Abstract
High-fructose corn syrup (HFCS) accounts for as much as 40% of caloric sweeteners used in the United States. Some studies have shown that short-term access to HFCS can cause increased body weight, but the findings are mixed. The current study examined both short- and long-term effects of HFCS on body weight, body fat, and circulating triglycerides. In Experiment 1, male Sprague-Dawley rats were maintained for short term (8 wks) on (1) 12-h/day of 8% HFCS, (2) 12-h/day 10% sucrose, (3) 24-h/day HFCS, all with ad libitum rodent chow, or (4) ad libitum chow alone. Rats with 12-h access to HFCS gained significantly more body weight than animals given equal access to 10% sucrose, even though they consumed the same number of total calories but fewer calories from HFCS than sucrose. In Experiment 2, the long-term effects of HFCS on body weight and obesogenic parameters, as well as gender differences, were explored. Over the course of 6 or 7 months, both male and female rats with access to HFCS gained significantly more body weight than control groups. This increase in body weight with HFCS was accompanied by an increase in adipose fat, notably in the abdominal region, and elevated circulating triglyceride levels. Translated to humans, these results suggest that excessive consumption of HFCS may contribute to the incidence of obesity.
Keywords: obesity, high-fructose corn syrup, sucrose, body weight, triglycerides, fat pad, rat
Introduction
The introduction of high-fructose corn syrup (HFCS) as a cost-effective sweetener in the American diet has gradually led to a great increase in its use. From 1970 to 1990, consumption of HFCS increased more than 1000% and currently accounts for 40% of all added caloric sweeteners (Bray et al., 2004; Bray, 2010). The increase in HFCS use was accompanied by a decline in sucrose use during the same time period (Anderson, 2007). One common source of HFCS is caloric beverages (i.e., soft-drinks, colas). It is also a primary ingredient in baked goods, many cereals, breads, canned fruits, jams and jellies, desserts, and fruit juices (Hanover and White, 1993). It is estimated that nearly 7% of daily caloric consumption in the United States is from HFCS, an estimate that has been labeled as conservative (Bray et al., 2004). Other studies indicate that over 10% of daily calories come from fructose, of which 75% (in adults) and 82% (in children) is attributed to added sweeteners rather than naturally occurring fructose (Vos et al., 2008). Given its prevalence in the American diet, it is crucial to understand the behavioral and physiological effects of dietary HFCS.
The rise in obesity that has occurred since the introduction of HFCS into the American diet suggested a link between the two (Bray, 2008; Elliott et al., 2002). However, many have refuted the conjecture that HFCS alone is at fault, suggesting that sugars in general are the problem (Melanson et al., 2008). Some studies indicate that HFCS and sucrose elicit similar post-metabolic profiles (Melanson et al., 2008; Stanhope et al., 2008), but there are differences in how these sugars are metabolized and utilized in the body. HFCS-55 is 55% fructose, 42% glucose and 3% higher saccharides (White, 2008). Meals high in fructose have been shown to reduce circulating insulin and leptin levels in women (Teff et al., 2004). Thus, intake of HFCS would presumably not produce the degree of insulin or leptin-induced satiety that would ensue with a meal of sucrose, potentially fueling overeating. Studies have shown that pure fructose leads to increased plasma free fatty acids, leptin, adiponectin, abdominal adipose tissue and impaired insulin sensitivity (Alzamendi et al., 2009; Melanson et al., 2008), as well as increased leptin resistance and exacerbated weight-gain in rats that are subsequently maintained on a high-fat diet (Shapiro et al., 2008). However, very few studies have actually tested HFCS, and the literature is deficient of long-term studies (Bray, 2010).
The question investigated here is whether or not a standard diet supplemented with HFCS can cause obesity in male and female out-bred rats.
Methods
Male and female Sprague-Dawley rats were obtained from Taconic Farms (Germantown, NY) and housed individually on a reversed 12-h light: 12-h dark cycle. The Princeton University Institutional Animal Care and Use Committee approved all procedures
Experiment 1: Male rats with short term (2 month) HFCS access
Weight-matched, male rats (300-375 g, n=10/group) were fed either (1) ad libitum chow, (2) 24-h HFCS and chow, (3) 12-h HFCS and ad libitum chow, or (4) 12-h sucrose with ad libitum chow for 8 weeks (2 months). We selected these schedules to allow comparison of intermittent and continuous access, as our previous publications show limited (12-h) access to sucrose precipitates binge-eating behavior (Avena et al., 2006). The 12-h groups had access to sugar (HFCS or sucrose) starting 4 h into the dark phase each day. These sugars were selected because they are the primary sweeteners in many soft-drinks. HFCS was an 8% solution (Nature’s Flavors®, Formula 55, v/v dissolved in tap water, 0.24 Kcal/mL), and sucrose was given as a 10% solution (Domino® Granulated Pure Cane Sugar, w/v, dissolved in tap water, 0.4 Kcal/mL). Standard rodent chow was provided to all groups (LabDiet #5001, PMI, St. Louis, MO, 3.02 kcal/g). All animals had water available ad libitum (see Table 1 for complete list of diets).
Table 1.
