Abstract
Palmitate increased AMPK (5′-AMP-activated protein kinase) activity, glucose utilization and 2-DOG (2-deoxyglucose) transport in rat adipocytes. All three effects were blocked by the AMPK inhibitor Compound C, leading to the conclusion that in response to an increase in long-chain NEFA (non-esterified fatty acid) concentration AMPK mediated an enhancement of adipocyte glucose transport, thereby providing increased glycerol 3-phosphate for FA (fatty acid) esterification to TAG (triacylglycerol). Activation of AMPK in response to palmitate was not due to an increase in the adipocyte AMP:ATP ratio. Glucose decreased AMPK activity and effects of palmitate and glucose on AMPK activity were antagonistic. While insulin had no effect on basal AMPK activity insulin did decrease AMPK activity in the presence of palmitate and also decreased the percentage effectiveness of palmitate to increase the transport of 2-DOG. It is suggested that activation of adipocyte AMPK by NEFA, as well as decreasing the activity of hormone-sensitive lipase, could modulate adipose tissue dynamics by increasing FA esterification and, under certain circumstances, FA synthesis.
Keywords: acute regulation, adipocyte, AMP kinase, fatty acid, glucose, insulin
Abbreviations: ACC, acetyl-CoA carboxylase; AICAR, 5-amino-4-imidazolecarboxamide-1-β-D-ribofuranoside; AMPK, 5′-AMP-activated protein kinase; 2-DOG, 2-deoxyglucose; FA, fatty acid; GLUT4, glucose transporter 4; LPL, lipoprotein lipase; NEFA, non-esterified fatty acid; PDH, pyruvate dehydrogenase; PI3K, phosphoinositide 3-kinase; TAG, triacylglycerol
INTRODUCTION
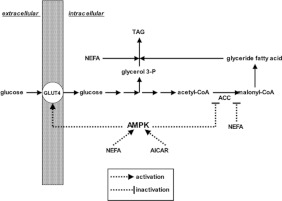
White adipocytes are important in mammalian energy homoeostasis since they store TAG (triacylglycerol), the body's main energy reserve. The long-chain NEFA (non-esterified fatty acid) precursors of adipocyte TAG are (i) derived from plasma lipoproteins, (ii) arise by de novo synthesis within adipocytes or (iii) are provided for re-esterification secondary to lipolysis. The other essential TAG precursor is glycerol 3-phosphate. Adipocytes have minimal expression of glycerol kinase and normally glyceroneogenesis from 3-carbon precursors is low [1]. Therefore cellular glycerol 3-phosphate is largely derived from plasma glucose whose entry into adipocytes is mainly facilitated by the insulin-activated glucose transporter GLUT4. Diagrams that describe in more detail the ‘glucose economy’ and the ‘FA (fatty acid) economy’ of adipocytes are shown in Supplementary Figures S1 and S2 (at http://www.bioscirep.org/bsr/033/bsr033e007add.htm).
The effect of insulin to stimulate glucose transport in skeletal or cardiac muscle cells and in adipocytes through increasing the abundance of GLUT4 at the cell surface is well known. Additionally, in skeletal muscle [2] and in cardiac myocytes [3] GLUT4 translocation and glucose transport are increased following activation of the AMPK (5′-AMP-activated protein kinase). The AMPK is a heterotrimeric (αβγ) serine/threonine protein kinase that is a key regulator of energy homoeostasis. AMPK is allosterically activated by an increase in the cellular AMP:ATP ratio which also promotes covalent activation of AMPK (at α-Thr172 in the activation loop of the catalytic subunit) by upstream AMPKs because binding of AMP to the kinase γ-subunit renders the α-Thr172 phosphorylation site a poorer substrate for protein phosphatases. Therefore AMPK is activated during cellular energy stress. Previously it has been realized that several neuroendocrine factors, including insulin, regulate AMPK phosphorylation/activity in tissue-specific ways [4,5] that are mainly independent of energy stress and changes in the AMP:ATP ratio. Also the AMPK system can register the availabilities of carbohydrate and lipid metabolic fuels under conditions where the AMP:ATP ratio is unchanged. Palmitate increases AMPK α-Thr172 phosphorylation and activity with subsequent phosphorylation of ACC (acetyl-CoA carboxylase) in heart [6] and in L6 skeletal muscle myotubes [7,8]–an effect that is also seen with other long-chain NEFAs, e.g. oleate [6] and linoleate [8]. By contrast AMPK α-Thr172 phosphorylation and activity as well as ACC phosphorylation are decreased by glucose in skeletal muscle [9] and cardiac myocytes [10]. In 2005, Daval et al. [11] noted ‘whereas the function of AMPK in liver and muscle has been well illustrated, its role in adipose tissue remains poorly documented and controversial’. cAMP-raising agents such as lipolytic hormones activate AMPK in adipocytes, an effect that is, at least in part, secondary to stimulation of lipolysis with the subsequent re-esterification of accumulated FAs resulting in an increase in the cellular AMP:ATP ratio [12,13]. Long-chain NEFAs also have an antilipolytic effect that may be secondary to activation of AMPK which phosphorylates hormone-sensitive lipase and decreases its activity [11,14,15]. The few studies that have addressed the potential role of AMPK as a regulator of glucose transport into adipocytes have yielded conflicting findings. In 3T3L-1 adipocytes activation of AMPK by AICAR (5-amino-4-imidazolecarboxamide-1-β-D-ribofuranoside) stimulated basal uptake of 2-DOG (2-deoxyglucose) [16] and promoted translocation to the plasma membrane of GLUT4 [17] while reducing insulin-stimulated 2-DOG uptake [16]. By contrast overexpression of a dominant negative AMPK mutant had no effect on AICAR-induced glucose transport [18]. In rodent, primary adipocytes AICAR decreased both basal and insulin-stimulated uptake of 2-DOG [19,20]. By contrast increases in both basal and insulin-stimulated glucose uptake in response to adiponectin were blocked by inhibitors of AMPK suggesting that AMPK may play a role in the stimulation of glucose uptake [21].
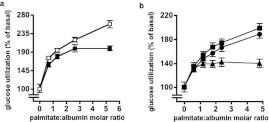
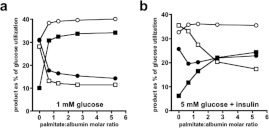
Data originating from this laboratory [22] can be used to show that palmitate increases glucose utilization by rat adipocytes (see Figure 1), an effect that is the direct opposite of the Randle cycle as proposed for muscle tissues. Other studies, subsequent to that of [22] have demonstrated stimulation of 2-DOG or 3-O-methylglucose transport into adipocytes by various long-chain NEFAs [23–27], but the mechanism initiating this NEFA effect has remained unexplained. Informed by recent advances in knowledge of the AMPK system, we have revisited this issue. Using palmitate as a representative long-chain NEFA, we found this to cause activation of adipocyte AMPK without any alteration of the cellular AMP:ATP ratio. Additionally we found that adipocyte AMPK activity can be decreased by glucose and we investigated the effect of insulin on AMPK activity in these cells.
Figure 1. Palmitate increased calculated rates of glucose utilization by adipocytes.
Values for FA, GG, lactate and pyruvate were taken from Table 3 of [22] (a) and from Table 9 and Figure 3 of [22] (b). Adipocytes (n=5 in all cases) were incubated for 1 h with 5% (w/v) BSA (a) or 2.75% (w/v) BSA (b) together with sodium palmitate. Abscissa values are expressed as palmitate:albumin molar ratios in order to allow comparison between these two experiments. Eqn (1.8) in the Supplementary Online data section (at http://www.bioscirep.org/bsr/033/bsr033e007add.htm) was used to calculate glucose utilization. (a) Adipocytes were from fed rats. Basal rates of glucose utilization with 5 mM glucose+0.2 μM insulin (filled symbols) were 10.4±0.8 μmol·h−1·100 μg of DNA−1 and with 1 mM glucose (open symbols) were 2.6±0.3 μmol·h−1·100 μg of DNA−1. (b) Basal rates of glucose utilization with 5 mM glucose+0.2 μM insulin were 8.9±0.7, 6.7±0.5 and 5.4±0.4 μmol·h−1·100 μg of DNA−1 for cells from fed (squares), 24 h-starved (circles) and 72 h-starved (triangles) rats. All tested additions of palmitate increased glucose utilization to at least the P<0.005 significance level.
