Abstract
Melanin-concentrating hormone (MCH) and neuropeptide Y (NPY) are orexigenic peptides found in hypothalamic neurons that project throughout the forebrain and hindbrain. The effects of fourth ventricle (4V) infusions of NPY (5 μg) and MCH (5 μg) on licking for water, 4 mM saccharin, and sucrose (0.1 and 1.0 M) solutions were compared to identify the contributions of each peptide to hindbrain-stimulated feeding. NPY increased mean meal size only for the sucrose solutions, suggesting that caloric feedback or taste quality is pertinent to the orexigenic effect; MCH infusions under identical testing conditions failed to produce increases for any tastant. A second experiment also observed no intake or licking effects after MCH doses up to 15 μg, supporting the conclusion that MCH-induced orexigenic responses require forebrain stimulation. A third experiment compared the 4V NPY results with those obtained after NPY infusions (5 μg) into the third ventricle (3V). In contrast to the effects observed after the 3V NPY injections and previously reported forebrain intracerebroventricular (ICV) NPY infusion studies, 4V NPY failed to increase meal frequency for any taste solution or ingestion rate in the early phases of the sucrose meals. Overall, 4V NPY responses were limited to intrameal behavioral processes, whereas forebrain ICV NPY stimulation elicited both consummatory and appetitive responses. The dissociation between MCH and NPY effects observed for 4V injections is consistent with reports that forebrain ICV injections of MCH and NPY produced nearly dichotomous effects on the pattern of licking microstructure, and, collectively, the results indicate that the two peptides have separate sites of feeding action in the brain.
Keywords: taste, feeding, microstructure, intake
Melanin-Concentrating Hormone (MCH) and neuropeptide Y (NPY) are orexigenic neuropeptides implicated in hypothalamic signaling cascades that modulate ingestive behavior and metabolism (62). Intracerebroventricular (ICV) and hypothalamic arcuate (ARC), dorsomedial, or paraventricular nucleus injections of either peptide have been shown to reliably increase food and/or water intake (1, 7, 9, 17–19, 31, 39, 48–51, 54, 55, 58, 59, 61). MCH-synthesizing neurons are limited to lateral hypothalamic and zona incerta regions (perifornical and juxtacapsular zones), although they project extensively throughout the brain (15, 37, 45, 60, 79, 82, 83, 85) and are considered potentially important in the downstream mediation of hypothalamic influences on feeding and metabolism (19, 59, 62, 66, 85). MCH neurons are sensitive to direct input by the NPY and proopiomelanocortin synthesizing neurons of the ARC, which, in turn, are responsive to ghrelin and leptin, hormones that influence metabolism, food intake, and body weight (40, 56, 66). Unlike MCH, extrahypothalamic NPY-synthesizing neurons have also been identified in caudal brain stem sites associated with feeding and autonomic processes (63, 84). Numerous brain sites exhibit dense staining for NPY receptors implicated in feeding, including Y1 receptor mRNA in the dorsal, lateral, and medial regions of the hindbrain nucleus of the solitary tract (NST) (16, 43, 46, 80). It is, therefore, not surprising that brain stem delivery of NPY and related NPY receptor compounds stimulate food intake with an efficacy equal to that observed for comparable doses used in hypothalamic or forebrain ICV NPY infusion studies (20, 21).
To date, a majority of the research concerning central neuropeptidergic feeding influences has been limited to analyses of the consumption of relatively vapid animal chow across large time frames (e.g., 2–4 h), parameters that neither distinguish treatment effects across taste and caloric dimensions, nor explore potential treatment influences on individual meals. Licking microstructure analyses provide advantages for the study of ingestive behavior: detailed analyses of the temporal dynamics of spout licking can be performed for a range of tastants that may be systematically varied in chemosensory and/or caloric properties across tests. Such evaluations also permit assessment of the relative contributions of orosensory and inhibitory postingestive feedback signals that conjointly influence the size of a given meal (7, 9, 24, 25, 73).
Recently, we characterized the effects of NPY and MCH delivery to the third ventricle (3V) on intake and licking microstructure (described below) for a range of taste solutions (water, saccharin, and sucrose solutions) that varied in palatability and caloric content (7, 9). If the MCH system at least partially mediates the influences of NPY, one may hypothesize that the patterns of licking responses to these two hyperphagic compounds would share some similarities, but this does not appear to be the case. While 3V or lateral ventricle (LV) applications of either NPY or MCH increase intake for many tastants, the behavioral underpinnings of these effects appear distinct. First, the intake increases produced by each peptide varies by the type of solution offered. NPY has been reported to increase meal size for caloric solutions and solid foods, but not for water or preferred noncaloric saccharin solutions (7, 21, 48); MCH, on the other hand, increases consumption of water and a variety of saccharin, sucrose, and ethanol solutions (19, 31, 33, 47, 61). Second, coadministration of small ICV doses of forebrain NPY and MCH produced no synergistic effect on chow intake (58). Third, whereas NPY treatments increased both meal size and meal frequency (30, 48, 51), MCH treatment failed to affect meal frequency in two of three studies (7, 47, 53). In addition, whereas intake was increased for the same type of solution (sucrose), the effects of each peptide on the licking patterns were dramatically different (7, 9). The analysis of 3V MCH and NPY injection responses indicated that NPY increased the number of licking bursts and it prolonged consumption time for sucrose but not water or saccharin solutions, whereas MCH increased intake for water and sucrose solutions through increases in early meal ingestion rate and mean burst lick size, with no effect on the number of licking bursts or the meal duration. Overall, the results suggested that NPY produced a diminution of inhibitory gut feedback, while MCH enhanced measures more commonly associated with gustatory evaluation (7, 9). Finally, while fourth ventricle (4V) infusions of NPY robustly increase chow intake, the only study to date to evaluate 4V infusions of MCH reported no increase in either chow or water consumption (85).
In the present study, the aims were twofold. First, we evaluated whether the behavioral processes underlying the previously documented orexigenic effects of forebrain MCH and NPY infusions were qualitatively and/or quantitatively different when MCH and NPY infusions were restricted to the hindbrain. We explicitly compared the 4V NPY results with the effects obtained after comparable 4V MCH treatment using testing conditions that were not only identical between drug groups, but also closely matched to those used in our laboratory's previously published forebrain ICV NPY and MCH studies (7, 9). Although 4V MCH infusions were recently reported to produce no effect on 1- to 2-h chow or water intake (85), a microstructural analysis of licking responses for a variety of tastants may be ideally suited to unearth potentially more subtle effects of hindbrain MCH stimulation on feeding within a meal. Second, we further examined the differences between responses to forebrain vs. hindbrain ICV NPY infusions through a comparison of the 4V NPY infusion responses to sucrose solutions with those obtained after 3V NPY infusion under otherwise identical testing conditions. Differences in the results obtained can be used to clarify the relative contributions of forebrain and hindbrain NPY-sensitive sites to ICV NPY-induced hyperphagia for taste solutions.
METHODS
Animals
Adult albino male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 367–423 g on the first day of the experiment were used. Rats were maintained individually in plastic tubs (48 × 25 × 15 cm) with wire lids on a 12:12-h light-dark schedule in a temperature-controlled room. Food (Purina rat chow 5001, Lab Diets, St. Louis, MO) and tap water were available ad libitum in the home cage, except where noted below. Rats were tested at the same time each day, between 6 and 8 h after lights on (0700), in a separate test cage.
Surgery
Rats were anesthetized (intraperitoneally) with a mixture of keta-mine HCl (66 mg/kg) and xylazine HCl (6 mg/kg). A 22-g guide cannula (Plastics One, Roanoke, VA) was stereotaxically implanted into either the 3V (coordinates relative to bregma: anteroposterior: –2.3 mm, mediolateral: 0 mm, dorsoventral: –7.5 mm from brain surface) or 4V (coordinates relative to lambda: anteroposterior: –2.5 mm, mediolateral: 0 mm, dorsoventral: –6.8 mm from brain surface), and fastened with dental acrylic and skull screws. The 28-g injection cannula extended 1 mm below the tip of the guide cannula, and an obturator cut flush to the guide tip was maintained in the guide cannula at all other times. After surgical recovery, correct placement for 3V cannulas was confirmed in 14 of 16 rats, as indicated by a minimum of 5-ml water consumption within 30 min of a 50 ng/5 μl cannula injection of angiotensin II (7, 9). Four rats in the 3V group died before experiment completion, and one did not exhibit habituation. Correct placements for rats fitted with 4V cannulas were confirmed by an increase in blood glucose after a cannula infusion of the antimetabolite, 5-thio-d-glucose (120 μg/2 μl; 2 μl/min; see Ref. 57). Tail blood was collected every 10 min beginning 20 min before the 5-thio-d-glucose injection, using a commercially available glucometer and test strips. Rats that did not exhibit at least a 50% increase in blood glucose within 30 min of injection were removed from the study (n = 17/47). After behavioral experiments were concluded, cannula placements were again confirmed. India ink (5 μl via 3V and 2 μl via 4V cannulas) was injected immediately following a lethal overdose of pentobarbital sodium (100 mg/kg). Rats were then transcardially perfused with isotonic saline followed by 10% formalin. The brain was removed, bisected midsaggitally, and inspected for ventricular ink perfusion. Data for rats with no ink perfusion of either the 3V or 4V were discarded (n = 2/39). Ink was never observed to have reached the 3V or LV after a 4V infusion. All procedures were approved by the Amherst College Institutional Animal Care and Use Committee.
Apparatus
Rats were taken from their home cages and tested in individual plastic tubs (48 × 25 × 15 cm). A drinking spout (3 mm orifice; Girton, Millville, PA) was introduced to the test chamber with the spout orifice positioned 4 cm from the floor and 0–1 mm behind a slit (8 × 28 mm) in a metal plate attached to the front of the cage. A lickometer (MS-108, DiLog Instruments, Tallahassee, FL) and PC computer were used to record licking: tongue contacts with the spout completed a circuit, which allowed the computer to record the time of each lick with 1-ms resolution. Files for each test session for each rat were saved for offline analysis.
Procedures
Experiment 1: Effects of 4V NPY or MCH on licking for a range of taste solutions
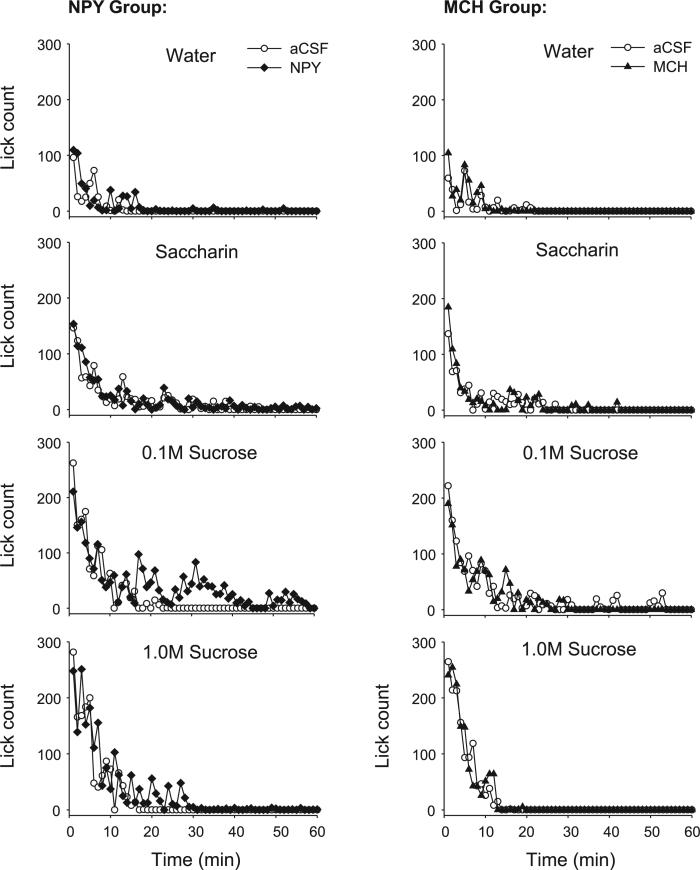
Rats were randomly divided into two groups: one scheduled to receive NPY injections and one scheduled to receive MCH injections. Groups were tested simultaneously under otherwise identical conditions. Before testing, rats were habituated in the test cage daily where they were free to ingest 0.5 M sucrose for 60 min. Habituation training continued until session intakes stabilized and exceeded 5 ml per session (2–5 sessions). For the experiment, responses to either 4V NPY or MCH paired with the vehicle control, artificial cerebrospinal fluid (aCSF; Harvard Apparatus, Holliston, MA), were tested at two concentrations of sucrose (0.1 and 1.0 M), one concentration of sodium saccharin (4 mM), and distilled water. These solutions produce systematic differences in gustatory and intake responses. Water and saccharin, while noncaloric, vary in palatability. Similarly, 0.1 and 1.0 M sucrose solutions often produce distinct licking microstructure profiles, even though these differences can result in the same level of intake consumption. The 1.0 M sucrose solution generates maximal gustatory responses and is avidly consumed, resulting in a high initial rate of ingestion, larger mean lick burst sizes, and a steeper slope of decline in ingestion rate, while 0.1 M sucrose solutions generate less vigorous responses, yielding slower initial ingestion rates, smaller bursts, and a flatter slope of decline in ingestion rate (see Ref. 24 for further discussion). Recently, our laboratory also determined that 4 mM saccharin solutions produced gustatory responses stronger than those for 0.03 M sucrose, but less than those for 0.3 M sucrose, as measured by initial lick rate and mean burst size responses (7). Therefore, we anticipated that a 4 mM saccharin solution would produce gustatory responses similar in palatability to 0.1 M sucrose, permitting a comparison of two solutions of comparable gustatory appeal but different caloric content.