Summary of experiments, diets and final body weight.
| Experiment | Diet | End Point Body Weight (g) |
|---|---|---|
| Experiment 1 Males: 8 wks |
1. 24-h HFCS + ad libitum Chow | 470±7 |
| 2.12-h HFCS + ad libitum Chow | 502±11* | |
| 3.12-h Sucrose + ad libitum Chow | 477±9 | |
| 4. Ad libitum Chow | 462±12 | |
| Experiment 2 Males: 6 mo |
1. 24-h HFCS + ad libitum Chow | 767±24* |
| 2. 12-h HFCS + ad libitum Chow | 718±28* | |
| 3. Ad libitum Chow | 616±36 | |
| Experiment 2 Females: 7 mo |
1. 24-h HFCS + ad libitum Chow | 355±12* |
| 2. 12-h HFCS + 12-h Chow | 323±9 | |
| 3. 12-h Sucrose + 12-h Chow | 333±10 | |
| 4. Ad libitum Chow | 328±10 |
Signifies statistical difference when compared to chow-fed control groups, p<0.05. See graphs for comparison of HFCS and sucrose groups.
HFCS, sucrose, and chow intakes were measured daily, and body weight was measured weekly. After 8 weeks on the diets, the rats were sacrificed via rapid decapitation and trunk blood was collected and assayed for blood glucose levels using the Analox GM7 Fast Enzymatic Metabolizer (Analox, Lunenburg, MA) as per the manufacturer’s instructions.
Experiment 2: male and female rats with long-term (6-7 months) access to HFCS
To determine the effects of long-term access to HFCS, male rats (initially 275-325g, n=8/group) were maintained on either (1) 24-h HFCS and chow, (2) 12-h HFCS and ad libitum chow, or (3) ad libitum chow (Table 1) for 6 months. Since we did not see effects of sucrose on body weight in Experiment 1 with males, we did not include sucrose groups in this long-term analysis in males. Access to chow was made a variable (12-h or ad libitum), to see if that had an effect on body weight. Measurements of HFCS and chow were taken as described in Experiment 1, and body weights were measured weekly for 6 months.
Female rats (150-200 g at the onset of the experiment, n=8/group) were also tested to determine if the findings applied to both sexes. These rats were maintained on either (1) 24-h -HFCS and ad libitum chow, (2) 12-h HFCS and 12-h chow, (3) 12-h sucrose and 12-h chow, or (4) ad libitum chow (table 1). In this study with females, we included a group with access to sucrose for comparison with HFCS, as well as 12-h access to chow, to determine if limited access to chow, in the presence of HFCS or sucrose, could affect body weight. All animals had water available ad libitum. Sucrose, HFCS, and chow intake were measured daily, as described in Experiment 1, and body weights were measured weekly for 7 months.
At the end of the experiment, animals from both the male and female studies were sacrificed by rapid decapitation at the end of the dark cycle. Trunk blood was collected and serum was analyzed, as per the manufacturers’ instructions, for triglycerides (TG) using enzymatic hydrolysis (Cayman Chemicals, kit #10010303) and insulin using Insulin ELISA kit (Calbiotech, Spring Valley, CA), per the manufacturers’ instructions. Unilateral body fat pads from three regions, abdominal, gonadal and intestinal, were collected and weighed individually and collectively by an observer blind to the experimental conditions.
Data from all groups were compared using ANOVAs, or repeated measures ANOVA, when appropriate, followed by post hoc pair-wise comparisons, when justified.
Results
Male rats with daily 12-h HFCS access gain more weight in 8 weeks than animals with equal access to sucrose
Animals with 12-h 8% HFCS access gained significantly more weight in 8 weeks than animals with 12-h 10% sucrose access (F(2,25)=3.42; p<0.05). Even though the 12-h HFCS group gained significantly more body weight, they were ingesting fewer calories from HFCS than the sucrose group was ingesting from sucrose (21.3 ± 2.0 Kcal HFCS vs. 31.3 ± 0.3 Kcal sucrose; F(1,16)=12.14; p<0.01). There was no overall difference in total caloric intake (sugar plus chow) among the sucrose group and two HFCS groups. Further, no difference was found in HFCS intake and total overall caloric intake in the groups given 12-h access versus 24-h access. Further, both groups consumed the same amount of HFCS on average (21.3 ± 2.0 Kcal HFCS in 12-h versus 20.1 ± 1.6 Kcal HFCS in 24-h), even though only the 12-h group showed a significant difference in body weight when compared with the control groups. There was no statistically significant difference in blood glucose levels among the groups.
Male rats with ad libitum HFCS for 6 months have increased body weight, abdominal fat and TG, compared to controls
Figure 1 shows that male rats with 12-h or 24-h access to HFCS with ad libitum chow gained significantly more weight than the control group with ad libitum chow alone (F(1,14)=5.07; p<0.05). The difference in body weight was significant by week 3 (F(2,21)=4.44; p<0.05). There was no significant difference in weight gain between the 12-h and 24-h HFCS groups (p>0.05); both gained more than the chow control rats. During the 6 months of study, the ad libitum chow group grew normally with a final weight that was 202% of their initial baseline body weight, whereas the 12-h HFCS group was 234% and the 24-h access HFCS group was 257% of baseline.
Figure 1.
