Abstract
Background
The late positive potential (LPP) is an event-related potential component that indexes selective attention toward motivationally salient information and is sensitive to emotional stimuli. Few studies have examined the LPP in children. Depression has been associated with reduced reactivity to negative and positive emotional stimuli, including reduced LPPs in response to emotional faces. The current study sought to identify the time course and scalp distribution of the LPP in response to emotional faces in young children and to determine whether reduced reactivity is observed among children at risk for depression.
Methods
Electrocortical reactivity to emotional faces was examined in a large sample of young children and as a function of maternal and paternal depression.
Results
In the overall sample, emotional faces were associated with increased positivities compared to neutral faces at occipital sites 200–600 ms after stimulus onset and at parietal sites 600–1,000 ms after stimulus onset. Children of mothers with a history of depressive disorders exhibited reduced differentiation in the early occipital LPP for emotional compared to neutral faces.
Conclusions
Results suggest that children as young as 6 years exhibit LPPs to emotional faces, and patterns of electrocortical reactivity to emotional stimuli may be associated with vulnerability to depressive disorders.
Keywords: Depression, vulnerability markers, emotion, psychophysiology
Introduction
In the last few decades, there has been growing interest in research on emotion, particularly with regard to individual differences in emotional reactivity and expression (Rottenberg & Johnson, 2007). Psychophysiological methods, such as event-related potentials (ERPs), are particularly well-suited to the study of emotion because they can measure aspects of emotion that may not be observed through behavior or subjective reports (Santerre & Allen, 2007). In addition, ERP methods are temporally sensitive, making them useful in identifying the time course of emotional reactions, and can be used across development (Nelson & McCleery, 2008).
Emotion research has the potential to increase understanding of the mechanisms underlying psychopathology, particularly emotional disorders such as depression, and to lead to improvements in intervention and prevention (Kring, 2010; Rottenberg & Johnson, 2007). To understand emotional processing in depression, it is important to consider the distinction between mood and emotion. Emotions are brief adaptive reactions to specific stimuli, while mood refers to longer, more general feeling states (Ekman, 1992). Although depression is associated with negative mood, experimental evidence suggests that it may also be characterized by disengagement from the environment and reductions in reactivity to emotional changes, a pattern referred to as emotion context insensitivity (ECI; Bylsma, Morris, & Rottenberg, 2008; Rottenberg, Gross, & Gotlib, 2005; Rottenberg & Vaughan, 2008). Evolutionarily, emotional withdrawal may play an adaptive role in depression, preventing continued engagement in goal-directed activities that may involve wasted effort or threat of harm (Nesse, 2000). Reduced reactivity to emotional stimuli in depression has been observed in studies using facial electromyography, startle reactivity, behavioral observation, self-reports and ERPs (Allen, Trinder, & Brennen, 1999; Deldin, Keller, Gergen, & Miller, 2001; Ellis, Beevers, & Wells, 2009; Foti, Olvet, Klein, & Hajcak, 2010).
Support for the ECI view of depression has included research using ERPs that are sensitive to emotion. In particular, the late positive potential (LPP) is a slow-wave ERP component that has consistently been shown to be larger for emotional compared to neutral stimuli. In adults, the LPP begins by 200 ms after stimulus onset, is maximal around centro-parietal electrode sites and persists for the duration of stimulus presentation (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Hajcak & Olvet, 2008). The LPP is thought to reflect selective processing of, and attentional allocation toward, emotional stimuli, and is correlated with the intensity of emotional stimuli (Schupp, Flaisch, Stockburger, & Junghöfer, 2006; Weinberg & Hajcak, 2010, 2011). Although many LPP studies use images from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008) to elicit emotional reactions, other studies have used words or emotional faces (Naumann, Bartussek, Diedrich, & Laufer, 1992; Schupp et al., 2004).
Recent work suggests that mood and anxiety disorders are associated with abnormalities in the LPP. For example, compared to healthy controls, depressed individuals have been shown to exhibit reduced LPPs in response to both threatening faces and faces depicting dermatological diseases (Foti et al., 2010; Kayser, Bruder, Tenke, Stewart, & Quitkin, 2000). Reductions in emotional reactivity may be specific to later ERP components, as major depressive disorder (MDD) has been associated with emotional modulation of earlier ERPs (i.e. the vertex positive potential) but not the LPP (Foti et al., 2010). In contrast to depression findings, there is some evidence that anxiety is associated with enhanced LPPs in response to aversive stimuli (e.g. MacNamara & Hajcak, 2010; Michalowski et al., 2009), suggesting that the LPP may index distinct patterns of emotional reactivity for specific affective disorders.
Although few studies have examined LPPs in youth, there is evidence that they are observable in both children and adolescents (Gao, Liu, Ding, & Guo, 2010; Hajcak & Dennis, 2009). Compared to the LPP in adults, the LPP in children appears to be maximal over more occipital sites and less protracted in time (Hajcak & Dennis, 2009). Previous work examining the LPP in children recorded electrocortical responses to IAPS pictures, so the nature of the LPP in response to other emotional stimuli remains unclear. In addition, research has yet to extend LPP work with children to developmental psychopathology research.
