the two normal weight participants excluded from all analyses because they had LNG values consistently below the level of detection, we excluded two additional participants for this analysis who had several LNG values below the level of detection, indicating inconsistent OC use throughout the study cycle, and one participant who had a persistent 38 mm ovarian cyst (with concomitant undetectable E2 levels) during the study cycle. We excluded these three additional participants because it would be inappropriate to relate the OC AUC, Cmax, and Cmin observed on a single day to their follicular activity throughout that cycle. All statistical significance levels (p-values) quoted are two-sided.
3. Results
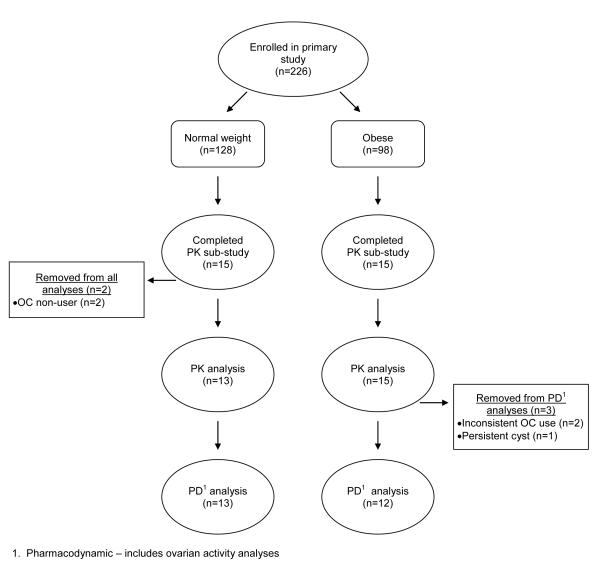
Thirty women participated in the 24-h PK study. Fig. 1 shows the flow of participants through each stage of the study. We excluded two participants because compliance checks showed that they were not taking the OC except for the PK day itself (OC non-user); we excluded these participants from all analyses because they would not have steady-state levels. Table 1 shows the baseline characteristics of the remaining 28 participants. The participants from the two BMI groups differ in body weight and BMI by design. The obese participants were more likely to be parous. None of the participants who smoked cigarettes reported smoking more than three cigarettes per day.
Fig 1.
Flowchart of participants in pharmocokinetic (PK) study
Table 1.
Baseline characteristics of study participants
| Variable | Normal weight (n = 13) |
Obese (n = 15) |
p-value |
|---|---|---|---|
|
| |||
| Height (cm) | 160 (159, 165) | 167 (162, 172) | 0.11 |
|
| |||
| Body weight (kg) | 59.4 (54.2, 62.2) | 94.8 (85.8, 99.1) | < 0.01 |
|
| |||
| BMI (kg/m2) | 22.4 (21.1, 23.7) | 33.5 (31.3, 35.7) | < 0.01 |
|
| |||
| Age | 24 (22,28) | 26 (24,30) | 0.34 |
|
| |||
| Race/ethnicity | |||
| Hispanic | 2 (15.4) | 6 (40.0) | |
| Non-Hispanic black | 1 (7.7) | 4 (26.7) | 0.20 |
| Non-Hispanic white | 7 (53.9) | 4 (26.7) | |
| Non-Hispanic Asian | 3 (23.1) | 1 (6.7) | |
|
| |||
| Education | |||
| Less than bachelor’s degree | 5 (38.5) | 9 (60.0) | 0.26 |
| Bachelor’s degree or more | 8 (61.5) | 6 (40.0) | |
|
| |||
| Ever been pregnant | 4 (30.1) | 7 (46.7) | 0.39 |
|
| |||
| Ever given birth | 0 | 5 (33.3) | 0.04 |
|
| |||
| Smokes cigarettes | 0 | 3 (20.0) | 0.23 |
Values are shown as median (Q1, Q3) or n (%).
