Abstract
AbstractOlfactory sensory deprivation during development has been shown to induce significant alterations in the neurophysiology of olfactory receptor neurons (ORNs), the primary sensory inputs to the brain’s olfactory bulb. Deprivation has also been shown to alter the neurochemistry of the adult olfactory system, but the physiological consequences of these changes are poorly understood. Here we used in vivo synaptopHluorin (spH) imaging to visualize odorant-evoked neurotransmitter release from ORNs in adult transgenic mice that underwent 4 weeks of unilateral olfactory deprivation. Deprivation reduced odorant-evoked spH signals compared with sham-occluded mice. Unexpectedly, this reduction was equivalent between ORNs on the open and plugged sides. Changes in odorant selectivity of glomerular subpopulations of ORNs were also observed, but only in ORNs on the open side of deprived mice. These results suggest that naris occlusion in adult mice produces substantial changes in primary olfactory processing which may reflect not only the decrease in olfactory stimulation on the occluded side but also the alteration of response properties on the intact side. We also observed a modest effect of true sham occlusions that included noseplug insertion and removal, suggesting that conventional noseplug techniques may have physiological effects independent of deprivation per se and thus require more careful controls than has been previously appreciated.
Key words: olfactory transduction, optical imaging, sensory deprivation, synaptopHluorin
Introduction
Changes in sensory environment can induce plasticity in both developing and adult sensory systems. Experimentally induced sensory deprivation has been an essential tool to study how overall levels of sensory input shape the structure and function of many different sensory brain regions, especially in sensory cortex (olfactory system: Brunjes 1985; Brunjes et al. 1985; Franks and Isaacson 2005; Kim et al. 2006; visual system: Weisel and Hubel 1965; Kirkwood et al. 1996; auditory system: Chang and Merzenich 2003; Zhou and Merzenich 2007; de Villers-Sidani et al. 2008; somatosensory system: Finnerty et al. 1999; Lendvai et al. 2000). However, only a few studies have investigated the effects of deprivation on the behavior of primary sensory neurons, including photoreceptors during development (Tian and Copenhagen 2001) and olfactory receptor neurons (ORNs) both during development and after brief deprivations in adults (Tyler et al. 2007; He et al. 2012). To date, this work has been restricted to in vitro assessment of synaptic transmission, leaving open the possibility of deprivation-induced changes in transduction of natural stimuli, neuronal firing properties, or other factors. Characterizing any deprivation-induced alterations in the neural response to a stimulus at the level of the primary receptor neurons is a critical step in understanding the observed plasticity of the downstream brain regions working to interpret this modified sensory signal.
In the mammalian olfactory system, odorant molecules bind to metabotropic olfactory receptors expressed in the cilia of ORNs in the nasal epithelium, which depolarizes the cell via a second-messenger cascade and evokes action potentials (Wilson and Mainen 2006). Each ORN expresses only one out of a repertoire of hundreds of receptor types and is thus selective for a subset of potential odorants (Malnic et al. 1999; Bozza et al. 2002; Grosmaitre et al. 2006; Grosmaitre et al. 2009). ORNs expressing the same receptor type project their axons ipsilaterally to the brain’s olfactory bulb, where they converge into 1 or 2 glomeruli on the bulbar surface (Mombaerts 2006). Presentation of an odorant in vivo induces neural activity in an odorant-dependent subset of ORNs (based on receptor expression) and thus drives synaptic input into a corresponding subset of olfactory bulb glomeruli which serves as the primary sensory representation of the odorant in the brain (Sharp et al. 1975; Johnson et al. 1999; Rubin and Katz 1999; Johnson and Leon 2000; Uchida et al. 2000; Bozza et al. 2004; Xu et al. 2003; Igarashi and Mori 2005; Cleland et al. 2007; Soucy et al. 2009). Here we use optical imaging from transgenic mice expressing the fluorescent exocytosis indicator synaptopHluorin in all mature ORNs (spH; Miesenböck et al. 1998; Bozza et al. 2004) to visualize odorant-evoked neurotransmitter release from ORNs into olfactory bulb glomeruli in vivo. Adult mice underwent unilateral naris occlusion using a removable noseplug for 4 weeks, and odorant-evoked neural activity in the olfactory bulb ipsilateral to the occlusion was compared with that of the contralateral bulb and to that in the olfactory bulbs of sham-occluded mice.
Materials and methods
Subjects
Imaging experiments used 17 male and female transgenic mice expressing the spH exocytosis indicator under the control of the olfactory marker protein (OMP) promoter (Bozza et al. 2004). Mice were F1 hybrids resulting from crosses between albino C57BL/6 mice homozygous for spH as previously reported (Czarnecki et al. 2011) with wild-type 129 strain mice, resulting in experimental subjects which were heterozygous for spH and OMP (Bozza et al. 2004). In mice expressing this transgene, odorant-evoked spH signals linearly indicate neurotransmitter release from ORN synaptic terminals (Wachowiak et al. 2005). Additional wild-type female C57BL/6 mice (Charles River Laboratories, Indianapolis, IN) were used in immunohistochemistry experiments to confirm deprivation efficacy. Respiration measurements from reversibly occluded mice were compared with that taken from additional naive wild-type female 129 mice. All subjects were adults between 6 and 15 weeks of age at the onset of use. Throughout experimentation, mice were single-housed in a temperature-controlled environment (22±5 °C) that was maintained on a 12:12h light–dark cycle (7:00 AM–7:00 PM), and food and water were provided ad libitum. All experiments were performed in accordance with protocols approved by the Rutgers University Institutional Animal Care and Use Committee.
Reversible unilateral naris occlusion
Removable noseplugs were constructed in the manner of Cummings et al. (1997) and are described in detail elsewhere (Cummings et al. 1997; Cummings and Brunjes 1997). Briefly, plugs were constructed out of polyethylene (PE) tubing (Becton Dickinson, Sparks, MD), chromic gut suture (MYCO Medical, Cary, NC), and braided silk suture (Surgical Specialties Corp, Reading PA). Plug length was varied (5 or 7mm) to account for differences in animal size, but plug diameter was always the same; the sizes of the PE tubing, chromic gut suture, and silk suture were PE10, 5-0, and 6-0, respectively.
