Abstract
In an environment filled with a complex spectrum of chemical stimuli, insects rely on the specificity of odorant receptors (ORs) to discern odorants of ecological importance. In nature, cyclic esters, or lactones, represent a common class of semiochemicals that exhibit a range of diversity through ring size and substituents, as well as stereochemistry. We have used heterologous expression to explore the lactone sensitivity of AgOr48, an odorant-sensitive OR from the principal malaria vector mosquito, Anopheles gambiae. Voltage clamp and calcium-imaging experiments revealed that AgOr48 is particularly sensitive to changes in the size of the lactone ring and in the length of the carbon chain substituent. In addition, the two enantiomers of a strong agonist, δ-decalactone, elicited significantly different potency values, implicating AgOr48 as an enantioselective odorant receptor. Investigation of the molecular receptive range of this lactone receptor may contribute to a greater understanding of ligand–OR interactions and provide insight into the chemical ecology of An. gambiae.
Key words: Anopheles gambiae, lactone, mosquito, odorant receptor, sugar-feeding
Introduction
Insect behaviors, especially those activated at long range, are largely dictated by olfactory cues in the environment (Gillot 2005). In these systems, multiple types of cell-surface chemoreceptors are responsible for the specific detection of odorant molecules and lead to signal transduction in sensory neurons (Kaupp 2010). Within the insect chemoreceptor families, the majority of studies have focused on odorant receptors (ORs). Insect ORs possess an inverted heptahelical topology relative to typical G-protein–coupled receptors and function as odorant-gated ion channels (Benton et al. 2006; Sato et al. 2008; Smart et al. 2008; Wicher et al. 2008; Jones et al. 2011). An extraordinarily conserved insect coreceptor, Orco, forms a heteromultimeric complex with a tuning OR subunit that imparts odorant specificity and other channel properties (Larsson et al. 2004; Pitts et al. 2004; Jones et al. 2005; Nichols et al. 2011; Pask et al. 2011; Nakagawa et al. 2012).
Although several studies have identified odorant ligands for numerous tuning ORs of various insects, the molecular mechanisms underlying ligand–OR activation, binding, and gating remain uncertain. Defining the molecular receptive range of an OR can provide insight into its interaction with an odorant ligand. Through functional screens with a wide range of odorants, the chemical characteristics (function groups, ring size, chain length, etc.) that are critical for receptor activation can be determined (Bestmann et al. 1987). Further, examination of the chemical properties can be used to support a pharmacophore model, a generalized ligand structure that provides insight into the presumed OR binding pocket.
In an effort to better understand the olfactory system of Anopheles gambiae, the principal afro-tropical malaria vector, heterologous screens using broad panels of odorant stimuli have deorphanized several of the An. gambiae ORs (AgOrs) (Xia et al. 2008; Carey et al. 2010; Wang et al. 2010). However lactones, or cyclic esters, which have been identified as important semiochemicals in mosquitoes and other insects were underrepresented in the odorant panels utilized in these studies. For example, erythro-6-acetoxy-5-hexadecanolide has been identified as the major component of oviposition pheromone in egg rafts of Culex pipiens fatigans (Laurence and Pickett 1982). In hermit beetles, Osmoderma eremita, males produce R-(+)-γ-decalactone as a sex pheromone, and are known for their fruity, peach-like odor profile (Svensson and Larsson 2008). In addition to these animal sources of lactones, numerous flower and fruit volatiles contain mixtures of lactones (Grab 1998). One study utilized several lactones identified in ripe peaches to elicit olfactory responses in the antennae of the fruit-piercing moth, Oraesia excavata (Tian et al. 2008). Also, volatile fractions from some monofloral honeys have been found to contain different lactone species, and honey is attractive to both male and female An. gambiae seeking a sugar meal (Moreira et al. 2002; Foster and Takken 2004). Taken together, animal- and plant-derived lactones may have a role in the chemical ecology of An. gambiae, specifically in the selection of sugar sources and oviposition sites.