MATERIALS AND METHODS
Isolation and incubation of adipocytes
Adipocytes were isolated in accordance with UK Home Office procedures by collagenase digestion of epididymal fat pads from male Sprague–Dawley rats (200–250 g). Adipocytes equivalent to 1/5th of one fat pad (typically 8–10×106 cells) were immediately incubated for 1 h in 4 ml of incubation medium consisting of Krebs–Henseleit bicarbonate buffer [pre-equilibrated with O2/CO2 (19:1)] containing FA-poor albumin (20 mg/ml) and other additions as indicated.
Glucose utilization by adipocytes
This was measured experimentally as the sum of the conversion of [U-14C]glucose to total lipid (FA+GG)+CO2 together with the formation of lactate+pyruvate [28] (note: FA, long-chain fatty acid synthesized de novo from glucose and converted into TAG; GG, the glyceroyl moiety of TAG formed from glucose). Additionally this was calculated from data in [22] using eqn (1.8), which is described and validated in the Supplementary Online data section (at http://www.bioscirep.org/bsr/033/bsr033e007add.htm).
2-DOG transport into adipocytes
After incubation for 1 h, 0.5 μCi of [3H]2-DOG was added to adipocytes to give a final concentration of 125 μM. After 10 min transport was terminated by addition of 50 μM cytochalasin B. Adipocytes were recovered by centrifugation and washed twice with incubation medium containing cytochalasin B and then solubilized in 0.1 M NaOH prior to scintillation counting. Cytochalsin B was added simultaneously with [3H]2-DOG in blank assays. Since the cells were washed under conditions where sugar transport was inhibited, 2 mM sodium pyruvate was present in the incubation and washing media in order to avoid compromising the cellular energy status.
Activity assay of adipocyte α-1 AMPK
Adipocytes were recovered by brief centrifugation and lysed in 1 ml of ice-cold 50 mM Tris/HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, 50 mM NaF, 5 mM Na4P2O7, 10% (v/v) glycerol, 1% (v/v) Triton X-100, 1 mM DTT (dithiothreitol), 1 mM PMSF, 1 mM benzamidine and SBTI (soya-bean trypsin inhibitor) (4 μg/ml). The lysate was centrifuged at 13000 g for 10 min at 4°C and aliquots of the supernatant were used for immunoprecipitation of α-1 AMPK followed by activity assay in the presence of 200 μM AMP using 200 μM SAMS peptide as a substrate [6].
Adipocyte adenine nucleotide contents
Adipocyte incubations were terminated with HClO4 and ATP, ADP and AMP were measured by ion-pair RP-HPLC (reverse-phase HPLC) [29].
Adipocyte DNA contents
DNA was measured by reaction with diphenylamine using calf thymus DNA as a standard. A total of 1 g of epididymal fat pad yielded 135±4 μg of adipocyte DNA (n=55).
Expression of results and statistical methods
Glucose utilization, 2-DOG transport and α-1 AMPK activity are expressed as μmol·h−1·100 μg of DNA−1, pmol·10 min−1·100 μg of DNA−1 and pmol·30 min−1·100 μg of DNA−1 respectively. Statistical significance was assessed by Student's t test for paired samples with P<0.05 indicating a significant effect. Values of n indicate the numbers of independent adipocyte preparations. Error bars in Figures indicate S.E.M.
RESULTS
Palmitate increased adipocyte glucose utilization, 2-DOG transport and AMPK activity
Using data for FA, GG and (lactate+pyruvate) from [22], rates of glucose utilization at a range of palmitate concentrations were calculated using Method 1 described in the Supplementary Online data. At palmitate:albumin molar ratios that encompass and exceed NEFA physiological levels in the systemic and adipose tissue circulations this FA increased glucose utilization by cells incubated with 1 mM glucose alone or with 5 mM glucose and insulin (Figure 1a). At 5 mM glucose with insulin this could be attributed to a ‘pull’ effect due to palmitate increasing utilization of glucose-derived glycerol 3-phosphate for TAG synthesis. However, with 1 mM glucose alone where glucose transport should have greater control strength over glucose utilization the stimulatory effect of palmitate was at least as large. This suggested that palmitate might also stimulate glucose utilization by a ‘push’ mechanism, i.e. at the level of glucose transport. Figure 1(b) shows that cells from 24-h starved rats had a similar percentage response to palmitate as in cells from fed rats even though their basal rate of glucose utililization was decreased. Cells from 72-h starved rats had a further decrease in their basal rate of glucose utilization and a decreased, though still significant, stimulation of glucose utilization.
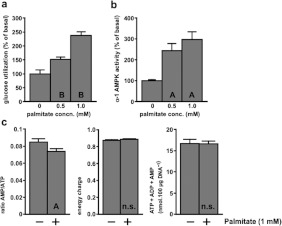
Direct measurements with adipocytes from fed rats incubated at 5 mM glucose without insulin confirmed an enhancement of glucose utilization by palmitate (Figure 2a). As shown in Figure 2(b), palmitate increased the activity of α-1 AMPK, the predominant form of AMPK in adipocytes [11,30]. Pooling all available measurements, we found that 1 mM palmitate increased glucose utilization by 92±11% (n=18, P<0.0005) and increased AMPK activity by 90±15% (n=25, P<0.0005). The activation of AMPK by 1 mM palmitate was not linked to an increase in the cellular AMP:ATP ratio (Figure 2c). In fact palmitate caused a significant 13% decrease in that ratio. No significant alterations in the ‘energy charge’ or in the total adenine nucleotide content were seen with palmitate.
Figure 2. Palmitate increased the experimentally measured rate of net glucose utilization and α-1 AMPK activity without increasing the AMP:ATP ratio.
Adipocytes were incubated for 1 h with 5 mM glucose and the indicated concentrations of sodium palmitate. n.s., A and B indicate P>0.05, <0.005, <0.0005 respectively compared with the basal state without palmitate. (a) Conversion of [U-14C]glucose into total lipid+CO2+(lactate+pyruvate). The basal rate of glucose utilization was 1.12±0.16 μmol·h−1·100 μg of DNA−1 (n=7). (b) α-1 AMPK activity. The basal activity was 16.4±2.0 pmol·30 min−1·100 μg of DNA−1 (n=6). (c) Measurements of ATP+ADP+AMP. Energy charge was calculated as (ATP+0.5×ADP)/(ATP+ADP+AMP) (n=15).
The uptake of 2-DOG (and its subsequent conversion into 2-DOG 6-phosphate) was measured as an index of glucose transport activity. It is customary to measure 2-DOG uptake in the absence of glucose or at a low glucose concentration to minimize the control strength of hexokinase relative to that for glucose transport. We always had some glucose present together with 2 mM pyruvate to ensure that the energy status of the cells was not compromised. At 1 mM glucose palmitate increased 2-DOG transport by 45% (Figure 3c). As expected, an increase in glucose from 1 to 5 mM decreased the basal rate of 2-DOG transport. However, the percentage increase in 2-DOG transport due to palmitate was similar at 1 and 5 mM glucose (Figures 3c and 3d).