Although the developmental course of ECI is not well understood, there is some evidence that it may be associated with vulnerability for depression, rather than a correlate of the disorder. If ECI is a vulnerability marker, it should be observable before the development of a depressive disorder and after remission (Ingram & Luxton, 2005). For instance, reduced electrodermal reactivity to loud sounds has been observed in individuals after recovery from depression (Iacono et al., 1984) and reduced affective modulation of startle responses has been observed during and following treatment for depression (Dichter, Tomarken, Shelton, & Sutton, 2004). As emotional reactivity plays a critical role in guiding adjustment to the environment and problem solving (Keltner & Gross, 1999), it is possible that patterns of ECI may precede the development of depression and increase risk for the disorder. If so, ECI should be more apparent among children at high risk for depression compared to those at lower risk.
Children with a history of parental depression are at an increased risk for developing the disorder themselves (Goodman et al., 2011; Hammen, 2009). Although both maternal and paternal depression are associated with increased risk in offspring, the association with maternal depression appears to be stronger, particularly in younger children (Connell & Goodman, 2002). Offspring of mothers with a history of depression also exhibit abnormalities in processing emotional information prior to the onset of symptoms. For example, children of depressed mothers display attentional biases for sad faces (Joormann, Talbot, & Gotlib, 2007; Kujawa et al., 2011) and memory biases for negative self-relevant information (Taylor & Ingram, 1999) compared to children of never depressed mothers. In addition, there is evidence for abnormal neural processing of rewards and losses in offspring of depressed mothers (Foti, Kotov, Klein, & Hajcak, 2011; Gotlib et al., 2010). However, little is known about emotional processing in offspring of depressed fathers.
The current study examined LPPs toemotional faces as a potential risk marker for depression. As the literature on LPPs in young children is limited and LPPs to faces have not previously been studied in children, our first aim was to determine whether the LPP in children is sensitive to emotional faces and to examine the time course and topography of these responses. Our second aim was to examine patterns of electrocortical reactivity to emotional stimuli among children with maternal and paternal histories of depression. Consistent with ECI and Foti et al.’s (2010) study of LPPs in depressed adults, we hypothesized that children of depressed mothers would exhibit reduced electrocortical reactivity to emotional faces. We also examined associations between paternal depression and electrocortical reactivity to emotional faces; however, these analyses were exploratory, given the limited research on vulnerability markers among children of depressed fathers.
Method
Participants
Participants were 415 children from a larger prospective study (Olino, Klein, Dyson, Rose, & Durbin, 2010). The initial sample (n = 559) was recruited through a commercial mailing list 3 years earlier. Children between 3 and 4 years of age living with an English-speaking biological parent and with no significant medical conditions or developmental disabilities were eligible. Families were invited to return for a psychophysiological assessment at age 6 and 415 families participated. Psychophysiological data from 70 children were excluded for poor quality, leaving 345 children for overall LPP analyses. For analyses of associations with parental depression, 3 children with a parental history of bipolar disorder and 8 children missing diagnostic data (obtained during the initial assessment) for one or both parents were excluded for a sample of 334. The overall sample was a mean age of 6.10 years (SD = 0.44). Participants were primarily middle class, with an average social class of 2.20 (SD = 0.90) according to Hollingshead’s Four Factor Index of Social Status (Hollingshead, 1975). The sample was 87.0% Caucasian, 8.1% Hispanic, 2.3% African American, 2.0% Asian American, and 0.6% from other ethnic/racial backgrounds.
Participants were divided into groups based on maternal and paternal history of MDD and/or dysthymic disorder (DD). Of participants included in analyses of parental depression, 116 had a mother with a history of depression and 62 had a father with a history of depression. Demographics are presented for each combination of maternal and paternal depression in Table 1. Overall, 188 children had no parental history of depressive disorders, 30 had only fathers with a history of depression, 84 had only mothers with a history of depression and 32 had two parents with a history of depression.
Table 1.
Parent and child characteristics by parental depression group
| No parental depression (N = 188) % |
Paternal depression only (N = 30) % |
Maternal depression only (N = 84) % |
Both parents depression (N = 32) % |
|
|---|---|---|---|---|
| Child Caucasian | 89.4 | 76.7 | 89.3 | 81.3 |
| Child female | 42.0 | 56.7 | 52.4 | 43.8 |
| Child ADHD | 2.2 | 6.7 | 7.2 | 12.9 |
| Child ODD | 7.5 | 10.0 | 9.6 | 16.1 |
| Child anxiety disorder | 13.4 | 16.7 | 19.3 | 9.7 |
| Parents married | 91.3 | 93.3 | 85.5 | 90.6 |
| Mother completed college | 60.3 | 60.0 | 60.2 | 71.9 |
| Father completed college | 66.3 | 60.0 | 61.7 | 43.8 |
| Parental history of MDD | 0.0 | 73.3 | 83.3 | 93.8 |
| Parental history of DD | 0.0 | 46.7 | 33.3 | 46.9 |
| Parental history of anxiety disorder | 34.0 | 63.3 | 59.5 | 81.3 |
| Parental history of substance use disorder | 43.1 | 46.7 | 61.9 | 75.0 |
|
M (SD)
|
M (SD)
|
M (SD)
|
M (SD)
|
|
| Child’s age | 6.09 (0.42) | 6.22 (0.46) | 6.07 (0.43) | 6.09 (0.53) |
| Mother’s age | 39.25 (4.14) | 39.23 (4.79) | 38.38 (4.85) | 38.75 (5.45) |
| Father’s age | 41.29 (5.26) | 41.83 (5.86) | 40.80 (5.92) | 41.32 (5.40) |
| CDI score | 7.36 (5.04) | 7.57 (6.03) | 6.94 (4.96) | 7.33 (4.63) |
| Parental depression age of onset | – | 23.17 (9.42) | 22.78 (8.33) | 18.47 (6.91) |
| Number of parental MDEs | – | 1.29 (0.56) | 1.79 (1.60) | 3.50 (4.22) |
| Median
|
Median
|
Median
|
Median
|
|
| Duration of longest parental MDE (weeks) | – | 6.0 | 16.0 | 14.5 |
ADHD, attention deficit hyperactivity disorder; ODD, oppositional defiant disorder; MDD, major depressive disorder; DD, dysthymic disorder; CDI, Child Depression Inventory; MDE, major depressive episode.
