Abstract
To investigate the role mitochondrial membrane lipids play in the actions of CR (calorie restriction), C57BL/6 mice were assigned to four groups (control and three 40% CR groups) and the CR groups were fed diets containing soya bean oil (also in the control diet), fish oil or lard. The fatty acid composition of the major mitochondrial phospholipid classes, proton leak and H2O2 production were measured in liver mitochondria following 1 month of CR. The results indicate that mitochondrial phospholipid fatty acids reflect the PUFA (polyunsaturated fatty acid) profile of the dietary lipid sources. CR significantly decreased the capacity of ROS (reactive oxygen species) production by Complex III but did not markedly alter proton leak and ETC (electron transport chain) enzyme activities. Within the CR regimens, the CR-fish group had decreased ROS production by both Complexes I and III, and increased proton leak when compared with the other CR groups. The CR-lard group showed the lowest proton leak compared with the other CR groups. The ETC enzyme activity measurements in the CR regimens showed that Complex I activity was decreased in both the CR-fish and CR-lard groups. Moreover, the CR-fish group also had lower Complex II activity compared with the other CR groups. These results indicate that dietary lipid composition does influence liver mitochondrial phospholipid composition, ROS production, proton leak and ETC enzyme activities in CR animals.
Keywords: aging, energy restriction, mitochondria, oxidative stress phospholipids, proton leak
Abbreviations: BHT, butylated hydroxytoluene; CL, cardiolipin; CR, calorie restriction; DCPIP, 2,6-dichlorophenol-indophenol; ETC, electron transport chain; MUFA, monounsaturated fatty acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PUFA, polyunsaturated fatty acid; RCR, respiratory control ratio; ROS, reactive oxygen species; TBARS, thiobarbituric acid-reactive substance; TPMP+, methyl-triphenyl-phosphonium
INTRODUCTION
CR (calorie restriction) has been shown to delay the onset of several age-related diseases and extend mean and maximum lifespan in a variety of species [1]. Although the beneficial effects of CR are well demonstrated, the underlying mechanisms are not entirely clear. The membrane theory of aging proposes that life span is inversely related to the level of unsaturation of membrane phospholipids [2,3], and it has been hypothesized that CR may retard aging by decreasing membrane fatty acid unsaturation [2,4]. In support of this idea, CR has been reported to decrease the level of long chain PUFAs (polyunsaturated fatty acids) in membranes from a variety of tissues [5–8]. The membrane theory of aging focuses on changes in fatty acid unsaturation as a way to alter susceptibility to lipid peroxidation, but changes in membrane fatty acid composition could also influence oxidative stress by altering membrane-linked processes such as ROS (reactive oxygen species) production and proton leak.
The free radical theory of aging proposes that aging is the result of oxidative damage caused by free radicals produced as a by product of oxidative metabolism [9]. The mitochondrial inner membrane ETC (electron transport chain) Complexes I and III are major cellular sites of ROS generation [10]. ROS production by these membrane bound complexes could be influenced by CR-induced alterations in membrane lipid composition. Several lines of evidence have shown that CR decreases mitochondrial ROS production in multiple tissues [11,12]. There is also some evidence that mitochondrial ROS production could be altered by changes in membrane lipid composition [13,14]. However, whether changes in membrane lipid composition are a major contributor to the decreased ROS production with CR still remains to be determined.
Mitochondrial proton leak is a major cellular energy consuming process that may also influence ROS production [15]. However, little is known about the influence of membrane lipids on CR-induced changes in proton leak. Studies have shown that mitochondrial proton leak is positively correlated with membrane unsaturation and n−3 PUFA content [16,17]. Studies have also reported that CR alters proton leak in liver [18,19]. However, it is unclear if alterations in membrane lipid composition with CR contribute to these changes in mitochondrial proton leak.
To determine the role membrane lipids play in the actions of CR, we manipulated mitochondrial membrane fatty acid composition by feeding CR animals diets that differ in lipid composition. The objective of the study was to determine if dietary lipid composition (fish oil, soya bean oil or lard) influences liver mitochondrial membrane composition, ROS production, proton leak and ETC enzyme activities in mice fed a CR diet for 1 month. Furthermore, this study will help determine if specific changes in membrane fatty acid composition are required for CR-induced alterations in mitochondrial ROS production, proton leak and ETC enzyme activities.
MATERIALS AND METHODS
Chemicals
All chemicals and reagents were purchased from Sigma Aldrich, except for protein assay kits (Bio-Rad) and BSA (MP Biochemicals).
Animals and diets
Male C57BL/6 mice were purchased from the Jackson Laboratory at 14-week of age. The mice were fed a commercial rodent chow diet (Harlan Teklad #7012) for 14 days, and then were randomly assigned into four dietary groups and fed modified AIN-93G purified diets. The control group was fed 95% of a pre-determined ad libitum intake. This slight restriction in food intake was initiated to prevent excessive weight gain during the study. The three CR dietary groups were maintained on 60% of the ad libitum intake, and fed diets that were identical except for the lipid sources. The dietary fat for the control group was soya bean oil (Control-soy). The dietary fats for the three CR group were soya bean oil (high in n−6 PUFAs; Super Store Industries), fish oil (high in n−3 PUFAs: 18% EPA (eicosapentaenoic acid), 12% DHA (docosahexaenoic acid); Jedwards International), or lard (high in saturated and MUFAs (monounsaturated fatty acids) and low in PUFAs; ConAgra Foods). To insure adequate linoleic acid levels, the CR-fish group was supplemented with soya bean oil (Table 1). The mice were fed the control or CR diets for 1 month. The fatty acid compositions of the dietary lipids are shown in Table 2. All mice were housed individually in a vivarium maintained at 22–24°C and 40–60% relative humidity with a 12 h light/12 h dark cycle and free access to water. All experimental procedures were approved by the University of California Institutional Animal Care and Use Committee.
Table 1. Diet composition (g/100 g of diet).
All diets contained AIN-93G mineral mix and AIN-93 vitamin mix obtained from Dyets
| Dietary group | |||
|---|---|---|---|
| Ingredients | Control/CR-Soy | CR-Fish | CR-Lard |
| Corn starch | 39.7 | 39.7 | 39.7 |
| Casein | 20.0 | 20.0 | 20.0 |
| Maltodextrin | 13.2 | 13.2 | 13.2 |
| Sucrose | 10.0 | 10.0 | 10.0 |
| Soybean oil | 7.0 | 1.0 | 0.0 |
| Fish oil | 0.0 | 6.0 | 0.0 |
| Lard | 0.0 | 0.0 | 7.0 |
| Cellulose | 5.0 | 5.0 | 5.0 |
| Mineral mix | 3.5 | 3.5 | 3.5 |
| Vitamin mix | 1.0 | 1.0 | 1.0 |
| l-Cystine | 0.3 | 0.3 | 0.3 |
| Choline bitartrate | 0.25 | 0.25 | 0.25 |
| t-Butylhydroquinone | 0.0014 | 0.0028 | 0.0014 |
Table 2. Major fatty acid composition of the diet lipids (percentage of total).
| Fatty Acid | Soybean oil | Fish oil | Lard |
|---|---|---|---|
| 14:0 | 0.1 | 7.5 | 1.4 |
| 16:0 | 10.2 | 16.5 | 23.7 |
| 16:1, n−7 | 0.1 | 9.6 | 0.0 |
| 18:0 | 4.0 | 3.2 | 15.0 |
| 18:1, n−9 | 21.2 | 8.7 | 39.2 |
| 18:1, n−7 | 0.0 | 2.9 | 0.0 |
| 18:2, n−6 | 55.0 | 1.3 | 15.2 |
| 18:3, n−3 | 8.0 | 0.1 | 0.5 |
| 18:4, n−3 | 0.0 | 3.0 | 0.1 |
| 20:4, n−6 | 0.0 | 0.9 | 0.2 |
| 20:5, n−3 | 0.0 | 17.7 | 0.0 |
| 22:6, n−3 | 0.0 | 10.3 | 0.0 |
| Others | 1.3 | 18.4 | 4.7 |
| Saturates | 14.8 | 28.3 | 40.3 |
| Total n−3 | 8.1 | 33.9 | 0.7 |
| Total n−6 | 55.0 | 3.2 | 16.0 |
| n−6/n−3 | 6.8 | 0.1 | 24.4 |
Isolation of mitochondria from mouse liver
At the end of 1 month of CR, mice were killed by cervical dislocation after an overnight fast. Body and liver weights were recorded and liver mitochondria were isolated by differential centrifugation as described previously [20]. All procedures were performed at 4°C. Briefly, the liver was rapidly removed and weighed after killing. The liver was then washed and finely minced in isolation medium (220 mM mannitol, 70 mM sucrose, 1 mM EDTA, and 20 mM Tris/HCl, pH 7.4, containing 0.1% fatty acid-free albumin). The minced tissue was gently homogenized in isolation medium (1:10, w/v) using a glass-Teflon motor-driven homogenizer at 500 rev./min for 2 min with six up and down strokes. Subsequently, the homogenate was centrifuged at 500 g (Beckman Coulter Model J2-21M) for 10 min to remove debris and nuclei. The retained supernatant was centrifuged at 10000 g for 10 min. The resulting pellet was washed twice with wash medium (220 mM mannitol, 70 mM sucrose, 1 mM EGTA and 20 mM Tris/HCl, pH 7.4) and centrifuged at 10000 g for 10 min. The final pellet was re-suspended in a minimal volume of the wash medium and used in the experiments. Portions of this final pellet suspension were used for H2O2, proton leak, ETC and lipid peroxidation assays, and the remainder was further purified on Percoll gradients and stored in liquid nitrogen to be used for lipid analysis.