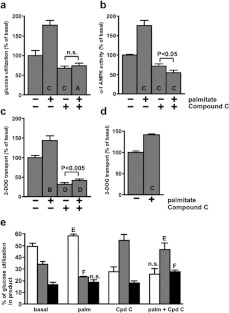
Figure 3. Compound C blocked the effect of palmitate on glucose utilization, α-1 AMPK activity and 2-DOG transport.
(a–d) Measurements were made in adipocytes incubated for 1 h with the indicated additions of 1 mM sodium palmitate or 50 μM Compound C. n.s., A, B, C and D indicate P>0.05, <0.025, <0.01, <0.005, <0.0005 respectively compared with the basal state without palmitate or Compound C. P values on the figures indicate the significance of the effect of palmitate in the presence of Compound C. (a) Conversion of 5 mM [U-14C]glucose to total lipid+CO2+(lactate+pyruvate). The basal rate of glucose utilization was 1.27±0.16 μmol·h−1·100 μg of DNA−1 (n=5). (b) α-1 AMPK activity in the presence of 5 mM glucose. The basal activity was 15.9±0.3 pmol·30 min−1·100 μg of DNA−1 (n=5). (c) Transport of [3H] 2-DOG in the presence of 1 mM glucose. The basal rate of transport was 123±7 pmol·10 min−1·100 μg of DNA−1 (n=6). (d) Transport of [3H] 2-DOG in the presence of 5 mM glucose. The basal rate of transport was 30.5±1.2 pmol·10 min−1·100 μg of DNA−1 (n=9). (e) The values are derived from the experiment shown in (a) and indicate the percentage of utilized glucose in the products total lipid (white bars), CO2 (grey bars) and lactate+pyruvate (black bars). n.s., E and F indicate the significance of the effects of palmitate relative to the control without palmitate (P>0.05, <0.025, <0.005, respectively). The significances of effects of Compound C are given in the text.
Palmitate (1 mM) in the presence of 2% albumin gives a NEFA:albumin ratio of 3.3 which is similar to that with 2 mM NEFA at physiological plasma albumin concentration. This is a high value for systemic plasma where NEFA concentration rarely exceeds 2 mM. However, as discussed below, higher NEFA:albumin ratios can be observed within the adipose tissue circulation [31]. There was an important technical necessity to have a relatively high initial palmitate in the incubations. This is because adipocytes from fed rats avidly convert exogenous NEFA to stored TAG. For example, it can be calculated from data used for the study of [22] in which rates of glucose metabolism per incubation flask were similar to the present study that adipocytes incubated for 1 h with 5 mM glucose removed 76% of available FA at 0.5 mM palmitate in the presence of 1% albumin. Insulin increased this removal to 87%. The percentage removal of FA was smaller at lower glucose concentrations or at higher albumin concentrations; e.g. at 5 mM glucose with insulin removal with 1 mM initial palmitate was 67% and 59% at 2.75% and 5% BSA, respectively. While the measurement of [U-14C]glucose utilization represents the integrated activity over 1 h, measurement of 2-DOG transport is a 10 min measurement made after 1 h of incubation and AMPK measurement is just a ‘snapshot’ at the 1 h point. Therefore it was essential to ensure the continued presence of NEFA to the end of the incubation period if those measurements were to have any meaning. Hence, the use of 1 mM palmitate.
Compound C attenuated the enhancements of glucose utilization, 2-DOG transport and AMPK activity by palmitate
Compound C is a cell-permeable reversible ATP-competitive inhibitor of AMPK (Ki=109 nM at 5 μM ATP) that substantially alters the conformation of the AMPK-activation loop [32]. It also decreases the α-Thr172 phosphorylation of AMPK [15] and has been shown in vivo to attenuate the effects of AICAR and metformin on AMPK activity [33]. Compound C abolished the enhancements of glucose utilization and AMPK activity by 1 mM palmitate (Figures 3a and 3b). In the presence of Compound C, which itself lowered the basal AMPK activity, palmitate actually caused a small but significant decrease in AMPK activity (Figure 3b). Compound C substantially lowered the basal rate of 2-DOG transport and largely decreased the enhancement of this by palmitate although a small but significant effect of palmitate was retained (Figure 3c).
Palmitate and Compound C altered the distribution of metabolic products originating from glucose (Figure 3e). Palmitate had a net ‘anabolic effect’, i.e. it increased the proportion of glucose converted into total lipid (FA+GG) relative to the catabolic products CO2, lactate and pyruvate. This ‘anabolic effect’ was consistently seen with 11 independent cell preparations in which 1 mM palmitate increased the percentage of glucose utilization for total lipid synthesis from 51.2±2.5% to 62.5±2.6% (P<0.0005). By contrast Compound C had a net ‘catabolic effect’ by decreasing the percentage of glucose utilization for total lipid synthesis (P<0.01) and increasing that for CO2 formation (P<0.025), while having no effect on the percentage converted into lactate+pyruvate. Additionally Compound C abolished the ‘anabolic effect’ of palmitate.
The results presented in Figures 1–3 together with the published literature lead to the following conclusions. (i) At least some of the increase in glucose utilization in response to palmitate is due to a ‘push’ mechanism through increased glucose transport. (ii) The palmitate effect appears to be confined to the transport/utilization of glucose and is not seen with non-glucose lipogenic precursors that enter adipocytes independently of GLUT4. For example, the utilization of fructose by adipocytes is unaffected by palmitate [28]. Most fructose transport is through GLUT5 that is entirely in the plasma membrane with no modulation of its membrane abundance by insulin [34]. Also the utilization of pyruvate that enters the cells via a monocarboxylate transporter is unaffected by palmitate [1]. (iii) The attenuation by Compound C of the effects of palmitate on glucose utilization, 2-DOG transport and on the palmitate ‘anabolic effect’ as well as its attenuation of the activation of AMPK by palmitate implies that AMPK is a mediator of these effects.
Glucose decreased adipocyte AMPK activity. Also glucose and palmitate attenuated each other's effects on AMPK activity
In the absence of palmitate increasing glucose from 1 to 5 mM decreased AMPK activity with higher glucose having no additional effect (Figure 4). Palmitate increased the concentration of glucose needed to achieve a maximal decrease in AMPK activity since the AMPK activity at 25 mM glucose was less than that at 5 mM glucose in the presence of palmitate (P<0.05). Palmitate increased AMPK activity at all glucose concentrations although the percentage increase due to palmitate was less at higher glucose concentrations (49±9%, 42±7%, 22±9% and 21±8% at 1, 5, 10 and 25 mM glucose respectively). In conclusion, glucose and palmitate attenuated each other's effects on AMPK activity.
Figure 4. Glucose decreased α-1 AMPK activity.
Adipocytes were incubated for 1 h with the indicated concentrations of glucose without (open symbol) or with 1 mM sodium palmitate (filled symbol). The values are expressed relative to the α-1 AMPK activity at 1 mM glucose in the absence of palmitate which was 11.7±0.7 pmol·30 min−1·100 μg of DNA−1 (n=6). A and B indicate P<0.005, <0.0005 respectively for effects of 5, 10 and 25 mM glucose versus 1 mM glucose. C–E indicate P<0.05, <0.025, <0.005 for the effect of palmitate at a given glucose concentration.
The combined effects of palmitate and insulin on glucose utilization, 2-DOG transport and AMPK activity
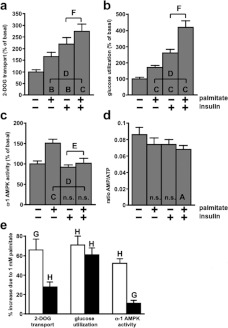
Insulin inactivates AMPK through an Akt [also known as PKB (protein kinase B)]-mediated phosphorylation of Ser485/491 in the AMPK α-1/α-2 subunits [35]. Therefore we investigated combined effects of palmitate and insulin. Palmitate, insulin and (insulin+palmitate) increased 2-DOG transport relative to the basal level by 63%, 119% and 175% respectively (Figure 5a). These effects showed no synergy, i.e. the combined effect was not significantly different from the sum of the individual effects (P>0.4). For glucose utilization (Figure 5b) palmitate, insulin and (insulin+palmitate) caused increases of 72%, 162% and 320%. Here, the combined effect was significantly greater than the sum of the individual effects (P<0.005). This synergy was not unexpected because, in addition to stimulating glucose transport, palmitate has a ‘pull’ effect on glucose utilization through being a co-substrate with glucose-derived glycerol 3-phosphate for TAG synthesis. An increase in glucose transport by insulin would be expected to potentiate this ‘pull’ effect.
