Abstract
For several decades, anesthetic gases have greatly enhanced the comfort and outcome for patients during surgery. The benefits of these agents have heavily outweighed the risks. In recent years, the attention towards their overall contribution to global climate change and the environment has increased. Anesthesia providers have a responsibility to minimize unnecessary atmospheric pollution by utilizing techniques that can lessen any adverse effects of these gases on the environment. Moreover, health care facilities that use anesthetic gases are accountable for ensuring that all anesthesia equipment, including the scavenging system, is effective and routinely maintained. Implementing preventive practices and simple strategies can promote the safest and most healthy environment.
Key Words: : Volatile anesthetics, Environmental pollution, Greenhouse warming potential, Ozone depletion potential
Anesthetics are commonly used during surgery with the aim of providing the patient with an experience free of sights, sounds, and any other unpleasant sensations. The major atmospheric effects that may arise from emission of volatile anesthetics are their contributions to ozone depletion in the stratosphere and to greenhouse warming in the troposphere.1 The purpose of this paper is to highlight the pertinent anesthetic gases and the anthropogenic impact of these agents on the environment. In addition, our intention is to present simple knowledge-based decisions and preventive strategies that may minimize deleterious effects of these gases on the environment.
ANESTHETIC AGENTS
During surgery involving general anesthesia, the patient is anesthetized with the use of intravenous and/or inhaled anesthetics. The most commonly used inhaled anesthetic agents include 2 different classes of chemicals: nitrous oxide (N2O) and volatile halogenated agents (vapors) (Table 1). N2O is supplied in a gas form. The halogenated agents are supplied as a liquid, which is then vaporized by the anesthesia machine into a gaseous state prior to its delivery to the patient. N2O and/or inhaled volatile agents are administered to the patient via a mask or breathing tube which is connected to a corrugated circuit to the anesthesia machine. The 3 most commonly used inhaled anesthetics—isoflurane, sevoflurane, and desflurane—undergo very little in vivo metabolism in clinical use.2 Less than 5% of these volatile anesthetics is metabolized by the patient.3 Because they undergo very little metabolic change inside the body, upon exhalation by the patient these agents remain in a form that may pollute the environment. Inhaled anesthetics are exhaled and scavenged by anesthesia machines with little or no additional degradation,4 and are typically vented into the outside environment without abatement as waste anesthetic gases (WAGs).
Table 1. .
Waste Anesthetic Gases and Vapors*

Nitrous Oxide
Since its discovery as an anesthetic in 1844, N2O has been one of the safest, most popular, and most useful adjuncts for surgical procedures.5 Whether administered in the dental office or the operating room, N2O has greatly enhanced the surgical experience for all parties for many decades. The use of N2O is quite appealing for anesthesia providers because of several advantageous properties. It serves as a significant analgesic, elevating the pain threshold during surgery.6 Also, when incorporated into the gas mixture administered to a patient, N2O reduces the necessary minimum alveolar concentration of other concomitantly used anesthetic gases (ie, isoflurane, desflurane, or sevoflurane).7 Consequently, a smaller amount of a volatile anesthetic agent is required to achieve the same level of anesthesia when N2O is added than when the volatile agent is used alone. Moreover, because N2O is eliminated from the body so quickly at the termination of the anesthetic, the reduced amount of volatile agent remaining also contributes to a more prompt recovery from general anesthesia, and adverse effects of the lower amounts of these potent anesthetic agents are decreased.