Rats were exposed to the same tastant for 90 min over 5 consecutive test days, with 4V injections on days 3 and 5 of each concentration block. Two nontest days intervened each of the four 5-day concentration test blocks. The four concentration blocks and drug order with those blocks were counterbalanced with a Latin square. On drug test days, rats in the NPY group received a 5 μg/2 μl cannula injection (1 μl/min) of either human NPY (American Peptide, Sunnyvale, CA; 1.17 nM) or the vehicle, aCSF, 15 min before intake testing. Rats in the MCH group received either rat MCH (5 μg/2 μl; American Peptide, Sunnyvale, CA; 2.1 nM) or aCSF under conditions identical to the NPY group. To permit comparisons with our forebrain ICV data, these doses were identical to those used in our laboratory's 3V infusion studies (7, 9) and by other investigators for LV, 3V, and 4V infusions (see Table 1). The 2-min infusions were made using a 10-μl Hamilton syringe in a programmable syringe pump (KD Scientific, model no. 100).
Table 1.
ICV injection studies evaluating an orexigenic effect of NPY or MCH on nutritive fluid consumption
| Study (Ref. No.) | Injection Site | NPY Dose, nM | MCH Dose, nM | Solutions Tested |
|---|---|---|---|---|
| Ammar et al. (4) | LV | 2* | 1.0 M sucrose (bottle) | |
| Ammar et al. (3) | LV | 2* | 1.0 M sucrose (bottle) | |
| Badia-Elder et al. (5) | LV | 2* | 0.07 M sucrose; 0.17 M ethanol | |
| Badia-Elder et al. (6) | LV | 1*, 2*, 5* | 0.15 M sucrose; 0.17 M ethanol | |
| Lynch et al. (49) | LV | 1* | 0.3, 0.6, 1.5 M sucrose | |
| Lynch et al. (50) | LV | 1* | Sweetened condensed milk | |
| Sederholm et al. (67) | LV | 2* | 1.0 M sucrose | |
| Slawecki et al. (71) | LV | 0.5, 1*, 3 | 0.03, 0.06, 0.15, 0.3 M sucrose; 0.04, 0.11, 0.22 M ethanol | |
| Sakamaki et al. (61) | LV | 6* | 0.015, 0.03, 0.06, 0.12, 0.26 M glucose and sucrose | |
| Baird et al. (7) | 3V | 1* | 0.03, 0.3, 1.0 M sucrose | |
| Katner et al. (42) | 3V | 0.5, 1, 3 | 0.015, 0.06 M sucrose; 0.22 M ethanol | |
| Seeley et al. (69) | 3V | 2* | 0.1 M sucrose (bottle) | |
| Torregrossa et al. (78) | 3V | 0.5*, 1*, 2* | 0.8 M sucrose | |
| Baird et al. (9) | 3V | 2* | 0.1, 1.0 M sucrose | |
| Benoit et al. (12) | 3V | 2* | 0.1 M sucrose | |
| Duncan et al. (31) | 3V | 0.4, 2, 4* | Saccharin/ethanol (4 mM/0.22 M); sucrose/quinine (0.5 M/0.35 mM) | |
| Corp et al. (21) | 4V | 0.2, 0.4, 1*, 2* | Chow† | |
| Corp et al. (20) | 4V | 0.02, 0.04, 0.2, 0.5*, 1* | Chow† | |
| Xu et al. (81) | 4V | 1* | Chow† | |
| Zheng et al. (85) | 4V | 4 | Chow† |
NPY, neuropeptide Y; MCH, melanin-concentrating hormone; LV, lateral ventricle; 3V, third ventricle; 4V, fourth ventricle. Intracerebroventricular doses ≥1 nM are rounded to nearest integer.
Dose at which a significant increase in intake was observed.
No prior studies have evaluated the effects of 4V NPY or MCH administration on nutritive fluid consumption. (Bottle) refers to those conditions within these studies where the solutions were ingested from a bottle spout rather than via intraoral delivery.
Experiment 2: 4V MCH dose-response analysis
To further evaluate the lack of MCH effects observed in experiment 1, a MCH dose-response curve was evaluated for 0.1 M sucrose consumption, the tastant for which 4V NPY effects were most robust. Rats were prepared and habituated as in experiment 1. For the experiment, rats received 0.1 M sucrose for six consecutive daily trials under conditions identical to experiment 1. On days 2, 4, and 6, rats received a 4V injection of either vehicle (aCSF), or 5 μg (2.1 nM) or 15 μg (6.3 nM) of MCH in counterbalanced order before testing, using procedures identical to experiment 1. The 6 nM dose corresponded to the strongest concentration that could be dissolved in vehicle and to the largest reported MCH concentration tested for a forebrain ICV injection (61).
Experiment 3: Comparison of 3V and 4V NPY infusion effects on sucrose consumption
To evaluate the relative contributions of hind-brain vs. forebrain ICV NPY infusions on licking for taste solutions, rats in this study were fitted with 3V cannulas. Rats were habituated as in experiment 1. The study design was identical to experiment 1 with the exception that only 0.1 and 1.0 M sucrose solutions were tested, and that the dose of NPY was delivered as 5 μg/5 μl (rather than 5 μg/2 μl) into the 3V. The results were then compared with the subset of results obtained for the 0.1 and 1.0 M sucrose solutions in experiment 1 after 4V NPY infusion. This study was also designed to closely replicate our laboratory's previous published (7) results on the effects of 3V NPY (5 μg/5 μl) infusions on sucrose solution consumption. That prior study tested responses for 0.03, 0.3, and 1.0 M sucrose solutions, while this study used 0.1 and 1.0 M sucrose solutions.
Data Analysis
Data were analyzed according to previously established analysis parameters, as follows (see Refs. 7, 9, and 74 for details). Meal analyses were limited to the first meal in the test session.
Meal size (ml) was calculated as the number of licks in the meal (first lick of the first burst to last lick of the last burst; Refs. 10, 75). The end of the meal was defined by a pause in licking greater than or equal to 10 min (74). Meal duration (min) was defined as the session time of the last lick in the meal minus the session time of the first lick in the meal. Average ingestion rate (licks/min) was calculated as the number of licks in the meal divided by meal duration. Lick volume (μl) was calculated by dividing the difference between pre- and posttest weights of the spout bottle by the total number of licks in the session.
The temporal distribution of licking was analyzed using a variety of custom-made programs (7–9). A licking burst was defined as two or more consecutive licks with no interlick interval (ILI) exceeding 1 s. Thus, pauses >1 s determined burst termination (75). Burst duration (s) was calculated by subtracting the session time of the first lick in the burst from the time of the last lick in that burst. Mean burst size (lick count) was calculated as the cumulative number of licks in all bursts in the meal divided by the number of bursts in the meal. To minimize artifact registrations due to nonlingual spout contacts, meal onset was defined as the first lick of the first burst containing at least three licks. Latency (s) was defined as the time between placement of the rat into the test cage and meal onset. Initial lick rate was the number of licks in the first minute of the meal.
ILIs were analyzed in several ways. The average within-burst ILI (ms) was determined by averaging all ILIs < 1 s. Because >95% of all ILIs in a meal are <250 ms (in rats) and are normally distributed below this cutoff (22, 74), the average duration of ILIs <250 ms was also determined. We also evaluated the relative proportion of ILIs < 250 ms as a percentage of all ILIs within bursts (ILIs < 1 s). Any significant variation in this proportion would also indicate a reciprocal variation in the proportion of ILIs ranging from 250 to 999 ms.
Pauses (s) were defined as ILIs ≥ 1 s. The mean pause duration (s) was defined as the meal duration minus the cumulative duration of bursts in the meal, divided by the number of meal pauses (number of bursts minus one). Percent pause duration (%) for the meal was the cumulative time of all pauses divided by the meal duration, multiplied by 100.
To measure licking progress over the course of the meal, the number of licks for each minute of the intake test was analyzed. Meals were also divided temporally into thirds, and the mean lick rate (licks/s converted to licks/min) and the number of bursts were calculated for each meal third (7, 9, 10, 74, 75).
Responses for each measure in experiments 1 and 3 across the 2 drug days (MCH/aCSF) for each of the two (experiment 3) or four (experiment 1) tastants were compared with two-way (drug × tastant) or three-way (drug × tastant × minute) repeated-measures ANOVAs using SPSS 11.5 software. Least significant difference pairwise comparisons and paired T-tests were used to explore significant main effects and interactions. A mixed-factors two-way ANOVA was also used to ensure comparable baseline conditions between experimental groups (group aCSF condition × tastant) in experiment 1. For experiment 2, results for the three drug levels (vehicle, 2 nM, and 6 nM) were analyzed using a one-way repeated-measures ANOVA. Responses in experiment 3 included the subset of responses for the matched sucrose solutions (0.1 and 1.0 M) in the 4V NPY group tested in experiment 1, using a three-way mixed-factors ANOVA (ICV location × drug × tastant). Difference scores (NPY minus aCSF) for each rat in the 3V and 4V groups were also compared using t-tests separately for each sucrose concentration to compare the effect magnitudes of NPY-induced response increases. The criterion for a statistically significant difference was P ≤ 0.05.