Figure 5. The effects of palmitate and insulin on glucose utilization, 2-DOG transport, α-1 AMPK activity and the cellular AMP:ATP ratio.
(a–d) Measurements were made in adipocytes incubated for 1 h with the indicated additions of sodium palmitate (1 mM) or insulin (10 nM). n.s, A, B and C indicate P>0.05, P<0.01, <0.005, <0.0005, respectively compared with the basal state. D indicates P<0.0005 for the effect of insulin in the presence of palmitate. E and F indicate P<0.005, <0.0005 respectively for the effect of palmitate in the presence of insulin. (a) Transport of [3H] 2-DOG in the presence of 1 mM glucose. The basal rate of transport was 148±14 pmol·10 min−1·100 μg of DNA−1 (n=9). (b) Conversion of 5 mM [U-14C]glucose into total lipid+CO2+(lactate+pyruvate). The basal rate of glucose utilization was 2.23±0.23 μmol·h−1·100 μg of DNA−1 (n=7). (c) α-1 AMPK activity in the presence of 5 mM glucose. The basal activity was 14.8±1.2 pmol·30 min−1·100 μg of DNA−1 (n=8). (d) AMP:ATP ratios in the presence of 5 mM glucose. The ‘energy charge’ in the basal state was 0.887±0.008 and with palmitate+insulin was 0.902±0.004 (P<0.005; n=5). (e) The values are derived from the experiments shown in (a–c) to indicate the percentage effects of palmitate on 2-DOG transport, glucose utilization and α-1 AMPK activity in the absence (white bars) or presence (black bars) of insulin. G and H indicate P<0.005 and <0.0005 respectively for effects of palmitate.
Insulin had no effect on basal AMPK activity but decreased AMPK activity when palmitate was present leading to a reduction in, but not a total ablation of, the activation of AMPK by palmitate (Figure 5c). Insulin had no effect on the cellular AMP:ATP ratio (Figure 5d). Compared with the basal state insulin+palmitate in combination caused a small but significant decrease in the AMP:ATP ratio (Figure 5d) and increased the ‘energy charge’ from 0.887±0.008 to 0.902±0.004 (n=5, P<0.005) without any change in the total content of adenine nucleotides (results not shown). These small changes in the cellular-energy status appeared to be insufficient to alter AMPK activity (Figure 5c).
Figure 5(e) presents results from Figures 5(a)–5(c) expressed as the percentage enhancements caused by palmitate in the presence and absence of insulin. When expressed in this manner, insulin was seen to diminish the percent effectiveness of palmitate to increase 2-DOG transport. This was not unexpected because activation of AMPK appeared to mediate the effect of palmitate on 2-DOG transport (Figure 3) and insulin diminished the percent effectiveness of palmitate to increase AMPK activity (Figure 5e). The activation of AMPK by palmitate in the presence of insulin was small. That this could be attributed to excessive removal of palmitate prior to the ‘snapshot’ measurement of AMPK activity at 1 h is most unlikely because in the presence of insulin palmitate increased 2-DOG transport as measured after 1 h (Figure 5a). A more detailed study of the effects of several concentrations of palmitate and insulin on 2-DOG transport and AMPK activity is necessary in order to establish whether such a small activation of AMPK is sufficient to cause the activation of 2-DOG transport by palmitate in the presence of insulin. By contrast with the effect on 2-DOG transport insulin had minimal effect on the percentage stimulation of glucose utilization by palmitate. We attribute this to palmitate's additional role as a co-substrate with glucose-derived glycerol 3-phosphate in TAG synthesis (the ‘pull’ effect).
DISCUSSION
As was observed previously in heart [6] and in L6 skeletal muscle myotubes [7,8] adipocyte AMPK was increased by long-chain NEFA, e.g. palmitate, oleate and linoleate. In the present study, we have just used palmitate as a representative long-chain NEFA. While it is possible that in adipocytes this effect is specifically confined to palmitate we expect that to be unlikely in view of the previously reported effects of a wider range of FAs. In previous studies, changes in AMPK activity were correlated with increased α-Thr172 phosphorylation of AMPK [6–8]. We attempted to make the same correlation but in our hands satisfactory measurements of α-Thr172 phosphorylation were prevented by carry-over of albumin from the incubation medium into cell extracts that interfered with the Western blotting procedure. We tried to obviate this by washing the cells in albumin-free medium prior to freeze-stopping but that delay in freeze-stopping caused large variations in the measurements of α-Thr172 phosphorylation. No significant change in total AMPK protein was observed after exposure to palmitate for 1 h (results not shown) and since the increase in AMPK activity with palmitate survived cell extraction and immunoprecipitation (procedures that would eliminate allosteric effects), we concluded that the increase in AMPK activity with palmitate must be due to covalent modification. As also was observed previously in heart and L6 skeletal muscle myotubes [6,7] the activation of adipocyte AMPK by NEFA occurred in the absence of any detectable change in the cellular AMP:ATP ratio. The mechanism of that effect is not fully understood, but a study in vitro has suggested that NEFA enhances the ability of the upstream AMPK LKB1 to phosphorylate AMPK α-Thr172 through an allosteric interaction of FA with the AMPK βγ-subunits [8]. An adenine nucleotide-independent activation of adipocyte AMPK in response to NEFA is contrary to the observation that lipolytic agents such as forskolin and isoprenaline increased the AMP:ATP ratio in 3T3-L1 adipocytes together with an increase in AMPK α-Thr172 phosphorylation [12]. Those changes were offset by orlistat and triacsin C and it was concluded that the ATP-dependent re-esterification of lipolysis-derived NEFA decreased the cellular energy state, leading to activation of AMPK. The contrast between our findings and those of [12] raises the following questions. (i) How representative of primary adipocytes are 3T3-L1 adipocytes; i.e. do those cell types differ in the response of their energy status to NEFA? (ii) Do exogenous NEFAs and those that are generated endogenously by lipolysis have different metabolic effects? As we discuss below, increases in exogenous and endogenous NEFA appear to have indistinguishable effects on glucose metabolism in primary adipocytes.