In initial odorant panel screenings of tuning ORs from An. gambiae, γ-decalactone elicited the strongest responses from Xenopus oocytes expressing AgOr48 and AgOrco, with much weaker responses from 2-nonanone and 1-octanol (Wang et al. 2010). AgOr30 and AgOr57 also responded to γ-decalactone, albeit with lower sensitivity; each of these AgOr’s also responded more strongly to several other agonists (Wang et al. 2010). AgOr48 is expressed in antennal structures during the larval and adult stages and was found to respond to another lactone, δ-undecalactone (Xia et al. 2008; Pitts et al. 2011; Jones et al. 2012). In this study, we further explored the molecular receptive range of AgOr48 by determining the effects of chain length, ring size, and chirality on agonist potency and have determined a preliminary pharmacophore.
Materials and methods
Chemicals
Supplier information and CAS numbers for purchased lactones are provided in Supplementary Table S1. All assay compounds were first diluted in dimethyl sulfoxide (DMSO) and subsequently diluted in assay buffer with a final DMSO concentration of 0.1%.
Synthesis of ε-undecalactone and ε-tridecalactone
General
All nonaqueous reactions were performed in flame-dried or oven-dried round-bottomed flasks under an atmosphere of argon. Stainless steel syringes or cannulae were used to transfer air- and moisture-sensitive liquids. Reaction temperatures were controlled using a thermocouple thermometer and analog hotplate stirrer. Reactions were conducted at room temperature (RT, ~23 °C) unless otherwise noted. Analytical thin-layer chromatography was performed on E. Merck silica gel 60 F254 plates and visualized using UV, ceric ammonium molybdate, potassium permanganate, and anisaldehyde stains. Yields were reported as isolated, spectroscopically pure compounds.
Materials
Solvents were obtained from an MBraun MB-SPS solvent system. Commercial reagents were used as received.
Procedure
To a solution of either 2-pentylcyclohexanone or 2- heptylcyclohexanone (100mg, 0.59 mmol) in 11.5mL of CH2Cl2 at 0 °C was added a solution of m-CPBA (205.4mg, 1.19 mmol) in 2mL of CH2Cl2. After 48h at RT, saturated NaHCO3 (50mL) was added and the resulting solution stirred for 10min. The aqueous layer was extracted with CH2Cl2 (3×50mL). The combined organic layers were dried (MgSO4) and concentrated to a residue. Each residue was purified by column chromatography with EtOAc/Hexane (1:4) to afford 35mg (32%) of ε-undecalactone or 49mg (45%) of ε-tridecalactone: 1H NMR data matched published data for each product (Fellous et al. 1994).
Cell culture, electrophysiology, and calcium imaging
The AgOr48 + AgOrco stable HEK cell line was previously generated (Jones et al. 2012). Whole-cell patch clamp recording from AgOr48 + AgOrco cells were performed using previously described methods (Jones et al. 2011). The calcium mobilization assays were performed using Fluo-4 AM dye and an FDSS6000 plate reader (Hamamatsu) as described previously (Jones et al. 2011). In a 384-well plate, each well contains an identical number of cells and is given a single agonist treatment to prevent desensitization due to repeated stimulations. Each of the 12 concentrations was done in tetraplicate. The generation of concentration response curves and the statistical analysis of the fitted curves values were completed in Prism 4 (GraphPad).
Results
In order to assay odorants of potential ecological relevance to An. gambiae, the panel used in this study represents lactones commonly found in nature. These consist of various lactone ring sizes, including five- (γ), six- (δ), and seven-(ε) membered rings; α- and β- lactones are less common due to the decreased stability of the three- and four-membered rings, respectively. Additionally, lactones with variation in the carbon side chain length were selected to determine the optimal chain length for agonist activity. Altogether, the selected panel of lactones examines the effects of ring size and side chain length on the efficiency of AgOr48 agonists.
