Abstract
Multiple assays are available to measure P450 activity in insects, including mosquitoes; however, each of these assays has drawbacks in terms of the number of mosquitoes required, specificity, sensitivity, cost, and/or time required to prepare active enzyme homogenates. In this study, a commercially available luminescent assay, P450-Glo, was modified and evaluated to measure P450 activity from the gut of a single larva after removal of the gut contents. We also compared this assay to an earlier developed fluorescent assay. After optimization of assay conditions, the P450-Glo assay held considerable promise to be used as an effective, inexpensive, high-throughput, and sensitive screening assay to measure P450 activities in single mosquitoes. Furthermore, we tested the utility of the single gut assay using the pyrethroid resistant Marin strain of Culex pipiens pipiens form molestus and the pyrethroid sensitive CQ-1 strain of Cx. pipiens quinqefasciatus. We observed on average 1.8-fold higher levels of P450 activity in the resistant mosquitoes in comparison to the sensitive mosquitoes. Additionally, consistent with our previous findings, distribution plots of P450 activity showed 33% of individual Marin mosquitoes had higher P450 activities than the highest activity displayed by a CQ-1 mosquito. The assay platform is highly flexible in terms of choice of tissue, method of preparation, isozyme specificity, and sample quantity and thus could easily be adapted to be used for other arthropod species.
Keywords: mosquito, Culex pipiens complex, cytochrome P450, luminescent assay, pyrethroid resistance
Enzymes belonging to the cytochrome P450 monooxy-genase supergene family have diverse functions in the synthesis of endogenous molecules such as steroids and in the defense against dissimilar xenobiotics of varied origins both in insects and mammals (Feyereisen 2006). The wide range of functions of these enzymes is paralleled by their genetic and structural diversity. In insects, cytochrome P450s are distributed in many tissues. From an applied perspective, these enzymes are important because of their potential role in mediating resistance against major classes of insecticides, although their activity is known to respond to stimuli other than insecticides or xenobiotics. The functional diversity brings forth significant difficulties in determining the levels of cytochrome P450s or associating these enzyme activities with insecticide resistance. Simple, rapid, and specific kinetic assays such as those used to measure carboxylesterase and glutathione-S-transferase activities that were developed decades ago are not available for measuring cytochrome P450 activity. However, the ability to quantify these enzymes from individual insects is important because single insect assays can identify and show the distribution of resistant individuals in a population that is not known to be resistant.
The involvement of cytochrome P450s in insecticide resistance was suggested by Kulkarni et al.(1974), who showed that the UV spectra in the range of 380- to 430-nm wavelength differed between resistant and susceptible house flies. However, accurate quantification of enzyme activity rather than quantification of the concentration of P450s in a sample yields useful information in regard to diagnosing insecticide resistance. For example, a recent modified heme assay that can be used in single mosquitoes gives a crude estimation of P450 activity but suffers from poor selectivity and sensitivity (Brogdon et al. 1997, Coleman and Hemingway 2007). For quantification of cytochrome P450 activity in insects, many assays that were originally used for mammalian homologs have been adapted to measure insect enzymes (Baars et al. 1977, Wheelock and Scott 1992). Multiple derivatives of coumarin, resorufin, and fluorescein in addition to benzopyrene have been used as substrates to measure cytochrome P450 activity in mammals and insects (Wheelock and Scott 1992, Donato et al. 2004). Recently, a microfluorometric method has been developed that uses individual Drosophila abdomens and the surrogate substrate ethoxycoumarin, although no ethoxy groups are present in pyrethroids (Desousa et al. 1995). Nevertheless, this fluorometric assay has the utility of requiring only one to four live mosquito larvae for detection (David et al. 2002, 2006, Boyer et al. 2006). Current assays also have the drawback of requiring significant preparation times. For example, authentic substrates such as permethrin and propoxur have been used to directly measure P450-based metabolism in pyrethroid- and carbamate-resistant larval mosquitoes or to study insecticide metabolism in cockroaches, potato beetle, or rodents (Shrivastava et al. 1970, Shono et al. 1978, 1979, Soderlund et al. 1987, Kasai et al. 1998). Although this is a “information rich” approach that avoids a surrogate substrate, obvious disadvantages include very low-throughput, labor-intensive preparation of samples from hundreds of individuals, the requirement of a priori knowledge of the potential metabolites of the insecticide tested, and the requirement for advanced instrumentation. Recently developed, two innovative approaches, one detecting methoxy-resorufin with an electrochemical sensor and the other the detoxification gene chip assay, also require expensive equipment and specialized knowledge (David et al. 2005, Jenkins et al. 2006).
Although it is predicted that quantitative reverse transcriptase (RT)-polymerase chain reaction (PCR)-based diagnostic assays are likely to be used more frequently, biochemical assays based on enzyme activity remain to be the most functionally relevant. In this regard, luminescence-based assays are proposed to be more sensitive and versatile than fluorescence-based assays (Cali et al. 2006). In this study, we explored and adapted a P450-Glo luminescent assay to detect cytochrome P450 activity in the gut of a single larval mosquito after removal of its lumen contents. A side by side comparison of this assay with that of Desousa et al. (1995) produced comparable results. We did not compare the P450-Glo assay with our previously described α-cyano ester-based fluorescent assay (Kang et al. 2005). The P450-Glo luminescent assay uses a low-cost, commercially available luminescent substrate (Luciferin-H). The assay can be completed in <1 h, depending on the assay enzyme source used. The use of this assay can be enhanced by detection based on individual insects that not only reduces sample preparation time but allows for examination of variations within a population.
Materials and Methods
Mosquito Strains and Maintenance
Two laboratory-reared strains (Marin and CQ-1) of Culex pipiens Say (Diptera:Culicidae) complex mosquitoes were used in this study (McAbee et al. 2004). Marin is a pyrethroid-resistant Cx. p. pipiens variety molestus Forskål (Diptera: Culicidae) that was field collected in Marin County, CA, in 2001. After field collection, Marin mosquitoes were colonized, and permethrin resistance was maintained by exposing fourth-instar larvae to permethrin every five generations as described previously (McAbee et al. 2004). CQ-1 is a pyrethroid-susceptible Cx. quinquefasciatus Say strain that was field collected in Merced County, CA, in the early 1950s. In this study, the Marin strain ranged from generations 87 to 101 (F87-F101). Marin and CQ-1 larvae were reared on a diet of ground rodent pellets (Purina Mills, Richmond, IN) at 27 ± 1°C under a 14:10 (L:D) photoperiod.
Larval Bioassays
Bioassays with varying concentrations of permethrin, pyrethrum, or deltamethrin were conducted on fourth-instar Marin F87 and CQ-1 larvae with or without the general P450 inhibitor piperonyl butoxide (PBO; 3 mg/liter for pyrethrum or 5 mg/liter for permethrin and deltamethrin) as described previously (McAbee et al. 2004). Analytical grade insecticides (Chem Service, West Chester, PA) and chemicals (Sigma-Aldrich, St. Louis, MO) were used in all experiments. LC50 values were calculated using POLO PC software (LeOra 1994).
