Abstract
Emerging evidence suggests that TLR (Toll-like receptor) 4 and downstream pathways [MAPKs (mitogen-activated protein kinases) and NF-κB (nuclear factor κB)] play an important role in the pathogenesis of insulin resistance. LPS (lipopolysaccharide) and saturated NEFA (non-esterified fatty acids) activate TLR4, and plasma concentrations of these TLR4 ligands are elevated in obesity and Type 2 diabetes. Our goals were to define the role of TLR4 on the insulin resistance caused by LPS and saturated NEFA, and to dissect the independent contribution of LPS and NEFA to the activation of TLR4-driven pathways by employing TAK-242, a specific inhibitor of TLR4. LPS caused robust activation of the MAPK and NF-κB pathways in L6 myotubes, along with impaired insulin signalling and glucose transport. TAK-242 completely prevented the inflammatory response (MAPK and NF-κB activation) caused by LPS, and, in turn, improved LPS-induced insulin resistance. Similar to LPS, stearate strongly activated MAPKs, although stimulation of the NF-κB axis was modest. As seen with LPS, the inflammatory response caused by stearate was accompanied by impaired insulin action. TAK-242 also blunted stearate-induced inflammation; yet, the protective effect conferred by TAK-242 was partial and observed only on MAPKs. Consequently, the insulin resistance caused by stearate was only partially improved by TAK-242. In summary, TAK-242 provides complete and partial protection against LPS- and NEFA-induced inflammation and insulin resistance, respectively. Thus, LPS-induced insulin resistance depends entirely on TLR4, whereas NEFA works through TLR4-dependent and -independent mechanisms to impair insulin action.
Keywords: endotoxin, mitogen-activated protein kinase (MAPK), nuclear factor κB (NF-κB), saturated non-esterified fatty acid (NEFA), Toll-like receptor 4 (TLR4).
Abbreviations: AP-1, activator protein-1; 2-DG, 2-deoxy-D[1,2-3H]glucose; DAG, diacylglycerol; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GSK, glycogen synthase kinase; IκB, inhibitory κB; IKK, IκB kinase; IL, interleukin; iNOS, inducible nitric oxide synthase; IRAK, IL-1-receptor-associated kinase; IRS, insulin receptor substrate; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MCP1, monocyte chemoattractant protein-1; MEMα, minimum essential medium α; MyD88, myeloid differentiation factor 88; NEFA, non-esterified fatty acid(s); NF-κB, nuclear factor κB; NOD2, nucleotide-binding oligomerization domain-2; PKC, protein kinase C; RT–PCR, reverse transcription–PCR; TIR, Toll/IL-1 receptor; TIRAP, TIR domain-containing adaptor protein; TLR, Toll-like receptor; TNF, tumour necrosis factor; TRAF-6, TNF-receptor-associated factor-6
INTRODUCTION
Insulin resistance is a characteristic feature of obesity and Type 2 diabetes mellitus. Skeletal muscle is the main site responsible for insulin-stimulated glucose disposal; therefore muscle is are an important target in the prevention and management of insulin resistance and Type 2 diabetes mellitus. Increasing evidence suggests that signalling pathways downstream of TLR4 (Toll-like receptor 4) play an important role in the pathogenesis of insulin resistance in muscle [1–4].
TLR4 is a member of the TLR family of pattern recognition receptors that generate innate immune responses to pathogens by activating a cascade of pro-inflammatory events [5]. LPS (lipopolysaccharide), an outer membrane component of Gram-negative bacteria, is a ligand and potent agonist of TLR4. The fatty acid component of LPS (lipid A) is sufficient to activate TLR4, and studies in monocytes suggest that saturated, but not unsaturated NEFA (non-esterified fatty acids), may also activate TLR4 and downstream signalling pathways [6,7]. When a ligand binds to TLR4 and its co-receptors CD14 and MD-2, the adaptor molecules TIRAP [TIR (Toll/IL-1 receptor) domain-containing adaptor protein], MyD88 (myeloid differentiation factor 88), IRAK [IL (interleukin)-1-receptor-associated kinase] and TRAF-6 [TNF (tumour necrosis factor)-receptor-associated factor-6] are recruited to the TIR domain of TLR4. This protein–protein interaction cascade enables downstream signalling through the IKK [IκB (inhibitory κB) kinase]–NF-κB (nuclear factor κB) complex and the MAPK (mitogen-activated protein kinase) pathways. The TLR4 signalling cascade described above has been linked to insulin resistance at a number of levels. For example, in cultured cells, activation of IKK [8] and the MAPKs, JNK (c-Jun N-terminal kinase) [9] and p38 [10], inhibit insulin signal transduction by increasing serine phosphorylation of IRS-1 (insulin receptor substrate 1). Activation of the MAPK and NF-κB pathways in muscle cells also induces transcription of proinflammatory genes (i.e. TNFα and IL-6) whose protein products induce insulin resistance [11,12]. In line with these in vitro findings, elevated gene and protein expression of TLR4 [4] as well as elevated NF-κB [4,13] and JNK signalling [14], have all been observed in skeletal muscles of the insulin-resistant individuals.
The mechanism(s) responsible for the increased TLR4 signalling observed in insulin-resistant conditions is not clear. One potential mechanism involves activation of TLR4 by saturated NEFA from a nutritional/metabolic origin. Plasma NEFA concentrations are increased in most people with obesity and Type 2 diabetes mellitus [15] and an experimental elevation of plasma NEFA levels (by systemic lipid infusion) reduces whole body insulin sensitivity in individuals without Type 2 diabetes mellitus [16]. Deletion or inhibition of TLR4 protects against the deleterious effects of saturated NEFA on NF-κB signalling and insulin action in muscle in vitro [1,4] and ex vivo [2]. Accordingly, in rodents TLR4 plays an essential role in the insulin resistance caused by acute systemic lipid infusion [1,17] and high-fat diet [2,3,17]. Recent studies have demonstrated that obese and Type 2 diabetic subjects have elevated plasma LPS concentrations [18,19]; thus, in addition to saturated NEFA, an increase in plasma LPS concentration could be another mechanism responsible for elevated TLR4 signalling in these individuals. Indeed, chronic elevation of circulating intestinal-generated LPS (i.e. metabolic endotoxemia) has been hypothesized to play a role in the pathophysiology of insulin resistance [20,21]. That hypothesis proposes that high fat-containing diets alter gut flora growth and intestinal wall permeability, elevating enterobacterial production and translocation of LPS into the systemic circulation [20,21].