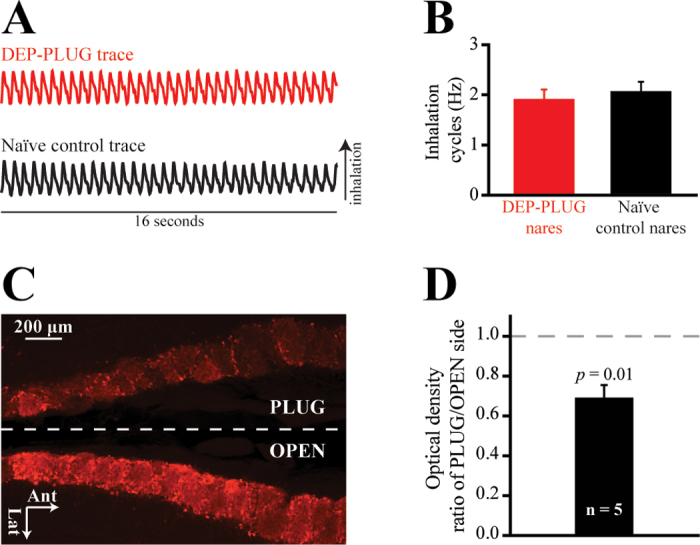
For deprivation (DEP group, N = 12: n = 6 in experiment 1 and n = 6 in experiment 2), adult mice were lightly anesthetized using isoflurane (Baxter Healthcare Corp, Deerfield, IL) and a noseplug was gently inserted into the left or right external naris with curved forceps. Plugged (PLUG) and open (OPEN) sides were counterbalanced across animals. This design permitted within-subjects comparisons between the PLUG and OPEN sides because the nasal septum separates the left and right nasal passages and ORNs project exclusively to the ipsilateral olfactory bulb. After a brief recovery (between 10 and 20min on a heating pad), mice were returned to the colony room in standard cages which were lined with white paper towels. Prior to switching back to standard bedding 2 days after the occlusion procedure, the paper towel lining was checked twice (at 24 and 48h) to determine if the plug had fallen out. After 4 weeks of unilateral naris occlusion, mice were lightly anesthetized using isoflurane and noseplugs were removed with curved forceps. In pilot experiments (data not shown), we observed no response to odorants in bulbs ipsilateral to the noseplug immediately after noseplug removal. Consequently, mice were given a 24-h recovery period after plug removal prior to imaging to ensure the restoration of airflow. As shown in Figure 1A, in a subset of mice the restoration of airflow was confirmed via intranasal thermocouple measurements. Respiration was recorded on the PLUG (re-opened) side in a subset of DEP mice (n = 2) and also on a single side in naive control mice (N = 2). A thermocouple (emtss-010g-12, Omega Engineering, Stamford, CT) was acutely implanted into the nasal bone after the manner of Wesson et al. (2008). Briefly, the sensor was lowered into the naris approximately 3mm anterior to the frontal-nasal fissure and 1mm lateral to the septal bone along the midline. The thermocouple signal was amplified ×1000, low-pass filtered at 1 Hz (model BMA-931, CWE, Inc), digitized at 100 Hz, and saved to a hard drive via Neuroplex software (RedShirtImaging). Respiration was recorded from each deeply anesthetized subject during 4 consecutive 16-s trials and the frequency of inhalations was quantified. As shown in Figure 1B, respiration frequency did not differ (P = 0.655) between deprived nares and unmanipulated control nares.
Figure 1.
Four weeks of unilateral naris occlusion is reversed 24h after plug removal and causes a relative reduction in periglomerular TH expression in the olfactory bulb ipsilateral to the occluded naris. (A) Representative thermocouple traces recorded from a DEP-PLUG airway (top trace, red) and from an airway of a naive control mouse (bottom trace, black). Inflections correspond with inhalations and deflections correspond with exhalations. (B) No difference was observed in the mean (±SEM) inhalation cycles which were recorded from DEP-PLUG nares (red) and naive control nares (black). (C) Representative horizontal section showing the posterior-medial edges of the OPEN and PLUG bulbs. Note that data quantification was performed across the entire bulb and only a portion of this section is displayed. (D) Mean (±SEM) ratio of TH expression on the PLUG side relative to that on the OPEN side.
Sham-occluded mice (SHAM group, N = 5) received identical treatment to occluded mice, including isoflurane anesthesia and insertion of a plug, but the plug was removed immediately after insertion. Sham-occluded mice later received a sham plug removal in which the mice were anesthetized and forceps inserted into the naris as if a plug had been present.
Surgical procedures
The surgery for implanting the cranial window above the dorsal surface of the olfactory bulbs was performed approximately 24h after reversal of unilateral olfactory sensory deprivation. Initially, mice were anesthetized via i.p. administration of a 10mg/mL pentobarbital (Sigma-Aldrich, St. Louis, MO) solution at a volume of 1mL/0.01kg, and additional boosters were administered as needed to maintain deep anesthesia throughout the duration of the imaging procedures. Between 0.3 and 0.5mL of 0.25% bupivacaine, HCl (Sigma-Aldrich, St. Louis, MO) was administered s.c. along the scalp to provide local anesthesia (varied based on animal size) and 0.1% atropine sulfate (Sigma-Aldrich, St. Louis, MO) was administered s.c. at a volume of 1mL/0.01kg to reduce mucous secretions. In addition, to reduce any inflammation in the re-opened naris which may have resulted from the plug removal procedure, up to 1mL/0.01kg of a 2mg/mL dexamethasone solution (VEDCO Inc, St. Joseph, MO) was administered s.c. Although the animals were anesthetized, body temperature was monitored via rectal probe thermometry and was maintained at 38±0.5 °C via a feedback-regulated heating pad (TC-1000, CWE Inc, Ardmore, PA). As previously reported (Czarnecki et al. 2011), the scalp was shaved, wiped down with a topical antiseptic bactericide (betadine solution), and then surgically removed. After preparing the skull by scraping and peeling back the periosteal membrane and then washing it with a 70% ethanol solution, the head was mounted in a custom head mount and the skull was fixed to a head bar using dental acrylic. The bone overlying both olfactory bulbs was thinned with a hand-held dental drill and became translucent when Ringer’s solution, containing 140mM NaCl, 5mM KCl, 1mM CaCl2, 1mM MgCl2, 10mM HEPES, and 10mM dextrose, was applied. A custom-sized glass cover slip was placed on top of the window and the preparation was moved from the stereomicroscope (Leica MZ12) to the imaging apparatus.
Optical recordings and imaging analysis
Odorant-evoked ORN synaptic output into glomeruli on the dorsal surface of the olfactory bulbs was imaged in OMP-spH mice, as previously described (Czarnecki et al. 2011; Czarnecki et al. 2012). Briefly, spH signals were recorded in vivo with a custom imaging apparatus using fluorescence epi-illumination on an Olympus BX51 microscope with a ×4 (0.28 NA) objective. Illumination was provided by either a 150W Xenon arc lamp (Optosource lamphouse, Cairn Research Ltd, Kent, UK) or a bright light-emitting diode (470nm wavelength, Thorlabs, Newton, NJ). SpH signals were visualized with a filter set containing HQ480/40 excitation, Q505LP dichroic, and HQ535/50 emission filters. Optical signals were acquired via a back-illuminated, monochrome CCD camera (NeuroCCD, SM-256, RedShirtImaging, Decatur, GA) at a pixel resolution and frame acquisition rate of 256×256 and 7 Hz, respectively. Data acquisition and shutter control were performed using Neuroplex software (RedShirtImaging).
A custom 8 channel, air dilution olfactometer was used to present a panel of up to 4 odorants known to activate glomeruli on the dorsal surface of the olfactory bulbs. Separate lines were used to avoid cross-contamination between odorants. All odorants, including n-butyl acetate (BA), methyl valerate (MV), 2-hexanone (2HEX), and trans-2-methyl-2-butenal (2M2B), were at 95−99% purity (Sigma-Aldrich, St. Louis, MO) and were diluted to a desired concentration in clean air by passing a nitrogen stream through a vial containing a single odorant. User-defined odorant dilution was performed using a mass flow controller (Aalborg, Orangeburg, NY) operating through custom software written for MatLab (Mathworks, Natick, MA). A photoionization detector (ppbRAE Plus, RAE Systems) was used to standardize odorant concentrations across all subjects. For the first imaging experiment (N = 11: SHAM group, n = 5; DEP group, n = 6), where 4 odorants were delivered at up to 3 concentrations between 0.5% and 6%, concentration is expressed as percent dilution of saturated vapor (Figures 2, 3, and 5), and for the second imaging experiment (N = 6, DEP group only), where BA was delivered at 9 concentrations spanning an almost 300-fold range, concentration is expressed in arbitrary units (a.u., Figure 4). The odorant delivery manifold was positioned approximately 2cm in front of the mouse’s nose and odorants were presented during blocks of 4−8 trials (6-s trial, minimum 60-s intertrial interval).