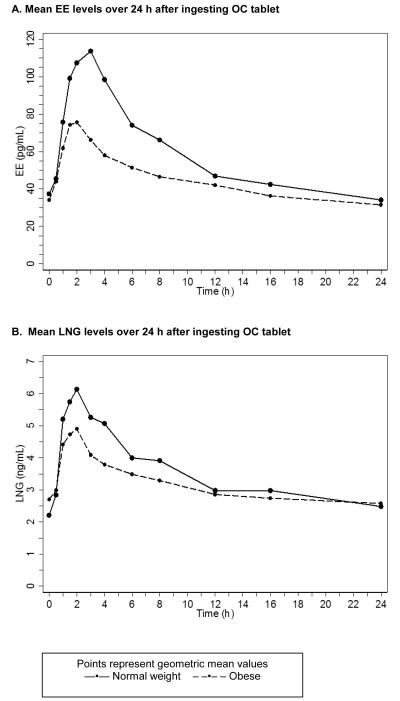
Fig. 2A and 2B show the geometric mean serum concentrations of EE and LNG of the obese and the normal weight participants. For both hormones, the curves are lower for the obese participants. Table 2 presents the AUC, Cmax, Tmax, elimination half-life, and Cmin estimates for each hormone, stratified by BMI group. For AUC, Cmax, and Cmin, the maximum values are about four times greater than the minimum values. The individual variability in the elimination half-life varies even more. To summarize the marked individual variability, Table 2 shows the interquartile ranges. For EE, the obese participants have a significantly lower AUC and Cmax and an earlier Tmax than the normal weight participants. For LNG, the AUC and Cmax differences between the groups are in the same direction, but smaller. The elimination half-life for LNG was markedly longer among the obese participants. While peak values of LNG, and especially EE, were higher among normal weight participants, the trough values (Cmin) were similar. Substituting body weight, percent body fat, or fat mass for BMI did not change the results for AUC and Cmax. We found no relationship between any of the PK estimates and ethnicity, parity, or smoking status. The normal weight and obese participants had similar values for SHBG (geometric mean 70.1 nmol/L and 66.6 nmol/L, respectively, p = 0.75).
Fig. 2.
Serum concentrations of EE and LNG in 13 normal weight and 15 obese study participants
Table 2.
OC pharmacokinetic parameter estimates in normal weight versus obese women
| Variable | Normal weight (n = 13) |
Obese (n = 15) |
p-value1 |
|---|---|---|---|
| EE | |||
| AUC2 (pg*h/mL) |
1413.7 (1042.5, 1917.1) | 1077.2 (749.9, 1547.6) | 0.04 |
| Cmax (pg/mL) |
129.5 (94.0, 178.4) | 85.7 (57.7, 127.3) | < 0.01 |
| Tmax (h) |
3.0 (2.0, 3.0) | 1.5 (1.0, 2.0) | 0.04 |
| Elimination half-life3 (h) |
28.0 (14.7, 53.3) | 30.4 (20.5, 45.1) | 0.19 |
| Cmin (pg/mL at t24) |
34.2 (23.6, 49.6) | 31.5 (21.4, 46.5) | 0.37 |
| LNG | |||
| AUC2 (ng*h/mL) |
85.8 (61.9, 119.0) | 79.9 (45.0, 142.0) | 0.66 |
| Cmax (ng/mL) |
7.0 (5.2, 9.4) | 5.6 (3.9, 8.1) | 0.19 |
| Tmax (h) |
2.0 (1.5, 2.0) | 1.5 (1.0, 2.0) | 0.18 |
| Elimination half-life3 (h) |
37.6 (18.9, 74.7) | 73.6 (39.5, 137.1) | < 0.01 |
| Cmin (ng/mL at t24) |
2.5 (1.5, 4.0) | 2.6 (1.5, 4.5) | 0.56 |
Tmax shown as median (Q1, Q3); other parameter estimates shown as geometric mean ± SD.
Wilcoxon rank-sum test.
AUC calculated from t0 to t24.
Elimination half-life calculated using values from t12, t16, and t24.
As shown in Table 3, there was some evidence of more follicular activity in the obese participants but the differences were not statistically significant. Table 3 indicates the proportion of participants in each BMI group with ovarian follicle diameters (FD) that were at least 8, 10, 13, or 18 mm in size. These proportions were calculated using the largest mean FD from any of the eight sonograms that each participant underwent. Because FD seen during the first week of the OC cycle may indicate ovarian activity that occurred during the preceding placebo week, we repeated this analysis excluding the sonograms obtained during the first week and the results were similar (data not shown). Finally, we repeated this analysis also excluding sonograms done during the final placebo week, and again the results were similar.
Table 3.
Maximum follicular diameter during one cycle of OC use in normal weight and obese women
| Maximum follicular diameter |
Normal weight (n = 13) | Obese (n = 12) | p-value |
|---|---|---|---|
| ≥ 8 mm | 5/13 = 0.38 | 8/12 = 0.67 | 0.16 |
| ≥ 10 mm | 4/13 = 0.31 | 5/12 = 0.42 | 0.69 |
| ≥ 13 mm | 3/13 = 0.23 | 4/12 = 0.33 | 0.67 |
| ≥ 18 mm | 2/13 = 0.15 | 3/12 = 0.25 | 0.64 |
Concentration of E2 was measured in serum from blood draws taken at all eight follow-up visits. Overall, maximum E2 levels were low in these participants (median = 16.0 pg/mL, Q1= 5.4 pg/mL, Q3 = 40.5 pg/mL), consistent with the expected ovarian suppression in OC users. Maximum serum concentration of E2 was higher in obese participants than in normal weight participants, on average (median = 18.5 pg/mL vs. median = 6.5 pg/mL, p = 0.08). We repeated this analysis excluding blood specimens obtained during the first week, and again excluding blood specimens obtained during the first week and the final placebo week, and the results were similar.