The finding that insulin-resistant animals [17] and human subjects [4] have enhanced TLR4 content and signalling in insulin-sensitive tissues, coupled with an increased concentration of two different TLR4 agonists: saturated NEFA [15] and LPS [18,19], suggest that TLR4 may have a causal input towards the development of insulin resistance. In this regard, pharmacological inhibitors of TLR4 signalling may be a useful strategy to enhance insulin sensitivity in insulin-resistant individuals. TAK-242 (resatorvid), a cyclohexene derivative, is a small-molecule inhibitor of TLR4 signalling, which was originally characterized as a novel anti-sepsis agent capable of inhibiting inflammatory mediator production [22]. Investigations into the mechanisms of action have shown that TAK-242 binds selectively to Cys747 in the TIR domain of TLR4 [23] and subsequently disrupts the ability of TLR4 to associate with TIRAP [24]. To date, TAK-242 is the only small-molecule compound reported to regulate protein–protein interactions between TLR4 and its adaptor molecules. TAK-242 has no binding affinity to other characterized TLRs [23,24]. The goal of the present study was to utilize TAK-242 to define the role of TLR4 on the insulin resistance caused by LPS and saturated NEFA, and to dissect the independent contribution of these endogenous TLR4 ligands to the activation of the MAPK and NF-κB pathways. L6 myotubes were used for this purpose as they are a bona fide cell culture system of muscle origin that responds to insulin. We hypothesized that TLR4 plays an important role on the effects caused by LPS and stearate, one of the most abundant saturated NEFA in human plasma, and that TAK-242 would protect against the inflammation and insulin resistance caused by these TLR4 agonists.
MATERIALS AND METHODS
Materials
MEMα (minimum essential medium α), FBS (fetal bovine serum), penicillin/streptomycin and trypsin/EDTA, PBS and Hepes buffer solution were obtained from Invitrogen. Blasticidin was from EMD Biosciences. TAK-242 was a gift from Takeda Pharmaceuticals. 2-DG (2-deoxy-D-[1,2-3H]glucose was obtained from PerkinElmer Life. LPS from Escherichia coli J5 (L5014), stearate (S4751), cytochalasin B, 2-DG, protease inhibitor cocktail and other chemicals, unless otherwise noted, were from Sigma. Insulin (Novolin-R) was obtained from Novo Nordisk. Reagents for PAGE were from Bio-Rad. Antibodies to phospho-Akt (Ser473), Akt, phospho-GSK (glycogen synthase kinase)-3α/β (Ser21/9), GSK-3α, GSK-3β, phospho-JNK (Thr183/Tyr185), JNK, phospho-NF-κB p65 (Ser536), phospho-p38 (Thr180/Tyr182), p38, phospho-IκBα (Ser32), IκBα, phospho-IRS-1 (Ser1101) and β-actin were from Cell Signaling Technology. Antibodies to TLR4 (M300), NF-κB p65 and phospho-IRS-1 (Ser307) were from Santa Cruz Biotechnology, whereas an antibody to total IRS-1 was from Invitrogen. ECL® (enhanced chemiluminescence) anti-rabbit IgG, HRP (horseradish peroxidase)-linked whole antibody and ECL® Plus Western Blotting Detection System was from GE Healthcare. The RNEasy Midi kit was from Qiagen. Reagents for QPCR (quantitative PCR) analysis, including Fast Advanced master mix and Taqman assays for detection of TNFα (Rn00562055_m1), IL6 (Rn01410330_m1), Ccl2 also known as MCP-1 (monocyte chemoattractant protein-1; Rn00580555_m1), TLR4 (Rn 00569848_m1) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (Rn01775763_g1) transcripts, were from Applied Biosystems. Nuclear extraction kits and DNA-binding ELISA kits for NF-κB p65 and phosphorylated c-Jun (Ser73) were from Active Motif.
Preparation of stearate and LPS solution
Stock solutions of TAK-242 were dissolved in DMSO to a final concentration of 100 mM, and were stored at −80°C. Prior to use, stock solutions were thawed and dissolved in MEMα to a final concentration of 1 μM. The 0.001% DMSO solution in MEMα served as a vehicle control. Stock solutions of stearate were prepared as described previously [25], and were stored at −80°C prior to use. Briefly, stearate was dissolved in a solvent of 0.1 M NaOH/70% (v/v) ethanol to a final concentration of 40 mM and heated to 70°C. Stock solutions were then further diluted to a final concentration of 400 μM in MEMα supplemented with 10% (v/v) FBS. The stearate solution was then allowed to incubate for 30 min at 37°C, which enabled stearate to complex with BSA present in the FBS. The final stearate/albumin ratio used in experiments was approximately 2.3:1. The same 0.1 M NaOH/70% ethanol/MEMα solution, without the addition of stearate, was used as a vehicle control.
Cell culture
L6-GLUT4myc (L6 myoblasts stably expressing GLUT4) [26] were maintained with α-MEM supplemented with 10% FBS, blasticidin S (2 μg/ml), and 1% (v/v) antibiotic/antimycotic solution (10000 units/ml penicillin G, 10 mg/ml streptomycin and 25 μg/ml amphotericin B) under 5% (v/v) CO2 at 37°C. To induce differentiation into myotubes, the percentage of FBS in the medium was reduced to 2%. All experiments were carried out 5–7 days later when >85% of the cells were differentiated as assessed visually through morphology changes and myotube formation. Dose-and time-response experiments were utilized to derive the maximum effective concentration (10, 100 and 1000 ng/ml) and stimulation time (10 min, 30 min, 1 h, 2 h, 6 h and 24 h) of LPS on inflammation (JNK phosphorylation). The dose/stimulation time of LPS used for all insulin signalling/sensitivity measurements was based on previously published data [27]. Cells were exposed to LPS (100 ng/ml) for 1 h (inflammatory assays) or 24 h (insulin signalling assays). Alternatively, cells were exposed to stearate (400 μM) or vehicle control for 1 or 6 h. The dose/stimulation time of stearate used for all experiments was based on published data [1,4] and our previous observations that insulin resistance is present after 6 h stearate stimulation. Prior to treatment with LPS or stearate, cells were pre-treated with TAK-242 (1 μM) or vehicle control for 1 h. TAK-242 remained in culture medium for the duration for the experiment. For measurement of insulin signalling proteins by immunoblotting and 2-DG transport (described below), cells were serum-deprived for 3 h followed by stimulation with/without insulin (5 nM, 10 min or 100 nM, 20 min).
