Abstract
We investigate acute effects of axial stretch, applied by carbon fibers (CFs), on diastolic Ca2± spark rate in rat isolated cardiomyocytes. CFs were attached either to both cell ends (to maximize the stretched region), or to the center and one end of the cell (to compare responses in stretched and nonstretched half-cells). Sarcomere length was increased by 8.01 ± 0.94% in the stretched cell fraction, and time series of XY confocal images were recorded to monitor diastolic Ca2± spark frequency and dynamics. Whole-cell stretch causes an acute increase of Ca2± spark rate (to 130.7 ± 6.4%) within 5 seconds, followed by a return to near background levels (to 104.4±5.1%) within 1 minute of sustained distension. Spark rate increased only in the stretched cell region, without significant differences in spark amplitude, time to peak, and decay time constants of sparks in stretched and nonstretched areas. Block of stretch-activated ion channels (2 gmol/L GsMTx-4), perfusion with Na±/Ca2±-free solution, and block of nitric oxide synthesis (1 mmol/L L-NAME) all had no effect on the stretch-induced acute increase in Ca2± spark rate. Conversely, interference with cytoskeletal integrity (2 hours of 10 gmol/L colchicine) abolished the response. Subsequent electron microscopic tomography confirmed the close approximation of microtubules with the T-tubular–sarcoplasmic reticulum complex (to within · 10−8m). In conclusion, axial stretch of rat cardiomyocytes acutely and transiently increases sarcoplasmic reticulum Ca2± spark rate via a mechanism that is independent of sarcolemmal stretch-activated ion channels, nitric oxide synthesis, or availability of extracellular calcium but that requires cytoskeletal integrity. The potential of microtubule-mediated modulation of ryanodine receptor function warrants further investigation.
Keywords: mechanoelectric feedback, ryanodine receptor, stretch-activated channel, nitric oxide, electron microscopic tomography
Cardiomyocyte Ca2± handling underlies the mechanical activity of the heart.1 In turn, cardiac Ca2± handling is affected by the mechanical environment.2 In situ, cardiomyocytes are exposed to dynamically changing mechanical conditions, making it difficult to dissociate the bidirectional interplay of Ca2± handling and mechanics. To obtain a better understanding of the dynamic integration of cardiac function, it is helpful to characterize in detail how the mechanical environment may affect individual facets of Ca2± handling in intact isolated cardiomyocytes, such as spontaneous diastolic Ca2± spark generation.
The sarcoplasmic reticulum (SR) is the major functional Ca2± store in cardiac myocytes with important roles in cardiac excitation-contraction coupling. Its Ca2± content is a key determinant of SR Ca2± release.3 In resting cells, SR Ca2± content ([Ca2±]SR) is determined by the balance between Ca2± uptake (via the sarco-/endoplasmic reticulum Ca2± ATPase) and Ca2± leak (largely in the form of Ca2± sparks: release events via type 2 ryanodine receptors [RyR2]4). This diastolic Ca2± balance may be negative (“rest-decay phenomenon”) or positive (“postrest potentiation”), depending on species. Rest decay is observed when the diastolic Ca2± leak from the SR exceeds reuptake, such asin cat, guinea pig, rabbit, or frog.5 In other species, such as humans, mouse, and rat, postrest potentiation occurs.6,7 Interestingly, stretch appears to reduce [Ca2±]SR compared with resting preparations at shorter length in species showing either type of overall diastolic Ca2± balance.8,9
Given their maintained high diastolic SR Ca2± levels, rat cells have become a key experimental model for the study of diastolic SR Ca2+ leak via observation of Ca2+ sparks.10,11 Single-cell studies into related phenomena have, by and large, been conducted in mechanically unloaded cells, so that the effects of axial stretch on SR Ca2+ handling are generally not well elucidated. One prior report indicated that axial stretch reduces overall SR Ca2+ load in guinea pig cardiomyocytes within seconds of stretch application, but individual release events (sparks) were not studied.9 Another study showed that stretch increases Ca2+ spark rate in rat cardiomyocytes via a nitric oxide (NO)-mediated pathway, but this was observed after prolonged exposure to the mechanical stimulus (10 minutes).12
In the present study, we investigated the acute effects on Ca2+ spark rate of diastolic stretch, applied axially to single intact rat ventricular cardiomyocytes. We found that this causes an acute and transient increase in Ca2+ spark rate, without affecting the dynamic behavior of individual sparks, via a pathway that requires cytoskeletal integrity.
Materials and Methods
Cell Preparation
Experiments were conducted in accordance with the guidelines of relevant institutional animal care and ethics regulations, and in agreement with the UK Home Office Animals (Scientific Procedures) Act of 1986. Adult rats were terminally anesthetized by injection of pentobarbital (100 mg · kg−1), followed by cardiac excision and enzymatic isolation of ventricular myocytes, as described elsewhere.13 Cardiomyocytes were stored in a normal Tyrode solution, containing (in mmol/L): NaCl 140, KCl 10, CaCl2 1.8, MgCl2 1, HEPES 5, glucose 11. Experiments were performed at room temperature (22°C).