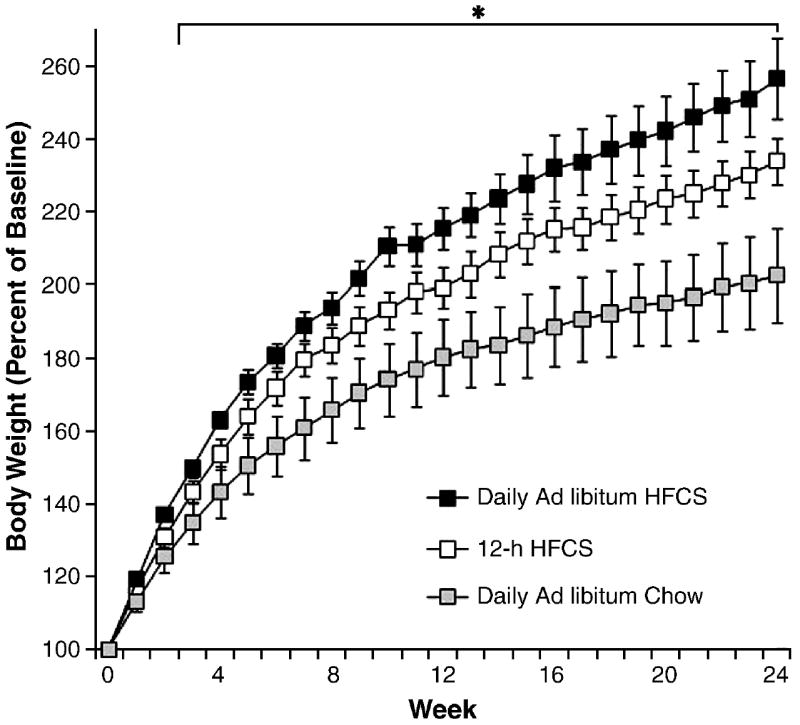
Body weight gain in male rats during 6 mo as percent of initial weight. Groups had daily 12-h access to HFCS and chow, 24-h access to HFCS and chow, or ad libitum chow. Males with access to HFCS gained significantly more weight over the duration of the experiment than animals with only chow access, reaching significance at week 3. *p<0.05. Values are means ± SEM.
As an indication of obesity, the rats with 24-h or 12-h HFCS had significantly heavier fat pads than control rats (F(4,35)=13.01; p<0.01; Fig. 4). Although all fat pads were heavier, this effect was most pronounced in the abdominal region (F(4,35)=8.36; p<0.05; Fig. 2).
Figure 4.
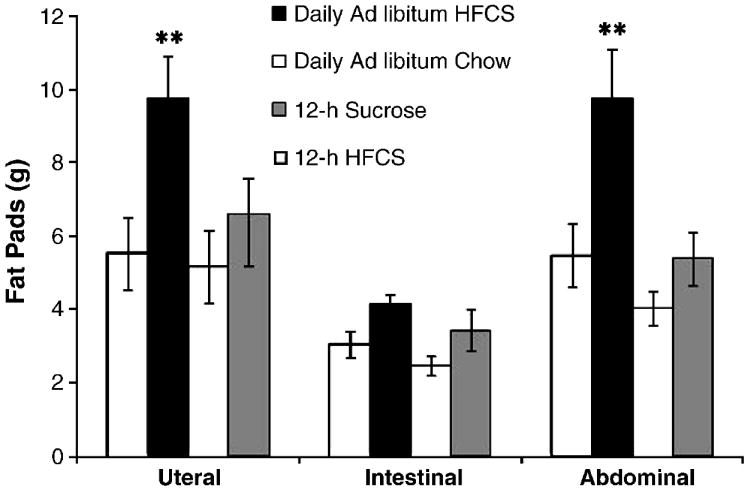
Females maintained on 24-h access to HFCS and chow showed increased abdominal and uteral fat pad weight. **p<0.05. Values are means ± SEM.
Figure 2.
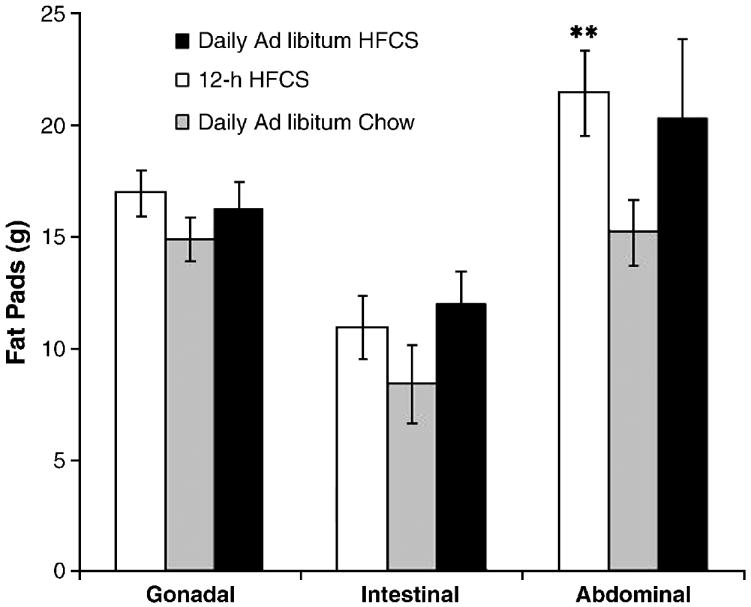
Males maintained on either 24- or 12-h HFCS diets showed increased abdominal fat pad weight. **p<0.05. Values are means ± SEM.
