Abstract
Recent studies suggest that oxidative stress and vascular dysfunction contribute to heart failure with preserved ejection fraction (HFPEF). In ‘salt-sensitive’ HFPEF animal models, diets low in sodium and high in potassium, calcium, magnesium, and antioxidants attenuate oxidative stress and cardiovascular damage. We hypothesized that the sodium-restricted Dietary Approaches to Stop Hypertension diet (DASH/SRD) would have similar effects in human hypertensive HFPEF. Thirteen patients with treated hypertension and compensated HFPEF consumed the DASH/SRD for 21 days (all food/most beverages provided). The DASH/SRD reduced clinic systolic (155 to 138 mmHg, p=.02) and diastolic BP (79 to 72 mmHg, p=.04), 24-hour ambulatory systolic (130 to 123 mmHg, p=.02) and diastolic BP (67 to 62 mmHg, p=.02), and carotid-femoral pulse wave velocity (12.4 to 11.0 m/s, p=.03). Urinary F2-isoprostanes decreased by 31% (209 to 144 pmol/mmol Cr, p=.02) despite increased urinary aldosterone excretion. The reduction in urinary F2-isoprostanes closely correlated with the reduction in urinary sodium excretion on the DASH/SRD. In this cohort of HFPEF patients with treated hypertension, the DASH/SRD reduced systemic blood pressure, arterial stiffness, and oxidative stress. These findings are characteristic of ‘salt-sensitive’ hypertension, a phenotype present in many HFPEF animal models, and suggest shared pathophysiological mechanisms linking these two conditions. Further dietary modification studies could provide insights into the development and progression of hypertensive HFPEF.
Keywords: diastolic heart failure, hypertension, dietary sodium, ambulatory blood pressure monitoring, vascular stiffness
Introduction
Heart failure with preserved ejection fraction (HFPEF) constitutes over half of heart failure (HF) in older adults, and its incidence and prevalence are increasing 1. In contrast to heart failure with reduced ejection fraction (HFREF), HFPEF treatment remains empirical with guidelines based largely on expert consensus 2. In part, therapeutic uncertainty reflects an incomplete understanding of how HFPEF develops and progresses. Systemic hypertension often contributes to characteristic cardiovascular remodeling, but the specific aspects of hypertension that predispose to HFPEF are undefined.
High dietary sodium intake predicts hypertension across populations 3, but only some individuals are ‘salt-sensitive,’ i.e. have blood pressure (BP) that changes with sodium intake 4. In salt-sensitive animal models, high-sodium feeding induces systemic hypertension, oxidative stress, and cardiovascular damage similar to human HFPEF 5–8. Factors that predict human salt-sensitive hypertension closely match HFPEF demographics 1, 4, 9, but no dietary modification studies have previously been reported in HFPEF.
The Dietary Approaches to Stop Hypertension (DASH) eating pattern is high in potassium, magnesium, calcium, and antioxidants 10. When combined with sodium restriction (DASH/SRD), this diet reduces BP, vascular stiffness, and systemic oxidative stress in salt-sensitive humans without HF 11. We hypothesized that three weeks of the DASH/SRD would have similar effects in HFPEF patients with treated hypertension.
Methods
This investigation conforms with the Declaration of Helsinki, was approved by the University of Michigan Institutional Review Board, followed institutional guidelines, and obtained written informed consent from all subjects.
Patient selection and study structure
The inclusion and exclusion criteria (see Table S1) were patterned after the 2007 European Society of Cardiology HFPEF diagnostic guidelines 12. All subjects had a history of systemic hypertension, left ventricular ejection fraction ≥ 50%, and evidence of ventricular diastolic dysfunction by catheterization, echocardiography, and/or neurohormonal measures. Specific exclusions related to the DASH/SRD included estimated glomerular filtration rate < 30 ml/min/1.73 m2, and current serum potassium > 5.0 mmol/L (or prior ≥ 6.0 mmol/L). Based on prior reports 11, we estimated that 12 subjects would give > 80% power to detect a 10 mmHg reduction in clinic systolic BP and a 20% reduction in urinary 8-epi-PGF2α-isoprostane levels (F2-isoprostanes).
The study took place over 25 days, with two days of testing prior to and following 21 days of DASH/SRD (see Figure S1 for timeline). On Day 1, patients presented on their habitual diet after their usual morning medications. Baseline diet was assessed with the Block food frequency questionnaire 13. Seated clinic BPs and six-minute walk testing were performed; patients returned home on their habitual diet for ambulatory BP measurement and 24-hour urinary collection. On Day 2, patients presented fasting and prior to taking morning antihypertensives; they underwent vascular testing, transthoracic echocardiography, and blood sampling.