Axial Stretch Technique
The CF technique used in this study has been described in detail elsewhere.14 In short, a pair of CFs was attached to a single isolated cardiomyocyte using two 3-axis miniature hydraulic manipulators (SM-28, Narishige, Tokyo, Japan), each mounted on separate computer-controlled piezoelectric translators (PZT; P-621.1CL, Physik Instrumente, Karlsruhe/Palmbach, Germany) of a custom-made railing system (IonOptix, Milton, Mass). Axial stretch was applied by PZT movement of CFs, graded to cause an increase in sarcomere length of =8% in the stretched portion of the cell. Larger strains increased the likelihood of either CF detachment or mechanical induction of Ca2+ waves (see “excess stretch wave” movie in the online data supplement, available at http://circres.ahajournals.org), both of which were exclusion criteria. Sarcomere length changes were confirmed via fast Fourier transformation of striation patterns in the confocal images (Figure 1). The Table summarizes the resultant changes in sarcomere length during half-cell stretch application, reconfirming that the target increase in sarcomere length was achieved in the stretched portion of the cell only, whereas the nonstretched cell side remained unaffected.
Figure 1.
Confocal images (raw data) of Fluo-4–loaded isolated cardiomyocytes with CFs attached, at initial length (top) and after application of axial stretch (bottom), using either the whole-cell (A) or the half-cell (B) stretch protocol. Arrows indicate actual position of CFs; gray levels in the cell are indicative of Ca2+ concentration. Note bright spots caused by Ca2+ sparks (bottom images), and striation pattern used to establish sarcomere length; scale bar=20 See “whole-cell-stretch” and “half-cell-stretch” movies in the online data supplement.
Table 1.
Changes in Sarcomere Length During Half-Cell Stretch Application (see Fig 1B)
| Sarcomere Length (Am) | |||
|---|---|---|---|
| Nonstretched Half-Cell |
Stretched Half-Cell |
||
| Before Stretch | During Stretch | Before Stretch | During Stretch |
| 1.874±0.024 | 1.874±0.024 | 1.877±0.032 | 2.027±0.035 |
The relative sarcomere length increase in the stretched half-cell is 8.01 ±0.94%, while there is no change in the nonstretched cell-portion. Data are mean±SEM; n=10.
Whole-Cell Stretch Protocol
To maximize the mechanically affected cell proportion, CFs were attached to each cell end (whole-cell stretch; Figure 1A). Ca2+ spark rate was compared during 5-second intervals, before application of stretch, immediately after onset of stretch, and at the end of 1 minute of stretch.
Half-Cell Stretch Protocol
One CF was attached to the center of the cell, and the other CF was attached to one end of the same cell. The central CF remained stationary, whereas the end-standing CF was used to apply stretch to half of the cell only, leaving the remainder of the cell relatively undisturbed (half-cell stretch; Figure 1B). Ca2+ sparks were counted in both the stretched and the nonstretched portion of the cell, for 5 seconds, immediately before and after application of stretch, and the percentage change in Ca2+ spark rate (“during stretch” divided by “prestretch” times 100) was assessed separately for each cell half.
Ca2+ Spark Measurements
Cells were loaded with Fluo-4 by 10 minutes of incubation with Fluo-4-acetoxymethyl-ester (Invitrogen, Carlsbad, Calif) and scanned using an argon ion laser beam for illumination at 488 nm. Emitted fluorescence was detected above 505 nm. XY confocal images were acquired every 20 to 30 ms in a time series using an LSM 510 confocal microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany). Automated analysis of images for Ca2+ spark locations was performed using custom routines (available from C.W.W. [ ward@son.umaryland.edu]), written in Interactive Data Language (IDL version 6.2). Some core-processing routines from previous work15,16 were modified and used for this XY time (XYT) series application. For each time point of the XYT series, a 5-frame running average was performed to create a parallel XYT (XYTp) image array. Following the manual identification of an area outside the cardiomyocyte to measure background fluorescence, a 4×4 boxcar filter was applied to each image. From this, the area containing the cardiomyocyte was empirically identified as being 1.5 SD greater than the background fluorescence, and the mean intensity (Fmean,tot) and SD of total fluorescence within the cardiomyocyte boundary were established. Potential spark locations were identified as contiguous pixel regions with an intensity of 2 SD above Fmean,tot. The XYTp image array was then reprocessed, to calculate mean intensity (Fmean,net) and SD of the net fluorescence in cardiomyocyte area outside potential spark locations. Thereafter, a AF representation (local fluorescence intensity minus Fmean,net) was constructed of each image, and Ca2 sparks were finally confirmed as contiguous pixel areas with a local intensity that exceeded Fmean,net by 3.8 SD.10 Ca2 spark frequency was analyzed, and, to obtain actual Ca2 spark rates, duplicate counts of sparks at any coordinate (ie, those that lasted throughout more than one of the contiguous frames) were subtracted.
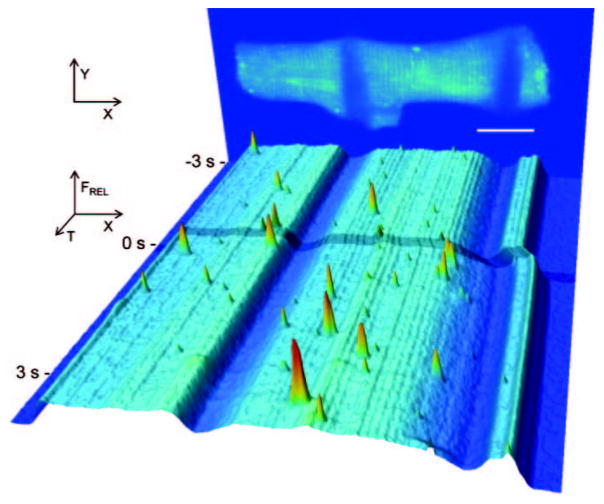
In addition, fast XYT series image acquisition (one 512×30 pixel frame captured every 1.5 to 2.5 ms) was performed during the half-cell stretch protocol, using an LSM 5-Live microscope to analyze spark dynamics. XY regions containing individual sparks (Figure 2A) were collapsed onto the x axis, to provide a 1D signal intensity line (pseudo line-scan image), and then all 1D pseudo line-scan traces were stacked in chronological order to create a 2D XT-sequence (Figure 2B, pseudo line-scan time plot). The time course of the signal at the center line of Figure 2B was then used to analyze spark amplitude, time to peak, and decay time constant of the spark (Figure 2C), also shown in a pseudocolor 3D surface plot (Figure 2D).