Procedure
Data were collected across two assessment periods. Diagnoses of parental psychopathology were made approximately 3 years prior to the psychophysiological assessment when the children first entered the study. Study procedures were approved by Stony Brook University’s Institutional Review Board. Written informed consent from parents and verbal assent from children were obtained at both assessments. At the second (age 6) assessment, parents completed a diagnostic interview to assess child psychopathology and children participated in the faces viewing task and completed a self-report measure of depression. At the start of the faces task, one set of practice faces was presented and the experimenter named each emotional expression as it appeared. During the experiment, the child was monitored through a camera inside the chamber.
Measures
Parental depression
When the children entered the study at age 3, both biological parents were interviewed using the Structured Clinical Interview for DSM–IV (SCID) nonpatient version (First, Spitzer, Gibbon, & Williams, 1996). Interviews were conducted by telephone, which yields similar results to face-to-face interviews (Rohde, Lewinsohn, & Seeley, 1997). Two Masters-level raters conducted the diagnostic interviews. To assess interrater reliability, another rater derived independent diagnoses from audiotapes of 30 interviews (Kappa for lifetime depressive disorder = .93). When SCID data were unavailable, family history information for the other parent was obtained from the index parent using a semistructured interview (Andreasen, Endicott, Spitzer, & Winokur, 1977). Diagnoses for 44 fathers were derived using the family history method.
Child psychopathology
During the second assessment at age 6, children completed the Child Depression Inventory (CDI; Kovacs, 1992), a self-report measure that assesses depressive symptoms in children. Although the CDI is generally used for children older than 7, it appears to be valid for children as young as 5 (Ialongo, Edelsohn, & Kellam, 2001). Because of the age of the children, the items were read aloud by a research assistant and two items related to school work were excluded. CDI data were missing for one child.
During the second assessment, parents also completed the Preschool Age Psychiatric Assessment (PAPA) (Egger, Ascher, & Angold, 1999), a structured, interviewer-based diagnostic interview. Assessments were conducted by advanced graduate students and Masters-level staff with a second rater independently rating audiotapes of 35 interviews (intraclass correlation coefficient for depressive symptom ratings = .95). PAPA data were missing for 4 children.
Faces task
Children were instructed to watch a series of emotional faces as they appeared on the screen. Emotional face pictures from the MacArthur Foundation Research Network on Early Experience and Brain Development stimulus set were used for the task (Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. Please contact Nim Tottenham at tott0006@tc.umn.edu for more information concerning the stimulus set.) Ten (5 male, 5 female) actors were selected from the database with sad, angry, fearful, happy and neutral faces included for each actor (50 images total). The task consisted of 150 trials divided into three blocks. Each image was randomly presented once in each block for a total of 30trials for each emotional face type. Eachtrial consisted of a fixation that varied randomly between 1,000 and 2,000 ms followed by the face presentation for 1,000 ms.
Psychophysiological data
The continuous electroencephalogram (EEG) was recorded during the task using the ActiveTwo Biosemi system, with recordings taken from a 32-channel cap (10/20 system). The electrooculogram generated from eye blinks and movement was recorded from electrodes attached to the face: two approximately 2 cm above and below the right eye, one approximately 2 cm to the right of the right eye and one approximately 2 cm to the left of the left eye. The ground electrode during data collection was formed by the Common Mode Sense active electrode and the Driven Right Leg passive electrode. All signals were digitized using ActiView (BioSemi, Amsterdam, the Netherlands) software. The EEG was sampled at 512 Hz. Offline analysis was performed using Brain Vision Analyzer (Brain Products, Gilching, Germany) software. Data were referenced to the average reference and band-pass filtered with cutoffs of 0.1 and 30 Hz. The EEG was segmented for each trial beginning 200 ms before stimulus presentation and continuing for 1,000 ms after stimulus onset. The EEG was corrected for blinks and eye movements using the procedures described by Gratton, Coles, and Donchin (1983). Semiautomated artifact rejection procedures were used to eliminate artifacts in each trial, with the following parameters: voltage step of more than 50 μV between sample points, a voltage difference of 300 μV within a trial, and activity < 0.5 μV within 100-ms intervals. Trials for each emotional face type were averaged together, with the activity in the window 200 ms before stimulus onset serving as the baseline. Visual inspection of the difference between neutral faces and the average of all emotional faces for all participants indicated that the LPP was maximal at occipital and parietal sites. To examine the time course and scalp distribution of effects, the LPP was scored as the mean amplitude at occipital (O1, O2 and Oz) and parietal (P3, P4 and Pz) sites in early (200–600 ms poststimulus onset) and late (600–1,000 ms poststimulus onset) windows.