Fractionation of mitochondria using Percoll gradients
To minimize sample contamination with broken plasma membrane or ER (endoplasmic reticulum) [21], a discontinuous Percoll gradient was used to further fractionate the isolated mitochondria for lipid analysis. This discontinuous gradient was achieved by using equal volumes of Percoll at 18, 30 and 60% (v/v) in a buffer of 0.225 M mannitol, 1 mM EGTA and 25 mM Hepes [14]. Resuspended mitochondria were loaded on the gradient and centrifuged at 8700 g for 1 h at 4°C. After the centrifugation, the mitochondrial fraction was isolated from the 30–60% interface and twice washed in isolation media without BSA and centrifuged at 10000 g for 10 min to remove Percoll. The purified mitochondria were reconstituted in a minimal volume of the isolation media and stored in liquid nitrogen until lipid analysis.
Protein assays
Protein concentrations of mitochondrial samples were determined using the BioRad protein assay method, with BSA as the standard.
Lipid extraction and fatty acid analysis
Percoll-purified mitochondria were used to determine the influence of dietary lipid manipulations on the fatty acid composition of PC (phosphatidylcholine), PE (phosphatidylethanolamine) and CL (cardiolipin). Specifically, the purified mitochondria were shipped on dry ice to Lipid Technologies LLC and analysed for phospholipid fatty acid composition. Lipids were extracted from the mitochondria using Bligh-Dyer extraction [22]. A mixture of mitochondria, chloroform/methanol (2:1, v/v) and water was prepared in order to recover the lipid in a chloroform layer. Lipid classes were separated by preparative TLC and the phospholipid fractions were scraped from the thin layer plates and methylated with boron trifluoride (10%) in excess methanol in an 80°C water bath for 90 min. The resulting fatty acid methyl esters were extracted with petroleum ether and water and stored frozen for separation and quantification by capillary GC analysis [23]. Results were expressed as the percentage of each individual fatty acid in relation to the total fatty acids of each lipid class.
H2O2 production by mouse liver mitochondria
The rate of mitochondrial H2O2 production was determined fluorimetrically (excitation 320 nm, emission 400 nm) at 37°C using PHPA (p-hydroxyphenylacetate; 500 μg), HRP (horseradish peroxidase) (4 units) and mitochondria (0.2 mg) suspended in incubation medium (10 mM potassium phosphate buffer, 154 mM KCl, 0.1 mM EGTA, 3 mM MgCl2, pH 7.4). Fluorescence was measured using a PerkinElmer LS 55 luminescence spectrometer equipped with a Peltier water heating system and a magnetic stirring sample compartment [24]. Measurements were completed with substrates alone (10 mM succinate or 10 mM pyruvate/5 mM malate), substrate and rotenone (5 μM), or substrate and antimycin A (5 μM). Rotenone (Complex I inhibitor) and antimycin A (Complex III inhibitor) maintain Complexes I and/or III of the ETC in a reduced state. The rates of H2O2 production were expressed as pmol H2O2 min−1·(mg of protein)−1. A standard curve generated over a range of H2O2 concentrations was used to determine the amount of H2O2 produced.
Measurement of mitochondrial oxygen consumption
Mitochondrial oxygen consumption [nmol O2·min−1·(mg of protein) −1] was measured in duplicate using a previously described method [25] with modifications. Mitochondrial respiration was monitored using a Clark-type oxygen electrode (Hansatech). All measurements were completed at 30°C using mitochondria (0.35 mg of mitochondrial protein/ml) calibrated with air-saturated incubation medium (145 mM KCl, 5 mM KH2PO4, 30 mM Hepes, 3 mM MgCl2 and 0.1 mM EGTA, pH 7.4). Respiration was initiated by adding 5 mM succinate (plus 5 μM rotenone) in the absence (state 4) and in the presence (state 3) of 500 μM ADP. RCRs (respiratory control ratios) were determined as state 3 divided by state 4 respiration rates. The RCR values for the four experimental groups were: Control-soy (4.0±0.2), CR-soy (3.9±0.1), CR-fish (3.5±0.1) and CR-lard (4.5±0.2). Within the CR groups, the RCR value from the CR-lard group was higher than both the CR-fish and CR-soy groups (P<0.05). However, there were no differences in RCR values between the Control-soy and any of the CR groups.
Measurement of proton leak kinetics
Mitochondrial membrane potential (ΔΨm) in non-phosphorylating conditions was assessed simultaneously with oxygen consumption measurements using a TPMP+ (methyl-triphenyl-phosphonium)-sensitive electrode. All measurements were completed in duplicate using mitochondria (0.35 mg/ml) in the above mentioned incubation medium. A TPMP+ (0–2.5 μM) standard curve was generated in each sample prior to the initiation of respiration and membrane potential measurements. Proton leak kinetics were determined by titrating the ETC with incremental additions of malonate (0.1–2.4 mM), an inhibitor of Complex II, in the presence of 5 mM succinate, 5 μM rotenone, oligomycin (8 μg/ml) and nigericin (0.08 μg/ml) [26]. Membrane potentials were calculated using a modified Nernst equation [26]. A TPMP+ binding correction for liver of 0.4 per μl/mg protein was used for membrane potential calculations [27].
Measurement of ETC activity
The activities of ETC Complexes I, II, III and IV were measured spectrophotometrically [14,28,29] using a PerkinElmer Lambda 25 UV/Vis spectrometer. All assays were performed at 30°C using 25 mM potassium phosphate buffer, pH 7.2 (assay buffer). The total volumes of reaction mixtures were 1 ml. Complex I (NADH-ubiquinone oxidoreductase) activity was measured at 340 nm (∊=6.22 mM−1·cm−1). The reaction mixture contained assay buffer, 5 mM MgCl2, 0.13 mM NADH, 65 μM ubiquinone-1, 2 mM KCN, 2.5 mg/ml BSA and 5 μg/ml antimycin A. The reaction was initiated by the addition of mitochondria (20–55 μg of protein) and the change in absorbance due to NADH oxidation in the presence of ubiquinone-1 was monitored for 1 min, after which 5 μg/ml rotenone was added and the reaction was monitored for a further 2 min. Complex I activity was calculated as the difference between the total enzymatic rate and the rotenone-sensitive rate. Complex II (succinate: ubiquinone oxidoreductase) activity was measured at 600 nm (∊=19.1 mM−1·cm−1) by following the decrease in absorbance due to the reduction of DCPIP (2,6-dichlorophenol-indophenol). Then 20 mM succinate and mitochondria (1–8 μg of protein) were added to the assay buffer and incubated for 20 min at 30°C. Subsequently, 5 mM MgCl2, 2 mM KCN, 5 μg/ml antimycin A, 5 μg/ml rotenone and 50 μM DCPIP were added, followed by the final addition of 65 μM ubiquinone-1 to initiate the reaction. The assay was monitored for 10 min. Complex III (ubiquinol:ferricytochrome c oxidoreductase) activity was determined at 550 nm (∊=19.1 mM−1·cm−1) by following the increase in absorbance due to the reduction of oxidized ferricytochrome c by decylubiquinol. The reaction mixture consisted of assay buffer, 5 mM MgCl2, 2 mM KCN, 5 μg/ml rotenone, 2.5 mg/ml BSA and 50 μM oxidized cytochrome c. Mitochondria (2–10 μg of protein) were added to the reaction mixture and the reaction was initiated by the addition of 60 μM decylubiquinol and absorbance was monitored for 60 s. The rate from the initial 30 s was taken for calculations of Complex III activity. The assay was also performed with the inclusion of 5 μg/ml antimycin A to distinguish between non-enzymic reduction of ferricytochrome c and that due to reduction by decylubiquinol. Decylubiquinol was prepared as previously described [30]. Complex IV (cytochrome c oxidase) activity was determined at 550 nm (∊=19.1 mM−1·cm−1) by following the decrease in absorbance due to the oxidation of ferrocytochrome c. The reaction mixture consisted of assay buffer and 15 μM ferrocytochrome c. The reaction was initiated by the addition of mitochondria (1–5 μg of protein) and the reaction was monitored for 2 min. The rates from the initial 30 s were taken for calculations of Complex IV activity.