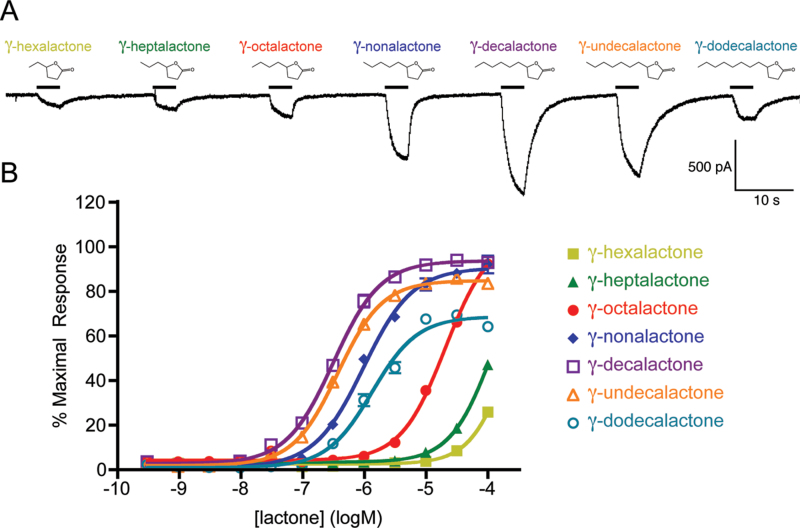
A panel of γ-lactones with side chains ranging from two to eight carbons was applied to AgOr48 + AgOrco-expressing HEK cells at a concentration of 1 µM (Figure 1A). In whole-cell patch clamp studies, larger current amplitudes were elicited by γ-lactones with five- to seven-carbon side chains, with γ-decalactone producing the largest. In these studies, repeated stimulation with the lactone panel resulted in decreased current amplitudes for some strong agonists (Supplementary Figure S1). Calcium mobilization assays were subsequently performed in order to generate concentration-response curves and remove the desensitization effects of repeated agonist application (Figure 1B). The general trend observed in the whole-cell recording of Figure 1A correlated with the concentration-response curve data, as γ-decalactone was the most potent agonist with an EC50 value of −6.49±0.02 logM. These observations suggest that with a γ-lactone ring structure, the six-carbon chain yields the optimal agonist activity.
Figure 1.
AgOr48-expressing HEK cells respond to γ-lactones. (A) Whole-cell recording of a AgOr48 + AgOrco cell responding to 1 µM concentrations of lactone. Holding potential is −60 mV. (B) Concentration-response curves generated from Ca++-imaging assays on AgOr48 + AgOrco cells (n = 4). Data points represent the mean ± SEM and percent maximal response is normalized to the plate standard, δ-decalactone. EC50 values from the curve fit can be found in Supplementary Table S1.
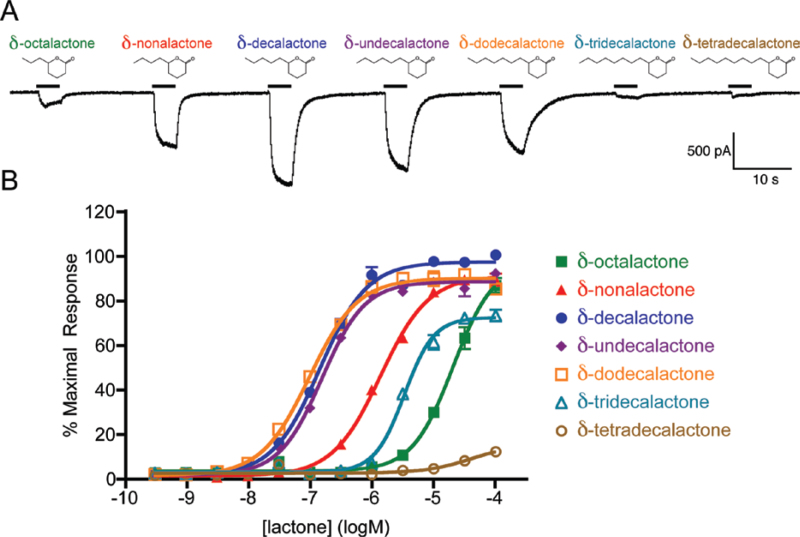
We next examined the responses to a series of δ-lactones with side chains of three to nine carbons, where several δ-lactones elicited robust currents in the AgOr48 + AgOrco cells (Figure 2A). The concentration-response curves from Ca++-imaging assays reveal three lactone agonists, δ-decalactone (−6.84±0.03 logM), δ-undecalactone (−6.81±0.03 logM), and δ-dodecalactone (−7.01±0.03 logM) that are clearly more potent than the other compounds (Figure 2B). As was the case with the γ-lactones, the five- to seven-carbon chain δ-lactones elicited the most potent AgOr48 responses.