Immunoblotting
Cells were lysed using lysis buffer (20 mM Tris/HCl, pH 7.5, 5 mM EDTA, 10 mM Na3PO4, 100 mM NaF, 2 mM Na3VO4, 1% Nonidet P40, 10 μM leupeptin, 3 mM benzamidine, 10 μg/ml aprotinin and 1 mM PMSF). Proteins were separated by SDS/PAGE (10% gel) and transferred to nitrocellulose membranes. Membranes were incubated overnight with primary antibodies of interest as indicated in the Figure legends. Detection of primary antibodies was performed using an appropriate peroxidase-conjugated IgG, and protein signals were visualized using ECL® reagents by exposure to Kodak autoradiographic film. Quantification of immunoblots was performed using ImageQuant software (Molecular Dynamics).
Quantitative RT–PCR (reverse transcription–PCR)
Total RNA was isolated using TRIzol® reagent (Sigma). One-step real-time RT–PCR was performed on an ABI-Prism-7900HT System using TaqMan One-Step RT–PCR Master Mix Reagents and Assay On-Demand primer/probes (Applied Biosystems). mRNA levels were normalized to GAPDH.
DNA-binding activity of nuclear transcription factor proteins
DNA-binding activity of NF-κB p65 and phosphorylated c-Jun (Ser73) was measured in nuclear extracts (10 and 0.5 μg, respectively) using an ELISA kit (Active Motif).
2-DG transport
L6 cell 2-DG transport was measured as described previously [28].
Statistical analysis
Data are presented as the means±S.E.M. Data were compared using one-way ANOVA with Tukey–Kramer multiple comparisons test (GraphPad Software). Significance was set at P<0.05.
RESULTS
LPS causes a time- and dose-dependent increase in JNK phosphorylation
First, we performed time- and dose-response experiments assessing the inflammatory response to several stimulation periods (10 min, 30 min, 1 h, 2 h, 6 h and 24 h) and doses of LPS (10, 100 and 1000 ng/ml). LPS caused a time-dependent increase in JNK phosphorylation over the first hour of stimulation, which was followed by a gradual decrease when measured at 6 and 24 h (Supplementary Figure S1 at http://www.bioscirep.org/bsr/033/bsr033e004add.htm). The maximum increase in JNK phosphorylation was observed with 100 ng/ml LPS, with no further increase achieved with 1 μg/ml LPS (Supplementary Figure S1). Based on these preliminary experiments, LPS (100 ng/ml) for 1 h was used for all further inflammatory measurements.
Effect of TAK-242 on LPS- and stearate-induced inflammatory responses
When a ligand binds to TLR4, the adaptor molecules TIRAP, MyD88, IRAK and TRAF-6 are sequentially recruited to the TIR domain of TLR4. This protein–protein interaction enables signalling through the IKK–NF-κB pathways. Phosphorylation by IKKβ targets IκBα for proteasomal degradation, which liberates NF-κB for translocation into the nucleus where it promotes the expression of numerous genes. TLR4 stimulation also leads to activation of the MAPK family, including JNK and p38, which may in turn regulate activation of the AP-1 (activator protein-1) transcription factor. Among the AP-1 family proteins, c-Jun is thought to play central roles in inflammatory responses [29].
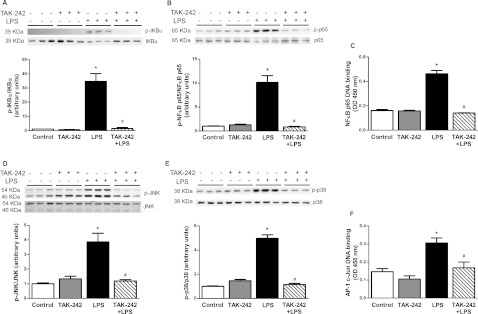
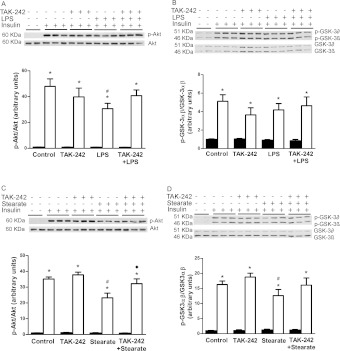
LPS treatment for 1 h caused a robust increase in the phosphorylation of IκBα (35-fold) and a concomitant reduction in cellular IκBα protein (Figure 1A), which was accompanied by an increase in the phosphorylation (10-fold, Figure 1B) and DNA binding (2.5-fold, Figure 1C) of NF-κB p65. Pre-treatment of myotubes with TAK-242 completely prevented the ability of LPS to increase IκBα phosphorylation, and, accordingly, cellular IκBα protein levels were maintained (Figure 1A). TAK-242 also fully prevented LPS-induced NF-κB p65 phosphorylation (Figure 1B) and DNA-binding (Figure 1C). Treatment of muscle cells with LPS caused a robust increase in the phosphorylation of JNK (3.9-fold, Figure 1D) and p38 (5.0-fold, Figure 1E), and these responses were completely blocked by TAK-242 (Figures 1D and 1E). Consistent with the effect of TAK-242 on LPS-induced JNK phosphorylation, TAK-242 completely prevented the increase (2.0-fold) in c-Jun DNA binding caused by LPS (Figure 1F).
Figure 1. Effect of TAK-242 on LPS-induced inflammatory responses.
L6 myotubes were incubated with/without TAK-242 (1 μM) for 1 h prior to LPS (100 ng/ml) for 1 h. Cells were lysed and equal amounts of total protein from each sample were immunoblotted with specific antibodies against phospho-IκBα (A), phospho-NF-κB p65 (B), phospho-JNK (D) and phospho-p38 (E). Data are expressed as a ratio of phosphorylated to total protein or total protein to β-actin (loading control) where appropriate, in arbitrary units as fold change from control. Representative immunoblots are shown above. Results are means±S.E.M. of three independent experiments performed in triplicate. NF-κB p65 (C) and c-Jun (F) DNA binding were measured in nuclear extracts by ELISA, n=3. *P<0.05 against control; #P<0.05 against LPS. P-, phospho.