Measurement of lipid peroxidation levels
Lipid peroxidation was measured by the TBARSs (thiobarbituric acid-reactive substances) test [31], except 0.07 mM per assay of BHT (butylated hydroxytoluene) was added to prevent artificial lipid peroxidation during the boiling step [32]. Specifically, 0.5 mg of mitochondrial protein in 1 ml volume was combined with a 2 ml solution of TCA–TBA–HCl reagent [15% (w/v) trichloracetic acid; 0.375% (w/v) thiobarbituric acid; 0.25 M hydrochloric acid]. BHT (0.07 mM) was added to the solution and mixed thoroughly. The solution was heated for 15 min in a boiling water bath. Subsequently, after cooling, the precipitate was removed by centrifugation at 1000 g for 10 min. The absorbance of the sample was determined at 535 nm against a blank that contained all the reagents minus the mitochondria. Results were calculated using an absorption coefficient of 1.56×105 M−1·cm−1 (535 nm) and expressed as nmol of TBARS/mg of protein.
Statistical analysis
All results were expressed as the means±S.E.M. Normality of the data distribution was checked using the Shapiro–Wilk test. Comparisons between the control and CR-soy group were used to determine the influence of CR on each variable of interest while comparisons among the three CR groups were used to determine the influence of dietary lipids on the variables of interest. Wilcoxon/Kruskal–Wallis tests or ANOVA were performed as appropriate using JMP software (SAS Institute). A post-hoc Tukey–Kramer test was performed to correct for multiple comparisons.
RESULTS
Liver and body weights
Body weights (in g) for each of the four diet groups were 27.7±0.4 (Control-soy), 22.5±0.3 (CR-fish), 22.3±0.3 (CR-lard) and 21.4±0.2 (CR-soy). Liver weights (in g) were 1.04±0.03 (Control-soy), 0.95±0.02 (CR-fish), 0.94±0.02 (CR-lard) and 0.89±0.01 (CR-soy). 1 month of CR (Control-soy versus CR-soy) produced a decrease (P<0.05) in body (22.7%) and liver (14.4%) weights. Within the CR groups, soya bean oil-fed CR mice had slightly lower body and liver weights (P<0.05) than the other groups.
Mitochondrial phospholipid fatty acid composition
Liver mitochondrial fatty acid composition of the phospholipids PC, PE and CL are presented in Tables 3–5 respectively. Lipid analysis showed that liver mitochondrial membrane lipids changed in a manner which reflected the dietary lipid sources.
Table 3. Fatty acid composition (%) of PC from liver mitochondria of mice consuming a control diet (Control-Soy, n=6), or CR diets containing soya bean oil (CR-Soy, n=6), lard (CR-Lard, n=7) or fish oil (CR-Fish, n=4).
Values are the percentages of total HUFA amount. Superscripts that do not share a common letter indicate a significant difference (P<0.05) between treatments. SFA, saturated fatty acid.
| Fatty acid | Control-Soy | CR-Soy | CR-Lard | CR-Fish |
|---|---|---|---|---|
| 14:0 | 0.43±0.06 | 0.59±0.14 | 0.36±0.07 | 0.35±0.04 |
| 16:0 | 31.2±1.0a | 27.8±0.5b | 27.2±0.4b | 32.0±1.1a |
| 18:0 | 11.6±0.6a | 13.7±0.3b | 12.4±0.6a,b | 11.9±0.4a,b |
| 20:0 | 0.14±0.02 | 0.10±0.02 | 0.08±0.02 | 0.15±0.06 |
| 24:0 | 0.06±0.02 | 0.21±0.06 | 0.13±0.03 | 0.22±0.09 |
| 16:1, n−7 | 1.7±0.1 | 1.9±0.2 | 2.0±0.1 | 1.7±0.1 |
| 18:1, n−7 | 1.4±0.1 | 2.1±0.1 | 1.7±0.5 | 1.0±0.1 |
| 18:1, n−9 | 9.1±0.7a | 13.0±1.0a | 18.7±2.0b | 9.0±0.6a |
| 22:1, n−9 | 0.02±0.01 | 0.03±0.01 | 0.14±0.06 | 0.11±0.03 |
| 18:2, n−6 | 17.9±0.7a | 15.5±1.2a | 10.8±0.7c | 6.9±0.5b |
| 18:3, n−6 | 0.37±0.04a | 0.29±0.03a,b | 0.23±0.02b | 0.19±0.02b |
| 20:3, n−6 | 1.5±0.3a | 1.4±0.1a | 1.6±0.1a | 0.42±0.04b |
| 20:4, n−6 | 11.3±0.4a | 9.4±0.4a | 12.0±1.1a | 4.2±0.2b |
| 22:4, n−6 | 0.06±0.01a,c | 0.10±0.01b,c | 0.14±0.02b | 0.003±0.002a |
| 22:5, n−6 | 0.16±0.06b | 0.27±0.09a,b | 0.57±0.09a | 0.13±0.07b |
| 18:3, n−3 | 0.26±0.04a | 0.26±0.01a | 0.10±0.03b | 0.10±0.01b |
| 20:5, n−3 | 0.24±0.03a,c | 0.37±0.04a | 0.13±0.01c | 8.9±0.6b |
| 22:5, n−3 | 0.27±0.04a | 0.28±0.04a | 0.15±0.03a | 1.4±0.1b |
| 22:6, n−3 | 7.9±0.6b | 7.0±0.8b | 6.9±0.5b | 17.2±1.3a |
| Unsaturation index | 152.2±3.9a | 140.6±5.6a | 147.2±6.4a | 202.1±10.4b |
| % SFA | 43.6±0.7b | 42.8±0.4b | 40.0±0.8a | 44.9±1.2b |
| % MUFA | 13.4±0.6a | 18.5±1.1c | 23.7±1.6b | 13.2±1.0a,c |
| % PUFA | 40.2±1.0a | 35.2±1.3a,b | 33.3±1.9b | 39.7±2.1a,b |
| % Total n−3 | 8.7±0.6b | 8.0±0.8b | 7.4±0.5b | 27.8±1.8a |
| % Total n−6 | 31.4±0.9a | 27.2±1.1a,b | 25.6±1.6b | 11.9±0.6c |
| HUFA | 22.1±0.8b | 19.4±1.2b | 22.2±1.6b | 32.7±2.0a |
| % n−3 HUFAa | 39.2±1.8a | 40.6±1.8a | 33.3±1.3c | 84.9±0.2b |
| % n−6 HUFAa | 60.6±1.8a,b | 59.1±1.7b | 65.2±1.2a | 15.2±0.2c |
Table 5. Fatty acid composition (%) of CL from liver mitochondria of mice consuming a control diet (Control-Soy, n=6), or CR diets containing lard (CR-Lard, n=7) soya bean oil (CR-Soy, n=6) or fish oil (CR-Fish, n=4).
Values are percentages of total HUFA amount. Superscripts that do not share a common letter indicate a significant difference (P<0.05) between treatments. ND, not detected; SFA, saturated fatty acid.