Figure 2.
Several δ-lactones gate the AgOr48 complex. (A) Current responses of an AgOr48 + AgOrco-expressing cell during application of various δ-lactones (1 µM). Holding potential is −60 mV. (B) Concentration-response curves generated from Ca++-imaging assays on AgOr48 + AgOrco cells (n = 4). Data points represent the mean ± SEM and percent maximal response is normalized to the plate standard, δ-decalactone. EC50 values from the curve fit can be found in Supplementary Table S1.
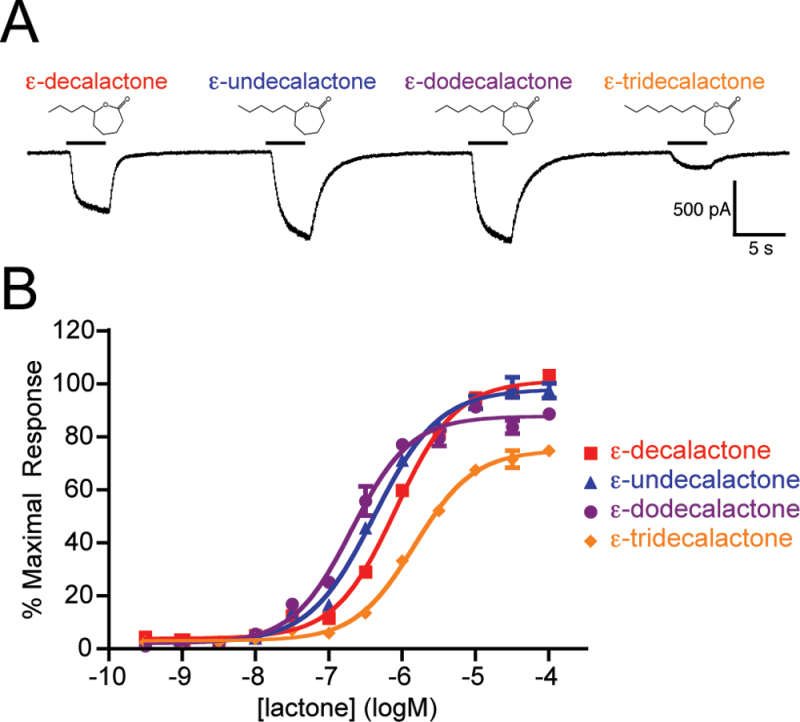
In order to test a series of ε-lactones, we synthesized both ε-undecalactone and ε-tridecalactone to supplement ε-decalactone and ε-dodecalactone, which are commercially available (see Materials and methods). Initial whole-cell recordings showed current responses to all ε-lactones at 1 µM, with the largest amplitudes resulting from ε-undecalactone and ε-dodecalactone (Figure 3A). The most potent ε-lactone was ε-dodecalactone, with an EC50 value of −6.69±0.04 logM (Figure 3B). Also, agonist potency decreased greatly with the addition of a single carbon to the side chain (ε-tridecalactone, −5.83±0.03 logM).
Figure 3.
The AgOr48 complex responds to ε-lactones. (A) Representative whole-cell currents of an AgOr48 + AgOrco cell during application of various ε-lactones (1 µM). Holding potential is −60 mV. (B) Concentration-response curves generated from Ca++-imaging assays on AgOr48 + AgOrco cells (n = 4). Data points represent the mean ± SEM and percent maximal response is normalized to the plate standard, δ-decalactone. EC50 values from the curve fit can be found in Supplementary Table S1.
