Abstract
Background
Glossina fuscipes fuscipes is the primary vector of trypanosomiasis in humans and livestock in Uganda. The Lake Victoria basin has been targeted for tsetse eradication using a rolling carpet initiative, from west to east, with four operational blocks (3 in Uganda and 1 in Kenya), under a Pan-African Tsetse and Trypanosomiasis Eradication Campaign (PATTEC). We screened tsetse flies from the three Ugandan PATTEC blocks for genetic diversity at 15 microsatellite loci from continental and offshore populations to provide empirical data to support this initiative.
Methods
We collected tsetse samples from 11 sites across the Lake Victoria basin in Uganda. We performed genetic analyses on 409 of the collected tsetse flies and added data collected for 278 individuals in a previous study. The flies were screened across 15 microsatellite loci and the resulting data were used to assess the temporal stability of populations, to analyze patterns of genetic exchange and structuring, to estimate dispersal rates and evaluate the sex bias in dispersal, as well as to estimate demographic parameters (NE and NC).
Results
We found that tsetse populations in this region were stable over 4-16 generations and belong to 4 genetic clusters. Two genetic clusters (1 and 2) corresponded approximately to PATTEC blocks 1 and 2, while the other two (3 and 4) fell within PATTEC block 3. Island populations grouped into the same genetic clusters as neighboring mainland sites, suggesting presence of gene flow between these sites. There was no evidence of the stretch of water separating islands from the mainland forming a significant barrier to dispersal. Dispersal rates ranged from 2.5 km per generation in cluster 1 to 14 km per generation in clusters 3 and 4. We found evidence of male-biased dispersal. Few breeders are successfully dispersing over large distances. Effective population size estimates were low (33–310 individuals), while census size estimates ranged from 1200 (cluster 1) to 4100 (clusters 3 and 4). We present here a novel technique that adapts an existing census size estimation method to sampling without replacement, the scheme used in sampling tsetse flies.
Conclusion
Our study suggests that different control strategies should be implemented for the three PATTEC blocks and that, given the high potential for re-invasion from island sites, mainland and offshore sites in each block should be targeted at the same time.
Keywords: Glossina fuscipes fuscipes, Tsetse, Tryaponosomiasis, Vector, Gene flow, Census size
Background
The tsetse fly (Glossina) is a major vector of trypanosomiasis throughout sub-Saharan Africa, causing extensive morbidity and mortality in humans and livestock [1,2]. It has been estimated that economic benefits to Africa from the eradication of tsetse could reach US$4.5 billion per year. Currently, no vaccines exist to prevent the disease and available drugs to treat HAT are expensive, can cause severe side-effects, and are difficult to administer in remote villages [3]. As a consequence, an effective alternative for controlling the disease is to target the tsetse vector [4,5]. A variety of methods to control tsetse populations are available, including habitat modification around homesteads, trapping, insecticide-treated targets, insecticide-treated cattle, and aerial or ground spraying. The release of sterile or transgenic insects has been either used or proposed as an additional control measure [4-9]. Tsetse control is implemented using two strategies: eradication and suppression. Eradication aims at eliminating fly populations from a given area, while the objective of suppression is to greatly reduce population size.
Genetic data provide a powerful tool to help identify appropriate vector control strategies, as they can be used to estimate spatial and temporal differentiation of populations, and patterns and extent of migration. These data, together with ecological and environmental, data can be used to customize vector control efforts according to local conditions and species in order to determine whether eradication or suppression is appropriate [10,11]. For example, studies of tsetse in Burkina Faso, Guinea and Senegal have identified populations that are sufficiently isolated to warrant attempts at complete eradication [10-12]. Studies elsewhere have documented relatively high levels of gene flow, necessitating integration of barriers into eradication schemes [13,14], or warranting an area-wide control effort that encompasses populations linked by gene flow [15,16]. Regional studies such as the one on G. palpalis palpalis in west and central Africa [17] have provided information that is useful for control efforts at a regional scale. Other population genetic studies have pointed to specific populations at a local level where control and detection methods need improvement [18].
In 2001, the Organization for African Unity (OAU) launched a new initiative, the Pan-African Tsetse and Trypanosomiasis Eradication Campaign (PATTEC) to eradicate the tsetse flies from a vast area of sub-Saharan Africa (~10 million km2, seven Glossina species) by first reducing populations using area-wide approaches (odor-baited traps, insecticide-treated targets, pour-ons and ultra-low-volume aerial spraying), followed by massive release of sterile males to ensure eradication [19]. In Uganda, phase I of this initiative has been initiated in the Lake Victoria basin, which is infested with Glossina fuscipes fuscipes (Gff). A program (Farming in Tsetse Controlled Areas, FITCA), which ended in 2004, reduced tsetse populations by 75% to 90% in the mainland sites of the target area, but the program did not include islands [20]. By 2009, a PATTEC baseline survey revealed that mainland tsetse populations had rebounded to the high levels prior to the FITCA initiative [21].
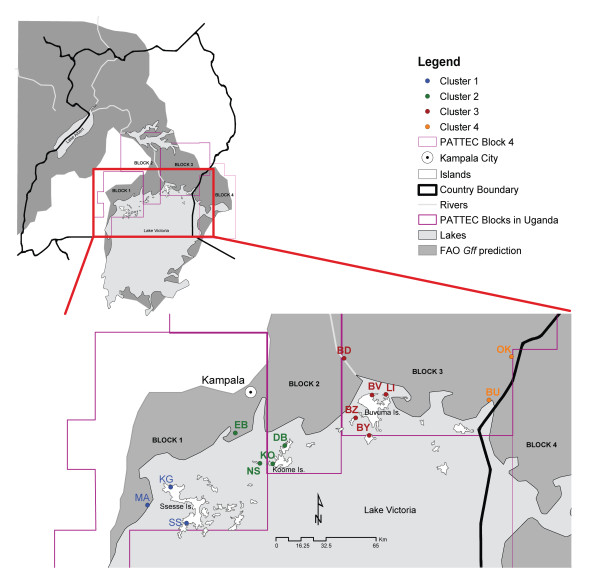
The PATTEC plan, unlike FITCA, includes islands and intends to eradicate Gff from the Lake Victoria basin progressively from west to east [22]. The basin has been partitioned into four operational blocks (3 in Uganda and 1 in Kenya) based on the Food and Agriculture Organization (FAO) predicted habitat suitability for Gff, natural barriers, major urban areas, international borders and drainage patterns [22,23]. Block 1 (Figure 1), targeted for Gff eradication during PATTEC phase 1, is the most isolated block due to the expansion of the city of Kampala and subsequent urbanization and habitat fragmentation of the surrounding area. Block 2 has been targeted for control to create a buffer between the eradication block and the rest of the Gff predicted range in this area of Uganda (Figure 1). Only vector population monitoring activities are planned for the other two blocks during phase 1. Upon successful eradication in block 1, block 2 would become the eradication target and so on until the whole basin is tsetse-free. To support the PATTEC initiative, the government of Uganda and the International Atomic Energy Agency (IAEA) is planning a trial eradication of Gff on a remote island in the region (IAEA Project UGA5033) because of previous success of tsetse eradication on islands in Equatorial Guinea [24] and Zanzibar [25].
Figure 1.
Map of sampling sites. Location of sampling sites (colored dots and location codes) is shown with reference to the three intervention blocks (purple contours) defined by PATTEC in Uganda. The fourth block in Kenya is also shown. Dot and location code color denotes the genetic cluster to which the majority of individuals at each site were assigned (blue – cluster 1, green – cluster 2, red – cluster 3, and orange – cluster 4). The inset in the upper left corner shows the location of sampling sites and PATTEC blocks with reference to the whole of Uganda and neighboring countries.
We used genetic variation at 15 microsatellite DNA loci to examine the genetic differentiation of Gff populations within and between the three Ugandan PATTEC blocks. We used these data to estimate effective population size (NE), to evaluate temporal stability over 4-16 generations, and to measure genetic exchange and dispersal rates within and between blocks. In view of the finding that high levels of genetic exchange occur among continental Ugandan populations of Gff separated by distances smaller than 100 km [16], we embarked upon an investigation of gene flow patterns at a finer scale and used the results to assess the validity of the PATTEC operational blocks with respect to vector control, to provide suggestions regarding control strategies in each block, and to evaluate the possibility of using Gff island populations for eradication trials.
Methods
Sampling
We sampled Gff at 3 continental localities across the Lake Victoria basin in Uganda (sites BD, EB, and MA: Table 1 and Figure 1) and at 8 localities from three groups of islands in Lake Victoria (sites BV, BY, BZ and LI from Buvuma islands; DB, NS and KO from Koome islands; and KG from Ssese islands, Table 1 and Figure 1). We used 409 tsetse flies from the above 11 sampling sites for genetic analyses. Several localities were sampled over two to three months both in the wet (April-June 2010) and the dry (February-March 2010 or February-March 2011) season (Table 1a). In addition to the data we collected from these 11 sites, we included data collected for 278 individuals sampled between the wet season of 2008 and the dry season of 2009: BU, OK [26] and SS (unpublished) (Table 1b). Assuming that Gff goes through approximately 8 generations per year [27,28], seasonal samples were about 4 generations apart. Tsetse flies were caught using biconical traps [29] and preserved individually in cryo-tubes containing 90% ethanol.
