Abstract
Previous studies of triclocarban suggest that its biotransformation could yield reactive metabolites that form protein adducts. Since the skin is the major route of triclocarban exposure, present work examined this possibility in cultured human keratinocytes. The results provide evidence for considerable biotransformation and protein adduct formation when cytochrome P450 activity is induced in the cells by TCDD, a model Ah receptor ligand. Since detecting low adduct levels in cells and tissues is difficult, we utilized the novel approach of accelerator mass spectrometry for this purpose. Exploiting the sensitivity of the method, we demonstrated that a substantial portion of triclocarban forms adducts with keratinocyte protein under the P450 inducing conditions employed.
Keywords: Accelerator mass spectrometry, Biotransformation, Cytochrome P450, Keratinocytes, Protein adducts, Reactive metabolites, TCDD, Triclocarban
INTRODUCTION
Triclocarban (TCC) is a common antimicrobial preservative in personal care products, particularly in bar soaps. Human exposure to TCC has come to public attention because it accumulates in the aquatic environment [1] and has biological effects on mammals. TCC is a potent inhibitor (IC50 24 ± 5 nM) of the human soluble epoxide hydrolase, an enzyme of the arachidonic cascade [2-4]. Similarly potent inhibitors have pronounced pharmacological effects on regulation of inflammation and pain [5]. Moreover, TCC may act as an endocrine disruptor at high concentration by enhancing the action of testosterone [6].
Bathing with TCC-containing soaps typically results in deposition of TCC on human skin of ≈0.3 μg/cm2 [7]. A substantial portion traverses the epidermal barrier, is absorbed and becomes systemically available [4, 8]. During showering with antibacterial soap, ≈0.6% of the applied amount of TCC is dermally absorbed and is detected almost exclusively as metabolites [4]. In the plasma of mammals, TCC is found primarily as its sulfated oxidative metabolites, particularly 2′-SO3-O-TCC. In the bile, glucuronic acid conjugates of hydroxylated metabolites dominate, and in human monkey and mouse urine primarily N-glucuronides are excreted [9, 10]. Thus, both oxidative phase I metabolism, catalyzed by cytochrome-P-450 monooxygenases, and phase II metabolism, catalyzed by sulfotransferases and UDP-glucuronlytransferases, are important. Since keratinocytes in the epidermis and in culture express substantial phase I and phase II activities [11, 12], this work explores the hypothesis that, upon dermal exposure, TCC undergoes biotransformation that produces potentially deleterious reactive metabolites. Use of accelerator mass spectrometry (AMS) offers the possibility to detect protein adducts of such metabolites with much greater sensitivity than with other methods [13-15].
MATERIALS AND METHODS
Generation and Analysis of Metabolites in Cell Culture
Human epidermal spontaneously immortalized keratinocytes (SIK) were cultured with feeder layer support in a DMEM-Ham’s F-12 mixture (2:1) as previously described [16]. For metabolite identification, near-confluent cultures were treated with or without 10 nM 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) for one day before addition of 2 μM TCC (1:1000 dilution of a 2 mM stock solution in DMSO). After 10 min or 20 h incubation, cultures were rinsed in 0.15 M sodium chloride - 10 mM sodium phosphate (pH 7.2), drained, recovered by scraping from the dishes in 0.5 ml of 10 mM Tris (pH 8) - 1 mM EDTA, sonicated and stored at -80°C in two equal aliquots. 50 μL of the homogenate were mixed with 200 μL of acetonitrile/acetic acid (99/2 v/v) containing 12.5 nM 13C6-TCC as internal standard and analyzed directly by online-SPE-LC-MS as previously described [17]. The second aliquot was dissolved in 2% SDS for protein assay using bicinchoninic acid (BCA, Pierce Chem Co, Rockford IL). For determination of protein binding, cells were incubated for 3, 8 and 24 h with 2 μM [14C]TCC (specific activity 0.04 Ci/mol), rinsed twice with phosphate buffered saline, dissolved in 2% SDS containing 25 mM DTE, and sulfhydryls were alkylated with iodoacetamide. The protein was isolated by Sephadex G-25 gel filtration in 0.1% SDS and precipitated by addition of 2.5 vol of ethanol. The pellet was rinsed 3X in 67% ethanol and then analyzed for 14C content.