Subjects were provided all food and beverages (except water, tea, and/or coffee) for the DASH/SRD beginning on Day 3, with a safety visit 4–6 days later. If orthostatic vital sign changes, overt hypovolemia, or worsening azotemia were observed, diuretics were withdrawn or reduced. Other antihypertensives were reduced or withdrawn only for systolic BP < 100 mmHg or severe orthostasis. Subjects were withdrawn for serum potassium > 5.7 mg/dL (mandated) or significant worsening of renal function (investigator discretion). On Days 24–25, patients had identical testing to Days 1–2.
Characteristics of study diet, tracking of dietary adherence
The study diet was prepared by research dietitians in a metabolic kitchen at the University of Michigan Clinical Research Unit. The intent was to match the DASH diet nutritional content and food types while maintaining a daily sodium intake of 50 mmol (1150 mg)/2100 kilocalories, the amount producing the largest BP reduction in the DASH-Sodium trial 10. Caloric content was adjusted for expected energy needs to maintain lean body mass. In patients with estimated glomerular filtration rate < 60 ml/min/1.73 m2, study diet potassium content was reduced from 4 to 3 g/day as suggested by the National Kidney Foundation 14.
Study food was packaged for storage, with pre-meal preparation (heating, etc.) completed at home by the patient. Subjects were advised not to ingest non-study foods or beverages, but were not required to consume all of the provided food. Adherence was assessed with a three-day food diary (at the midpoint) and pre-post DASH/SRD 24-hour urinary sodium and potassium excretion.
Blood pressure, laboratory, cardiovascular, and functional testing
Two seated clinic BPs were obtained per Joint National Committee (Seventh Report) recommendations 15. Ambulatory BP monitoring was performed per British Society of Hypertension guidelines 16 using the Spacelabs 90207 monitor (Spacelabs Healthcare, Issaquah, WA). Patient-reported sleep and wake times separated recordings into nighttime and daytime BPs. As in previous HF studies, the ‘non-dipping’ BP pattern was defined as a nighttime-to-daytime systolic BP ratio of ≥ 0.917.
All laboratory testing was performed in the University of Michigan Health System clinical laboratory except urinary F2-isoprostanes, measured with a commercially available ELISA (Cayman Chemicals, Ann Arbor, MI).
Transthoracic echocardiography used the Acuson Sequoia C512 (Siemens USA, Malvern, PA) and carotid-femoral pulse wave velocity the Sphygmocor tonometer (AtCor Medical, Itasca, IL). Six-minute walk testing was performed per American Thoracic Society guidelines with Borg scores for dyspnea and fatigue.
Statistical analysis
Data are presented as the mean ± SD or median (IQR). Analysis was performed using STATA 10.0 (STATACorp, College Station, TX). Paired t-tests and Wilcoxon rank-sum tests were used for continuous and ordinal variables, respectively, with p < 0.05 considered statistically significant.
Results
Patient characteristics
The characteristics of the 14 enrolled participants (of 22 screened) are shown in Table 1. In addition to meeting study inclusion criteria, 13 of 14 subjects also fulfilled 2007 European Society of Cardiology HFPEF diagnostic guidelines (seven by catheterization and six via neurohormonal and/or echocardiographic criteria) 12. Participants were primarily obese post-menopausal women with multiple comorbidities, and most had been previously hospitalized for decompensated HFPEF.
Table 1.
Baseline demographics and clinical characteristics of subjects
| Demographics | ||
|
| ||
| Age (years) | 72 ± 10 | |
| Gender (# female/male) | 13/1 | |
| BMI (kg/m2) | 35.5 ± 7.9 | |
|
| ||
| Baseline characteristics | n (%) | Comments |
|
| ||
| Hypertension | 14 (100%) | No known secondary causes |
| Coronary artery disease | 5 (36%) | 1 with prior CABG, 1 with prior PCI |
| Diabetes mellitus | 6 (43%) | All type II; Hgb A1c 6.8 ± 0.8% |
| Chronic kidney disease | 14 (100%) | eGFR 60 ± 17 ml/min/1.73 m2 |
| Stage II (eGFR 60–90) | 6 (43%) | |
| Stage III (eGFR < 60) | 8 (57%) | |
| Anemia | 6 (43%) | Hematocrit 38 ± 5% |
| NYHA class III | 12 (86%) | |
| Prior heart failure hospitalization | 10 (71%) | |
| On chronic loop diuretic therapy | 11 (79%) | |
| On 3 antihypertensives * | 10 (71%) | |
Abbreviations: BMI: body mass index; CABG: coronary artery bypass grafting; NYHA: New York Heart Association; eGFR: estimated glomerular filtration rate per Modification of Diet in Renal Disease; PCI: percutaneous coronary intervention
Thirteen patients completed the study; one was withdrawn due to serum potassium of 5.9 mmol/L at the safety visit. This subject’s electrolytes and renal function data are included in calculated pre-post mean values.