Table 1.
Sample collection information and genetic summary statistics
| Population | Code | N | Longitude | Latitude | Season | Collection Date |
Seasonal and Total |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean AR | Mean HO | Mean HE | Mean FIS | |||||||||||
|
(a) New sites for this study | ||||||||||||||
| Budondo |
BD |
35 |
33.1209 |
0.5208 |
dry |
March, 2011 |
4.867 |
4.867 |
0.516 |
0.516 |
0.513 |
0.513 |
−0.005 |
−0.005 |
| Buvuma Is. |
BV |
21 |
33.2788 |
0.1368 |
dry |
Feb, 2010 |
3.867 |
4.800 |
0.446 |
0.477 |
0.476 |
0.506 |
0.076 |
0.057 |
| Buvuma Is. |
BV |
39 |
33.2788 |
0.1368 |
wet |
June, 2010 |
4.400 |
0.493 |
0.513 |
0.035 |
||||
| Bugaya Is. |
BY |
27 |
33.2684 |
0.0675 |
dry |
Feb, 2010 |
4.067 |
4.867 |
0.499 |
0.486 |
0.496 |
0.497 |
−0.017 |
0.011 |
| Bugaya Is. |
BY |
35 |
33.2684 |
0.0675 |
wet |
June, 2010 |
4.400 |
0.476 |
0.490 |
0.018 |
||||
| Buziri Is. |
BZ |
7 |
33.1883 |
0.1716 |
dry |
Feb, 2010 |
2.933 |
3.600 |
0.441 |
0.454 |
0.460 |
0.477 |
0.064 |
0.051 |
| Buziri Is. |
BZ |
11 |
33.1883 |
0.1716 |
wet |
June, 2010 |
3.133 |
0.461 |
0.465 |
0.021 |
||||
| Lingira Is. |
LI |
29 |
33.3532 |
0.3168 |
dry |
Feb, 2010 |
4.933 |
5.200 |
0.519 |
0.509 |
0.511 |
0.509 |
−0.021 |
0.002 |
| Lingira Is. |
LI |
17 |
33.3532 |
0.3168 |
wet |
June, 2010 |
4.000 |
0.494 |
0.492 |
0.012 |
||||
| Damba Is. |
DB |
32 |
32.7659 |
0.0127 |
wet |
Apr, 2010 |
3.067 |
3.067 |
0.394 |
0.394 |
0.394 |
0.394 |
0.015 |
0.015 |
| Koome Is. |
KO |
40 |
32.6879 |
−0.0911 |
wet |
Apr, 2010 |
3.267 |
3.267 |
0.318 |
0.318 |
0.360 |
0.360 |
0.187 |
0.187 |
| Nsazi Is. |
NS |
16 |
32.6296 |
−0.0956 |
wet |
Apr, 2010 |
3.000 |
3.000 |
0.322 |
0.322 |
0.340 |
0.340 |
0.114 |
0.114 |
| Entebbe |
EB |
35 |
32.4852 |
0.0823 |
dry |
March, 2011 |
3.667 |
3.667 |
0.413 |
0.413 |
0.418 |
0.418 |
0.006 |
0.006 |
| Kalangala Is. |
KG |
32 |
32.1083 |
−0.2267 |
wet |
March, 2010 |
3.000 |
3.000 |
0.288 |
0.288 |
0.302 |
0.302 |
0.098 |
0.098 |
| Masaka |
MA |
33 |
31.9852 |
−0.3564 |
dry |
March, 2011 |
2.867 |
2.867 |
0.329 |
0.329 |
0.296 |
0.296 |
−0.098 |
−0.098 |
|
(b) Sites from previous studies | ||||||||||||||
| Busime |
BU |
39 |
33.9711 |
0.2508 |
wet |
March, 2008 |
4.733 |
5.400 |
0.486 |
0.486 |
0.507 |
0.502 |
0.027 |
|
| Busime |
BU |
40 |
33.9711 |
0.2508 |
wet |
March, 2009 |
4.467 |
0.494 |
0.499 |
0.047 |
||||
| Busime |
BU |
40 |
33.9711 |
0.2508 |
dry |
Oct, 2009 |
4.200 |
0.479 |
0.487 |
0.012 |
||||
| Okame |
OK |
39 |
33.3532 |
0.3168 |
wet |
March, 2008 |
4.000 |
4.533 |
0.483 |
0.516 |
0.529 |
0.535 |
0.083 |
0.032 |
| Okame |
OK |
40 |
33.3532 |
0.3168 |
wet |
March, 2009 |
3.933 |
0.541 |
0.532 |
−0.010 |
||||
| Okame |
OK |
39 |
33.3532 |
0.3168 |
dry |
Oct, 2009 |
4.133 |
0.524 |
0.532 |
0.012 |
||||
| Ssese Is. | SS | 40 | 32.1691 | −0.5022 | wet | Apr, 2009 | 3.467 | 3.467 | 0.333 | 0.333 | 0.332 | 0.332 | −0.016 | −0.016 |
Summary statistics are based on 15 microsatellite loci, both for (a) recently collected tsetse specimens and (b) flies collected for previous studies. N – number of individuals analyzed. Mean across all loci of AR – allelic richness, HO – observed heterozygosity, HE – expected heterozygosity and FIS – inbreeding coefficient. AR, HO, HE and FIS are reported for the dry and the wet season separately (seasonal), and for both seasons (total).
Data collection
DNA was extracted from tsetse legs using the PrepGEM Insect DNA extraction kit (ZYGEM Corp Ltd, Hamilton, New Zealand) as per the protocol provided by the manufacturer. We collected genotypic data across 18 microsatellite loci (see Additional file 1: Supplementary Material for technical details), including the 13 loci used in Beadell et al. [16]. The other 5 (GmmA06, GmmB20, GmmD15, GmmL03, GmmL11) were selected among the loci described in Hyseni et al. [30]. Due to low amplification and scorability of large alleles, locus Pgp17 was excluded from all analyses. We also excluded locus GpC5b, because it was monomorphic for all populations, excepting BZ and NS (in both cases, FIS = 1), and locus GmmD15, because it was monomorphic in all of the 11 sites we sampled. Thus, we only used 15 loci for all subsequent genetic analyses.
Genetic analyses
We used Genepop 4.1[31] to test for deviation from Hardy-Weinberg equilibrium (HWE) and also test for linkage disequilibrium (LD). For loci with fewer than four alleles, the complete enumeration method [32] was used. All other loci were tested using the Guo and Thompson [33] Markov chain method with 100,000 dememorizations, 1,000 batches and 10,000 iterations per batch. We also used Genepop to carry out global tests across loci for heterozygote deficiency and heterozygote excess. Significance values were adjusted for multiple testing (HWE) and comparisons (LD) using the Benjamini-Hochberg method [34] with a false discovery rate of 0.05. Summary statistics, including allele frequencies, allelic richness, observed heterozygosity (HO), expected heterozygosity (HE) and the inbreeding coefficient (FIS) were calculated using the program Genalex 6.41[35]. In order to assess the statistical significance of genetic differentiation between temporal samples, we used Genepop to perform Fisher’s exact test on both microsatellite alleles and genotypes.
We used the model-based Bayesian clustering method implemented in Structure 2.3.3[36] to determine the genetic structure present among Gff populations in the Lake Victoria basin (details in Additional file 1: Supplementary Material). In addition to the Bayesian clustering implemented in Structure, we used a two-tiered multivariate ordination analysis, which makes no assumptions about deviations from Hardy-Weinberg and linkage equilibrium. This multivariate procedure, discriminant analysis of principal components (DAPC), has been shown to perform better than the Bayesian clustering approach when hierarchical and clinal structure is present in the data [37]. We used the adegenet package [38] in R [39] for the DAPC (details in Additional file 1: Supplementary Material).
In order to quantify the genetic heterogeneity of Lake Victoria populations, we computed pairwise FST values [40] among sampling localities and among genetic clusters. FST values were obtained using the program Arlequin 3.5[41] and their significance computed via 10,000 permutations. FST describes the genetic structure produced by non-random distribution of individuals among subpopulations relative to the total population. However, our sample may contain multiple hierarchical levels of genetic differentiation, such as genetic clusters of populations with further partitioning within clusters, including potential genetic structure arising from isolation of island from mainland populations. In order to determine the contribution of different hierarchical levels to the observed genetic structure, we estimated hierarchical F-statistics using the method described in Yang [42] (details in Additional file 1: Supplementary Material) and implemented in the R package hierfstat[43].
To determine whether the genetic heterogeneity of Gff around Lake Victoria could be attributed to differences in dispersal ability between male and female flies, we performed t-tests on pairwise relatedness between individuals within genetic groups. Relatedness was computed using maximum likelihood estimation [44] implemented in Kingroup 2[45]. Sex-biased dispersal was also assessed using three tests [46] implemented in Fstat 2.9.4[47] (details in Additional file 1: Supplementary Material).
Isolation by distance (IBD) and dispersal were evaluated using Rousset’s procedure [48] within genetic groups, using both a one-dimensional (1D) and a two-dimensional (2D) stepping-stone model. We compared the two models in order to determine differences in dispersal ability along Lake Victoria depending on whether movement happens along a line or across a surface (details in Additional file 1: Supplementary Material).