AMS Analysis
Each protein pellet was placed in a quartz tube (~6×30 mm, 4 mm i.d.) nested inside two borosilicate glass culture tubes (10×75 mm in 12×100 mm) and dried overnight in a vacuum centrifuge. An excess of CuO (≈40 mg) was added and the inner quartz vials were transferred to quartz combustion tubes, evacuated and sealed. The samples were combusted at 900°C for 3.5 h to oxidize completely all carbon to CO2 and then reduced to filamentous carbon as previously described [18]. Carbon samples were packed into aluminum sample holders, and carbon isotope ratios were measured on the compact 1-MV AMS spectrometer at the Lawrence Livermore National Laboratory centered around a National Electrostatics Corporation 3SDH-1 accelerator [19]. Typical AMS measurement times were 3-5 min/sample, with a counting precision of 0.8 – 1.4 % and a standard deviation among 3-7 measurements of 1-3%. The 14C/13C ratios of the unknowns were normalized to measurements of four identically prepared standards of known isotope concentration (Australian National University Sucrose) and converted to units of pmol TCC/mg protein [20].
RESULTS
TCC was rapidly absorbed by the epidermal cells. After 10 min, the intracellular TCC concentration reached 1.21 ± 0.27 nmol/mg protein and increased by 20 h incubation to a level of 2.04 ± 0.27 nmol/mg protein. LC-MS/MS analysis revealed that a small portion of the absorbed TCC was metabolized by SIK. After 24 h, 4.6 ± 1.6 pmol/mg protein 2′OH-TCC was found, along with lower concentrations of the other known mono-hydroxylated TCC metabolites, 3′OH-TCC (1.9 ± 0.7 pmol/mg protein) and 6-OH-TCC (0.4 ± 0.7 pmol/mg protein). The glucuronide of 2′OH-TCC was the second most abundant detected metabolite (Figure 1). Preincubation with 10 nM TCCD dramatically augmented metabolism of TCC in the keratinocytes, and the relative conversion (cellular metabolite concentration versus cellular TCC concentration) increased from ≈ 0.5 % to 15% (Figure 1). In line with previous findings that TCC is not an AhR agonist [21], this finding indicates that the CYP isoforms responsible for TCC metabolism (likely CYP1A1 and CYP1B1) are inducible by AhR agonists but are not well induced by TCC alone. After TCDD induction, unconjugated 2′OH-TCC remained the major metabolite with a concentration of 165 ± 28 pmol/mg protein, far exceeding the concentration of its conjugates. At 5.91 ± 28 pmol/mg protein, the recently described oxidative metabolite 3,4-dichloro-4’-hydroxy-carbanilide (DHC) [22] was also formed in substantial amounts. 2′-SO3-O-TCC levels were <1 pmol/mg protein, suggesting that 2′OH-TCC is a poor substrate for the major sulfotransferase (SULT2B1b) reported in epidermis [11] and expressed in SIK [16]. Additionally, formation of 6-OH-Gluc-TCC was detected, whereas no evidence for direct glucuronidation of TCC to the major urine N-glucuronide metabolites of TCC [4] was found.
FIGURE 1.