Selection of Mosquitoes for P450 Activity Assays
Late fourth-instar larval mosquitoes were used for all P450 activity assays. Larvae were selected by identifying individuals having gone through apolysis and excretion of a visibly dark red or purple pupal cuticle under the clear larval cuticle. Only individuals in this stage without visible sclerotization of the pupal respiratory trumpets were selected and assayed. This was done to rule out possibilities of stage- and age-specific related variations in P450 activity. The guts of mosquitoes chosen after sclerotization of the pupal respiratory trumpets showed less structural integrity, and activity assays run with them were inconclusive (data not shown).
Preparation of Tissues for P450 Activity Assays
Microsomes and tissue homogenates were prepared from various sources as described in Table 1. The tissues were dissected from larval mosquitoes under ice-cold phosphate-balanced salt (PBS) buffer, pH 6.2, and transferred to ice-cold homogenization buffer (HB: 0.1 M sodium phosphate, pH 7.5, containing 1 mM EDTA, 1 mM phenylmethylsulphonyl fluoride [PMSF], 0.1 mM dithiothreitol [DTT], and 1 mM 1-phenyl-2-thiourea [PTU]). The PMSF and PTU were dissolved in ethanol and added to the HB such that concentration of ethanol never exceeded 1% (vol: vol) of the total buffer volume. All of the enzyme preparations were used within 8 h of tissue dissection.
Table 1.
Cytochrome P450 activity of various enzyme preparations from the Marin colony measured using Lu-H or 7-EC as substrates
| Type of preparation | Abbreviation | Enzyme source |
n per preparation |
Assay time (min)a |
Protein (µg) per mosquito (mean ± SD) |
Activityb | Activity per assay timec |
|---|---|---|---|---|---|---|---|
| Microsome | Mgut | Cleared gutd | 100 | 290 | 1.92 (0.578) | 209 | 0.72 |
| Homogenate (Lu-H) | Hwhole | Whole larva | 30 | 55 | 79.4 (11.7) | –e | – |
| Hheadless | Headless larva | 30 | 70 | 63.2 (11.5) | – | – | |
| Hcarcass | Carcassf | 30 | 70 | 33.2 (1.20) | – | – | |
| Hhead | Head | 30 | 70 | 15.1 (1.28) | – | – | |
| Hgut | Cleared gut | 30 | 108 | 28.7 (3.43) | 68.9 | 0.64 | |
| Hcaecae | Caecae | 30 | 108 | 10.3 (1.03) | 16.8 | 0.16 | |
| Hventriculus | Ventriculus | 30 | 108 | 11.8 (0.883) | 71.8 | 0.66 | |
| Htubules | Malpighian tubules | 30 | 108 | 6.70 (1.01) | 25.2 | 0.23 | |
| S-Hgut | Cleared gut | 27 | 100 | 31.2 (2.12) | 33.5g | 0.34 | |
| Whole tissue | Sgut(Lu-H) | Cleared gut | 40 | 85 | 36.1 (5.73)h | 112g | 1.3 |
| Whole tissue | Sgut(7-EC) | Cleared gut | 22 | 280 | 36.1 (5.73)h | 1,840g | 6.6 |
| Whole larva | Slive(Lu-H) | Live larva | 40 | 55 | 89.2 (2.38)h | 0.964g | 0.0175 |
| Whole larva | Slive(7-EC) | Live larva | 119 | 250 | 89.2 (2.38)h | 117g | 0.47 |
| Whole larva (Lu-H) | Sdead | Dead larva | 6 | 250 | 89.2 (2.38)h | – | – |
| Blank | Bwater | Larval panwater | NA | 55 | NA | – | – |
The 7-EC substrate was used for two preparations (whole tissue and whole larvae).
Does not include buffer and reagent preparation or luminescent signal stabilization time.
Pmoles substrate (luciferin or 7-OH coumarin) per minute per microgram protein.
Activity per assay time was calculated by dividing activity reading by assay time.
Cleared gut: midgut (gastric cecae + ventriculus) and malpighian tubules cleared of food content.
Activity not detectable with Lu-H at high protein concentration or with single dead larvae.
Carcass: headless and gutless body.
Individual mosquitoes assayed, average activity per individual larvae is reported.
Protein measurements from single cleared gut/whole larvae homogenates without centrifugation.
–, no activity detected; NA, not applicable.
The microsomes were prepared from the midgut (gastric cecae and ventriculus) and malpighian tubules of 100 late fourth instars that were cleared of gut contents following the method of Kasai et al. (1998). The cleared guts were combined in a 1.8-ml micro-centrifuge tube containing 100 µl of ice-cold HB and transferred to a 15-ml glass dounce tissue homognizer (Wheaton, Millville, NJ) containing 11.9 ml of ice-cold HB for homogenization (12 ml total volume). After homogenization, the homogenate was subjected to two centrifugation steps at 4°C. The initial centrifugation was performed at 10,000 × g for 15 min to remove insoluble debris. A second centrifugation at 110,000 × g for 1 h was performed to pellet the microsomes. The microsomal pellet was resuspended in 100 µl of ice-cold resuspension buffer (HB without PTU). Protein concentration was measured in triplicate by the BCA method of Smith et al. (Smith et al. 1985) using bovine serum albumin as a standard.
The tissue homogenates were prepared from the tissues of a batch of 30 or individual fourth instars (S-Hgut). The tissues were homogenized in a 1.8-ml microcentrifuge tube containing various volumes of HB using a microfuge tube pestle (USA Scientific, Woodland, CA). After homogenization, the homogenate was centrifuged at 1,000 × g for 5 min (2 min for S-Hgut preparations) at 4°C to remove large debris. The supernatant was transferred to a new 1.8-ml microfuge tube and protein concentration was determined as described above. Subsequently, the supernatant was diluted with HB to give a final protein concentration of 0.5–4 mg/ml (i.e., a protein concentration that was within the linear range of the P450 activity assay, data not shown). Whole, cleared guts without homogenization were dissected as described above and placed individually in a well of a 96-well plate (on ice and covered with aluminum foil) containing 50 µl of HB for use in the P450 activity assays.
In pilot experiments, individual whole larvae and guts of Marin and CQ1 mosquitoes that were of similar age were weighed. Because no differences in weights were found, the amount of protein in these samples were quantified and were used to calculate specific activity in Tables 1 and 2.
Table 2.