We used two methods to test for individual migrants between geographically neighboring genetic clusters (clusters 1 and 2, clusters 2 and 3, and clusters 3 and 4). In the first approach, we used the software Geneclass 2.0[49] to compute the likelihood of individual assignment based on regional allele frequencies [50,51]. In the second approach, we used Flock 2.0[52] to assign genetically similar individuals to k partitions (details in Additional file 1: Supplementary Material).
We estimated effective and census population sizes for each genetic group. The geographic distance between the genetic groups should reduce the bias in estimation of effective population size (NE) that could be introduced by migration, which influences linkage disequilibrium as well as temporal methods of NE estimation [53]. NE was computed using Waples and Do’s LD method implemented in the program LDNe[54]. We also used two temporal methods to estimate NE: 1) a Bayesian algorithm based on coalescence and implemented in the program TM3[55] and 2) a pseudo-likelihood method [56] implemented in MLNE. Census size (NC) was computed via a sequential Bayesian method [57] adapted from Gazey and Staley [58] using an R software script [59]. This method applies to a sampling scheme with replacement (non-invasive genetic sampling). Tsetse sampling, however, was done without replacement. We designed a method to account for the difference in sampling, which allowed us to utilize the adapted Gazey-Staley method [57,58] to estimate NC (details in Additional file 1: Supplementary Material).
Results
We observed the lowest HO and HE values in KG (0.29 and 0.30, respectively) and the highest in OK (0.54 and 0.53, respectively), while mean allelic richness across the 15 loci ranged from 2.87 in MA to 5.40 in BU (Table 1). The highest FIS value was observed in KO (0.187). After applying the Benjamini-Hochberg false discovery rate procedure [34] to the multiple testing of HWE, significant deviation from HWE was only observed in KO, which was due to significant heterozygote deficit. After applying the same correction for multiple comparisons of linkage between loci, no evidence of significant LD was found.
Temporal stability
We examined the temporal stability of the samples for which temporal collections were available. Pairwise FST values between different sampling seasons revealed temporal homogeneity, i.e. temporal samples from the same sites were not significantly genetically differentiated. The smallest difference was observed between BU seasonal samples (FST = -0.001, P = 0.63) and highest in BY (FST = 0.003, P = 0.24). This was consistent with the similarity in allele frequencies between seasons observed in Additional file 2: Figure S1. We also carried out Fisher’s exact test on microsatellite alleles and genotypes and found no significant differences between temporal samples ( Additional file 3: Table S1).
Patterns of genetic differentiation
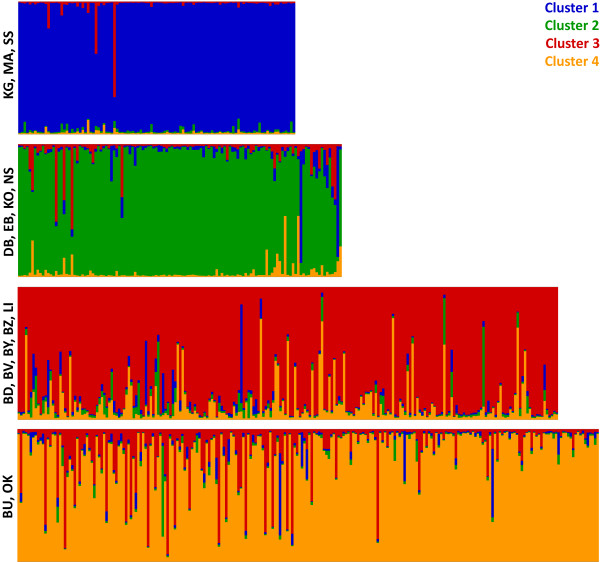
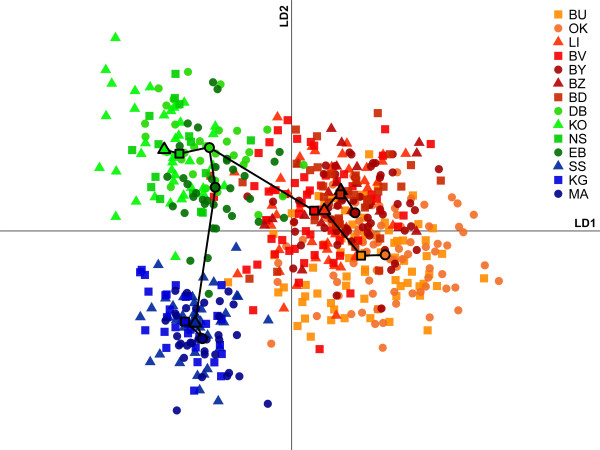
Using the Evanno criterion of ΔK, the results of the Bayesian analysis in Structure identified four distinct genetic clusters (Figure 2). The DAPC approach (Figure 3), concordantly, detected four clusters comprising the same populations. Clusters 1 and 2 are within PATTEC blocks 1 and 2. However, one continental site and one island site within block 1 (EB and NS) are genetically closer to the continental and island sites in cluster 2 than the other samples from block 1. Cluster 3 includes the Buvuma islands and adjacent mainland sites and cluster 4 includes two inland sites to the east (BU and OK). Both clusters are within PATTEC block 3. While the two westernmost clusters (1 and 2) are genetically distinct, from each other (FST = 0.184, Additional file 4: Table S2) and from clusters 3 and 4 (FST = 0.124-0.191, Additional file 4: Table S2), there is a great amount of gene flow between clusters 3 and 4. The FST value between clusters 3 and 4 (FST =0.036), while significantly different, is much lower than among other clusters. The extent of gene flow between clusters 3 and 4 is also evident from posterior probabilities of assignment obtained from Structure (Figure 2) as well as from the DAPC method (Figure 3; posterior probabilities not shown).
Figure 2.
Bayesian clustering. Probability of assignment of individuals from 14 sampling sites (abbreviated with two-letter codes; see Table 1) to each of the 4 identified clusters (blue – cluster 1, green – cluster 2, red – cluster 3, and orange – cluster 4) is denoted by the color composition of individual vertical bars.
Figure 3.
Discriminant analysis of principal components. Following selection of 15 principal components using a-score optimization, two linear discriminants (LD1 and LD2) were used to plot tsetse individuals represented in orange (cluster 4), red (cluster 3), green (cluster 2) and blue (cluster 1). Also shown is a minimum spanning tree between the centroids of the 14 populations.
The analysis of hierarchical F-statistics (Table 2) revealed only one significant hierarchical level of genetic structure, i.e. the subdivision into three genetic groups (group 1 = cluster 1, group 2 = cluster 2, group 3 = clusters 3 and 4; F3/T = 0.114, P = 0.001; Table 2a). Subdivision of group 3 into clusters 3 and 4 did not contribute significantly to the observed genetic structure (F4/3 = 0.019, P = 0.072; Table 2a). Island areas with nearby mainland sites were also analyzed separately in order to look at the contribution of distance between mainland and island sites to the genetic structuring of each cluster. Tsetse flies from island sites were not significantly isolated from mainland flies within the same cluster (cluster 1: FIM/T = −0.017, P = 1.000; cluster 2: FIM/T = 0.042, P = 0.259; cluster 3: FIM/T = 0.009, P = 0.204; Table 2b-d). Consistent with this finding, pairwise FST values ( Additional file 5: Table S3) showed that absence of significant differentiation does not carry the proviso that the compared sites be situated on the same island or that the comparison not be between island and mainland sites. For instance, in group 1, the island site, SS, was not significantly different from the mainland site, MA (FST = 0.007; Additional file 5: Table S3), but it was significantly different from the other island site, KG (FST = 0.024; Additional file 5: Table S3).
Table 2.
Hierarchical F-statistics
|
(a) Entire sample | ||||
|---|---|---|---|---|
| 3 Regions | 4 Clusters | Populations | Individuals | |
|
Total |
0.114 (F3/T) |
0.130 (F4/T) |
0.151 (FPop/T) |
0.173 |
|
3 Regions |
|
0.019 (F4/3) |
0.043 (FPop/3) |
0.067 |
|
4 Clusters |
|
|
0.024 (FPop/4) |
0.049 |
|
Populations |
|
|
|
0.026 |
|
(b) Cluster 1 | ||||
| |
Island/Mainland |
Populations |
Individuals |
|
|
Total |
−0.017 (FIM/T) |
0.009 (FPop/T) |
0.001 |
|
|
Island/Mainland |
|
0.025 (FPop/IM) |
0.017 |
|
|
Populations |
|
|
−0.009 |
|
|
(c) Cluster 2 | ||||
| |
Island/Mainland |
Populations |
Individuals |
|
|
Total |
0.042 (FIM/T) |
0.071 (FPop/T) |
0.123 |
|
|
Island/Mainland |
|
0.030 (FPop/IM) |
0.084 |
|
|
Populations |
|
|
0.055 |
|
|
(d) Cluster 3 | ||||
| |
Island/Mainland |
Populations |
Individuals |
|
|
Total |
0.009 (FIM/T) |
0.021 (FPop/T) |
0.048 |
|
|
Island/Mainland |
|
0.012 (FPop/IM) |
0.039 |
|
| Populations | 0.028 | |||
(a) Hierarchical F-statistics computations were carried out for the entire sample based on 3 hierarchical levels: (1) populations (sampling sites), (2) populations grouped into four clusters, and (3) clusters 3 and 4 grouped together. (b)-(d) Hierarchical F-statistics were computed separately for clusters 1–3 (containing populations from Ssese, Koome and Buvuma islands, respectively), based on 2 levels: (1) populations, and (2) populations grouped into island and mainland.