RESULTS
Experiments 1 and 2: 4V NPY and MCH Effects on Licking for a Range of Taste Solutions
Whole meal measures
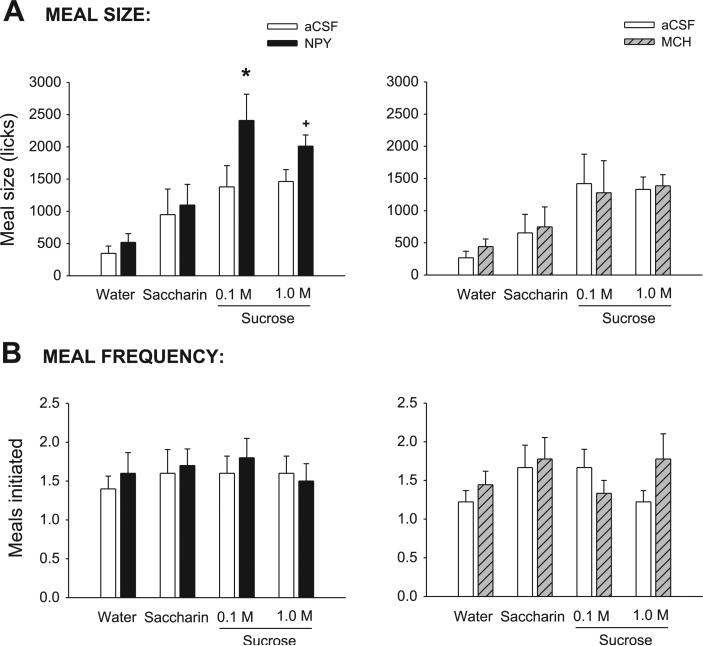
Consistent with previous studies (7, 9, 25, 74), meal size for both drug groups varied across tastants: meal size was smallest for water and largest for the two sucrose concentrations, as supported by a significant main effect for the tastant term in the ANOVA (Table 2). Meal size for any given tastant under control conditions was comparable across groups (Fig. 1A), as indicated by a nonsignificant between-subjects effect of group [F(1,17) = 0.13, P = 0.72], and the lack of a tastant × group interaction [F(3,51) = 0.23, P = 0.87]. For each drug group, there was also no significant difference in meal size between the vehicle condition and the preceding noninjection test day (both P > 0.09). As expected, NPY significantly increased meal size. The mean volume intakes under NPY were significantly greater than αCSF conditions [F(1, 8) 13.07, P = 0.007]. Volume intakes after NPY were as follows: water (32 ml), saccharin (7.0 ml), 0.1 M sucrose (14.8 ml), and 1.0 M sucrose (12.3 ml). NPY also increases meal size as measured by lick count, as indicated by a significant main effect of drug (Table 2); however, this result was only observed for the 0.1 M sucrose solution, supported by a statistically significant interaction term (Table 2) and pairwise comparisons (Fig. 1A). NPY nearly doubled meal size for the 0.1 M sucrose solution, with a smaller, marginally significant 38% increase for the 1.0 M sucrose solution. In contrast, MCH treatment failed to increase meal size by any discernable amount, as clearly indicated in Fig. 1A (right) and by nonsignificant drug and interaction terms (Table 2). Mean volume intakes after MCH were not significantly different from aCSF conditions [F(1,9) = 0.24, P = 0.63]. Volume intakes after MCH were as follows: water (2.7 ml), saccharin (4.3 ml), 0.1 M sucrose (7.2 ml), and 1.0 M sucrose (9.2 ml). This result was replicated in experiment 2. Here, neither the 2 nM nor the 6 nM MCH concentration produced a significant increase in meal size (Table 3). In addition, in experiment 2, there were no significant differences across MCH concentrations for any of the licking microstructure measures reported below (see Table 3).
Table 2.
ANOVA values for licking measures across tastant concentration and drug conditions
| 4V NPY |
4V MCH |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug |
Tastant Concn |
Interaction |
Drug |
Tastant Concn |
Interaction |
|||||||
| Measure | F(1,8) | P | F(3,24) | P | F(3,24) | P | F(1,9) | P | F(3,27) | P | F(3,27) | P |
| Meal size | 19.53 | 0.001* | 11.69 | 0.001* | 3.21 | 0.04* | 0.32 | 0.59 | 5.76 | 0.004* | 0.61 | 0.62 |
| Meals initiated | 1.46 | 0.26 | 2.53 | 0.08 | 0.91 | 0.45 | 0.38 | 0.56 | 0.24 | 0.57 | 0.25 | 0.86 |
| Meal duration | 7.60 | 0.03* | 3.53 | 0.03* | 2.55 | 0.08 | 0.29 | 0.60 | 2.55 | 0.07 | 1.59 | 0.22 |
| Average ingestion rate | 0.56 | 0.48 | 6.31 | 0.003* | 0.91 | 0.45 | 0.21 | 0.66 | 3.21 | 0.04* | 0.01 | 0.99 |
| Lick volume | 8.34 | 0.02* | 3.08 | 0.05* | 3.76 | 0.02* | 0.76 | 0.41 | 1.24 | 0.32 | 0.78 | 0.52 |
| First-minute lick count | 0.93 | 0.36 | 12.11 | 0.001* | 1.25 | 0.31 | 0.33 | 0.58 | 8.32 | 0.001* | 1.00 | 0.41 |
| ILIs < 1 s, ms | 0.11 | 0.75 | 10.10 | 0.001* | 0.46 | 0.71 | 0.20 | 0.66 | 3.22 | 0.04* | 1.75 | 0.18 |
| Burst count | 8.42 | 0.02* | 3.15 | 0.04* | 2.98 | 0.05* | 0.82 | 0.39 | 2.02 | 0.13 | 2.79 | 0.08 |
| Mean burst size | 2.26 | 0.17 | 2.99 | 0.05* | 1.98 | 0.14 | 0.09 | 0.77 | 5.31 | 0.01* | 0.90 | 0.45 |
ILI, interlick interval.
P met the criterion for statistical significance, which was set at P ≤ 0.05.
Fig. 1.
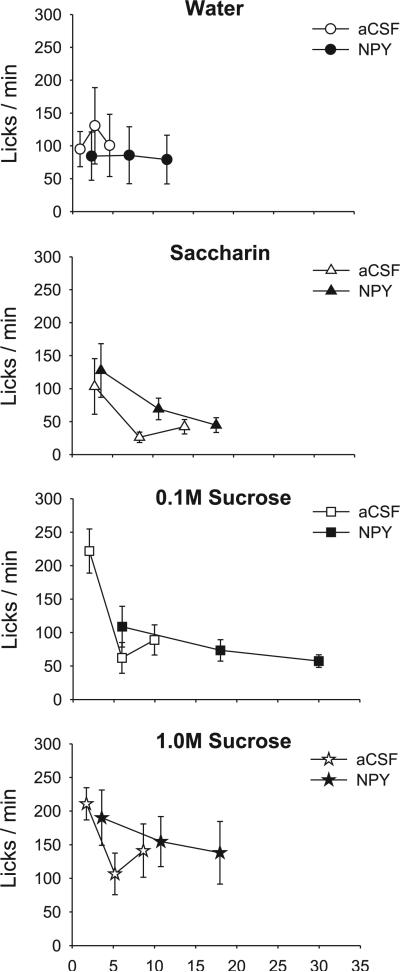
A, left: mean (+SE) meal size (licks) values for artificial cerebrospinal fluid- (aCSF; open bars) and neuropeptide Y-treated (NPY; solid bars) fourth ventricle (4V) cannula-fitted rats (n = 9), ingesting water, saccharin, and two concentrations of sucrose. Right: same results for aCSF- (open bars) and melanin-concentrating hormone (MCH; hatched bars)-treated rats (n = 10). B, left: mean number of meals initiated for the test solutions in 4V cannula-fitted rats treated with aCSF (open bars) or NPY (solid bars). Right: same results for aCSF-(open bars) and MCH-treated (hatched bars) rats. *P < 0.05; +P < 0.07.
Table 3.
Licking measures across MCH dose conditions (0.1 M sucrose)
| ANOVA |
|||||
|---|---|---|---|---|---|
| Measure | 0 nM aCSF | 2 nM MCH | 6 nM MCH | F(2,16) | P |
| Meal size, no. licks | 1,496±224 | 1,672±249 | 1,826±345 | 0.80 | 0.47 |
| Meal size, ml | 8.51±1.51 | 9.68±1.55 | 10.74±1.84 | 1.31 | 0.30 |
| Meal duration, min | 16.74±1.93 | 13.30±1.34 | 16.84±3.62 | 0.51 | 0.61 |
| Average ingestion rate, licks/min | 96.36±17.04 | 127.08±11.35 | 152.15±31.49 | 1.71 | 0.21 |
| Lick volume, μl/lick | 5.72±0.51 | 5.68±0.21 | 6.18±0.41 | 0.48 | 0.63 |
| First-minute lick count | 157.00±33.03 | 195.44±39.78 | 201.89±26.99 | 2.82 | 0.09 |
| ILIs < 1 s, ms | 149.82±1.97 | 152.45±2.06 | 151.81±2.92 | 0.32 | 0.73 |
| Burst count | 21.56±2.73 | 28.33±4.00 | 28.67±5.62 | 0.70 | 0.51 |
| Mean burst size, licks/burst | 71.26±7.37 | 66.96±9.46 | 71.37±10.52 | 0.09 | 0.91 |
Values are means ± SE. aCSF, artificial cerebrospinal fluid (vehicle).
Contrary to previous findings using forebrain NPY delivery (see also experiment 3), 4V NPY administration in this study failed to produce any increase in the number of meals initiated (Table 2), nor did meal initiation vary across tastant conditions (Table 2; Fig. 1B). Similarly, 4V MCH treatment produced no distinguishable effect on meal initiation frequency (Table 2; see Fig. 1B). No interaction terms were significant for either peptide group (all P > 0.24).
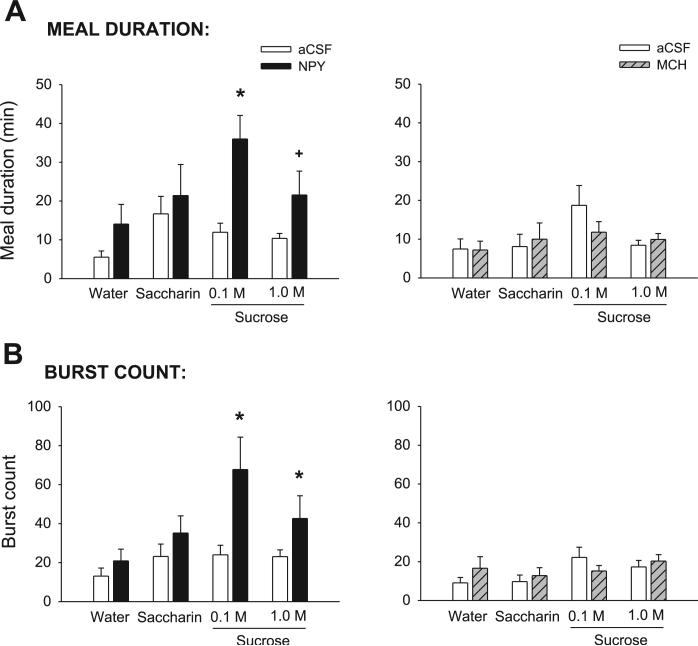
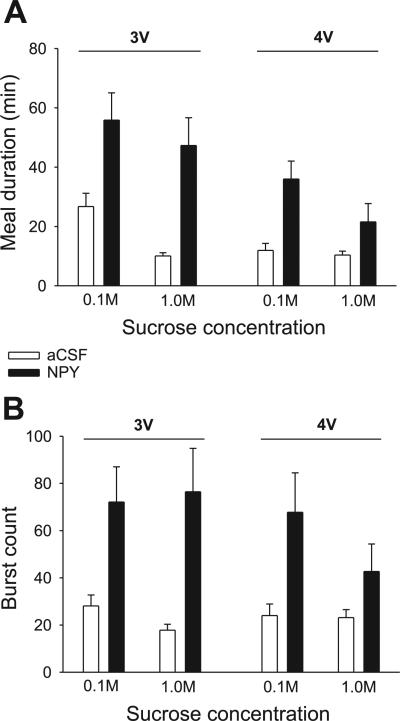
Meal duration varied significantly across tastant conditions in the NPY group (Table 2; Fig. 2A, left), with no significant differences in the MCH group (Table 2; Fig. 2B, right). After NPY treatment, meal duration was more than tripled for the 0.1 M sucrose solution and more than doubled for the 1.0 M sucrose solution, supported by a significant main effect of drug (Table 2). The drug × tastant interaction term did not reach statistical criterion (P = 0.08). Consistent with previous reports (9, 53) for 3V MCH treatment, there was no effect of 4V MCH on any meal duration measures (Table 2; Fig. 2A, right).
Fig. 2.
A, left: mean (+SE) meal duration (min) values for aCSF- (open bars) and NPY-treated (solid bars) 4V cannula-fitted rats (n = 9), ingesting water, saccharin, and two concentrations of sucrose. Right: same results for aCSF- (open bars) and MCH-treated (hatched bars) rats (n = 10). B, left: mean number bursts in the meal for 4V cannula-fitted rats treated with aCSF (open bars) or NPY (solid bars). Right: same results for aCSF- (open bars) and MCH-treated (hatched bars) rats. *P < 0.05; +P < 0.07.