Results
Participant characteristics
Demographics of each group are presented in Table 1. No children in any of the groups met full criteria for MDD or DD based on the criteria described in the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2000). The groups did not significantly differ on rates of oppositional defiant disorder or anxiety disorders, maternal, paternal or child age, child sex, CDI score, ethnicity, proportion of mothers or fathers completing college or proportion of married parents. The groups significantly differed on rates of child attention deficit hyperactivity disorder, χ2(3) = 8.53, p < .05, parental alcohol/substance use disorders, χ2(3) = 16.31, p < .01, and parental anxiety disorders, χ2(3) = 36.15, p < .001.
For children with a history of maternal or paternal depression, parental depression characteristics are presented in Table 1. For children with two parents with a history of depression, the parent with the earliest age of onset, greatest number of episodes and longest duration was used to calculate averages. The groups did not significantly differ on major depressive episode duration, but significant differences were found with regard to age of onset, F(2, 141) = 3.57, p < .05, and number of major depressive episodes, F(2, 116) = 6.62, p < .01.
LPP in young children
Given the paucity of studies of the LPP in young children, and the absence of data on the LPP in response to emotional faces, the first aim of the study was to determine whether emotional expressions were associated with an increased positivity relative to neutral facial expressions and to ascertain the time course and scalp distribution of these effects. The LPP was examined at parietal and occipital sites in two time windows: 200–600 and 600–1,000 ms after stimulus onset. Greenhouse–Geisser procedures were used to correct for significant differences in sphericity and Bonferroni procedures were used to correct for multiple comparisons. A 5 (emotion of face) × 2 (time window) × 2 (electrode site) repeated measures analysis of variance (ANOVA) was performed. The main effects of Emotion, F(4, 1376) = 4.54, p < .01, Time, F(1, 344) = 339.62, p < .001, and Electrode site, F(1, 344) = 1654.42, p < .001, were all significant, as were the Emotion × Time, F(4, 1376) = 4.95, p < .01, Emotion × Electrode site, F(4, 1376) = 5.67, p < .001, and Time × Electrode site, F(1, 344) = 1512.06, p < .001 interactions. All effects were qualified by the significant Emotion × Time × Electrode site interaction, F(4, 1376) = 19.16, p < .001. Two-way ANOVAs were then calculated to examine the Emotion × Time effects at each set of electrodes.
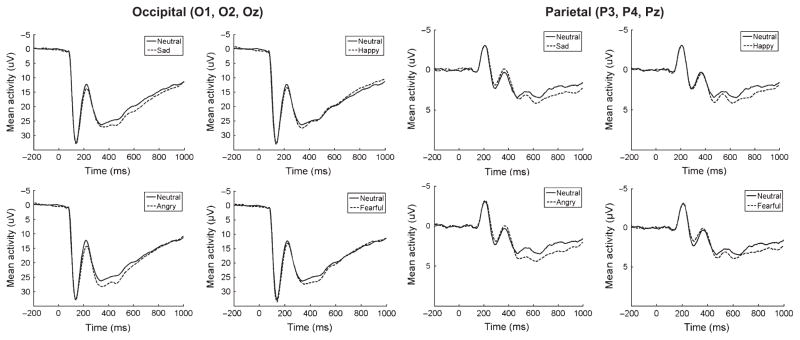
At occipital sites, the main effects of Emotion, F(4, 1376) = 6.15, p < .001, and Time, F(1, 344) = 835.59, p < .001, were significant but were qualified by a significant Emotion × Time interaction, F(4, 1376) = 11.06, p < .001. A one-way ANOVA in the early time window revealed a significant effect of Emotion, F(4, 1376) = 9.31, p < .001, and planned comparisons indicated that angry, F(1, 344) = 33.74, p < .001, fearful, F(1, 344) = 8.80, p < .01, and sad, F(1, 344) = 14.61, p < .001, faces are associated with significant positivities compared to neutral faces at occipital sites in the early time window. Happy faces did not significantly differ from neutral, F(1, 344) = 1.59, p > .05. A one-way ANOVA in the late time window also revealed a significant effect of Emotion, F(4, 1376) = 5.29, p < .001, and planned comparisons indicated that when using Bonferroni corrected p values (p = .0125), sad faces approached significance, F(1, 344) = 5.99, p = .015, but neither angry, F(1, 344) = 0.45, p > .05, fearful, F(1, 344) = 0.12, p > .05, or happy, F(1, 344) = 4.43, p = .04, faces significantly differed from neutral at occipital sites in the later time window.
At parietal sites, the two-way ANOVA revealed that the main effect of Emotion was not significant, F(4, 1376) = 1.75, p > .05; however, there was a significant effect of Time, F(1, 344) = 117.07, p < .001, and Emotion × Time interaction, F(4, 1376) = 6.45, p < .001. A one-way ANOVA in the early time window revealed that the effect of Emotion was not significant, F(4, 1376) = 0.97, p > .05. A one-way ANOVA in the late time window revealed a significant effect of Emotion, F(4, 1376) = 3.49, p < .01, and planned comparisons indicated that when using Bonferroni corrected p values (p = .0125), fearful faces approached significance, F(1, 344) = 5.82, p = .016, and angry, F(1, 344) = 10.40, p = .001, sad, F(1, 344) = 8.75, p < .01, and happy, F(1, 344) = 6.62, p = .011, faces were all significantly more positive than neutral.
