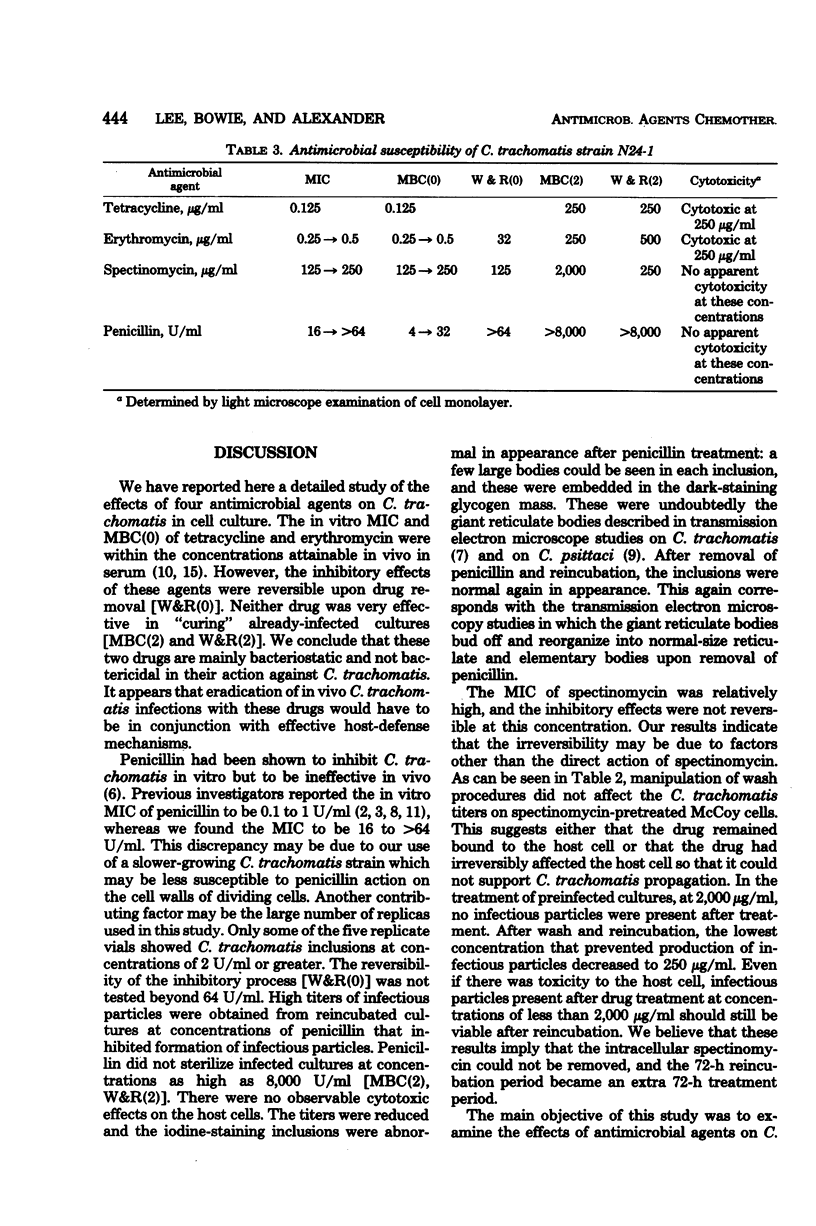
Abstract
The antimicrobial susceptibility of a low-laboratory-passage, slow-growing, genital Chlamydia trachomatis strain was studied by five different procedures with the use of McCoy cells pretreated with 5-iodo-2-deoxyuridine. The effects of antimicrobial agents when added to cultures on day 0 or day 2 after inoculation with C. trachomatis and the effects of washing and reincubating treated cultures in antimicrobial-free media were investigated. Tetracycline and erythromycin inhibited C. trachomatis growth at concentrations attainable in human serum, although their actions were reversible and significantly higher concentrations were needed to "cure" 48-h infected cultures. On a weight basis, spectinomycin was relatively ineffective in inhibiting C. trachomatis growth. The minimal inhibitory concentration of penicillin measured by our assay procedures was higher than that reported by other investigators. The five assay procedures used in this study were reproducible, and our results indicate that we can obtain more pertinent information about the efficacy of an antimicrobial agent in controlling C. trachomatis growth by using a combination of these assays than by simple minimal inhibitory concentration determinations, as had been previously described by other investigators. In addition, we failed to demonstrate changes in tetracycline susceptibility of C. trachomatis isolates from two patients who had received tetracycline therapy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNKOPF H., MASHIAH P., BECKER Y. Susceptibility of a trachoma agent grown in FL cell cultures to antibiotics and a sulfa drug. Proc Soc Exp Biol Med. 1962 Oct;111:61–67. doi: 10.3181/00379727-111-27706. [DOI] [PubMed] [Google Scholar]
- Gordon F. B., Dressler H. R., Quan A. L., McQuilkin W. T., Thomas J. I. Effect of ionizing irradiation on susceptibility of McCoy cell cultures to Chlamydia trachomatis. Appl Microbiol. 1972 Jan;23(1):123–129. doi: 10.1128/am.23.1.123-129.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon F. B., Harper I. A., Quan A. L., Treharne J. D., Dwyer R. S., Garland J. A. Detection of Chlamydia (Bedsonia) in certain infections of man. I. Laboratory procedures: comparison of yolk sac and cell culture for detection and isolation. J Infect Dis. 1969 Oct;120(4):451–462. doi: 10.1093/infdis/120.4.451. [DOI] [PubMed] [Google Scholar]
- Kramer M. J., Gordon F. B. Ultrastructural analysis of the effects of penicillin and chlortetracycline on the development of a genital tract Chlamydia. Infect Immun. 1971 Feb;3(2):333–341. doi: 10.1128/iai.3.2.333-341.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Grayston J. T. Antimicrobial activity of several antibiotics and a sulfonamide against Chlamydia trachomatis organisms in cell culture. Antimicrob Agents Chemother. 1977 Jul;12(1):80–83. doi: 10.1128/aac.12.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A., Manire G. P. Electron microscopic observations on the effects of penicillin on the morphology of Chlamydia psittaci. J Bacteriol. 1970 Jan;101(1):278–285. doi: 10.1128/jb.101.1.278-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway G. L., Owen J. M., Oriel J. D. A method for testing the antibiotic susceptibility of Chlamydia trachomatis in a cell culture system. J Antimicrob Chemother. 1976 Mar;2(1):71–76. doi: 10.1093/jac/2.1.71. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Kuo C. C., Grayston J. T. A simplified method for immunological typing of trachoma-inclusion conjunctivitis-lymphogranuloma venereum organisms. Infect Immun. 1973 Mar;7(3):356–360. doi: 10.1128/iai.7.3.356-360.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth B. B., Alexander E. R. Isolation of Chlamydia trachomatis by use of 5-iodo-2-deoxyuridine-treated cells. Appl Microbiol. 1974 May;27(5):912–916. doi: 10.1128/am.27.5.912-916.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