In both the NPY and MCH groups, the average ingestion rate tended to vary across tastants, with the fastest rate of ingestion expressed for the 1.0 M sucrose condition. This was supported by a significant main effect for the tastant term in both groups (Table 2). As suggested by a doubling of intake but a tripling of meal duration, the average ingestion rate under NPY declined by 37% for the 0.1 M sucrose condition (data not shown). However, there was no significant main effect of drug, nor was there a significant interaction for the NPY group or the MCH group (Table 2).
There were no effects of tastant or drug on lick volume in the MCH group (Table 2). In the NPY group, however, signifi-cant differences were observed (Table 2). Further analysis indicated that this was due to some very small meals, mostly for water, which produced inaccurate overestimates of lick volume. When the analysis was restricted to meals in which rats expressed at least 100 licks for water, no significant differences were observed [drug: F(1,4) = 5.90, P = 0.08; tastant: F(3,12) = 0.92, P = 0.46; interaction: F(3,12) = 0.74, P = 0.55].
Licking microstructure
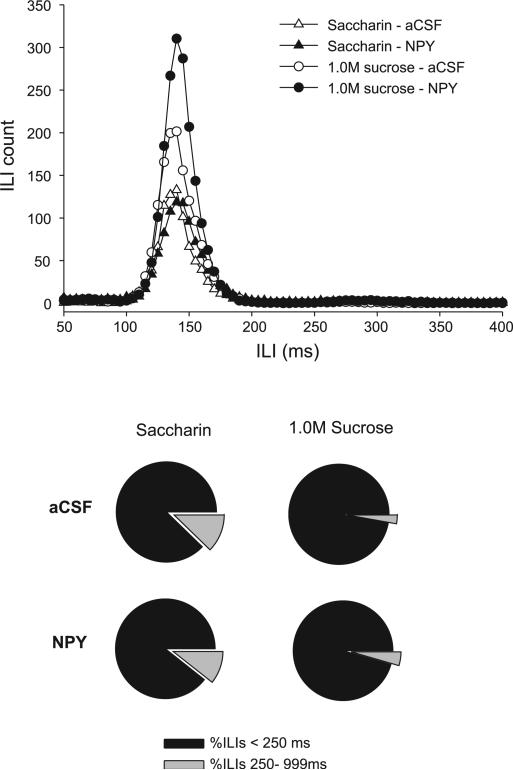
Rats licked ~17% faster within bursts for the sucrose solutions compared with the water and saccharin solutions, as supported by a significant main effect of tastant for the NPY group (Table 2) and the MCH group (Table 2, paired comparisons: P = 0.03). However, when the analysis was limited to ILIs < 250 ms, this tastant effect was lost [NPY: F(3,24) = 0.18, P = 0.91; MCH: F(3,27) = 1.32, P = 0.29; Fig. 3, top], indicating that the difference across tastants was due to increases in ILIs ranging from 250 to 999 ms. These differences are commonly attributed to missed lick contacts or brief orofacial taste reactivity movements (e.g., tongue protrusions, see Refs. 10, 22). The proportion of ILIs < 250 ms was significantly smaller for the water and saccharin concentrations compared with the sucrose solutions [NPY group: F(3,24) = 7.04, P < 0.001; comparisons: P < 0.05; MCH group: F(3,27) = 2.95, P = 0.05; comparisons: P < 0.04; see Fig. 3, bottom for examples]. Importantly, there were no significant effects of either NPY or MCH for all of the measures described above, as indicated by the lack of any significant effects for the drug terms and the interaction terms (all P > 0.26; Fig. 3).
Fig. 3.
Top: mean interlick interval (ILI) count for ILIs ranging from 50 to 400 ms in 4V cannula-fitted rats (n = 9), ingesting saccharin (triangles) or 1.0 M sucrose (circles) after either aCSF (open symbols) or NPY (solid symbols) treatment. Although NPY increased the number of ILIs (number of licks), there were no horizontal shifts in the curve for any of the tastants tested. Bottom: relative proportions (%) of all ILIs within bursts for saccharin and 1.0 M sucrose solutions after aCSF and NPY treatment. Although rats exhibited proportionally more long ILIs (250–999 ms) for saccharin than for sucrose, these distributions were not affected by NPY treatment.
Similar to the observations reported following forebrain infusions (7, 50, 78), NPY increased meal size through an increase in the number of bursts rather than the size of bursts. NPY nearly tripled the number of bursts for 0.1 M sucrose, and it increased the burst count for the 1.0 M sucrose solution by 85% (Fig. 2B, left). There was a significant main effect of the drug and the tastant terms (Table 2) and a statistically significant interaction term (Table 2). The differences in water and saccharin conditions were not statistically significant (P > 0.22). In contrast to NPY, MCH had no effect on mean burst count (Table 2).
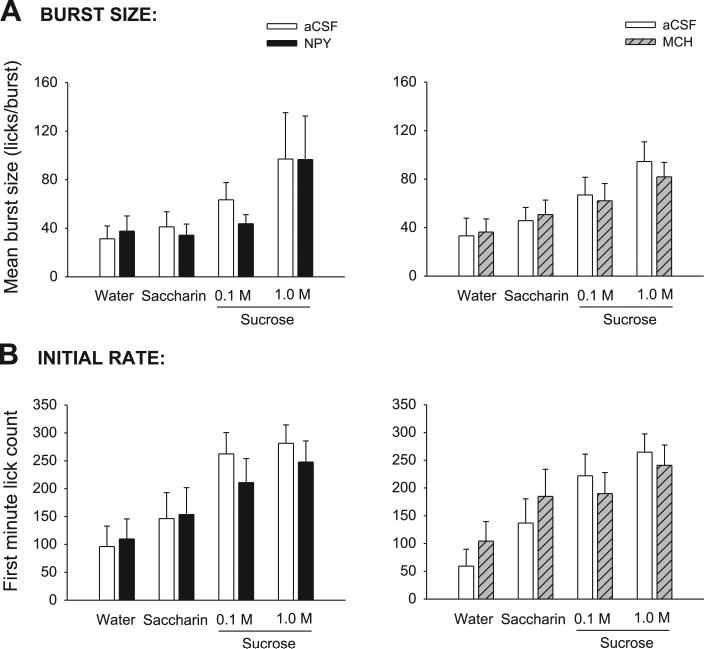
As expected, the mean size of bursts varied significantly across tastants, exhibiting comparable monotonic increases under aCSF conditions in both the NPY and MCH groups when tastants were placed in the order of water, saccharin, and 0.1 and 1.0 M sucrose (Table 2; Fig. 4A). However, there was no significant main effect of drug for either treatment group and no significant interaction term (Table 2). While the failure of NPY to increase burst size is consistent with previous reports after forebrain ICV NPY stimulation, the failure of MCH to affect burst size observed here stands in contrast to findings noted after 3V administration (7, 9, 50, 78). This failure of MCH was also observed when bursts were expressed in terms of their mean duration (s/burst) rather than mean size [F(1,9) = 0.00, P = 0.99], while the monotonic increase in burst duration across tastants was conserved [F(3,27) = 4.54, P = 0.01; data not shown].
Fig. 4.
A, left: mean (+SE) burst size (licks/burst) values for aCSF- (open bars) and NPY-treated (solid bars) 4V cannula-fitted rats (n = 9), ingesting water, saccharin, and two concentrations of sucrose. Right: same results for aCSF- (open bars) and MCH-treated (hatched bars) rats (n = 10). B, left: mean number of licks in the first minute of the meal for 4V cannula-fitted rats (n = 9) treated with aCSF (open bars) or NPY (solid bars). Right: same results for aCSF- (open bars) and MCH-treated (hatched bars) rats (n = 10).
Consistent with the pattern observed for mean burst size, the number of licks expressed in the first minute of the meal for both the NPY and MCH groups increased linearly across tastants when they were ordered from water to 1.0 M sucrose (Fig. 4B). Accordingly, the tastant terms were robustly statistically significant (Table 2). However, as also observed for mean burst size, there were no significant differences for any of the drug conditions, and no significant interactions (P > 0.36).
As NPY had no effect on mean burst size, it is possible that the increase in sucrose meal duration produced by NPY was due to increases in the duration of pauses between bursts. Notably, neither NPY nor MCH had any effect on this pause measure, as indicated by the lack of significant main drug effects or interaction terms (P > 0.43). Similarly, no signifi-cant drug or interaction terms were observed for the percentage of pause time measures for either group (P > 0.08; data not shown). Therefore, the increase in meal duration was restricted to the increase in the number of bursts and their subsequent pauses.
Ingestion rate
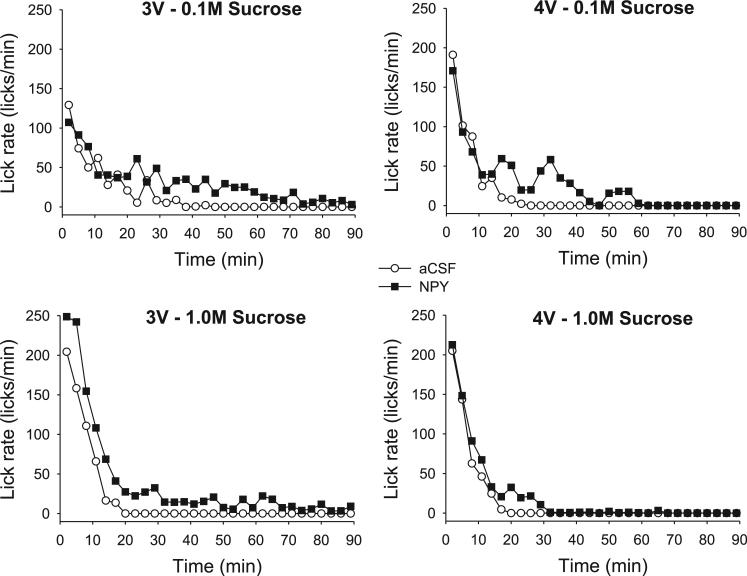
Previous reports indicated that both forebrain MCH and NPY injections increased ingestion rate early in the meal, while NPY also prolonged meals at a slow but sustained rate (7, 9, 50, 78). The lack of effect of either drug on the first-minute lick count in this study suggested that neither drug increased ingestion rate early in the meal, although NPY appeared to prolong meals for the sucrose solutions (Fig. 5). Because the group curves depicted in Fig. 5 include subject attrition due to differential meal termination times (7, 9, 41), we statistically evaluated licking rate in the first 5 min of the meals for the saccharin and sucrose solutions, which were portions of the meals when all rats were actively ingesting (8, 41). This analysis also indicated that neither NPY nor MCH increased early meal ingestion rate, as there were no significant drug or drug × minute interaction terms [NPY group: drug: F(1,8) = 0.07, P = 0.80; interaction: F(4,32) = 1.89, P = 0.18; MCH group: drug: F(1,9) = 0.99, P = 0.35; interaction: F(4,36) = 0.32, P = 0.86]. Ingestion rates did, however, tend to uniformly decline over the first 5 min, as indicated by a significant minute term for both groups [NPY group: F(4,32) = 7.24, P < 0.001; MCH group: F(4,36) = 15.07, P < 0.001].
Fig. 5.
Left: mean ingestion rates (licks/min) for each minute of the intake test in 4V cannula-fitted rats (n = 9), ingesting water, saccharin, and two sucrose concentrations, after aCSF (open symbols) or NPY (solid symbols) treatment. Right: same results plotted for 4V cannula-fitted rats (n = 10) after aCSF (open symbols) or MCH (solid symbols) treatment.
To explore the effect of each peptide on ingestion rate in later phases of the meal, meals were also temporally divided into thirds. The results support the conclusion drawn from the group curves: NPY tended to extend meals at a slow, sustained ingestion rate, particularly for the 0.1 M sucrose solution, where there was a significant drug × meal third interaction [F(2,16) = 6.85, P = 0.007; see Fig. 6]. By comparison, MCH did not affect the ingestion rate across meal thirds (P > 0.23; data not shown).
Fig. 6.