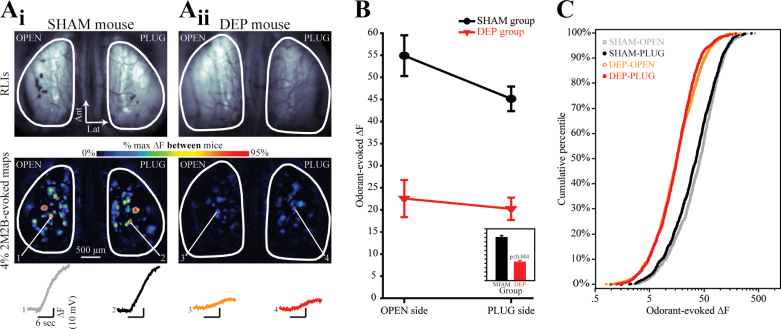
Figure 2.
The magnitude of odorant-evoked nerve output is suppressed equally in both the OPEN and PLUG sides of DEP mice, as compared with the OPEN and PLUG sides of SHAM controls. (A) Resting light images (RLIs) of the dorsal olfactory bulbs through the cranial window (top row) and pseudocolored difference maps (bottom row) from a SHAM mouse (A i) and a DEP mouse (A ii). RLIs are scaled individually to control for differences in windows. Numbered callouts indicate traces showing the change in fluorescence in the corresponding glomerulus which was evoked by a 6-s presentation of 4% 2M2B. (B) Mean (±SEM) odorant-evoked change in fluorescence (∆F) plotted as a function of side for SHAM and DEP groups. The inset displays the main effect of group and is scaled to the same y-axis. (C) The four distributions (separated by group and side) of observed ∆F values are shown as cumulative probability plots.
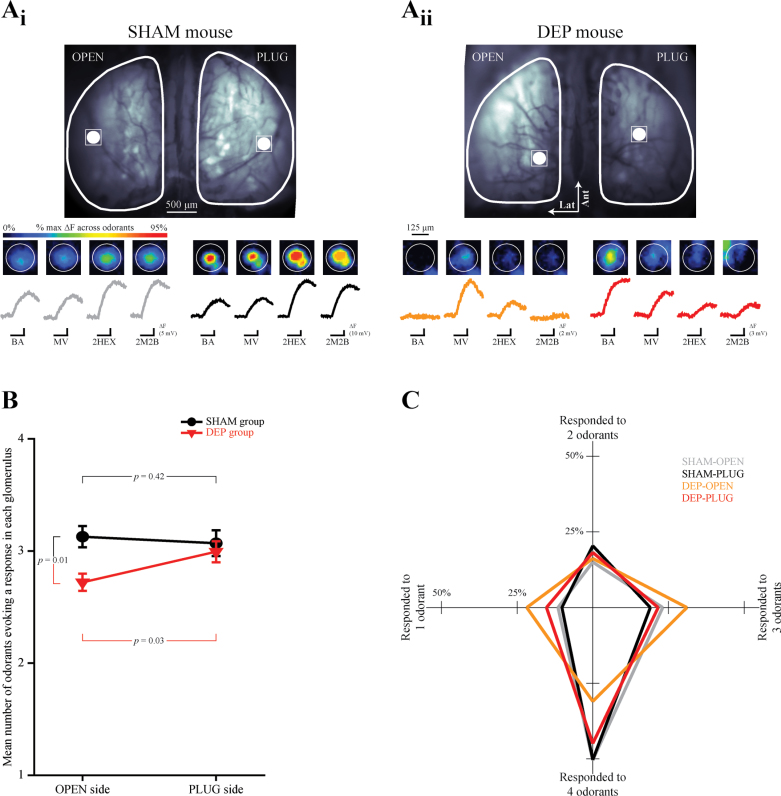
Figure 5.
Glomerular response selectivity in the OPEN side of DEP mice is increased. (A) A single glomerulus’ location is identified on the OPEN and PLUG sides in the resting fluorescence images from SHAM (A i) and DEP (A ii) mice and magnified in the pseudocolored images which show 4 odorant-evoked responses. Black scale bars indicate the 6-s odorant presentation corresponding to each trace. Each set of 4 traces is scaled relative to the maximum evoked amplitude across odorants, as indicated by the evoked fluorescence (ΔF) scale bars for each trace set. (B) The mean (±SEM) number of odorants responded to (min = 1, max = 4) plotted as a function of side for SHAM and DEP groups. (C) The percent of glomeruli from OPEN and PLUG sides of SHAM and DEP groups which responded to 1, 2, 3, or 4 odorants. The shape of each plot represents the overall response selectivity of each population of responding glomeruli.
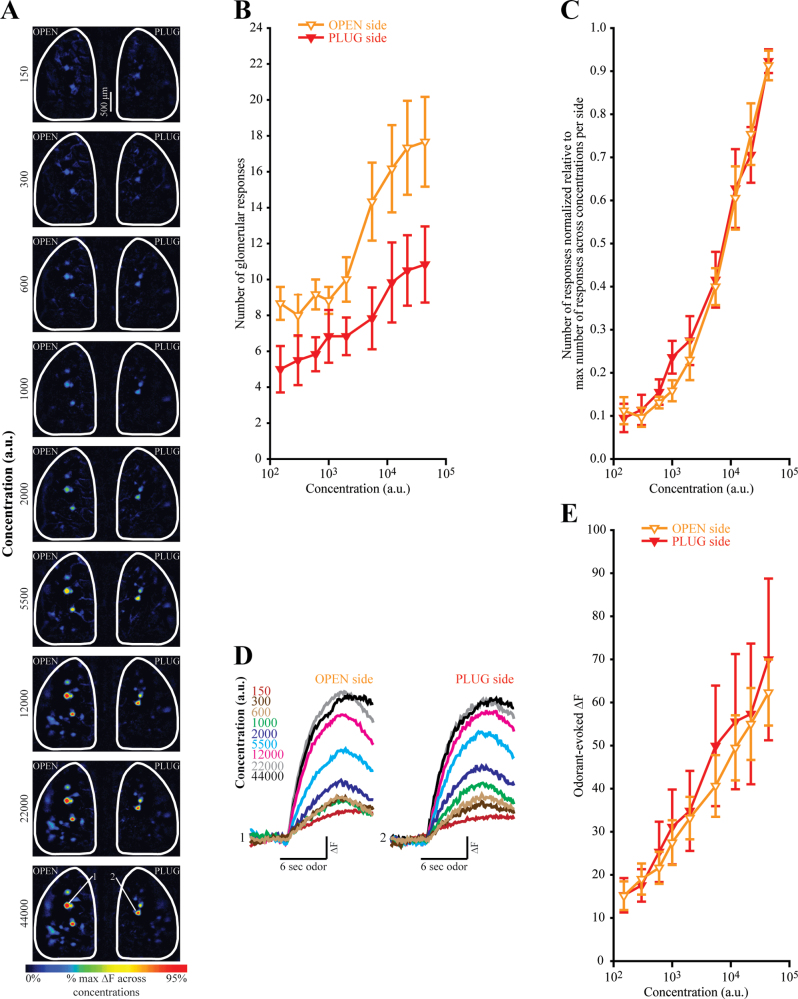
Figure 4.
(A) Pseudocolored difference maps evoked by an almost 300-fold range of BA concentrations (shown in arbitrary units, a.u.) in a DEP mouse. (B) Absolute number of BA-evoked responses plotted as a function of concentration for the OPEN and PLUG sides of DEP mice. (C) Normalized number of responses plotted as a function concentration. Data are normalized relative to the maximum number of BA-evoked responses across all 9 concentrations within each olfactory bulb of each mouse. (D) Sets of traces correspond to 2 glomeruli shown across 9 concentrations in (A) with the callouts indicated in the bottom map. Response amplitudes from both glomeruli are scaled to the same maximum. (E) Odorant-evoked change in fluorescence (∆F) plotted as a function of concentration for the OPEN and PLUG sides of DEP mice. All data are displayed as the mean (±SEM).
