Abstract
The purpose of this study was to test the null hypothesis that children with environmental tobacco smoke (ETS) exposure (also known as passive smoke exposure) do not demonstrate an increased likelihood of adverse respiratory events during or while recovering from general anesthesia administered for treatment of early childhood caries. Parents of children (ages 19 months–12 years) preparing to receive general anesthesia for the purpose of dental restorative procedures were interviewed regarding the child's risk for ETS. Children were observed during and after the procedure by a standardized dentist anesthesiologist and postanesthesia care unit nurse who independently recorded severity of 6 types of adverse respiratory events—coughing, laryngospasm, bronchospasm, breath holding, hypersecretion, and airway obstruction. Data from 99 children were analyzed. The children for whom ETS was reported were significantly older than their ETS-free counterparts (P = .03). If the primary caregiver smoked, there was a significantly higher incidence of smoking by other members of the family (P < .0001) as well as smoking in the house (P < .0005). There were no significant differences between the adverse respiratory outcomes of the ETS (+) and ETS (−) groups. The ETS (+) children did have significantly longer recovery times (P < .0001) despite not having significantly more dental caries (P = .38) or longer procedure times. ETS is a poor indicator of post–general anesthesia respiratory morbidity in children being treated for early childhood caries.
Key Words: Passive smoke exposure, Dental caries
Environmental tobacco smoke (ETS) is defined as the gaseous by-product of burning tobacco products and is also referred to as “passive” or “secondhand” smoke. It has been further defined as 15% mainstream smoke and 85% sidestream smoke from a smoldering cigarette.1 It is estimated that there are upwards of 4000 chemical compounds (some of which are carcinogenic and/or toxic) within ETS. In a developing airway, there may be significant airway remodeling (reduced maximal expiratory flow and forced expiratory volume) as well as marked increase in airway irritation.1
Previous research has detailed negative health consequences of ETS to include increased likelihood of respiratory infections, middle ear infections, asthma onset and severity, sudden infant death syndrome, and even dental caries.2–5 Jones and Bhattacharyya (2006) demonstrated that children who are exposed to ETS at home are more likely to experience adverse respiratory events while under or recovering from general anesthesia (GA) for a wide variety of procedures.6 However, another study in 2006 noted that although healthy children with a positive history of ETS did have lower preoperative peak expiratory flow rate, their recovery from anesthesia was unaffected.7 To the authors' knowledge, no study has examined ETS exposure exclusively in patients receiving general anesthesia for dental procedures. General anesthesia is gaining increasing popularity as a modality to treat early childhood caries. In 2005, Eaton et al commented on the shift of general anesthesia from one of the least acceptable modalities of treatment for dental disease to the third most desirable behind tell-show-do and nitrous oxide.12 Currently, 31 of 50 states have legislation that lists criteria for dentistry for children under GA, and in many instances, there are age-based criteria.9,10 The primary aim of this study was to evaluate the likelihood for post–GA respiratory morbidity in a pediatric dental population with parent-reported ETS.
METHODS
This institutional review board–approved, double-blinded, randomized case cohort study was conducted at the Nationwide Children's Hospital Dental Surgery Center. Caregivers gave written consent for participation prior to the recording of observations. Subjects included in this study were limited to children from 18 months to 12 years old presenting for the purpose of having restorative dental work completed while under GA. These children were referred for GA for 1 of 3 reasons: age, scope of treatment, or history of poor cooperation. Patients were excluded if they presented with a history of respiratory disease (eg, cystic fibrosis, diagnosed asthma, respiratory syncytial virus, or bronchiolitis).
Following consent to participate, the parent or guardian accompanying the child was interviewed to determine the degree of the child's ETS exposure. All interviews were conducted by 1 of 3 calibrated/standardized study staff (S.T., R.S., or B.C.). The interviewer asked the following questions:
How often is the child at home rather than at school or day care? Who is the child's primary caretaker?
Does the primary caretaker smoke? If yes, then does the caretaker smoke in the house, and how many cigarettes does the caretaker smoke per day?
Does anyone else who lives in the house smoke? If yes, how many cigarettes are smoked by persons other than the primary caretaker?
Does any person, resident or visitor, smoke inside the house? If yes, how many people other than the primary caretaker smoke inside the house?
The preceding questions were selected based on the results of a study by Groner et al demonstrating that a reasonably reliable estimate of a child's smoke exposure can be determined based on the answers to these questions.10 Respondents were scored (0) if they did not smoke and (1) if they did.