Study diet and adherence
A sample one-day DASH/SRD study menu is shown in Table S2. All subjects reported excellent DASH/SRD adherence per three-day food diaries, corroborated by increased urinary potassium excretion and halving of urinary sodium excretion from baseline in the 12 participants who were not on self-adjusted diuretic regimens (Table 2). A comparison of caloric and major nutrient intake between baseline and DASH/SRD diet is shown in Table S3. The mean weight loss during the study was 3.8 pounds.
Table 2.
Electrolytes and renal function pre- and post-dietary intervention
| Parameter | Pre | Post | p |
|---|---|---|---|
| Serum sodium (mmol/L)* | 141 ± 3 | 139 ± 3 | .07 |
| Serum potassium (mmol/L)* | 4.5 ± 0.4 | 4.6 ± 0.6 | .46 |
| Serum creatinine (mg/dL)* | 1.0 ± 0.2 | 1.2 ± 0.3 | .02 |
| Blood urea nitrogen (mg/dL)* | 28 ± 13 | 41 ± 24 | .03 |
| 24-hour creatinine clearance (ml/min/1.73 m2) | 66 ± 35 | 57 ± 30 | .11 |
| Serum cystatin C (mg/dL) | 1.34 | 1.41 | .65 |
| 24-hour urinary sodium (mg/24 hr) | 3353 ± 1593 | 1478 ± 933 | <.001 |
| 24-hour urinary potassium (mg/24 hr) | 2284 ± 793 | 2925 ± 1024 | .04 |
One subject was withdrawn at safety visit for elevated serum potassium (see text); safety visit values for these parameters are included in ‘Post’ column
Blood pressure and medication changes
Baseline medications and changes during the study are shown in Table S4. All clinic and ambulatory BP recordings were adequate for analysis. In most subjects, baseline 24-hour monitoring demonstrated well-controlled BP consistent with HFPEF guidelines (systolic BP < 130 mmHg) 2. The DASH/SRD significantly lowered clinic and 24-hour BP (Table 3). The number of patients with above-goal BP significantly decreased and there was a trend toward fewer subjects with non-dipping blood pressure following DASH/SRD.
Table 3.
Blood pressure pre- and post-dietary intervention
| Clinic BP (mmHg) | Pre | Post | p |
|---|---|---|---|
| Systolic | 155 ± 29 | 138 ± 22 | .02 |
| Diastolic | 79 ± 15 | 72 ± 8 | .04 |
| Clinic systolic ≥ 130 mmHg | 11/13 | 7/13 | .01 |
|
| |||
| 24-hour ambulatory BP (mmHg) | Pre | Post | p |
|
| |||
| Systolic | 130 ± 4 | 123 ± 4 | .02 |
| Diastolic | 67 ± 3 | 62 ± 3 | .02 |
| BP non-dippers | 10/13 | 7/13 | .08 |
| 24-hour systolic ≥ 130 mmHg | 7/13 | 4/13 | .02 |
Abbreviations: BP, blood pressure
Laboratory studies
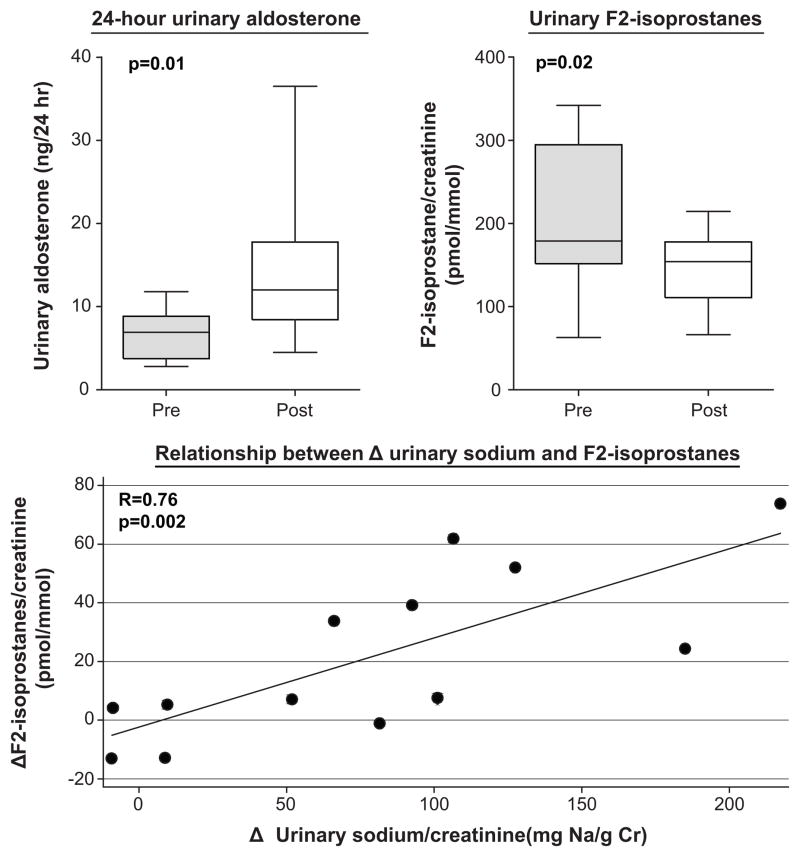
Serum sodium and measured creatinine clearance trended lower, blood urea nitrogen increased, and serum cystatin C was unchanged (Table 2). Urinary aldosterone excretion increased, but BNP and urinary F2-isoprostanes significantly declined (Table 2 and Figure, top panels). The change in urinary F2-isoprostanes correlated closely (r=0.76, p=.002) with the reduction in urinary sodium excretion on the DASH/SRD (Figure, bottom panel), but not with urinary aldosterone (r=−0.19, p=0.57). Upon exploratory stepwise regression, changes in urinary sodium excretion on DASH/SRD predicted urinary F2-isoprostane changes independent of baseline urinary sodium excretion and F2-isoprostane levels and weight change during the study.