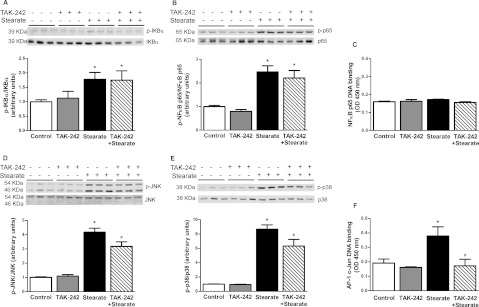
Treatment with stearate for 1 h (results not shown) and 6 h (Figure 2A) increased phosphorylation of IκBα by 70 and 80% respectively. Stimulation with stearate for 1 h (results not shown) and 6 h (Figure 2B) also increased NF-κB p65 phosphorylation (2.0- and 1.5-fold increase respectively). Interestingly, the increase in NF-κB p65 phosphorylation was not accompanied by enhanced DNA binding (Figure 2C). This finding was unexpected, although a previous study demonstrated that NF-κB p65 phosphorylation and transcriptional activity can occur in the absence of any effect on nuclear translocation or DNA binding of NF-κB [30]. Another notable finding was the lack of IκBα degradation in response to stearate. Moreover, and in contrast with its restoring effect on LPS-induced IκBα and NF-κB p65 phosphorylation, TAK-242 had no effect on stearate-induced IκBα (Figure 2A) or NF-κB p65 phosphorylation (Figure 2B). Treatment of cells with stearate for 1 h (results not shown) and 6 h (Figure 2D) increased phosphorylation of JNK (2.5- and 4.2-fold, respectively), whereas p38 phosphorylation was increased (8.7-fold) by stearate at 6 h (Figure 2E) but not 1 h (results not shown). A concomitant increase in c-Jun DNA-binding was observed after 6 h stearate treatment (2-fold, Figure 2F). TAK-242 had a partial inhibitory effect on 1 h (22% reduction, results not shown) and 6 h (32% reduction, Figure 2D) stearate-induced JNK phosphorylation, whereas c-Jun DNA binding was totally blocked by TAK-242 (Figure 2F). A partial inhibitory effect of TAK-242 on p38 phosphorylation was also observed (37% reduction, Figure 2E). In summary, LPS and stearate share some, but not all, features of activation of the NF-κB pathway, and TAK-242 prevented all of the effects of LPS but only some of those of stearate.
Figure 2. Effect of TAK-242 on stearate-induced inflammatory responses.
L6 myotubes were incubated with/without TAK-242 (1 μM) for 1 h prior to stearate (400 μM) for 6 h. Cells were lysed and equal amounts of total protein from each sample were immunoblotted with specific antibodies against phospho-IκBα (A), phospho-NF-κB p65 (B), phospho-JNK (D) and phospho-p38 (E). Data are expressed as a ratio of phosphorylated to total protein or total protein to β-actin (loading control) where appropriate, in arbitrary units as fold change from control. Representative immunoblots are shown above. Results are means±S.E.M. of three independent experiments performed in triplicate. NF-κB p65 (C) and c-Jun (F) DNA binding were measured in nuclear extracts by ELISA, n=3. *P<0.05 against control; #P<0.05 against stearate. P-, phospho.
TAK-242 protects against LPS- and stearate-induced inflammatory gene expression
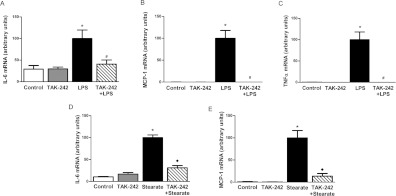
NF-κB and AP-1 are transcription factors downstream of TLR4 that are implicated in the inducible expression of a variety of genes. Classic genes implicated in the pathogenesis of insulin resistance in muscle include IL-6, MCP-1 and TNFα. Thus, we investigated the ability of TAK-242 to protect against expression of these inflammatory genes by LPS and stearate. In line with activation of the MAPK and NF-κB signalling pathways, LPS caused a robust increase in IL-6 mRNA expression (3.5-fold) and TAK-242 blocked this effect (Figure 3A). MCP-1 and TNFα mRNA expression was detected only in cells stimulated with LPS (Figures 3B and 3C); LPS caused a robust increase in expression of these inflammatory genes, and TAK-242 completely prevented these effects. With regard to stearate, treatment for 6 h caused a robust increase (10-fold) in IL-6 mRNA expression and TAK-242 blocked this effect almost completely (Figure 3D). MCP-1 mRNA was detected in cells stimulated with stearate for 6 h but was undetected in control and TAK-242 treated cells, which suggests that TAK-242 was able to completely block the effects of stearate on MCP-1 gene expression (Figure 3E). No increase in IL-6 or MCP-1 mRNA expression was observed in cells stimulated with stearate for 1 h (results not shown). Moreover, TNFα could not be detected in cells with either 1 or 6 h stearate stimulation (results not shown). In summary, although the pattern of activation of inflammatory genes by LPS and stearate overlaps only partially, TAK-242 completely blocked the ability of LPS and stearate to induce the expression of key genes implicated in the pathogenesis of insulin resistance.
Figure 3. Effect of TAK-242 on inflammatory gene expression.
L6 myotubes were incubated with/without TAK-242 (1 μM) for 1 h prior to LPS (100 ng/ml) for 1 h (A–C) or stearate (400 μM) for 6 h (D and E). RNA was extracted from cells and IL-6 (A and D), MCP-1 (B and E) and TNFα (C) mRNA expression was determined by quantitative real-time RT–PCR as described in the Materials and methods section. Data are in arbitrary units normalized to 100 in LPS/stearate stimulated cells. Results are means±S.E.M. of three independent experiments performed in triplicate. *P<0.05 against control; #P<0.05 against LPS; ◆P<0.05 against stearate.
TAK-242 protects against the negative effects of LPS and stearate on insulin signalling transduction
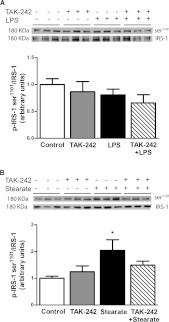
Serine phosphorylation of IRS-1 impairs insulin signalling by reducing tyrosine phosphorylation of IRS-1 by the insulin receptor β subunit. Some studies propose that phosphorylation of IRS-1 at Ser307 (Ser312 in human IRS-1) by JNK [9] and IKKβ [8] is a mechanism by which NEFA and cytokines impair insulin signalling. Phosphorylation of IRS-1 at Ser1101 also impairs tyrosine-phosphorylation of IRS-1 and downstream insulin signalling at the level of Akt and AS160 [31]. Concomitant with impairments in Akt phosphorylation, insulin-stimulated GSK-3 phosphorylation is reduced, which results in impaired activation of glycogen synthase [32]. In addition to direct negative effects of the IKK–NF-κB and MAPK pathways on insulin-signalling intermediates, gene products of these pathways (TNFα and MCP-1) may also further impair insulin signalling [11]. Considering that TAK-242 blocked LPS- and stearate-induced inflammatory responses, we explored whether TAK-242 protects against impairments in insulin signalling caused by these stimuli. LPS (24 h) had no effect on phosphorylation of IRS-1 at Ser307 (results not shown) or Ser1101 (Figure 4A). Rather, LPS reduced (approximately 36%) insulin-signalling downstream, at the level of Akt phosphorylation on Ser473 (Figure 5A). There was also a tendency for reduced (approximately 18%) insulin-stimulated GSK-3α/β phosphorylation; however, this did not reach statistical significance (Figure 5B). These inhibitory effects of LPS on insulin signalling were prevented by pre-treating cells with TAK-242 (Figures 5A and 5B). In contrast with LPS, treatment with stearate (6 h) increased phosphorylation of IRS-1 at Ser1101 (2-fold, Figure 4B), whereas it failed to affect Ser307 (results not shown). Stearate also reduced insulin-stimulated Akt (approximately 20%, Figure 5C) and GSK-3α/β (approximately 25%, Figure 5D) phosphorylation. Notably, the ability of stearate to increase IRS-1 phosphorylation at Ser1101 (Figure 4B), and to reduce insulin-stimulated Akt (Figure 5C) and GSK-3α/β phosphorylation (Figure 5D) was no longer observed when cells were pre-treated with TAK-242. Stearate also reduced insulin signalling downstream of Akt to its substrate AS160, which was similarly prevented by TAK-242 (results not shown).