Mean (±SE) licking rate (licks/min) across meal thirds for NPY- (solid symbols) and aCSF-treated (open symbols) 4V cannula-fitted rats (n = 9). Meals were temporally divided into thirds, and the mean ingestion rates (licks/min) associated with each meal third are presented for rats ingesting water, saccharin, and two sucrose solutions. The mean lick rate for each meal third is plotted at the temporal midpoint of each meal third (i.e., at 1/6th, 3/6ths, and 5/6ths of the average meal duration). Note that all meals begin at the same time, minute 1. The figure shows that, for sucrose concentrations, NPY significantly prolonged meals with a slow rate of ingestion in the final third of the meal.
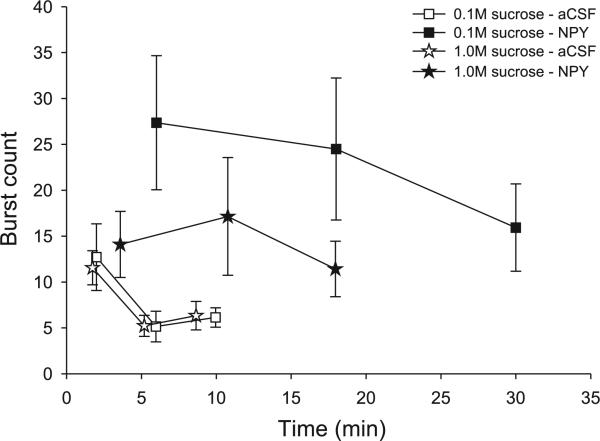
Since NPY increased meal size through an increase in both burst number and meal duration, we also evaluated the distribution of burst counts across meal thirds. In this analysis, burst counts declined from the first to second meal thirds under control conditions (Fig. 7). After NPY, rats showed less decline in burst counts in later meal phases, supported by a significant main effect of drug for the 0.1 M sucrose condition [F(1,8) = 9.31, P = 0.02] and a significant drug × meal-third interaction for the 1.0 M sucrose condition [F(2,16) = 3.68, P = 0.05]. As expected, the comparable analyses for the MCH group lacked any significant differences (all P > 0.16).
Fig. 7.
Comparison of burst counts across meal thirds for NPY- (solid symbols) and aCSF-treated (open symbols) 4V cannula-fitted rats (n = 9) ingesting sucrose. The mean for each meal third is plotted at the temporal midpoint of each meal third, (i.e., at 1/6th, 3/6ths, and 5/6ths of the average meal duration). Note that all meals begin at the same time, minute 1. NPY increased the number of bursts in the meal in a manner that was evenly distributed across meal thirds. Rats showed less relative decline in the number of bursts across the meal thirds compared with aCSF conditions.
Experiment 3: 3V vs. 4V NPY Infusion Effects on Sucrose Consumption
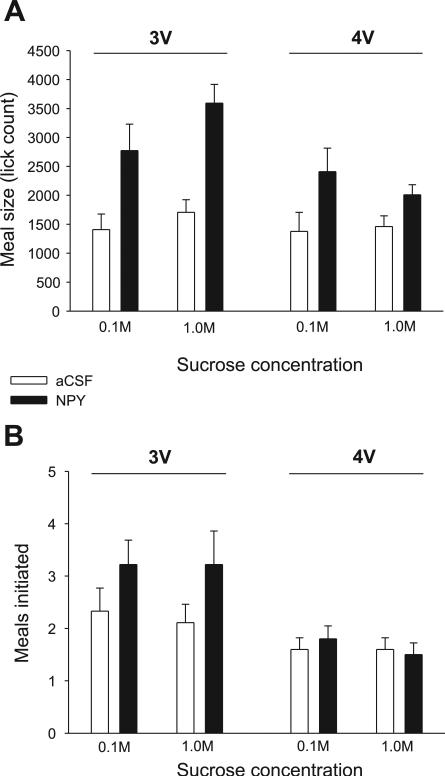
The previously reported effects of 3V NPY on licking for sucrose (7) were replicated. NPY delivered to the forebrain ventricle more than doubled meal size for both sucrose solutions, as indicated by a significant main effect of drug, with no significant drug × concentration interaction term (Fig. 8, left; Table 4). The increases for sucrose were mediated by statistically significant two- to threefold increases in meal duration and burst count (Fig. 9, left; Table 4). 3V NPY also significantly reduced the average ingestion rate and the mean burst size for 1.0 M sucrose, as indicated by a significant drug × concentration interaction term (Table 4), which is consistent with our laboratory's previous findings for 1.0 vs. 0.3 and 0.03 M sucrose solutions (see Ref. 7). Also consistent with our laboratory's previous report for sucrose solutions, 3V NPY did not markedly increase the initial rate of licking, but it did significantly increase in the number of meals initiated during the intake test (Fig. 8; Table 4). Analysis of lick rate in the first 5 min of the meal indicated that 3V NPY increased the overall rate of licking in this period for the 1.0 M sucrose solution [F(1,8) = 6.31, P = 0.036], but not for the 0.1 M sucrose solution [F(1,8) = 0.04, P = 0.95; see Fig. 10].
Fig. 8.
A: mean (+SE) meal size (licks) values for aCSF- (open bars) and NPY-treated (solid bars) rats fitted with either third ventricle (3V) (n = 9) or 4V (n = 9) cannulas that ingested 0.1 and 1.0 M sucrose solutions. B: mean (+SE) number of meals initiated during the 90-min test period for rats fitted with either 3V (n = 9) or 4V (n = 9) cannulas that were treated with aCSF (open bars) or NPY (solid bars).
Table 4.
Licking measures for sucrose solutions after forebrain (3V) NPY infusions
| ANOVA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 M Sucrose |
1.0 M Sucrose |
Drug |
Concn. |
Interaction |
||||||
| Measure | aCSF | NPY | aCSF | NPY | F(1,8) | P | F(1,8) | P | F(1,8) | P |
| Meals initiated, no. | 2.33±0.44 | 3.22±0.46 | 2.11±0.35 | 3.22±0.64 | 5.54 | 0.05* | 0.18 | 0.68 | 0.03 | 0.87 |
| Meal lick count, no. | 1,408±269 | 2,772±457 | 1,706±218 | 3,592±324 | 41.69 | 0.001* | 2.91 | 0.13 | 0.79 | 0.40 |
| Meal size, ml | 6.67±1.25 | 12.23±2.13 | 8.45±1.17 | 16.49±1.99 | 30.68 | 0.001* | 3.26 | 0.10 | 0.67 | 0.43 |
| Meal duration, min | 26.74±4.45 | 55.86±9.20 | 10.05±1.10 | 47.30±9.37 | 26.51 | 0.001* | 2.03 | 0.19 | 0.31 | 0.59 |
| Mean ingestion rate, licks/min | 56.95±8.71 | 55.21±8.97 | 173.61±17.90 | 99.87±18.36 | 15.54 | 0.01* | 22.94 | 0.001* | 10.17 | 0.02* |
| Lick volume, μl | 4.75±0.17 | 4.36±0.31 | 4.99±0.32 | 4.56±0.38 | 11.45 | 0.01* | 0.56 | 0.47 | 0.05 | 0.83 |
| First-minute lick rate | 141.56±39.21 | 103.44±23.98 | 214.44±24.07 | 213.44±29.72 | 0.35 | 0.57 | 17.31 | 0.01* | 0.63 | 0.45 |
| Latency, s | 116.21±78.02 | 36.28±12.12 | 33.28±19.58 | 13.28±7.55 | 1.60 | 0.24 | 1.61 | 0.24 | 0.48 | 0.51 |
| Burst count, no. | 28.11±4.64 | 72.11±14.92 | 17.78±2.57 | 76.44±18.41 | 10.19 | 0.02* | 0.12 | 0.74 | 1.04 | 0.34 |
| Mean burst size, no. licks | 46.72± 8.52 | 48.32±9.79 | 110.67±20.22 | 60.83±10.95 | 7.30 | 0.03* | 17.85 | 0.01* | 8.55 | 0.02* |
| Mean pause duration, s | 50.40±9.66 | 44.67±3.39 | 30.49±10.28 | 36.78±12.13 | 0.01 | 0.97 | 1.80 | 0.22 | 0.49 | 0.51 |
Values are means ± SE. 0.1 and 1.0 M sucrose are the tastants. aCSF and NPY are the drug conditions.
P met the criterion for statistical significance, which was set at P ≤ 0.05.
Fig. 9.
A: mean (+SE) meal duration (min) values for aCSF- (open bars) and NPY-treated (solid bars) rats fitted with either 3V (n = 9) or 4V (n = 9) cannulas, that ingested 0.1 and 1.0 M sucrose solutions. B: mean (+SE) number of licking bursts in the meal for rats fitted with either 3V (n = 9) or 4V (n = 9) cannulas that were treated with aCSF (open bars) or NPY (solid bars).
Fig. 10.
Mean ingestion rates (licks/min) in rats fitted with either 3V (n = 9) or 4V (n = 9) cannulas, that ingested 0.1 M sucrose (top) or 1.0 M sucrose (bottom), after aCSF (open symbols) or NPY (solid symbols) treatment. Symbols indicate the average rate of licking (licks/min) for successive 3-min periods. NPY significantly prolonged meals with a slow and sustained ingestion rate. 3V NPY also increased ingestion rate in the early phases of 1.0 M sucrose meals.
Responses for 0.1 and 1.0 M sucrose solutions in the 3V NPY group were next compared with those for the 4V NPY group using a three-way ANOVA (drug × ICV group × concentration). While meal lick count was increased by NPY in both groups, a statistically significant drug × ICV group interaction indicated that more 1.0 M sucrose was consumed after 3V NPY [F(1,16) = 8.27, P = 0.01] than after 4V NPY (see Fig. 8). This was confirmed by separate t-tests comparing the difference scores (NPY minus aCSF) for each ICV group at each concentration. The net increase in meal size for 1.0 M sucrose after NPY was significantly greater in the 3V group compared with the difference scores for the 4V group [t(16) = 3.06, P = 0.007]. No such group difference was observed for the 0.1 M sucrose solution [t(16) = 0.65, P = 0.52]. This greater increase for 1.0 M sucrose consumption was mediated through greater effects of NPY on meal duration rather than on burst count (see Fig. 9). For the burst count measure, only the main drug term was statistically significant [F(1,16) = 9.79, P = 0.001]; there was no significant main effect of ICV group [F(1,16) = 0.92, P = 0.35], nor were any other main or interaction terms statistically significant. In addition, t-tests comparing difference scores in the 1.0 M condition did not reach the level of statistical significance [t(16) = 1.91, P = 0.08]. In contrast, there was a statistically significant main effect for the ICV group for the meal duration values [F(1,16) = 14.03, P < 0.02], in addition to a robust main drug term [F(1,16) = 44.78, P < 0.001]. The drug × group interaction term approached but did not reach statistical significance [F(1,16) = 4.69, P = 0.057], and no other interaction terms were significant. As with the meal lick count measures, t-tests comparing the drug-vehicle difference scores for each ICV group indicated that NPY more profoundly extended meal duration in the 3V group for the 1.0 M sucrose concentration [t(16) = 2.38, P = 0.03; see also Fig. 10], whereas the NPY-induced increases in meal duration for 0.1 M sucrose were comparable between 3V and 4V groups [t(16) = 0.46, P = 0.61].
DISCUSSION
The effects of NPY and MCH on meal feeding appear to depend on the tastant quality and the anatomical site of stimulation. 4V NPY increased meal size for 0.1 and 1.0 M sucrose solutions by >35%, but it was without effect on water or 4 mM saccharin consumption. This outcome is consistent with the findings of experiment 3 and with our laboratory's previous report that 3V NPY injections under identical testing conditions increased meal size for 0.03, 0.1, 0.3, and 1.0 M sucrose, but not for saccharin or water solutions (7), with two noteworthy differences. First, where 3V NPY increased meal frequency for water, saccharin, and sucrose solutions (Ref. 7 and experiment 3), 4V NPY delivery did not. Second, the sucrose meal size increases induced by 3V NPY in experiment 3 and our laboratory's previous study (7) were greater than those observed after 4V NPY. Moreover, while previous studies have reported that 3V MCH infusions increased meal size for water and sucrose solutions (9, 31, 47, 61), in the present study, under testing conditions identical to our laboratory's recent 3V MCH infusion study (9), 4V MCH application failed to increase intake of either water or sucrose (Fig. 1A). Therefore, the meal size effects of MCH treatment appear to require forebrain stimulation.