However, this widely used gas has some potentially undesirable effects, which may affect health care workers and the environment. Because N2O is minimally metabolized in humans (with an approximate rate of 0.004%), it retains its pharmacological effect when exhaled into the room by the patient, and can pose an intoxicating and prolonged exposure hazard to operating room personnel if the room is poorly ventilated.8 It has been estimated that more than 250,000 health care professionals in the United States who work in hospitals, operating rooms, dental offices, and veterinary clinics are potentially exposed to WAGs and are at risk of an occupational illness.9 These agents are also a recognized greenhouse gas, accounting for around 6% of the heating effect of greenhouse gases in the atmosphere. N2O also causes ozone depletion.10 N2O emissions from all of its various environmental sources are currently the single most important ozone-depleting substance emission and are expected to remain the largest throughout the 21st century.11
Halogenated Agents
Currently, the 3 most commonly used halogenated inhalational anesthetics used for surgery—isoflurane, sevoflurane, and desflurane—are recognized greenhouse gases.1,12 All volatile anesthetics are halogenated chlorofluorocarbons (halothane, enflurane, isoflurane) or fluorinated hydrocarbons (sevoflurane and desflurane) and are thus potentially damaging to the earth's ozone layer.13 They also contribute to global warming. The bromide-containing agent halothane is the most destructive against ozone, although it is rarely used in the United States today. Isoflurane and enflurane (which contains only chloride and fluoride ion substitutions) have a lesser impact.12
Most of the organic anesthetic gases remain for a long time in the atmosphere, where they have the potential to act as greenhouse gases. Published atmospheric lifetimes range between 1.4 and 21.4 years for sevoflurane and desflurane, respectively.1,12 Recently, estimates have been made that focus upon the accretion of these anesthetic gases emanating from hospitals. Worldwide yearly sales of inhaled anesthetics total in the millions of liters, given that a busy midsize US hospital might purchase approximately 1000 L of inhaled anesthetics per year.14 Assuming an average 4.78 metric tons of CO2 emissions/passenger car/year in the United States,15 this would be the equivalent of approximately 100 to 1200 passenger car emissions/year/midsized hospital, depending on which inhaled anesthetics were used.
Intravenous Anesthetics
Over the last few decades, there has been a significant increase in use of intravenous agents for sedation and general anesthesia among anesthesia providers.16 As the trend in surgery continues to move towards shorter hospital stays and more ambulatory cases,17 anesthetics that facilitate a rapid recovery have gained greater preference. Because of their favorable characteristics such as rapid onset, quick recovery, and minimal side effects, intravenous agents are now used extensively in hospitals and ambulatory surgery centers.18 Unfortunately, metabolic products of these agents can be released into the hospital's sewage system. It is possible that degradation products such as phenol from propofol are produced, but it is not known whether there is a considerable buildup in the food chain.19
ENVIRONMENTAL CONTAMINATION PATHWAYS
There are 2 major pathways for the undesirable leakage of anesthesia gases into the operating room environment: anesthetic techniques and the anesthesia machine delivery system (Table 2).20 An example of the former occurs when applying a face mask to the patient during the induction of general anesthesia. In these situations, trace quantities of gas seepage around the mask are unavoidable.21 However, the amount magnifies with the use of a poorly fitting mask, especially when used for a patient with a difficult airway, and when high gas concentrations are utilized, commonly with pediatric patients. Moreover, immediately prior to intubation of the patient's trachea, the anesthesia provider may set the mask and anesthesia circuit off to one side, while the inhalational gas inadvertently continues to flow. Because the circuit is no longer connected to the patient, WAGs freely escape into the atmosphere.22 Another improper technique is the failure to turn off all of the flow control valves (oxygen, nitrous, air) and/or vaporizer when the circuit is disconnected from the patient at the end of a case, or while these valves remain open between cases.20 Also, upon refilling the anesthesia machine's vaporizer, spillage of the liquid inhalational anesthetic contaminates the workplace.23 One more hazardous anesthetic practice occurs upon hastening the patient's emergence from anesthesia, when a practitioner may elect to flush the anesthesia circuit of residual gases at the end of a procedure. If the gas flow is not entirely flushed into a scavenging system, operating room pollution of anesthetic gases ensues.22 Other anesthetic-related causes include an inappropriately underinflated cuff of an endotracheal tube or laryngeal mask airway and the use of an uncuffed endotracheal tube.20
Table 2. .