We hypothesized that ovarian activity as indicated by the maximum FD and E2 levels should be inversely associated with serum levels of EE on an individual level because EE suppresses follicle stimulating hormone (FSH). We used rank correlation to assess the strength of the association between AUC, Cmax, and Cmin and maximum FD as well as the maximum E2 during the study cycle (Table 4). All PK estimates of EE were associated with a smaller FD and a lower E2, although some of these associations did not achieve statistical significance. The relationships were similar when assessing the maximum FD from all study days or when limited to Days 7-28 or Days 7-21 only (data not shown). Because individual levels of LNG and EE are correlated (r = 0.48; p < 0.01), the LNG PK values were also related to FD and to E2; however, these associations were weaker than those described for EE and were not statistically significant (data not shown).
Table 4.
EE PK estimates and measures of ovarian activity during one cycle of OC use
| Maximum follicular diameter (mm) (n = 25) |
Maximum E2 (pg/mL) (n = 25) |
|||
|---|---|---|---|---|
| Variable | Correlation coefficient1 |
p-value1 | Correlation coefficient1 |
p-value1 |
| AUC2 EE (pg*h/mL) |
−0.40 | 0.05 | −0.41 | 0.04 |
| Cmax EE (pg/mL) |
−0.22 | 0.29 | −0.35 | 0.09 |
| Cmin EE (pg/mL at t24) |
−0.44 | 0.03 | −0.37 | 0.07 |
Spearman rank correlation.
AUC calculated from t0 to t24.
In this study, any serum P levels of 3 ng/mL or greater were presumed a priori to indicate ovulation. Only one normal weight participant, who took the assigned pills consistently, according to the LNG compliance checks, ovulated based on a serum P = 10.1 ng/mL at cycle Day 10 and 12.4 ng/mL at Day 13. In the PK analyses, this participant had EE AUC and Cmax levels in the middle and had EE elimination half-life and Cmin values in the lowest quartile; her LNG levels were all in the lowest quartile, including the second-lowest LNG Cmin value of all participants. We also identified two obese participants who missed multiple (but not all) OCs based on the LNG compliance checks; one of them ovulated. Mean endometrial thickness during the study cycle was similar for obese and normal weight participants (6.9 mm vs. 6.3 mm, respectively, p =0.68).
4. Discussion
This PK study shows that the peak circulating levels of OC hormones are lower among obese (BMI 30-39.9) than among normal weight (BMI 19.0-24.9) women using a monophasic 28-day pill regimen containing 30 mcg EE and 150 mcg LNG. The differences shown in Fig. 2A and 2B are greater for EE than for LNG. While the peak values are clearly higher in normal weight participants than obese participants, all levels after 12 h, in particular the trough levels (i.e., those at t24), do not differ by BMI status. This small study had inadequate statistical power to compare the PK values by ethnicity, parity or other factors of interest.
Which PK parameter is most important for drug effectiveness? The answer varies by drug; peak levels, total levels, and trough or minimum levels are most important for different drugs. Among contraceptive hormones, the importance of the minimum LNG value was seen in a PK study of women using an LNG contraceptive implant where heavier women had somewhat lower steady-state levels of LNG, but regardless of body weight, women with LNG levels above 0.3 ng/mL did not become pregnant [8]. A seven-cycle study of a monthly injectable contraceptive containing medroxyprogesterone acetate and estradiol cypionate, found a significant inverse relation between body weight and peak MPA levels, but Day 0 and Day 8 trough levels were similar regardless of body weight; the authors concluded that the peak PK differences had no effect on contraceptive efficacy because the trough concentrations were sufficient to suppress ovulation [7]. Taken together with our data, these results suggest that, despite peak PK differences, the contraceptive efficacy of OCs in obese women will not be impaired as the observed minimum LNG concentrations in obese women are similar to those in normal weight women, and remain well above the minimum value needed to suppress ovulation.
All hormone values shown here reflect total circulating levels, and do not account for differences in binding. As much as half of LNG is bound to SHBG, therefore obesity-related decreases in SHBG could mitigate the observed differences in LNG. Neither our results nor those of Edelman et al. [9] in a recent similar study found any obesity-related differences in SHBG among women using OCs, so the salience of this issue is uncertain. Another unanswered question is whether any of the PK values might be related to adverse effects during OC use. Consistent with other authors, we found at least a four-fold difference between the maximum and minimum values of EE and LNG for both AUC and Cmax among our participants [13]. To our knowledge, no one has assessed whether reported side effects during OC use are associated with individual PK results. For other drugs, such as antibiotics, we routinely measure peak and trough levels to maintain a therapeutic level while avoiding toxicity, and we then titrate the individual dose based on the PK results. Our approach to oral contraceptives, which are given in a single daily tablet with a single dose for all users, is simple, but may not give optimal results for individual users.