However, while both forebrain and hindbrain NPY treatments increased meal size, the effects were distinct in other respects. Several investigators have reported that forebrain NPY treatment increases appetitive behaviors, such as exploration, spout approaches, and meal frequency, in addition to meal size or hourly food consumption (4, 7, 30, 48, 51, 72; note Refs. 4, 7, 76 for some exceptions). These effects of forebrain NPY treatment contrast with the report that 4V NPY infusions do not increase exploratory behavior (21). In this study, we replicated these findings, as 3V NPY significantly increased meal frequency, while 4V NPY did not (Fig. 8B). The present results contribute to a growing body of evidence that suggests that hindbrain NPY effects may be limited to the intrameal processes that underlie the consumption of the sucrose solutions.
Effects of NPY on Licking Microstructure
The inspection of meal microstructure revealed additional differences regarding the effects of 3V vs. 4V NPY delivery on the pattern of ingestion within a meal. Previous meal pattern studies have reported that NPY delivered to the forebrain primarily increases meal size by prolonging meal duration (48, 51, 76). To date, three studies have evaluated intrameal licking microstructure after forebrain ICV NPY administration, and all report relatively consistent observations (7, 50, 78), with additional replication of those results also obtained in experiment 3. Collectively, the previous studies and experiment 3 indicated that forebrain NPY infusions increased meal duration and the number of licking bursts, and that 3V NPY reduced the rate of decay in ingestion rate for either sweetened milk or a range of sucrose solutions (0.03–1.0M).1 Two of these studies also reported that NPY increased ingestion rate early in the meal (7, 78), and experiment 3 found a similar result for the 1.0 M but not the 0.1 M sucrose solution. In the present study, a 4V NPY infusion reproduced the majority of these intrameal effects, with some noted exceptions. As with forebrain injections, 4V NPY increased meal burst count and the meal duration, but the previously reported increases in initial and/or early-meal ingestion rate after forebrain infusions were not observed. There was no effect of 4V NPY on ingestion rate through the first 5 min of the meal for any solution tested (e.g., Fig. 10, also cf. Fig. 5 with Fig. 5 in Ref. 7 or Fig. 2 in Ref. 78), and it is unlikely that this failure was due to a ceiling effect, since the initial rate of ingestion for both sucrose solutions tended to decline after 4V NPY infusions (see Figs. 5 and 10).
It is possible that the absence of a 4V NPY effect on early-meal ingestion rate represents a failure of 4V NPY to stimulate one or more anticipatory or appetitive processes that are evoked for palatable solutions after forebrain NPY stimulation. For example, food deprivation has been shown to increase the initial rate of ingestion for various sucrose solution concentrations (25, 74), and both forebrain NPY and food deprivation increase willingness to work for food, as indicated by an increase in break point on a progressive ratio schedule (39). However, as noted, the initial rate of ingestion also covaries with orosensory properties of tastants (e.g., Fig. 4B; Refs. 7, 9, 25, 74). It would be worthwhile in future studies to evaluate whether progressive ratio or other instrumental responses for food access are increased after 4V NPY delivery. Overall, it appears that hindbrain sites sensitive to 4V NPY stimulation are not involved in the processes that underlie the early meal avidity observed after forebrain NPY infusions.
Previous studies have noted that forebrain ICV NPY delivery produces a two-part effect on lick rate such that ingestion rate early in the meal is increased (7, 50, 78), and the meal is prolonged with a slower ingestion rate in later phases (7, 78). Similar results were observed in experiment 3, where meal duration was prolonged for both sucrose solutions, while early-meal ingestion rate was increased for the 1.0 M sucrose solution but not for the 0.1 M sucrose solution. After 4V NPY injections (see Figs. 5–7), no ingestion rate increases in early phases of the meal were observed for any taste solution, but the later phase effects were reproduced. As ingestion rate declined and control meals ended, both 3V and 4V NPY meals continued at a slow, sustained pace (Fig. 10). The present results appear to support our laboratory's previous speculation that NPY stimulation influences ingestion rate through at least two separable processes (7), as early- and late-meal ingestion rate effects appeared to be dissociated, depending on the site of NPY stimulation and type of solution consumed.
The lack of 4V NPY influences on early-meal ingestion rate may have partially contributed to the more modest increases in consumption relative to forebrain NPY injections. Compared with 4V NPY conditions, 3V NPY induced a significantly greater meal size increase (roughly doubled) for the 1.0 M sucrose solution in experiment 3, an effect size that was consistent with our laboratory's previous 3V NPY injection study (Ref. 7; see also Fig. 8). A comparison of 3V and 4V NPY meal microstructure measures showed that increases in the meal duration after 3V NPY were also commensurately greater than those after 4V NPY, whereas burst count increases were, on average, greater but not significantly more so after the 3V vs. the 4V NPY infusions (Fig. 9). These results suggest that forebrain NPY-stimulated sites also likely contributed to processes that prolonged individual meals.
The present findings correspond with the hypothesis that ICV NPY reduces the gain of inhibitory postingestive feedback. 4V NPY effects were limited to measures of licking microstructure that are commonly associated with gastrointestinal manipulations, namely later meal ingestion rate, meal duration, and burst count (see Refs. 22, 23, 26–29, 32, 65), and the effects were also specific to caloric taste solutions. In support, NPY has also been reported to suppress electrophysiological gastric distension responses in the NST (64), and alter myoelectrical activity in the small intestine and proximal stomach after delivery to the fore-brain (33, 77), and induce gastric relaxation after a 4V injection (44). Nevertheless, a recent report indicated that 3V NPY treatment increased ingestion in sham-feeding rats, a behavioral preparation in which postingestive feedback during feeding is removed by diverting ingesta through an open gastric fistula (78). Those results indicate that postingestive feedback modulation is not necessary to mediate intake increases after 3V NPY injection. However, the sham-feeding results are not inconsistent with the observed appetitive behavioral effects that are also observed after forebrain NPY treatment, including increases in meal frequency and exploratory behavior, including spout approaches (7, 30, 48, 51). Furthermore, NPY effects on consumption are clearly influenced by orosensory factors. NPY has little effect on the meal size for water (Ref. 7 and Fig. 1A) or consumption of caloric, but less preferred, corn starch (35). Furthermore, NPY produces a conditioned preference for sweetened, but not for unsweetened, Kool-Aid solutions (49, 70). Interestingly, neither forebrain nor hind-brain ICV NPY treatments increased meal size for the noncaloric tastant saccharin, although an increase in saccharin intake over 2-to 4-h periods in which multiple meals could be expressed has been reported (7, 33, 49). Saccharin was preferred over water in this study and in our laboratory's previous study, over 0.03 M sucrose, suggesting that the effects of NPY on meal size also covary with the postingestive cues provided by ingesta (Ref. 7, and this study)2 or possibly differences in taste quality between saccharin and sucrose. Although NPY meal size effects may be limited to tastants that are preferred and caloric, it does not appear that NPY modulates gustatory hedonic evaluation per se, as NPY has little effect on ingestive oromotor responses (taste reactivity) to brief intraoral infusions of water or sucrose solutions (11, 14, 67). Furthermore, the increases in initial ingestion rate after 3V NPY treatment (where observed) were not accompanied by increases in the mean size of licking bursts (7, 50, 78): these two measures normally vary in tandem when rats are offered tastants of increasing palatability (e.g., Fig. 4) or those that are naturally or conditioned to be aversive (7, 9, 10, 25, 74, 75).
Effects of MCH on Licking Microstructure
Previous reports indicate that forebrain ICV NPY and MCH treatments produce distinct effects on feeding: MCH has been reported to increase meal size for water, sucrose, saccharin/ethanol, and sucrose/quinine HCl mixtures, whereas NPY was shown to increase both meal size and frequency, but the meal size effects were limited to caloric sucrose solutions (7, 9, 31, 50, 61). Furthermore, 3V MCH affected licking measures associated with gustatory evaluation, whereas NPY affected measures more commonly associated with postingestive feedback. Namely, where NPY produced either an absent or suppressive effect on the mean size of licking bursts, 3V MCH delivery under comparable conditions significantly increased burst length, first-minute lick count, ingestion rate in the first 5 min, and, in a separate experiment, brief-access licking for water and sucrose, but not aversive quinine HCl concentrations (9). Finally, 3V NPY greatly increased the number of bursts (as much as fourfold) in the meal and significantly extended meal duration (experiment 3; Refs. 7, 50, 78), whereas 3V MCH treatment produced no such results (9). These data are also strongly consistent with the recent report that forebrain ICV NPY (0.5 nM) and orexin-A (3 nM) modulated proximal gastric motor responses, but forebrain ICV MCH (3 nM) infusions produced no such effect under the same conditions (33).
The effects of 4V MCH also contrasted with the reported effects of forebrain application of MCH. There was no effect of any concentration of MCH on meal size, initial ingestion rate, or the mean burst size (Refs. 9, 53, 61). Indeed, there was no effect trend after 4V MCH for any of the measures in this study, as indicated in Tables 2 and 3, Figs. 1, 2, and 4, and by the very low F values (many <1) for the majority of statistical comparisons. This lack of MCH effect is interesting, given that the licking microstructure analysis is ideally suited to detect subtle effects of a treatment on licking behavior, influences that might not necessarily impact composite measures such as meal size or hourly consumption (see Refs. 23, 85). Moreover, under control conditions, clear and consistent monotonic increases in the initial rate of ingestion and the mean burst size were obtained across taste solutions (Fig. 4), providing an ideal baseline response curve against which any potential gustatory effects of hindbrain MCH could be observed. Finally, the significant effects of 4V NPY obtained under identical testing conditions may be considered a positive control condition that rules out procedure or apparatus artifacts. We conclude, therefore, that the reported effects of MCH on gustatory hedonic responses and on feeding require direct forebrain stimulation by MCH.
Sites of NPY and MCH Action
The present results strongly support the conclusion that ventricular injections of NPY and MCH have separate sites of action. The results support and strengthen the conclusions of previous behavioral analyses, which indicated divergent effects of the two peptides on ingestive microstructure after forebrain ventricle delivery (discussed above). These data also support a growing body of literature that shows that feeding is controlled by multiple systems distributed throughout the forebrain and hindbrain (e.g., Refs. 36, 68). For example, hypothalamic NPY systems are not essential for the mediation of exogenous NPY-induced feeding, as NPY-related lesions of the ARC, paraventricular nucleus, or basal hypothalamus fail to prevent either hyperphagic responses to ICV NPY (2, 17, 52, 54) or the attainment of normal levels of food intake, body weight, and adiposity in adulthood (13, 38).
It has been noted that 4V infusions favor stimulation of hindbrain sites without forebrain stimulation, as the caudal flow of cerebrospinal fluid minimizes back-diffusion of infusates. Consequently, forebrain ICV infusions of angiotensin II are potently dipsogenic, but comparable 4V infusions are without effect (20), 4V ink injections do not stain forebrain ventricles (see methods and Ref. 85), and the failure of 4V MCH (up to 6 nM) to influence any measures of licking in this study may be considered further evidence.
The failure of a range of MCH concentrations to influence any measures of licking after 4V infusions in this study is somewhat surprising, as there are extensive MCH-immunore-active processes throughout viscerotopic areas of caudal NST and dorsal motor nucleus of the vagus, with some fibers in close apposition to NST cells that expressed c-fos immunore-activity after a nutritive gastric preload (85). Nevertheless, the results are consistent with the finding that forebrain ICV MCH did not modify gastric motor responses (33) and that 4V MCH (4 nM) did not increase 2-h intake of water or rat chow (85), therefore emphasizing the role of forebrain sites of action on sensory/behavioral processes that are not directly related to postingestive feedback processing. Recently, MCH injections into the nucleus accumbens were shown to influence feeding, which raises the possibility that forebrain MCH influences the perceived reward or incentive value of food stimuli (34). This hypothesis is consistent with the finding that 3V MCH enhanced brief-access taste responses for accepted and palatable tastants, but not aversive tastants, and reports that MCH more effectively increases consumption of palatable food (53; see also Ref. 9 for discussion).