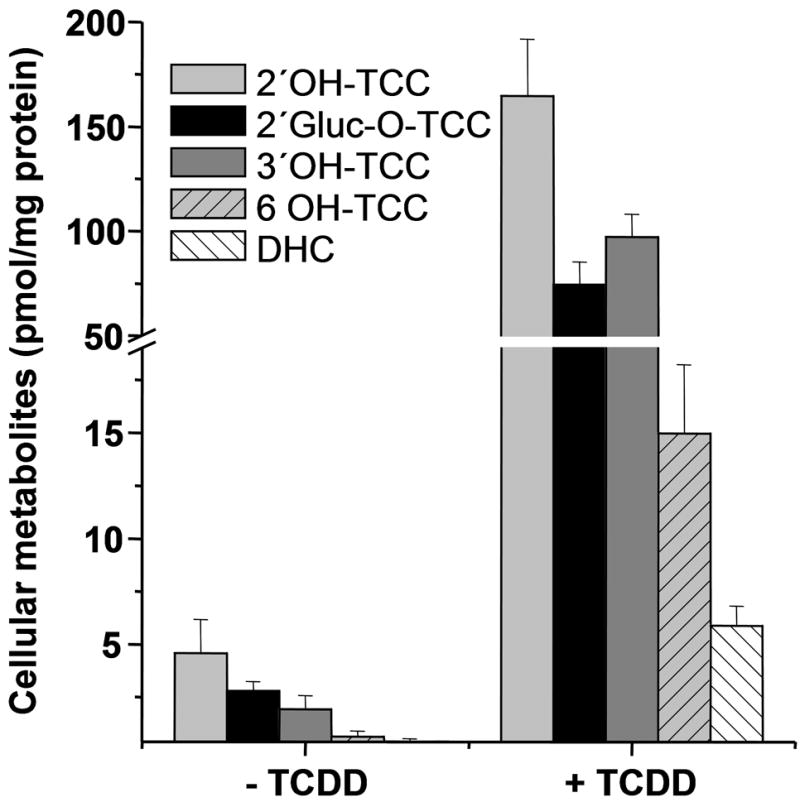
TCC biotransformation by human keratinocytes. Parallel SIK cultures pretreated one day ± 10 nM TCDD as indicated were then treated for 20 hr with 2 μM TCC. The metabolite levels detected were normalized to protein concentration (3.6 ± 0.4 mg/ml without TCDD; 4.1 ± 0.3 mg/ml after TCDD treatment). DHC, 3,4-dichloro-4’-hydroxy-carbanilide. Each experiment was conducted in triplicate, and values are presented as means ± SD.
Since TCC can be metabolized in epidermal keratinocytes, it is anticipated to be biotransformed during dermal absorption from personal care products. The observed metabolites are consistent with those detected in mammals with 2′OH-TCC as the major oxidative metabolite [9]. By contrast to the metabolite patterns in blood, bile and urine, the amount of unconjugated oxidative metabolites of TCC exceeded by far the level of conjugated (phase II) species. Nearly two fold more 2′OH-TCC was formed than 2′-Gluc-O-TCC with or without TCCD pretreatment (Figure 1). Because further oxidation of 2′OH-TCC can yield a reactive quinone-imine [22], we hypothesized that TCC metabolism in keratinocytes can lead to protein adduct formation. As shown in Figure 2, up to 23 ± 2 pmol TCC adducts/mg protein were detected after 24 h incubation in the presence of TCDD. As illustrated, protein adducts increased in a time dependent manner, but only very low adduct formation of about 1 pmol/mg protein, slightly above the background signal, was detected in the absence of TCDD, in line with the low metabolic conversion of TCC (Fig 1).
FIGURE 2.
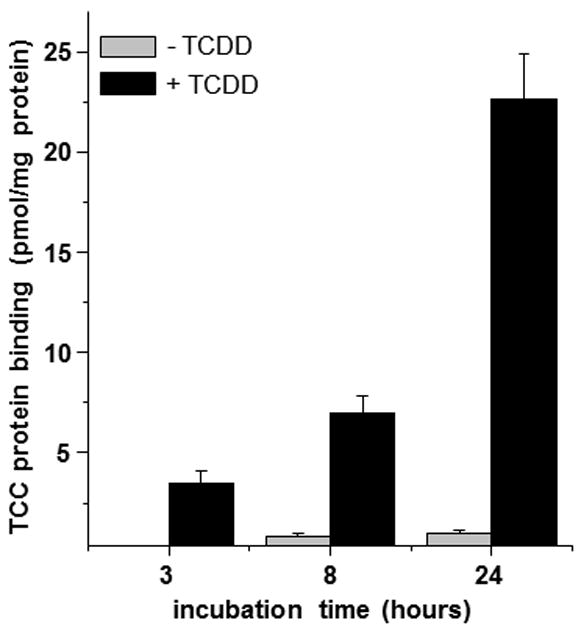
Covalent protein adduct formation by TCC (2 μM) in spontaneously immortalized keratinocytes (SIK) determined by accelerator mass spectrometry. A background value of 0.5 pmol/mg (0.5 h incubation without TCDD induction) has been subtracted. Each experiment was conducted in triplicate and values are presented as means ± SD.