Figure. Aldosterone and oxidative stress pre- and post-dietary intervention.
Whiskers: minimum/maximum values, line: median value, box: interquartile range
Cardiovascular and functional studies
All patients had normal left ventricular ejection fraction by transthoracic echocardiography (69 ± 6%; Simpson’s method in 11, estimated in two). The mean left atrial diameter was 42 mm. At baseline, six patients had grade II and four had grade I diastolic dysfunction, with two indeterminate (due to atrial fibrillation and suboptimal Doppler signal quality respectively) 18 and one ‘normal’ (with recent pulmonary capillary wedge pressure of 35 mmHg and BNP of 455 pg/mL). Twelve subjects had adequate signals for lateral and nine for septal mitral annulus tissue Doppler velocities; the DASH/SRD did not significantly alter E/e′ ratio (lateral 12 ± 2 to 11 ± 2, p=.45; septal 10 ± 3 to 9 ± 2, p=.14)
Twelve subjects had paired carotid-femoral pulse wave velocities (in one subject, data could not be obtained due to technical factors). Following DASH/SRD, pulse wave velocity remained stable in four, decreased by < 1.0 m/s in six, and increased by > 1.0 m/s in two participants. Pulse wave velocity increased by 6.7 m/s (50%) in one subject, nearly four standard deviations above the change in the other 11 patients. After excluding this extreme outlier, average pulse wave velocity significantly declined post-DASH/SRD (12.4 ± 3.0 to 11.0 ± 2.2 m/s, p=.03). Changes in pulse wave velocity did not significantly correlate with changes in systolic BP.
The baseline 6-minute walk test distance was similar to other outpatient HF cohorts, and modestly increased following DASH/SRD (313 ± 86 to 337 ± 91 m, p=.006). Borg dyspnea scores after the walk test improved slightly post-DASH/SRD [3.5 (2.5–5) to 3 (2–3), p=.049], but fatigue scores were unchanged [3 (1–4) to 3 (2–3), p = .43]. The number of patients reporting New York Heart Association Class III symptoms trended lower post-DASH/SRD (11/13 to 8/13, p=.17).
Discussion
Our study is the first to investigate the physiological effects of dietary modification in hypertensive HFPEF. As hypothesized, the DASH/SRD significantly reduced clinic BP, ambulatory BP, and arterial stiffness. Despite an expected increase in aldosterone production, urinary F2-isoprostane levels significantly declined in parallel to urinary sodium excretion following DASH/SRD.
Potential links between diet, salt-sensitive hypertension, and HFPEF
Dietary sodium ‘indiscretion’ frequently precipitates HF decompensation, particularly in elderly patients. However, dietary sodium reduction can increase neurohormonal activation, worsen renal function, and increase hospitalizations in patients with compensated HFREF 19. The possibility that dietary patterns contribute to HFPEF pathophysiology has not been extensively considered.
In the first National Health and Nutrition Examination Survey, high dietary sodium intake predicted incident HF only in overweight and obese respondents 20. In a large cohort of postmenopausal women, the most common HFPEF demographic, incident HF was inversely related to DASH diet pattern adherence 21. Hospitalized HFPEF patients instructed to reduce dietary sodium at hospital discharge were less likely to be readmitted within 30 days 9. These studies suggest that dietary factors influence the pathogenesis of HFPEF.
Over three-quarters of HFPEF patients have systemic hypertension 1, 9. Chronic high dietary sodium intake correlates with hypertension prevalence across disparate populations 3, and many advocate population-based dietary sodium restriction to prevent hypertension and its complications. However, only approximately 25% of normotensives and 30–50% of hypertensives are ‘salt-sensitive’, as defined by short-term BP changes between sodium-replete and deplete states 4. The BP threshold for salt-sensitivity, measurement protocols, and reproducibility of the sodium-BP response vary in the literature 4, 22.