Conclusions and Caveats
Ventricle applications of NPY and MCH produce nearly dichotomous patterns of ingestive behavior that are further dissociated based on the locus of stimulation and the type of tastant offered. Consequently, these two peptides appear unlikely to share a final common pathway. It also appears that the influences of NPY on consummatory (intrameal) feeding do not depend on MCH function, since 4V NPY but not 4V MCH increased feeding, and that direct lateral hypothalamic-brain stem MCH projections (79, 85) and visceromotor processes (33) are likely not relevant to the established effects of fore-brain MCH treatment on feeding. Stronger support could be provided by the application of MCH antagonists to either the 4V or hindbrain parenchymal sites, applied separately and conjointly with NPY. Furthermore, ICV administration of any compound can never truly mimic the quantitative, spatial, and temporal dynamics of the natural system; therefore, a contribution of hindbrain MCH systems to feeding cannot be completely excluded. It is clear, however, that the ingestive effects elicited by forebrain MCH ICV stimulation are not mediated by direct access of the peptide to the ventricular hindbrain.
Acknowledgments
GRANTS
These studies were supported by National Institutes of Health Grant DC007389 to J. P. Baird, an Amherst College Dean of Faculty research award to C. Rios and a Howard Hughes Medical Institute student research fellowship to A. Tran.
Footnotes
The analysis of Ref. 50 reported the number and length of consecutive 10-s periods in which one or more licks occurred. This approach is analogous to a burst analysis using a 10-s pause criterion (see Ref. 74).
Ref. 49 reported that LV NPY increased 4-h consumption of one of three concentrations of saccharin tested. As 3V NPY in our hands increased saccharin meal frequency but not meal size, we speculated that the 4-h intake increase was due to LV NPY effects on meal frequency for saccharin (see discussion in Ref. 7).
REFERENCES
- 1.Abbott CR, Kennedy AR, Wren AM, Rossi M, Murphy KG, Seal LJ, Todd JF, Ghatei MA, Small CJ, Bloom SR. Identification of hypothalamic nuclei involved in the orexigenic effect of melanin-concentrating hormone. Endocrinology. 2003;144:3943–3949. doi: 10.1210/en.2003-0149. [DOI] [PubMed] [Google Scholar]
- 2.Abe M, Saito M, Shimazu T. Neuropeptide Y in the specific hypothalamic nuclei of rats treated neonatally with monosodium glutamate. Brain Res Bull. 1990;24:289–291. doi: 10.1016/0361-9230(90)90218-o. [DOI] [PubMed] [Google Scholar]
- 3.Ammar AA, Nergardh R, Fredholm BB, Brodin U, Sodersten P. Intake inhibition by NPY and CCK-8: a challenge of the notion of NPY as an “Orexigen”. Behav Brain Res. 2005;161:82–87. doi: 10.1016/j.bbr.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Ammar AA, Sederholm F, Saito TR, Scheurink AJ, Johnson AE, Sodersten P. NPY-leptin: opposing effects on appetitive and consummatory ingestive behavior and sexual behavior. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1627–R1633. doi: 10.1152/ajpregu.2000.278.6.R1627. [DOI] [PubMed] [Google Scholar]
- 5.Badia-Elder NE, Stewart RB, Powrozek TA, Murphy JM, Li TK. Effects of neuropeptide Y on sucrose and ethanol intake and on anxiety-like behavior in high alcohol drinking (HAD) and low alcohol drinking (LAD) rats. Alcohol Clin Exp Res. 2003;27:894–899. doi: 10.1097/01.ALC.0000071929.17974.DA. [DOI] [PubMed] [Google Scholar]
- 6.Badia-Elder NE, Stewart RB, Powrozek TA, Roy KF, Murphy JM, Li TK. Effect of neuropeptide Y (NPY) on oral ethanol intake in Wistar, alcohol-preferring (P), and -nonpreferring (NP) rats. Alcohol Clin Exp Res. 2001;25:386–390. [PubMed] [Google Scholar]
- 7.Baird JP, Gray NE, Fischer SG. Effects of neuropeptide Y on feeding microstructure: dissociation of appetitive and consummatory actions. Behav Neurosci. 2006;120:937–951. doi: 10.1037/0735-7044.120.4.937. [DOI] [PubMed] [Google Scholar]
- 8.Baird JP, Grill HJ, Kaplan JM. Effect of hepatic glucose infusion on glucose intake and licking microstructure in deprived and nondeprived rats. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1136–R1143. doi: 10.1152/ajpregu.1999.277.4.R1136. [DOI] [PubMed] [Google Scholar]
- 9.Baird JP, Rios C, Gray NE, Walsh CE, Fischer SG, Pecora AL. Effects of melanin-concentrating hormone on licking microstructure and brief-access taste responses. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1265–R1274. doi: 10.1152/ajpregu.00143.2006. [DOI] [PubMed] [Google Scholar]
- 10.Baird JP, St. John SJ, Nguyen EA. Temporal and qualitative dynamics of conditioned taste aversion processing: combined generalization testing and licking microstructure analysis. Behav Neurosci. 2005;119:983–1003. doi: 10.1037/0735-7044.119.4.983. [DOI] [PubMed] [Google Scholar]
- 11.Baird JP, Travers JB, Travers S. Effects of ICV NPY on taste reactivity (Abstract). Chem Senses. 2000;25:600. [Google Scholar]
- 12.Benoit SC, Clegg DJ, Woods SC, Seeley RJ. The role of previous exposure in the appetitive and consummatory effects of orexigenic neuropeptides. Peptides. 2005;26:751–757. doi: 10.1016/j.peptides.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Bergen HT, Mizuno TM, Taylor J, Mobbs CV. Hyperphagia and weight gain after gold-thioglucose: relation to hypothalamic neuropeptide Y and proopiomelanocortin. Endocrinology. 1998;139:4483–4488. doi: 10.1210/endo.139.11.6324. [DOI] [PubMed] [Google Scholar]
- 14.Berridge KC. Measuring hedonic impact in animals and infants: micro-structure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 15.Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 16.Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci USA. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bugarith K, Dinh TT, Li AJ, Speth RC, Ritter S. Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology. 2005;146:1179–1191. doi: 10.1210/en.2004-1166. [DOI] [PubMed] [Google Scholar]
- 18.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 19.Clegg DJ, Air EL, Benoit SC, Sakai RS, Seeley RJ, Woods SC. Intraventricular melanin-concentrating hormone stimulates water intake independent of food intake. Am J Physiol Regul Integr Comp Physiol. 2003;284:R494–R499. doi: 10.1152/ajpregu.00399.2002. [DOI] [PubMed] [Google Scholar]
- 20.Corp ES, McQuade J, Krasnicki S, Conze DB. Feeding after fourth ventricular administration of neuropeptide Y receptor agonists in rats. Peptides. 2001;22:493–499. doi: 10.1016/s0196-9781(01)00359-x. [DOI] [PubMed] [Google Scholar]
- 21.Corp ES, Melville LD, Greenberg D, Gibbs J, Smith GP. Effect of fourth ventricular neuropeptide Y and peptide YY on ingestive and other behaviors. Am J Physiol Regul Integr Comp Physiol. 1990;259:R317–R323. doi: 10.1152/ajpregu.1990.259.2.R317. [DOI] [PubMed] [Google Scholar]
- 22.Davis JD. Deterministic and probabilistic control of the behavior of rats ingesting liquid diets. Am J Physiol Regul Integr Comp Physiol. 1996;270:R793–R800. doi: 10.1152/ajpregu.1996.270.4.R793. [DOI] [PubMed] [Google Scholar]
- 23.Davis JD. A model for the control of ingestion–20 years later. Prog Psychobiol Physiol Psychol. 1998;17:127–173. [Google Scholar]
- 24.Davis JD, Levine MW. A model for the control of ingestion. Psychol Rev. 1977;84:379–412. [PubMed] [Google Scholar]
- 25.Davis JD, Perez MC. Food deprivation- and palatability-induced micro-structural changes in ingestive behavior. Am J Physiol Regul Integr Comp Physiol. 1993;264:R97–R103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- 26.Davis JD, Smith GP, Kung TM. Abdominal vagotomy alters the structure of the ingestive behavior of rats ingesting liquid diets. Behav Neurosci. 1994;108:767–779. [PubMed] [Google Scholar]
- 27.Davis JD, Smith GP, Kung TM. Abdominal vagotomy attenuates the inhibiting effect of mannitol on the ingestive behavior of rats. Behav Neurosci. 1995;109:161–167. [PubMed] [Google Scholar]
- 28.Davis JD, Smith GP, Sayler JL. Closing the pylorus decreases the size of large meals in the rat. Physiol Behav. 1998;63:191–196. doi: 10.1016/s0031-9384(97)00420-4. [DOI] [PubMed] [Google Scholar]
- 29.Davis JD, Smith GP, Sayler JL. Reduction of intake in the rat due to gastric filling. Am J Physiol Regul Integr Comp Physiol. 1997;272:R1599–R1605. doi: 10.1152/ajpregu.1997.272.5.R1599. [DOI] [PubMed] [Google Scholar]
- 30.Day DE, Keen-Rhinehart E, Bartness TJ. Role of NPY and its receptor subtypes in foraging, food hoarding, and food intake by Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2005;289:R29–R36. doi: 10.1152/ajpregu.00853.2004. [DOI] [PubMed] [Google Scholar]
- 31.Duncan EA, Proulx K, Woods SC. Central administration of melanin-concentrating hormone increases alcohol and sucrose/quinine intake in rats. Alcohol Clin Exp Res. 2005;29:958–964. doi: 10.1097/01.alc.0000167741.42353.10. [DOI] [PubMed] [Google Scholar]
- 32.Eisen S, Davis JD, Rauhofer E, Smith GP. Gastric negative feedback produced by volume and nutrient during a meal in rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1201–R1214. doi: 10.1152/ajpregu.2001.281.4.R1201. [DOI] [PubMed] [Google Scholar]
- 33.Furudono Y, Ando C, Yamamoto C, Kobashi M, Yamamoto T. Involvement of specific orexigenic neuropeptides in sweetener-induced overconsumption in rats. Behav Brain Res. 2006;175:241–248. doi: 10.1016/j.bbr.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 34.Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glass MJ, Cleary JP, Billington CJ, Levine AS. Role of carbohydrate type on diet selection in neuropeptide Y-stimulated rats. Am J Physiol Regul Integr Comp Physiol. 1997;273:R2040–R2045. doi: 10.1152/ajpregu.1997.273.6.R2040. [DOI] [PubMed] [Google Scholar]
- 36.Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front Neuroendocrinol. 2002;23:2–40. doi: 10.1006/frne.2001.0224. [DOI] [PubMed] [Google Scholar]
- 37.Hervieu GJ, Cluderay JE, Harrison D, Meakin J, Maycox P, Nasir S, Leslie RA. The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, slc-1, in the central nervous system of the rat. Eur J Neurosci. 2000;12:1194–1216. doi: 10.1046/j.1460-9568.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- 38.Hollopeter G, Erickson JC, Seeley RJ, Marsh DJ, Palmiter RD. Response of neuropeptide Y-deficient mice to feeding effectors. Regul Pept. 1998;75–76:383–389. doi: 10.1016/s0167-0115(98)00092-5. [DOI] [PubMed] [Google Scholar]
- 39.Jewett DC, Cleary J, Levine AS, Schaal DW, Thompson T. Effects of neuropeptide Y, insulin, 2-deoxyglucose, and food deprivation on food-motivated behavior. Psychopharmacology (Berl) 1995;120:267–271. doi: 10.1007/BF02311173. [DOI] [PubMed] [Google Scholar]