DISCUSSION
This study demonstrates that TCC oxidative conversion leads to reactive intermediates that can bind covalently to protein, a phenomenon clearly manifest upon induction of metabolizing enzymes. It is noteworthy that the low level of TCC adducts produced a substantial 14C signal, which was only ≈ 20x fold higher than the natural 14C/C concentration of 1.2×10-12. Due to the high sensitivity of AMS, quantitative information about very low protein adducts of TCC can be obtained enabling investigation of their formation mechanistically. The relatively low protein adduct formation of TCC and high sensitivity of AMS are well suited for obtaining quantitative information on the distribution of protein adducts in vivo, especially if individual proteins are isolated to identify specific targets.
Human epidermal cells cultured in the Rheinwald-Green system provide a close approximation to natural epidermis [23]. The SIK line, a minimally deviated epidermal model, has been utilized previously to demonstrate the importance of CYP induction to produce toxic effects of agents that are themselves poor inducers [24]. The reactivity of a chemical to produce a complete antigen by covalently adducting carrier protein is the major factor in producing allergic skin sensitization [25]. Among the numerous compounds for which the importance of epidermal cytochrome P450 activity is known to activate non-reactive chemicals is the common rubber constituent and known sensitizer diphenylthiourea [26]. In the absence of P450 induction, the low level of TCC-protein adducts suggests only a low probability of adverse effects in keratinocytes. However, the substantial level of protein adducts with TCDD treatment raises the possibility of skin sensitization in special circumstances. P450 inducers such as Ah receptor agonists [27], for which TCDD in this work serves as a proxy, are widely encountered in tobacco smoke, pharmaceuticals, food constituents and other consumer products, as well as in environmental pollutants, and even from the skin microflora [28]. The unrecognized possibility that TCC, with wide human exposure to the integument, contributes to the burden on society of allergic contact dermatitis or other toxic effects, particularly in individuals exposed to ubiquitous Ah receptor agonists, merits further investigation. Moreover, in view of the ability of Langerhans cells to participate in activation of chemicals in the epidermis [29], present work could underestimate the risk posed by TCC.
Acknowledgments
We thank Qin Qin and Miranda Sarachine Falso for excellent technical assistance. This work was supported by NIH grants R01 ES002710, P42 ES004699, NCRR RR13461 and 8 P41 GM103483-14, NIOSH grant PHS OH07550 and the German Academic Exchange Service (NHS). BDH is a senior fellow of the American Asthma Society. Work was performed in part under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344.