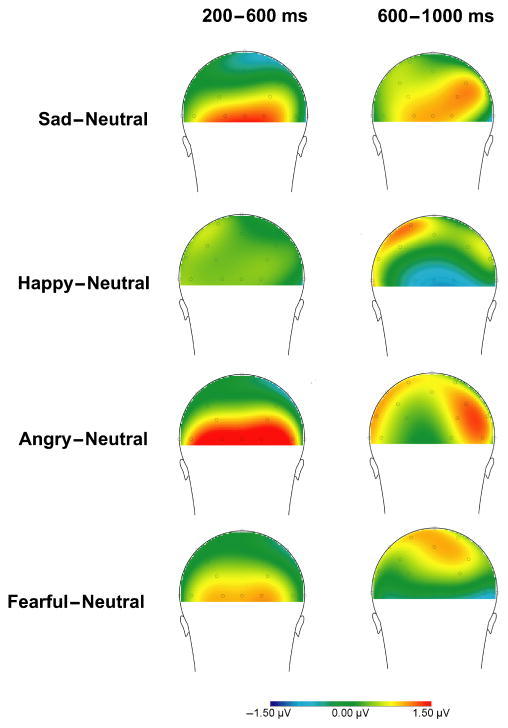
These results indicate that in response to emotional faces, occipitally maximal positivities in children begin around 200 ms after stimulus onset and are observable for all emotional faces except for happy (Figure 1). Around 600 ms after stimulus onset, positivities appear maximal over parietal sites; however, as shown in Figure 2, the later time window is associated with a less consistent, organized response compared to the early time window. Given these findings, analyses of parental depression focused on early (200–600 ms) activity at occipital sites and late (600– 1,000 ms) activity at parietal sites.
Figure 1.
Overall event-related potentials (negative up) in response to sad, happy, angry and fearful faces averaged across O1, O2 and Oz (left) and P3, P4 and Pz (right). Occipital and parietal event-related potentials are presented on different scales
Figure 2.
Overall scalp distributions of the difference in mean activity following emotional faces compared to mean activity following neutral faces at 200–600 (left) and 600–1,000 ms (right) after stimulus onset
Associations of LPP with parental depression
As we hypothesized that risk for depression would be linked to reduced differentiation between emotional and neutral stimuli, difference scores were calculated for each participant’s reactivity to each emotional face type compared to neutral. As the analyses of the overall sample identified two components that appear distinct both in time course and scalp distribution (i.e. an earlier occipital effect and a later parietal effect), we examined the effect of parental depression separately for each of these components. Two 4 (difference score for each emotional face) × 2 (paternal depression group) × 2 (maternal depression group) mixed-model ANOVAs were performed. To examine the main and interaction effects of maternal and paternal depression, maternal and paternal depression histories were included as separate independent variables.
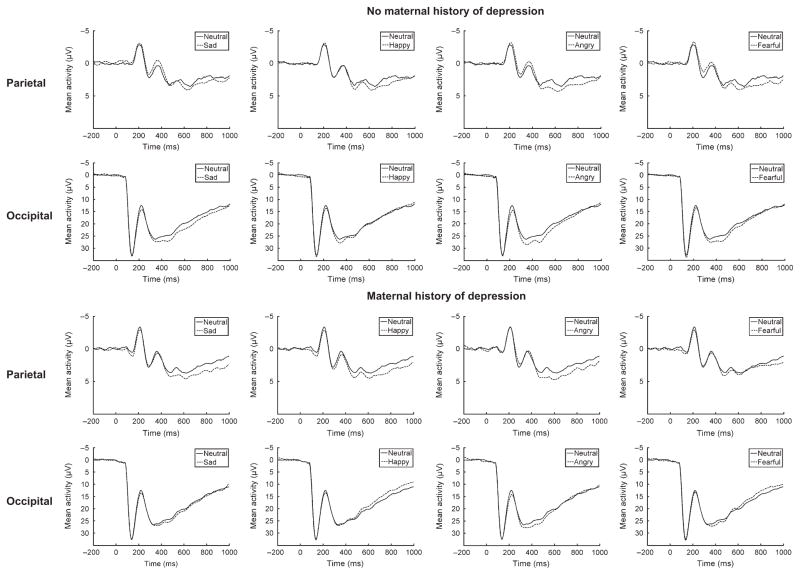
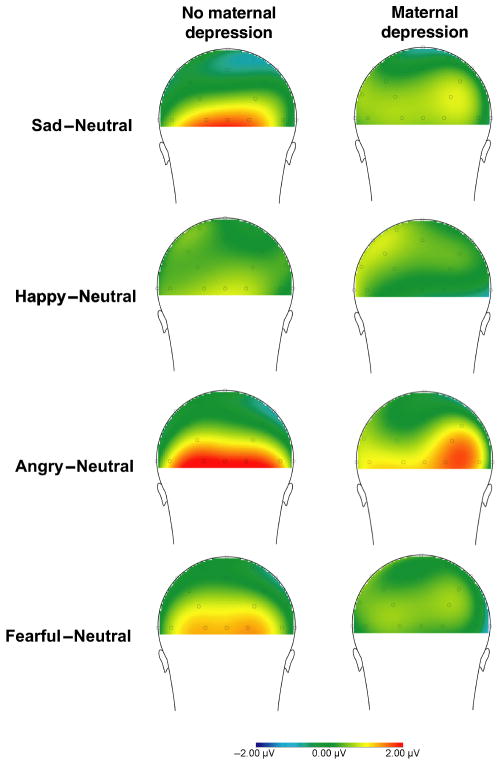
For the earlier occipital component, the overall effect of Emotion, F(3, 990) = 5.91, p < .01, was significant. In addition, the main effect of Maternal Depression was significant, F(1, 330) = 5.64, p < .05, partial η2 = .02, suggesting that there is less differentiation between neutral and emotional faces for children of mothers with a history of depression compared to those with no maternal depression (see Figures 3 and 4). The main effect of Paternal Depression was not significant, F(1, 330) = 0.02, p > .05. The Maternal × Paternal Depression interaction approached significance, F(1, 330) = 3.37, p = .07, but the Emotion × Paternal Depression, F(3, 990) = 1.33, p > .05, Emotion × Maternal Depression, F(3, 990) = 0.39, p > .05, and Emotion × Paternal Depression × Maternal Depression, F(3, 990) = 1.18, p > .05, interactions were not significant.