The observed PK differences in OC users are less important than direct indicators of pregnancy risk, such as ovulation during OC use. This PK study is much too small to assess differences in ovulation by obesity, but we did compare follicular development, which is a necessary prelude to ovulation. We found minimal, non-significant differences in follicular size between normal weight and obese women. A larger study is nonetheless needed to compare follicular development and ovulation rates in normal weight versus obese OC users [14].
Acknowledgements
This study was a sub-study within a clinical trial supported by NIH Grant: R01 HD04578. Duramed Pharmaceuticals donated oral contraceptives and supported the pharmacokinetic assay analyses. Progesterone and estradiol analyses were supported by NIH CTSA Grant: 1 UL1 RR024156-03.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dinger JC, Cronin M, Mohner S, Schellschmidt I, Minh TD, Westhoff C. Oral contraceptive effectiveness according to body mass index, weight, age, and other factors. Am J Obstet Gynecol. 2009;201:e1–9. doi: 10.1016/j.ajog.2009.03.017. [DOI] [PubMed] [Google Scholar]
- [2].Trussell J, Schwarz EB, Guthrie K. Obesity and oral contraceptive pill failure. Contraception. 2009;79:334–8. doi: 10.1016/j.contraception.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Weshtoff C, Reape K, Shu H. Impact of body weight on observed pregnancy rates with a low-dose estrogen, 91-day extended regimen oral contraceptive. Contraception. 2009;80:196. [Google Scholar]
- [4].Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39:215–31. doi: 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- [5].Cheymol G. Drug pharmacokinetics in the obese. Fund & Clin Pharmacol. 1988;2:239–56. doi: 10.1111/j.1472-8206.1988.tb00635.x. [DOI] [PubMed] [Google Scholar]
- [6].Huber J. Pharmacokinetics of Implanon: an integrated analysis. Contraception. 1998;58:85S–90S. doi: 10.1016/s0010-7824(98)00120-6. [DOI] [PubMed] [Google Scholar]
- [7].Rahimy MH, Cromie MA, Hopkins NK, Tong DM. Lunelle(TM) monthly contraceptive injection (medroxyprogesterone acetate and estradiol cypionate injectable suspension): effects of body weight and injection sites on pharmacokinetics. Contraception. 1999;60:201–8. doi: 10.1016/s0010-7824(99)00085-2. [DOI] [PubMed] [Google Scholar]
- [8].Sivin I, Wan L, Ranta S, et al. Levonorgestrel concentrations during 7 years of continuous use of Jadelle contraceptive implants. Contraception. 2001;64:43–9. doi: 10.1016/s0010-7824(01)00226-8. [DOI] [PubMed] [Google Scholar]
- [9].Edelman AB, Carlson NE, Cherala G, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009;80:119–27. doi: 10.1016/j.contraception.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].World Health Organization Department of Reproductive Health and Research . Medical eligibility criteria for contraceptive use. Third edition 2004. [Google Scholar]
- [11].Price TM, Dupuis RE, Carr BR, Stanczyk FZ, Lobo RA, Droegemueller W. Single- and multiple-dose pharmacokinetics of a low-dose oral contraceptive in women with chronic renal failure undergoing peritoneal dialysis. Am J Obstet Gynecol. 1993;168:1400–6. doi: 10.1016/s0002-9378(11)90772-8. [DOI] [PubMed] [Google Scholar]
- [12].Stanczyk FZ, Hiroi M, Goebelsmann U, Brenner PF, Lumkin ME, Mishell DR., Jr. Radioimmunoassay of serum d-norgestrel in women following oral and intravaginal administration. Contraception. 1975;12:279–98. doi: 10.1016/0010-7824(75)90088-8. [DOI] [PubMed] [Google Scholar]
- [13].Goldzieher JW, Stanczyk FZ. Oral contraceptives and individual variability of circulating levels of ethinyl estradiol and progestins. Contraception. 2008;78:4–9. doi: 10.1016/j.contraception.2008.02.020. [DOI] [PubMed] [Google Scholar]
- [14].Westhoff C, Torgal A, Mayeda ER, Lerner J, Paik M. Ovarian suppression during oral contraceptive use in normal-weight and obese women. Contraception. 2009;80:210. doi: 10.1097/AOG.0b013e3181e79440. [DOI] [PubMed] [Google Scholar]