Case Report
The patient is a 92 year old white male with significant history of anemia of chronic disease with probable hemolysis from metallic aortic valve, requiring anticoagulation with warfarin. He also suffered from gastric antral vascular ectasia and chronic kidney disease, leading to continuous bleeding. He received periodic blood transfusions as well as Epoetin alpha and Darbepoetin alpha. Seven years prior to treatment, he was noted to have marginal zone lymphoma, stage IV-A (CD 20 positive). Ten months prior to treatment, he was noted to have an exophytic growth on his scalp. Biopsy revealed melanoma, with a Breslow thickness of at least 6.1 mm, 20 mitosis per high powered field, extensive ulceration and angiolymphatic invasion. The lesion was negative for microsatellites. Thus, the tumor was staged as AJCC staging pT4bN0. Staging workup at this time using PET/CT revealed bilateral cervical adenopathy and pulmonary nodules less than 1 cm in diameter. The lesion was excised, but rapidly recurred. The patient received radiation therapy to his scalp lesion, followed by regression of the scalp lesion.
At the time of presentation, six months after radiation therapy, he presented with three crusted lesions in the scalp, ranging in size from 1.5 to 4.5cm in diameter (Figure 1a). The lesions were anesthetized, debulked, and curetted, followed by cryotherapy of the base of each lesion for 30 seconds with a rapid movement, so as to rapidly obtain frosting. The halo was maintained by rapid movement of the cryotherapy apparatus to keep the halo for 30 seconds, followed by a 2.5 minute thaw (Figure 1b). The size of the halo corresponded to the size of the debulked lesions. Upon thawing, 1% gentian violet was applied to the wounds, and the patient was instructed to apply gentian violet and imiquimod to the base of the lesions daily. This was accompanied by a brisk inflammatory response. At the time of re-examination, four months after the procedure, no clinical recurrence was noted. The largest lesion was replaced by a depressed scar (Figure 1c).
Figure 1.
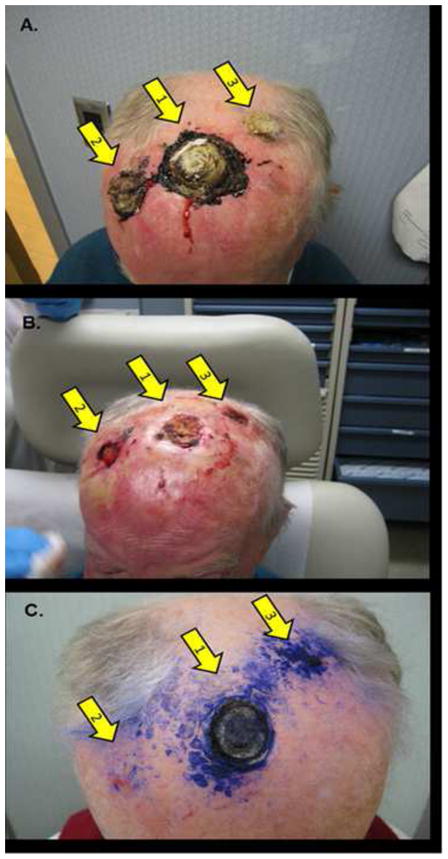
Cutaneous Melanoma Metastases. Clinical appearance of the patient at presentation a), immediately after treatment b) and four months after treatment c). Arrows mark the original tumor site a,b) and at follow-up c).
Discussion
Cutaneous metastasis is a common sequelae of advanced melanoma. We present a novel palliative method for the treatment of this complication in a patient with significant comorbidities, including advanced age, anticoagulation for a metallic valve, chronic anemia, macular degeneration, and prior history of hematopoetic malignancy (high grade lymphoma, Waldenstroms macroglobulinemia). We do not claim to have cured the patient, as he likely has distant disease not amenable to local treatment. However, the lack of recurrence after six months is remarkable for the following reasons. When the patient had a solitary lesion, recurrence after both surgery and radiation therapy occurred before four months, but we have not observed recurrence yet in this patient. Second, our therapy most likely did not eradicate every single melanoma cell on the scalp. Given that the original tumors were highly mitotic, it is likely that residual tumor cells were left. The lack of recurrence points towards a potential immune role in tumor elimination. Third, we observed a brisk inflammatory response to the combination of gentian violet and imiquimod. Given that gentian violet is usually anti-inflammatory, we were surprised by this response. One potential explanation is that gentian violet is an angiogenesis inhibitor, and angiogenesis inhibitors have been found to potentiate immune responses due to reversal of vascular endothelial growth factor (VEGF) mediated inhibition of dendritic cell maturation 1.