Fluorescence imaging data were analyzed as described previously (Czarnecki et al. 2011). Briefly, 4−8 trials were averaged to improve the signal-to-noise ratio of the data. To correct for photobleaching, blank (no-odorant) trials were subtracted from odorant trials. To measure odorant-evoked spH signals for traces from each pixel overlying an identified glomerulus, the average fluorescence of 15 frames immediately prior to odorant onset were subtracted from the average fluorescence of 15 frames centered above the peak inflection. All data were high-pass filtered with a Gaussian spatial filter through software written for MatLab (Mathworks, Natick, MA). Glomerular responses were first-hand selected and then confirmed statistically; when a glomerulus’ average response to an odorant was more than 3 standard errors (SEs) greater than 0, it was counted as a response.
Statistical analysis
All data were processed in Neuroplex and Matlab and then exported to SPSS 17.0 for statistical analysis and to SigmaPlot 11.0 and STATVIEW for graphing. Data from the first imaging experiment (shown in Figures 2, 3, and 5) were pooled across odorants and concentrations. These data were then analyzed via mixed-model analysis of variances (ANOVAs; with side as a within-subjects factor and group as a between-subjects factor) and appropriate post-hoc tests to evaluate measures of central tendency. The data were also analyzed with the nonparametric Kolmogorov-Smirnov (K-S) and Mann–Whitney (M-W) U tests to evaluate overall distributions. Data from the second imaging experiment (shown in Figure 4) were analyzed via 2-way repeated-measures ANOVAs (with side and concentration as within-subjects factors) and t-tests for planned post-hoc comparisons. The overall α value was set to 0.05 for all analyses and, where applicable, was Bonferroni-corrected to compensate for the number of pairwise comparisons, as noted below.
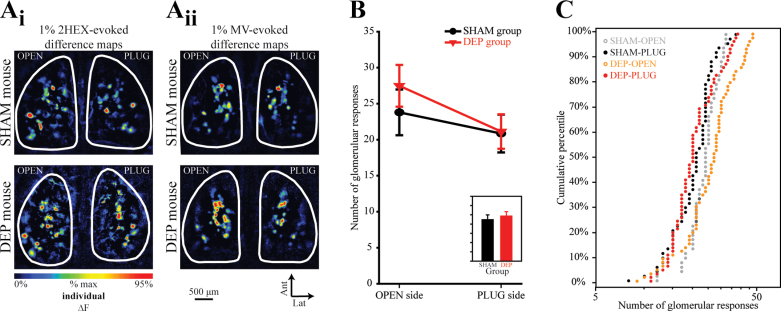
Figure 3.
The number of glomeruli receiving measurable ORN input increases in the OPEN side of DEP mice. (A) Pseudocolored difference maps evoked by 1% 2HEX (A i) and 1% MV (A ii) in SHAM mice (top row) and DEP mice (bottom row). (B) The mean (±SEM) number of odorant-evoked responses plotted as a function of side for SHAM and DEP groups. The inset displays the main effect of group (P > 0.05, n.s.) and is scaled to the same y-axis. (C) The 4 distributions (separated by group and side) of observed response numbers are shown as cumulative probability plots.
Histological procedures and analysis
Adult olfactory deprivation causes a reduction in periglomerular tyrosine hydroxylase (TH) expression (Baker et al. 1993). To validate the deprivation technique used here, TH expression was assessed via immunohistochemistry in periglomerular interneurons of PLUG and OPEN bulbs from DEP mice. Olfactory bulb histology and immunohistochemistry were performed as previously reported (Moberly et al. 2012). Briefly, mice were intracardially perfused with 0.1M phosphate-buffered saline (PBS) solution followed by 4% paraformaldehyde either following imaging procedures or following plug removal procedures (no differences were observed between these time points). Brains were removed, postfixed in 4% paraformaldehyde, and then transferred to PBS for a minimum of 24h prior to sectioning. Both olfactory bulbs were sectioned horizontally on a Vibratome at 50 µm. Sections were incubated in solutions containing primary antibody against TH (a 1:1000 dilution of rabbit antimouse TH, Millipore, #AB152) and 10 µg/mL of fluorophore-tagged secondary antibody (goat antirabbit IgG H + L conjugated to AlexaFluor568, Invitrogen, #A-11011). Sections were mounted on glass slides in a ProLong Gold antifade reagent (Invitrogen) containing 4′,6-diamidino-2-phenylindole (DAPI, nuclear counterstain).
Images were acquired at 4× magnification via an Olympus BX series fluorescence microscope with a Jenoptik MFcool CCD camera and were then analyzed using ImageJ software. DAPI fluorescence was first used to identify regions of interest. The optical density of identified regions was then quantified in the corre sponding AlexaFluor568 fluorescent image. Experimenters were blind while performing all histological procedures and quantifications.
Periglomerular TH immunoreactivity was reduced by reversible, unilateral naris occlusion (Figure 1C), consistent with earlier studies using the same (Cummings et al. 1997) or alternative (Baker et al. 1993; Cho et al. 1996) methods of occlusion. Relative to contralateral (OPEN) bulbs, there was a 31% (±7%) reduction in TH expression in ipsilateral (PLUG) bulbs (one-sample t-test, t df = 4, = −4.554, P = 0.01), as shown in Figure 1D. Thus, the efficacy of the occlusion method used here is comparable with that of methods used in earlier research (Baker et al. 1993; Cummings et al. 1997).
Results
Unilateral sensory deprivation reduces the magnitude of odorant-evoked synaptic input from ORNs to olfactory bulb glomeruli on both plugged and open sides equally
To evaluate the physiological consequences of olfactory sensory deprivation, 11 OMP-spH mice were randomly assigned to undergo either a reversible unilateral deprivation via a noseplug inserted into the external naris (DEP group, n = 6) or a sham procedure in which the plug was inserted but then immediately removed (SHAM group, n = 5). After 4 weeks of deprivation, noseplug removal was performed (DEP group) or simulated (SHAM group). The following day, each mouse was anesthetized and neurotransmitter release from ORNs into olfactory bulb glomeruli was visualized via optical imaging of spH signals bilaterally through an implanted cranial window. Up to 4 odorants were presented at up to 3 concentrations each during this imaging experiment. For each mouse, the odorant-evoked increase in fluorescence was calculated for each glomerulus based on difference maps of the baseline fluorescence subtracted from the fluorescence following odorant presentation (Figure 2A). These spH responses, which indicate the total neurotransmitter release from ORNs during odorant presentation, were analyzed by mixed-model ANOVA.
As shown in Figure 2, there was a large main effect of group such that the observed spH signals were about half as large in DEP mice as in SHAM mice (F 1,9 = 78.30; P < 0.001; η p 2 = 0.897, Figure 2B, inset). Surprisingly, there was no main effect of side (F 1,9 = 2.28; P = 0.165; η p 2 = 0.202) and no significant side by group interaction (F 1,9 = 0.86; P = 0.087; η p 2 = 0.087) as shown in Figure 2B. Following up on this analysis, the overall distributions of response amplitudes in each group were compared using cumulative frequency histograms and nonparametric tests (Figure 2C). For both PLUG and OPEN olfactory bulbs, glomerular responses observed in SHAM mice were larger than those in corresponding bulbs of DEP mice across the full range of response amplitudes as shown in Figure 2C (α = 0.0125 for the following comparisons: PLUG sides, K-S, Z = 9.15, P < 0.001 and M-W U, Z = 19.543, P < 0.001; OPEN sides, K-S Z = 10.795, P < 0.001 and M-W U, Z = 23.599, P < 0.001). Within the DEP group, the distribution of response amplitudes was not different between the PLUG and OPEN sides (K-S, Z = 1.593, P = 0.013 and M-W U, Z = 0.826, P = 0.409; α = 0.0125). Within the SHAM group, the distribution of responses on the PLUG side was slightly but significantly shifted toward smaller responses across the distribution (K-S, Z = 2.101, P < 0.001 and M-W U, Z = 3.697, P < 0.001; α = 0.0125). This difference presumably shows an effect of the sham plug removal, which includes brief anesthesia and the insertion of forceps deep into the nasal passages on the PLUG side.