Causes of Operating Room Contamination*
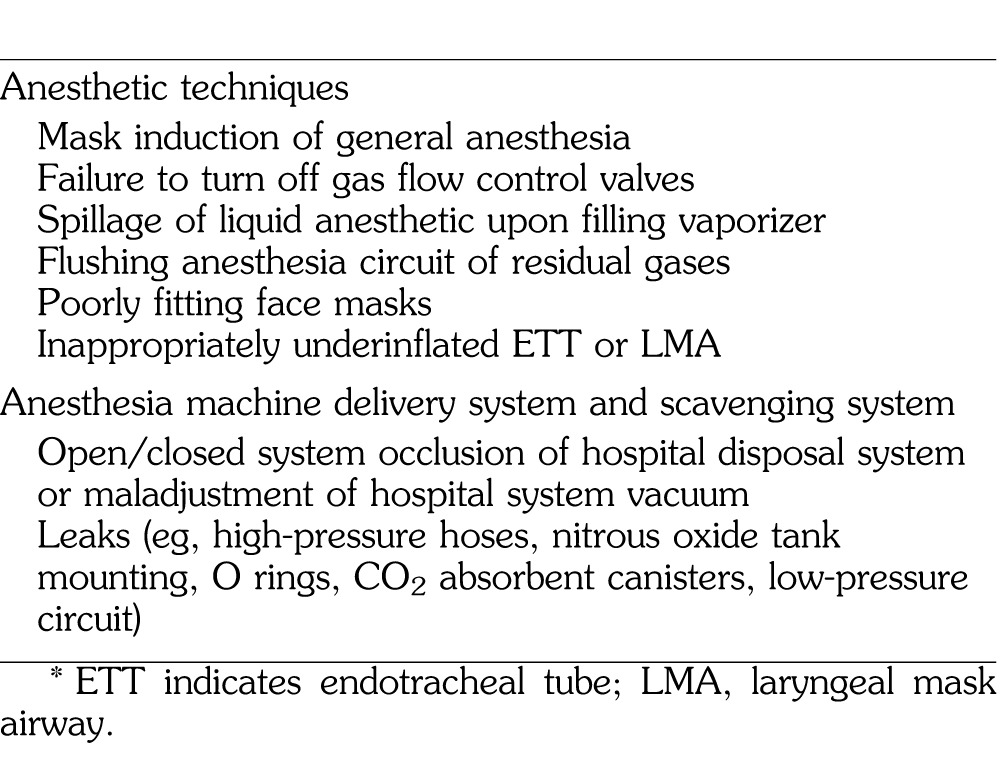
It should be noted that the anesthesia provider is not solely responsible for the atmospheric contamination in the operating room. Exposure measurements taken in operating rooms during the clinical administration of inhaled anesthetics indicate that waste gases can escape into the room from various components of the anesthesia delivery system.22 Potential leak sources include tank valves, high- and low-pressure machine connections, and any connection along the breathing circuit, as well as defects in rubber and plastic tubing, hoses, reservoir bags, and ventilator bellows.23 Also, an improperly functioning (or absent) scavenging system will lead to operating room contamination.22
Scavenging systems are designed to collect gases and vapors that are vented from the breathing circuit. The gases are then redirected to a safe area (directly exhausted outside of the facility) or a dedicated WAG disposal system.23 Currently, gas scavenging is the most practical engineering control for removing WAGs. Levels of WAGs can be further reduced when gas scavenging is combined with other recommended control procedures such as dilution ventilation. An effective room heating, ventilation, and air conditioning system, when used in combination with an anesthetic gas scavenging system, should reduce, although not entirely eliminate, the contaminating anesthetic gases. If excessive concentrations of anesthetic gases are present, then airflow should be increased in the room to allow for more air mixing and further dilution of the anesthetic gases.
PREVENTION
Wherever N2O or a volatile anesthetic is administered, a continuous-flow fresh air ventilation system or scavenger system must be used to prevent waste gas accumulation. It is the responsibility of each institution to organize and document a program of maintenance and checking of all anesthetic equipment, including the scavenging system.21 Additionally, eliminating the use of an anesthesia technique that could elevate contamination levels in the operating room is another effective means to prevent WAGs.22 Employing the following anesthetic practices will minimize contamination of the environment: reducing gas flow during mask inductions; turning off excessive gas flows and vaporizers following an anesthetic; careful filling of vaporizers; judicious checking of all connections along the anesthesia circuit and at the machine; and using properly fitting face masks and inflated endotracheal tube and laryngeal mask airway cuffs.21
Reducing maintenance flows of anesthetic gases to 1–2 L/min rather than 4–6 L/min can also minimize environmental pollution.
Ryan and Nielsen14 suggest several approaches that anesthesiologists can use to minimize their environmental impact when delivering inhaled anesthetics. First, the authors propose avoiding N2O as a carrier gas unless there is a clinical reason to prefer it. Second, it is suggested to avoid unnecessarily high fresh gas flow rates, particularly when using desflurane. Desflurane has a 10-year “lifetime” in the atmosphere, compared with 3.6 years for isoflurane and 1.2 years for sevoflurane. The authors calculated that desflurane has about 26 times the global warming potential of sevoflurane and 13 times the potential of isoflurane. Using desflurane for 1 hour is equivalent to 235–470 miles of driving, according to the study.14 The optimal (lowest environmental impact) fresh gas flow rate has not yet been established. It would appear that the lowest fresh gas flow possible would be best for the environment, because it would minimize anesthetic use.