Serum assays revealed that the groups with 24-h or 12-h HFCS had elevated TG levels compared to ad libitum chow-fed controls (24-h HFCS= 201±29 mg/dL, 12-h HFCS= 195±29 mg/dL, ad libitum chow= 147±11 mg/dL TG; t(13)= 2.18; p<0.05). There were no differences among groups in serum insulin levels.
Female rats with 7 months of HFCS access gain significantly more body weight, have more abdominal fat and elevated TG levels compared with chow- and sucrose-fed controls
As seen in Figure 3, female rats with 24-h access to HFCS for 7 months gained more body weight than chow- and sucrose-fed controls (F(1,14)=8.74, p<0.01). Difference in body weight compared to ad libitum chow-fed controls was seen as early as week 5, and it reached statistical significance at week 24 (p<0.05). There was also a statistically significant difference in body weight, with 24-h HFCS rats weighing more than sucrose-fed rats at week 25 (F(4,35)=4.24, p<0.05). During the 7-month experimental period, the females with ad libitum chow gained a normal amount of weight, 177% from their initial baseline body weight. At the end of the study, the 12-h HFCS and sucrose groups were 183% of baseline, and the group with 24-h access to HFCS weighed the most, on average, ending the experiment at 200% of baseline.
Figure 3.
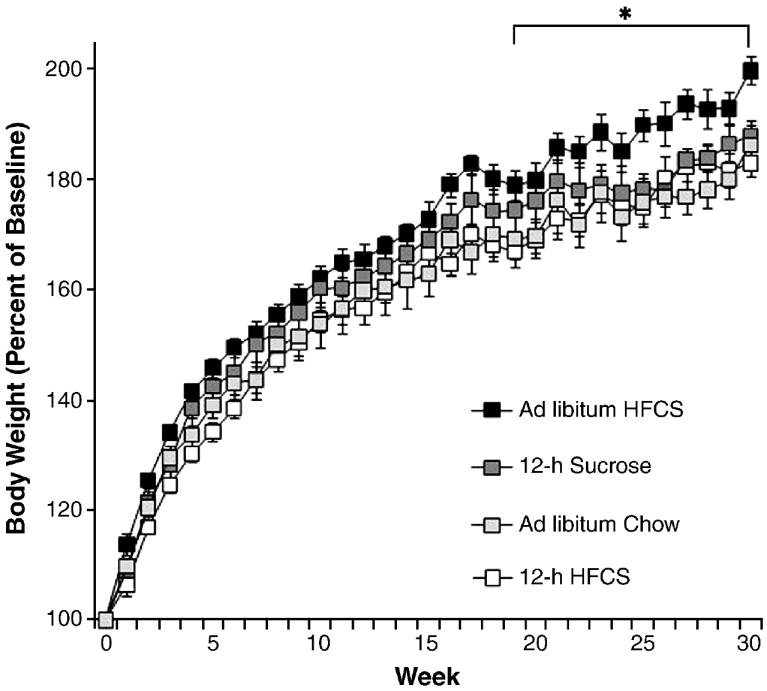
Body weight gain in female rats during 7 mo as percent of initial weight in rats with 12-h access to HFCS and chow, 24-h access to HFCS and chow, 12-h sucrose and chow, or ad libitum chow. Females with 24-h access to HFCS gained significantly more weight over the duration of the experiment than animals with sucrose access or chow access, data reached significance at week 19. *p<0.05. Values are means ± SEM.
Figure 4 shows that females with 24-h access to HFCS for 7 months had increased overall fat pad weight compared to chow-fed controls (F(4,35)=7.06; p<0.01). When analyzed separately both the fat pads surrounding the uterus (F(4,35)=4.83, p<0.01) and the abdomen (F(4,35)=5.59, p<0.01) were significantly heavier in 24-h access HFCS rats compared to ad libitum chow-fed controls.
After 7 months of access, the 24-h access HFCS group had significantly elevated TG levels compared to both ad libitum chow-fed controls and rats maintained on 12-h sucrose (24-h HFCS= 225±36 mg/dL, 12-h sucrose= 128±16 mg/dL, ad libitum chow-fed controls= 153±15 mg/dL; F(2,17)=4.03; p<0.05). No difference was found in TG levels for the 12-h HFCS group (128±7 mg/dL) when compared to chow-fed controls. There were no differences found among the groups in serum insulin levels.
Discussion
In Experiment 1 (short-term study, 8 wk), male rats with access to HFCS drank less total volume and ingested fewer calories in the form of HFCS (mean= 18.0 Kcal) than the animals with identical access to a sucrose solution (mean= 27.3 Kcal), but the HFCS rats, never the less, became overweight. In these males, both 24-h and 12-h access to HFCS led to increased body weight. In Experiment 2 (long-term study, 6-7 months), HFCS caused obesity greater than that of chow in both male and female rats. This increase in body weight was accompanied by an increase in fat accrual and circulating levels of TG, shows that this increase in body weight is reflective of obesity.
HFCS and Body Weight
While increased body weight alone does not necessarily represent obesity, the cooccurrence of obesogenic parameters such as increased body fat accrual and increased TG levels, lends support for the label of an obese status. In the current study, long term access to HFCS in rats led to obesity, while sucrose did not. We have previously shown that rats are able to adjust for the excess calories obtained when consuming 10% sucrose by taking in fewer calories of chow and thereby maintaining a normal body weight (Avena et al., 2008). Similarly, Jurgens found that mice with 73 days of access to a frucose solution showed increased adiposity, while mice given access to a 10% sucrose solution did not (Jurgens et al., 2005). This might be an effect of concentration of sucrose, as others have shown that at higher concentrations of sucrose (i.e., 32%) rats can become overweight (Ackroff et al., 2007; Light et al., 2009; Lindqvist et al., 2008,).