The findings in Figure 3 accord with reports that activation of AMPK by AICAR in 3T3L-1 adipocytes [16] or by adiponectin in rat adipocytes [21] led to increased transport of 2-DOG. However, our findings are at variance with observations that AICAR decreased glucose utilization by rat primary adipocytes in the absence and presence of insulin [19,20]. We offer the following explanation for this. The major anabolic products that adipocytes manufacture from glucose are FA and GG. GG esterifies newly synthesized FA and NEFA generated exogenously by LPL (lipoprotein lipase). GG also re-esterifies some lipolytic NEFA. Without the provision of some exogenous or lipolytic NEFA, the proportion of glucose utilization that is committed to FA synthesis under basal conditions normally is comparable with or higher than that committed to GG formation. Insulin increases the FA:GG ratio. However, as shown in Figure 6, provision of NEFA appreciably reverses this proportionality–an effect that can be seen over a wide range of glucose concentrations with or without insulin (Supplementary Figure S8 at http://www.bioscirep.org/bsr/033/bsr033e007add.htm). In the studies of [19,20] exogenous NEFAs were not added to the adipocyte incubations. Under those conditions potential enhancement of glucose utilization due to stimulation of glucose transport activity by AICAR/AMPK could be counteracted by a decrease in glucose utilization for FA synthesis as a result of AMPK phosphorylating and inactivating ACC. The greater the proportion of net glucose utilization for FA synthesis, the greater could be this counteractive effect. Therefore AICAR could decrease net glucose utilization, particularly when insulin is present and FA synthesis is high. However, with NEFA present stimulation of glucose transport by AICAR/AMPK would be accompanied by increased glucose utilization for GG formation and the enhancement of glucose utilization ought to predominate over a decreased percentage glucose utilization for FA synthesis with a net increase in glucose utilization being the outcome. We suggest that inactivation of ACC through its AMPK phosphorylation site is only one contributor to the rate of FA synthesis that is actually observed (rather than the percentage of glucose utilization committed to FA synthesis). Other contributing factors may be (i) increased provision of its acetyl-CoA substrate secondary to increased glucose transport and (ii) inhibition of ACC at higher NEFA concentrations–presumably due to an increased cellular level of fatty acyl-CoA. These suggestions are summarized in Figure 7 and are supported by the following. With 1 mM glucose in the absence of insulin where glucose transport is likely to have appreciable control strength over glucose utilization palmitate substantially decreased the percentage of glucose committed to FA synthesis but the absolute decrease in this process was small (Supplementary Table S1a and Figure S9 at http://www.bioscirep.org/bsr/033/bsr033e007add.htm). By contrast, with 5 mM glucose and insulin, although palmitate decreased the percentage of glucose committed to FA synthesis, lower concentrations of palmitate actually increased FA synthesis (Supplementary Table S1b and Figure S9). These data are not at variance with the notion that provided there is sufficient intracellular glucose provision an enhancement of glucose transport by NEFA could generate sufficient extra acetyl-CoA to counteract the reduction of the catalytic potential of ACC due to its phosphorylation by AMPK. The decline in the increase in FA synthesis at higher concentrations of palmitate could be due to allosteric inhibition of ACC by fatty acyl-CoA. At very low glucose concentrations (i.e. 0.3 or 0.6 mM) palmitate decreased FA synthesis but at 2.5 and 5 mM FA synthesis was increased by palmitate in the absence or presence of insulin (Supplementary Table S2 at http://www.bioscirep.org/bsr/033/bsr033e007add.htm). This further illustrates how glucose provision can dictate the effect of palmitate on FA synthesis. The possible physiological significance of an activation of FA synthesis by NEFA is discussed below. Supplementary Tables S1 and S2 also demonstrate the substantial increase that palmitate brings about in increasing both the absolute rate of GG formation and the percentage contribution of glucose utilization to that process. NEFAs are as much a part of the environment of the adipocyte as glucose because these are provided in the fed state by LPL and in the fasted state by lipolysis. As far as we are aware the effect of AICAR on adipocyte glucose utilization and transport in the presence of exogenous NEFA has not been investigated. In principle AICAR and NEFAs could have additive effects since ZMP (AICAR monophosphate) derived from AICAR interacts allosterically with AMPK to decrease its propensity for dephosphorylation by protein phosphatases [36], whereas NEFAs are proposed to increase AMPK's propensity for phosphorylation by LKB1 [8].
Figure 6. Effect of palmitate on the distribution of glucose-derived metabolic products in adipocytes from fed rats.
Data from Table 3 of [22] were used as in Figure 1(a) to calculate glucose utilization. Eqn (1.1) in the Supplementary Online data section (at http://www.bioscirep.org/bsr/033/bsr033e007add.htm) was then used to derive values for CO2 formation. All values were then expressed as percentages of glucose utilization. Each data point is the mean of five independent experiments. (a) Incubation with 1 mM glucose. (b) Incubation with 5 mM glucose+0.2 μM insulin. FA: open squares; GG: filled squares; CO2: open circles; (lactate+pyruvate): filled circles.
Figure 7. Scheme suggesting how the availability of NEFA could alter the metabolic fate of glucose and so alter the net effect of AICAR on glucose utilization by adipocytes.
(i) Both NEFA and AICAR cause α-Thr172 phosphorylation and activation of AMPK. (ii) AMPK increases glucose transport by an undefined mechanism which then provides glycerol 3-phosphate for TAG synthesis and also provides the acetyl-CoA precursor for de novo biosynthesis of FA. However AMPK phosphorylates and inactivates ACC, thereby decreasing the potential for FA biosynthesis. (iii) Provision of NEFA results in increased generation of fatty acyl-CoA thioesters which are (a) precursors for TAG synthesis and (b) allosteric inhibitors of ACC. (iv) The net result is that at minimal NEFA availability glucose utilization for FA biosynthesis can predominate over its conversion into GG (via glycerol 3-phosphate) and activation of AMPK by AICAR would be expected to decrease net glucose utilization. However, at higher NEFA availability glucose percentage conversion to glyceride glycerol becomes more significant and its percentage use for FA biosynthesis is diminished. Under these conditions AICAR would be expected to increase net glucose utilization.
Our conclusion that the increase in 2-DOG transport in response to palmitate was mediated by AMPK is also at variance with the study of [19] which showed that 2-DOG transport in primary adipocytes was decreased by AICAR. However, those authors measured 2-DOG transport into adipocytes preincubated for 1 h in a glucose-free medium–a procedure that ought to activate AMPK even in the absence of AICAR. In our study 2 mM pyruvate was present in the 2-DOG transport studies so that the presence of 1 mM glucose did not compromise the energy status of the cells. Those authors [19] also studied the uptake of 30 μM FA by adipocytes during a 1–5 min transport assay and found that prior exposure to AICAR for 1 h decreased FA transport. It is difficult to assess whether our findings might be at variance with that study because the control strength of NEFA transport over NEFA utilization for TAG synthesis was not established.
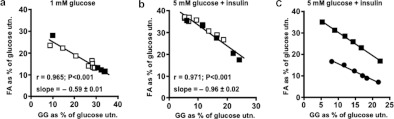
The effect of palmitate to increase the percentage of glucose utilization for GG formation while decreasing that for FA synthesis is considered further in Figure 8 which shows that these values were inversely related in a linear fashion. This was true for cells incubated with 1 mM glucose (Figure 8a) or with 5 mM glucose+insulin (Figure 8b), although the slope of the regression line was lower in the former case. A similar linear relationship was seen with cells from fasted rats but with a lower slope than for cells from fed animals (Figure 8c). The expression of the FA and GG values as percentages of net glucose utilization rather than as their absolute values is a normalization procedure that allows comparison across separate experiments of the effects of exogenous palmitate with those due to NEFA generated endogenously in response to adrenaline. The percentage plots of FA compared with GG for these two conditions were found to superimpose, implying that the origin of the NEFA was immaterial to the observed effects on glucose metabolism.
Figure 8. The percentages of utilized glucose that are converted into FA and into GG by adipocytes are negatively correlated in a linear fashion.
(a, b) Adipocytes from fed rats were incubated for 1 h with 5% (w/v) BSA and glucose or insulin (0.2 μM) as indicated together with 0.5–4.0 mM sodium palmitate (data from Table 3 of [22]) or 0.05–5 μM adrenaline (data from Table 4 of [22]). Eqn (1.8) from the Supplementary Online data section (at http://www.bioscirep.org/bsr/033/bsr033e007add.htm) was used to calculate glucose utilization. Filled symbols: with palmitate (n=5); open symbols: with adrenaline (n=4). The slopes of the linear regression lines in (a, b) are significantly different (P<0.001). (c) Data from Table 9 and Figure 3 of [22] for adipocytes incubated with 2.75% (w/v) BSA for 1 h with 5 mM glucose+insulin (0.2 μM) together with 0.25–2.0 mM sodium palmitate were used to calculate glucose utilization as in Figure 1(b). Squares: cells from fed rats. The regression line is y=−1.072+0.035 (r=0.999, P<0.001). Circles: cells from 24 h-starved rats. The regression line is y=−0.752+0.06 (r=0.991, P<0.001). The slopes of these two regression lines were significantly different (P<0.01). A similar plot for cells from 72 h-starved rats is not feasible because glucose incorporation into FA was very small.