Following the interview, the child was escorted to the GA suite and was induced using an inhalational technique with sevoflurane in oxygen. Intravenous access was established and, following a 2 mg/kg bolus of propofol (Diprivan, AstraZeneca), the trachea was intubated. The planned dental procedures were then completed. During the induction and throughout the procedure, the blinded dentist anesthesiologist observed and recorded signs of respiratory events in each of 6 categories: breath holding, laryngospasm, bronchospasm, airway obstruction, hypersecretion, and coughing. For each type of adverse event experienced by the child, the anesthesiologist also rated the severity of the event on scale of 0 to 3, 0 being the absence of an event, 3 being the most severe manifestation of that event. At the conclusion of the procedure, the patient was carefully suctioned and extubated deep in the GA suite. The patient was allowed to recover until he or she met criteria for phase I recovery. The patient was then transported to an adjacent phase II recovery unit where he or she was supervised by a blinded postanesthesia care unit nurse who used the same rating system to record the presence or absence as well as the severity of any adverse respiratory events. The dentist anesthesiologist and postanesthesia care unit nurse rated the adverse events independently.
SAS and JMP systems were used to statistically analyze the data. Descriptive statistics were reported as frequency of responses for each question. Categorical variables were analyzed using the Fisher exact test. Means were analyzed using a 2-tailed t test. The level of statistical significance was set at .05.
RESULTS
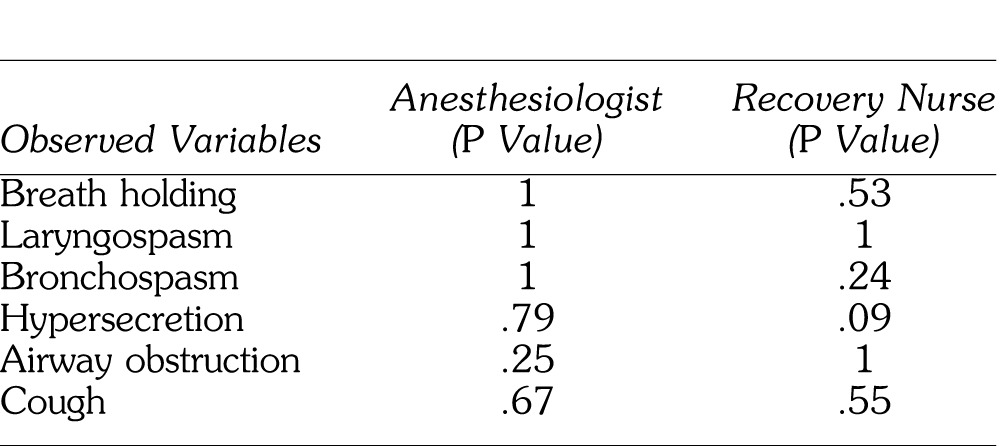
Data were collected from 99 children with a mean age of 58.1 months (±25.7 months). The subjects were gender balanced, with 48 males and 51 females overall. In addition there was a gender balance in the ETS (+) and ETS (−) groups. Within the entire cohort, 83 subjects were American Society of Anesthesiologists (ASA) I, with 16 ASA II. All of the ASA II patients were classified so because of a neurobehavioral disorder (attention-deficit/hyperactivity disorder or autism). Forty-eight primary caregivers reported not smoking at all, and 51 reported smoking to some degree. The children for whom ETS was reported were significantly older than their ETS-free counterparts (P = .03). If the primary caregiver smoked, there was a significantly higher incidence of smoking by other members of the family (P < .0001) as well as smoking in the house (P < .0005). There were no differences in amount of time spent in day care between the 2 groups (P = .21). There were no significant differences between the adverse respiratory outcomes of the ETS (+) and ETS (−) groups as assessed by either the dentist anesthesiologist or the recovery nurse. (See Table.)
When health status (measured by ASA rating) was the predictor variable, the data demonstrated no significant difference in age of children (P = .192), or any of the respiratory variables. When dental disease was assessed, the overall mean number of carious teeth among subjects was 9.2 (±4.5). There was no significant difference between the ETS (+) and ETS (−) groups (P = .384). The mean overall procedure time (defined from throat pack in to throat pack out) time was 77.1 min (±24.7 min). The ETS (−) children had a significantly longer procedure time (P = .028). However, the ETS (+) group spent significantly more time in the phase II recovery unit (P < .0001) before final discharge.
DISCUSSION
Previous research supports the fact that exposure to cigarette smoke in the home increases a child's likelihood of developing dental caries. This was not the case in our study, as the overall number of carious lesions of the ETS (+) group (9.7 ± 4.6) was not significantly different from that of the ETS (−) group (8.9 ± 4.5). This is interesting when noting that the ETS (+) group was significantly older than the ETS (−) group. In many cases, extensive decay warrants treatment under GA for one of several reasons. If the child's behavior makes it too difficult to safely complete the procedures awake, or if the parents would otherwise need to make many long trips to complete the treatment, GA might be the most practical and even the most cost-effective means for achieving the treatment goals. Although GA is overwhelmingly safe, it is not a “risk-free” treatment modality, with reports of associated morbidity and mortality throughout the literature.
An interesting finding from this study dealt with the time associated with treatment and recovery. Despite having no differences in number of carious teeth and a significantly shorter procedure time (10 minutes shorter compared to ETS (−) group), the ETS (+) group spent a significantly longer amount of time in the recovery unit (a mean of 11 minutes more). We did not collect data to explain this, however, as there is a standardized recovery nurse, and standardized discharge criteria; it is not unreasonable to assume the ETS (+) group took longer to achieve discharge criteria.