Despite these limitations, the salt-sensitive phenotype remains a useful construct to understand hypertensive cardiovascular disease. As in animal models 5–8, independent of total BP load salt-sensitive humans develop cardiovascular abnormalities that are implicated in HFPEF. These include left ventricular hypertrophy and diastolic dysfunction, large-artery stiffening, and endothelial dysfunction. Clinical predictors of salt-sensitive BP include advanced age, the post-menopausal state, hypertension, abdominal obesity, insulin resistance, and chronic renal insufficiency 4; these demographics match those in large HFPEF cohorts 1, 9.
Blood pressure effects of DASH-SRD
The magnitude of BP reduction following DASH/SRD in this study was greater than in untreated hypertensives 10 and prehypertensives 11, comparable to adding an antihypertensive drug 23, and occurred despite treatment with multiple antihypertensive agents in most subjects. By the end of the study, significantly more HFPEF patients met the guideline-recommended systolic blood pressure goal 2. Moreover, in four of 13 subjects antihypertensives and/or diuretics were reduced shortly after starting the DASH/SRD (Table S3).
As expected 23, the 24-hour BP reduction was smaller than in clinic BP but still notable. The BP ‘non-dipping’ pattern reflects impaired renal sodium handling and tracks closely with clinically demonstrated salt-sensitive BP 24. Non-dipping BP doubles the risk of incident HF in older adults, independent of overall BP load and other clinical predictors 25, and in HFREF patients increases cardiovascular hospitalization and death17. Ambulatory BP has not previously been reported in HFPEF. In this cohort, most patients were non-dippers despite diuretic treatment. The DASH/SRD restored normal BP dipping in three subjects, consistent with the effects of dietary sodium reduction in hypertensives without HF 24.
Cardiovascular and functional effects of DASH-SRD
The classic conceptual model of HFPEF has expanded beyond ventricular diastolic dysfunction to encompass abnormalities in multiple cardiovascular domains. In particular, increased large-artery stiffness likely contributes to exercise intolerance and dyspnea in HFPEF. In this cohort, elevated pulse pressure and carotid-femoral pulse-wave velocity at baseline indicated significant large-artery stiffness. Previous studies demonstrated reduced arterial stiffness after three months of sodium restriction in postmenopausal women 26 and following three weeks of the DASH/SRD in younger subjects with clinically confirmed salt-sensitive BP 11. Our findings are consistent with these results, and were accompanied by a modest improvement in 6-minute walk distance and post-exercise dyspnea.
Echocardiographic diastolic function 18 did not significantly change following the DASH/SRD. The short study duration was unlikely to produce structural myocardial changes, and standard parameters such as E/e′ ratio may not be accurate enough to reflect serial differences in diastolic function or filling pressures in HFPEF 27. More recently developed measures, such as diastolic strain rate, could potentially delineate subtle changes in diastolic function with dietary modification.
Aldosterone and oxidative stress effects of DASH/SRD
In salt-sensitive animals, high sodium feeding induces oxidative stress, which drives immune cell activation, perivascular inflammation, and fibrosis 5–8. In these models, maintenance of normal or low dietary sodium intake largely prevents hypertension and end-organ pathology. Dietary supplementation with potassium, magnesium, calcium, and/or antioxidants reduces oxidative stress, inflammation, and cardiovascular damage even if high sodium intake continues 5, 28, 29. The DASH/SRD, low in sodium and high in potassium, magnesium, calcium, and antioxidants, reduces systemic oxidative stress in salt-sensitive humans but does not in ‘salt-resistant’ persons, i.e. those whose BP changes little in response to sodium intake 11, 30.
Given reduced sodium and increased potassium intake, we expected that the DASH/SRD would increase aldosterone, generally viewed as a strong pro-oxidant stimulus 5, 31. In salt-sensitive experimental models, genomic and non-genomic mineralocorticoid effects often mediate end-organ damage. However, high mineralocorticoid levels in these animals do not cause severe adverse effects unless accompanied by elevated oxidative stress 5, 31. In this study, urinary F2-isoprostanes did not correlate with aldosterone excretion; rather, they were closely related to the reductions achieved in urinary sodium excretion on the DASH-SRD. Our findings are consistent with a prior study in salt-sensitive humans without HF where the DASH/SRD reduced systemic oxidative stress despite more than doubling serum aldosterone levels11.
In HFREF, systemic oxidative stress predicts functional class, increases during decompensation, and decreases following HF treatment 32, 33. In this cohort, the baseline urinary F2-isoprostane excretion was comparable to that reported in HFREF patients 32 and decreased by over 30% following the DASH/SRD. In hypertensives without HF, increased systemic oxidative stress predicts reduced exercise capacity, ventricular hypertrophy and diastolic dysfunction, and endothelial dysfunction 34, 35. Venous endothelial biopsy directly links oxidative stress and vascular dysfunction in older adults with metabolic syndrome, a demographic precursor to HFPEF 36. In addition, heightened oxidative stress is present in the myocardium of HFPEF patients and is thought to drive cytokine production and myofibroblast differentiation 37. These observations imply that oxidative stress is a key etiological component of HFPEF; our findings suggest a potential method to ameliorate this mechanism.