| Fatty acids | Control-Soy | CR-Soy | CR-Lard | CR-Fish |
|---|---|---|---|---|
| 16:0 | 7.3±1.0 | 5.0±0.5 | 7.1±1.3 | 4.0±0.6 |
| 18:0 | 2.6±0.2 | 2.4±0.1 | 3.6±0.9 | 2.3±0.2 |
| 20:0 | 0.03±0.01a | 0.05±0.002a | 0.10±0.03a | 0.25±0.03b |
| 24:0 | 0.24±0.03a | 0.20±0.01a | 0.05±0.02b | 0.22±0.02a |
| 16:1, n−7 | 2.8±0.3 | 2.8±0.2 | 4.0±0.5 | 2.9±0.2 |
| 18:1, n−7 | 4.6±0.1a | 4.0±0.6a,b,c | 5.4±0.2c | 3.9±0.1b |
| 18:1, n−9 | 9.4±0.5a | 10.2±0.3a | 15.8±1.0c | 7.5±0.3b |
| 22:1, n−9 | ND | ND | ND | ND |
| 18:2, n−6 | 53.2±1.1a | 60.0±1.7c | 47.7±3.8a,b | 43.8±1.0b |
| 18:3, n−6 | 1.68±0.06a | 0.18±0.01c | 0.56±0.17b | 0.45±0.05b |
| 20:3, n−6 | 1.47±0.06a | 1.08±0.05c | 1.56±0.11a | 0.17±0.03b |
| 20:4, n−6 | 1.2±0.2a | 1.2±0.1a | 1.4±0.1a | 2.8±0.2b |
| 22:4, n−6 | ND | ND | ND | ND |
| 22:5, n−6 | 0.09±0.04a | 0.42±0.06c | 0.60±0.06b | 0.03±0.02a |
| 18:3, n−3 | 0.46±0.02a | 0.77±0.03c | 0.21±0.05b | 0.23±0.01b |
| 20:5, n−3 | 0.16±0.04a | 0.18±0.01a | 0.05±0.02c | 6.19±0.14b |
| 22:5, n−3 | 0.39±0.06a | 0.44±0.02a | 0.15±0.03c | 0.75±0.10b |
| 22:6, n−3 | 3.0±0.2a | 2.8±0.2a | 2.8±0.2a | 10.6±0.4b |
| Unsaturation index | 168.0±0.7a | 174.8±1.3c | 160.0±7.2a,c | 220.1±1.2b |
| % SFA | 10.4±1.1 | 7.9±0.6 | 11.0±2.2 | 7.0±0.8 |
| % MUFA | 20.7±1.4a | 19.2±1.3a | 27.9±1.5b | 16.9±0.5a |
| % PUFA | 62.6±1.0a | 68.1±1.5c | 56.2±3.7a,b | 66.1±0.8b,c |
| % Total n−3 | 4.1±0.3b,c | 4.5±0.2c | 3.5±0.2b | 18.8±0.4a |
| % Total n−6 | 58.4±1.1a,c | 63.6±1.6a | 52.7±3.6b,c | 47.3±1.1b |
| HUFA | 8.6±0.4a | 7.5±0.3a | 7.9±0.3a | 22.3±0.4b |
| % n−3 HUFAa | 48.2±1.9a | 60.2±1.5c | 43.8±2.2a | 84.5±1.0b |
| % n−6 HUFAa | 51.8±1.9a | 39.8±1.5c | 56.2±2.2a | 15.5±1.0b |
Table 4. Fatty acid composition (%) of PE from liver mitochondria of mice consuming a control diet (Control-Soy, n=6), or CR diets containing soya bean oil (CR-Soy, n=6), lard (CR-Lard, n=7) or fish oil (CR-Fish, n=4).
Values are percentages of total HUFA amount. Superscripts that do not share a common letter indicate a significant difference (P<0.05) between treatments. ND, not detected. SFA, saturated fatty acid.
| Fatty acid | Control-Soy | CR-Soy | CR-Lard | CR-Fish |
|---|---|---|---|---|
| 14:0 | 1.3±0.1a | 2.1±0.6a,b | 1.7±0.3a,b | 4.4±1.5b |
| 16:0 | 8.4±0.9 | 7.6±0.5 | 7.5±0.3 | 8.0±1.3 |
| 18:0 | 35.4±2.1 | 33.1±1.7 | 34.5±1.3 | 31.3±2.6 |
| 20:0 | 0.21±0.05a,b | 0.10±0.01b | 0.14±0.02b | 0.29±0.06a |
| 24:0 | ND | ND | ND | ND |
| 16:1, n−7 | 0.66±0.07a | 0.70±0.09a,b | 1.02±0.15a,b | 0.91±0.10b |
| 18:1, n−7 | 1.00±0.18a,b | 1.19±0.09b | 1.30±0.13b | 0.84±0.04a |
| 18:1, n−9 | 4.6±0.7 | 5.4±0.8 | 6.1±0.5 | 4.0±0.8 |
| 22:1, n−9 | 0.45±0.15 | 0.11±0.02 | 0.09±0.03 | 0.24±0.11 |
| 18:2, n−6 | 6.8±0.6 | 6.8±1.5 | 5.7±1.0 | 5.1±1.1 |
| 18:3, n−6 | 0.21±0.02a | 0.19±0.02a,b | 0.15±0.01b | 0.25±0.03a |
| 20:3, n−6 | 0.98±0.26 | 1.13±0.22 | 0.88±0.20 | 0.63±0.07 |
| 20:4, n−6 | 27.5±1.1a | 31.1±1.5a | 27.2±0.6a | 17.3±1.6b |
| 22:4, n−6 | 0.08±0.02 | 0.12±0.04 | 0.22±0.08 | 0.04±0.02 |
| 22:5, n−6 | 0.07±0.05a | 0.15±0.07a,b | 0.20±0.07a,b | 0.30±0.06b |
| 18:3, n−3 | 0.16±0.06 | 0.10±0.03 | 0.10±0.02 | 0.13±0.04 |
| 20:5, n−3 | 0.45±0.17a | 0.22±0.08a | 0.27±0.10a | 6.08±0.37b |
| 22:5, n−3 | 0.34±0.03a | 0.37±0.03a | 0.13±0.04c | 1.74±0.12b |
| 22:6, n−3 | 1.9±0.3a | 1.2±0.1a | 1.2±0.2a | 7.4±0.7b |
| Unsaturation index | 153.5±4.5a | 170.0±4.8a,b | 156.1±6.6a,b | 177.0±9.6b |
| % SFA | 48.3±1.1 | 44.4±1.7 | 45.7±0.8 | 46.1±2.1 |
| % MUFA | 7.7±0.1a,b | 9.2±0.8a,b | 9.8±0.7b | 7.3±0.6a |
| % PUFA | 39.0±0.9 | 43.1±1.5 | 37.1±2.6 | 39.5±2.0 |
| % Total n−3 | 3.0±0.3a | 2.0±0.1a,b | 1.8±0.3b | 15.8±1.0c |
| % Total n−6 | 35.8±0.7a | 39.8±1.4b | 34.2±2.5a,b | 23.6±1.1c |
| HUFA | 32.1±1.2a | 36.1±1.0b | 31.6±0.2a | 34.5±2.3a,b |
| % n−3 HUFAa | 9.3±0.9a | 5.5±0.4c | 6.1±1.0c | 46.1±2.1b |
| % n−6 HUFAa | 90.1±0.9a | 93.2±0.2a | 90.5±1.1a | 53.7±2.1b |
Control-soy against CR-soy
When examining the effect of CR on mitochondrial phospholipid fatty acid composition, it was observed that most of the fatty acid changes occurred in CL. In CL, HUFA (highly unsaturated fatty acid) composition was altered by CR towards an increase (P<0.05) in n−3 fatty acids and a decrease (P<0.05) in n−6 fatty acids. This was due to a CR-induced increase (P<0.05) in 18:3, n−3 and concomitant decreases (P<0.05) in 18:3, n−6 and 20:3, n−6. CR also resulted in an increase (P<0.05) in unsaturation index along with increases (P<0.05) in 18:3, n−3 and 22:5, n−6. CR resulted in increased (P<0.05) MUFA content of PC. There were no significant changes in fatty acid composition with CR in PE.
Comparisons between CR groups
To examine the effects of dietary lipids on the mitochondrial membrane fatty acid composition of CR mice, comparisons were made between the three CR dietary groups. Compared with the soy and lard groups, fish oil resulted in higher (P<0.05) n−3 fatty acid and lower (P<0.05) n−6 fatty acid content in all three phospholipids. The fish oil group had higher (P<0.05) 20:5, n−3, 22:5, n−3 and 22:6, n−3 than the other diet groups in all phospholipids. Moreover, fish oil increased (P<0.05) unsaturation index in PC and CL compared with the soy and lard groups. Soya bean oil increased (P<0.05) 18:2, n−6 and 18:3, n−3 content in PC and CL compared with the fish oil and lard groups. Soya bean oil also increased (P<0.05) 20:5, n−3 content in PC compared with the lard group. Lard increased (P<0.05) MUFA content in PC and CL compared with both the soy and fish oil groups. Lard also decreased (P<0.05) 22:5, n−3 content in PE and CL compared with the other diet groups.
Mitochondrial H2O2 production
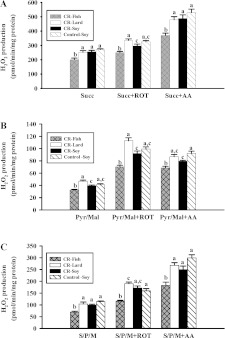
The influence of CR and dietary lipid composition on mitochondrial H2O2 production is summarized in Figure 1.
Figure 1. H2O2 production in liver mitochondria from mice consuming a control diet (Control-Soy, n=9), or CR diets containing lard (CR-Lard, n=7), soya bean oil (CR-Soy, n=10) or fish oil (CR-Fish, n=8).