The data presented here suggest that rats with access to HFCS do not maintain a normal body weight. In a related study, adolescent female rats with access to HFCS showed increased body weight and fat pad weight compared to controls after 8 weeks of access. However, unlike the current study, there was no difference in body weight between HFCS and sucrose consuming rats, although a more rapid weight gain was noted in HFCS-consuming animals compared to fructose- and sucrose-consuming animals (Light et al., 2009).
Given the relationship in humans between the intake of calorically sweetened beverages and obesity (Olsen and Heitmann, 2009) as well as the development of increased risk of heart disease (Fung et al., 2009), several studies have addressed the effects of pure fructose, using it to represent the HFCS seen in the American diet. These studies indicate an increase in weight gain on diets rich in fructose (Stanhope et al., 2009). Compared to glucose-sweetened beverages, fructose-sweetened beverages lead to decreased circulating glucose, insulin and leptin concentrations, and elevated TG (Stanhope et al., 2008). Other studies have confirmed the effects of fructose on leptin, insulin and serum lipids (Abdel-Sayed et al., 2008; Adams et al., 2008; Faeh et al., 2005; Le et al., 2006; Le et al., 2009; Swarbrick et al., 2008; Teff et al., 2004; Teff et al., 2009). A correlation between body fat and circulating TG has been well established (Hollister et al., 1967) and a combination of abdominal adiposity and elevated TG has been linked with higher mortality rates (Bengtsson et al., 1993). Studies of pure fructose fed to laboratory animals show increased plasma free fatty acids, leptin, adiponectin, and abdominal adipose tissue, as well as impaired insulin sensitivity (Alzamendi et al., 2009; Melanson et al., 2008). Increased leptin resistance and weight gain have been shown when rats have access to fructose and then are subsequently maintained on a high-fat diet (Shapiro et al., 2008). Access to a high concentration of fructose, sucrose or glucose (23% w/v) leads to caloric over consumption and weight gain (Lindqvist et al., 2008).
HFCS and Fat Accrual
Obesity is characterized not just by an increase in body weight, but also by changes in body composition and certain hormone levels (Clegg et al., 2006; Dourmashkin et al., 2006). An increase in body-fat accrual is a key indicator of obesity (Clegg et al., 2006; Tamashiro et al., 2007). Light et al. (2009) report that rats with access to HFCS have increased fat pad accrual, while rats similarly fed sucrose do not. The significantly heavier abdominal fat pads in the HFCS-consuming groups in Experiment 2 support this finding, and demonstrate obesity in the present study.
Abdominal obesity in humans is considered the most dangerous form of fat accrual, leading to impaired health and diminished longevity (Seidell et al., 1989). Several studies compare the effects of acute sucrose and HFCS on weight gain and metabolic profiles in humans (Akhavan and Anderson, 2007; Melanson et al., 2008; Stanhope et al., 2008), and most conclude that sucrose and HFCS similarly affect the body in the short term (Stanhope et al., 2008). However, little is known of the long-term effects of HFCS consumption on fat accrual. The preclinical data in Experiment 2 suggest that long-term exposure to HFCS compared with sucrose differentially affects body fat accrual.
HFCS and TG
Rats with HFCS-access gain more weight than sucrose-consuming rats, even when ingesting fewer calories from their respective sugars. One important factor might be that the HFCS-induced weight gain is accompanied with hyper-triglyceridemia. Given the known role of TG in fat accrual (Owen et al., 1979), the elevated TG levels observed in the rats fed HFCS in the present study might account, in part, for the deposition of body fat. Further, a recent study confirms changes in plasma lipid profile, without indications of weight gain, after 10 weeks of intermittent access to either fructose or a 12.5% HFCS solution (Figlewicz et al., 2009). Storage of excess body fat subsequently can lead to chronic changes in leptin, insulin and corticosterone. In obese animals, leptin and insulin insensitivity can ensue, with the loss of hormonal satiety signals (Strader and Woods, 2005). Fructose consumption by adult rats has been shown to produce diminished glucose tolerance and insulin sensitivity as well as elevated TG, cholesterol, and body fat (de Moura et al., 2009; Elliott et al., 2002). Additionally, in the presence of obesity, elevated TG levels are commonly associated with a clustering of metabolic risk factors known as the metabolic syndrome (Grundy et al., 2004). Further adverse effects precipitated by increased fructose intake include negative effects on cardiovascular and kidney functions (Abdullah et al., 2009; Alzamendi et al., 2009; Lindqvist et al., 2008; Nguyen et al., 2009).
Previous research has suggested that an elevated level of circulating TG is, in part, responsible for an increase in high-fat intake (Chang et al., 2007). Therefore by chronically elevating serum TG levels, HFCS may create a propensity towards fat intake and fat deposition. This could work to induce leptin and insulin resistance. Taken together, leptin or insulin resistance and elevated TG serum levels promulgate food over-consumption and contribute to the corresponding obesity.