Figure 2.
Analysis of Ca2 spark dynamics. A, Part of a time series of XY spark recordings, with normalized calcium fluorescence intensity [FNORM=([F/F0]−1)/([FMAX/F0]−1)] rendered in pseudocolor (blue=0; red=1). B, Time course image of signal intensity distribution of the same time series, where the Y dimension of each frame was collapsed to form individual maximum intensity line (mimicking line-scan data) that were then arranged chronologically to form an XT plot. C, Relative signal intensity (F/F0) profile through Ca2 spark maximum, with indication of analyzed parameters. D, Three-dimensional surface plot of normalized XT image intensity data. Solid scale bars=5 jim; dotted scale bar=20 ms.
Electron Microscopy and Tomography
Spatial interrelation of microtubules with the T-tubular–SR membrane system that contains RyR2 was further investigated by electron tomography (ET). The principle of ET has been described in detail elsewhere.17 In short, adult rat ventricular cardiomyocytes were fixed in PBS containing 2% glutaraldehyde for 40 minutes and postfixed in 1% OsO4 for 10 minutes. Cells were then dehydrated in acetone and embedded in Epon-Araldite resin (Electron Microscopy Sciences, Hatfield, Pa). Sections (250 nm) were cut and transferred onto ET grids. Colloidal gold particles (15 nm) were added to both surfaces of the sections as fiducial markers for use during subsequent image stack alignment. Preparations were imaged with a Tecnai TF30 microscope operating at 300 kV (FEI Company, Eindhoven, The Netherlands), with images captured on an Ultrascan 4K CCD camera (GATAN Inc, Pleasanton, Calif). At the nominal magnification of ×23 000, projected image dimension is 1.02× 1.02 nm2 per camera pixel, providing a Nyquist resolution of 2.04 nm in the XY plane. Resolution in the Z direction of the sample is affected by the highest possible tilt angle a in the tomogram and cannot be better than the [XY resolution] × [sin(amax)]−1(for a detailed discussion of the technique and its limitations, see Lucic et al18). This theoretical maximum resolution, however, does not take into account the effects of cell isolation and fixation on subcellular structural integrity, or the challenges involved in distinguishing biological detail in densely populated cells such as cardiomyocytes. Physical resolution is therefore not to be confused with the ability to resolve and properly interpret biologically relevant detail, which, here, is in the order of 4 to 5 nm. With this technique, it was possible to track with confidence the T-tubular–SR membrane system in the 3D ET data sets, as well as continuous microtubular structures.
For dual-axis tilt series imaging, the specimen holder was tilted from ±60° to −60° at 1° intervals (121 images collected); the specimen was then rotated by 90° in the XY plane of the holder, and another ±60° to −60° tilt series was taken. The images from each tilt-series were aligned (by fiducial marker tracking) and back-projected to generate 2 single full-thickness reconstructed volumes (tomograms), which were then combined to generate a single high-resolution 3D reconstruction of the original partial cell volume. Tomograms were processed using the IMOD software19 to generate 3D models of the relevant structures of interest. Microtubules were modeled as tubes with a diameter of 24 nm (shown in green in relevant figures and animations), whereas SR and T-tubular membranes were modeled by red and yellow contours (respectively) along the bilayer projection delimiting these distinct compartments, traced for each tomographic slice (see “3D-EM-tomography” movie in the online data supplement). The model was smoothed and meshed to obtain the final 3D representation, where spatial relationships among microtubules, SR, and T-tubules were analyzed.
Statistics
All values are presented as means ± standard error of means. Paired Student t test and 2-way ANOVA were used for statistical assessment, where appropriate. A probability value of less than 0.05 was considered to indicate a significant difference between means.
Results
Figure 3 summarizes the result of stretch-induced changes in Ca2± spark rate with the whole-cell stretch protocol. Data are normalized to Ca2± spark rate in the same cell just before stretch application. Axial stretch caused an acute increase in Ca2± spark rate to 130.7±6.4% of control (n=8, P<0.01), followed by return to background levels (104.4±5.1%) within 1 minute of maintained diastolic distension (see “whole-cell-stretch” movie in the online data supplement).
Figure 3.
Ca2± spark rates observed with the whole-cell stretch protocol. Ca2± spark rate increases immediately (within the first 5-second bin) after application of about 8% axial stretch, and returns to control levels (within 1 minute) in the presence of maintained stretch. Data are normalized to spark rate in the same cell before stretch application. *P<0.05 vs control.