Unilateral sensory deprivation increases the number of olfactory bulb glomeruli receiving odorant-evoked synaptic input from ORNs contralateral to the noseplug
For each olfactory bulb, the number of glomeruli receiving detectable synaptic input from the olfactory nerve was quantified for each odorant presented in the experiment described above, and these measurements were analyzed by ANOVA as above. As shown in Figure 3B (inset), there was no significant main effect of group (F 1,9 = 0.31, P = 0.589, η p 2 = 0.034) or significant group by side interaction (F 1,9 = 0.975; P = 0.349; η p 2 = 0.098), but there was a significant main effect of side (F 1,9 = 7.24; P = 0.025, η p 2 = 0.446). To follow up on this analysis, we compared the distributions of the numbers of glomeruli receiving odorant-evoked ORN input across groups, pooling across odorants and concentrations (Figure 3C). The number of glomeruli receiving measurable input during our odorant panel on the PLUG side of DEP mice was identical to that on the PLUG side of SHAM mice (Figure 3A and 3B; K-S, Z = 0.88, P = 0.417 and M-W U, Z = 0.44, P = 0.663; α = 0.0125), suggesting that deprivation did not affect the number of responsive glomeruli on the PLUG side. As shown in Figure 3A and 3B, comparing between sides in DEP mice (note that this is a within-subjects comparison), the number of glomeruli receiving measurable input during our odorant panel was clearly greater on the OPEN side than the PLUG side (K-S, Z = 2.25, P < 0.001 and M-W U, Z = 3.92, P < 0.001; α = 0.0125). Comparing the distribution of responses between the OPEN bulbs in DEP and SHAM mice (Figure 3C, orange vs. gray), the OPEN bulbs in DEP mice consistently exhibit more glomeruli receiving input in the top half of the distribution, which corresponds to trials on which larger numbers of glomerular responses were evoked (for overall distribution: K-S, Z = 1.37, P = 0.046; M-W U, Z = 1.97, P = 0.049; α = 0.0125). These data suggested that 1) deprivation does not affect the number of glomeruli receiving odorant-evoked synaptic input from ORNs in olfactory-deprived or sham-deprived olfactory bulbs, 2) that deprivation could cause an increase in the number of responsive glomeruli in olfactory bulbs on the OPEN side, and 3) that rather than being an increase of fixed size (which would have shifted the entire distribution towards larger numbers of responses), the increase could be proportional to the number of glomeruli responding.
To test the hypothesis that deprivation induces a proportional increase in the number of glomeruli that respond to an odorant in the OPEN side, we repeated the experiment using a single odorant over a broad range of concentrations. Because additional glomeruli are recruited into the odorant representation at higher concentrations (Malnic et al. 1999; Rubin and Katz 1999; Meister and Bonhoeffer 2001; Spors and Grinvald 2002), this experiment permitted the comparison of the effects of deprivation on the number of activated glomeruli across a range of total response numbers.
Six additional OMP-spH mice were naris occluded for 4 weeks and then (24h after plug removal) underwent a bilateral imaging session in which ORN inputs to the bulb were evoked by 9 different concentrations of BA over an almost 300-fold range (Figure 4), as described above. As expected, there was a significant main effect of concentration (F 8,40 = 22.12, P < 0.001, η p 2 = 0.816) such that the absolute number of glomerular responses increased across concentrations (Figure 4B). There was no main effect of side (F 1,5 = 3.306, P = 0.129, η p 2 = 0.398). Importantly, there was a significant concentration × side interaction (F 8,40 = 3.45, P = 0.004, η p 2 = 0.408). This confirms the previous finding that the difference in number of evoked responses between OPEN and PLUG sides is greatest when larger numbers of glomeruli are responding.
Notably, the size of the increase seemed proportional to the absolute number of responses across concentrations (Figure 4B). To test this proportionality statistically, data were normalized to the maximum number of glomeruli to respond to any odorant concentration within each olfactory bulb (Figure 4C). If the increase is indeed proportional, this normalization should equalize the number of responses between OPEN and PLUG bulbs. As shown in Figure 4C, the normalized concentration-response curves overlap almost perfectly. Normalization eliminates the statistical interaction between side and concentration (F 8,40 = 0.818, P = 0.592, η p 2 = 0.141) while preserving the main effect of concentration (F 8,40 = 69.135, P < 0.001, η p 2 = 0.933), as predicted. Side continues to have no significant main effect (F 1,5 = 0.468, P = 0.524, η p 2 = 0.086).
The magnitudes of glomerular responses were also measured in this experiment and were comparable between OPEN and PLUG bulbs at all concentrations tested (Figure 4D and 4E), consistent with the previous results (Figure 2). There was no main effect of side (F 1,5 = 0.241,P = 0.644, η p 2 = 0.046) nor a significant interaction of deprivation state with concentration (F 8,40 = 0.586, P = 0.783, η p 2 = 0.105). The main effect of concentration was of course significant (F 8,40 = 12.318, P < 0.001, η p 2 = 0.711).
Unilateral deprivation increases the odorant-response selectivity of ORN populations contralateral to the noseplug
The population of ORNs expressing a given odorant receptor (and projecting to an individual glomerulus in the olfactory bulb) typically responds to a range of odorants determined by the receptor identity (Zhao et al. 1998; Malnic et al. 1999; Bozza et al. 2002; Grosmaitre et al. 2006). Conversely, a given odorant typically activates a range of ORN populations and drives input to multiple olfactory bulb glomeruli in a concentration-dependent manner (see, e.g., Figure 4). The change in the number of glomeruli responding to a given odorant in olfactory bulbs on the OPEN side thus raises the question of whether the ORNs in these bulbs might have an altered odorant-response profile. To test this hypothesis (as presented in Figure 5), we examined the odorant selectivity of individual glomeruli in the olfactory bulbs of SHAM and DEP mice across the 4 odorants presented in the experiment in Figures 2 and 3.
Each individual responding glomerulus was categorized as responding to 1, 2, 3, or 4 odorants in the panel based on the statistical criterion of an average increase in fluorescence at least 3 standard errors above 0 across trials. Then, the values (ranging from 1 to 4) from individual glomeruli were averaged together for each olfactory bulb (N = 20) of each mouse (N = 10). As shown in Figure 5B, this measure of selectivity (number of odorants evoking a response in each glomerulus) was then analyzed with a 2-way mixed ANOVA, with group (DEP or SHAM) and side (PLUG or OPEN) as factors, which revealed a significant group by side interaction (F 1,8 = 7.325, P = 0.027, η p 2 = 0.478). Post-hoc comparison revealed that glomeruli in the olfactory bulb on the OPEN side of deprived mice responded to significantly fewer odorants than glomeruli on the DEP side of the same animals (paired t-test, t df = 5 = −3.046, P = 0.029) and also responded to significantly fewer odorants than glomeruli on the OPEN side of SHAM mice (independent-groups t-test, t df = 8 = 3.327, P = 0.01). Interestingly, response selectivity of glomeruli on the PLUG side of SHAM control mice did not differ from that of the PLUG sides of the DEP group (independent-groups t-test, t df = 8 = 0.51, P = 0.624) or from the OPEN sides in the same (SHAM) mice (paired t-test, t df = 3 = 0.342, P = 0.418).