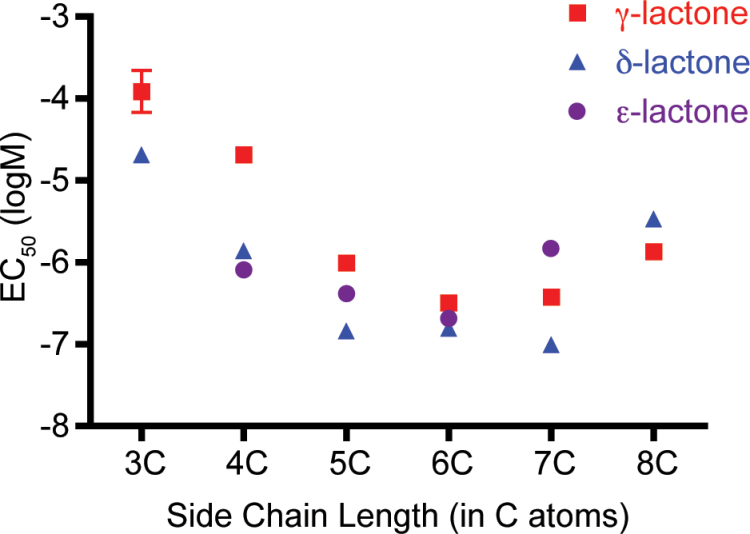
These studies of the molecular receptive range of AgOr48 to lactone agonists facilitate the construction of an initial pharmacophore model. From the EC50 values of each agonist, it appears that lactones with a six-carbon chain are the most potent in gating the AgOr48 complex (Figure 4). Additionally, the δ-lactone ring offers the most flexibility in chain length in terms of potency, with the five- or seven-carbon chain δ-lactones displaying near-equal potencies. It is also interesting to note that AgOr48 displays relatively high sensitivity (≤1 µM) to several of the lactones in the panel.
Figure 4.
Lactone potency depends on side chain length and ring size. Plot of EC50 values by number of carbons in the side chain and the lactone ring structure. Data points represent mean ± SEM, and exact values can be found in Supplementary Table S1.
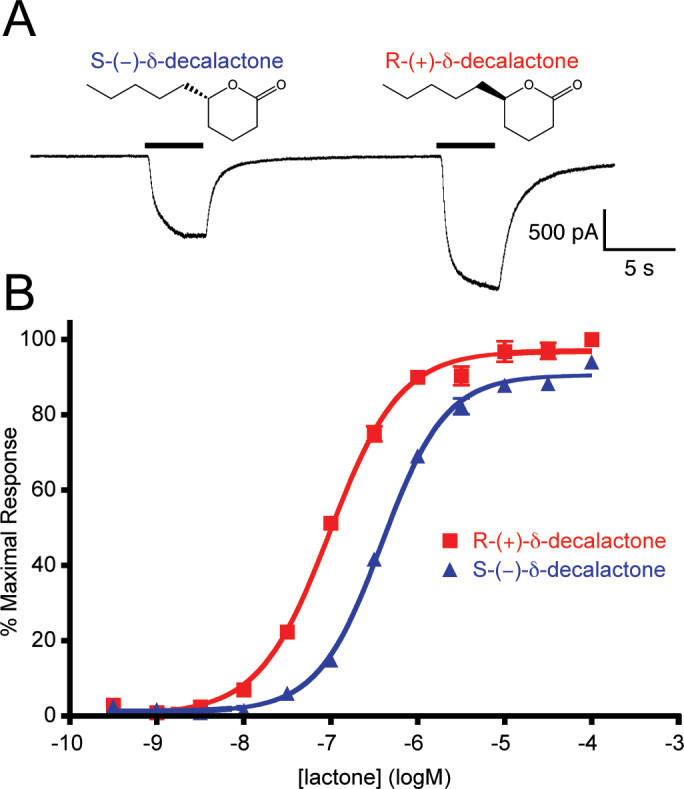
Because the carbon that links the side chain to the lactone ring is chiral, we next determined if AgOr48 is enantioselective, and thus able to differentiate between the R-(+) and S-(−) forms of a lactone agonist. AaOr8, an enantioselective mosquito OR from Aedes aegypti, is ~100-fold more sensitive to R-(−)-1-octen-3-ol than the S-(+) form (Bohbot and Dickens 2009). Both enantiomers of the strong AgOr48 agonist, δ-decalactone, were commercially available and elicited differential currents at equimolar concentrations (Figure 5A). Indeed, these differences were observed in the concentration-response curves where R-(+)-δ-decalactone (EC50 −7.01±0.02 logM) was a significantly more potent AgOr48 agonist than the S-(−) enantiomer (EC50 −6.41±0.02 logM) (Figure 5B). These results demonstrate that the AgOr48 complex is able to effectively discriminate between these chiral lactone agonists.