Safety
The DASH/SRD has not previously been studied in HF or chronic kidney disease (100% of our cohort). Four patients developed orthostasis requiring medication adjustment, and one was withdrawn for elevated serum potassium despite DASH/SRD modification as suggested by the National Kidney Foundation 14. In contrast to a prior study of sodium reduction in HFREF 19, where high-dose loop diuretics were continued, we adjusted diuretics at a safety visit based on clinical status. Although no subject was removed from participation due to renal dysfunction, blood urea nitrogen levels increased and creatinine clearance trended lower following DASH/SRD (Table 2). These changes are consistent with plasma volume reduction, also suggested by the modest weight loss on the isocaloric study diet. The lack of change in cystatin C implies that the DASH/SRD did not cause renal injury. Nonetheless, significant renal dysfunction can occur during sodium restriction in HF 19, and this short-term study highlights the importance of close renal monitoring during dietary modification.
Study limitations
Our results are hypothesis-generating and not necessarily generalizable to all HFPEF patients, such as those with non-hypertensive etiology or different demographics. The study group was enriched in characteristics that predict salt-sensitivity (e.g. advanced age, diabetes, obesity, renal insufficiency, post-menopausal state) 4 and as such closely resembled patients in large HFPEF cohort studies 1, 9. In this small cohort, we were unable to determine whether specific factors contributed disproportionately to the salt-sensitive phenotype. Since extracardiac comorbidities contribute to the pathophysiology of HFPEF 38, defining these relationships is an important area for future research.
In this pilot study, we used dietary intervention to reveal similarities between the salt-sensitive phenotype and hypertensive HFPEF. Our study is analogous to an early study of exercise training in HFPEF demonstrating safety and proof of concept 39 that subsequently led to larger randomized trials 40. To place our results in context, the effect sizes for blood pressure and oxidative stress were consistent with previous DASH diet studies in non-HF cohorts, which showed no clinic or ambulatory BP changes with habitual or control diets 41, 42. In particular, the observed difference in 24-hour blood pressure in this study is unlikely to be due to a placebo effect 23, 41. Nonetheless, our findings should now be verified and expanded upon in the context of a randomized trial.
We do not know which specific DASH/SRD components (e.g. sodium reduction, increased potassium, antioxidant intake) contributed to the observed effects. We did not hospitalize subjects to formally control dietary intake. However, we provided all food and beverages for the DASH/SRD, and urinary measures and food diaries indicated excellent dietary adherence. It is unknown whether our short-term results could be sustained in a cohort with complete freedom of food choice. Future, longer-term studies could include ongoing dietary education and other measures to support adherence.
Supplementary Material
Perspectives.
HFPEF is a major public health challenge currently without evidence-based therapy, largely due to incomplete understanding of its pathophysiology. Experimental models and HFPEF epidemiology suggest that dietary patterns and salt-sensitive hypertension play important roles. In this cohort of patients with hypertensive HFPEF, three weeks of the sodium-restricted DASH diet significantly reduced clinic and ambulatory BP, arterial stiffness, and oxidative stress. These observations are characteristic of salt-sensitive hypertension, a phenotype present in nearly all animal models of HFPEF. Further dietary modification studies could provide insights into the development and progression of hypertensive HFPEF.
Novelty and Significance.
What is New?
Recent studies implicate oxidative stress and vascular dysfunction as key factors in human hypertensive HFPEF
No prior dietary modification studies have been reported in hypertensive HFPEF
What is Relevant?
Animal models of HFPEF display a salt-sensitive phenotype, with diet-induced oxidative stress driving perivascular inflammation and fibrosis
The demographics and comorbidities commonly found in HFPEF are strongly associated with blood pressure salt-sensitivity.
Summary
In HFPEF patients with treated hypertension, the sodium-restricted DASH diet reduced blood pressure, arterial stiffness, and systemic oxidative stress
These observations suggest links between human hypertensive HFPEF and the salt-sensitive blood pressure phenotype
Acknowledgments
The authors would like to acknowledge Theresa Han-Markey, M.S., R.D., Debra Peterman, B.S., R.D., Laura Foess-Wood, B.S., Cynthia Boersma, B.S., Martha Hartdegen, B.S., Elizabeth Meurer, M.S., R.D., and the Michigan Clinical Research Unit staff for their invaluable assistance.