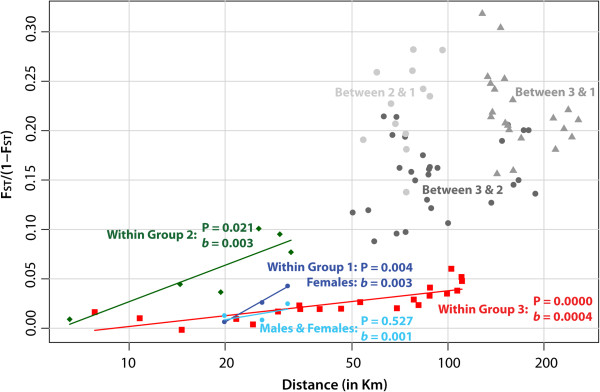
Having delineated three genetic groups through multiple methods, we regressed linearized FST (i.e., FST/(1-FST)) values against geographic distance to evaluate the occurrence of isolation by distance (IBD) within these groups (Figure 4). We detected significant IBD within groups 2 and 3 (1D: P = 0.021, P = 0.000; 2D: P = 0.022, P = 0.000). IBD within group 1 was not significant when males were included in the model (1D: P = 0.527; 2D: P = 0.568). We did, however, find significant isolation by distance among females in group 1 (1D: P = 0.004; 2D: P = 0.044). In addition to the local patterns within groups, global linearized FST patterns between groups revealed that group 1 is as isolated from the more adjacent group 2 as it is from group 3 (Figure 4), which reiterated the DAPC results (Figure 3).
Figure 4.
Isolation by distance. Isolation by distance between sites within clusters 1, 2 and the group of clusters 3 and 4. Linearized FST (i.e., FST/(1-FST)) values were regressed against geographic distance (in kilometers). Slopes (b) and P-values are shown. For cluster 1, two isolation-by-distance models are pictured (light blue = both male and female individuals; dark blue = females only). In addition to populations within clusters (blue = cluster 1, green = cluster 2, red = clusters 3 and 4), populations belonging to different clusters were regressed, separately, and are depicted in three shades of grey.
Dispersal and migration
We tested for sex-biased dispersal within the three genetic groups using four methods (Table 3). Three of the four methods showed evidence of male-biased sex dispersal. The FST-based method did not reveal significant differences between sexes, possibly because of the reduced power of this method for low dispersal rates [46]. In group 3, male-biased dispersal was only supported by mAIc (P = 0.012, Table 3). Evidence for male-biased dispersal was stronger in group 1 (vAIc and mPr were significantly different between males and females) and group 2 (mAIc, vAIc and mPr were significant).
Table 3.
Sex-biased dispersal
| FST | mAIC | vAIC | mPr | ||
|---|---|---|---|---|---|
|
Clusters 3 & 4 |
F (202) |
0.028 |
0.476 |
20.05 |
0.031 |
| |
M (179) |
0.028 |
−0.537 |
17.20 |
0.033 |
| |
P-value |
0.991 |
0.012 |
0.261 |
0.255 |
|
Cluster 2 |
F (71) |
0.061 |
0.706 |
11.64 |
0.115 |
| |
M (52) |
0.076 |
−0.964 |
22.15 |
0.137 |
| |
P-value |
0.617 |
0.024 |
0.025 |
0.001 |
|
Cluster 1 |
F (61) |
0.020 |
0.541 |
17.19 |
0.099 |
| |
M (44) |
0.013 |
−0.750 |
35.52 |
0.252 |
| P-value | 0.931 | 0.144 | 0.033 | 0.000 |
Female and male dispersal were compared within three groups: (1) cluster 1, (2) cluster 2, and (3) clusters 3 and 4. Sex differences in dispersal were evaluated using four different statistics, FST, mAIc, vAIc (mean and variance, respectively, of the Favre et al. [60] assignment index) and mPr (mean pairwise relatedness (based on Konovalov and Heg [44]). P-values in bold type are significant.
We calculated per generation dispersal (σ), dispersal surface (σ2), Wright’s neighborhood size (WN) and migration rate (m), using both 1D and 2D stepping-stone models (Table 4). In groups 1 and 2, dispersal distance σ (group 1 (females): 1D = 3.9 km, 2D = 2.5 km; group 2: 1D = 4.5 km, 2D = 3.6 km), dispersal surface σ2 (2D: 6.2 km2 and 12.9 km2), and neighborhood size (13 and 19 individuals) were similar to each other. These estimates were higher for group 3 samples (2D: σ2 = 200.1 km2, WN = 64 and σ = 14.1 km). Migration rate estimates per generation were also higher in group 3 (0.033) than the other two genetic groups (0.013 in group 1 (females) and 0.025 in group 2).
Table 4.
Population size, migration and dispersal estimates
| |
Linkage Diseq. |
Temporal (MLNE) |
Temporal (TM3) |
Mean NC |
95% HPD |
|||
|---|---|---|---|---|---|---|---|---|
| |
(LDNe) |
(Likelihood) |
(Bayesian) |
|||||
| Mean NE | (95% CI) | Mean NE | (95% CI) | Mean NE | (95% CI) | |||
| Clusters 3 & 4 |
310 |
(258–380) |
243 |
(181–327) |
240 |
(223–250) |
4121 |
(2422–6109) |
| Cluster 2 |
121 |
(88–180) |
45 |
(35–61) |
33 |
(22–47) |
1199 |
(470–2191) |
| Cluster 1 |
157 |
(98–326) |
212 |
(106–611) |
178 |
(105–285) |
1299 |
(412–2621) |
| |
NE/NC |
σ (km) |
σ (km) |
σ2(km2) |
m |
WN |
|
|
|
(1D) |
(2D) |
(2D) |
(2D) |
(2D) |
|
|
||
|
Based on LDNe estimates |
|
|
||||||
| Clusters 3 & 4 |
0.075 |
15.16 |
14.14 |
200.06 |
0.033 |
64 |
|
|
| Cluster 2 |
0.101 |
4.53 |
3.59 |
12.92 |
0.025 |
19 |
|
|
| Cluster 1 | 0.121 | 3.94 | 2.49 | 6.21 | 0.013 | 13 | ||
Estimates were computed for clusters 1, 2 and the group comprising clusters 3 and 4. Effective population size (NE) was computed using both a linkage disequilibrium method (in LDNe) and two temporal methods (a likelihood approach implemented in MLNE and a Bayesian approach implemented in TM3). Mean NE and parametric 95% confidence intervals are shown. Census size (NC) was computed using a sequential Bayesian method [51]. Mean NC and 95% highest probability density (HPD) intervals are shown. Dispersal distance (σ) was estimated using both a one-dimensional (1D) (FST/(1-FST) ~ a + bGD; GD = geographic distance in kilometers) and a two-dimensional (2D) (FST/(1-FST) ~ a + bln(GD); ln(GD) = log of geographic distance) isolation-by-distance model. Dispersal surface (σ2), migration (m) and Wright’s neighborhood size (WN) estimates from the 2D model are also reported. σ, σ2, m and WN in cluster 1 were only computed for female individuals (the model was not significant when males were considered) and are shown in italic type.
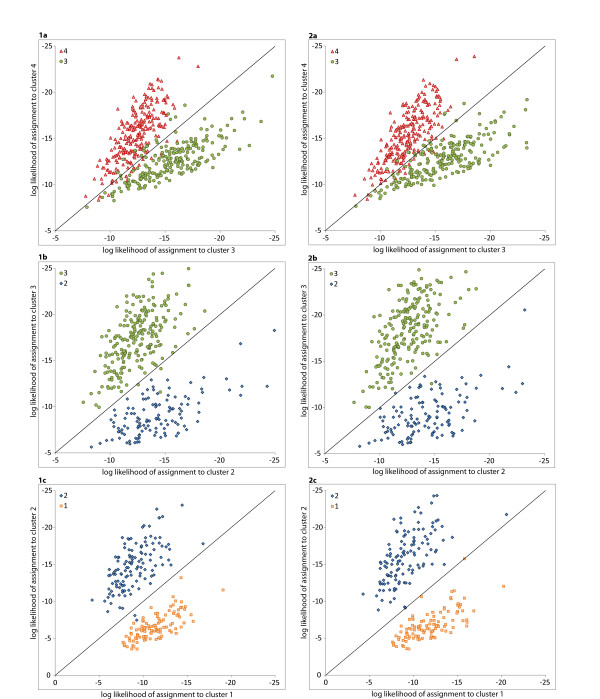
Figure 5 shows the scatterplots of log-likelihood of assignment of individuals to their cluster of origin and neighboring clusters using Flock and Geneclass. Migrant detection using these two methods was largely congruent with assignment of individuals to clusters using Structure (Figure 2) and DAPC (Figure 3; posterior probabilities not shown). Using log-likelihood ratios <0.5, we identified two migrants from cluster 1 in cluster 2 and four migrants from cluster 2 in clusters 3 and 4. The genetic exchange between clusters 3 and 4 was much higher, with 14 migrants from Buvuma islands in the BU-OK region and 12 individuals who migrated in the other direction. These two clusters shared 26 to 56 migrants for log-likelihood ratios <0.5 to <1.0, respectively.