References
- 1.Chalew TEA, Halden RU. Environmental exposure of aquatic and terrestrial biota to triclosan and triclocarban. J Am Water Res Assn. 2009;45:4–13. doi: 10.1111/j.1752-1688.2008.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morisseau C, Merzlikin O, Lin A, He G, Feng W, Padilla I, Denison MS, Pessah IN, Hammock BD. Toxicology in the fast lane: application of high-throughput bioassays to detect modulation of key enzymes and receptors. Environ Hlth Perspect. 2009;117:1867–1872. doi: 10.1289/ehp.0900834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schebb NH, Huby M, Morisseau C, Hwang SH, Hammock BD. Development of an online SPE-LC-MS-based assay using endogenous substrate for investigation of soluble epoxide hydrolase (sEH) inhibitors. Anal Bioanal Chem. 2011;400:1359–1366. doi: 10.1007/s00216-011-4861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schebb NH, Inceoglu B, Ahn KC, Morisseau C, Gee SJ, Hammock BD. Investigation of human exposure to triclocarban after showering and preliminary evaluation of its biological effects. Environ Sci Technol. 2011;45:3109–3015. doi: 10.1021/es103650m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inceoglu B, Wagner K, Schebb NH, Morisseau C, Jinks S, Ulu A, Hegedus C, Rose T, Brosnan R, Hammock BD. Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP. Proc Natl Acad Sci USA. 2011;108:5093–5097. doi: 10.1073/pnas.1101073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Ahn KC, Gee NA, Ahmed MI, Duleba AJ, Zhao L, Gee SJ, Hammock BD, Lasley BL. Triclocarban enhances testosterone action: a new type of endocrine disruptor? Endocrino. 2008;149:1173–1179. doi: 10.1210/en.2007-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.North-Root H, Corbin N, Demetrulias JL. Skin deposition and penetration of triclocarban. Dermatol. 1985;6:141–152. [Google Scholar]
- 8.Scharpf LJ, Hill ID, Maibach HI. Percutaneous penetration and disposition of triclocarban in man: body showering. Arch Environ Health. 1975;30:7–14. doi: 10.1080/00039896.1975.10666624. [DOI] [PubMed] [Google Scholar]
- 9.Birch CG, Hiles RA, Eichhold TH, Jeffcoat AR, Handy RW, Hill JM, Willis SL, Hess TR, Wall ME. Biotransformation products of 3,4,4’-trichlorocarbanilide in rat, monkey, and man. Drug Metab Dispos. 1978;6:169–176. [PubMed] [Google Scholar]
- 10.Schebb NH, Franze B, Maul R, Ranganathan A, Hammock BD. In vitro glucuronidation of the antibacterial triclocarban and its oxidative metabolites. Drug Metab Dispos. 2012;40:25–31. doi: 10.1124/dmd.111.042283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luu-The V, Duche D, Ferraris C, Meunier JR, Leclaire J, Labrie F. Expression profiles of phases 1 and 2 metabolizing enzymes in human skin and the reconstructed skin models Episkin and full thickness model from Episkin. J Steroid Biochem Mol Biol. 2009;116:178–186. doi: 10.1016/j.jsbmb.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Hu T, Khambatta ZS, Hayden PJ, Bolmarcich J, Binder RL, Robinson MK, Carr GJ, Tiesman JP, Jarrold BB, Osborne R, Reichling TD, Nemeth ST, Aardema MJ. Xenobiotic metabolism gene expression in the EpiDerm™ in vitro 3D human epidermis model compared to human skin. Toxicol in Vitro. 2010;24:1450–1463. doi: 10.1016/j.tiv.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Kwok ESC, Buchholz BA, Vogel JS, Turtletaub KW, Eastmond DA. Dose-dependent binding of ortho-phenylphenol to protein but not DNA in the urinary bladder of male F344 rats. Toxicol Appl Pharmacol. 1999;159:18–24. doi: 10.1006/taap.1999.8722. [DOI] [PubMed] [Google Scholar]
- 14.Brown K, Dingley KH, Turtletaub KW. Accelerator mass spectrometry for biomedical research. Meth Enzymol. 2005;402:423–443. doi: 10.1016/S0076-6879(05)02014-8. [DOI] [PubMed] [Google Scholar]