All measurements were completed on freshly isolated mitochondria. H2O2 production was monitored in mitochondria respiring on succinate (A), pyruvate/malate (B) or succinate plus pyruvate/malate (C). All comparisons were made between mitochondria from different diet groups respiring on the same substrate or substrate plus inhibitor combination. Scale bars that do not share a common letter are different (P<0.05). AA, Antimycin A; ROT, Rotenone.
Control-soy against CR-soy
There were no differences (P>0.10) in H2O2 production between the control-soy and CR-soy groups for mitochondria respiring on succinate. Succinate (a Complex II-substrate) was used to assess H2O2 production at Complex I through electron backflow and at Complex III through forward electron transport. There were also no differences (P>0.10) in H2O2 production between the control-soy and CR-soy groups for mitochondria respiring on succinate plus rotenone or succinate plus antimycin A. Succinate plus rotenone (Complex I inhibitor) was used to assess H2O2 production after blocking backflow into Complex I. Succinate plus antimycin A (Complex III inhibitor) was used to promote maximum H2O2 production by maintaining both Complex I (via backflow) and Complex III in a reduced state. There were no differences (P>0.10) in H2O2 production between control-soy and CR-soy groups for mitochondria respiring on pyruvate/malate (Complex I-linked substrates) alone or with rotenone. Rotenone was added to maximize ROS production from Complex I by blocking electron transport to coenzyme Q and maintaining Complex I in a reduced state. There was a decrease (P<0.05) in H2O2 production in the CR-soy compared with control-soy group when mitochondria were respiring on pyruvate/malate plus antimycin A. Antimycin A blocks electron flow through Complex III and stimulates ROS production from both Complexes I and III by maintaining the respiratory chain components prior to centre i of Complex III in a reduced state [33]. There were no differences (P>0.10) between the two groups when mitochondria were respiring on pyruvate/malate/succinate alone or these substrates plus the inhibitors. However, there was a trend towards a decrease in H2O2 production in the CR-soy compared with control-soy group for mitochondria respiring on pyruvate/malate/succinate plus antimycin A. Overall, the results of these studies suggest that CR induces a decrease in capacity for ROS production from Complex III. This reflects the fact that H2O2 production was decreased in CR compared with control-soy mitochondria when Complex III was inhibited (antimycin A), but not when Complex I alone was inhibited (rotenone).
Comparisons between CR groups
The CR-fish group had decreased (P<0.05) H2O2 production compared with the CR-soy and CR-lard groups for all substrates and substrate/inhibitor combinations. In addition, H2O2 production was increased (P<0.05) in the CR-lard group compared with the other CR groups when mitochondria were respiring on succinate plus rotenone or pyruvate/malate plus rotenone. The comparisons between the three CR groups indicate that liver mitochondrial ROS production can be influenced by dietary lipid composition. In particular, a decrease in ROS production was associated with mitochondria containing phospholipids enriched in n−3 PUFAs.
Mitochondrial proton leak kinetics
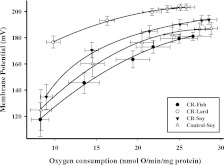
Mitochondrial proton leak kinetics are plotted in Figure 2.
Figure 2. Proton leak kinetics curves in liver mitochondria at the end of 1 month CR from mice consuming a control diet (Control-Soy, n=7), or CR diets containing lard (CR-Lard, n=8), soya bean oil (CR-Soy, n=8) or fish oil (CR-Fish, n=7).
Leak-dependent respiration and membrane potential were measured simultaneously using a Clark-type electrode and a TPMP+-sensitive electrode, respectively. The furthest point on the right in each panel represents state 4 respiration. All measurements were completed using mitochondria (0.35 mg/ml) in incubation medium (145 mM KCl, 5 mM KH2PO4, 30 mM Hepes, 3 mM MgCl2 and 0.1 mM EGTA, pH 7.4). A TPMP+ (0–2.5 μM) standard curve was generated in each sample prior to the initiation of respiration and membrane potential measurements. All assays were performed in the presence of 5 mM succinate, 5 μM rotenone, oligomycin (8 μg/ml) and nigericin (0.08 μg/ml) and the curves were generated by incremental additions of malonate (0.1–2.4 mM).
Control-soy against CR-soy
State 4 oxygen consumption and membrane potential (furthest points to the right on the proton leak kinetics plot; Figure 2) were not significantly different (P>0.05) between the CR-soy and control groups.
Comparisons between CR groups
When assessing the effects of dietary lipids on proton leak kinetics within the three CR groups, we observed that rates of proton leak were lowest in the CR-lard and highest in CR-fish group, as indicated by the fact that at similar oxygen consumption levels, membrane potential was highest (P<0.05) in the CR-lard group and lowest (P<0.05) in the CR-fish group compared with the other CR groups.
Lipid peroxidation in liver mitochondria
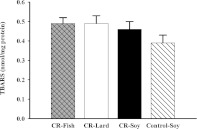
TBARS were measured to assess liver mitochondrial lipid peroxidation in the four groups of mice (Figure 3).
Figure 3. TBARS (nmol/mg protein) in liver mitochondria at the end of 1 month CR from mice consuming a control diet (Control-Soy, n=8), or CR diets containing lard (CR-Lard, n=8), soya bean oil (CR-Soy, n=8) or fish oil (CR-Fish, n=8).
Control-soy against CR-soy
There were no differences in TBARS levels between the CR-soy and control-soy groups at 1 month of CR.
Comparisons between CR groups
There were no differences in mitochondrial TBARS levels among the three CR groups. These results indicate that liver mitochondrial lipid peroxidation is not altered by dietary lipid source in mice following 1 month of CR.
Measurement of mitochondrial ETC enzyme activities
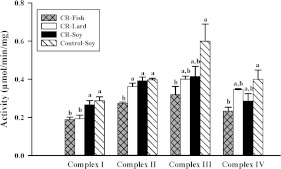
The influence of CR and dietary lipid composition on liver mitochondrial ETC enzyme activities is shown Figure 4.
Figure 4. Activities of ETC complexes in liver mitochondria from mice (n=8 per group) consuming a control diet (Control-Soy) or CR diets containing lard (CR-Lard), soya bean oil (CR-Soy) or fish oil (CR-Fish).
All measurements were completed at 30°C and activities are expressed as μmol·min−1·(mg of mitochondrial protein)−1. For each ETC enzyme, scale bars that do not share a common letter indicate a significant difference (P<0.05) between diet groups.
Control-soy against CR-soy
CR did not alter (P>0.10) the activities of ETC enzymes.
Comparisons between CR groups
The CR-fish group had lower (P<0.05) Complex I activity than the CR-soy group and lower (P<0.05) Complex II activity than both the CR-soy and CR-lard groups. A decrease (P<0.05) in Complex I activity in the CR-lard group was the only difference between the CR-lard and CR-soy groups. These results indicate that dietary lipids can differentially influence the activities of ETC enzymes in mitochondria from CR mice.
DISCUSSION
Effect of dietary fats and CR on mitochondrial membrane phospholipid fatty acid composition
It has been proposed that CR may prolong life span and retard aging by altering biological membrane phospholipids toward a decreased degree of unsaturation, and subsequently produce membranes that are resistant to oxidative damage [4]. However, it is conceivable that CR-induced changes in membrane composition could also influence aging by altering membrane-linked processes, such as ROS production or proton leak. The objective of this study was to determine if mitochondrial phospholipid fatty acid composition influences alterations in mitochondrial ROS production, proton leak and ETC activities with CR. To investigate how membrane fatty acid composition affects liver mitochondria from CR mice, we used different sources of dietary lipids to alter mitochondrial membrane phospholipid fatty acid composition. Several lines of evidence have shown that dietary lipids can alter the fatty acid composition of liver mitochondrial membranes [13,34,35]. However, to our knowledge, there have been no studies using dietary lipids to manipulate liver mitochondrial membrane fatty acid composition in CR animals. The results of the present study show that liver mitochondrial membrane fatty acid composition can be significantly altered in CR mice by dietary lipid composition. Specifically, at 1 month of CR, lard increased the MUFA content in both PC and CL, fish oil dramatically increased n−3 PUFA levels in all phospholipids and soya bean oil increased 18:2, n−6 content in both PC and CL. These results are consistent with our previous study in skeletal muscle mitochondria which showed that mitochondrial phospholipid fatty acid composition is readily altered by dietary lipid source in CR mice [36]. However, these mitochondrial results contrast with a previous study which found that CR blunted the effects of dietary fats on plasma membrane composition [37]. In that study, relatively modest changes in fatty acid composition were observed for some phospholipids when comparing fish oil, safflower oil or beef tallow [37]. The differences between the two studies are likely due to differences in the fatty acid composition of plasma and mitochondrial membranes, with plasma membranes containing a very high amount of saturated fatty acids [37] compared with mitochondrial membranes. This relatively high amount of saturated fatty acids in plasma membrane phospholipids would likely limit the magnitude of changes in PUFAs that could be produced by diet, and may explain why diet produced much larger changes in phospholipid fatty acid composition in liver mitochondria from CR animals.