Gender Differences
In the present study, both male and female rats gained an excessive amount of weight when maintained on 24-h access to HFCS, however, the males gained significantly more weight than females, and at a faster pace. This is impressive given that male Sprague-Dawley rats have a steadily rising growth curve that can obscure obesity. Females normally show more gradual gain after adolescence such that extra weight gain is often obvious (see chow-fed controls in Fig. 1; Hoebel and Teitelbaum, 1966; Hubert et al., 2000). In the present study, male rats maintained on 12-h access to HFCS also gained significantly more weight than chow-fed controls, while female rats maintained on 12-h access did not. It is possible that this can be accounted for by the fact that these males had ad libitum chow, while the female had 12-h access to chow. It is possible that the lack of chow for 12-h daily suppressed weight gain and TG levels that might have otherwise been elevated in the female 12-h HFCS access group. This would indicate an effect of diet rather than a gender difference.
Differences in Sugars and Metabolism
HFCS-55, which is commonly used in processed food and drinks, is 55% fructose, 42% glucose and 3% higher saccharides (Corn Refiners Association, 2009; White, 2008). Given that sucrose is a disaccharide, which is metabolized to one fructose and one glucose molecule (Caspary, 1992), it has been argued that there is little difference between fructose and sucrose, since both provide about 50% fructose and 50% glucose in the blood stream; and until recently, there was no evidence that HFCS contributes to long-term weight gain beyond what sucrose contributes (Forshee et al., 2007). However, the present study suggests that HFCS and sucrose can have different effects on body weight and obesogenic measures.
HFCS is different than sucrose in many ways. First, HFCS-55 has proportionately slightly more fructose than sucrose (White, 2008). Second, fructose is absorbed further down the intestine than glucose, with much of the metabolism occurring in the liver, where it is converted to fructose-1-phsophate, a precursor to the backbone of the triglyceride molecule (Havel, 2005). Third, fructose is metabolically broken down before it reaches the rate-limiting enzyme (phosphofructokinase), thereby supplying the body with an unregulated source of three-carbon molecules. These molecules are transformed into glycerol and fatty acids, which are eventually taken up by adipose tissue, leading to additional adiposity (Hallfrisch, 1990). And fourth, HFCS causes aberrant insulin functioning, in that it bypasses the insulin-driven satiety system (Curry, 1989). Where as circulating glucose increases insulin release from the pancreas (Vilsboll et al., 2003), fructose does this less efficiently, because cells in the pancreas lack the fructose transporter (Curry, 1989; Sato et al., 1996). Typically, insulin released by dietary sucrose inhibits eating and increases leptin release (Saad et al., 1998), which in turn further inhibits food intake. As previously discussed, meals of HFCS have been shown to reduce circulating insulin and leptin levels (Teff et al., 2004). Thus, fructose intake might not result in the degree of satiety that would normally ensue with a meal of glucose or sucrose, and this could contribute to increased body weight.
Conclusion
In summary, rats maintained on a diet rich in HFCS for 6 or 7 months show abnormal weight gain, increased circulating TG and augmented fat deposition. All of these factors indicate obesity. Thus, over consumption of HFCS could very well be a major factor in the “obesity epidemic,” which correlates with the upsurge in the use of HFCS.
Acknowledgments
We thank Aimee Chen for her assistance in preparing the manuscript. The research was supported by USPHS grant AA-12882 (BGH) and DK-079793 (NMA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Sayed A, Binnert C, Le KA, Bortolotti M, Schneiter P, Tappy L. A high-fructose diet impairs basal and stress-mediated lipid metabolism in healthy male subjects. Br J Nutr. 2008;100:393–399. doi: 10.1017/S000711450789547X. [DOI] [PubMed] [Google Scholar]
- Abdullah MM, Riediger NN, Chen Q, Zhao Z, Azordegan N, Xu Z, Fischer G, Othman RA, Pierce GN, Tappia PS, Zou J, Moghadasian MH. Effects of long-term consumption of a high-fructose diet on conventional cardiovascular risk factors in Sprague-Dawley rats. Mol Cell Biochem. 2009;327:247–256. doi: 10.1007/s11010-009-0063-z. [DOI] [PubMed] [Google Scholar]
- Ackroff K, Bonacchi K, Magee M, Yiin YM, Graves JV, Sclafani A. Obesity by choice revisited: effects of food availability, flavor variety and nutrient composition on energy intake. Physiol Behav. 2007;92:468–478. doi: 10.1016/j.physbeh.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackroff K, Touzani K, Peets TK, Sclafani A. Flavor preferences conditioned by intragastric fructose and glucose: differences in reinforcement potency. Physiol Behav. 2001;72:691–703. doi: 10.1016/s0031-9384(01)00442-5. [DOI] [PubMed] [Google Scholar]