Figure 4. Effect of TAK-242 on IRS-1 phosphorylation.
L6 myotubes were incubated with/without TAK-242 (1 μM) for 1 h prior to LPS (100 ng/ml) for 1 h (A) or stearate (400 μM) for 6 h (B). Cells were lysed and equal amounts of total protein from each sample were immunoblotted with specific antibodies against phospho-IRS-1 on Ser1101 (p-IRS-1 Ser1101) and total IRS-1. Data are expressed as a ratio of phosphorylated to total protein, in arbitrary units as fold change from control. Representative immunoblots are shown above. Results are means±S.E.M. of two independent experiments performed in triplicate. *P<0.05 against control.
Figure 5. Effect of TAK-242 on insulin-stimulated phosphorylation of Akt and GSK-3α/β.
L6 myotubes were incubated with/without TAK-242 (1 μM) for 1 h prior to LPS (100 ng/ml) for 1 h (A and B) or stearate (400 μM) for 6 h (C and D), then with/without insulin (5 nM) for 10 min. Cells were lysed and equal amounts of total protein from each sample were immunoblotted with specific antibodies against phospho-Akt (A and C) and phospho-GSK-3α/β (B and D). Data are expressed as a ratio of phosphorylated to total protein, in arbitrary units as fold change from control. Results are means±S.E.M. of three independent experiments performed in triplicate. *P<0.05 against basal; #P<0.05 against insulin-stimulated control; ◆P<0.05 against insulin-stimulated stearate. P-, phospho.
TAK-242 protects against LPS- and stearateinduced reduction in glucose transport
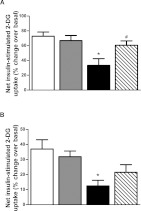
Insulin signalling through IRS-1 and Akt leads to enhanced glucose transport by increasing GLUT4 translocation to the cell membrane. Pre-treatment with LPS for 24 h reduced insulin-stimulated glucose transport by approximately 25% (Figure 6A). TAK-242 completely prevented the ability of LPS to reduce insulin-stimulated glucose transport (Figure 6A). Stearate also reduced (approximately 20%) insulin-stimulated glucose transport (Figure 6B). TAK-242 partially restored insulin-stimulated glucose transport, such that the reduction by stearate was no longer statistically significant (Figure 6B).
Figure 6. Effect of TAK-242 on insulin-stimulated 2-DG uptake.
L6 myotubes were incubated with/without TAK-242 (1 μM) for 1 h prior to treatment with LPS (100 ng/ml) for 1 h (A) or stearate (400 μM) for 6 h (B). Following 2 h of serum starvation, cells were stimulated in the presence or absence of insulin (100 nM for 20 min) and then assayed for 2-DG uptake. Data are in arbitrary units as percentage change over basal 2-DG uptake. Results are means±S.E.M. of four independent experiments performed in triplicate. *P<0.05 against control; #P<0.05 against LPS.
DISCUSSION
There is growing recognition that insulin-resistant (obese and Type 2 diabetic) individuals are subject to systemic low-grade inflammation [33]. There is also increasing evidence suggesting that signalling through TLR4 contributes to the inflammatory state and insulin resistance observed in these subjects [2,4]. Two important hypotheses have emerged to explain this notion. Proponents of the ‘metabolic endotoxemia’ theory suggest that sustained elevation of circulating LPS, a result of perturbations in the gut microbiota and increased intestinal permeability of LPS, leads to increased activation of TLR4 [20,21]. The second theory proposes that chronic TLR4 activation is a consequence of elevated circulating NEFA [2,4,17], secondary to increased NEFA release from an enlarged adipose tissue mass and/or reduced NEFA oxidation/clearance [34]. Moreover, there are data suggesting that saturated NEFA may act in synergy with LPS to promote inflammation and insulin resistance in obesity and Type 2 diabetes mellitus [35]. In this regard, pharmacological inhibitors of TLR4 signalling may be useful strategies to protect against insulin resistance in muscle. By utilizing the selective TLR4 inhibitor, TAK-242, in the present study we show that LPS-induced inflammation and insulin resistance is dependent entirely on TLR4, while saturated NEFA work partially through TLR4 to induce these deleterious effects. Moreover, our systematic analysis of inflammatory and insulin signals reveal similarities and differences in the actions of the TLR4 agonist LPS and the saturated fatty acid, stearate.
TAK-242 completely prevented LPS-induced inflammation and insulin resistance in muscle cells. Reduced signalling through the NF-κB and MAPK pathways followed by restored insulin-stimulated Akt phosphorylation provide a mechanism for the ability of TAK-242 to protect against LPS-induced insulin resistance. Our findings are in accordance with the study of Holland et al. [3], in which the insulin resistance caused by LPS ex vivo was dependent on TLR4-mediated IKKβ activation and ceramide production. They are also concordant with Pilon et al. [27], who found that mice lacking the inflammatory cytokine-mediated enzyme, iNOS (inducible nitric oxide synthase), were protected from LPS-induced insulin resistance in muscle, a result of diminished nitrosylation of IRS-1. Increased expression of SOCS (suppressor of cytokine signalling) in muscle also participates in LPS-induced insulin resistance through negative feedback loops in cytokine signalling [36]. Since LPS increases cytokine expression in muscle (iNOS, TNFα, IL-6 etc.) via a TLR4-dependent mechanism [37], these data lend additional evidence that TLR4 is required for LPS-induced insulin resistance in muscle in vitro.