Imiquimod has previously been used as monotherapy for both lentigo maligna and melanoma metastases, with mixed results. In treatment of patients with lentigo maligna, regression of thin lesions are sometimes seen, but nodules of invasive tumor are often left behind 2, 3. Similar findings have been observed in metastatic nodules 4. The reasons for failure of imiquimod monotherapy in melanoma are poorly understood, but we hypothesize two potential mechanisms. The first is of delivery. It is a major challenge for a topically applied drug to reach parts of either a lentigo maligna or a dermal melanoma nodule. Second is the possibility that imiquimod may select for populations of melanoma cells that are resistant to cytokine therapy, or that preexisting populations of resistant cells are present in the primary tumor. Both of these have been seen in other forms of cancer. Our procedure differs from imiquimod monotherapy in two important ways. First, we combine it with gentian violet, a topical angiogenesis inhibitor, and this may potentiate an immune response broader than that of imiquimod alone, for the reasons described above. Of interest, brilliant green, which exhibits only slight structural differences from gentian violet, downregulates the expression of the immunosuppressive molecule B7H1 on glioma cells (Parsa and Arbiser, unpublished data). Second, surgical debulking may remove populations of inherently resistant melanoma cells with stem cell like characteristics, and removes the immunosuppressive environment seen in hypoxic solid tumors. Our method of therapy has advantages over current treatment of cutaneous metastases of melanoma in that it is easy to perform in the office setting, is less expensive than other treatments, and has very little morbidity compared with systemic interferon, limb perfusion, or radical surgery.
In summary, we describe a novel method of treatment of cutaneous melanoma metastasis. This method is useful in the case when surgery is not an option, and can be easily performed in the office setting. The benefit is likely mediated in part through an immune response, given the inflammation we noted after therapy. Given that melanoma is known to be an immunoresponsive tumor in certain instances, our treatment may work in part through immune activation.
Figure 2.
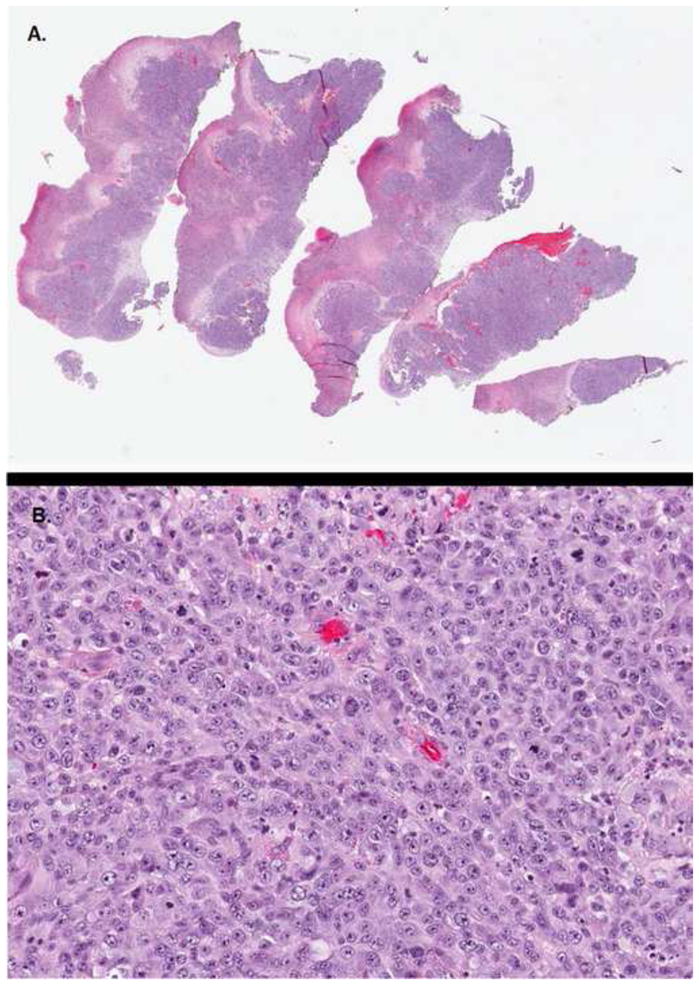
Cutaneous Melanoma Metastases. Histology of a representative tumor removed during palliative debulking. 2a) represents a low powered view and 2b) represents a high powered view. Note the numerous mitoses in 2b).
Acknowledgments
Funding/Support: This study was supported in part by: JLA was supported by the grant RO1 AR47901and P30 AR42687 Emory Skin Disease Research Core Center Grant from the National Institutes of Health, as well as funds from the Rabinowitch-Davis Foundation for Melanoma Research and the Betty Minsk Foundation for Melanoma Research.
Footnotes
Financial Disclosure:
None reported
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Perry BN, Govindarajan B, Bhandarkar SS, Knaus UG, Valo M, Sturk C, et al. Pharmacologic Blockade of Angiopoietin-2 Is Efficacious against Model Hemangiomas in Mice. J Invest Dermatol. 2006 Oct;126(10):2316–22. doi: 10.1038/sj.jid.5700413. [DOI] [PubMed] [Google Scholar]
- 2.Woodmansee CS, McCall MW. Recurrence of lentigo maligna and development of invasive melanoma after treatment of lentigo maligna with imiquimod. Dermatol Surg. 2009 Aug;35(8):1286–9. doi: 10.1111/j.1524-4725.2009.01227.x. [DOI] [PubMed] [Google Scholar]
- 3.Fisher GH, Lang PG. Treatment of melanoma in situ on sun-damaged skin with topical 5% imiquimod cream complicated by the development of invasive disease. Arch Dermatol. 2003 Jul;139(7):945–7. doi: 10.1001/archderm.139.7.945. [DOI] [PubMed] [Google Scholar]
- 4.Turza K, Dengel LT, Harris RC, Patterson JW, White K, Grosh WW, et al. Effectiveness of imiquimod limited to dermal melanoma metastases, with simultaneous resistance of subcutaneous metastasis. J Cutan Pathol. 2010 Jan;37(1):94–8. doi: 10.1111/j.1600-0560.2009.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