Figure 3.
Event-related potentials (negative up) at parietal and occipital sites for children with no maternal history of depression (top) and children with a maternal history of depression (bottom). A significant effect of maternal depression was found for the difference between emotional and neutral faces at occipital but not parietal sites
Figure 4.
Scalp distributions of the mean activity following emotional faces compared to mean activity following neutral faces 200–600 ms after stimulus onset for children with no maternal history of depression (left) and children with a maternal history of depression (right)
For the later parietal component, the main effects of Emotion, F(3, 990) = 0.18, p > .05, Maternal Depression group, F(1, 330) = 0.06, p > .05, and Paternal Depression, F(1, 330) = 0.73, p > .05 were not significant. In addition, the Emotion × Paternal Depression, F(3, 990) = 1.52, p > .05, Emotion × Maternal Depression, F(3, 990) = 1.24, p > .05, Maternal Depression × Paternal Depression, F(1, 330) = 0.43, p > .05, and Emotion × Paternal Depression × Maternal Depression, F(3, 990) = 0.49, p > .05, interactions were not significant.
These results suggest that maternal depression is associated with reduced differentiation between emotional and neutral stimuli but only in the earlier and occipitally maximal LPP (see Figure 4); moreover, the effect of parental depression appears specific to maternal depression. See Supporting Information for ERPS for each parental depression group. To ensure that differences cannot be accounted for by other parental or child diagnoses, the analyses were repeated with parental substance use disorder, parental anxiety disorder and child attention deficit hyperactivity disorder included as covariates. The main effect of maternal depression remained significant, F(1, 321) = 4.73, p < .05.
Discussion
The results of the current study suggest that LPPs are observable in children in response to emotional faces, and include both an early occipital and later parietal differentiation. In the later time window, the scalp distribution appeared less consistent across emotional face types, indicating that the organized centroparietal positivities observed in adults may not fully develop until later childhood. It will be important to examine older children to delineate the trajectory of developmental changes in the LPP response. Among adults, Foti et al. (2010) found that only angry and fearful faces elicited significantly more positive reactivity compared to neutral faces. Consistent with Foti et al., happy faces only elicited later positivities in our sample; however, sad faces did elicit a larger LPP than neutral faces among children.
These results also suggest that children with a maternal history of depression fail to exhibit the same patterns of electrocortical reactivity to emotional stimuli observed in children with no maternal history of depression. Consistent with ECI theory, children at higher risk for depression exhibited reduced reactivity to emotional stimuli compared with children at lower risk for depression; however, these patterns were specific to early emotional reactivity in the occipital regions. No differences were found in later, more parietal components. Importantly, patterns of ECI were observed only among children with maternal histories of depression, with no significant effect found for paternal depression. ECI may be more apparent among children of depressed mothers because of the stronger link between risk and maternal depression (e.g. Connell & Goodman, 2002) or because paternal and maternal depression are associated with qualitatively distinct patterns of emotional processing. Future work is needed to further examine these possibilities.
Several previous studies have observed reduced electrocortical reactivity to emotional compared to neutral stimuli among depressed adults (e.g. Foti et al., 2010; Kayser et al., 2000). The present study extends these findings and suggests that these patterns of hyporeactivity to emotional stimuli may be vulnerability markers that precede the onset of mood disorders. Interestingly, these patterns seem to be observable in children as young as 6 years old, long before the age of greatest risk for the onset of MDD. It is possible that maladaptive patterns of emotional reactivity early in life may lead to difficulty adapting to the environment (e.g. Keltner & Gross, 1999) and contribute to risk for depression, although prospective research is needed to determine whether patterns of ECI in childhood predict depression in later life. While one interpretation of these data is that an attenuated neural response to emotional faces is a vulnerability marker for depression, it is also possible that reduced emotional reactivity is only a correlate of maternal depression and is not directly linked to the later development of depression in offspring. Only prospective studies can tease apart these possibilities.
One limitation of the current study was the use of a passive picture viewing paradigm. Although these paradigms are common in adult work on the LPP, additional behavioral or eye-tracking measures would have bolstered our interpretation of these data. As we did not assess the children’s ability to discriminate emotional faces, we also cannot rule out the possibility that group differences in recognition of emotional faces may have contributed to the results. In addition, parental diagnoses of depression were made approximately 3 years prior to the psychophysiological assessment; thus, parents in the control group may have experienced a depressive episode in the intervening period, although this would likely weaken support of the research hypothesis. While sustained positivities were observed in response to emotional faces in this sample, the magnitude of the difference between emotional and neutral faces was relatively small compared to those observed in studies using IAPS images (e.g. Hajcak & Dennis, 2009), suggesting that emotional faces may be less effective in eliciting LPPs than IAPS images. Consistent with this possibility, faces have been rated as less arousing than IAPS images (Britton, Taylor, Sudheimer, & Liberzon, 2006). Relatively small differences between emotional and neutral stimuli in the overall sample may have limited the magnitude of the effects of parental depression on offspring LPPs.