To more richly display these results, the distributions of glomerular selectivities are plotted in Figure 5C such that for each side by group population, the percentage of glomeruli that responded to 1, 2, 3, or 4 odorants is depicted as the distance from a common origin. The shapes of the resulting plots reflect their distributions of odorant selectivities. Note that the plot for the DEP-OPEN bulbs is notably skewed towards fewer odorants, reflecting an increase in selectivity.
Discussion
In this study, we found that 4 weeks of unilateral olfactory deprivation during adulthood altered the primary neural responses to odorants after naris reopening in mice. The magnitude of total odorant-evoked ORN synaptic input to olfactory bulb glomeruli was greatly reduced by deprivation, but surprisingly this reduction was comparable between the olfactory bulb on the plugged side and on the open side across odorants and concentrations. Despite the smaller response magnitudes, the number of glomeruli receiving detectable ORN synaptic input was not different between sensory-deprived and sham-deprived olfactory bulbs. However, in mice that underwent deprivation, the olfactory bulb contralateral to the plug exhibited a proportional increase in the number of glomeruli receiving detectable odorant-evoked synaptic input from ORNs across a broad range of concentrations. These glomeruli also exhibited a change in their odorant-response profile such that they were more odorant-selective than their counterparts on the plugged side.
The present in vivo results provide important physiological context to the wealth of literature on the neurochemical, morphological, behavioral, and in vitro consequences of olfactory sensory deprivation. Previous reports have revealed a cascade of seemingly compensatory responses observed in adult ORNs following early postnatal naris occlusion. Adenylate cyclase type III and phosphodiesterase type IV are upregulated in ORNs ipsilateral to the occlusion (Coppola et al. 2006) in a manner that suggests a compensatory increase in the gain of olfactory transduction, while epithelial electroolfactograms (Waggener and Coppola 2007) and recordings from individual ORNs reveal an increase in the amplitude of population-level neural responses to odorants ipsilateral to deprivation (He et al. 2012). ORN terminals exhibit an increase in both the probability of release and quantal content in an olfactory bulb slice preparation from both neonatally occluded and briefly deprived adults rats (Tyler et al. 2007), and odorant-evoked uptake of 2-deoxyglucose is enhanced in the olfactory bulb glomeruli of neonatally occluded rats (Guthrie et al. 1990). These results have been interpreted as evidence of homeostatic plasticity such that neural responses are enhanced to compensate for the reduction in primary sensory input (Coppola 2012; Coppola and Waggener 2012). However, the present results indicate that in adult mice, a period of deprivation substantially reduces the amplitude of odorant-evoked ORN synaptic input to the brain (Figure 2). This suggests that the compensatory responses observed in neonatally occluded mice may be unique to early development and not indicative of adult function. That said, previous reports have shown decreases in the odorant selectivity of mitral and tufted cells which might reflect changes in intrabulbar circuitry after 60 days of naris occlusion in young adult rats (Wilson and Sullivan 1995), suggesting that compensatory responses may still occur downstream of the ORNs (Saghatelyan et al. 2005).
Naris occlusion is often viewed as the olfactory analog of visual deprivation via eyelid suture (Weisel and Hubel 1965). However, the two techniques are not strictly equivalent because naris occlusion forces all nasal airflow to pass through the contralateral nasal passage while closing one eyelid does not change the light levels in the contralateral eye. In light of the recent discovery that some ORNs are mechanosensitive (Grosmaitre et al. 2007), naris occlusion is potentially confounded a priori by the elimination of airflow (in addition to olfactory stimuli) on the occluded side and by the presumed increase in airflow on the contralateral side. The present results demonstrate that unilateral naris occlusion via noseplug had large effects on both olfactory bulbs. This confirms that the bulb on the open side can be affected by occlusion just as much as the bulb on the plugged side.
With neonatal deprivation, the expression of OMP and transduction proteins such as adenylate cyclase III in ORNs increases on the closed side and decreases on the open side (Coppola 2012), which would likely lead to an increase in ORN sensitivity to odorants (Lee et al. 2011). However, the present data do not exhibit corresponding changes in the ORN response to odorants, instead showing a comparable decrease in odorant-evoked ORN response amplitudes on both sides (Figure 2). As noted above, it is possible that mice deprived in adulthood do not exhibit the same changes in protein expression or perhaps that these effects are counteracted in vivo by changes in other aspects of the system such as energy metabolism or mucus composition. It is intriguing but unexpected that we observed a large decrease in spH response amplitude in DEP-OPEN bulbs. The constant airflow through the nasal passages on the opened side may have caused this reduction through either an adaptive downregulation of ORN responsivity in reaction to the chronic odorant exposure or possibly adaptation of mechanosensitive ORNs (Grosmaitre et al. 2007) in reaction to the chronic stimulation. Alternatively, because the spH signals reflect total ORN neurotransmitter release throughout the entire 6-s odorant presentation, it is possible that this reduction reflected a more rapid or more complete adaptation to the odorant rather than a decrease in peak spike rate.
The reduction in odorant-evoked response amplitude and modest increase in odorant selectivity in ORNs in DEP-OPEN bulbs is consistent with the effects of odorant exposure (Buonviso and Chaput 2000; Mandairon and Linster 2009). Brief odorant exposures can increase the selectivity of mitral cells (Fletcher and Wilson 2003) or shift the pattern of some mitral cell responses from increases in firing rate to decreases in firing rate (Buonviso and Chaput 2000). The present results thus suggest that the effects observed in DEP-OPEN bulbs may reflect increased exposure to odors caused by forcing all airflow through that side.
The proportional increase in the number of glomeruli responding to an odorant observed in olfactory bulbs contralateral to the noseplug (Figure 4) is unexpected and in contrast to the increased odorant selectivity of responsive glomeruli (Figure 5). This seeming contradiction can potentially be explained by differential effects across subpopulations of ORNs (Cavallin et al. 2010), some of which become more selective (presumably as a result of increased odorant exposure; Buonviso and Chaput 2000) and others of which begin to respond broadly across odorants. One model for the latter effect would be the presence of some number of broadly tuned ORN populations (such as SR1-expressing ORNs, see Grosmaitre et al. 2009) in DEP-OPEN sides that begin responding to odorants after the 1-month deprivation period. Such an effect could occur through activity-dependent alterations in inhibitory bulbar circuitry (Parrish-Aungst et al. 2011; Lau and Murthy 2012) or through the relief of tonic presynaptic inhibition that unmasks previously subthreshold responses in some glomeruli but not others (McGann et al. 2005; Pírez and Wachowiak 2008). Alternatively, the endogenous turnover of ORNs over the 1-month occlusion period could permit changes in odorant-receptor expression. For instance, olfactory deprivation can cause individual ORNs to express more than one odorant receptor (Tian and Ma 2008), which would be expected to increase the range of odorants a given ORN population responds to, especially if different ORNs within a given glomerulus select different secondary receptors. Second, olfactory deprivation has been shown to reduce the pruning of ORN projections to “incorrect” glomeruli during development (Zou et al. 2004). On the timescale assessed here, it is thus possible that the increase in the number of glomerular responses reflects the addition of “miswired” adult-born ORNs which are not pruned away during the deprivation period. Finally, it is possible that the increase in the number of glomeruli receiving odorant-evoked ORN input that we observed in DEP-OPEN bulbs is caused by the interactive effects of odorant deprivation and exposure. Specifically, deprivation increases proliferation in the olfactory epithelium on the open side (Suh et al. 2006) and long-term odorant exposure can increase the number of supernumerary glomeruli (Valle-Leija et al. 2012). Thus, the increase in adult-born ORNs on the open side together with the increased odorant exposure on that side may give rise to glomerular maps which integrate additional odorant-specific glomeruli.