Figure 5.
Migrants. Scatterplots of log-likelihood of assignment of individuals to the cluster of origin and neighboring clusters. Each dot represents an individual and its color denotes the individual’s cluster of origin (red = cluster 4, green = cluster 3, blue = cluster 2 and orange = cluster 1; plots 1a-1c: Flock results, and 2a-2c: Geneclass results). Diagonal lines represent a log-likelihood ratio of 0. Migrants are represented by dots found across diagonals, away from their cluster of origin.
Population size
Estimates of population size were computed for each genetic group. Table 4 shows effective population size (NE) estimates computed using both a linkage disequilibrium method (LDNe) and two temporal methods (a likelihood approach implemented in MLNE and a Bayesian approach implemented in TM3). Table 4 also reports the estimated census size (NC).
NE and NC were estimated for a dataset consisting of 12, 4, and 16 generations of flies from groups 1, 2 and 3, respectively. For the temporal methods only the first and the last samplings were used for NE estimation, as the increased sampling interval might decrease the bias caused by overlapping generations and age structure [53]. With the exception of flies in group 1, the estimates obtained with the LDNe and temporal methods were very similar (Table 4). The lower temporal estimates for group 1 could be due to the higher substructure (FST between EB and other populations in the region was 0.07-0.09). We used NE estimates obtained via the LD method to calculate dispersal. NC was much higher in group 3 (4,121; Table 4) than elsewhere (1,199 in group 2 and 1,299 in group 1). The NE/NC ratio was lowest (0.075) in group 3; this ratio was higher in group 2 (0.101) and highest in group 1 (0.121).
Discussion
Temporal stability
The genetic data we collected for 14 sampling sites (Table 1 and Figure 1), which span up to 16 generations, showed that Gff populations are genetically stable over multiple seasons. Recent work on mainland sites in Uganda on the same species corroborates this result [26]. The significant genetic non-differentiation over several generations seems counterintuitive, given seasonal fluctuations in Gff abundance [61,62]. If this fluctuation is due to actual population reduction, however, 16 generations might not be enough time for genetic drift to produce differentiation, even at low NE values (33–310 individuals), especially if migration alleviates the effects of drift. Seasonal fluctuations in population size could also be a reflection of the low efficiency of trapping devices used to monitor fly populations [63]. Gff is susceptible to high temperatures [64] and is known to inhabit the most humid habitats [65,66]. The reduced fly catches during the dry season could thus be due to refuge-seeking behavior whereby flies hide under bushes that cannot be easily accessed by traps. This behavior is shared by other riverine tsetse species [67,68]. Reduced movement during the dry season has been reported for G. tachinoides in northern Nigeria, which, like Gff, belongs to the palpalis group [69].
At the offshore island sites BY, BZ, and LI (Table 1), where temperatures are constantly lower and humidity is relatively high due to high rainfall and high evaporation rates throughout the year [70], dry and wet season fly catches are comparable in size. An exception to this is the low dry season tsetse abundance on the Buvuma islands (BV; Table 1). Strong dry-season winds, which are known to impact Gff activity [71,72] and commonly occur on Buvuma, might be responsible for the low fly densities recorded there. Windy conditions have also been reported to affect tsetse movement in Nigeria [73]. Additionally, given the genetic similarity between flies on the Buvuma islands and flies found in BY, BZ and LI, it is likely that Buvuma flies seek respite in adjacent habitats from harsh local dry season conditions.
Patterns of genetic differentiation and dispersal within and between clusters
Clustering (Figure 2) and multivariate (Figure 3) analyses detected four genetic clusters of tsetse populations in the Ugandan Lake Victoria basin. Based on DAPC (Figure 3), FST ( Additional file 4: Table S2), hierarchical F-statistics (Table 2) and IBD analyses (Figure 4), levels of genetic differentiation varied among clusters; while flies from clusters 1 and 2 were genetically distinct from each other and flies from clusters 3 or 4, the latter two clusters exchanged a large amount of genetic information. This was also confirmed by individual likelihood assignment tests, which identified numerous migrants (26 to 56) between clusters 3 and 4 (Figure 5). Clusters 1 and 2 (i.e., groups 1 and 2) correspond approximately to blocks 1 and 2, respectively, which were identified by PATTEC for eradication and suppression. However, two sites in block 1 are genetically closer to sites from block 2 (Figure 1). Clusters 3 and 4 (i.e., group 3) fall within block 3 targeted for initial monitoring in the first phase of the rolling carpet initiative.
The high level of genetic connectivity between the two easternmost clusters (clusters 3 and 4) could be facilitated by the presence of suitable Gff habitat, i.e., riparian and lacustrine thickets, tree canopies with understory vegetation, or patches of banana and Lantana camara[74-76]. These thickets have been greatly reduced through human settlement and other human activities in the areas that make up clusters 1 and 2. The higher level of isolation of tsetse flies observed in cluster 1 could be a function of increased habitat fragmentation and the intensive tsetse control activities dating back to the 1960s. In this area, high levels of deforestation have left only small gallery forests along the lakeshore. The growth of the city of Kampala could be an additional physical barrier to gene flow between flies from clusters 1 and 2. Increasing habitat fragmentation caused by human encroachment has been reported to have a major impact on the distribution, densities and structuring of riverine tsetse species [13,77,78].
Within clusters, island populations were not genetically differentiated from the mainland ones (Table 2 and Additional file 3: Table S1). Thus, the water body separating island and mainland sites (cluster 1: 19.9 km; cluster 2: 25.5 km; cluster 3: 34.4 km) does not act as a barrier to gene flow. Frequent human movements between islands and the mainland (fishing boats and daily ferry transport) may contribute to passive dispersal [79]. The extent of genetic connectivity of fly populations in this study is congruent with the general finding of other genetic studies on Gff[16,26] and other riverine species of tsetse [13,14,17,80]. In G. p. gambiensis, however, Solano et al.[81] report very low levels of migration between coastal sites and the Loos islands, situated 30 km off the coast of Guinea. Although this distance is not considerably greater than the distance separating adjacent island and mainland sites in our study (19.9-34.4 km), the differences in gene flow may be attributable to differences in the intensity of boat traffic, and thus more opportunity for passive dispersal of tsetse in the Lake Victoria region than off the coast of Guinea.
Population size and dispersal
Population size estimates were calculated for three groups, which we defined as genetically distinct units using multiple analyses. The effective population size estimates (NE) were quite concordant among the linkage and temporal methods, ranging from 33 to 121 in group 2, 157 to 212 in group 1 and 240 to 310 in group 3. The NC estimated for group 3 was much higher than the NC estimates for groups 1 and 2. Despite the large difference in NC estimates, the NE estimates were similarly low in all three groups. The NE/NC ratio was, therefore, lowest in group 3 (Table 4). This ratio is affected by family-correlated survival (i.e. how families survive as a unit [82,83]) and variance in family size at high NC values [83,84]. In terms of control, this implies that reducing population size alone without eradication would not constitute a permanent solution because NE levels could be maintained, or even increased when NC is reduced because of a decrease in variance of family size and increase in survival of families as units. The robustness of NE in the face of population reduction can be seen in group 1, which has been subjected to intensive control efforts in the past; despite the low NC, NE was relatively high (the NE/NC ratio was as high as 0.121 compared to 0.101 in group 2 and 0.075 in group 3).
Regression of linearized FST against geographic distance revealed significant IBD within groups 2 and 3, as well as females in group 1. Group 1 comprised only three sampling sites and, thus, we only had three data points for the regression. Despite the scarcity of data points, we observed a significant IBD pattern for the less mobile females by removing the better-dispersing male individuals from the model. Dispersal distances (σ) were similar between the 1D and 2D IBD models. These distances were approximately 14–15 km for flies in group 3 and lower in the other two groups, ranging from 2.5 to 4.5 km (Table 4). Based on the 2D estimates, the dispersal surfaces (σ2) within groups 1, 2 and 3 were 6.2, 12.9 and 200.1 km2, respectively. Similarly, migration rates per generation (m) within the three units ranged from 0.01 in group 1 to 0.03 in group 3. Flies in group 3 cover greater distances and disperse over a wider surface area per generation, suggesting that conditions for movement are more favorable in this region, possibly due to environmental factors.
These genetically derived dispersal distances and migration rates are very similar to estimates of dispersal rates for Gff based on mark-release-recapture (MRR) studies, which are about 14.2 km per generation given the movement estimate of 338 m/day [85]. Our genetic estimates are also similar to MRR estimates for other riverine species, such as G. palpalis gambiensis and G. tachinoides[13,86]. MRR data do not necessarily correlate with genetic data, as was observed for flies from the morsitans group [87], suggesting that although habitat fragmentation reduces dispersal capacity, it may not impact levels of intraspecific genetic cohesiveness and that its effect is species-dependent. The relatively low dispersal rates in groups 1 and 2, as compared to group 3 and MRR estimates for Gff suggest that the dispersal capacity of flies is reduced in these areas, probably due to habitat loss and control efforts. The reduction in dispersal capacity has had an effect on genetic cohesiveness in the region and led to the differentiation into distinct genetic groups.