Differences and similarities between LPS- and stearate-induced inflammatory responses
Although both LPS and stearate activated inflammatory responses, they shared only partial aspects of these responses. Both LPS and stearate elevated the phosphorylation of JNK, p38, IκBα and NF-κB p65, and induced IL-6 and MCP-1 gene expression, but only LPS reduced IκBα mass, p65 binding to DNA, and TNFα gene expression. Compared with its full-spectrum action on LPS effects, TAK-242 conferred partial protection against stearate-induced inflammation and insulin resistance. The TLR4 inhibitor prevented stearate effects on phosphorylation of JNK and p38 MAPK, but did not prevent phosphorylation of IκBα or NF-κB p65. These non-TLR4 mediated inflammatory responses to stearate may potentially arise through activation of other innate immune receptors that engage the NF-κB signalling pathway. Indeed, direct activation of the cytosolic pattern recognition receptor, NOD2 (nucleotide-binding oligomerization domain-2), by its agonist muramyl dipeptide, activated inflammatory signals and dampened insulin action in muscle cells [38]. Future studies should reveal whether stearate acts through this or other inflammation sensors.
Differences and similarities between LPS- and stearate-induced insulin resistance
Previous studies have shown that JNK-mediated IRS-1 phosphorylation on serine residues is an important mechanism mediating inflammatory-mediated insulin resistance [9,10,39,40]. This action is thought to occur through phosphorylation of IRS-1 on Ser307. On the other hand, IRS-1 phosphorylation on Ser1101 is mediated by novel PKCs (protein kinase Cs) and also contributes to insulin resistance by reducing tyrosine phosphorylation of IRS-1 [31,41]. In the present study, increased stearate-induced JNK phosphorylation and c-Jun DNA binding occurred with a concomitant increase in IRS-1 phosphorylation on Ser1101, but not Ser307. TAK-242 reduced IRS-1 Ser1101 phosphorylation and subsequently restored insulin-stimulated Akt phosphorylation and glucose transport. Our observations are in good agreement with previous studies, which have shown that inhibition or absence of TLR4 confers partial protection against the detrimental effects of saturated NEFA on insulin action [1,2,4,17]. The incomplete protection against NEFA-induced inflammation and insulin resistance by TLR4 inhibition using TAK-242 suggests that TLR4-independent mechanisms also mediate the effects of saturated NEFA. In addition to the possible contribution of other inflammation sensors such as NOD2, direct metabolic action occurs through augmented intracellular pools of DAGs (diacylglycerols) [1] and ceramides [42] in response to elevated NEFA. DAGs activate PKCs, which have been linked to inhibition of insulin action via increased phosphorylation of IRS-1 on serine residues [31,43], while ceramides work downstream on Akt to cause insulin resistance [3,44]. Notably, recent findings suggest that TLR4 is an upstream signalling component required for saturated NEFA-induced ceramide biosynthesis in muscle [3]. It is possible that one or more of these TLR4-independent mechanisms act synergistically with the TLR4 signalling pathway such that inhibition of one pathway is not sufficient to completely protect against lipid-induced insulin resistance.
Potential mechanisms underlying restoration of insulin action by TAK-242
Previous studies have shown that inhibition or absence of MAPKs [45] or NF-κB [46] protects against insulin resistance in vitro and in vivo. JNK [9,39], p38 [10] and the upstream regulator of NF-κB, IKKβ [8], may promote insulin resistance by phosphorylation of IRS-1 at serine residues. IKKβ may also inhibit insulin signalling intermediates downstream of IRS-1 at the level of PI3K (phosphoinositide 3-kinase) and Akt [46]. In the present study, both LPS and stearate increased JNK and p38 phosphorylation. However, only stearate increased IRS-1 phosphorylation at Ser1101. It is possible that LPS works on other IRS-1 serine phosphorylation sites to induce insulin resistance. Another plausible explanation is that the brief increase in JNK phosphorylation by LPS (which peaks at 1 h) does not endure long enough to promote insulin resistance through IRS-1 (at 24 h). Rather, the more persistent increase in signalling through the NF-κB pathway by LPS may act downstream on Akt phosphorylation to cause insulin resistance. Collectively, our findings suggest that restoration of insulin-stimulated Akt phosphorylation and glucose transport by TAK-242 may be a direct result of reduced signalling through the LPS-activated NF-κB and MAPK pathways. Conversely, TAK-242-mediated reductions in stearate-induced MAPK signalling may account for the observed restoration of IRS-1 phosphorylation on Ser1101, and subsequently the increased insulin-stimulated Akt phosphorylation and glucose transport.
LPS and stearate may also work through TLR4 at the transcriptional level to cause insulin resistance. Cytokines expressed locally in muscle function in an autocrine/paracrine manner to regulate insulin action [47]. TNFα and MCP-1 can directly cause insulin resistance in muscle in vitro, and they may further activate the JNK and NF-κB pathways through a feed-forward mechanism [47]. In the present study, the increase in NF-κB DNA binding caused by LPS was associated with increased gene expression of IL-6, TNFα and MCP-1. Surprisingly, stearate did not increase NF-κB DNA binding. Rather, stearate increased DNA binding of c-Jun and this occurred in association with increased MCP-1 and IL-6 gene expression. TAK-242 completely prevented LPS-induced NF-κB DNA binding, and, accordingly, gene expression of TNFα, IL-6 and MCP-1 was blocked. TAK-242 also blocked stearate-induced DNA binding of phosphorylated c-Jun, and, in turn, blunted mRNA expression of IL-6 and MCP-1. Taken together, the data indicate that, in muscle cells, LPS regulates gene expression via the NF-κB and MAPK pathways, and this effect is entirely dependent on TLR4. In contrast, stearate has a more pronounced effect to regulate gene expression through the MAPKs over the NF-κB axis, and this response is partially mediated by TLR4.
It is noteworthy that the concentration of LPS and stearate used in the present study were supraphysiologic. Nonetheless, data from the present study demonstrate that TAK-242 is a useful experimental tool for the study of the physiological significance of TLR4 in muscle. TAK-242 reached Phase III clinical trials as an antisepsis agent following promising preclinical efficacy of TAK-242 in cell and animal models [22]. However, clinical studies were discontinued in 2009 due to a failure to suppress cytokine levels in patients with severe sepsis [48]. Severe sepsis is an acute severe inflammatory state in which plasma levels of LPS may increase approximately 100-fold [49]. In contrast, insulin resistance is characterized by a state of chronic low-grade inflammation, whereby circulating TLR4 ligands (LPS and NEFA) are modestly increased by 2- to 3-fold [15,20]. Although TAK-242 may be ineffective to protect against severe sepsis and septic shock, TLR4 inhibitors may be a valuable therapy for low-grade inflammatory diseases such as obesity and Type 2 diabetes mellitus.