A study in QS mice has shown that changes in mitochondrial phospholipid fatty acid composition depend on the magnitude and duration of CR [38]. The results of our study indicate that phospholipid classes do not show uniform changes in fatty acid composition at 1 month of CR, and many of these fatty acid changes may not be apparent when measuring total phospholipid composition. It has been previously reported that moderate to severe CR for 1 month in mice produces an increase in liver mitochondrial MUFA content [38], and the results of our study would suggest that these changes are entirely due to alterations in mitochondrial PC fatty acid composition. Additional studies are needed to determine the mechanisms and physiological consequences of the phospholipid-specific changes in fatty acid composition induced by CR.
Effect of dietary fats and CR on mitochondrial ROS production by liver mitochondria
CR may play a role in the retardation of aging by decreasing mitochondrial ROS production. In support of this idea, it has been shown that CR decreases mitochondrial ROS production in a variety of tissues [11,12]. However, the influence of short-term CR on liver ROS production is not entirely clear. In the present study, 1 month of CR induced only subtle changes in ROS production. CR did not decrease ROS production in liver mitochondria respiring on substrate alone or substrate plus rotenone (an inhibitor of Complex I), and a decrease in ROS production was only observed with CR when liver mitochondria were respiring on pyruvate/maleate plus antimycin A (an inhibitor of Complex III). These results suggest that CR may decrease capacity for ROS production from Complex III. There is no consensus in the literature about the influence of short-term CR on liver ROS production. Studies in rats have reported that ROS production is not decreased in liver mitochondria following 1 month [26] or 6–7 weeks [39] of CR. In contrast, it has also been reported that ROS production is decreased at 6 weeks of CR in rat liver mitochondria respiring on pyruvate/malate [40]. The reason for the differences between studies is not entirely clear, although diet composition should be considered as a contributory factor. In particular, we recently reported that capacity for ROS production by Complex I of the ETC is decreased in mouse liver mitochondria following 2 months of CR [41]. This finding differs from the current study where CR primarily influences capacity for ROS production from Complex III. There were no differences in the strain of mouse, housing conditions or CR protocols between these studies. However, diet was different with the present study using an AIN93G purified diet while the previous study [41] used a chow diet (7012 Harlan Teklad). Thus, it appears that diet composition may have a major influence on mitochondrial ROS production and should be considered as a factor when comparing results between studies.
Little is known about the influence of dietary lipid composition on ROS production in CR animals. Complexes I and III, sites of mitochondrial ROS production [42,43], are components of the membrane-bound ETC and it follows that diet-induced alterations in mitochondrial phospholipid fatty acid composition could influence ROS production by these complexes. The results of the present study support the idea that phospholipid fatty acid composition may have a major influence on mitochondrial ROS production. Under all substrate (succinate, or pyruvate/malate, or pyruvate/malate/succinate) and substrate plus inhibitor conditions, mitochondrial H2O2 production was significantly lower in the CR-fish compared with the other CR groups. These results suggest fish oil consumption may change mitochondrial phospholipid fatty acid composition in a manner that decrease ROS production from both Complexes I and III. A few studies have investigated the influence on ROS production of n−3 PUFA enriched liver mitochondrial membranes from ad libitum-fed animals [13,14]. When combinations of inhibitors were used to chemically dissect the ETC for sites of ROS production, it was observed that n−3 PUFA enriched liver mitochondria from fish oil fed rats had lower H2O2 production when respiring on succinate alone or on either succinate or pyruvate/malate in the presence of antimycin A compared with mitochondria from corn oil fed rats [13]. Hagopian et al. [14] also observed that n−3 PUFA enriched liver mitochondria from fat-1 mice had lower H2O2 production when respiring on either succinate or succinate/glutamate/malate compared with control mice. The results of the present study are in accordance with this previous work indicating that ROS production is decreased in n−3 PUFA enriched liver mitochondria.
Effect of dietary fats and CR on liver mitochondrial proton leak
Mitochondrial proton leak is a process where protons bypass the ATP synthase and cross the mitochondrial inner membrane to re-enter the matrix. Overall proton leak is thought to consist of basal H+ leak that is unregulated and inducible H+ leak that is regulated by either the uncoupling proteins or the AMP/ANT pathway [44]. The mechanism of the basal proton leak is not fully understood. This membrane property could be influenced by CR and mitochondrial membrane fatty acid composition. It has been shown that basal proton leak increases with age [45,46] and it has been proposed that CR may oppose age-related increases in proton leak and improve mitochondrial efficiency by inducing a sustained decrease in proton leak [47]. However, studies in liver have produced mixed results with CR inducing a decrease [18], increase [19,48] or no change [26,49] in mitochondrial proton leak. Our results are in agreement with studies indicating that liver mitochondrial proton leak is not altered with short-term CR [26,49]. All of the studies that have reported changes in proton leak with CR [18,19,48] have been of longer duration than the present study and additional work is needed to determine possible time courses for changes in proton leak with CR.
In the inner mitochondrial membrane, lipid–lipid and lipid–protein interactions are potential sites for basal proton leak. A study investigating proton leak in liposomes prepared from rat liver mitochondria concluded that only 5% of proton leak occurs through the phospholipid bilayer [50], although interactions between proteins and membrane fatty acids may have a substantial impact on proton leak. In support of this idea, studies have found that unsaturation index and n−3 PUFAs of inner mitochondrial membranes are positively correlated with proton permeability in liver mitochondria [16,17,51]. In agreement with these findings, the results of the present study showed that unsaturation index and n−3 PUFAs were significantly higher in mitochondrial membranes from the CR-fish compared with the other CR groups and mitochondria from the CR-fish group also had increased proton leak compared with the other CR regimens. It is likely that the increase in proton leak observed in the CR-fish versus other CR groups was due to mitochondrial changes induced by both CR and dietary lipids, since liver mitochondrial proton leak was not increased in previous studies with rats fed ad libitum a fish oil diet [13] or ad libitum fed fat-1 mice, which are capable of synthesizing n−3 fatty acids [14].
Within the three CR groups, the CR-lard group showed the lowest proton leak as indicated by the highest membrane potentials at given oxygen consumption levels. Our present study showed that lard increased (P<0.05) MUFA content in PC and CL compared with the other CR groups. These results are consistent with comparative studies which have reported that liver mitochondrial proton leak is inversely associated with level of MUFAs in mitochondrial phospholipids [50,51]. Whether the increased MUFA content in PC and CL is responsible for the decrease in mitochondrial proton leak in the CR-lard group requires further investigation. Nonetheless, the results of the present study indicate that dietary lipid composition has a major influence on mitochondrial proton leak in CR animals.
Effect of dietary fats and CR on liver mitochondrial ETC enzyme activities
A few studies have investigated the influence of long-term CR on muscle ETC enzyme activities. It has been reported that the activities of ETC Complexes I, III and IV are 33–64% lower in muscle mitochondria from CR compared with control mice at 10 months of age (or ~26 weeks of CR) [52] while at older age (20 months), CR mice did not experience the age-related decrease in ETC enzyme activity observed in control animals. Young adult rats (8–10 months old) on CR for 4.5–6.5 months were also reported to have lower Complex IV activity compared with their age-matched ad libitum controls [53]. However, to our knowledge, there have been no studies investigating the influence of CR on ETC enzyme activity in liver. The results from the present study indicate that 1 month of CR did not significantly alter ETC enzyme activities. There are two major factors which could contribute to differences in results between studies investigating the influence of CR on ETC enzyme activity. First, it is possible that liver and skeletal muscle show different responses to CR. Secondly, duration of CR appears to have an impact on ETC enzyme activity and results observed with short-term (1 month) CR may be very different from those observed following several months of CR. Nonetheless, the results of the present study indicate that short-term CR does not significantly alter liver mitochondrial ETC enzyme activities.