In addition to inverting the relationship between glucose conversion to FA and GG, calculations leading to Supplementary Figures S10–S13 (available at http://www.bioscirep.org/bsr/033/bsr033e007add.htm) show that palmitate had other effects on glucose metabolism. These were: (i) increased conversion of glucose carbon to TCA (trichloroacetic acid) cycle CO2; (ii) increased net cytosolic usage of NADH with increased usage for GG formation but decreased usage for lactate and FA formation; (iii) an increase in the ratio of mitochondrial:cytosolic ATP formation that is needed to satisfy the conversion of glucose carbon into FA and GG; and (iv) a decrease in the percentage contribution of PDH (pyruvate dehydrogenase) to mitochondrial NADH formation. Supplementary Figures S10–S13 show that adrenaline had similar effects to palmitate in this regard. Supplementary Figure S14 (available at http://www.bioscirep.org/bsr/033/bsr033e007add.htm) shows a normalization that allowed comparison of the effects of palmitate and adrenaline. In all cases, data from the palmitate and the adrenaline experiments were superimposed, again indicating that the origin of the NEFA was immaterial to the resulting effects on glucose metabolism.
Activation of AMPK by NEFA in catabolic tissues such as heart and skeletal muscle has been proposed to be a feedforward mechanism which activates the β-oxidation of FAs [6,8]. The same cannot apply to white adipocytes where the rate of FA β-oxidation is so low that it is greatly exceeded by the capacity of the cells to convert NEFA into TAG [37] and where palmitate has minimal effect on the 14C/3H ratio in de novo synthesized FAs when adipocytes are incubated with 3H2O together with either [U-14C]glucose or [U-14C]pyruvate [22]–indicating that palmitate makes little contribution to the acetyl-CoA pool. For adipocytes, we propose that increased NEFA, through an AMPK-mediated increase in glucose transport activity that leads to increased glycerol 3-phosphate formation, causes a feedforward activation of NEFA disposal into cellular TAG (Figure 9). If that is so, ‘classical’ generalizations about AMPK being a driver of catabolic events and suppressor of biosynthetic processes may not be universally applicable. From the data used to calculate glucose utilization in Figure 3(a) it can be shown that Compound C decreased conversion of glucose into total lipid (FA+GG) by 78±6%, to (lactate+pyruvate) by 39±1% and to CO2 by 29±2%, i.e. inactivation of AMPK selectively increased glucose catabolism relative to its anabolic usage.
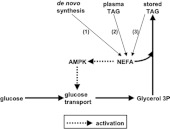
Figure 9. A hypothetical process that involves AMPK in a feed-forward activation of TAG synthesis by adipocytes.
This proposes that NEFA in adipocytes derived from (1) the de novo synthesis of FAs, (2) from the activity of LPL or (3) from lipolytic turnover of existing stored TAG can activate AMPK. This then increases the transport into adipocytes of glucose which is a precursor for glycerol 3-phosphate, thereby facilitating the synthesis or resynthesis of stored TAG.
How an increase in glucose concentration decreases AMPK activity is unresolved. In cardiomyocytes, this may be mediated by a pentose phosphate pathway intermediate [10]. Whether the same applies to adipocytes has not been investigated.
How activation of AMPK mediates increased glucose transport activity in adipocytes is unresolved. Phosphorylation of the rab GAP (GTPase-activating protein) AS160 (TBC1D4) by the PI3K (phosphoinositide 3-kinase)/Akt pathway is necessary for the stimulation of GLUT4 exocytosis by insulin [38], but is not required for inhibition of GLUT4 endocytosis by insulin [39]. In cardiac myocytes activation of AMPK leads to increased glucose transport. This activation pathway is independent of PI3K and causes decreased endocytosis of GLUT4 without affecting GLUT4 exocytosis [3]. As shown in Supplementary Figure S15 (at http://www.bioscirep.org/bsr/033/bsr033e007add.htm) palmitate had no effect on adipocyte AS160 phosphorylation. Future studies to investigate whether palmitate decreased adipocyte GLUT4 endocytosis as a result of activation of AMPK would be interesting.
We are aware of few studies of the response of adipocyte AMPK activity to insulin. Our finding that insulin had no effect on basal AMPK activity while palmitate appeared to facilitate inactivation of AMPK by insulin was in contrast with observations that in perfused rodent hearts inactivation of AMPK by insulin occurred in the absence of NEFA but was abolished by palmitate [6,40]. Gaidhu et al. [19] found that insulin had no effect on the basal level of AMPK α-Thr172 phosphorylation in freshly isolated rat adipocytes, whereas the same laboratory subsequently demonstrated a decrease in basal AMPK phosphorylation in adipocytes that had been cultured for 24 h [20], suggesting that the response to insulin of adipocyte basal AMPK phosphorylation/activity may be dependent on experimental conditions (e.g. the extent of NEFA accumulation). Insulin decreased AMPK phosphorylation that had been elevated in response to forskolin in 3T3-L1 adipocytes [41] or in response to isoprenaline in rat primary adipocytes [13]. Whether those insulin effects were secondary to the antilipolytic action of insulin (thereby decreasing NEFA accumulation and subsequent AMPK activation) or were caused by NEFA directly facilitating the insulin effect on AMPK is unclear. It is noteworthy that insulin had no effect on adipocyte AMPK α-Thr172 phosphorylation after treatment with AICAR [19,20].
What are the physiological implications of this study? Palmitate increased adipocyte glucose utilization at palmitate:albumin ratios that are within the normal physiological range (Figure 1). However, 1 mM palmitate (palmitate:albumin=3.3) was initially present in incubations leading to measurements of AMPK activity and we have explained the necessity to use this concentration which could be considered to be above the normal physiological range for systemic plasma. However, it is more relevant to make comparisons with NEFA concentrations in the adipose tissue circulation. Hodgetts et al. [31] have reported NEFA levels in subcutaneous adipose tissue of the human abdominal wall–a depot that appears to behave similarly to the bulk of body adipose tissue. In overnight fasted subjects at rest they observed NEFA levels in the tissue's venous drainage of 1.1 mM (NEFA:albumin=1.72). After exercise this peaked at a median of 3.8 mM (NEFA:albumin=5.89) with a highest recorded NEFA:albumin ratio of 7.04. Peak median values after exercise in arterialized blood from a dorsal vein draining a hand (representative of systemic plasma) were 1.85 mM (NEFA:albumin=2.87) with a highest recorded value of 2.16 mM (NEFA:albumin=3.35). Our use of 1 mM palmitate with 2% albumin therefore creates conditions that correspond to those seen in venous plasma in the adipose tissue circulation during exercise and it is noteworthy that Park et al. [42] found that exercise increased AMPK activity and decreased ACC activity in rat epididymal adipose tissue. The notion that increased supply of NEFAs increases glucose uptake and utilization is the direct opposite of the Randle cycle as conceived for muscle tissues. However, a fundamental feature of the Randle cycle is the β-oxidation of the increased NEFA supply [43]. We suggest that the low propensity for β-oxidation in adipocytes [37] due to (i) the very high activity of TAG synthesizing enzymes and (ii) the low activity of CPT-1 (carnitine palmitoyltransferase-1) in adipocyte mitochondria [44] could minimize any tendency for a Randle cycle-mediated decrease in glucose transport and utilization. Also, as a consequence of this low propensity for β-oxidation, adipocyte PDH could be less susceptible to inactivation in response to an increase in NEFA supply. This in turn may contribute to the ability of palmitate to increase FA synthesis–provided there is adequate glucose availability to provide the necessary acetyl-CoA. Such an increase in FA synthesis could enhance the propensity for TAG formation and deposition in adipocytes when LPL is active and is generating FAs from lipoprotein TAG in the adipose tissue capillary bed. Our study has other possible physiological implications as follows. (i) If under ‘mobilizing conditions’ lipolysis exceeded vascular removal of NEFA from the adipose tissue capillary bed, activation of adipocyte AMPK by NEFA could modulate NEFA release by (a) phosphorylation and inhibition of hormone-sensitive lipase [11] and by (b) increasing glucose transport leading to NEFA re-esterification. (ii) Enhancement by NEFA of the inactivation of AMPK in response to glucose may expand the range over which AMPK activity can change during the fed/fasted transition. (iii) NEFA makes AMPK activity moderately responsive to glucose under hyperglycaemic conditions, an observation that is relative to diabetes. (iv) The production and/or release by adipocytes of adipokines such as leptin, adiponectin and visfatin is regulated by AMPK [45–49]. The present study suggests ways by which NEFA and glucose could influence cytokine production through regulation of AMPK.