Suppression should be followed by eradication in all three groups. While eradication would be harder to implement in group 3 because of the comparatively larger population size, larger dispersal distance and surface per generation, even the best suppression efforts would be much more difficult to maintain over time. Thus, the better option would be for an eradication campaign to follow an initial suppression phase post-haste, much more rapidly in group 3 than the other two groups.
The dispersal of flies across our study area showed evidence of sex bias. While male-biased dispersal in group 3 was only supported by mAIc, evidence for male-biased dispersal was stronger in groups 1 and 2. The finding of male-biased dispersal is very valuable for sterile insect technique (SIT) vector control efforts. The release of sterile male flies, as the better dispersing sex, holds the promise of sterile individuals being able to efficiently compete with wild breeders.
Conclusion
The findings of this study have reaffirmed the importance of gathering genetic data prior to implementing area-wide tsetse vector control operations. The high levels of genetic mixing between islands and mainland sites suggest that these sites should be treated at the same time. The boundaries of the PATTEC blocks need to be modified to reflect the genetic composition of fly populations (i.e., three genetic groups). Fly populations from the two westernmost groups (1 and 2) are relatively isolated from flies in group 3, suggesting that suppression followed by eradication measures can be effective in these regions, but only if area-wide approaches include both island and mainland sites. For group 3, high levels of gene flow, which translate to a large dispersal surface (σ2 = 200 km2), as well as a very large census size and the potential for the NE/NC ratio to increase in the case of population size reduction, all suggest that suppression alone is not likely to produce desirable results in the long run and that it should be coupled with eradication. Additionally, following the initial suppression phase, eradication would have to ensue faster for group 3 than the other two groups in order to overcome the comparatively higher dispersal capacity of flies and prevent re-infestation. The high dispersal rate in group 3 is a strong argument against the Buvuma islands being a suitable location to evaluate eradication protocols, and that the Ssese islands are a better target for this purpose, provided mainland sites are also included. The finding of male-biased dispersal of tsetse populations in this region ensures that eradication efforts involving SIT are likely to be successful.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SA, AC, LMO, CM and JOO designed the study. ABK and LMO collected samples in Uganda. CH and ABK performed the lab work. CH carried out the statistical analyses. ABK provided background and ecological information. CH and ABK wrote the initial draft of the manuscript. CH, AC, SA, and CM revised the manuscript. SA and AC jointly supervised the work at Yale University. All authors read and approved the final manuscript.
Supplementary Material
Figure S1. Temporal allele frequencies. Frequencies of alleles (above 0.05) across 15 loci are shown for the group of BU and OK samples (wet season of 2008 – dark blue, wet season of 2009 – blue, dry season of 2009 – light blue), and BV, BY, BZ and LI samples grouped together (dry season of 2010 – dark red, wet season of 2010 – light red).
Table S1. Fisher’s exact differentiation test . For each site or group of sites (BU-OK and BV-BY-BZ-LI), tsetse collected at different sampling times (wet and dry seasons; see Table 1) were tested for differences in allelic frequencies (genic differentiation) and genotypic frequencies. P (genic) and P (genotypic) – probability of non-differentiation.
Table S2. Pairwise FST values. FST values were computed for 4 clusters, averaged across 15 loci based on Weir and Cockerham [40]. FST values are reported in the lower diagonal and significance (α = 0.05) in the upper diagonal (‘-’: not significant; ‘+’: significant).
Table S3. Pairwise FST values. FST values were computed for 14 sampling sites, averaged across 15 loci based on Weir and Cockerham [40]. FST values are reported in the lower diagonal and significance (α = 0.05) in the upper diagonal (‘-’: not significant; ‘+’: significant). Non-significant FST values are represented in bold italic type.
Contributor Information
Chaz Hyseni, Email: chaz.hyseni@aya.yale.edu.
Agapitus B Kato, Email: agapituskato@gmail.com.
Loyce M Okedi, Email: lmokedi@gmail.com.
Charles Masembe, Email: cmasembe@gmail.com.
Johnson O Ouma, Email: joouma@gmail.com.
Serap Aksoy, Email: serap.aksoy@yale.edu.
Adalgisa Caccone, Email: adalgisa.caccone@yale.edu.
Acknowledgements
This study was supported by grants from NIH (R01 AI068932 and D43 TW007391) to SA, AC and LMO, and WHO-TDR (A80132) to AC and LMO. The research was accomplished while ABK was a Fogarty Research Fellow at Yale University. We are thankful for the support of Drs. Vincent Muwanika and Anne M. Akol (Makerere University, Uganda), PATTEC Uganda’s STATFA Project and Mukono District Administration. We are grateful to the technical staff of NaLIRRI for excellent assistance with field sampling.
References
- Dyer NA, Lawton SP, Ravel S, Choi KS, Lehane MJ, Robinson AS, Okedi LM, Hall MJR, Solano P, Donnelly MJ. Molecular phylogenetics of tsetse flies (Diptera: Glossinidae) based on mitochondrial (COI, 16S, ND2) and nuclear ribosomal DNA sequences, with an emphasis on the palpalis group. Mol Phylogenet Evol. 2008;49:227–239. doi: 10.1016/j.ympev.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Leak SGA, Leak SGA. Tsetse biology and ecology: their role in the epidemiology and control of trypanosomosis. Wallingford: CABI; 1999. [Google Scholar]
- Budd L. Economic analysis. Aylesford: DFID; 1999. DFID-funded tsetse and trypanosome research and development since 1980. Vol. 2. [Google Scholar]
- Aksoy S, Maudlin I, Dale C, Robinson AS, O’Neill SL. Prospects for control of African trypanosomiasis by tsetse vector manipulation. Trends Parasitol. 2001;17:29–35. doi: 10.1016/S1471-4922(00)01850-X. [DOI] [PubMed] [Google Scholar]
- Aksoy S. Control of tsetse flies and trypanosomes using molecular genetics. Vet Parasitol. 2003;115:125–145. doi: 10.1016/S0304-4017(03)00203-6. [DOI] [PubMed] [Google Scholar]
- Torr SJ, Hargrove JW, Vale GA. Towards a rational policy for dealing with tsetse. Trends Parasitol. 2005;21:537–541. doi: 10.1016/j.pt.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Vreysen MJB, Marc JB. Prospects for area-wide integrated control of tsetse flies (Diptera: Glossinidae) and trypanosomosis in sub-Saharan Africa. RSEA. 2006;65:1–21. [Google Scholar]
- Schofield CJ, Kabayo JP. Trypanosomiasis vector control in Africa and Latin America. Parasit Vectors. 2008;1:24. doi: 10.1186/1756-3305-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreysen MJB, Robinson AS. Ionising radiation and area-wide management of insect pests to promote sustainable agriculture. A review. Agron Sustain Dev. 2011;31:233–250. doi: 10.1051/agro/2010009. [DOI] [Google Scholar]
- Solano P, Kaba D, Ravel S, Dyer NA, Sall B, Vreysen MJ, Seck MT, Darbyshir H, Gardes L, Donnelly MJ. et al. Population genetics as a tool to select tsetse control strategies: suppression or eradication of Glossina palpalis gambiensis in the Niayes of Senegal. PLoS Negl Trop Dis. 2010;4:e692. doi: 10.1371/journal.pntd.0000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano P, Ravel S, de Meeus T. How can tsetse population genetics contribute to African trypanosomiasis control? Trends Parasitol. 2010;26:255–263. doi: 10.1016/j.pt.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Kagbadouno M, Camara M, Bouyer J, Hervouet JP, Courtin F, Jamonneau V, Morifaso O, Kaba D, Solano P. Tsetse elimination: its interest and feasibility in the historical sleeping sickness focus of Loos islands, Guinea. Parasite. 2009;16:29–35. doi: 10.1051/parasite/2009161029. [DOI] [PubMed] [Google Scholar]
- Bouyer J, Balenghien T, Ravel S, Vial L, Sidibé I, Thévenon S, Solano P, Meeûs T. Population sizes and dispersal pattern of tsetse flies: rolling on the river? Mol Ecol. 2009;18:2787–2797. doi: 10.1111/j.1365-294X.2009.04233.x. [DOI] [PubMed] [Google Scholar]