Funding:
This work was supported by the National Institutes of Health [1K23HL109176 to S.L.H. and UL1RR024986 to the University of Michigan] and the Innovations in Cardiovascular Medicine Award from the University of Michigan Cardiovascular Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Conflicts of interest:
None declared.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction.[see comment] New Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Lindenfeld J, Albert N, Boehmer J, Collins S, Ezekowitz J, Givertz M, Katz S, Klapholz M, Moser D, Rogers J, Starling R, Stevenson W, Tang WHW, Teerlink J, Walsh M. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Stamler J, Rose G, Stamler R, Elliott P, Dyer A, Marmot M. INTERSALT study findings: public health and medical care implications. Hypertension. 1989;14:570–577. doi: 10.1161/01.hyp.14.5.570. [DOI] [PubMed] [Google Scholar]
- 4.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 5.Bayorh MA, Mann G, Walton M, Eatman D. Effects of enalapril, tempol, and eplerenone on salt-induced hypertension in Dahl salt-sensitive rats. Clin Exp Hypertens. 2006;28:121–132. doi: 10.1080/10641960500468276. [DOI] [PubMed] [Google Scholar]
- 6.Klotz S, Hay I, Zhang G, Maurer M, Wang J, Burkhoff D. Development of heart failure in chronic hypertensive Dahl rats: focus on heart failure with preserved ejection fraction. Hypertension. 2006;47:901–911. doi: 10.1161/01.HYP.0000215579.81408.8e. [DOI] [PubMed] [Google Scholar]
- 7.Matsui H, Ando K, Kawarazaki H, Nagae A, Fujita M, Shimosawa T, Nagase M, Fujita T. Salt excess causes left ventricular diastolic dysfunction in rats with metabolic disorder. Hypertension. 2008;52:287–294. doi: 10.1161/HYPERTENSIONAHA.108.111815. [DOI] [PubMed] [Google Scholar]
- 8.Ahn J, Varagic J, Slama M, Susic D, Frohlich ED. Cardiac structural and functional responses to salt loading in SHR. Am J Physiol Heart Circ. 2004;287:H767–772. doi: 10.1152/ajpheart.00047.2004. [DOI] [PubMed] [Google Scholar]
- 9.Hummel SL, DeFranco AC, Skorcz S, Montoye CK, Koelling TM. Recommendation of low-salt diet and short-term outcomes in heart failure with preserved systolic function. Am J Med. 2009;122:1029–1036. doi: 10.1016/j.amjmed.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG, Karanja N, Lin PH. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. New Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 11.Al-Solaiman Y, Jesri A, Zhao Y, Morrow JD, Egan BM. Low-sodium DASH reduces oxidative stress and improves vascular function in salt-sensitive humans. J Hum Hypertens. 2009;23:826–835. doi: 10.1038/jhh.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 13.Ard JD, Coffman CJ, Lin P-H, Svetkey LP. One-year follow-up study of blood pressure and dietary patterns in Dietary Approaches to Stop Hypertension (DASH)-Sodium participants. Am J Hypertens. 2004;17:1156–1162. doi: 10.1016/j.amjhyper.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Foundation. Clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43 (suppl 1):S1–S290. [PubMed] [Google Scholar]
- 15.Chobanian A, Bakris G, Black H, Cushman W, Green L, Izzo J, Jones D, Materson B, Oparil S, Wright J, Roccella E. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien E, Coats A, Owens P, Petrie J, Padfield PL, Littler WA, de Swiet M, Mee F. Use and interpretation of ambulatory blood pressure monitoring: recommendations of the British Hypertension Society. BMJ. 2000;320:1128–1134. doi: 10.1136/bmj.320.7242.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin J, Kline S, Moore M, Gong Y, Bhanderi V, Schmalfuss CM, Johnson JA, Schofield RS. Association of diurnal blood pressure pattern with risk of hospitalization or death in men with heart failure. J Card Fail. 2007;13:656–662. doi: 10.1016/j.cardfail.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bursi F. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 19.Paterna S, Gaspare P, Fasullo S, Sarullo FM, Di Pasquale P. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend? Clin Sci (Lond) 2008;114:221–230. doi: 10.1042/CS20070193. [DOI] [PubMed] [Google Scholar]
- 20.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Dietary sodium intake and incidence of congestive heart failure in overweight us men and women: first National Health And Nutrition Examination Survey epidemiologic follow-up study.[see comment] Arch Intern Med. 2002;162:1619–1624. doi: 10.1001/archinte.162.14.1619. [DOI] [PubMed] [Google Scholar]
- 21.Levitan EB, Wolk A, Mittleman MA. Consistency with the DASH diet and incidence of heart failure. Arch Intern Med. 2009;169:851–857. doi: 10.1001/archinternmed.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Sierra A. Lack of correlation between two methods for the assessment of salt sensitivity in essential hypertension. J Hum Hypertens. 2002;16:255–260. doi: 10.1038/sj.jhh.1001375. [DOI] [PubMed] [Google Scholar]