OMP expression is developmentally regulated such that it is expressed only in mature ORNs (Graziadei et al. 1980), at which point it can convey an increase in odorant selectivity (Lee et al. 2011). After neonatal olfactory deprivation, OMP expression is reduced in the olfactory epithelium on the side opposite the occlusion (Coppola et al. 2006), although it is not clear if this represents a downregulation of OMP expression within mature ORNs or the addition of a population of immature, potentially less odorant-selective ORNs. If such a decrease occurred in our adult-deprived mice, it could potentially explain the increase in the number of glomeruli (ORN populations) stimulated by a given odorant in DEP-OPEN bulbs (Figures 3 and 4).
The results in Figure 2 demonstrate the importance of careful sham controls in naris occlusion studies. The majority of naris occlusion studies use control animals which are either unmanipulated (e.g., Cummings and Belluscio 2010) or have received sham occlusions on the outside of the snout (e.g., Wilson and Sullivan 1995) which do not actually affect the nasal passages. Such designs do not control for potential irritation or inflammation associated with the occlusion itself, independent of olfactory deprivation per se. However, here we find modest but significant effects of the noseplug insertion and removal in our sham mice, which had a noseplug actually inserted and then immediately removed during the initial “occlusion” and then experienced a simulated removal in which forceps were inserted into the nasal passages in a manner comparable with the occluded mice. This difference suggests that conventional techniques for the mechanical occlusion of the olfactory system may have confounding effects besides mere removal of sensory stimuli.
The present results illustrate that experience-dependent plasticity can produce substantial changes in sensory codes as early as the synaptic output of the receptor neurons themselves. In other sensory systems, the primary sensory neurons are less experimentally accessible in vivo, and investigations of experience-dependent plasticity have been largely confined to downstream processing, especially in sensory cortices. The current findings suggest that primary sensory neurons can change in complex and stimulus-selective ways (beyond just stronger-weaker) that could be essential to interpreting subsequent sensory information processing.
Funding
This work was supported by the National Institute on Deafness and other communication disorders at the National Institutes of Health [R00 DC009442 to J.P.M.].
Acknowledgements
We thank Daniel Wesson for sharing his protocols for noseplug construction and intranasal thermocouple implantation.
References
- Baker H, Morel K, Stone DM, Maruniak JA. 1993. Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res. 614(1–2):109–116 [DOI] [PubMed] [Google Scholar]
- Bozza T, Feinstein P, Zheng C, Mombaerts P. 2002. Odorant receptor expression defines functional units in the mouse olfactory system. J Neurosci. 22(8):3033–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza T, McGann JP, Mombaerts P, Wachowiak M. 2004. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 42(1):9–21 [DOI] [PubMed] [Google Scholar]
- Brunjes PC. 1985. Unilateral odor deprivation: time course of changes in laminar volume. Brain Res Bull. 14(3):233–237 [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Smith-Crafts LK, McCarty R. 1985. Unilateral odor deprivation: effects on the development of olfactory bulb catecholamines and behavior. Brain Res. 354(1):1–6 [DOI] [PubMed] [Google Scholar]
- Buonviso N, Chaput M. 2000. Olfactory experience decreases responsiveness of the olfactory bulb in the adult rat. Neuroscience. 95(2):325–332 [DOI] [PubMed] [Google Scholar]
- Cavallin MA, Powell K, Biju KC, Fadool DA. 2010. State-dependent sculpting of olfactory sensory neurons is attributed to sensory enrichment, odor deprivation, and aging. Neurosci Lett. 483(2):90–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. 2003. Environmental noise retards auditory cortical development. Science. 300(5618):498–502 [DOI] [PubMed] [Google Scholar]
- Cho JY, Min N, Franzen L, Baker H. 1996. Rapid down-regulation of tyrosine hydroxylase expression in the olfactory bulb of naris-occluded adult rats. J Comp Neurol. 369(2):264–276 [DOI] [PubMed] [Google Scholar]
- Cleland TA, Johnson BA, Leon M, Linster C. 2007. Relational representation in the olfactory system. Proc Natl Acad Sci USA. 104(6):1953–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola DM. 2012. Studies of olfactory system neural plasticity: the contribution of the unilateral naris occlusion technique. Neural Plast. 2012: 351752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola DM, Waggener CT. 2012. The effects of unilateral naris occlusion on gene expression profiles in mouse olfactory mucosa. J Mol Neurosci. 47(3):604–618 [DOI] [PubMed] [Google Scholar]
- Coppola DM, Waguespack AM, Reems MR, Butman ML, Cherry JA. 2006. Naris occlusion alters transductory protein immunoreactivity in olfactory epithelium. Histol Histopathol. 21(5):487–501 [DOI] [PubMed] [Google Scholar]
- Cummings DM, Belluscio L. 2010. Continuous neural plasticity in the olfactory intrabulbar circuitry. J Neurosci. 30(27):9172–9180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DM, Brunjes PC. 1997. The effects of variable periods of functional deprivation on olfactory bulb development in rats. Exp Neurol. 148(1):360–366 [DOI] [PubMed] [Google Scholar]
- Cummings DM, Henning HE, Brunjes PC. 1997. Olfactory bulb recovery after early sensory deprivation. J Neurosci. 17(19):7433–7440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki LA, Moberly AH, Rubinstein T, Turkel DJ, Pottackal J, McGann JP. 2011. In vivo visualization of olfactory pathophysiology induced by intranasal cadmium instillation in mice. Neurotoxicology. 32(4):441–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki LA, Moberly AH, Turkel DJ, Rubinstein T, Pottackal J, Rosenthal MC, McCandlish EF, Buckley B, McGann JP. 2012. Functional rehabilitation of cadmium-induced neurotoxicity despite persistent peripheral pathophysiology in the olfactory system. Toxicol Sci. 126(2):534–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Simpson KL, Lu YF, Lin RC, Merzenich MM. 2008. Manipulating critical period closure across different sectors of the primary auditory cortex. Nat Neurosci. 11(8):957–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerty GT, Roberts LS, Connors BW. 1999. Sensory experience modifies the short-term dynamics of neocortical synapses. Nature. 400(6742):367–371 [DOI] [PubMed] [Google Scholar]
- Fletcher ML, Wilson DA. 2003. Olfactory bulb mitral-tufted cell plasticity: odorant-specific tuning reflects previous odorant exposure. J Neurosci. 23(17):6946–6955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks KM, Isaacson JS. 2005. Synapse-specific downregulation of NMDA receptors by early experience: a critical period for plasticity of sensory input to olfactory cortex. Neuron. 47(1):101–114 [DOI] [PubMed] [Google Scholar]
- Graziadei GA, Stanley RS, Graziadei PP. 1980. The olfactory marker protein in the olfactory system of the mouse during development. Neuroscience. 5(7):1239–1252 [DOI] [PubMed] [Google Scholar]