Figure 5.
Enantiomers of δ-decalactone display different agonist potencies on AgOr48 cells. (A) A current recording from an AgOr48 + AgOrco cell during a 1 µM application of S-(−)-δ-decalactone and R-(+)-δ-decalactone. Holding potential is −60 mV. (B) Concentration-response curves generated from Ca++-imaging assays on AgOr48 + AgOrco cells (n = 4). Data points represent the mean ± SEM and percent maximal response is normalized to the plate standard, δ-decalactone. The EC50 values from the curve fit differ significantly between the R- and S-enantiomers (P < 0.0001, F test) and can be found can be found in Supplementary Table S1.
Discussion
We have used an odorant panel of straight-chain lactones to explore the molecular receptive range of AgOr48 and have identified several additional potent agonists. From these data, we can conclude that AgOr48 is most sensitive to lactones with a five- to seven-carbon side chain, and that the six-membered δ-lactone ring structure typically results in more potent agonists. It should be noted that several ketones and alcohols are capable of eliciting less potent responses from AgOr48, and it remains unclear whether this occurs at the same site as lactone recognition (Carey et al 2010; Wang et al. 2010). Furthermore, AgOr48 appears to be enantioselective, with a preference towards the R-(+) configuration of δ-decalactone. These differences are not as sizeable when compared with the previously observed enantioselective mosquito OR, but suggest that the lactone-binding site of AgOr48 is capable of discriminating the two side chain positions on the lactone ring (Bohbot and Dickens 2009). In addition, the R-(+) configuration of the larger straight-chain lactones (five carbons and above) appears to be more abundant in several fruit species (Bernreuther et al 1989). The resulting preliminary pharmacophore for AgOr48 lactone agonists consists of a six-membered lactone ring (± one carbon) with a side chain of five to seven carbons in an R-(+) conformation at the chiral center.
Our initial hypothesis was that the specificity of AgOr48 to lactones might play a role in the chemical ecology of An. gambiae. We were specifically interested in nectar-feeding and oviposition, as several of the strong lactone agonists identified here are naturally abundant in a range of plant flowers and fruits (Grab 1998). This would be consistent with our hypothesis that lactones function as semiochemicals, signaling a potential sugar source for An. gambiae. Indeed, several fruits that have recently been associated with field-caught An. gambiae contain many of these lactones in their volatile profiles. One study observed an abundance of An. gambiae males associated with Mangifera indica, the common mango tree, and observed attraction to M. indica odor using a Y-shaped olfactometer (Gouagna et al. 2010). The aroma of M. indica is largely dependent on a mixture of several straight chain γ- and δ-lactones, many of which display agonist activity against AgOr48 (Wilson et al. 1990; Grab 1998). Another field study in Mali found that the most attractive fruits to both male and female An. gambiae were guava (Psidium guajava) and honey melon (Cucumis melo) (Müller et al. 2010). In studies analyzing the volatile constituents of these fruits, γ-decalactone has been implicated as a major component of guava odor, and the skin and pulp of the honey melon contain several γ- and δ-lactones (Grab 1998; Aubert and Pitrat 2006). Taken together, although we recognize that AgOr48 may be part of a larger suite of dedicated ORs that cooperatively discriminate among structurally divergent lactones, it is likely that the lactone specificity of AgOr48 plays a role in the attraction of adult An. gambiae to sugar sources.
AgOr48 displayed high sensitivity (≤1 µM) to nine of the tested lactones, and this may play a significant role in detecting mixtures of lactones from fruit volatiles. In nature, lactone-containing fruit volatiles typically consist of complex odor blends that are comprised of several compound species in distinct ratios, rather than a single lactone (Bernreuther et al. 1989). Olfactory-driven behaviors in insects are often driven by blends rather than single odorants, as observed in field experiments with O. excavata (Tian et al. 2008). Although the moth responded robustly to several individual lactones in electroantennograms, which can be used to assess peripheral signaling, only the mixture of lactones was effective in capturing O. excavate in field traps. Similarly, complex blends of lactones, as well as other odorants, are likely required for An. gambiae in locating a sugar source.