In summary, the present study is the first to characterize the selective TLR4 inhibitor, TAK-242, as a modulator of insulin action and glucose metabolism in a muscle system. Collectively, our findings reveal that LPS-induced inflammation and insulin resistance are fully mediated by TLR4, whereas saturated NEFA work partially through TLR4 to induce inflammation and insulin resistance. Given the key role that TLR4 and downstream signalling pathways play in regulating inflammation and insulin resistance in muscle [1–4], inhibition of TLR4-mediated signalling is an appealing target for therapeutic development.
Online data
AUTHOR CONTRIBUTION
Sophie Hussey and Nicolas Musi designed the study. Sophie Hussey, Hanyu Liang, Sheila Costford, Alicia Sanchez-Avila and Brain Ely helped in conducting the experiments/data collection. Sophie Hussey and Sheila Costford analysed the data. Sophie Hussey, Sheila Costford, Amira Klip, Ralph DeFronzo and Nicolas Musi wrote the paper.
FUNDING
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) [grant numbers RO1-DK80157 and RO1-DK089229 (to N.M.), and RO1-DK24092 (to R.A.D.)] and the Canadian Institutes of Health Research [grant number MT12601 (to A.K.)].
References
- 1.Radin M. S., Sinha S., Bhatt B. A., Dedousis N., O’Doherty R. M. Inhibition or deletion of the lipopolysaccharide receptor Toll-like receptor-4 confers partial protection against lipid-induced insulin resistance in rodent skeletal muscle. Diabetologia. 2008;51:336–346. doi: 10.1007/s00125-007-0861-3. [DOI] [PubMed] [Google Scholar]
- 2.Tsukumo D. M., Carvalho-Filho M. A., Carvalheira J. B., Prada P. O., Hirabara S. M., Schenka A. A., Araujo E. P., Vassallo J., Curi R., Velloso L. A., Saad M. J. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 3.Holland W. L., Bikman B. T., Wang L. P., Yuguang G., Sargent K. M., Bulchand S., Knotts T. A., Shui G., Clegg D. J., Wenk M. R., et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest. 2011;121:1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reyna S. M., Ghosh S., Tantiwong P., Meka C. S., Eagan P., Jenkinson C. P., Cersosimo E., DeFronzo R. A., Coletta D. K., Sriwijitkamol A., Musi N. Elevated Toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. 2008;57:2595–2602. doi: 10.2337/db08-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 6.Lee J. Y., Sohn K. H., Rhee S. H., Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 7.Lee J. Y., Plakidas A., Lee W. H., Heikkinen A., Chanmugam P., Bray G., Hwang D. H. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 2003;44:479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Gao Z., Hwang D., Bataille F., Lefevre M., York D., Quon M. J., Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor κB kinase complex. J. Biol. Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 9.Aguirre V., Uchida T., Yenush L., Davis R., White M. F. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J. Biol. Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 10.Hemi R., Yochananov Y., Barhod E., Kasher-Meron M., Karasik A., Tirosh A., Kanety H. p38 mitogen-activated protein kinase-dependent transactivation of ErbB receptor family: a novel common mechanism for stress-induced IRS-1 serine phosphorylation and insulin resistance. Diabetes. 2011;60:1134–1145. doi: 10.2337/db09-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Aguila L. F., Claffey K. P., Kirwan J. P. TNFα impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am. J. Physiol. 1999;276:E849–E855. doi: 10.1152/ajpendo.1999.276.5.E849. [DOI] [PubMed] [Google Scholar]
- 12.Kim H. J., Higashimori T., Park S. Y., Choi H., Dong J., Kim Y. J., Noh H. L., Cho Y. R., Cline G., Kim Y. B., Kim J. K. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes. 2004;53:1060–1067. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- 13.Sriwijitkamol A., Christ-Roberts C., Berria R., Eagan P., Pratipanawatr T., DeFronzo R. A., Mandarino L. J., Musi N. Reduced skeletal muscle inhibitor of κB β content is associated with insulin resistance in subjects with type 2 diabetes: reversal by exercise training. Diabetes. 2006;55:760–767. doi: 10.2337/diabetes.55.03.06.db05-0677. [DOI] [PubMed] [Google Scholar]
- 14.Bandyopadhyay G. K., Yu J. G., Ofrecio J., Olefsky J. M. Increased p85/55/50 expression and decreased phosphotidylinositol 3-kinase activity in insulin-resistant human skeletal muscle. Diabetes. 2005;54:2351–2359. doi: 10.2337/diabetes.54.8.2351. [DOI] [PubMed] [Google Scholar]
- 15.Reaven G. M., Chen Y. D. Role of abnormal free fatty acid metabolism in the development of non-insulin-dependent diabetes mellitus. Am. J. Med. 1988;85:106–112. doi: 10.1016/0002-9343(88)90402-0. [DOI] [PubMed] [Google Scholar]
- 16.Boden G., Jadali F., White J., Liang Y., Mozzoli M., Chen X., Coleman E., Smith C. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J. Clin. Invest. 1991;88:960–966. doi: 10.1172/JCI115399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., Flier J. S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creely S. J., McTernan P. G., Kusminski C. M., Fisher M., Da Silva N. F., Khanolkar M., Evans M., Harte A. L., Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007;292:E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 19.Al-Attas O. S., Al-Daghri N. M., Al-Rubeaan K., da Silva N. F., Sabico S. L., Kumar S., McTernan P. G., Harte A. L. Changes in endotoxin levels in T2DM subjects on anti-diabetic therapies. Cardiovasc. Diabetol. 2009;8:20. doi: 10.1186/1475-2840-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cani P. D., Amar J., Iglesias M. A., Poggi M., Knauf C., Bastelica D., Neyrinck A. M., Fava F., Tuohy K. M., Chabo C., et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 21.Cani P. D., Bibiloni R., Knauf C., Waget A., Neyrinck A. M., Delzenne N. M., Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 22.Yamada M., Ichikawa T., Ii M., Sunamoto M., Itoh K., Tamura N., Kitazaki T. Discovery of novel and potent small-molecule inhibitors of NO and cytokine production as antisepsis agents: synthesis and biological activity of alkyl 6-(N-substituted sulfamoyl)cyclohex-1-ene-1-carboxylate. J. Med. Chem. 2005;48:7457–7467. doi: 10.1021/jm050623t. [DOI] [PubMed] [Google Scholar]