There is considerable evidence that CL associates with ETC enzymes and the fatty acid composition of CL can alter the activities of ETC enzymes [54]. In particular, the level of linoleic acid in CL has been shown to influence ETC activity [54]. Thus, it is likely that changes in mitochondrial phospholipid fatty acid composition, especially in CL, would influence the activities of ETC complexes. To our knowledge, the present study is the first to investigate the influence of dietary lipid composition on ETC enzyme activities in CR animals. Our fatty acid data showed that CL fatty acid profiles can be altered by dietary lipids in CR animals. It has previously been reported that the activities of Complexes I and IV are increased in skeletal muscle in aged rats consuming a diet which increases CL linoleic acid levels and decreases the levels n−3 fatty acids [55]. In the present study, the CR-soy group had higher levels of linoleic acid (18:2, n−6) and lower levels of n−3 fatty acids in CL than the CR-fish group, and the enzyme activities of Complexes I and II were also higher in the CR-soy compared with CR-fish group. However, Complex IV activity was not significantly different between the CR-soy and CR-fish groups. These results suggest that liver and skeletal muscle mitochondrial ETC enzyme activities respond differently to changes in CL fatty acid composition which result in decreased linoleic acid and increases in highly unsaturated n−3 fatty acids. Additional studies are needed to determine which specific fatty acids in the CR-fish group are responsible for the decreased ETC enzyme activities observed in this group.
Conclusions
The results from our study indicate that liver mitochondrial phospholipid fatty acid composition in CR mice is strongly influenced by dietary lipid composition. The mitochondrial phospholipid membrane compositions reflected the dietary fatty acid composition. In addition to altering mitochondrial phospholipid fatty acid composition, dietary lipid composition also influenced ROS production, proton leak and ETC enzyme activities in CR mice. The results of this study have implications for designing dietary interventions that maximize the effects of CR. Future studies are needed to determine the optimum dietary lipid composition to decrease ROS production or induce other mitochondrial changes in CR animals.
AUTHOR CONTRIBUTION
Yana Chen and Kevork Hagopian performed all of the experiments, except lipid extraction and fatty acid analysis. Douglas Bibus extracted the lipids and analysed the fatty acids. José Villalba, Guillermo López-Lluch, Plácido Navas and Jon Ramsey designed the experiments. Kyoungmi Kim, Yana Chen and Jon Ramsey analysed the data. Roger McDonald and Jon Ramsey designed the diets. Yana Chen, Kevork Hagopian, José Villalba, Guillermo López-Lluch, Plácido Navas and Jon Ramsey wrote the paper.
FUNDING
This work was supported by the National Institutes of Health [grant numbers R01 AG028125 and P01 AG025532].
References
- 1.Weindruch R., Sohal R. S. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N. Engl. J. Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pamplona R., Barja G., Portero-Otin M. Membrane fatty acid unsaturation, protection against oxidative stress, and maximum life span: a homeoviscous-longevity adaptation? Ann. N. Y. Acad. Sci. 2002;959:475–490. doi: 10.1111/j.1749-6632.2002.tb02118.x. [DOI] [PubMed] [Google Scholar]
- 3.Hulbert A. J. Life, death and membrane bilayers. J. Exp. Biol. 2003;206:2303–2311. doi: 10.1242/jeb.00399. [DOI] [PubMed] [Google Scholar]
- 4.Yu B. P., Lim B. O., Sugano M. Dietary restriction downregulates free radical and lipid peroxide production: plausible mechanism for elongation of life span. J. Nutr. Sci. Vitaminol. (Tokyo) 2002;48:257–264. doi: 10.3177/jnsv.48.257. [DOI] [PubMed] [Google Scholar]
- 5.Laganiere S., Fernandes G. Study on the lipid composition of aging Fischer-344 rat lymphoid cells: effect of long-term calorie restriction. Lipids. 1991;26:472–478. doi: 10.1007/BF02536075. [DOI] [PubMed] [Google Scholar]
- 6.Venkatraman J., Fernandes G. Modulation of age-related alterations in membrane composition and receptor-associated immune functions by food restriction in Fischer 344 rats. Mech. Ageing Dev. 1992;63:27–44. doi: 10.1016/0047-6374(92)90014-5. [DOI] [PubMed] [Google Scholar]
- 7.Tacconi M. T., Lligona L., Salmona M., Pitsikas N., Algeri S. Aging and food restriction: effect on lipids of cerebral cortex. Neurobiol. Aging. 1991;12:55–59. doi: 10.1016/0197-4580(91)90039-m. [DOI] [PubMed] [Google Scholar]
- 8.Lee J., Yu B. P., Herlihy J. T. Modulation of cardiac mitochondrial membrane fluidity by age and calorie intake. Free Radical Biol. Med. 1999;26:260–265. doi: 10.1016/s0891-5849(98)00195-6. [DOI] [PubMed] [Google Scholar]
- 9.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 10.Barja G. Free radicals and aging. Trends Neurosci. 2004;27:595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Gredilla R., Barja G. Minireview: the role of oxidative stress in relation to caloric restriction and longevity. Endocrinology. 2005;146:3713–3717. doi: 10.1210/en.2005-0378. [DOI] [PubMed] [Google Scholar]
- 12.Sohal R. S., Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsey J. J., Harper M. E., Humble S. J., Koomson E. K., Ram J. J., Bevilacqua L., Hagopian K. Influence of mitochondrial membrane fatty acid composition on proton leak and H2O2 production in liver. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2005;140:99–108. doi: 10.1016/j.cbpc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Hagopian K., Weber K. L., Hwee D. T., Van Eenennaam A. L., Lopez-Lluch G., Villalba J. M., Buron I., Navas P., German J. B., Watkins S. M., et al. Complex I-associated hydrogen peroxide production is decreased and electron transport chain enzyme activities are altered in n−3 enriched fat-1 mice. PLoS One. 2010;5:e12696. doi: 10.1371/journal.pone.0012696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jastroch M., Divakaruni A. S., Mookerjee S., Treberg J. R., Brand M. D. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67. doi: 10.1042/bse0470053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brookes P. S., Buckingham J. A., Tenreiro A. M., Hulbert A. J., Brand M. D. The proton permeability of the inner membrane of liver mitochondria from ectothermic and endothermic vertebrates and from obese rats: correlations with standard metabolic rate and phospholipid fatty acid composition. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 1998;119:325–334. doi: 10.1016/s0305-0491(97)00357-x. [DOI] [PubMed] [Google Scholar]
- 17.Porter R. K., Hulbert A. J., Brand M. D. Allometry of mitochondrial proton leak: influence of membrane surface area and fatty acid composition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1996;271:R1550–R1560. doi: 10.1152/ajpregu.1996.271.6.R1550. [DOI] [PubMed] [Google Scholar]
- 18.Hagopian K., Harper M. E., Ram J. J., Humble S. J., Weindruch R., Ramsey J. J. Long-term calorie restriction reduces proton leak and hydrogen peroxide production in liver mitochondria. Am. J. Physiol. Endocrinol. Metab. 2005;288:E674–E684. doi: 10.1152/ajpendo.00382.2004. [DOI] [PubMed] [Google Scholar]
- 19.Lambert A. J., Merry B. J. Effect of caloric restriction on mitochondrial reactive oxygen species production and bioenergetics: reversal by insulin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R71–R79. doi: 10.1152/ajpregu.00341.2003. [DOI] [PubMed] [Google Scholar]
- 20.Venditti P., De Rosa R., Di Meo S. Effect of cold-induced hyperthyroidism on H2O2 production and susceptibility to stress conditions of rat liver mitochondria. Free Radical Biol. Med. 2004;36:348–358. doi: 10.1016/j.freeradbiomed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Arvier M., Lagoutte L., Johnson G., Dumas J. F., Sion B., Grizard G., Malthiery Y., Simard G., Ritz P. Adenine nucleotide translocator promotes oxidative phosphorylation and mild uncoupling in mitochondria after dexamethasone treatment. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1320–E1324. doi: 10.1152/ajpendo.00138.2007. [DOI] [PubMed] [Google Scholar]