RECYCLED ANESTHETICS
The development of new methods of capturing anesthetic gases for reuse, rather than releasing them into the atmosphere, can reduce the environmental impact. Researchers have developed a recycling system, called the Dynamic Gas Scavenging System, which collects and reuses 99% of anesthetic gases without chemically altering them in the process. Anesthetic Gas Reclamation, LLC, created the technology, and Vanderbilt University Medical Center has been instrumental in its development by providing a testing site in 4 operating rooms. The system is designed to work with any anesthesia machine, costs $20,000, and can serve up to 8 operating rooms. The exhaust system is activated only when the patient exhales and used anesthetic appears. Energy savings also result because the vacuum pump runs only 10% of the time.24
Another company that seeks to capture the unused anesthetic before it is released into the environment is Blue-Zone Technologies Ltd, with its canister system, Deltasorb. The Deltasorb selectively captures the inhalation anesthetics before they enter the atmosphere through a filtration process. The self-sterilizing drugs are then extracted, liquefied, and used as raw materials to produce bulk anesthetic drugs. They can be sold back into the market to large pharmaceutical companies at less than what it costs the companies to manufacture them. Hospitals are charged a monthly service fee that covers the exchange of Deltasorb canisters and monthly reports detailing how much anesthetic is captured in terms of prevented emissions. Hospitals can also qualify for potential carbon credit offsets, based on their reductions.25
CONCLUSIONS
Over the years there have been significant improvements in the control of environmental contamination by anesthetic gases. These have been accomplished through the use and improved design of scavenging systems, installation of more effective general ventilation systems, and an increased attention to equipment maintenance and leak detection, as well as careful anesthetic practice. However, the environmental impact of these anesthetic gases remains a concern.
The implications of inhaled anesthetics currently used during surgery warrant further study for their overall contribution to global climate change and the environment. Release of anesthetic gases into the atmosphere presents a relatively small problem in comparison with other sources of ozone-depleting chemicals and greenhouse gases. However, anesthesia providers should be prudent by minimizing unnecessary atmospheric pollution. This goal can be accomplished by reexamining the relative impact of their practice patterns and employing techniques that would lessen the hazards. In addition, any facility that utilizes anesthetic gases has a responsibility to put into effect a maintenance program that routinely checks all anesthesia equipment, including the scavenging system. Vigilance, education, and the implementation of these strategies can optimize the healthy environments of an operating room and the atmosphere.
REFERENCES
- 1.Langbein T, Sonntag H, Trapp D et al. Volatile anaesthetics and the atmosphere: atmospheric lifetimes and atmospheric effects of halothane, enflurane, isoflurane, desflurane and sevoflurane. Br J Anaesth. 1999;82:66–73. doi: 10.1093/bja/82.1.66. [DOI] [PubMed] [Google Scholar]
- 2.Shiraishi Y, Ikeda K. Uptake and biotransformation of sevoflurane in humans: a comparative study of sevoflurane with halothane, enflurane, and isoflurane. J Clin Anesth. 1990;2:381–386. doi: 10.1016/0952-8180(90)90024-w. [DOI] [PubMed] [Google Scholar]
- 3.Hospital anesthetic gas discharges and the environment: prevent the vent. Canadian Centre for Pollution Prevention Web site. Available at: http://www.c2p2online.com/documents/FINAL%202%20pg%20Fact%20Sheet.pdf. January 2005. Accessed November 10, 2010. [Google Scholar]
- 4.McHaourab A, Arain SR, Ebert TJ. Lack of degradation of sevoflurane by a new carbon dioxide absorbent in humans. Anesthesiology. 2001;94:1007–1009. doi: 10.1097/00000542-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Maloney WJ, Maloney MP, Wells Horace. and his significant contributions to the discovery of anesthesia. J Mass Dent Soc. 2009;58:18–19. [PubMed] [Google Scholar]