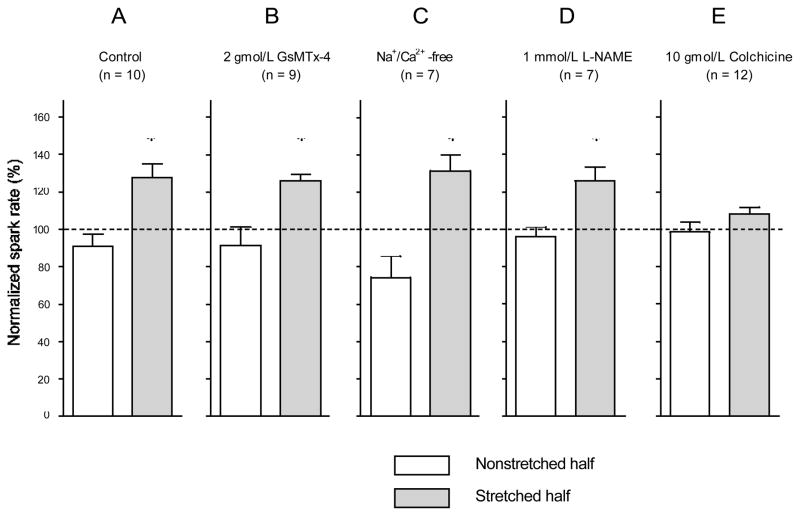
Figure 4 illustrates an example of the stretch-induced increase in Ca2± spark rate observed during application of half-cell stretch to a ventricular cardiomyocyte. Quantitative results are summarized in Figure 5A. Data from both the stretched and nonstretched cell half are normalized to the Ca2± spark rate in the corresponding cell area before application of stretch. Ca2± spark rate increased to 128.2±7.2% (n=10, P<0.01) in the stretched part of the cell, whereas in the nonstretched half, it was not statistically different from control (91.1±6.7%; see “half-cell-stretch” movie in the online data supplement).
Figure 4.
Time course of relative Fluo-4 signal intensity in a rat ventricular resting cardiomyocyte, illustrating dynamic changes in spatially resolved Ca2± concentration before and during half-cell stretch. The panel at the back shows an image of the cell, averaged from 10 confocal XY scans, before stretch application. Low signal intensity areas, overlapping the cell body, reveal CF positions before stretch (scale bar=20 Am). For the front, fluorescence intensity in each confocal XY scan was added along the y axis and plotted as a pseudo-3D XT sequence of relative calcium fluorescence (note sarcomere-related modulation of fluorescence intensity). Stretch was applied at 0 second by lateral movement of the CF near the cell end (right) (shading across XT plot indicates period of CF movement), stretching sarcomeres in the affected area by −.8%, whereas sarcomere length in the unaffected cell region (left cell half) remains unchanged.
Figure 5.
Ca2+ spark rate during the first 5 seconds of stretch, applied to one half of the cell only. Data for the stretched and the non-stretched cell half are normalized to spark rates in the same area during the 5 seconds before stretch application. Responses in control conditions (A) and after treatment of cells with the following compounds are shown: 2 Amol/L GsMTx-4 (B); Na+/Ca2+-free solution (C), 1 mmol/L L-NAME (D), 10 Amol/L colchicine (E). Stretch caused a significant increase in Ca2+ spark rate in the stretched part of the cell in all experimental conditions, except after preincubation (for 2 hours) with colchicine. Number of observations in brackets; *P<0.05 (no significant differences were observed under any experimental conditions in the nonstretched cell region between spark rate before and after application of stretch to the other cell half).
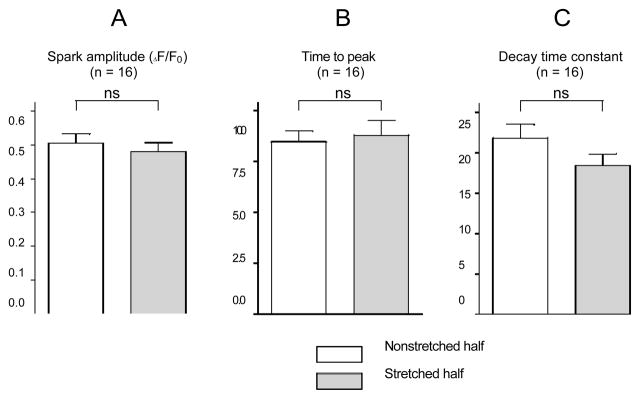
Stretch affected neither spark amplitude (AF/Fo=0.50± 0.03 in stretched versus 0.48±0.03 in nonstretched half-cell, n=16), nor time to peak (8.8±0.7 ms in stretched versus 8.5±0.5 ms in nonstretched half-cell, n=16) and decay time constant (18.2±1.4 ms in stretched versus 21.6±1.7 ms in nonstretched half-cell, n=16); all parameters assessed in sparks obtained during synchronous recording of stretched and non-stretched segments of the same cell (Figure 6).
Figure 6.
Parameters describing Ca2+ spark dynamics during application of half-cell stretch. No significant differences were observed between sparks in the stretched and nonstretched cell areas in relative (F/F0) spark amplitude (A), time to peak (B), and decay time constant (C). Number of observations shown in brackets; ns indicates nonsignificant.
Ca2+ spark rate may be augmented by extracellular Ca2+ influx causing Ca2+-induced Ca2+ release via RyR2. To investigate a possible involvement of extracellular Ca2+ influx via stretch-activated ion channels (SAC), we performed the half-cell stretch protocol after 10 minutes preincubation with 2 gmol/L of the SAC blocker GsMTx-4. As illustrated in Figure 5B, GsMTx-4 did not affect the acute stretch-induced increase of Ca2± spark rate (126.2 ± 3.7% in stretched versus 91.5 ± 10.1% in nonstretched half-cell; P=0.012 and P>0.05, respectively; n=9).