Cytochrome P450 activities of various enzyme source preparations with three luminescent substrates and one fluorescent substrate using Marin and CQ-1 larvae
| P450 activity (pmolluciferin/min/µ g protein) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Enzyme sourcea |
Luciferin-BE |
Luciferin-H |
Luciferin-Me |
||||||
| Marin | CQ-1 | AR | Marin | CQ-1 | AR | Marin | CQ-1 | AR | |
| Mgutb | 990 (32.6) | 1,180 (57.9) | 0.84 | 209 (12.4) | 128 (14.0) | 1.6 | 495 (43.4) | 394 (37.2) | 1.3 |
| Hgutc | 371 (40.2) | 426 (110) | 0.87 | 68.9 (11.1)d,e | 46.0 (7.96) | 1.5 | 149 (20.1) | 120 (11.4) | 1.2 |
| Hcaecaec | 117 (12.2) | 1.84 (68.9) | 0.64 | 16.8 (3.21) | 14.4 (6.76) | 1.2 | 49.9 (14.9) | 57.5 (27.8) | 0.87 |
| Hventriculusc | 349 (46.1)d,f | 268 (28.3) | 18.3 | 71.8 (19.8)d,g | 26.2 (9.09) | 2.7 | 213 (4.53)d,h | 93.5 (20.8) | 2.3 |
| Htubulesc | 43.6 (18.3) | 59.6 (16.3) | 0.73 | 25.2 (5.51) | 26.2 (8.73) | 0.96 | 32.1 (9.10) | 33.8 (6.06) | 0.95 |
| S-Hguti | — | — | — | 33.5 (15.1)jk | 24.6 (8.35) | 1.8 | — | — | — |
| Sgut(Lu-H)i | — | — | — | 112 (58.3)j,l | 62.3 (28.3) | 1.8 | — | — | — |
| Sgut(7-EC)i | — | — | — | 1,840m (543)j,n | 1,440m (321) | 1.3 | — | — | — |
| Slive(Lu-H)i | — | — | — | 0.964 (0.348)j,o | 0.760 (0.209) | 1.3 | — | — | — |
| Slive(7-EC)i | — | — | — | 117m (91.5)j,p | 77.4m (52.4) | 1.5 | — | — | — |
Values are mean (SD).
See Table 1.
Activities reported as the average of six replicate wells per substrate from individual mosquito gut microsomal preparations.
Activities reported as the average of at least three homogenates from larvae of different filial generations.
Marin mean significantly higher than CQ-1 by Student's t-test (Sigmastat 2004).
t = 2.91; df = 4; P = 0.04.
t = 3.01; df = 6; P = 0.02.
t = 4.20; df = 6; P = 0.006.
t = 4.82; df = 6; P = 0.003.
Activities reported as the average of n single preparations. See Table 1 for n for Marin preparations, n of CQ-1 = 30, 39, 24, 51, and 115 for S-Hgut, S-Hgut(H)), Sgut(7-Ec), Slive(H), and Slive(7-EC) preparations, respectively.
Marin mean significantly higher than CQ-1 by Mann-Whitney test (Sigmastat 2004).
T = 920 P = 0.03.
T = 1104; P ≤ 0.001.
Pmoles 7-OH coumarin per minute per microgram protein.
T = 626; P = 0.003.
T = 2202; P = 0.004.
T = 11466; P ≤ 0.001.
AR, activity ratio of Marin/CQ-1 for each preparation; –, not tested.
P450 Activity Assays
Luminescent P450 activity assays were performed in all-white 96-well plates (Thermo Fisher Scientifc, Hudson, NH) using the commercially available P450-Glo (Promega, Madison, WI) substrates Luciferin-Be (Lu-Be), Luciferin-H (Lu-H), or Luciferin-Me (Lu-Me). For all microsome and homogenate preparations (Table 1), each experimental well (50 µl total reaction volume) contained 2.5 µl of enzyme suspension source (1.25–10.0 µg/well final protein concentration), 25 µl of an NADPH generating system (1.3 mM NADP+, 3.3 mM glucose-6-phosphate, 0.4 U/ml glucose-6-phosphate dehydrogenase, and 3.3 mM MgCl2 final concentrations; BD Biosciences, San Jose, CA), 9 µl of an 0.4 M KPO4 buffer (pH 7.4; 72 mM final concentration), 12.5 µl of double-deionized water (dd H2O), and 1 µl of the appropriate substrate (50 µM final concentration).
Two assays were used to measure P450 activity from live individual whole larvae and whole gut preparations. Sgut(H) preparations were assayed similarly to those above, except an additional 27.5 µl of dd H2O replaced the enzyme suspension source and NADPH generating system. Slive(H) preparations were assayed as described in individual wells (100 µl total reaction volume) containing 18 µl of 0.4 M KPO4 buffer (pH 7.4; 72 mM final concentration), 81 µl of ddH2O, and 1 µl of substrate (50 µM final concentration). The fluorescent substrate 7-ethoxycoumarin (7-EC) was also used to measure P450 activity in black 96-well medium-binding plates (Greiner, Monroe, NC) according to the method of Boyer et al. (2006). For both Sgut(7-EC) and Slive(7-EC) preparations, individual reaction wells (100 µl total reaction volume) contained 0.4 mM 7-EC in 50 mM sodium phosphate buffer, pH 7.2.
After initiation of P450 activity assays by addition of appropriate substrate to each well, luminescent reactions were incubated at 24°C for 45 min (or 240-min Slive(H) preparations), and the reaction was stopped by the addition of 50 µl of luciferin detection reagent (Promega). After the addition of luciferin detection reagent, the reactions were incubated at 24°C for an additional 20 min after which luminescence was quantified using a Spectrafluor Plus spectrophotometer (Tecan, San Jose, CA) with an integration time of 1 s and gain of 150. When live insects were used in the assay, the insects were removed from the well immediately before measuring luminescence. The average relative luminescence unit (RLU) values from three wells containing all assay components except for the protein source was used as a blank. Luminescence readings that were not at least two-fold higher than the blank were considered nondetectable. A standard luciferin curve (0–800 nmol/well) was generated and used to convert RFU values to nmoles luciferin produced.
For 7-EC-based P450 activity assays, reaction wells were incubated at 30°C for 240 min, and the reaction was stopped by addition of a mixture containing equal volumes of a 10−4 M glycine buffer, pH 10.4, and ethanol. After removal of larvae, the fluorescence of the reaction medium was measured immediately by a Spectrafluor Plus Fluorometer (Tecan, Research Triangle Park, NC) with 380-nm excitation and 480-nm emission filters. The average RLU values from three wells containing all assay components except for the protein source were used as a blank. RFU values were converted to the production of 7-hydroxycoumarin (7-OH) by comparison to a 7-OH standard curve.
Statistical Analysis
Microsome and batch homogenate preparation data were analyzed by the Student’s t-test using Sigmastat software. Single mosquito assays data were analyzed by the Mann-Whitney rank sum test using Sigmastat software (Sigmastat 2004).