- Grosmaitre X, Fuss SH, Lee AC, Adipietro KA, Matsunami H, Mombaerts P, Ma M. 2009. SR1, a mouse odorant receptor with an unusually broad response profile. J Neurosci. 29(46):14545–14552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosmaitre X, Santarelli LC, Tan J, Luo M, Ma M. 2007. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat Neurosci. 10(3):348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosmaitre X, Vassalli A, Mombaerts P, Shepherd GM, Ma M. 2006. Odorant responses of olfactory sensory neurons expressing the odorant receptor MOR23: a patch clamp analysis in gene-targeted mice Proc Natl Acad Sci USA. 103: 1970–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie KM, Wilson DA, Leon M. 1990. Early unilateral deprivation modifies olfactory bulb function. J Neurosci. 10(10):3402–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Tian H, Lee AC, Ma M. 2012. Postnatal experience modulates functional properties of mouse olfactory sensory neurons Eur J Neurosci. doi:10.1111/j.1460-9568.2012.08170.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi KM, Mori K. 2005. Spatial representation of hydrocarbon odorants in the ventrolateral zones of the rat olfactory bulb. J Neurophysiol. 93(2):1007–1019 [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. 2000. Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. J Comp Neurol. 422(4):496–509 [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Hingco EE, Pham KL, Leon M. 1999. Multidimensional chemotopic responses to n-aliphatic acid odorants in the rat olfactory bulb. J Comp Neurol. 409(4):529–548 [PubMed] [Google Scholar]
- Kim HH, Puche AC, Margolis FL. 2006. Odorant deprivation reversibly modulates transsynaptic changes in the NR2B-mediated CREB pathway in mouse piriform cortex. J Neurosci. 26(37):9548–9559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MC, Bear MF. 1996. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 381(6582):526–528 [DOI] [PubMed] [Google Scholar]
- Lau CG, Murthy VN. 2012. Activity-dependent regulation of inhibition via GAD67. J Neurosci. 32(25):8521–8531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, He J, Ma M. 2011. Olfactory marker protein is critical for functional maturation of olfactory sensory neurons and development of mother preference. J Neurosci. 31(8):2974–2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendvai B, Stern EA, Chen B, Svoboda K. 2000. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 404(6780):876–881 [DOI] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. 1999. Combinatorial receptor codes for odors. Cell. 96(5):713–723 [DOI] [PubMed] [Google Scholar]
- Mandairon N, Linster C. 2009. Odor perception and olfactory bulb plasticity in adult mammals. J Neurophysiol. 101(5):2204–2209 [DOI] [PubMed] [Google Scholar]
- McGann JP, Pírez N, Gainey MA, Muratore C, Elias AS, Wachowiak M. 2005. Odorant representations are modulated by intra- but not interglomerular presynaptic inhibition of olfactory sensory neurons. Neuron. 48(6):1039–1053 [DOI] [PubMed] [Google Scholar]
- Meister M, Bonhoeffer T. 2001. Tuning and topography in an odor map on the rat olfactory bulb. J Neurosci. 21(4):1351–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenböck G, De Angelis DA, Rothman JE. 1998. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 394(6689):192–195 [DOI] [PubMed] [Google Scholar]
- Moberly AH, Czarnecki LA, Pottackal J, Rubinstein T, Turkel DJ, Kass MD, McGann JP. 2012. Intranasal exposure to manganese disrupts neurotransmitter release from glutamatergic synapses in the central nervous system in vivo. Neurotoxicology. http://dx.doi.org/10.1016/j.neuro .2012.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P. 2006. Axonal wiring in the mouse olfactory system. Annu Rev Cell Dev Biol. 22: 713–737 [DOI] [PubMed] [Google Scholar]
- Parrish-Aungst S, Kiyokage E, Szabo G, Yanagawa Y, Shipley MT, Puche AC. 2011. Sensory experience selectively regulates transmitter synthesis enzymes in interglomerular circuits. Brain Res. 1382: 70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pírez N, Wachowiak M. 2008. In vivo modulation of sensory input to the olfactory bulb by tonic and activity-dependent presynaptic inhibition of receptor neurons. J Neurosci. 28(25):6360–6371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BD, Katz LC. 1999. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 23(3):499–511 [DOI] [PubMed] [Google Scholar]
- Saghatelyan A, Roux P, Migliore M, Rochefort C, Desmaisons D, Charneau P, Shepherd GM, Lledo PM. 2005. Activity-dependent adjustments of the inhibitory network in the olfactory bulb following early postnatal deprivation. Neuron. 46(1):103–116 [DOI] [PubMed] [Google Scholar]
- Sharp FR, Kauer JS, Shepherd GM. 1975. Local sites of activity-related glucose metabolism in rat olfactory bulb during olfactory stimulation. Brain Res. 98(3):596–600 [DOI] [PubMed] [Google Scholar]
- Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. 2009. Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci. 12(2):210–220 [DOI] [PubMed] [Google Scholar]
- Spors H, Grinvald A. 2002. Spatio-temporal dynamics of odor representations in the mammalian olfactory bulb. Neuron. 34(2):301–315 [DOI] [PubMed] [Google Scholar]
- Suh KS, Kim SY, Bae YC, Ronnett GV, Moon C. 2006. Effects of unilateral naris occlusion on the olfactory epithelium of adult mice. Neuroreport. 17(11):1139–1142 [DOI] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. 2001. Visual deprivation alters development of synaptic function in inner retina after eye opening. Neuron. 32(3):439–449 [DOI] [PubMed] [Google Scholar]
- Tian H, Ma M. 2008. Activity plays a role in eliminating olfactory sensory neurons expressing multiple odorant receptors in the mouse septal organ. Mol Cell Neurosci. 38(4):484–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Petzold GC, Pal SK, Murthy VN. 2007. Experience-dependent modification of primary sensory synapses in the mammalian olfactory bulb. J Neurosci. 27(35):9427–9438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Takahashi YK, Tanifuji M, Mori K. 2000. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 3(10):1035–1043 [DOI] [PubMed] [Google Scholar]
- Valle-Leija P, Blanco-Hernández E, Drucker-Colín R, Gutiérrez-Ospina G, Vidaltamayo R. 2012. Supernumerary formation of olfactory glomeruli induced by chronic odorant exposure: a constructivist expression of neural plasticity. PLoS ONE. 7(4):e35358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, McGann JP, Heyward PM, Shao Z, Puche AC, Shipley MT. 2005. Inhibition [corrected] of olfactory receptor neuron input to olfactory bulb glomeruli mediated by suppression of presynaptic calcium influx. J Neurophysiol. 94(4):2700–2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggener CT, Coppola DM. 2007. Naris occlusion alters the electro-olfactogram: evidence for compensatory plasticity in the olfactory system. Neurosci Lett. 427(2):112–116 [DOI] [PubMed] [Google Scholar]
- Weisel TN, Hubel DH. 1965. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens J Neurophysiol. 26: 1003–1017 [DOI] [PubMed] [Google Scholar]
- Wesson DW, Donahou TN, Johnson MO, Wachowiak M. 2008. Sniffing behavior of mice during performance in odor-guided tasks. Chem Senses. 33(7):581–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. 1995. The D2 antagonist spiperone mimics the effects of olfactory deprivation on mitral/tufted cell odor response patterns. J Neurosci. 15(8):5574–5581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Mainen ZF. 2006. Early events in olfactory processing. Annu Rev Neurosci. 29: 163–201 [DOI] [PubMed] [Google Scholar]
- Xu F, Liu N, Kida I, Rothman DL, Hyder F, Shepherd GM. 2003. Odor maps of aldehydes and esters revealed by functional MRI in the glomerular layer of the mouse olfactory bulb. Proc Natl Acad Sci USA. 100(19):11029–11034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Ivic L, Otaki JM, Hashimoto M, Mikoshiba K, Firestein S. 1998. Functional expression of a mammalian odorant receptor. Science. 279(5348):237–242 [DOI] [PubMed] [Google Scholar]
- Zhou X, Merzenich MM. 2007. Intensive training in adult rats refines A1 representations degraded in an early postnatal critical period Proc Nat Acad Sci USA. 104: 15935–15940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou DJ, Feinstein P, Rivers AL, Mathews GA, Kim A, Greer CA, Mombaerts P, Firestein S. 2004. Postnatal refinement of peripheral olfactory projections. Science. 304(5679):1976–1979 [DOI] [PubMed] [Google Scholar]