- 15.Buchholz BA, Haack KW, Sporty JL, Buckpitt AR, Morin D. Free flow electrophoresis separation and AMS quantitation of C-naphthalene-protein adducts. Nucl Instrum Methods Phys Res B. 2010;268:1324–1327. doi: 10.1016/j.nimb.2009.10.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rea MA, Zhou L, Qin Q, Barrandon Y, Easley K, Gungner S, Phillips MA, Holland W, Gumerlock PH, Rocke DM, Rice RH. Spontaneous immortalization of human epidermal cells with naturally elevated telomerase. J Invest Dermatol. 2006;126:2507–2515. doi: 10.1038/sj.jid.5700424. [DOI] [PubMed] [Google Scholar]
- 17.Schebb NH, Flores I, Kurobe T, Franze B, Ranganathan A, Hammock BD, Teh SJ. Bioconcentration, metabolism and excretion of triclocarban in larval Qurt medaka (Oryzias latipes) Aquat Toxicol. 2011;105:448–454. doi: 10.1016/j.aquatox.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ognibene TJ, Bench G, Vogel JS, Peaslee GF, Murov S. A high-throughput method for the conversion of CO2 obtained from biochemical samples to graphite in septa-sealed vials for quantification of 14C via accelerator mass spectrometry. Analyt Chem. 2003;75:2192–2196. doi: 10.1021/ac026334j. [DOI] [PubMed] [Google Scholar]
- 19.Ognibene TJ, Bench G, Brown TA, Peaslee GF, Vogel JS. A new accelerator mass spectrometry system for C-14-quantification of biochemical samples. Int J Mass Spectrom. 2002;218:255–264. [Google Scholar]
- 20.Vogel JS, Love AH. Quantitating isotopic molecular labels with accelerator mass spectrometry. Meth Enzymol. 2005;402:402–422. doi: 10.1016/S0076-6879(05)02013-6. [DOI] [PubMed] [Google Scholar]
- 21.Ahn KC, Zhao B, Chen J, Cherednichenko G, Sanmarti E, Denison MS, Lasley B, Pessah IN, Kültz D, Chang DP, Gee SJ, Hammock BD. In vitro biological activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: receptor-based bioassay screens. Environ Hlth Perspect. 2008;116:1203–1210. doi: 10.1289/ehp.11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumann A, Lohmann W, Rose T, Ahn KC, Hammock BD, Karst U, Schebb NH. Electrochemistry-mass spectrometry unveils the formation of reactive triclocarban metabolites. Drug Metab Dispos. 2010;38:2130–2138. doi: 10.1124/dmd.110.034546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green H. The keratinocyte as differentiated cell type. Harvey Lect. 1979;74:101–138. [PubMed] [Google Scholar]
- 24.Walsh AA, deGraffenried LA, Rice RH. 2,3,7,8-Tetrachlorodibeno-p-dioxin sensitization of cultured human epidermal cells to carcinogenic heterocyclic amine toxicity. Carcinogenesis. 1995;16:2187–2191. doi: 10.1093/carcin/16.9.2187. [DOI] [PubMed] [Google Scholar]
- 25.Roberts DW, Aptula AO. Determinants of skin sensitisation potential. J Appl Toxicol. 2008;28:377–387. doi: 10.1002/jat.1289. [DOI] [PubMed] [Google Scholar]
- 26.Samuelsson K, Bergstrom MA, Jonsson CA, Westman G, Karlberg A-T. Diphenylthiourea, a common rubber chemical, is bioactivated to potent skin sensitizers. Chem Res Toxicol. 2011;24:35–44. doi: 10.1021/tx100241z. [DOI] [PubMed] [Google Scholar]
- 27.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Ann Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 28.Gaitanis G, Velegraki A, Magiatis P, Pappas P, Bassukas ID. Could Malassezia yeasts be implicated in skin carcinogenesis through the production of aryl-hydrocarbon receptor ligands? Med Hypothese. 2011;77:47–51. doi: 10.1016/j.mehy.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Modi BG, Neustadter J, Binda E, Lewis J, Filler RB, Roberts SJ, Kwong BY, Reddy S, Overton JD, Galan A, Tigelaar R, Cai L, Fu P, Shlomchik M, Kaplan DH, Hayday A, Girardi M. Langerhans cells facilitate epithelial DNA damage and squamous cell carcinoma. Science. 2012;335:104–108. doi: 10.1126/science.1211600. [DOI] [PMC free article] [PubMed] [Google Scholar]