Because mechanically induced Ca2± entry into cardiomyocytes could occur via other channels or transporters,20,21 we assessed possible roles of trans-sarcolemmal Ca2± influx using the half-cell stretch protocol in Na±/Ca2±-free solution (0NC; containing [in mmol/L] LiCl 140, KCl 10, EGTA 10, MgCl2 1, HEPES 5, glucose 11). The perfusate was switched from normal Tyrode to 0NC 5 seconds before stretch application to record control spark rate. As shown in Figure 5C, the 0NC environment did not abolish the acute stretch-induced increase in Ca2± spark rate (131.7±8.4% in stretched versus 74.3±11.2% in nonstretched half-cell; P<0.01 and P>0.05, respectively; n=8).
To probe a possible involvement of NO in the acute response of Ca2± spark rate to stretch, we performed the half-cell stretch protocol after 10 minutes preincubation with 1 mmol/L NG-nitro-L-arginine methyl ester (L-NAME) to block NO synthase. As shown in Figure 5D, this intervention did not abolish the increase in Ca2± spark rate in the stretched part of the cell (126.6±7.3% in stretched versus 96.6±7.0% in nonstretched half-cell; P<0.01 and P>0.05, respectively; n=7).
The possible involvement of cytoskeletal structures in the transmission of mechanical cues from cell surface to the T-tubular–SR complex was assessed by preincubation of cells, for 2 hours, with 10 gmol/L colchicine, which prevents microtubule polymerization. This protocol has previously been shown to cause a significant reduction in microtubular integrity in rat cardiomyocytes, reducing/3-tubulin–specific fluorescence by 38% to 43%.22,23 Colchicine did not affect the control spark rate before stretch application (12 383±553 sparks · mm−2 · sec−1 in control, n=10; versus 12 600±852 sparks · mm−2 · sec−1 in colchicine-treated cells, n=12), suggesting that nonspecific drug effects had little impact, if any, on the observed parameter. As shown in Figure 5E, after colchicine pretreatment no significant changes in Ca2± spark rate were observed on acute axial distension (108.9±3.5% in stretched versus 99.0±5.2% in nonstretched half-cell, n=12).
The spatial interrelation of microtubules with the T-tubular–SR membrane complex was further investigated in 3D, using ET. Figure 7 shows 2 representative XY tomographic sections (taken in the plane of the preparation, 14.25 nm apart; Figure 7A and 7B). These reconstructed sections, which are qualitatively similar to the kind of data obtained in transmission ET, highlight the relative ease with which one can identify extended membrane structures (such as the T-tubular–SR membrane complex, highlighted in yellow and red, respectively, in Figure 7C), compared to the difficulty of tracking filamentous structures that proceed at an oblique angle relative to the imaging plane (such as microtubules). One advantage of ET is that one can “cut” the 3D imaging data set in any desired plane, for example coaligned with a microtubule of interest (Figure 7D). In addition, the ability to track membranes and filamentous structures throughout a physically connected 3D space allows one to reconstruct spatially accurate 3D models of microtubules, SR, and T-tubular membranes (Figure 7E). Microtubules regularly traverse the T-tubular–SR membrane complex, which contains the cytoplasmic domain of RyR2 (arrowheads in Figure 7C). In the studied examples, microtubules approach SR and T-tubular membranes to within 7 and 13 nm, respectively, suggesting spatial proximity that is close enough to support mechanical interaction.
Figure 7.
Example of ET sections and 3D model of a T-tubular–SR membrane complex with associated microtubule from a rat ventricular cardiomyocyte. A and B show 2 virtual XY sections of the 3D ET data, taken at a planar distance of 14.25 nm. C shows the same XY section as A, with added indication of the traced T-tubular membrane (T-tub) (yellow outline), SR (red), and a microtubule (MT) (green). Gray densities between T-tubular and SR membranes (arrowheads) are indicative of the position of ryanodine receptors. D takes advantage of the 3D nature of ET data, presenting a virtual section aligned with the axis of a microtubule in the ET stack (relative position of the XY image planes shown in A and B indicated by arrows). E illustrates the 3D model, extracted from this data set, showing details of the spatial interrelation between microtubule and T-tubular–SR system. The microtubule passes through gaps between SR and T-tubular membranes and approaches them to within 7 and 13 nm, respectively). M indicates mitochondria; mf, myofilaments; Z, z-line. Scale bar: 200 nm. See “3D-EM-tomography” movie in the online data supplement.
Discussion
Diastolic stretch gives rise to an acute and transient increase in Ca2± spark rate, without changing the dynamic properties of individual sparks. Using the whole-cell stretch protocol (which has the advantage of maximizing the observation area), it was established that the stretch-induced increase in Ca2± spark rate (to 130.7±6.4% of control) occurs acutely, within the first 5 seconds (minimum observation period with sufficient power to conduct statistical analysis), and that this increase is of a transient nature, with spark rate returning to near control levels (104.4±5.1%) within 1 minute. This response cannot be explained by spatial rearrangement of RyR2, even though constant volume behavior predicts radial compression (by approximately −4%) during axial strain (by ±8%). Because the total number of RyR2 in the stretched cell portion does not change either, net density of RyR2 in any subvolume that is large compared to the dimensions of a single sarcomere is unlikely to show a significant change. In addition, the transient nature of the observed spark rate response (in the presence of maintained stretch) makes it unlikely that spatial rearrangement of RyR2 plays a significant role in this context.
A possible explanation for the initial increase in spark rate would be that axial cell stretch causes membrane depolarization, promoting Ca2± influx that could stimulate sparks. However, the local nature of the stretch-induced increase in Ca2± spark rate, established in half-cell stretch experiments (Figures 4 and 5), argues against any mechanism of inherently whole-cell nature.