This study extends our characterization of AgOr48 as a lactone receptor, capable of activation by several straight-chain lactones at relatively low concentrations. Although its exact role in An. gambiae chemical ecology is still unknown, we suggest that AgOr48 plays a role in locating lactone-containing sugar sources. Finally, we have investigated the molecular receptive range and proposed a preliminary lactone pharmacophore of AgOr48, which provides further insight into the mechanisms of odorant–OR interaction.
Supplementary material
Supplementary material can be found at http://www.chemse.oxfordjournals.org/
Funding
This work was supported by the National Institutes of Health [AI056402] and the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative [VCTR121] to L.J.Z. G.M.P is supported by the National Institute on Deafness and Other Communication Disorders at the National Institutes of Health through an NRSA F31 [DC011989].
Supplementary Material
Acknowledgements
We thank Dr Robert Taylor for technical assistance with Ca++-imaging experiments, Drs Gray Camp and Pingxi Xu for the generation of the AgOr48 cell line and the initial functional testing. We also thank members of the Zwiebel laboratory for critical discussions regarding this work.
References
- Aubert C, Pitrat M. 2006. Volatile compounds in the skin and pulp of Queen Anne’s pocket melon. J Agric Food Chem. 54: 8177–8182 [DOI] [PubMed] [Google Scholar]
- Benton R, Sachse S, Michnick SW, Vosshall LB. 2006. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 4: e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernreuther A, Christoph N, Schreier P. 1989. Determination of the enantiomeric composition of γ-lactones in complex natural matrices using multidimensional capillary gas chromatography. J Chrom. 481: 363–367 [Google Scholar]
- Bestmann HJ, Cai-Hong W, Dohla B, Li-Kedong, Kaissling KE. 1987. Functional group recognition of pheromone molecules by sensory cells of Antheraea polyphemus and Antheraea pernyi (Lepidoptera: Saturniidae) Z. Naturforsch. 42C: 435–441 [Google Scholar]
- Bohbot JD, Dickens JC. 2009. Characterization of an enantioselective odorant receptor in the yellow fever mosquito Aedes aegypti. PLoS ONE. 4: e7032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AF, Wang G, Su C-Y, Zwiebel LJ, Carlson JR. 2010. Odorant reception in the malaria mosquito Anopheles gambiae Nature. 464: 66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous R, Lizzani-Cuvelier L, Loiseau MA, Sassy E. 1994. Resolution of racemicε-lactones. Tetrahedron: Asymmetry. 5: 343–346 [Google Scholar]
- Foster WA, Takken W. 2004. Nectar-related vs. human-related volatiles: behavioural response and choice by female and male Anopheles gambiae (Diptera: Culicidae) between emergence and first feeding Bull Entomol Res. 94: 145–157 [DOI] [PubMed] [Google Scholar]
- Gillot C. 2005. Entomology. 3rd ed. Springer; Dordrecht, The Netherlands. [Google Scholar]
- Gouagna LC, Poueme RS, Dabiré KR, Ouédraogo JB, Fontenille D, Simard F. 2010. Patterns of sugar feeding and host plant preferences in adult males of An. gambiae (Diptera: Culicidae). J Vector Ecol. 35: 267–276 [DOI] [PubMed] [Google Scholar]
- Grab W. 1998.. Blended flavourings. In: Ziegler E, Ziegler H, editors. Flavourings: production, composition, applications, regulations. Weinheim, Germany: Wiley-VCH; p. 347–386. [Google Scholar]
- Jones PL, Pask GM, Rinker DC, Zwiebel LJ. 2011. Functional agonism of insect odorant receptor ion channels. Proc Natl Acad Sci. 108: 8821–8825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Pask GM, Romaine IM, Taylor RW, Reid PR, Waterson AG, Sulikowski GA, Zwiebel LJ. 2012. Allosteric antagonism of insect odorant receptor ion channels. PLoS ONE. 7: e30304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WD, Nguyen TA, Kloss B, Lee KJ, Vosshall LB. 2005. Functional conservation of an insect odorant receptor gene across 250 million years of evolution. Curr Biol. 15: R119–R121 [DOI] [PubMed] [Google Scholar]