Conclusion
The LPP appears to be a useful tool for measuring emotional reactivity in young children, and differences in reactivity to emotional compared to neutral facial expressions appear to be attenuated in children with maternal histories of depression. These results raise the possibility that electrocortical reactivity to emotional stimuli may be a vulnerability marker for depression. Additional work is needed to determine whether maladaptive emotional processing in childhood predicts the development of psychopathology later in life.
Key points.
In adults, depression has been associated with reduced electrocortical reactivity in response to emotional stimuli.
The late positive potential (LPP), an event-related potential component sensitive to emotional stimuli, is observable in 5- to 7-year-old children in response to emotional faces at occipital sites 200–600 ms after stimulus onset and parietal sites 600–1,000 ms after stimulus onset.
Children with a maternal history of depressive disorders show reduced differentiation between reactivity to emotional and neutral faces at occipital sites compared to children with no maternal history of depressive disorders.
Reduced early LPPs to emotional faces may be a vulnerability marker for depression.
Acknowledgments
This work was supported by the following grants: National Institute of Mental Health grant RO1 MH069942 (DNK) and GCRC Grant M01-RR10710 to Stony Brook University from the National Center for Research Resources.
Footnotes
Conflict of interest statement: No conflicts declared.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1. ERPs (negative up) at occipital sites depicting the effect of emotion for each parental history group.
Figure S2. ERPs (negative up) at parietal sites depicting the effect of emotion for each parental history group.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Allen NB, Trinder J, Brennen C. Affective startle modulation in clinical depression: Preliminary findings. Biological Psychiatry. 1999;46:542–550. doi: 10.1016/s0006-3223(99)00025-6. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. Rev. [Google Scholar]
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria: Reliability and validity. Archives of General Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Britton JC, Taylor SF, Sudheimer KD, Liberzon I. Facial expressions and complex IAPS pictures: Common and differential networks. NeuroImage. 2006;31:906–919. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 2008;28:676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Connell AM, Goodman SH. The association between psychopathology in fathers versus mothers and children’s internalizing and externalizing behavior problems: A meta-analysis. Psychological Bulletin. 2002;128:746–773. doi: 10.1037/0033-2909.128.5.746. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Deldin PJ, Keller J, Gergen JA, Miller GA. Cognitive bias and emotion in neuropsychological models of depression. Cognition and Emotion. 2001;15:787–802. [Google Scholar]
- Dichter GS, Tomarken AJ, Shelton RC, Sutton SK. Early- and late-onset startle modulation in unipolar depression. Psychophysiology. 2004;41:433–440. doi: 10.1111/j.1469-8986.00162.x. [DOI] [PubMed] [Google Scholar]
- Egger HK, Ascher BH, Angold A. Unpublished Interview Schedule. Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Science, Duke University Medical Center; 1999. The Preschool Age Psychiatric Assessment: Version 1.1. [Google Scholar]
- Ekman P. An argument for basic emotions. Cognition and Emotion. 1992;6:169–200. [Google Scholar]
- Ellis AJ, Beevers CG, Wells TT. Emotional dysregulation in dysphoria: Support for emotion context insensitivity in response to performance-based feedback. Journal of Behavior Therapy and Experimental Psychiatry. 2009;40:443–454. doi: 10.1016/j.jbtep.2009.05.002. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-IV Axis I Disorders - Non-patient editions. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Foti D, Kotov R, Klein DN, Hajcak G. Abnormal neural sensitivity to monetary gain versus loss among adolescents at risk for depression. Journal of Abnormal Child Psychology. 2011;39(7):913–924. doi: 10.1007/s10802-011-9503-9. [DOI] [PubMed] [Google Scholar]
- Foti D, Olvet DM, Klein DN, Hajcak G. Reduced electrocortical response to threatening faces in major depressive disorder. Depression and Anxiety. 2010;27:813–820. doi: 10.1002/da.20712. [DOI] [PubMed] [Google Scholar]
- Gao PX, Liu HJ, Ding N, Guo DJ. An event-related- potential study of emotional processing in adolescence. Acta Psychologica Sinica. 2010;42:342–351. [Google Scholar]
- Goodman S, Rouse M, Connell A, Broth M, Hall C, Heyward D. Maternal depression and child psychopathology: A meta-analytic review. Clinical Child and Family Psychology Review. 2011;14:1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Archives of General Psychiatry. 2010;67:380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Dennis TA. Brain potentials during affective picture processing in children. Biological Psychology. 2009;80:333–338. doi: 10.1016/j.biopsycho.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Olvet DM. The persistence of attention to emotion: Brain potentials during and after picture presentation. Emotion. 2008;8:250–255. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- Hammen CL. Children of depressed parents. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2. New York: Guilford; 2009. pp. 275–297. [Google Scholar]