Results
The P450-Glo assay was developed by Promega, and assay parameters including accuracy, precision, sensitivity, and selectivity were not further investigated except for temperature. Our preliminary studies found no differences in activity between room temperature (24°C) and the suggested temperature. We ran assays at room temperature to negate the need for an incubator. The remainder of the study focused on examining the activity of this commercially available assay on different enzyme sources (tissues, homogenates, and whole larvae) derived from mosquitoes. The summary of tissues tested, detected activities, and preparation details including time required to prepare and run the assays are given in Table 1. For these evaluations, we decided to focus on examining different forms of tissue preparation using the Lu-H, P450-Glo substrate because of the known possibility of P450-mediated hydroxylation reaction to occur on pyrethroids. The 7-EC P450 activity was used for comparative purposes on whole gut tissue and whole larvae. We first conducted evaluations using generally recommended microsomal preparations from the gut and then shifted toward using homogenates prepared from multiple and single mosquito tissues and whole mosquitoes. In tissue homogenates, we identified specific regions of high P450 activity. In samples where P450 activity was present, no luminescence was detected in the absence of the NADPH generating system, suggesting that the luminescence is generated by cytochrome P450 activity. The P450-Glo assay using the Lu-H substrate produced activity readings from a variety of mosquito tissue sources that did not have to be prepared using a lengthy microsomal extraction procedure (Table 1). Readings were even obtained from tissue and whole larva without any homogenization (Table 1). Highly sensitive readings with as little preparation were obtained with unhomogenized cleared guts using both the P450-Glo Lu-H substrate and 7-EC assay. The7-EC assay, however, is not a good surrogate for pyrethroids because it requires an O-dealkylation reaction, which is not a mechanism proposed for pyrethroid oxidation (Shono et al. 1978, 1979, Soderlund et al. 1987). The Lu-H substrate is a better pyrethroid surrogate because it undergoes hydroxylation of the terminal benzene ring to generate functional luciferin, which is similar to the pyrethroid oxidation process. For this reason, we decided to continue our comparative studies between pyrethroid-susceptible and –resistant mosquitoes using the Lu-H substrate, although the assay results for the other two Lu-substrates are given for comparison.
As indicated in Table 1, time to run the assays differed greatly between the substrate used and type of sample preparation. These times represent the total time required for dissection, extraction, incubations, and reading the results. The durations for the remaining preparations such as mosquito collection and data analysis were excluded because they were assumed equal. The fastest type of preparation that yielded significant levels of activity was Sgut (Lu-H). Crude homogenates prepared from 30 cleared whole guts and also from just the ventriculus had high levels of activity but required a longer time to dissect. (Table 1, activity per assay time). The 7-EC substrate resulted in highest levels of activity but required much longer incubation (Table 1). Larvae that were killed before assay, and larval pan water did not produce any activity with the Lu-H substrate.
Detection of Microsomal P450 Activity
The P450 activity of Marin and CQ-1 colonies using three luciferin substrates compared with 7-EC is shown in Table 2. Activity was consistently detected in microsomes that were prepared from cleared guts (Mgut) of both Marin and CQ-1 larvae (Table 2). The Mgut preparations from both Marin and CQ-1 showed activity (pmol luciferin/min/µg) toward all luminescent substrates with a rank order of Lu-Be > lu-Me > Lu-H. However, Lu-Be and Lu-Me are surrogate substrates of dealkylation reactions, whereas the Lu-H substrate is a surrogate for hydroxylation reactions. Therefore, we would expect the Lu-H substrate to correlate more significantly with pyrethroid resistance. Marin larval gut microsomes (Mgut) had 1.6-fold higher specific activity than the microsomal preparation from CQ-1 larvae for the Lu-H substrate (Table 1).
Detection and Localization of P450 Activity in Batch Homogenates
The preparation of microsomes is labor and time intensive and requires a relatively large number of mosquitoes. Therefore, we studied if P450 activities could be detected in partial and whole homogenates of guts dissected out of mosquitoes. Homogenates of whole larvae (Hwhole), headless larvae (Hheadless), larval carcasses (Hcarcass), or larval heads (Hheads) from either Marin (Table 1) or CQ-1 (data not provided) mosquitoes did not have detectable activities. This could possibly be attributed to the presence of factors that inhibit the P450 enzymes in eyes and gut contents of mosquito larvae (Schonbrod and Terriere 1971, Kasai et al. 1998). When simple homogenates were prepared from Marin or CQ-1 cleared guts (Hgut), significant P450 activity was detected (Table 2). Homogenates of resistant Marin colony cleared guts displayed higher levels of activity with the Lu-H substrate. P450 activity was not detected in the absence of the NADPH generating system.
To test whether a specific region of the gut had the highest P450 activity readings, homogenates were prepared from three distinct regions of the gut: caeca (Hcaecae), ventriculus (Hventriculus), and malpighian tubules (Htubules). All preparations from both Marin and CQ-1 larvae were active, but the Hventriculus preparations showed the highest activity with all three of the substrates tested (Table 2). The Hcaecae and Htubules preparations showed activities that were ≈50–70% lower than that found in the Hventriculus preparation. Marin ventriculus homogenates were 2.7-fold more active than CQ-1 preparations for Lu-H substrate, and mean activity values were significantly different from each other. Interestingly, there were no statistically significant differences in P450 activity between the Marin and CQ-1 strains for the Hcaecae and Htubules preparations regardless of the substrate used.
Detection of P450 Activity in Individual Mosquitoes
P450 activity was detectable on homogenates prepared from batches of 30 larval guts, but it would be even more convenient if activities could be detected on gut homogenate (S-Hgut) and whole guts from single mosquito larvae (Sgut and Slive). These evaluations were performed using the Lu-H and 7-EC substrates on both pyrethroid resistant (Marin) and susceptible (CQ1) mosquitoes. Lu-BE and Lu-Me activity levels were not included in this comparison because they likely do not measure pyrethroid metabolizing P450’s.
Based on Mann-Whitney rank sum test analysis, the distribution of the P450 Lu-H activity of the Marin S-Hgut preparations (14–66 pmol luciferin/min/µg) was significantly (P = 0.02) higher (1.4-fold) than readings from CQ-1 S-Hgut preparations (12–45 pmol luciferin/min/µg; Fig. 1.). No significant differences were detected in the mean protein concentrations obtained from Marin or CQ-1 S-Hgut preparations (data not shown). P450 Lu-H activity of a single, whole, cleared gut (Sgut) from Marin larvae (112 ± 58.3 pmol luciferin/min/µg protein) was significantly (P = 0.0001) higher (1.8-fold) than that of CQ-1 Sgut preparations (62.3 ± 28.3 pmol luciferin/min/µg protein; Table 2). This was slightly higher than the 1.5-fold difference found with P450 Lu-H using homogenates prepared from 30 cleared larval guts as the enzyme source (Table 2). Single cleared gut preparations also yielded detectable readings using the 7-EC substrate, although the difference between Marin and CQ-1 (1.3-fold) activities were not as large as with P450-Glo Lu-H assays.