Alternatively, stretch could increase transsarcolemmal Ca2± influx, perhaps via SAC, to an extent that might be small enough to have no effect on the membrane potential, while still acting locally to promote SR Ca2± release events. However, the lack of an effect of either GsMTx-4 exposure (Figure 5B) or perfusion with 0NC solution (Figure 5C) suggests that any contribution of trans-sarcolemmal Ca2± fluxes to the acute stretch-induced increase in Ca2± spark rate must be negligible.
This may be different during sustained axial stretch where, as previously illustrated by Gannier et al, an increase in resting [Ca2±]i may occur via a streptomycin-sensitive mechanism (streptomycin also blocks SAC24), perhaps involving Ca2± influx via SAC, or secondary effects of Na± influx via SAC on the Na±/Ca2± exchanger activity.25,26 This may contribute to an involvement of SAC in the slow force response to stretch (which is accompanied by an increase in [Ca2±]i transients).27,28 However, these mechanisms appear to require time periods of 5 minutes or more to affect cell function. In contrast, the acute increase in Ca2± spark rate observed here occurs in resting cells, within the first 5 seconds of stretch application, and even in the absence of extracellular Ca2.
Similar time constraints appear to apply to NO-mediated effects of stretch on Ca2 spark rate, which have been reported on exposure of rat cardiomyocytes to 10 minutes stretch.12 The acute stretch-induced increase in Ca2 spark rate observed here is of a transient nature (Figure 3) and involves different mechanisms, as it is not blocked by preincubation with L-NAME (Figure 5D). In common with the previously reported late effects of stretch on Ca2 spark activity,12 our data show no changes in spark amplitude, time to peak, or decay time constant (Figure 6). This suggests that stretch is unlikely to act via an increase in the Ca2 conductance of individual RyR2 or in the number of RyR2 recruited in a spark event cluster.
An alternative explanation is that axial cell stretch, via a hitherto unidentified pathway, increases the open-probability of RyR2. This could cause an acute increase in Ca2 spark rate, enhancing SR Ca2 leak, and thereby causing partial depletion of the SR (as Ca2, released into the dyadic cleft, will only partially be pumped back into SR, and partially extruded from the cell via Na/Ca2 exchanger). Such early stretch-induced reduction in [Ca2]SR has been reported before.8,9 Because [Ca2]SR is an important driver of Ca2 spark rate,4 this would allow the cell to return to near-equilibrium Ca2 spark rates, once the opposing effects of stretch and SR Ca2 load on RyR2 open probability have balanced out.
Several studies have discussed an involvement of the cytoskeleton in Ca2 handling, partially with contradictory results.22,23,29 Most recently, cardiomyocytes from the murine model of Duchene’s muscular dystrophy (ie, the dystrophin null MDX mouse) have been reported to respond to increased mechanical loads (whether applied as axial stretch by CFs30 or via osmotic swelling31) with an augmentation in SR Ca2 release. Of particular interest, in this context, is the observation that among the compensatory adaptations in the MDX heart there is an 1.4-fold increase in/3-tubulin,32 which, based on our findings, may strongly contribute to mechanically-promoted SR Ca2 release in this disease model.
Our findings highlight that microtubule integrity is obligatory for the acute stretch-induced increase in Ca2+ spark rate (Figure 5E). The actual mechanisms underlying this involvement of the cytoskeleton are not clear. Based on the close proximity of microtubules with SR and T-tubular membranes (10−8 m; Figure 7), one might speculate on the possibility of physical transmission of stress or strain from sarcolemmal CF attachment points to RyR2 or membrane areas near RyR2 via microtubules. As major force-bearing components of the nonsarcomeric cytoskeleton, microtubules contribute to cardiomyocyte stiffness during axial compression (when microtubules “buckle,” contributing to passive load and cell recoil), although they appear not to affect tensile or viscoelastic behavior during axial elongation (which is best explained by their ability to translocate in the direction of positive strain).33–36 Microtubules are laterally enforced, both by the cytosolic viscosity and by direct elastic cytoskeletal links, as can be illustrated by the observation that neighboring microtubules in cardiomyocytes “often buckle … in a coordinated manner, both temporally and spatially in phase.”37 Such coordinated buckling of microtubules in cardiomyocytes has been observed over distances in the 10−6 m region, highlighting the plausibility of mechanical interference between microtubules and the T-tubular–SR membrane complex.
It is possible, therefore, that microtubules mechanically interfere with the SR in a way that may affect RyR2 open probability in a manner akin to SAC activation. The approximately 102 RyR2 receptors within the Ca2+ release unit are thought to interact with each other mechanically, contributing to the coordinated generation and termination of Ca2+ sparks,38 and it is only a modest extension of this notion to suggest that RyR2 gating may be mechanically influenced by microtubule-mediated perturbation of the T-tubular–SR membrane complex. Similar deformation-induced increases in Ca2+ spark rate have been observed in the depth of atrial myocytes during sarcolemmal fluid-jet stimulation.39 Also, RyR2 mechanosensitivity could underlie the fluid-pressure induced increase in Ca2+-induced Ca2+ releasability from the SR, observed in rat ventricular cardiomyocytes.40 Alternatively, there may be mitochondria-mediated responses,39 or currently unknown effects of fast-acting local signal transduction pathways that are relevant for RyR2 function and affected by the cytoskeleton.41
In conclusion, axial stretch of rat cardiomyocytes acutely and transiently increases Ca2+ spark rate via pathways that are independent of SAC, NO, and transsarcolemmal Ca2+ influx but that do require cytoskeletal integrity. The mechanisms, interplay, and functional relevance of acute and late stretch effects on Ca2+ spark rate, as well as the interrelation of cytoskeletal elements withCa2+ handling cell structures, form worthwhile targets for further elucidation.