- 23.Mancia G, Parati G. Office compared with ambulatory blood pressure in assessing response to antihypertensive treatment: a meta-analysis. J Hypertens. 2004;22:435–445. doi: 10.1097/00004872-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Sachdeva A, Weder AB. Nocturnal sodium excretion, blood pressure dipping, and sodium sensitivity. Hypertension. 2006;48:527–533. doi: 10.1161/01.HYP.0000240268.37379.7c. [DOI] [PubMed] [Google Scholar]
- 25.Ingelsson E, Bjorklund-Bodegard K, Lind L, Arnlov J, Sundstrom J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA. 2006;295:2859–2866. doi: 10.1001/jama.295.24.2859. [DOI] [PubMed] [Google Scholar]
- 26.Seals DR, Tanaka H, Clevenger CM, Monahan KD, Reiling MJ, Hiatt WR, Davy KP, DeSouza CA. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: role of arterial stiffness. J Am Coll Cardiol. 2001;38:506–513. doi: 10.1016/s0735-1097(01)01348-1. [DOI] [PubMed] [Google Scholar]
- 27.Dumesnil JG, Pibarot P. Doppler assessment of diastolic function at rest and during exercise: distinguishing myth from reality. J Am Soc Echocardiogr. 2009;22:350–353. doi: 10.1016/j.echo.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Goodwin K, Ahokas R, Bhattacharya S, Sun Y, Gerling I, Weber K. Preventing oxidative stress in rats with aldosteronism by calcitriol and dietary calcium and magnesium supplements. Am J Med Sci. 2006;332:73–78. doi: 10.1097/00000441-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Seymour EM, Singer AAM, Bennink MR, Parikh RV, Kirakosyan A, Kaufman PB, Bolling SF. Chronic intake of a phytochemical-enriched diet reduces cardiac fibrosis and diastolic dysfunction caused by prolonged salt-sensitive hypertension. J Gerontol A Biol Sci Med Sci. 2008;63:1034–1042. doi: 10.1093/gerona/63.10.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laffer CL, Bolterman RJ, Romero JC, Elijovich F. Effect of salt on isoprostanes in salt-sensitive essential hypertension. Hypertension. 2006;47:434–440. doi: 10.1161/01.HYP.0000202480.06735.82. [DOI] [PubMed] [Google Scholar]
- 31.Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS, Stockstill RL, Hughson MD, Manning RD., Jr NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1858–1865. doi: 10.1152/ajpregu.90650.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cracowski JL, FT, Marpeau C, Baguet JP, Stanke-Labesque F, Mallon JM, Bessard G. Increased formation of F2-isoprostanes in patients with severe heart failure. Heart. 2000;84:439–440. doi: 10.1136/heart.84.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colombo PC, Banchs JE, Celaj S, Talreja A, Lachmann J, Malla S, DuBois NB, Ashton AW, Latif F, Jorde UP, Ware JA, LeJemtel TH. Endothelial cell activation in patients with decompensated heart failure. Circulation. 2005;111:58–62. doi: 10.1161/01.CIR.0000151611.89232.3B. [DOI] [PubMed] [Google Scholar]
- 34.Dekleva M, Celic V, Kostic N, Pencic B, Ivanovic AM, Caparevic Z. Left ventricular diastolic dysfunction is related to oxidative stress and exercise capacity in hypertensive patients with preserved systolic function. Cardiology. 2007;108:62–70. doi: 10.1159/000095883. [DOI] [PubMed] [Google Scholar]
- 35.Yugar-Toledo JC, Bonalume Tácito LH, Ferreira-Melo SE, Sousa W, Consolin-Colombo F, Irigoyen MC, Franchini K, Coelho OR, Moreno H. Low-renin (volume dependent) mild-hypertensive patients have impaired flow-mediated and glyceryl-trinitrate stimulated vascular reactivity. Circ J. 2005;69:1380–1385. doi: 10.1253/circj.69.1380. [DOI] [PubMed] [Google Scholar]
- 36.Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-{kappa}b activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–1292. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss H-P, Tschöpe C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction/clinical perspective. Circ Heart Fail. 2011;4:44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 38.Abramov D, He K-L, Wang J, Burkhoff D, Maurer MS. The impact of extra cardiac comorbidities on pressure volume relations in heart failure and preserved ejection fraction. J Card Fail. 2011;17:547–555. doi: 10.1016/j.cardfail.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smart N, Haluska B, Jeffriess L, Marwick TH. Exercise training in systolic and diastolic dysfunction: Effects on cardiac function, functional capacity, and quality of life. Am Heart J. 2007;153:530–536. doi: 10.1016/j.ahj.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Kitzman D, Brubaker P, Morgan T, Stewart K, Little W. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore TJ, Vollmer WM, Appel LJ, Sacks FM, Svetkey LP, Vogt TM, Conlin PR, Simons-Morton DG, Carter-Edwards L, Harsha DW. Effect of dietary patterns on ambulatory blood pressure: results from the Dietary Approaches to Stop Hypertension (DASH) trial. Hypertension. 1999;34:472–477. doi: 10.1161/01.hyp.34.3.472. [DOI] [PubMed] [Google Scholar]
- 42.Lopes HF, Martin KL, Nashar K, Morrow JD, Goodfriend TL, Egan BM. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension. 2003;41:422–430. doi: 10.1161/01.HYP.0000053450.19998.11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.