Online data
AUTHOR CONTRIBUTION
Abdel Hebbachi performed the experimental work shown in Figures 2–5. The data from a previous publication [22] that were used in Figures 1, 6 and 8 together with data used for Supplementary Figures S3–S14 were from experiments performed by David Saggerson (Supplementary references as shown). David Saggerson was involved in the conception and design of the project, in the analysis and interpretation of the results and in writing the paper.
FUNDING
This work was supported by Diabetes UK [grant number 07/0003528].
References
- 1.Saggerson E. D., Tomassi G. The regulation of glyceride synthesis from pyruvate in isolated fat cells. The effects of palmitate and altered dietary status. Eur. J. Biochem. 1971;23:109–117. doi: 10.1111/j.1432-1033.1971.tb01597.x. [DOI] [PubMed] [Google Scholar]
- 2.Kurth-Kraczek E. J, Hirshman M. F., Goodyear L. J., Winder W. W. 5′-AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48:1667–1671. doi: 10.2337/diabetes.48.8.1667. [DOI] [PubMed] [Google Scholar]
- 3.Yang J., Holman G. D. Insulin and contraction stimulate exocytosis, but increased AMP-activated protein kinase activity resulting from oxidative metabolism stress slows endocytosis of GLUT4 in cardiomyocytes. J. Biol. Chem. 2005;280:4070–4078. doi: 10.1074/jbc.M410213200. [DOI] [PubMed] [Google Scholar]
- 4.Kola B., Boscaro M., Rutter G. A., Grossman A. B., Korbonits M. Expanding role of AMPK in endocrinology. Trends Endocrinol. Metab. 2006;17:205–215. doi: 10.1016/j.tem.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Hutchinson D. S., Summers R. J., Bengtsson T. Regulation of AMP-activated protein kinase activity by G-protein coupled receptors: potential utility in treatment of diabetes and heart disease. Pharmacol. Ther. 2008;119:291–310. doi: 10.1016/j.pharmthera.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Clark H., Carling D., Saggerson D. Covalent activation of heart AMP-activated protein kinase in response to physiological concentrations of long-chain fatty acids. Eur. J. Biochem. 2004;271:2215–2224. doi: 10.1111/j.1432-1033.2004.04151.x. [DOI] [PubMed] [Google Scholar]
- 7.Fediuc S., Gaidhu M. P., Ceddia R. B. Regulation of AMP-activated protein kinase and acetyl-CoA carboxylase phosphorylation by palmitate in skeletal muscle cells. J. Lipid Res. 2006;47:412–420. doi: 10.1194/jlr.M500438-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Watt M. J., Steinberg G. R., Chen Z. P., Kemp B. E., Febbraio M. A. Fatty acids stimulate AMP-activated protein kinase and enhance fatty acid oxidation in L6 myotubes. J. Physiol. 2006;574:139–147. doi: 10.1113/jphysiol.2006.107318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itani S. I., Saha A. K., Kurowski T. G., Coffin H. R., Tornheim K., Ruderman N. B. Glucose autoregulates its uptake in skeletal muscle: involvement of AMP-activated protein kinase. Diabetes. 2003;52:1635–1640. doi: 10.2337/diabetes.52.7.1635. [DOI] [PubMed] [Google Scholar]
- 10.Tabidi I., Saggerson E. D. Inactivation of the AMP-activated protein kinase by glucose in cardiac myocytes. A role for the pentose phosphate pathway. Biosci. Rep. 2012;32:229–239. doi: 10.1042/BSR20110075. [DOI] [PubMed] [Google Scholar]
- 11.Daval M., Diot-Dupuy F., Bazin R., Hainauly I., Viollet B., Vaulont S., Hajduch E., Ferre P., Foufelle F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J. Biol. Chem. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- 12.Gauthier M. S., Miyoshi H., Souza S. C., Cacicedo J. M., Saha A. K., Greenberg A. S., Ruderman N. B. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J. Biol. Chem. 2008;283:16514–16524. doi: 10.1074/jbc.M708177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omar B., Zmuda-Trzebiatowska E., Manganiello V., Goransson O., Degerman E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell. Signalling. 2009;21:760–766. doi: 10.1016/j.cellsig.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan J. E., Brocklehurst K. J., Marley A. E., Carey F., Carling D., Beri R. K. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1993;353:33–36. doi: 10.1016/0014-5793(94)01006-4. [DOI] [PubMed] [Google Scholar]
- 15.Anthony N. M., Gaidhu M. P., Ceddia R. B. Regulation of visceral and subcutaneous lipolysis by acute AICAR-induced AMPK activation. Obesity. 2009;17:1312–1317. doi: 10.1038/oby.2008.645. [DOI] [PubMed] [Google Scholar]
- 16.Salt I. P., Connell J. M., Gould G. W. 5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR) inhibits insulin-stimulated glucose transport in 3T3-L1 adipocytes. Diabetes. 2000;49:1649–1656. doi: 10.2337/diabetes.49.10.1649. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi S., Katahira H., Ozawa S., Nakamichi Y., Tanaka T., Shimoyama T., Takahashi K., Yoshimoto K., Imaizumi M. O., Nagamatsu S., Ishida H. Activators of AMP-activated protein kinase enhance GLUT4 translocation and its glucose transport activity in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2005;289:E643–E649. doi: 10.1152/ajpendo.00456.2004. [DOI] [PubMed] [Google Scholar]
- 18.Sakoda H., Ogihara T., Anai M., Fujishiro M., Ono H., Onishi Y., Katagiri H., Abe M., Fukushima Y., Shojima, et al. Activation of AMPK is essential for AICAR-induced glucose uptake by skeletal muscle but not adipocytes. Am. J. Physiol. Endocrinol. Metab. 2002;282:E1239–E1244. doi: 10.1152/ajpendo.00455.2001. [DOI] [PubMed] [Google Scholar]
- 19.Gaidhu M. P., Fediuc S., Ceddia R. B. 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside-induced AMP-activated protein kinase phosphorylation inhibits basal and insulin-stimulated glucose uptake, lipid synthesis and fatty acid oxidation in isolated rat adipocytes. J. Biol. Chem. 2006;281:25956–25964. doi: 10.1074/jbc.M602992200. [DOI] [PubMed] [Google Scholar]
- 20.Gaidhu M. P., Perry R. L., Noor F., Ceddia R. B. Disruption of AMPK α1 signalling prevents AICAR-induced inhibition of AS160/TBC1D4 phosphorylation and glucose uptake in primary rat adipocytes. Mol. Endocrinol. 2010;24:1434–1440. doi: 10.1210/me.2009-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X., Motoshima H., Mahadev K., Stalker T. J., Scalia R., Goldstein B. J. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52:1355–1363. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]
- 22.Saggerson E. D. The regulation of glyceride synthesis in isolated white-fat cells. The effects of palmitate and lipolytic agents. Biochem. J. 1972;128:1057–1067. doi: 10.1042/bj1281057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukherjee S. P., Mikherjee C., Yeager M. D., Lynn W. S. Stimulation of glucose transport and oxidation in adipocytes by fatty acids: evidence for a regulatory role in the cellular response to insulin. Biochem. Biophys. Res. Commun. 1980;94:682–689. doi: 10.1016/0006-291x(80)91286-3. [DOI] [PubMed] [Google Scholar]