- Adams SH, Stanhope KL, Grant RW, Cummings BP, Havel PJ. Metabolic and endocrine profiles in response to systemic infusion of fructose and glucose in rhesus macaques. Endocrinology. 2008;149:3002–3008. doi: 10.1210/en.2007-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhavan T, Anderson GH. Effects of glucose-to-fructose ratios in solutions on subjective satiety, food intake, and satiety hormones in young men. Am J Clin Nutr. 2007;86:1354–1363. doi: 10.1093/ajcn/86.5.1354. [DOI] [PubMed] [Google Scholar]
- Alzamendi A, Giovambattista A, Raschia A, Madrid V, Gaillard RC, Rebolledo O, Gagliardino JJ, Spinedi E. Fructose-rich diet-induced abdominal adipose tissue endocrine dysfunction in normal male rats. Endocrine. 2009;35:227–232. doi: 10.1007/s12020-008-9143-1. [DOI] [PubMed] [Google Scholar]
- Anderson GH. Much ado about high-fructose corn syrup in beverages: the meat of the matter. Am J Clin Nutr. 2007;86:1577–1578. doi: 10.1093/ajcn/86.5.1577. [DOI] [PubMed] [Google Scholar]
- Avena NM, Bocarsly ME, Kim A, Rada P, Hoebel BG. After daily bingeing on a sucrose solution, prolonged food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol Behav. 2008 doi: 10.1016/j.physbeh.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Sugar bingeing in rats. Curr Protoc Neurosci. 2006;Chapter 9(Unit9):23C. doi: 10.1002/0471142301.ns0923cs36. [DOI] [PubMed] [Google Scholar]
- Bengtsson C, Bjorkelund C, Lapidus L, Lissner L. Associations of serum lipid concentrations and obesity with mortality in women: 20 year follow up of participants in prospective population study in Gothenburg, Sweden. BMJ. 1993;307:1385–1388. doi: 10.1136/bmj.307.6916.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray G. Fructose: should we worry? Int J Obes (Lond) 2008;32:S127–131. doi: 10.1038/ijo.2008.248. [DOI] [PubMed] [Google Scholar]
- Bray GA. Soft drink consumption and obesity: it is all about fructose. Curr Opin Lipidol. 20010 doi: 10.1097/MOL.0b013e3283346ca2. [DOI] [PubMed] [Google Scholar]
- Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- Caspary WF. Physiology and pathophysiology of intestinal absorption. Am J Clin Nutr. 1992;55:299S–308S. doi: 10.1093/ajcn/55.1.299s. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Gaysinskaya V, Marwil Z, Leibowitz SF. Dietary fat stimulates endogenous enkephalin and dynorphin in the paraventricular nucleus: role of circulating triglycerides. Am J Physiol Endocrino l Metab. 2007;292:E561–570. doi: 10.1152/ajpendo.00087.2006. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- Corn Refiners Association. SweetSurprise.com. 1701 Pennsylvania Ave, NW, Suite 950, Washington, DC: 2009. Questions & Answers about High Fructose Corn Syrup. [Google Scholar]
- Curry DL. Effects of mannose and fructose on the synthesis and secretion of insulin. Pancreas. 1989;4:2–9. doi: 10.1097/00006676-198902000-00002. [DOI] [PubMed] [Google Scholar]
- de Moura RF, Ribeiro C, de Oliveira JA, Stevanato E, de Mello MA. Metabolic syndrome signs in Wistar rats submitted to different high-fructose ingestion protocols. Br J Nutr. 2009;101:1178–1184. doi: 10.1017/S0007114508066774. [DOI] [PubMed] [Google Scholar]
- Dourmashkin JT, Chang GQ, Hill JO, Gayles EC, Fried SK, Leibowitz SF. Model for predicting and phenotyping at normal weight the long-term propensity for obesity in Sprague-Dawley rats. Physiol Behav. 2006;87:666–678. doi: 10.1016/j.physbeh.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76:911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- Faeh D, Minehira K, Schwarz JM, Periasamy R, Park S, Tappy L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes. 2005;54:1907–1913. doi: 10.2337/diabetes.54.7.1907. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Ioannou G, Bennett Jay J, Kittleson S, Savard C, Roth CL. Effect of moderate intake of sweeteners on metabolic health in the rat. Physiol Behav. 2009;98:618–624. doi: 10.1016/j.physbeh.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forshee RA, Storey ML, Allison DB, Glinsmann WH, Hein GL, Lineback DR, Miller SA, Nicklas TA, Weaver GA, White JS. A critical examination of the evidence relating high fructose corn syrup and weight gain. Crit Rev Food Sci Nutr. 2007;47:561–582. doi: 10.1080/10408390600846457. [DOI] [PubMed] [Google Scholar]
- Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89:1037–1042. doi: 10.3945/ajcn.2008.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24:e13–18. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Hallfrisch J. Metabolic effects of dietary fructose. FASEB J. 1990;4:2652–2660. doi: 10.1096/fasebj.4.9.2189777. [DOI] [PubMed] [Google Scholar]
- Hanover LM, White JS. Manufacturing, composition, and applications of fructose. Am J Clin Nutr. 1993;58:724S–732S. doi: 10.1093/ajcn/58.5.724S. [DOI] [PubMed] [Google Scholar]
- Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Teitelbaum P. Weight regulation in normal and hypothalamic hyperphagic rats. J Comp Physiol Psychol. 1966;61:189–193. doi: 10.1037/h0023126. [DOI] [PubMed] [Google Scholar]
- Hollister LE, Overall JE, Snow HL. Relationship of obesity to serum triglyceride, cholesterol, and uric acid, and to plasma-glucose levels. Am J Clin Nutr. 1967;20:777–782. doi: 10.1093/ajcn/20.7.777. [DOI] [PubMed] [Google Scholar]