- Koné N, Bouyer J, Ravel S, Vreysen MJB, Domagni KT, Causse S, Solano P, Meeûs T. Contrasting population structures of two vectors of African trypanosomoses in Burkina Faso: consequences for control. PLoS Negl Trop Dis. 2011;5:e1217. doi: 10.1371/journal.pntd.0001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abila PP, Slotman MA, Parmakelis A, Dion KB, Robinson AS, Muwanika VB, Enyaru JC, Okedi LM, Aksoy S, Caccone A. High levels of genetic differentiation between Ugandan Glossina fuscipes fuscipes populations separated by Lake Kyoga. PLoS Negl Trop Dis. 2008;2:e242. doi: 10.1371/journal.pntd.0000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadell JS, Hyseni C, Abila PP, Azabo R, Enyaru JC, Ouma JO, Mohammed YO, Okedi LM, Aksoy S, Caccone A. Phylogeography and population structure of Glossina fuscipes fuscipes in Uganda: implications for control of tsetse. PLoS Negl Trop Dis. 2010;4:e636. doi: 10.1371/journal.pntd.0000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanekou TT, Melachio TT, Simo G, Ravel S, Meeûs T, Causse S, Solano P, Lutumba P, Asonganyi T, Njiokou F. Population genetics of Glossina palpalis palpalis from central African sleeping sickness foci. Parasit Vectors. 2011;4:140. doi: 10.1186/1756-3305-4-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouma JO, Beadell JS, Hyseni C, Okedi LM, Krafsur ES, Aksoy S, Caccone A. Genetic diversity and population structure of Glossina pallidipes in Uganda and western Kenya. Parasit Vectors. 2011;4:122. doi: 10.1186/1756-3305-4-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabayo JP. Aiming to eliminate tsetse from Africa. Trends Parasitol. 2002;18:473–475. doi: 10.1016/S1471-4922(02)02371-1. [DOI] [PubMed] [Google Scholar]
- Agrisystems Limited. End of project evaluation study for FITCA Regional and the national components of five countries Kenya, Uganda, Tanzania, Rwanda and Ethiopia. Aylesbury: East Africa European Commission; 2005. [Google Scholar]
- Grant I. Creation of sustainable tsetse and trypanosomiasis free areas in East and West Africa. Environmental risk assessment report: the use of the sequential aerosol technique for the eradication of tsetse populations in Uganda. Boughton: Cybister Environmental Protection; 2009. [Google Scholar]
- Luyimbazi F. Detailed work plan/action plan for the collection of entomological baseline data. Integrated area-wide program for the creation of sustainable tsetse and trypanosomiasis free areas in the Lake Victoria basin. Entebbe: Ministry of Agriculture, Animal Industry and Fisheries; 2006. [Google Scholar]
- Wint W. Kilometre resolution tsetse fly distribution maps for the Lake Victoria basin and West Africa. Vienna: IAEA; 2001. [Google Scholar]
- da Costa BFB. Sleeping sickness: a record of four years’ war against it in Principe, Portuguese West Africa. Lisbon: Baillière, Tindall and Cox; 1916. [Google Scholar]
- Vreysen MJ, Saleh KM, Ali MY, Abdulla AM, Zhu ZR, Juma KG, Dyck VA, Msangi AR, Mkonyi PA, Feldmann HU. Glossina austeni (Diptera: Glossinidae) eradicated on the island of Unguja, Zanzibar, using the sterile insect technique. J Econ Entomol. 2000;93:123–135. doi: 10.1603/0022-0493-93.1.123. [DOI] [PubMed] [Google Scholar]
- Echodu R, Beadell JS, Okedi LM, Hyseni C, Aksoy S, Caccone A. Temporal stability of Glossina fuscipes fuscipes populations in Uganda. Parasit Vectors. 2011;4:19. doi: 10.1186/1756-3305-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove JW. Extinction probabilities and times to extinction for populations of tsetse flies Glossina spp. (Diptera: Glossinidae) subjected to various control measures. Bull Entomol Res. 2005;95:13–21. doi: 10.1079/ber2004335. [DOI] [PubMed] [Google Scholar]
- Krafsur ES, Marquez JG, Ouma JO. Structure of some East African Glossina fuscipes fuscipes populations. Med Vet Entomol. 2008;22:222–227. doi: 10.1111/j.1365-2915.2008.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challier A, Laveissiere C. A new trap for capturing Glossina flies (Diptera: Muscidae), description and field trials. Cah ORSTOM Entomol Med Parasitol. 1973;11:251–262. [Google Scholar]
- Molecular Ecology Resources Primer Development Consortium. Permanent genetic resources added to Molecular Ecology Resources Database 1 December 2010–31 January 2011. Mol Ecol Resour. 2011;11:586–589. doi: 10.1111/j.1755-0998.2011.03004.x. [DOI] [PubMed] [Google Scholar]
- Rousset F. Genepop'007: a complete reimplementation of the Genepop software for Windows and Linux. Mol Ecol Resour. 2008;8:103–106. doi: 10.1111/j.1471-8286.2007.01931.x. [DOI] [PubMed] [Google Scholar]
- Louis EJ, Dempster ER. An exact test for Hardy-Weinberg and multiple alleles. Biometrics. 1987;43:805–811. doi: 10.2307/2531534. [DOI] [PubMed] [Google Scholar]
- Guo SW, Thompson EA. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics. 1992;48:361–372. doi: 10.2307/2532296. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Peakall R, Smouse PE. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes. 2006;6:288–295. doi: 10.1111/j.1471-8286.2005.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jombart T, Devillard S, Balloux F. Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet. 2010;11:94. doi: 10.1186/1471-2156-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jombart T. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics. 2008;24:1403–1405. doi: 10.1093/bioinformatics/btn129. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Weir BS, Cockerham CC. Estimating F-Statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.2307/2408641. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Lischer HEL. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Yang R-C. Estimating hierarchical F-statistics. Evolution. 1998;52:950–956. doi: 10.2307/2411227. [DOI] [PubMed] [Google Scholar]
- Goudet J. Hierfstat: estimation and tests of hierarchical F-statistics. Vienna: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Konovalov DA, Heg DIK. TECHNICAL ADVANCES: a maximum-likelihood relatedness estimator allowing for negative relatedness values. Mol Ecol Resour. 2008;8:256–263. doi: 10.1111/j.1471-8286.2007.01940.x. [DOI] [PubMed] [Google Scholar]
- Konovalov DA, Manning C, Henshaw MT. KINGROUP: a program for pedigree relationship reconstruction and kin group assignments using genetic markers. Mol Ecol Notes. 2004;4:779–782. doi: 10.1111/j.1471-8286.2004.00796.x. [DOI] [Google Scholar]
- Goudet J, Perrin N, Waser P. Tests for sex-biased dispersal using bi-parentally inherited genetic markers. Mol Ecol. 2002;11:1103–1114. doi: 10.1046/j.1365-294X.2002.01496.x. [DOI] [PubMed] [Google Scholar]
- Goudet J. FSTAT (Version 1.2): a computer program to calculate F-statistics. J Hered. 1995;86:485–486. [Google Scholar]
- Rousset F. Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics. 1997;145:1219–1228. doi: 10.1093/genetics/145.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piry S, Alapetite A, Cornuet JM, Paetkau D, Baudouin L, Estoup A. GENECLASS2: a software for genetic assignment and first-generation migrant detection. J Hered. 2004;95:536–539. doi: 10.1093/jhered/esh074. [DOI] [PubMed] [Google Scholar]
- Paetkau D, Calvert W, Stirling I, Strobeck C. Microsatellite analysis of population structure in Canadian polar bears. Mol Ecol. 1995;4:347–354. doi: 10.1111/j.1365-294X.1995.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Paetkau D, Slade R, Burden M, Estoup A. Genetic assignment methods for the direct, real-time estimation of migration rate: a simulation-based exploration of accuracy and power. Mol Ecol. 2004;13:55–65. doi: 10.1046/j.1365-294X.2004.02008.x. [DOI] [PubMed] [Google Scholar]
- Duchesne P, Turgeon J. FLOCK: a method for quick mapping of admixture without source samples. Mol Ecol Resour. 2009;9:1333–1344. doi: 10.1111/j.1755-0998.2009.02571.x. [DOI] [PubMed] [Google Scholar]
- Luikart G, Ryman N, Tallmon DA, Schwartz MK, Allendorf FW. Estimation of census and effective population sizes: the increasing usefulness of DNA-based approaches. Conserv Genet. 2010;11:355–373. doi: 10.1007/s10592-010-0050-7. [DOI] [Google Scholar]
- Waples RS, Do C. LDNE: a program for estimating effective population size from data on linkage disequilibrium. Mol Ecol Resour. 2008;8:753–756. doi: 10.1111/j.1755-0998.2007.02061.x. [DOI] [PubMed] [Google Scholar]
- Berthier P, Beaumont MA, Cornuet JM, Luikart G. Likelihood-based estimation of the effective population size using temporal changes in allele frequencies: A genealogical approach. Genetics. 2002;160:741–751. doi: 10.1093/genetics/160.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JL, Whitlock MC. Estimating effective population size and migration rates from genetic samples over space and time. Genetics. 2003;163:429–446. doi: 10.1093/genetics/163.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit E, Valiere N. Estimating population size with noninvasive capture-mark-recapture data. Conserv Biol. 2006;20:1062–1073. doi: 10.1111/j.1523-1739.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- Gazey WJ, Staley MJ. Population estimation from mark-recapture experiments using a sequential Bayes algorithm. Ecology. 1986;67:941–951. doi: 10.2307/1939816. [DOI] [Google Scholar]