- Hollingshead AB. Unpublished manuscript. Yale University; New Haven: 1975. Four factor index of social status. [Google Scholar]
- Iacono WG, Peloquin LJ, Lykken DT, Haroian KP, Valentine RH, Tuason VB. Electrodermal activity in euthymic patients with affective disorders: One-year retest stability and the effects of stimulus intensity and significance. Journal of Abnormal Psychology. 1984;93:304–311. doi: 10.1037//0021-843x.93.3.304. [DOI] [PubMed] [Google Scholar]
- Ialongo NS, Edelsohn G, Kellam SG. A further look at the prognostic power of young children’s reports of depressed mood. Child Development. 2001;72:736–747. doi: 10.1111/1467-8624.00312. [DOI] [PubMed] [Google Scholar]
- Ingram RE, Luxton DD. Vulnerability-stress models. In: Hankin BL, Abela JRZ, editors. Development of psychopathology: A vulnerability-stress perspective. Thousand Oaks, CA: Sage; 2005. pp. 32–46. [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Kayser J, Bruder GE, Tenke CE, Stewart JW, Quitkin FM. Event-related potentials (ERPs) to hemifield presentations of emotional stimuli: Differences between depressed patients and healthy adults in P3 amplitude and asymmetry. International Journal of Psychophysiology. 2000;36:211–236. doi: 10.1016/s0167-8760(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Keltner D, Gross JJ. Functional accounts of emotions. Cognition and Emotion. 1999;13:467–480. [Google Scholar]
- Kovacs M. Children’s Depression Inventory. Toronto, ON: Multi-Health Systems; 1992. [Google Scholar]
- Kring AM. The future of emotion research in the study of psychopathology. Emotion Review. 2010;2:225–228. [Google Scholar]
- Kujawa AJ, Torpey D, Kim J, Hajcak G, Rose S, Gotlib IH, Klein DN. Attentional biases for emotional faces in young children of mothers with chronic or recurrent depression. Journal of Abnormal Child Psychology. 2011;39:125–135. doi: 10.1007/s10802-010-9438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Tech Rep A-8. Gainesville: University of Florida; 2008. International Affective Picture System (IAPS): Affective ratings of pictures and instructional manual. [Google Scholar]
- MacNamara A, Hajcak G. Distinct electrocortical and behavioral evidence for increased attention to threat in generalized anxiety disorder. Depression and Anxiety. 2010;27:234–243. doi: 10.1002/da.20679. [DOI] [PubMed] [Google Scholar]
- Michalowski JM, Melzig CA, Weike AI, Stockburger J, Schupp HT, Hamm AO. Brain dynamics in spider-phobic individuals exposed to phobia-relevant and other emotional stimuli. Emotion. 2009;9:306–315. doi: 10.1037/a0015550. [DOI] [PubMed] [Google Scholar]
- Naumann E, Bartussek D, Diedrich O, Laufer ME. Assessing cognitive and affective information processing functions of the brain by means of the late positive complex of the event-related potential. Journal of Psychophysiology. 1992;6:285–298. [Google Scholar]
- Nelson CA, McCleery JP. Use of event-related potentials in the study of typical and atypical development. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:1252–1261. doi: 10.1097/CHI.0b013e318185a6d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse RM. Is depression an adaptation? Archives of General Psychiatry. 2000;57:14–20. doi: 10.1001/archpsyc.57.1.14. [DOI] [PubMed] [Google Scholar]
- Olino TM, Klein DN, Dyson MW, Rose SA, Durbin CE. Temperamental emotionality in preschool-aged children and depressive disorders in parents: Associations in a large community sample. Journal of Abnormal Psychology. 2010;119:468–478. doi: 10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR. Comparability of telephone and face-to-face interviews in assessing axis I and II disorders. American Journal of Psychiatry. 1997;154(11):1593–1598. doi: 10.1176/ajp.154.11.1593. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology. 2005;114:627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Johnson SL. Emotion and psychopathology: Bridging affective and clinical science. Washington, DC: American Psychological Association; 2007. [Google Scholar]
- Rottenberg J, Vaughan C. Emotion expression in depression: Emerging evidence for emotion context-insensitivity. In: Vingerhoets A, Nyklíček I, Denollet J, editors. Emotion regulation: Conceptual and clinical issues. New York: Springer; 2008. pp. 125–139. [Google Scholar]
- Santerre C, Allen JJB. Methods for studying the psychophysiology of emotion. In: Rottenberg J, Johnson SL, editors. Emotion and psychopathology: Bridging affective and clinical science. Washington, DC: American Psychological Association; 2007. pp. 53–79. [Google Scholar]
- Schupp HT, Flaisch T, Stockburger J, Junghöfer M. Emotion and attention: Event-related brain potential studies. Progress in Brain Research. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Öhman A, Junghöfer M, Weike AI, Stockburger J, Hamm AO. The facilitated processing of threatening faces: An ERP analysis. Emotion. 2004;4:189–200. doi: 10.1037/1528-3542.4.2.189. [DOI] [PubMed] [Google Scholar]
- Taylor L, Ingram RE. Cognitive reactivity and depressotypic information processing in children of depressed mothers. Journal of Abnormal Psychology. 1999;108:202–210. doi: 10.1037//0021-843x.108.2.202. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: The time-course of neural activity elicited by specific picture content. Emotion. 2010;10:767–782. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. The late positive potential predicts subsequent interference with target processing. Journal of Cognitive Neuroscience. 2011;23(10):2994–3007. doi: 10.1162/jocn.2011.21630. [DOI] [PubMed] [Google Scholar]