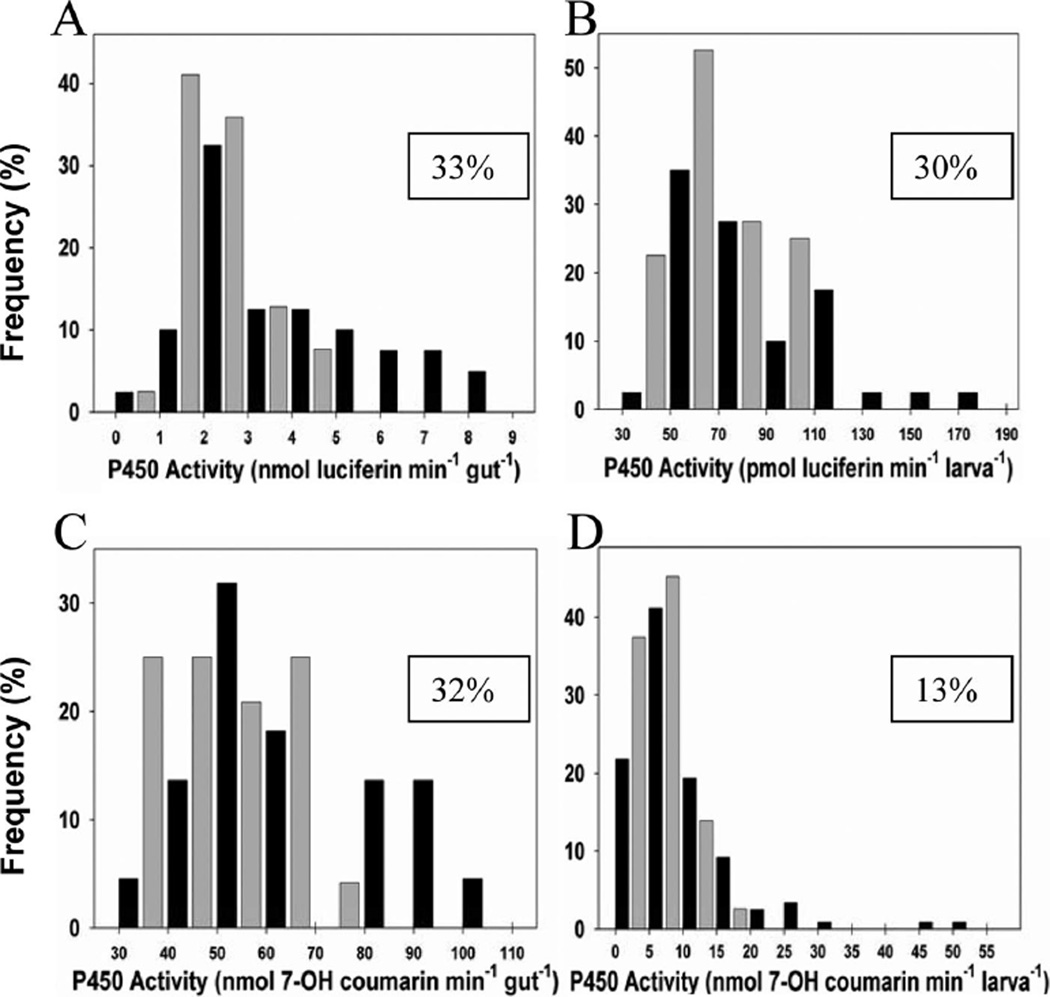
Fig. 1.
Frequency distribution diagrams of P450 activity in single-mosquito preparations of pyrethroid-resistant Marin (black bars) or pyrethroid-sensitive CQ-1 (gray bars) larvae using the luminescent substrate Lu-H (top) and fluorescent substrate 7-EC (bottom). Significant differences were found between Marin and CQ-1 using the Mann-Whitney rank sums test from (A) Sgut (single cleared gut, Lu-H substrate), (B) Slive(H) (single live larvae, Lu-H substrate), (C) Sgut (single cleared gut, 7-EC substrate), and (D) Slive (single live larvae, 7-EC substrate) preparations. See Tables 1 and 2 for number of individual assays performed (n) for each colony per preparation and associated test statistics. Boxed values in plot area are the percentage of individual Marin preparations found to have higher P450 activity than any of those from CQ-1 for each preparation.
We were also able to show that P450 Lu-H activity could be detected in individual live larva (Slive), which eliminates the need for gut dissections and reduces sample preparation time by even more. P450 Lu-H activity, which could only barely be detected, showed that live Marin individual activity levels were significantly (P = 0.01) higher than that of the live CQ-1 individuals (Table 2). The average P450 activity of live Marin individuals was 1.3 times higher than that of live CQ-1 individuals (Table 2). Activity readings were much higher with the fluorescent 7-EC substrate compared with P450 Lu-H readings and they too were higher in Marin than CQ-1 live larvae (Table 2).
The ability to biochemically measure P450 activities on individual mosquito guts and live larvae creates opportunities to examine variation among individual mosquitoes in colonies and wild populations. Both the fluorescent 7-EC and luminescent Lu-H substrates showed considerable variation in P450 activities among individual Marin (n = 40, Table 1) and CQ-1 (n = 51, Table 2) mosquitoes tested (Fig. 1). In the case of single cleared guts, 33 and 32% of the Marin mosquito larvae had higher values than the highest CQ-1 value using the luminescent Lu-H and fluorescent 7-EC substrates, respectively. The P450-Glo Lu-H assay, however, detected over twice as many live larvae with activity levels higher than the most active CQ-1 than the 7-EC assay (Fig. 1; 30 versus 13%).