- 6.Karmarkar SW, Bottum KM, Tischkau SA. Considerations for the use of anesthetics in neurotoxicity studies. Comp Med. 2010;60:256–262. [PMC free article] [PubMed] [Google Scholar]
- 7.Kihara S, Yaguchi Y, Inomata S et al. Influence of nitrous oxide on minimum alveolar concentration of sevoflurane for laryngeal mask insertion in children. Anesthesiology. 2009;99:1055–1058. doi: 10.1097/00000542-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Dale O, Brown BR., Jr Clinical pharmacokinetics of the inhalational anaesthetics. Clin Pharmacokinet. 1987;12:145–167. doi: 10.2165/00003088-198712030-00001. [DOI] [PubMed] [Google Scholar]
- 9.Waste anesthetic gases fact sheet No. OSHA 91-38. United States Dept of Labor Occupational Safety and Health Administration Web site. Available at: http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=FACT_SHEETS&p_id=128. 1991. Accessed November 10, 2010. [Google Scholar]
- 10.Ravishankara AR, Daniel JS, Portmann RW. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science. 2009;326:123–125. doi: 10.1126/science.1176985. [DOI] [PubMed] [Google Scholar]
- 11.Chipperfield M. Atmospheric science: nitrous oxide delays ozone recovery. Nat Geosci. 2009;2:742–743. [Google Scholar]
- 12.Brown AC, Canosa-Mas CE, Parr AD, Pierce JM, Wayne RP. Tropospheric lifetimes of halogenated anesthetics. Nature. 1989;341:635–637. doi: 10.1038/341635a0. [DOI] [PubMed] [Google Scholar]
- 13.Irwin MG, Trinh T, Yao C. Occupational exposure to anaesthetic gases: a role for TIVA. Expert Opin Drug Saf. 2009;8:473–483. doi: 10.1517/14740330903003778. [DOI] [PubMed] [Google Scholar]
- 14.Ryan MS, Nielsen CJ. Global warming potential of inhaled anesthetics: application to clinical use. Anesth Analg. 2010;111:92–98. doi: 10.1213/ANE.0b013e3181e058d7. [DOI] [PubMed] [Google Scholar]
- 15.Emissions facts: greenhouse gas emissions from a typical passenger vehicle. United States Environmental Protection Agency Web site. Available at: http://www.epa.gov/oms/climate/420f05004.htm#key. 2005. Accessed November 10, 2010. [Google Scholar]
- 16.Coulthard P, Craig D. Conscious sedation. Dent Update. 1997;24:376–381. [PubMed] [Google Scholar]
- 17.Diez R-Labajo A. Office-based surgery and anesthesia. Curr Opin Anaesthesiol. 1998;11:612–621. [PubMed] [Google Scholar]
- 18.Waugaman WR, Foster SD. New advances in anesthesia. Nurs Clin North Am. 1991;26:451–461. [PubMed] [Google Scholar]
- 19.Byhahn C, Wilke HJ, Westpphal K. Occupational exposure to volatile anaesthetics: epidemiology and approaches to reducing the problem. CNS Drugs. 2001;15:197–215. doi: 10.2165/00023210-200115030-00004. [DOI] [PubMed] [Google Scholar]
- 20.Occupational and safety and health guideline for nitrous oxide. United States Dept of Labor Occupational Safety and Health Administration Web site. Available at: http://www.osha.gov/SLTC/healthguidelines/nitrousoxide/recognition.html. 2002. Accessed November 10, 2010. [Google Scholar]
- 21.American Society of Anesthesiologists. Occupational disease among operating room personnel: a national study. Report of an ad hoc committee on the effects of trace anesthetics on the health of operating room personnel. Anesthesiology. 1974;41:321–40. [PubMed] [Google Scholar]
- 22.Guidelines for protecting the safety and health of health care workers. National Institute for Occupational Safety and Health Publication 88-119. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/niosh/docs/88-119/. September 1988. Accessed November 10, 2010. [Google Scholar]
- 23.American Society for Testing and Materials. Standard specification for anesthesia equipment: scavenging systems for anesthetic gases. West Conshohocken, Pa: American Society for Testing and Materials; 1991:1343–1391. Document F. [Google Scholar]
- 24.Recycled anesthetic technology saves dollars, environment. Vanderbilt. magazine Web site. Available at: http://www.vanderbilt.edu/magazines/vanderbilt-magazine/2009/03/recycled-anesthetic-technology-saves-dollars-environment/. 2009. Accessed November 10, 2010. [Google Scholar]
- 25.A remedy for the operating room. Blue-Zone Technologies Ltd Web site. Available at: http://www.bluezone.ca/site05/Home/tabid/36/Default.aspx. 2009. Accessed November 10, 2010. [Google Scholar]