The same caregiver behaviors that increase the child's odds of experiencing dental decay have been suggested to possibly also result in an increased likelihood of that child experiencing an adverse respiratory event during or following the GA administered to facilitate treatment of their decay.3 Our study did not suggest this, as there were no significant differences in occurrences or severity of adverse respiratory events between the ETS (+) and ETS (−) groups. It should be noted that all children presenting for treatment under GA in the Dental Surgery Center are required to have a preoperative history and physical examination completed by their primary care physician, and potentially, this prevents children who are symptomatic in either group from progressing to treatment. Thus, both the ETS (+) and ETS (−) groups treated were otherwise healthy. This may have “selected out” those children for whom ETS exposure may have resulted in more significant morbidity. In addition, much of the previous ETS literature has been conducted in a hospital operating room setting, and although the Dental Surgery Center is housed within a large tertiary-care urban children's hospital, it is an autonomous ambulatory setting with specific patient requirements. Data from this study seem to suggest that when all other health variables are largely equivalent, ETS is not of clinical significance in an ambulatory surgery center population.
In addition to an increased risk of dental caries, ETS exposure has already been shown to put children at risk for sudden infant death syndrome, respiratory and middle ear infections, and acute exacerbations of asthma. Exposure to environmental smoke while in utero may be even more detrimental to a child's health according to one study.11 Although it would be of use to collect in utero ETS data, caregiver recall bias could possibly leave the data highly subject to spurious interpretations.
CONCLUSION
ETS exposure did not significantly contribute to intraoperative or postoperative adverse respiratory events during GA for early childhood caries.
Table.
Dentist Anesthesiologist and Recovery Nurse Observed Variables
REFERENCES
- 1.Avsar A, Darka O, Topaloglu B, Bek Y. Association of passive smoking with caries and related salivary biomarkers in young children. Arch Oral Biol. 2008;53:969–974. doi: 10.1016/j.archoralbio.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Kum-Nji P, Mangrem C, Wells P, Herrod H. Is environmental tobacco smoke exposure a risk factor for acute gastroenteritis in young children? Clin Pediatr. 2009;48:756–762. doi: 10.1177/0009922809332591. [DOI] [PubMed] [Google Scholar]
- 3.Borchers A, Keen C, Gershwin M. Smoking cessation: significance and implications for children. Clin Rev Allergy Immunol. 2008;34:231–249. doi: 10.1007/s12016-007-8040-3. [DOI] [PubMed] [Google Scholar]
- 4.Horak E, Morass B, Ulmer H. Association between environmental tobacco smoke exposure and wheezing disorders in Austrian preschool children. Swiss Med Wkly. 2007;137:608–613. doi: 10.4414/smw.2007.11870. [DOI] [PubMed] [Google Scholar]
- 5.Harmanci K, Bakirtas A, Turktas I. Factors affecting bronchial hyperreactivity in asthmatic children. J Asthma. 2008;45:730–734. doi: 10.1080/02770900802385992. [DOI] [PubMed] [Google Scholar]
- 6.Jones DT, Bhattacharyya N. Passive Smoke Exposure as a risk factor for airway complications during outpatient pediatric procedures. Otolaryngol Head Neck Surg. 2006;135:12–6. doi: 10.1016/j.otohns.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Hoppenbrouwers L, Jara A, Declerck D. Parental smoking behavior and caries experience in preschool children. Community Dent Oral Epidemiol. 2008;36:249–257. doi: 10.1111/j.1600-0528.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- 8.Kallio K, Jokinen E, Hamalainen M, et al. Decreased aortic elasticity in healthy 11-year-old children exposed to tobacco smoke. Pediatrics. 2009;123:e267–e273. doi: 10.1542/peds.2008-2659. [DOI] [PubMed] [Google Scholar]
- 9.Skolnick E, Vomvolakis M, Buck K, Mannino S, Lena S. Exposure to environmental tobacco smoke and the risk of adverse respiratory events in children receiving general anesthesia. Anesthesiology. 1998;88:1144–1153. doi: 10.1097/00000542-199805000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Groner J, Hoshaw-Woodard S, Koren G, Klein J, Castile R. Screening for children's exposure to environmental tobacco smoke in a pediatric primary care setting. Arch Pediatr Adolesc Med. 2005;159:450–455. doi: 10.1001/archpedi.159.5.450. [DOI] [PubMed] [Google Scholar]
- 11.Kumar R. Prenatal factors and the development of asthma. Curr Opin Pediatr. 2008;20:682–687. doi: 10.1097/MOP.0b013e3283154f26. [DOI] [PubMed] [Google Scholar]
- 12.Eaton JJ, McTigue DJ, Fields HW, Jr, Beck M. Attitudes of contemporary parents towards behavior management techniques in pediatric dentistry. Pediatr Dent. 2005;27:107–113. [PubMed] [Google Scholar]