- 24.Joost H. G., Steinfelder H. J. Insulin-like stimulation of glucose transport is isolated adipocytes by fatty acids. Biochem. Biophys. Res. Commun. 1985;128:1358–1363. doi: 10.1016/0006-291x(85)91090-3. [DOI] [PubMed] [Google Scholar]
- 25.Hardy R. W., Ladenson J. H., Henriksen E. J., Holloszy J. O., McDonald J. M. Palmitate stimulates glucose transport in rat adipocytes by a mechanism involving translocation of the insulin sensitive glucose transporter (GLUT4) Biochem. Biophys. Res. Commun. 1991;177:343–349. doi: 10.1016/0006-291x(91)91989-p. [DOI] [PubMed] [Google Scholar]
- 26.Usui I., Haruta T., Takata Y., Iwata M., Uno T., Takano A., Ueno E., Ishibashi O., Ishihara H., Wada, et al. Differential effects of palmitate on glucose uptake in rat-1 fibroblasts and 3T3-L1 adipocytes. Horm. Metab. Res. 1999;31:546–552. doi: 10.1055/s-2007-978793. [DOI] [PubMed] [Google Scholar]
- 27.Murer E., Boden G., Gyda M., Deluca F. Effects of oleate and insulin on glucose uptake, oxidation and glucose transporter proteins in rat adipocytes. Diabetes. 1992;41:1063–1068. doi: 10.2337/diab.41.9.1063. [DOI] [PubMed] [Google Scholar]
- 28.Sooranna S. R., Saggerson E. D. Studies on the role of insulin in regulation of glyceride synthesis in rat epididymal adipose tissue. Biochem. J. 1975;150:441–451. doi: 10.1042/bj1500441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sellevold O. F., Jynge P., Aarstad K. High performance liquid chromatography: a rapid isocratic method for determination of creatine compounds and adenine nucleotides in myocardial tissue. J. Mol. Cell. Cardiol. 1986;18:517–527. doi: 10.1016/s0022-2828(86)80917-8. [DOI] [PubMed] [Google Scholar]
- 30.Lihn A. S., Jessen N., Pedersen S. B., Lund S., Richelsen B. AICAR stimulates adiponectin and inhibits cytokines in adipose tissue. Biochem. Biophys. Res. Commun. 2004;316:853–858. doi: 10.1016/j.bbrc.2004.02.139. [DOI] [PubMed] [Google Scholar]
- 31.Hodgetts V., Coppack S. W., Frayn K. N., Hockaday T. D. R. Factors controlling fat mobilization from human subcutaneous adipose tissue during exercise. J. Appl. Physiol. 1991;71:445–451. doi: 10.1152/jappl.1991.71.2.445. [DOI] [PubMed] [Google Scholar]
- 32.Handa N., Takagi T., Saijo S., Kishishita S., Takaya D., Toyama M., Terada T., Shirouzu M., Suzuki A., Lee S., et al. Structural basis for compound C inhibition of the human AMP-activated protein kinase α2 subunit kinase domain. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011;67:480–487. doi: 10.1107/S0907444911010201. [DOI] [PubMed] [Google Scholar]
- 33.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hajduch E., Darakhshan F., Hundal H. S. Fructose uptake in rat adipocytes: GLUT5 expression and the effects of streptozotocin-induced diabetes. Diabetologia. 1998;41:821–828. doi: 10.1007/s001250050993. [DOI] [PubMed] [Google Scholar]
- 35.Horman S., Vertommen D., Heath R., Neumann D., Mouton D., Woods A., Schlattner U., Wallimann U., Carling D., Hue L., Rider M. H. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase α-subunits in heart via hierarchical phosphorylation of Ser485/491. J. Biol. Chem. 2006;281:5335–5540. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 36.Sanders M. J., Grondin P. O., Hegarty B. D., Snowden M. A., Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem. J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harper R. D., Saggerson E. D. Factors affecting fatty acid oxidation in fat cells isolated from white adipose tissue. J. Lipid Res. 1976;17:516–526. [PubMed] [Google Scholar]
- 38.Bai L., Wang Y., Fan J., Chen Y., Qu A., James D. E., Xu T. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 2007;5:47–57. doi: 10.1016/j.cmet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Zeigerer A., McBrayer M. K., McGraw T. E. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol. Biol. Cell. 2004;15:4406–4415. doi: 10.1091/mbc.E04-04-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folmes C. D., Clanachan A. S., Lopaschuk G. D. Fatty acids attenuate insulin regulation of 5′-AMP-activated protein kinase and insulin cardioprotection after ischemia. Circ. Res. 2006;99:61–68. doi: 10.1161/01.RES.0000229656.05244.11. [DOI] [PubMed] [Google Scholar]
- 41.Yin W., Mu J., Birnbaum M. J. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3-L1 adipocytes. J. Biol. Chem. 2003;278:43074–43080. doi: 10.1074/jbc.M308484200. [DOI] [PubMed] [Google Scholar]
- 42.Park H., Kaushik V. K., Constant S., Prentki M., Przybytkowski E., Ruderman N. B., Saha A. K. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J. Biol. Chem. 2002;277:32571–32577. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- 43.Hue L., Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am. J. Physiol. Endocrinol. Metab. 2009;297:E578–E591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saggerson E. D., Carpenter C. A. The effect of malonyl-CoA on overt and latent carnitine acyltransferase activities in rat liver and adipocyte mitochondria. Biochem. J. 1983;210:591–597. doi: 10.1042/bj2100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huypens P., Quartier E., Pipeleers D., Van de Casteele M. Metformin reduces adiponectin protein expression and release in 3T3-L1 adipocytes involving activation of AMP activated protein kinase. Eur. J. Pharmacol. 2005;518:90–95. doi: 10.1016/j.ejphar.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 46.Giri S., Rattan R., Haq E., Khan M., Yasmin R., Won J. S., Key L., Singh A. K., Singh I. AICAR inhibits adipocyte differentiation in 3T3L1 and restores metabolic alterations in diet-induced obesity mice model. Nutr. Metab. 2006;3:31–50. doi: 10.1186/1743-7075-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu L., Isobe K., Zeng Q., Suzukawa K., Takekoshi K., Kawakami Y. β-Adrenoceptor agonists downregulate adiponectin, but upregulate adiponectin receptor 2 and tumor necrosis factor-α expression in adipocytes. Eur. J. Pharmacol. 2007;569:155–162. doi: 10.1016/j.ejphar.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Lorente-Cebrián S., Bustos M., Marti A., Martinez J. A., Moreno-Aliaga M. J. Eicosapentaenoic acid stimulates AMP-activated protein kinase and increases visfatin secretion in cultured murine adipocytes. Clin. Sci. 2009;117:243–249. doi: 10.1042/CS20090020. [DOI] [PubMed] [Google Scholar]
- 49.Lee M. J., Fried S. K. Integration of hormonal and nutrient signals that regulate leptin synthesis and secretion. Am. J. Physiol. Endocrinol. Metab. 2009;296:E1230–E1238. doi: 10.1152/ajpendo.90927.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.