Supplementary Material
Acknowledgments
We thank Dr Judith Sheldon (Oxford Brookes University) and Dr Eileen O’Toole (University of Colorado at Boulder) for bioimaging support, as well as Philip Cobden (University of Oxford), Dr Terry B. Rogers (University of Maryland), Dr Joshua Lynch, and Derek Zachman (University of Colorado at Boulder) for provision of isolated cardiomyocytes.
Sources of Funding
This study was supported British Heart Foundation grant BHF-04087 (to P.K.); NIH/National Center for Research Resources grant RR000592 (A.H.); NIH grants R01-HL081106, R01-HL36974, and P01-HL67849 (to W.J.L.); NIH grant R03-AR053318 (to C.W.); the UK Biotechnology and Biological Sciences Research Council (UK-Japan Partnership Award [to P.K. and G.I.]). G.I. received training support from Eisai Co Ltd. P.C. holds a Junior Research Fellowship at Christ Church College Oxford. P.K. is a British Heart Foundation Senior Research Fellow (grant BHF-17414).
Footnotes
Disclosures
None.
References
- 1.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 2.Calaghan SC, White E. The role of calcium in the response of cardiac muscle to stretch. Prog Biophys Mol Biol. 1999;71:59–90. doi: 10.1016/s0079-6107(98)00037-6. [DOI] [PubMed] [Google Scholar]
- 3.Diaz ME, O’Neill SC, Eisner DA. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res. 2004;94:650–656. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- 4.Satoh H, Blatter LA, Bers DM. Effects of [Ca2+]i SR Ca2+ load, and reston Ca2+ spark frequency in ventricular myocytes. Am J Physiol. 1997;272:H657–H668. doi: 10.1152/ajpheart.1997.272.2.H657. [DOI] [PubMed] [Google Scholar]
- 5.Bers DM. SR Ca loading in cardiac muscle preparations based on rapid-cooling contractures. Am J Physiol. 1989;256:C109–C120. doi: 10.1152/ajpcell.1989.256.1.C109. [DOI] [PubMed] [Google Scholar]
- 6.Pieske B, Sutterlin M, Schmidt-Schweda S, Minami K, Meyer M, Olschewski M, Holubarsch C, Just H, Hasenfuss G. Diminished post-rest potentiation of contractile force in human dilated cardiomyopathy. Functional evidence for alterations in intracellular Ca2+ handling. J Clin Invest. 1996;98:764–776. doi: 10.1172/JCI118849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shattock MJ, Bers DM. Rat vs. rabbit ventricle: Ca2+ flux and intracellular Na+ assessed by ion-selective microelectrodes. Am J Physiol. 1989;256:C813–C822. doi: 10.1152/ajpcell.1989.256.4.C813. [DOI] [PubMed] [Google Scholar]
- 8.Gamble J, Taylor PB, Kenno KA. Myocardial stretch alters twitch characteristics and Ca2+ loading of sarcoplasmic reticulum in rat ventricular muscle. Cardiovasc Res. 1992;26:865–870. doi: 10.1093/cvr/26.9.865. [DOI] [PubMed] [Google Scholar]
- 9.Iribe G, Kohl P. Axial stretch enhances sarcoplasmic reticulum Ca2+ leak and cellular Ca2+ reuptake in guinea pig ventricular myocytes: Experiments and models. Prog Biophys Mol Biol. 2008;97:298–311. doi: 10.1016/j.pbiomolbio.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 11.Diaz ME, Graham HK, O’Neill SC, Trafford AW, Eisner DA. The control of sarcoplasmic reticulum Ca2+ content in cardiac muscle. Cell Calcium. 2005;38:391–396. doi: 10.1016/j.ceca.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Petroff MG, Kim SH, Pepe S, Dessy C, Marban E, Balligand JL, Sollott SJ. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- 13.Mitra R, Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985;249:H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- 14.Iribe G, Helmes M, Kohl P. Force-length relations in isolated intact cardiomyocytes subjected to dynamic changes in mechanical load. Am J Physiol. 2007;292:H1487–1497. doi: 10.1152/ajpheart.00909.2006. [DOI] [PubMed] [Google Scholar]
- 15.Cheng H, Song LS, Shirokova N, Gonzalez A, Lakatta EG, Rios E, Stern MD. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys J. 1999;76:606–617. doi: 10.1016/S0006-3495(99)77229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chun LG, Ward CW, Schneider MF. Ca2+ sparks are initiated by Ca2+ entry in embryonic mouse skeletal muscle and decrease in frequency postnatally. Am J Physiol. 2003;285:C686–C697. doi: 10.1152/ajpcell.00072.2003. [DOI] [PubMed] [Google Scholar]
- 17.McIntosh R, Nicastro D, Mastronarde D. New views of cells in 3D: an introduction to electron tomography. Trends Cell Biol. 2005;15:43–51. doi: 10.1016/j.tcb.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Lucic V, Forster F, Baumeister W. Structural studies by electron tomography: from cells to molecules. Annu Rev Biochem. 2005;74:833–865. doi: 10.1146/annurev.biochem.73.011303.074112. [DOI] [PubMed] [Google Scholar]