Larval Sensitivity to Pyrethroids
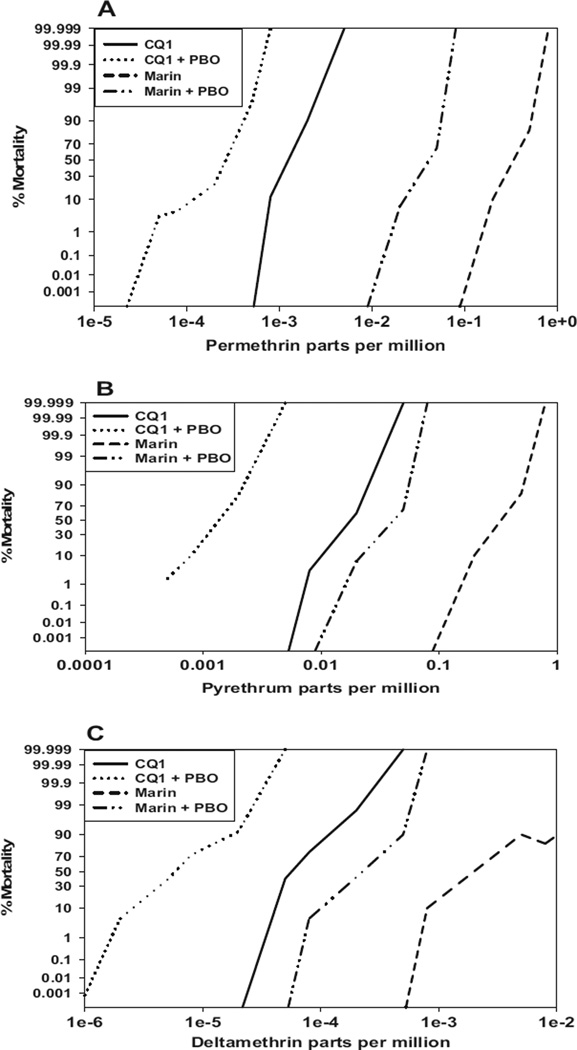
To confirm the pyrethroid resistance status of Marin colony mosquitoes, a dose-response study was performed on larvae from both Marin (generation 87, F87) and CQ-1 colonies exposed to permethrin, pyrethrum, and deltamethrin. The Marin colony showed resistance ratios (RR = LC50 of Marin/LC50 of CQ-1) of 186, 19, and 40 for permethrin, pyrethrum, and deltamethrin, respectively, compared with susceptible control CQ-1 larvae (Fig. 2). These resistance ratios were significantly higher than those of Marin larvae when the colony was first established for permethrin or pyrethrum (RR of 18.3 and 3.3, respectively) or at F4 for deltamethrin (RR of 12; McAbee et al. 2004). The synergist PBO increased the sensitivity of Marin larvae (F87) to permethrin, pyrethrum, and deltamethrin by 28-, 8-, and 11-fold, respectively (Fig. 2). However, even in the presence of PBO, Marin larvae showed a resistance ratio of 9, 2, and 4 for permethrin, pyrethrum, and deltamethrin, respectively, compared with CQ-1 larvae, suggesting target site insensitivity or another detoxification mechanism may contribute to pyrethroid/pyrethrum resistance in Marin mosquitoes (Table 3). No mortality was observed in control experiments in which Marin or CQ-1 larvae were untreated or treated with acetone only.
Fig. 2.
Susceptibility profiles of fourth-instar Marin and CQ-1 strain Cx. pipiens complex mosquitoes to permethin (A), pyrethrum (B), and deltamethrin (C) in the presence and absence of the synergist piperonyl butoxide.
Table 3.
LC50 values of CQ1 and Marin F87 colonies to permethrin, pyrethrum, and deltamethrin with and without the synergist PBO
| Permethrin | Permethrin + PBO |
Pyrethrum | Pyrethrum + PBO |
Deltamethrin | Deltamethrin +PBO |
|
|---|---|---|---|---|---|---|
| CQ1 | ||||||
| LC50 = | 0.0013 | 0.00023 | 0.01768 | 0.00136 | 0.00006 | 0.00001 |
| g = | 0.04616 | 0.08334 | 0.06625 | 0.04489 | 0.07975 | 0.03772 |
| Lower = | 0.00113 | 0.00019 | 0.01574 | 0.00121 | 0.00005 | 0.00001 |
| Upper = | 0.0014 | 0.00029 | 0.01991 | 0.00153 | 0.00007 | 0.00001 |
| slope = | 6.432 ± 0.705 | 4.006 ± 0.365 | 5.683 ± 0.746 | 5.335 ± 0.577 | 4.719 ± 0.600 | 3.193 ± 0.316 |
| Marin F87 | ||||||
| LC50 = | 0.24133 | 0.01126 | 0.33234 | 0.03923 | 0.00237 | 0.00022 |
| g = | 0.06771 | 0.07081 | 0.04538 | 0.04919 | 0.04381 | 0.07746 |
| Lower = | 0.18499 | 0.00924 | 0.29655 | 0.035 | 0.00192 | 0.00018 |
| Upper = | 0.32199 | 0.01388 | 0.36975 | 0.0435 | 0.00291 | 0.00027 |
| slope = | 2.197 ± 0.193 | 3.418 ± 0.326 | 6.020 ± 0.654 | 5.740 ± 0.650 | 2.622 ± 0.216 | 4.019 ± 0.379 |
| Resistance ratio | 185.64 | 8.66 | 18.80 | 2.22 | 39.50 | 3.67 |
Discussion
Bioassays are commonly used as a reliable and quantitative measure of insecticide resistance levels within an insect population. However, biochemical assays performed in the field or laboratory with ease could provide quantitative and qualitative information on the mechanism(s) of insecticide resistance that can subsequently be used to design and implement resistance management schemes. Here we studied if the commercially available P450-Glo assay kit could be adapted to monitor mosquito cytochrome P450 activity for evaluating pyrethroid resistance caused by increased cytochrome P450 activity. The cytochrome P450s are heme-thiolate enzymes that are known to collectively catalyze at least 60 chemically distinct reactions (Feyereisen 2005). Because of their diversity, P450s can be involved in the metabolism of virtually all synthetic chemical insecticides (Agosin 1985, Hodgson 1985, Berge et al. 1998). The P450s that are overexpressed in insecticide resistant insects are generally found in the insect CYP6 and CYP4 families (David et al. 2005, Feyereisen 2005, Muller et al. 2007). In terms of structure and mode of action, the insect CYP6 and CYP4 families are homologous to the vertebrate CYP3 and CYP4 families, respectively (Feyereisen 2006). Members of the vertebrate CYP4 family do metabolize the P450-Glo substrates Lu-Be and Lu-Me. The Lu-H substrate is not specifically metabolized by P450s in either the vertebrate CYP6 or CYP4 family; however, it has been shown to be preferentially degraded by the vertebrate CYP2C9, a P450 that metabolizes pyrethroids in humans (Godin et al. 2007). The most common mechanism of pyrethroid oxidation in insects is hydroxylation of a terminal benzene ring (Shono et al. 1978, 1979, Soderlund et al. 1987). The Lu-H substrate must undergo hydroxylation of the terminal benzene ring to generate functional luciferin. The Lu-Be and Lu-Me substrates, however, must undergo an O-dealkylation reaction similar to fluorometric P450 substrates such as alkoxyresorufins and alkoxycoumarins undergo (Ullrich and Weber 1972, Mayer et al. 1977). Therefore, assays using resorufin or coumarin based substrates should be evaluated with caution. In our study, the mosquito larvae used were suspected to have higher levels of cytochrome P450 activity than a susceptible laboratory strain, CQ-1. This was confirmed with a synergist bioassay. The susceptibility of Marin larvae to exposure of three pyrethroids were significantly increased in the presence of piperonyl butoxide, a known P450 inhibitor.
Our results clearly indicate that the luminescent P450-Glo assay is amenable to be used for monitoring mosquito cytochrome P450 activity in several different formats. We tested the assay platform by using a series of preparations that are progressively less time consuming to prepare. The assay performed well when microsomal preparations were used, which clearly showed differences in P450 activity levels between the Marin and CQ-1 mosquitoes. The lack of luminescence in these samples in the absence of a NADPH regenerating system strongly suggests that the measured signal is attributable to cytochrome P450 activity. Interestingly, the use of microsomal preparation did not necessarily produce higher levels of specific activity. This indicates that the longer time invested in microsomal preparations is not necessary for the luminescent assay procedures.