- Puechmaille SJ, Petit EJ. Empirical evaluation of non-invasive capture-mark-recapture estimation of population size based on a single sampling session. J Appl Ecol. 2007;44:843–852. doi: 10.1111/j.1365-2664.2007.01321.x. [DOI] [Google Scholar]
- Favre L, Balloux F, Goudet J, Perrin N. Female-biased dispersal in the monogamous mammal Crocidura russula: Evidence from field data and microsatellite patterns. Proc Biol Sci. 1997;264:127–132. doi: 10.1098/rspb.1997.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katunguka-Rwakishaya E, Kabagambe EK. Tsetse survey in Mukono district, south-east Uganda: population structure, distribution and blood meal status. Trop Anim Health Prod. 1996;28:151–157. [PubMed] [Google Scholar]
- Bonomi A, Bassetti F, Gabrieli P, Beadell J, Falchetto M, Scolari F, Gomulski LM, Regazzini E, Ouma JO, Caccone A. et al. Polyandry is a common event in wild populations of the tsetse fly Glossina fuscipes fuscipes and may impact population reduction measures. PLoS Negl Trop Dis. 2011;5:e1190. doi: 10.1371/journal.pntd.0001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak SGA, Ejigu D, Vreysen MJB. Collection of entomological baselinedata for tsetse area-wideintegrated pest management programmes. Rome: FAO; 2008. [Google Scholar]
- Hargrove JW, Brady J. Activity rhythms of tsetse flies (Glossina spp) (Diptera: Glossinidae) at low and high temperatures in nature. Bull Entomol Res. 1992;82:321–326. doi: 10.1017/S0007485300041092. [DOI] [Google Scholar]
- Dyer NA, Ravel S, Choi KS, Darby AC, Causse S, Kapitano B, Hall MJR, Steen K, Lutumba P, Madinga J. others. Cryptic diversity within the major trypanosomiasis vector Glossina fuscipes revealed by molecular markers. PLoS Negl Trop Dis. 2011;5:e1266. doi: 10.1371/journal.pntd.0001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock JN. Training manual for tsetse control personnel. Volume I. Tsetse biology, systematics and distribution; techniques. Rome: FAO; 1982. [Google Scholar]
- Cuisance D. Evolution sous l'action de la secheresse d'une population isolee de Glossina tachinoides West. (region du Bas Logone, Tchad) Rev Elev Med Vet Pays Trop. 1974;27:95–107. [PubMed] [Google Scholar]
- Lambrecht FL. Field studies of Glossina morsitans Westw. (Dipt.: Glossinidae) in relation to Rhodesian sleeping sickness in N’gamiland, Botswana. Bull Entomol Res. 1972;62:183–193. doi: 10.1017/S0007485300047623. [DOI] [Google Scholar]
- Dransfield RD, Brightwell R, Onah J, Okolo CJ. Population dynamics of Glossina morsitans submorsitans Newstead and G. tachinoides Westwood (Diptera: Glossinidae) in sub-Sudan savanna in northern Nigeria. I. Sampling methodology for adults and seasonal changes in numbers caught in different vegetation types. Bull Entomol Res. 1982;72:175–192. doi: 10.1017/S0007485300010488. [DOI] [Google Scholar]
- Kendall RL. An ecological history of the Lake Victoria basin. Ecol Monogr. 1969;39:121–176. doi: 10.2307/1950740. [DOI] [Google Scholar]
- Carpenter GDH. Second report on the bionomics of Glossina fuscipes (palpalis) of Uganda. Rep Sleep Sickn Commn R Soc. 1913;14:1–37. [Google Scholar]
- Ogwal LM, Kalyebi A, Kaddu JB. The diurnal activity, movement and trypanosome infection rates of Glossina fuscipes fuscipes (Diptera: Glossinidae) in Buvuma Island, Lake Victoria, Uganda. Insect Sci. 2007;14:477–484. doi: 10.1111/j.1744-7917.2007.00176.x. [DOI] [Google Scholar]
- Molyneux DH, Baldry DAT, Fairhurst C. Tsetse movement in wind fields - possible epidemiological and entomological implications for trypanosomiasis and its control. Acta Trop. 1979;36:53–65. [PubMed] [Google Scholar]
- Okoth JO, Kapaata R. A study of the resting sites of Glossina fuscipes fuscipes (Newstead) in relation to Lantana camara thickets and coffee and banana plantations in the sleeping sickness epidemic focus, Busoga, Uganda. Int J Trop Insect Sci. 1987;8:57–60. doi: 10.1017/S1742758400006962. [DOI] [Google Scholar]
- Kuzoe FAS, Schofield CJ. Strategic review of traps and targets for tsetse and African trypanosomiasis control. GenevaL: UNICEF/UNDP/World Bank/WHO; 2004. [Google Scholar]
- Syed Z, Guerin PM. Tsetse flies are attracted to the invasive plant Lantana camara. J Insect Physiol. 2004;50:43–50. doi: 10.1016/j.jinsphys.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Bouyer J, Guerrini L, Cesar J, de la Rocque S, Cuisance D. A phyto-sociological analysis of the distribution of riverine tsetse flies in Burkina Faso. Med Vet Entomol. 2005;19:372–378. doi: 10.1111/j.1365-2915.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- Guerrini L, Bord JP, Ducheyne E, Bouyer J. Fragmentation analysis for prediction of suitable habitat for vectors: example of riverine tsetse flies in Burkina Faso. J Med Entomol. 2008;45:1180–1186. doi: 10.1603/0022-2585(2008)45[1180:FAFPOS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ogwal LM, Kangwagye TN. In: Panel Proceedings Series. Offori ED, Vloedt AMV, editor. Vienna: IAEA; 1990. Population dynamics of Glossina fuscipes fuscipes on Buvuma Island, Lake Victoria, Uganda; pp. 67–77. [Google Scholar]
- Bouyer J, Ravel S, Guerrini L, Dujardin JP, Sidibé I, Vreysen MJB, Solano P, Meeûs T. Population structure of Glossina palpalis gambiensis (Diptera: Glossinidae) between river basins in Burkina Faso: consequences for area-wide integrated pest management. Infect Genet Evol. 2010;10:321–328. doi: 10.1016/j.meegid.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Solano P, Ravel S, Bouyer J, Camara M, Kagbadouno MS, Dyer N, Gardes L, Herault D, Donnelly MJ, Meeûs T. The population structure of Glossina palpalis gambiensis from island and continental locations in coastal Guinea. PLoS Negl Trop Dis. 2009;3:e392. doi: 10.1371/journal.pntd.0000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waples RS. Evaluating the effect of stage-specific survivorship on the Ne/N ratio. Mol Ecol. 2002;11:1029–1037. doi: 10.1046/j.1365-294X.2002.01504.x. [DOI] [PubMed] [Google Scholar]
- Hedrick P. Large variance in reproductive success and the Ne/N ratio. Evolution. 2005;59:1596–1599. [PubMed] [Google Scholar]
- Pray LA, Goodnight CJ, Stevens L, Schwartz JM, Yan G. The effect of population size on effective population size: an empirical study in the red flour beetle Tribolium castaneum. Genet Res. 1996;68:151–155. doi: 10.1017/S0016672300034030. [DOI] [Google Scholar]
- Rogers D. Study of a natural population of Glossina fuscipes fuscipes Newstead and a model of fly movement. J Anim Ecol. 1977;46:309–330. doi: 10.2307/3962. [DOI] [Google Scholar]
- Cuisance D, Février J, Dejardin J, Filledier J, Cuisance D, Février J, Dejardin J, Filledier J. Dispersion linéaire de Glossina palpalis gambiensis et G. tachinoides dans une galerie forestière en zone soudano-guinéenne (Burkina Faso) Rev Elev Med Vet Pays Trop. 1985;38:153–172. [Google Scholar]
- Krafsur ES, Endsley MA. Microsatellite diversities and gene flow in the tsetse fly, Glossina morsitans s.l. Med Vet Entomol. 2002;16:292–300. doi: 10.1046/j.1365-2915.2002.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin-Ganache I, Raposo M, Raymond M, Deschepper CF. M13-tailed primers improve the readability and usability of microsatellite analyses performed with two different allele-sizing methods. Biotechniques. 2001;31:24–27. [PubMed] [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Temporal allele frequencies. Frequencies of alleles (above 0.05) across 15 loci are shown for the group of BU and OK samples (wet season of 2008 – dark blue, wet season of 2009 – blue, dry season of 2009 – light blue), and BV, BY, BZ and LI samples grouped together (dry season of 2010 – dark red, wet season of 2010 – light red).
Table S1. Fisher’s exact differentiation test . For each site or group of sites (BU-OK and BV-BY-BZ-LI), tsetse collected at different sampling times (wet and dry seasons; see Table 1) were tested for differences in allelic frequencies (genic differentiation) and genotypic frequencies. P (genic) and P (genotypic) – probability of non-differentiation.
Table S2. Pairwise FST values. FST values were computed for 4 clusters, averaged across 15 loci based on Weir and Cockerham [40]. FST values are reported in the lower diagonal and significance (α = 0.05) in the upper diagonal (‘-’: not significant; ‘+’: significant).
Table S3. Pairwise FST values. FST values were computed for 14 sampling sites, averaged across 15 loci based on Weir and Cockerham [40]. FST values are reported in the lower diagonal and significance (α = 0.05) in the upper diagonal (‘-’: not significant; ‘+’: significant). Non-significant FST values are represented in bold italic type.