- Hubert MF, Laroque P, Gillet JP, Keenan KP. The effects of diet, ad Libitum feeding, and moderate and severe dietary restriction on body weight, survival, clinical pathology parameters, and cause of death in control Sprague-Dawley rats. Toxicol Sci. 2000;58:195–207. doi: 10.1093/toxsci/58.1.195. [DOI] [PubMed] [Google Scholar]
- Jurgens H, Haass W, Castaneda TR, Schurmann A, Koebnick C, Dombrowski F, Otto B, Nawrocki AR, Scherer PE, Spranger J, Ristow M, Joost HG, Havel PJ, Tschop MH. Consuming fructose-sweetened beverages increases body adiposity in mice. Obes Res. 2005;13:1146–1156. doi: 10.1038/oby.2005.136. [DOI] [PubMed] [Google Scholar]
- Le KA, Faeh D, Stettler R, Ith M, Kreis R, Vermathen P, Boesch C, Ravussin E, Tappy L. A 4-wk highfructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr. 2006;84:1374–1379. doi: 10.1093/ajcn/84.6.1374. [DOI] [PubMed] [Google Scholar]
- Le KA, Ith M, Kreis R, Faeh D, Bortolotti M, Tran C, Boesch C, Tappy L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr. 2009;89:1760–1765. doi: 10.3945/ajcn.2008.27336. [DOI] [PubMed] [Google Scholar]
- Light HR, Tsanzi E, Gigliotti J, Morgan K, Tou JC. The type of caloric sweetener added to water influences weight gain, fat mass, and reproduction in growing Sprague-Dawley female rats. Exp Biol Med (Maywood) 2009;234:651–661. doi: 10.3181/0812-RM-368. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Baelemans A, Erlanson-Albertsson C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul Pept. 2008;150:26–32. doi: 10.1016/j.regpep.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Melanson KJ, Angelopoulos TJ, Nguyen V, Zukley L, Lowndes J, Rippe JM. High-fructose corn syrup, energy intake, and appetite regulation. Am J Clin Nutr. 2008;88:1738S–1744S. doi: 10.3945/ajcn.2008.25825E. [DOI] [PubMed] [Google Scholar]
- Olsen NJ, Heitmann BL. Intake of calorically sweetened beverages and obesity. Obes Rev. 2009;10:68–75. doi: 10.1111/j.1467-789X.2008.00523.x. [DOI] [PubMed] [Google Scholar]
- Owen OE, Reichard GA, Jr, Patel MS, Boden G. Energy metabolism in feasting and fasting. Adv Exp Med Biol. 1979;111:169–188. doi: 10.1007/978-1-4757-0734-2_8. [DOI] [PubMed] [Google Scholar]
- Saad MF, Khan A, Sharma A, Michael R, Riad-Gabriel MG, Boyadjian R, Jinagouda SD, Steil GM, Kamdar V. Physiological insulinemia acutely modulates plasma leptin. Diabetes. 1998;47:544–549. doi: 10.2337/diabetes.47.4.544. [DOI] [PubMed] [Google Scholar]
- Sato Y, Ito T, Udaka N, Kanisawa M, Noguchi Y, Cushman SW, Satoh S. Immunohistochemical localization of facilitated-diffusion glucose transporters in rat pancreatic islets. Tissue Cell. 1996;28:637–643. doi: 10.1016/s0040-8166(96)80067-x. [DOI] [PubMed] [Google Scholar]
- Seidell JC, Hautvast JG, Deurenberg P. Overweight: fat distribution and health risks. Epidemiological observations. A review. Infusionstherapie. 1989;16:276–281. doi: 10.1159/000222401. [DOI] [PubMed] [Google Scholar]
- Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1370–1375. doi: 10.1152/ajpregu.00195.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhope KL, Griffen SC, Bair BR, Swarbrick MM, Keim NL, Havel PJ. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucosesweetened beverages with meals. Am J Clin Nutr. 2008;87:1194–1203. doi: 10.1093/ajcn/87.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ. Consuming fructosesweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128:175–191. doi: 10.1053/j.gastro.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Swarbrick MM, Stanhope KL, Elliott SS, Graham JL, Krauss RM, Christiansen MP, Griffen SC, Keim NL, Havel PJ. Consumption of fructose-sweetened beverages for 10 weeks increases postprandial triacylglycerol and apolipoprotein-B concentrations in overweight and obese women. Br J Nutr. 2008;100:947–952. doi: 10.1017/S0007114508968252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KL, Hegeman MA, Nguyen MM, Melhorn SJ, Ma LY, Woods SC, Sakai RR. Dynamic body weight and body composition changes in response to subordination stress. Physiol Behav. 2007;91:440–448. doi: 10.1016/j.physbeh.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D’Alessio D, Havel PJ. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–2972. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- Teff KL, Grudziak J, Townsend RR, Dunn TN, Grant RW, Adams SH, Keim NL, Cummings BP, Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009;94:1562–1569. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilsboll T, Krarup T, Madsbad S, Holst JJ. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul Pept. 2003;114:115–121. doi: 10.1016/s0167-0115(03)00111-3. [DOI] [PubMed] [Google Scholar]
- Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med. 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- White JS. Straight talk about high-fructose corn syrup: what it is and what it ain’t. The American journal of clinical nutrition. 2008;88:1716S–1721S. doi: 10.3945/ajcn.2008.25825B. [DOI] [PubMed] [Google Scholar]