Consistent with previous reports, whole larvae homogenates were devoid of activity even when very high levels of protein can be detected in the wells. When guts of larvae are dissected, cleared of their content, pooled, and homogenized, significant levels of activity were observed that elicited differences between pyrethroid-resistant and -susceptible mosquitoes. Within the midguts, we identified a region of higher P450 activity. When the midguts were dissected into the cecae, the ventriculus, and the malphigian tubules, only the ventriculus preparations between the Marin and CQ-1 colonies showed differences. Although we observed the highest difference between the Marin and CQ-1 larvae in the ventriculi preparations of pooled larvae, this step clearly did not provide any time savings. However, the identification of ventriculus as a source of high P450 activity shows the flexibility of the assay platform.
Finally, we tested whether individual larvae or tissues dissected from individual larvae could be used as the enzyme source. Tissue homogenates or whole cleared guts from individual larvae of both the Marin and the CQ-1 strains had much higher activity readings than live larvae. However, live larvae still produced distinguishable readings. This offers considerable promise for use as a low-cost, quick to perform, higher-throughput and more field deployable assay format. On the whole, the fluorescent 7-EC assay showed higher activity readings than the P450-Glo assay system regardless of enzyme source. There are several possibilities for this difference. There may be differential penetration of the 7-EC and P450-Glo substrates through mosquito gut tissue. Larval cuticle, may also block some of the Lu-H luminescent signal. It is also possible that either the fluorescent or the luminescent product could be further metabolized to nondetectable material. Differences in concentrations of the 7-EC (400 µM for the fluorescent assay) and P450 Glo Lu-H substrates (50 µM for luminescent) necessary to perform the assays could also account for this difference. Lower luminescent assay substrate concentrations were used to ensure that the luminescent signal to noise ratio readings were better than those of the fluorescent assay.
Both the P450 Glo Lu-H and 7-EC assays showed considerable variations in activity readings between individuals of Marin and CQ-1 larvae. The data in Tables 1 and 2 were expressed as specific activity (protein−1), whereas the data in Fig. 1 were expressed as activity from individual whole larvae without standardizing for protein content. The P450-Glo assay was more sensitive in differentiating more Marin live larvae with higher activities than CQ-1 live larvae when the frequencies of higher activity were compared. This is not unexpected because as explained earlier the Lu-H substrate is chemically a more relevant surrogate to mimic P450 mediated pyrethroid metabolism than 7-EC and Lu-BE and Lu-ME substrates. Variations in P450 activity between individuals of the same colony and especially the broader and higher variation noted among Marin individuals reflect tremendous heterozygosity in P450 activity. After several years of colonization and exposure to permethrin, the Marin colony is fixed for the knockdown-resistant kdr mutant that confers pyrethroid target site insensitivity in Culex mosquitoes (McAbee et al. 2004). We are under the impression that heterozygosity for higher levels of resistance to pyrethroids also exist in this colony and that these higher resistant properties of some individuals is conferred by other additional mechanisms such by P450 enzyme-mediated detoxification.
Overall, the P450-Glo platform required less time and effort to quantify P450 activity and may be a suitable assay for quantifying pyrethroid degrading cytochrome P450s. The activity level of individual mosquito whole guts were only two times lower than the microsomal preparation of the same tissue from a batch of 30 larvae. Interestingly, the ratio of activity between the Marin and CQ-1 larvae were comparable when either microsomes or whole cleared guts were used. This clearly indicates that microsomal preparations are not essential for monitoring resistance related P450 activity in the Promega P450-Glo assay system, and furthermore, this system shows promise for detecting P450 activity on even live larvae. The fluorescent 7-EC activity assays overall were more sensitive for detection of P450 activities than the luminescent P450 Glo platform. If further studies show that the 7-EC activity assay system does indeed detect t pyrethroid metabolizing P450s, we strongly advocate that the luminescent assay alone should not be relied on for detection of resistance.
Implementation of biochemical assays that are representative of insecticide resistance status in vector insects remain a challenge. The goal of accurately, rapidly, and cost effectively diagnosing or monitoring resistance development in the field is accumulating but has still not been reached. However, incremental advances in methodology like the ones reported here that require less instrumentation to prepare samples and to measure activities should significantly enhance our ability to monitor insecticide resistance and modify chemical pest control tactics.
Acknowledgments
We thank A. G. Schaefer for help with experiments and anonymous reviewers for useful comments. This work was funded by NIH/NIAID U01 Grant AI068855 and NIEHS Superfund Basic Research Program Grant P42 ES04699.
References Cited
- Agosin M. Role of microsomal oxidations in insecticide degradation. In: Kerkut GA, Gilbert LI, editors. Comprehensive insect physiology, biochemistry, and pharmacology. Oxford, United Kingdom: Pergamon; 1985. pp. 647–712. [Google Scholar]
- Baars AJ, Zijlstra JA, Vogel E, Breimer DD. The occurrence of cytochrome P-450 and aryl hydrocarbon hydroxylase activity in drosophila melanogaster microsomes, and the importance of this metabolizing capacity for the screening of carcinogenic and mutagenic properties of foreign compounds. Mutat. Res. Fundamental Molec. Mechanisms Mutagenesis. 1977;44:257–267. doi: 10.1016/0027-5107(77)90083-5. [DOI] [PubMed] [Google Scholar]
- Berge JB, Feyereisen R, Amichot M. Cytochrome P450 monooxygenases and insecticide resistance in insects. Philo. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1998;353:1701–1705. doi: 10.1098/rstb.1998.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer S, David JP, Rey D, Lemperiere G, Ravanel P. Response of Aedes aegypti (Diptera : Culicidae) larvae to three xenobiotic exposures: larval tolerance and detoxifying enzyme activities. Environ. Toxicol. Chem. 2006;25:470–476. doi: 10.1897/05-267r2.1. [DOI] [PubMed] [Google Scholar]