- 19.Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 20.Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- 21.Kupittayanant P, Trafford AW, Diaz ME, Eisner DA. A mechanism distinct from the L-type Ca current or Na-Ca exchange contributes to Ca entry in rat ventricular myocytes. Cell Calcium. 2006;39:417–423. doi: 10.1016/j.ceca.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Calaghan SC, Le Guennec JY, White E. Modulation of Ca2 signaling by microtubule disruption in rat ventricular myocytes and its dependence on the ruptured patch-clamp configuration. Circ Res. 2001;88:e32–e37. doi: 10.1161/01.res.88.4.e32. [DOI] [PubMed] [Google Scholar]
- 23.Gomez AM, Kerfant BG, Vassort G. Microtubule disruption modulates Ca2 signaling in rat cardiac myocytes. Circ Res. 2000;86:30–36. doi: 10.1161/01.res.86.1.30. [DOI] [PubMed] [Google Scholar]
- 24.Belus A, White E. Effects of streptomycin sulphate on I(CaL), I(Kr) and I(Ks) in guinea-pig ventricular myocytes. Eur J Pharmacol. 2002;445:171–178. doi: 10.1016/s0014-2999(02)01791-0. [DOI] [PubMed] [Google Scholar]
- 25.Gannier F, White E, Garnier, Le Guennec JY. A possible mechanism for large stretch-induced increase in [Ca2]i in isolated guinea-pig ventricular myocytes. Cardiovasc Res. 1996;32:158–167. [PubMed] [Google Scholar]
- 26.Gannier F, White E, Lacampagne A, Garnier D, Le Guennec JY. Streptomycin reverses a large stretch induced increases in [Ca2]i in isolated guinea pig ventricular myocytes. Cardiovasc Res. 1994;28:1193–1198. doi: 10.1093/cvr/28.8.1193. [DOI] [PubMed] [Google Scholar]
- 27.Calaghan S, White E. Activation of Na -H exchange and stretch-activated channels underlies the slow inotropic response to stretch in myocytes and muscle from the rat heart. J Physiol. 2004;559:205–214. doi: 10.1113/jphysiol.2004.069021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ennis IL, Garciarena CD, Perez NG, Dulce RA, Camilion de Hurtado MC, Cingolani HE. Endothelin isoforms and the response to myocardial stretch. Am J Physiol. 2005;288:H2925–H2930. doi: 10.1152/ajpheart.01202.2004. [DOI] [PubMed] [Google Scholar]
- 29.Leach RN, Desai JC, Orchard CH. Effect of cytoskeleton disruptors on L-type Ca channel distribution in rat ventricular myocytes. Cell Calcium. 2005;38:515–526. doi: 10.1016/j.ceca.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Yasuda S, Townsend D, Michele DE, Favre EG, Day SM, Metzger JM. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature. 2005;436:1025–1029. doi: 10.1038/nature03844. [DOI] [PubMed] [Google Scholar]
- 31.Jung C, Martins AS, Niggli E, Shirokova N. Dystrophic cardiomyopathy: amplification of cellular damage by Ca2 signalling and reactive oxygen species-generating pathways. Cardiovasc Res. 2008;77:766–773. doi: 10.1093/cvr/cvm089. [DOI] [PubMed] [Google Scholar]
- 32.Wilding JR, Schneider JE, Sang AE, Davies KE, Neubauer S, Clarke K. Dystrophin- and MLP-deficient mouse hearts: marked differences in morphology and function, but similar accumulation of cytoskeletal proteins. FASEB J. 2005;19:79–81. doi: 10.1096/fj.04-1731fje. [DOI] [PubMed] [Google Scholar]
- 33.Cooper Gt. Cardiocyte cytoskeleton in hypertrophied myocardium. Heart Failure Rev. 2000;5:187–201. doi: 10.1023/A:1009836918377. [DOI] [PubMed] [Google Scholar]
- 34.Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68:1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishimura S, Nagai S, Katoh M, Yamashita H, Saeki Y, Okada J, Hisada T, Nagai R, Sugiura S. Microtubules modulate the stiffness of cardiomyocytes against shear stress. Circ Res. 2006;98:81–87. doi: 10.1161/01.RES.0000197785.51819.e8. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto S, Tsutsui H, Takahashi M, Ishibashi Y, Tagawa H, Imanaka-Yoshida K, Saeki Y, Takeshita A. Role of microtubules in the viscoelastic properties of isolated cardiac muscle. J Mol Cell Cardiol. 1998;30:1841–1853. doi: 10.1006/jmcc.1998.0747. [DOI] [PubMed] [Google Scholar]
- 37.Brangwynne CP, MacKintosh FC, Kumar S, Geisse NA, Talbot J, Mahadevan L, Parker KK, Ingber DE, Weitz DA. Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J Cell Biol. 2006;173:733–741. doi: 10.1083/jcb.200601060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobie EA, Dilly KW, dos Santos CJ, Lederer WJ, Jafri MS. Termination of cardiac Ca2 sparks: an investigative mathematical model of calcium-induced calcium release. Biophys J. 2002;83:59–78. doi: 10.1016/s0006-3495(02)75149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belmonte S, Morad M. ‘Pressure-flow’-triggered intracellular Ca2 transients in rat cardiac myocytes: possible mechanisms and role of mitochondria. J Physiol. 2008;586:1379–1397. doi: 10.1113/jphysiol.2007.149294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S, Kim JC, Li Y, Son MJ, Woo SH. Fluid pressure modulates L-type Ca2 channel via enhancement of Ca2 -induced Ca2 release in rat ventricular myocytes. Am J Physiol. 2008;294:C966–C976. doi: 10.1152/ajpcell.00381.2007. [DOI] [PubMed] [Google Scholar]
- 41.Ingber DE. Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Biol. 2008;97:163–179. doi: 10.1016/j.pbiomolbio.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.