- 40.Jobst EE, Enriori PJ, Cowley MA. The electrophysiology of feeding circuits. Trends Endocrinol Metab. 2004;15:488–499. doi: 10.1016/j.tem.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan JM, Baird JP, Grill HJ. Dissociation of licking and volume intake controls in rats ingesting glucose and maltodextrin. Behav Neurosci. 2001;115:188–195. doi: 10.1037/0735-7044.115.1.188. [DOI] [PubMed] [Google Scholar]
- 42.Katner SN, Slawecki CJ, Ehlers CL. Neuropeptide Y administration into the third ventricle does not increase sucrose or ethanol self-administration but does affect the cortical EEG and increases food intake. Psychopharmacology (Berl) 2002;160:146–154. doi: 10.1007/s00213-001-0950-9. [DOI] [PubMed] [Google Scholar]
- 43.Kishi T, Aschkenasi CJ, Choi BJ, Lopez ME, Lee CE, Liu H, Hollenberg AN, Friedman JM, Elmquist JK. Neuropeptide Y Y1 receptor mRNA in rodent brain: distribution and colocalization with melanocortin-4 receptor. J Comp Neurol. 2005;482:217–243. doi: 10.1002/cne.20432. [DOI] [PubMed] [Google Scholar]
- 44.Kobashi M, Shimatani Y, Shirota K, Xuan SY, Mitoh Y, Matsuo R. Central neuropeptide Y induces proximal stomach relaxation via Y1 receptors in the dorsal vagal complex of the rat. Am J Physiol Regul Integr Comp Physiol. 2006;290:R290–R297. doi: 10.1152/ajpregu.00423.2005. [DOI] [PubMed] [Google Scholar]
- 45.Kokkotou EG, Tritos NA, Mastaitis JW, Slieker L, Maratos-Flier E. Melanin-concentrating hormone receptor is a target of leptin action in the mouse brain. Endocrinology. 2001;142:680–686. doi: 10.1210/endo.142.2.7981. [DOI] [PubMed] [Google Scholar]
- 46.Kopp J, Xu ZQ, Zhang X, Pedrazzini T, Herzog H, Kresse A, Wong H, Walsh JH, Hokfelt T. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience. 2002;111:443–532. doi: 10.1016/s0306-4522(01)00463-8. [DOI] [PubMed] [Google Scholar]
- 47.Kowalski TJ, Farley C, Cohen-Williams ME, Varty G, Spar BD. Melanin-concentrating hormone-1 receptor antagonism decreases feeding by reducing meal size. Eur J Pharmacol. 2004;497:41–47. doi: 10.1016/j.ejphar.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 48.Leibowitz SF, Alexander JT. Analysis of neuropeptide Y-induced feeding: dissociation of Y1 and Y2 receptor effects on natural meal patterns. Peptides. 1991;12:1251–1260. doi: 10.1016/0196-9781(91)90203-2. [DOI] [PubMed] [Google Scholar]
- 49.Lynch WC, Grace M, Billington CJ, Levine AS. Effects of neuropeptide Y on ingestion of flavored solutions in nondeprived rats. Physiol Behav. 1993;54:877–880. doi: 10.1016/0031-9384(93)90295-q. [DOI] [PubMed] [Google Scholar]
- 50.Lynch WC, Hart P, Babcock AM. Neuropeptide Y attenuates satiety: evidence from a detailed analysis of patterns ingestion. Brain Res. 1994;636:28–34. doi: 10.1016/0006-8993(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 51.Marin Bivens CL, Thomas WJ, Stanley BG. Similar feeding patterns are induced by perifornical neuropeptide Y injection and by food deprivation. Brain Res. 1998;782:271–280. doi: 10.1016/s0006-8993(97)01289-4. [DOI] [PubMed] [Google Scholar]
- 52.Meister B, Ceccatelli S, Hokfelt T, Anden NE, Anden M, Theodorsson E. Neurotransmitters, neuropeptides and binding sites in the rat medio-basal hypothalamus: effects of monosodium glutamate (MSG) lesions. Exp Brain Res. 1989;76:343–368. doi: 10.1007/BF00247894. [DOI] [PubMed] [Google Scholar]
- 53.Morens C, Norregaard P, Receveur JM, van Dijk G, Scheurink AJ. Effects of MCH and a MCH1-receptor antagonist on (palatable) food and water intake. Brain Res. 2005;1062:32–38. doi: 10.1016/j.brainres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Morley JE, Flood JF. The effect of neuropeptide Y on drinking in mice. Brain Res. 1989;494:129–137. doi: 10.1016/0006-8993(89)90151-0. [DOI] [PubMed] [Google Scholar]
- 55.Morley JE, Levine AS, Gosnell BA, Kneip J, Grace M. Effect of neuropeptide Y on ingestive behaviors in the rat. Am J Physiol Regul Integr Comp Physiol. 1987;252:R599–R609. doi: 10.1152/ajpregu.1987.252.3.R599. [DOI] [PubMed] [Google Scholar]
- 56.Niimi M, Sato M, Taminato T. Neuropeptide Y in central control of feeding and interactions with orexin and leptin. Endocrine. 2001;14:269–273. doi: 10.1385/ENDO:14:2:269. [DOI] [PubMed] [Google Scholar]
- 57.Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- 58.Sahu A. Interactions of neuropeptide Y, hypocretin-I (orexin A) and melanin-concentrating hormone on feeding in rats. Brain Res. 2002;944:232–238. doi: 10.1016/s0006-8993(02)02941-4. [DOI] [PubMed] [Google Scholar]
- 59.Sahu A. Leptin decreases food intake induced by melanin-concentrating hormone (MCH), galanin (GAL) and neuropeptide Y (NPY) in the rat. Endocrinology. 1998;139:4739–4742. doi: 10.1210/endo.139.11.6432. [DOI] [PubMed] [Google Scholar]
- 60.Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001;435:26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- 61.Sakamaki R, Uemoto M, Inui A, Asakawa A, Ueno N, Ishibashi C, Hirono S, Yukioka H, Kato A, Shinfuku N, Kasuga M, Katsuura G. Melanin-concentrating hormone enhances sucrose intake. Int J Mol Med. 2005;15:1033–1039. [PubMed] [Google Scholar]
- 62.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 63.Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol. 1985;241:138–153. doi: 10.1002/cne.902410203. [DOI] [PubMed] [Google Scholar]
- 64.Schwartz GJ, Moran TH. Leptin and neuropeptide Y have opposing modulatory effects on nucleus of the solitary tract neurophysiological responses to gastric loads: implications for the control of food intake. Endocrinology. 2002;143:3779–3784. doi: 10.1210/en.2002-220352. [DOI] [PubMed] [Google Scholar]
- 65.Schwartz GJ, Salorio CF, Skoglund C, Moran TH. Gut vagal afferent lesions increase meal size but do not block gastric preload-induced feeding suppression. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1623–R1629. doi: 10.1152/ajpregu.1999.276.6.R1623. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 67.Sederholm F, Ammar AA, Sodersten P. Intake inhibition by NPY: role of appetitive ingestive behavior and aversion. Physiol Behav. 2002;75:567–575. doi: 10.1016/s0031-9384(02)00648-0. [DOI] [PubMed] [Google Scholar]
- 68.Seeley RJ, Grill HJ, Kaplan JM. Neurological dissociation of gastrointestinal and metabolic contributions to meal size control. Behav Neurosci. 1994;108:347–352. [PubMed] [Google Scholar]
- 69.Seeley RJ, Payne CJ, Woods SC. Neuropeptide Y fails to increase intraoral intake in rats. Am J Physiol Regul Integr Comp Physiol. 1995;268:R423–R427. doi: 10.1152/ajpregu.1995.268.2.R423. [DOI] [PubMed] [Google Scholar]
- 70.Sipols AJ, Brief DJ, Ginter KL, Saghafi S, Woods SC. Neuropeptide Y paradoxically increases food intake yet causes conditioned flavor aversions. Physiol Behav. 1992;51:1257–1260. doi: 10.1016/0031-9384(92)90317-u. [DOI] [PubMed] [Google Scholar]
- 71.Slawecki CJ, Betancourt M, Walpole T, Ehlers CL. Increases in sucrose consumption, but not ethanol consumption, following ICV NPY administration. Pharmacol Biochem Behav. 2000;66:591–594. doi: 10.1016/s0091-3057(00)00215-x. [DOI] [PubMed] [Google Scholar]
- 72.Smialowski A, Lewinska-Gastol L, Smialowska M. The behavioural effects of neuropeptide Y (NPY) injection into the rat brain frontal cortex. Neuropeptides. 1992;21:153–156. doi: 10.1016/0143-4179(92)90038-x. [DOI] [PubMed] [Google Scholar]
- 73.Smith GP. Satiation: From Gut to Brain. Oxford University Press; New York: 1998. [Google Scholar]
- 74.Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the micro-structure of licking behavior in the rat. Behav Neurosci. 1998;112:678–694. doi: 10.1037//0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- 75.Spector AC, St. John SJ. Role of taste in the microstructure of quinine ingestion by rats. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1687–R1703. doi: 10.1152/ajpregu.1998.274.6.R1687. [DOI] [PubMed] [Google Scholar]
- 76.Stricker-Krongrad A, Max JP, Musse N, Nicolas JP, Burlet C, Beck B. Increased threshold concentrations of neuropeptide Y for a stimulatory effect on food intake in obese Zucker rats–changes in the microstructure of the feeding behavior. Brain Res. 1994;660:162–166. doi: 10.1016/0006-8993(94)90851-6. [DOI] [PubMed] [Google Scholar]
- 77.Thomson HJ, Geoghegan JG, Farouk M, Saperstein LA, Chung K, Meyers WC, Pappas TN. Exogenous neuropeptide Y blocks myoelectric activity in the upper gastrointestinal tract of starved dogs. Brain neuropeptide Y converts a fasting pattern of myoelectric activity to a fed pattern. Scand J Gastroenterol. 1993;28:469–474. doi: 10.3109/00365529309098251. [DOI] [PubMed] [Google Scholar]
- 78.Torregrossa AM, Davis JD, Smith GP. Orosensory stimulation is sufficient and postingestive negative feedback is not necessary for neuropeptide Y to increase sucrose intake. Physiol Behav. 2006;87:773–780. doi: 10.1016/j.physbeh.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 79.Touzani K, Tramu G, Nahon JL, Velley L. Hypothalamic melanin-concentrating hormone and alpha-neoendorphin-immunoreactive neurons project to the medial part of the rat parabrachial area. Neuroscience. 1993;53:865–876. doi: 10.1016/0306-4522(93)90631-o. [DOI] [PubMed] [Google Scholar]
- 80.Wolak ML, DeJoseph MR, Cator AD, Mokashi AS, Brownfield MS, Urban JH. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol. 2003;464:285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- 81.Xu B, Li BH, Rowland NE, Kalra SP. Neuropeptide Y injection into the fourth cerebroventricle stimulates c-Fos expression in the paraventricular nucleus and other nuclei in the forebrain: effect of food consumption. Brain Res. 1995;698:227–231. doi: 10.1016/0006-8993(95)00905-6. [DOI] [PubMed] [Google Scholar]
- 82.Zamir N, Skofitsch G, Bannon MJ, Jacobowitz DM. Melanin-concentrating hormone: unique peptide neuronal system in the rat brain and pituitary gland. Proc Natl Acad Sci USA. 1986;83:1528–1531. doi: 10.1073/pnas.83.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zamir N, Skofitsch G, Jacobowitz DM. Distribution of immunoreactive melanin-concentrating hormone in the central nervous system of the rat. Brain Res. 1986;373:240–245. doi: 10.1016/0006-8993(86)90337-9. [DOI] [PubMed] [Google Scholar]
- 84.Zardetto-Smith AM, Gray TS. Catecholamine and NPY efferents from the ventrolateral medulla to the amygdala in the rat. Brain Res Bull. 1995;38:253–260. doi: 10.1016/0361-9230(95)00097-x. [DOI] [PubMed] [Google Scholar]
- 85.Zheng H, Patterson LM, Morrison C, Banfield BW, Randall JA, Browning KN, Travagli RA, Berthoud HR. Melanin concentrating hormone innervation of caudal brainstem areas involved in gastrointestinal functions and energy balance. Neuroscience. 2005;135:611–625. doi: 10.1016/j.neuroscience.2005.06.055. [DOI] [PubMed] [Google Scholar]