- 22.Bligh E. G., Dyer W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 23.Forsythe C. E., Phinney S. D., Feinman R. D., Volk B. M., Freidenreich D., Quann E., Ballard K., Puglisi M. J., Maresh C. M., Kraemer W. J., et al. Limited effect of dietary saturated fat on plasma saturated fat in the context of a low carbohydrate diet. Lipids. 2010;45:947–962. doi: 10.1007/s11745-010-3467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyslop P. A., Sklar L. A. A quantitative fluorimetric assay for the determination of oxidant production by polymorphonuclear leukocytes: its use in the simultaneous fluorimetric assay of cellular activation processes. Anal. Biochem. 1984;141:280–286. doi: 10.1016/0003-2697(84)90457-3. [DOI] [PubMed] [Google Scholar]
- 25.Venditti P., Pamplona R., Portero-Otin M., De Rosa R., Di Meo S. Effect of experimental and cold exposure induced hyperthyroidism on H2O2 production and susceptibility to oxidative stress of rat liver mitochondria. Arch. Biochem. Biophys. 2006;447:11–22. doi: 10.1016/j.abb.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Ramsey J. J., Hagopian K., Kenny T. M., Koomson E. K., Bevilacqua L., Weindruch R., Harper M. E. Proton leak and hydrogen peroxide production in liver mitochondria from energy-restricted rats. Am. J. Physiol. Endocrinol. Metab. 2004;286:E31–E40. doi: 10.1152/ajpendo.00283.2003. [DOI] [PubMed] [Google Scholar]
- 27.Rolfe D. F., Hulbert A. J., Brand M. D. Characteristics of mitochondrial proton leak and control of oxidative phosphorylation in the major oxygen-consuming tissues of the rat. Biochim. Biophys. Acta. 1994;1188:405–416. doi: 10.1016/0005-2728(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 28.Birch-Machin M. A., Briggs H. L., Saborido A. A., Bindoff L. A., Turnbull D. M. An evaluation of the measurement of the activities of complexes I-IV in the respiratory chain of human skeletal muscle mitochondria. Biochem. Med. Metab. Biol. 1994;51:35–42. doi: 10.1006/bmmb.1994.1004. [DOI] [PubMed] [Google Scholar]
- 29.Kwong L. K., Sohal R. S. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch. Biochem. Biophys. 2000;373:16–22. doi: 10.1006/abbi.1999.1495. [DOI] [PubMed] [Google Scholar]
- 30.Trounce I. A., Kim Y. L., Jun A. S., Wallace D. C. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 1996;264:484–509. doi: 10.1016/s0076-6879(96)64044-0. [DOI] [PubMed] [Google Scholar]
- 31.Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 32.Guerrero A., Pamplona R., Portero-Otin M., Barja G., Lopez-Torres M. Effect of thyroid status on lipid composition and peroxidation in the mouse liver. Free Radical Biol. Med. 1999;26:73–80. doi: 10.1016/s0891-5849(98)00173-7. [DOI] [PubMed] [Google Scholar]
- 33.Lambert A. J., Brand M. D. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I) J. Biol. Chem. 2004;279:39414–39420. doi: 10.1074/jbc.M406576200. [DOI] [PubMed] [Google Scholar]
- 34.Tahin Q. S., Blum M., Carafoli E. The fatty acid composition of subcellular membranes of rat liver, heart, and brain: diet-induced modifications. Eur. J. Biochem. 1981;121:5–13. doi: 10.1111/j.1432-1033.1981.tb06421.x. [DOI] [PubMed] [Google Scholar]
- 35.Quiles J. L., Martinez E., Ibanez S., Ochoa J. J., Martin Y., Lopez-Frias M., Huertas J. R., Mataix J. Ageing-related tissue-specific alterations in mitochondrial composition and function are modulated by dietary fat type in the rat. J. Bioenerg. Biomembr. 2002;34:517–524. doi: 10.1023/a:1022530512096. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y., Hagopian K., McDonald R. B., Bibus D., Lopez-Lluch G., Villalba J. M., Navas P., Ramsey J. J. The influence of dietary lipid composition on skeletal muscle mitochondria from mice following 1 month of calorie restriction. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:1121–1131. doi: 10.1093/gerona/gls113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cha M. C., Jones P. J. Energy restriction dilutes the changes related to dietary fat type in membrane phospholipid fatty acid composition in rats. Metabolism. 2000;49:977–983. doi: 10.1053/meta.2000.7725. [DOI] [PubMed] [Google Scholar]
- 38.Faulks S. C., Turner N., Else P. L., Hulbert A. J. Calorie restriction in mice: effects on body composition, daily activity, metabolic rate, mitochondrial reactive oxygen species production, and membrane fatty acid composition. J. Gerontol. A: Biol. Sci. Med. Sci. 2006;61:781–794. doi: 10.1093/gerona/61.8.781. [DOI] [PubMed] [Google Scholar]
- 39.Gomez J., Caro P., Naudi A., Portero-Otin M., Pamplona R., Barja G. Effect of 8.5% and 25% caloric restriction on mitochondrial free radical production and oxidative stress in rat liver. Biogerontology. 2007;8:555–566. doi: 10.1007/s10522-007-9099-1. [DOI] [PubMed] [Google Scholar]
- 40.Gredilla R., Barja G., Lopez-Torres M. Effect of short-term caloric restriction on H2O2 production and oxidative DNA damage in rat liver mitochondria and location of the free radical source. J. Bioenerg. Biomembr. 2001;33:279–287. doi: 10.1023/a:1010603206190. [DOI] [PubMed] [Google Scholar]
- 41.Hagopian K., Chen Y., Simmons Domer K., Soo Hoo R., Bentley T., McDonald R. B., Ramsey J. J. Caloric restriction influences hydrogen peroxide generation in mitochondrial sub-populations from mouse liver. J. Bioenerg. Biomembr. 2011;43:227–236. doi: 10.1007/s10863-011-9353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andreyev A. Y., Kushnareva Y. E., Starkov A. A. Mitochondrial metabolism of reactive oxygen species. Biochemistry. 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 43.Lambert A. J., Brand M. D. Reactive oxygen species production by mitochondria. Methods Mol. Biol. 2009;554:165–181. doi: 10.1007/978-1-59745-521-3_11. [DOI] [PubMed] [Google Scholar]
- 44.Brookes P. S. Mitochondrial H+ leak and ROS generation: an odd couple. Free Radical Biol. Med. 2005;38:12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Lal S. B., Ramsey J. J., Monemdjou S., Weindruch R., Harper M. E. Effects of caloric restriction on skeletal muscle mitochondrial proton leak in aging rats. J. Gerontol. A: Biol. Sci. Med. Sci. 2001;56:B116–B122. doi: 10.1093/gerona/56.3.b116. [DOI] [PubMed] [Google Scholar]
- 46.Harper M. E., Monemdjou S., Ramsey J. J., Weindruch R. Age-related increase in mitochondrial proton leak and decrease in ATP turnover reactions in mouse hepatocytes. Am. J. Physiol. Endocrinol. Metab. 1998;275:E197–E206. doi: 10.1152/ajpendo.1998.275.2.E197. [DOI] [PubMed] [Google Scholar]
- 47.Ramsey J. J., Harper M. E., Weindruch R. Restriction of energy intake, energy expenditure, and aging. Free Radical Biol. Med. 2000;29:946–968. doi: 10.1016/s0891-5849(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 48.Ash C. E., Merry B. J. The molecular basis by which dietary restricted feeding reduces mitochondrial reactive oxygen species generation. Mech. Ageing Dev. 2011;132:43–54. doi: 10.1016/j.mad.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Lambert A. J., Merry B. J. Lack of effect of caloric restriction on bioenergetics and reactive oxygen species production in intact rat hepatocytes. J. Gerontol. A: Biol. Sci. Med. Sci. 2005;60:175–180. doi: 10.1093/gerona/60.2.175. [DOI] [PubMed] [Google Scholar]
- 50.Brookes P. S., Rolfe D. F., Brand M. D. The proton permeability of liposomes made from mitochondrial inner membrane phospholipids: comparison with isolated mitochondria. J. Membr. Biol. 1997;155:167–174. doi: 10.1007/s002329900168. [DOI] [PubMed] [Google Scholar]
- 51.Brand M. D., Couture P., Hulbert A. J. Liposomes from mammalian liver mitochondria are more polyunsaturated and leakier to protons than those from reptiles. Comp. Biochem. Physiol. Biochem. Mol. Biol. 1994;108:181–188. doi: 10.1016/0305-0491(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 52.Desai V. G., Weindruch R., Hart R. W., Feuers R. J. Influences of age and dietary restriction on gastrocnemius electron transport system activities in mice. Arch. Biochem. Biophys. 1996;333:145–151. doi: 10.1006/abbi.1996.0375. [DOI] [PubMed] [Google Scholar]
- 53.Hepple R. T., Baker D. J., McConkey M., Murynka T., Norris R. Caloric restriction protects mitochondrial function with aging in skeletal and cardiac muscles. Rejuvenation Res. 2006;9:219–222. doi: 10.1089/rej.2006.9.219. [DOI] [PubMed] [Google Scholar]
- 54.Hoch F. L. Cardiolipins and biomembrane function. Biochim. Biophys. Acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- 55.Bronnikov G. E., Kulagina T. P., Aripovsky A. V. Dietary supplementation of old rats with hydrogenated peanut oil restores activities of mitochondrial respiratory complexes in skeletal muscles. Biochemistry. 2010;75:1491–1497. doi: 10.1134/s0006297910120102. [DOI] [PubMed] [Google Scholar]