- 23.Kawamoto T., Ii M., Kitazaki T., Iizawa Y., Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur. J. Pharmacol. 2008;584:40–48. doi: 10.1016/j.ejphar.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 24.Matsunaga N., Tsuchimori N., Matsumoto T., Ii M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol. Pharmacol. 2011;79:34–41. doi: 10.1124/mol.110.068064. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz E. A., Zhang W. Y., Karnik S. K., Borwege S., Anand V. R., Laine P. S., Su Y., Reaven P. D. Nutrient modification of the innate immune response: a novel mechanism by which saturated fatty acids greatly amplify monocyte inflammation. Arterioscler. Thromb. Vasc. Biol. 2010;30:802–808. doi: 10.1161/ATVBAHA.109.201681. [DOI] [PubMed] [Google Scholar]
- 26.Ueyama A., Yaworsky K. L., Wang Q., Ebina Y., Klip A. GLUT-4myc ectopic expression in L6 myoblasts generates a GLUT-4-specific pool conferring insulin sensitivity. Am. J. Physiol. 1999;277:E572–E578. doi: 10.1152/ajpendo.1999.277.3.E572. [DOI] [PubMed] [Google Scholar]
- 27.Pilon G., Charbonneau A., White P. J., Dallaire P., Perreault M., Kapur S., Marette A. Endotoxin mediated-iNOS induction causes insulin resistance via ONOO induced tyrosine nitration of IRS-1 in skeletal muscle. PLoS ONE. 2010;5:e15912. doi: 10.1371/journal.pone.0015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somwar R., Kim D. Y., Sweeney G., Huang C., Niu W., Lador C., Ramlal T., Klip A. GLUT4 translocation precedes the stimulation of glucose uptake by insulin in muscle cells: potential activation of GLUT4 via p38 mitogen-activated protein kinase. Biochem. J. 2001;359:639–649. doi: 10.1042/0264-6021:3590639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-Jun mediated by MAP kinases. Nature. 1991;353:670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 30.Wang D., Baldwin A. S., Jr Activation of nuclear factor-κB-dependent transcription by tumor necrosis factor-α is mediated through phosphorylation of RelA/p65 on serine 529. J. Biol. Chem. 1998;273:29411–29416. doi: 10.1074/jbc.273.45.29411. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Soos T. J., Li X., Wu J., Degennaro M., Sun X., Littman D. R., Birnbaum M. J., Polakiewicz R. D. Protein kinase C Θ inhibits insulin signaling by phosphorylating IRS-1 at Ser1101. J. Biol. Chem. 2004;279:45304–45307. doi: 10.1074/jbc.C400186200. [DOI] [PubMed] [Google Scholar]
- 32.Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 33.Hotamisligil G. S. Inflammatory pathways and insulin action. Int. J. Obes. Relat. Metab. Disord. 2003;27(Suppl. 3):S53–S55. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- 34.Bjorntorp P., Bergman H., Varnauskas E. Plasma free fatty acid turnover rate in obesity. Acta Med. Scand. 1969;185:351–356. doi: 10.1111/j.0954-6820.1969.tb07347.x. [DOI] [PubMed] [Google Scholar]
- 35.Andreasen A. S., Kelly M., Berg R. M., Moller K., Pedersen B. K. Type 2 diabetes is associated with altered NF-κB DNA binding activity, JNK phosphorylation, and AMPK phosphorylation in skeletal muscle after LPS. PLoS ONE. 2011;6:e23999. doi: 10.1371/journal.pone.0023999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueki K., Kondo T., Tseng Y. H., Kahn C. R. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc. Natl. Acad. Sci. U.S.A. 2004;101:10422–10427. doi: 10.1073/pnas.0402511101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frost R. A., Nystrom G. J., Lang C. H. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R698–R709. doi: 10.1152/ajpregu.00039.2002. [DOI] [PubMed] [Google Scholar]
- 38.Tamrakar A. K., Schertzer J. D., Chiu T. T., Foley K. P., Bilan P. J., Philpott D. J., Klip A. NOD2 activation induces muscle cell-autonomous innate immune responses and insulin resistance. Endocrinology. 2010;151:5624–5637. doi: 10.1210/en.2010-0437. [DOI] [PubMed] [Google Scholar]
- 39.Werner E. D., Lee J., Hansen L., Yuan M., Shoelson S. E. Insulin resistance due to phosphorylation of insulin receptor substrate-1 at serine 302. J. Biol. Chem. 2004;279:35298–35305. doi: 10.1074/jbc.M405203200. [DOI] [PubMed] [Google Scholar]
- 40.de Alvaro C., Teruel T., Hernandez R., Lorenzo M. Tumor necrosis factor α produces insulin resistance in skeletal muscle by activation of inhibitor κB kinase in a p38 MAPK-dependent manner. J. Biol. Chem. 2004;279:17070–17078. doi: 10.1074/jbc.M312021200. [DOI] [PubMed] [Google Scholar]
- 41.Kewalramani G., Fink L. N., Asadi F., Klip A. Palmitate-activated macrophages confer insulin resistance to muscle cells by a mechanism involving protein kinase Cζ and ∊. PLoS ONE. 2011;6:e26947. doi: 10.1371/journal.pone.0026947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitz-Peiffer C., Craig D. L., Biden T. J. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J. Biol. Chem. 1999;274:24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 43.Yu C., Chen Y., Cline G. W., Zhang D., Zong H., Wang Y., Bergeron R., Kim J. K., Cushman S. W., Cooney G. J., et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 44.Summers S. A., Garza L. A., Zhou H., Birnbaum M. J. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 1998;18:5457–5464. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirosumi J., Tuncman G., Chang L., Gorgun C. Z., Uysal K. T., Maeda K., Karin M., Hotamisligil G. S. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 46.Sinha S., Perdomo G., Brown N. F., O’Doherty R. M. Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor κB. J. Biol. Chem. 2004;279:41294–41301. doi: 10.1074/jbc.M406514200. [DOI] [PubMed] [Google Scholar]
- 47.Shoelson S. E., Lee J., Goldfine A. B. Inflammation and insulin resistance. J. Clin. Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rice T. W., Wheeler A. P., Bernard G. R., Vincent J. L., Angus D. C., Aikawa N., Demeyer I., Sainati S., Amlot N., Cao C., et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit. Care Med. 2010;38:1685–1694. doi: 10.1097/CCM.0b013e3181e7c5c9. [DOI] [PubMed] [Google Scholar]
- 49.Opal S. M., Scannon P. J., Vincent J. L., White M., Carroll S. F., Palardy J. E., Parejo N. A., Pribble J. P., Lemke J. H. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 1999;180:1584–1589. doi: 10.1086/315093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.