- Brogdon WG, McAllister JC, Vulule J. Heme peroxidase activity measured in single mosquitoes identifies individuals expressing an elevated oxidase for in- secticide resistance. J. Am. Mosq. Control Assoc. 1997;13:233–237. [PubMed] [Google Scholar]
- Cali JJ, Ma D, Sobol M, Simpson DJ, Frackman S, Good TD, Daily WJ, Liu D. Luminogenic cytochrome P450 assays. Expert Opin. Drug Metab. Tox. 2006;2:629–645. doi: 10.1517/17425255.2.4.629. [DOI] [PubMed] [Google Scholar]
- Coleman M, Hemingway J. Insecticide resistance monitoring and evaluation in disease transmitting mosquitoes. J. Pest. Sci. 2007;32:69–76. [Google Scholar]
- David J-P, Rey D, Cuany A, Bride JM, Meyran JC. Larvicidal properties of decomposed leaf litter in the subalpine mosquito breeding sites. Environ. Toxicol. Chem. 2002;21:62–66. [PubMed] [Google Scholar]
- David J-P, Strode C, Vontas J, Nikou D, Vaughan A, Pignatelli PM, Louis C, Hemingway J, Ranson H. The Anopheles gambiae detoxification chip: a highly specific microarray to study metabolic-based insecticide resistance in malaria vectors. P. Natl. Acad. Sci. USA. 2005;102:4080–4084. doi: 10.1073/pnas.0409348102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David JP, Boyer S, Mesneau A, Ball A, Ranson H, Dauphin-Villemant C. Involvement of cytochrome P450 monooxygenases in the response of mosquito larvae to dietary plant xenobiotics. Insect Biochem. Molec. Biol. 2006;36:410–420. doi: 10.1016/j.ibmb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Desousa GA, Cuany A, Brun A, Amichot M, Rahmani R, Berge JB. A microfluorometric method for measuring ethoxycoumarin-O-deethylase activity on individual Drosophila melanogaster abdomens: interest for screening resistance in insect populations. Anal. Biochem. 1995;229:86–91. doi: 10.1006/abio.1995.1382. [DOI] [PubMed] [Google Scholar]
- Donato MT, Jimenez N, Castell JV, Gomez-Lechon MJ. Fluoresence-based assays for screening nine cytochrome P450 activities in intact cells expressing individual human P450 enzymes. Drug Metab. Dispos. 2004;32:699–706. doi: 10.1124/dmd.32.7.699. [DOI] [PubMed] [Google Scholar]
- Feyereisen R. Insect cytochrome P450. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive molecular insect science. Amsterdam, The Netherlands: Elsevier; 2005. pp. 1–77. [Google Scholar]
- Feyereisen R. Evolution of insect P450. Biochem. Soc. Trans. 2006;34:1252–1255. doi: 10.1042/BST0341252. [DOI] [PubMed] [Google Scholar]
- Godin SJ, Crow JA, Scollon EJ, Hughes MF, DeVito MJ, Ross MK. Identification of rat and human cytochrome P450 isoforms and a rat serum esterase that metabolize the pyrethroid insecticides deltamethrin and esfenvalerate. Drug Metab. Dispos. 2007;35:1664–1671. doi: 10.1124/dmd.107.015388. [DOI] [PubMed] [Google Scholar]
- Hodgson E. Microsomal mono-oxygenases, pp. 225-322. In: Kerkut GA, Gilbert LI, editors. Comprehensive insect physiology, biochemistry, and pharmacology. Elmsford, NY: Pergamon; 1985. [Google Scholar]
- Jenkins ATA, Dash H-A, Boundy S, Halliwell CM, ffrench-Constant RH. Methoxy-resorufin ether as an electrochemically active biological probe for cytochrome P450 O-demethylation. Bioelectrochemistry. 2006;68:67–71. doi: 10.1016/j.bioelechem.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Kang K-D, Jones PD, Huang H, Zhang R, Mostovich LA, Wheelock CE, Watanabe T, Gulyaeva LF, Hammock BD. Evaluation of [alpha]-cyano ethers as fluorescent substrates for assay of cytochrome P450 enzyme activity. Anal. Biochem. 2005;344:183–192. doi: 10.1016/j.ab.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai S, Weerashinghe IS, Shono T. P450 monooxygenases are an important mechanism of permethrin resistance in Culex quinquefasciatus Say larvae. Arch. Insect Biochem. Physiol. 1998;37:47–56. [Google Scholar]
- Kulkarni AP, Mailman RB, Baker RC, Hodgson E. Cytochrome-P-450 difference spectra: type-Ii interactions in insecticide-resistant and insecticide-susceptible houseflies. Drug Metab. Dispos. 1974;2:309–320. [PubMed] [Google Scholar]
- LeOra . POLO-PC probit and logit analysis user’s gude. Berkley, CA: LeOra; 1994. [Google Scholar]
- Mayer RT, Jermyn JW, Burke MD, Prough RA. Methoxyresorufin as a substrate for fluorometric assay of insect microsomal O-dealkylases. Pest. Biochem. Physiol. 1977;7:349–354. [Google Scholar]
- McAbee RD, Kang KD, Stanich MA, Christiansen JA, Wheelock CE, Inman AD, Hammock BD, Cornel AJ. Pyrethroid tolerance in Culex pipiens pipiens var molestus from Marin County, California. Pest Manag. Sci. 2004;60:359–368. doi: 10.1002/ps.799. [DOI] [PubMed] [Google Scholar]
- Muller P, Donnelly MJ, Ranson H. Transcription profiling of a recently colonised pyrethroid resistant Anopheles gambiae strain from Ghana. BMC Genomics. 2007;8:1–12. doi: 10.1186/1471-2164-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbrod D, Terriere LC. Eye pigments as inhibitors of microsomal aldrin epoxidase in house fly (Diptera-Muscidae) J. Econ. Entomol. 1971;64:44–45. [Google Scholar]
- Shono T, Unai T, Casida JE. Metabolism of permethrin isomers in American cockroach adults, house-fly adults, and cabbage-looper larvae. Pest. Biochem. Physiol. 1978;9:96–106. [Google Scholar]
- Shono T, Ohsawa K, Casida JE. Metabolism of trans-permethrin and cis-permethrin, trans-cypermethrin, and cis-cypermethrin, and decamethrin by microsomal enzymes. J. Agric. Food Chem. 1979;27:316–325. doi: 10.1021/jf60222a059. [DOI] [PubMed] [Google Scholar]
- Shrivastava SP, Georghio GP, Metcalf RL, Fukuto TR. Carbamate resistance in mosquitos: metabolism of propoxur by susceptible and resistant larvae of Culex pipiens fatigans. Bull. WHO. 1970;42:931–942. [PMC free article] [PubMed] [Google Scholar]
- Sigmastat. Version 3.0. SYSTAT Software. Richmond, CA: 2004. [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Soderlund DM, Hessney CW, Jiang M. Metabolism of fenvalerate by resistant Colorado potato beetles. J. Agric. Food Chem. 1987;35:100–105. [Google Scholar]
- Ullrich V, Weber P. O-Dealkylation of 7-ethoxycoumarin by liver microsomes: direct fluorometric test. Hoppe-Seylers Zeitschrift Physiol. Chem. 1972;353:1171–1177. doi: 10.1515/bchm2.1972.353.2.1171. [DOI] [PubMed] [Google Scholar]
- Wheelock GD, Scott JG. Anti-P4501pr antiserum inhibits specific monooxygenase activities in Lpr house-fly microsomes. J. Exp. Zool. 1992;264:153–158. doi: 10.1002/jez.1402640206. [DOI] [PubMed] [Google Scholar]