- Kaupp UB. 2010. Olfactory signalling in vertebrates and insects: differences and commonalities. Nat Rev Neurosci. 11: 188–200 [DOI] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. 2004. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 43: 703–714 [DOI] [PubMed] [Google Scholar]
- Laurence BR, Pickett JA. 1982. Erythro-6-acetoxy-5-hexadecanolide, the major component of a mosquito oviposition attractant pheromone. J Chem Soc, Chem Commun. 1: 59–60 [DOI] [PubMed] [Google Scholar]
- Moreira RF, Trugo LC, Pietroluongo M, De Maria CA. 2002. Flavor composition of cashew (Anacardium occidentale) and marmeleiro (Croton species) honeys. J Agric Food Chem. 50: 7616–7621 [DOI] [PubMed] [Google Scholar]
- Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, Doumbia S, Schlein Y. 2010. Field experiments of Anopheles gambiae attraction to local fruits/seedpods and flowering plants in Mali to optimize strategies for malaria vector control in Africa using attractive toxic sugar bait methods. Malar J. 9: 262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Pellegrino M, Sato K, Vosshall LB, Touhara K. 2012. Amino acid residues contributing to function of the heteromeric insect olfactory receptor complex. PLoS ONE. 7: e32372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols AS, Chen S, Luetje CW. 2011. Subunit contributions to insect olfactory receptor function: channel block and odorant recognition. Chem Senses. 36: 781–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pask GM, Jones PL, Rützler M, Rinker DC, Zwiebel LJ. 2011. Heteromeric Anopheline odorant receptors exhibit distinct channel properties. PLoS ONE. 6: e28774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Fox AN, Zwiebel LJ. 2004. A highly conserved candidate chemoreceptor expressed in both olfactory and gustatory tissues in the malaria vector Anopheles gambiae. Proc Natl Acad Sci. 101: 5058–5063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Rinker DC, Jones PL, Rokas A, Zwiebel LJ. 2011. Transcriptome profiling of chemosensory appendages in the malaria vector Anopheles gambiae reveals tissue- and sex-specific signatures of odor coding. BMC Genomics. 12: 271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. 2008. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 452: 1002–1006 [DOI] [PubMed] [Google Scholar]
- Smart R, Kiely A, Beale M, Vargas E, Carraher C, Kralicek AV, Christie DL, Chen C, Newcomb RD, Warr CG. 2008. Drosophila odorant receptors are novel seven transmembrane domain proteins that can signal independently of heterotrimeric G proteins. Insect Biochem Mol Biol. 38: 770–780 [DOI] [PubMed] [Google Scholar]
- Svensson GP, Larsson MC. 2008. Enantiomeric specificity in a pheromone-kairomone system of two threatened saproxylic beetles, Osmoderma eremita and Elater ferrugineus. J Chem Ecol. 34: 189–197 [DOI] [PubMed] [Google Scholar]
- Tian R, Izumi Y, Sonoda S, Yoshida H, Takanashi T, Nakamuta K, Tsumuki H. 2008. Electroantennographic responses and field attraction to peach fruit odors in the fruit-piercing moth, Oraesia excavata (Butler) (Lepidoptera: Noctuidae). Appl Entomol Zool. 43: 265–269 [Google Scholar]
- Wang G, Carey AF, Carlson JR, Zwiebel LJ. 2010. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci. 107: 4418–4423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher D, Schäfer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. 2008. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 452: 1007–1011 [DOI] [PubMed] [Google Scholar]
- Wilson C, Shaw P, Knight R. 1990.. Importance of some lactones and 2, 5-dimethyl-4-hydroxy-3 (2H)-furanone to mango (Mangifera indica L.) aroma. J Agric Food Chem. 38: 1556–1559 [Google Scholar]
- Xia Y, Wang G, Buscariollo D, Pitts RJ, Wenger H, Zwiebel LJ. 2008. The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae. Proc Natl Acad Sci. 105: 6433–6438 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.