Abstract
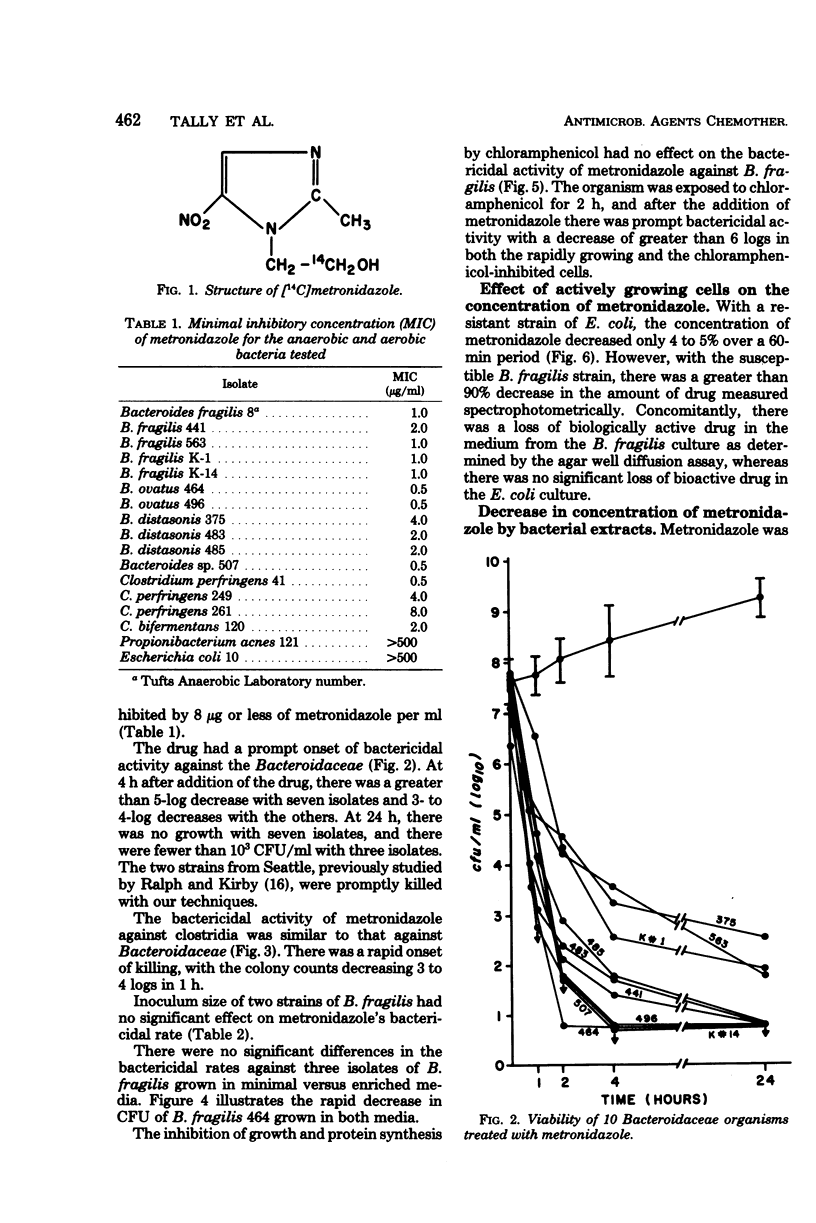
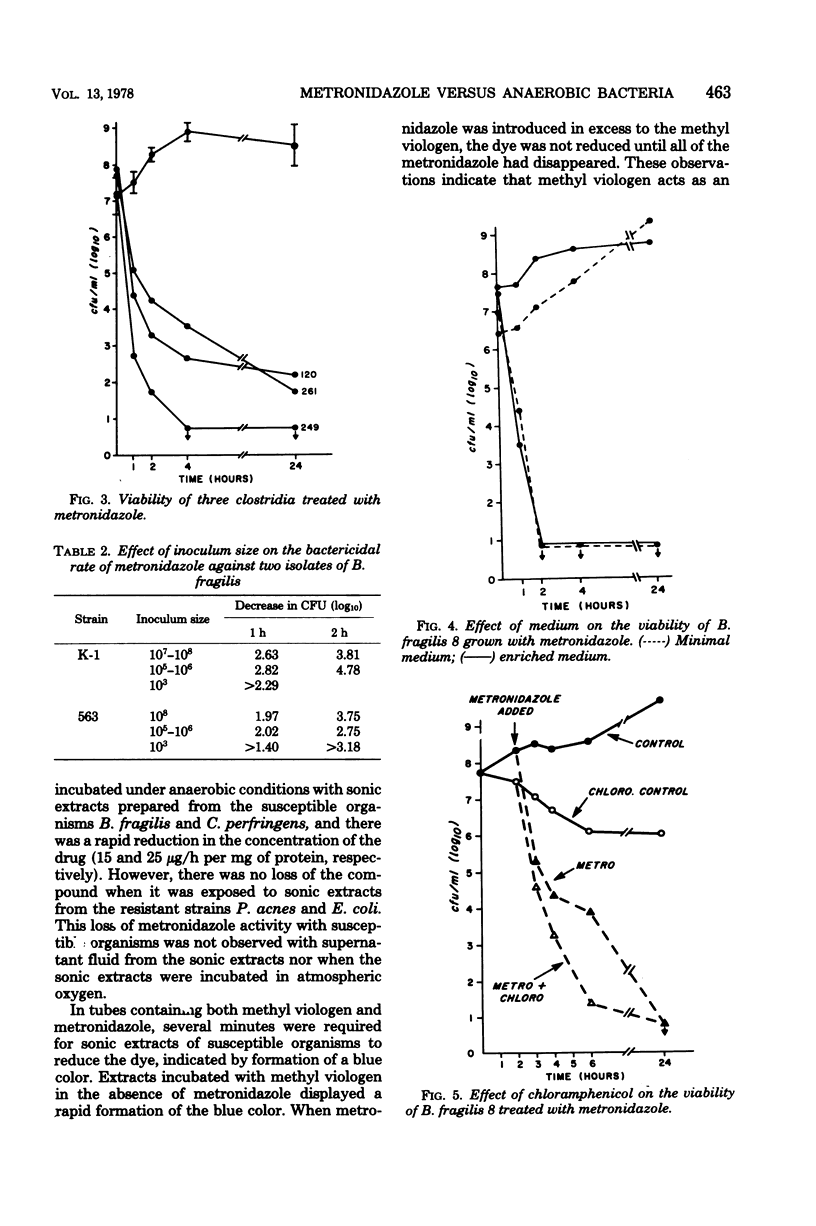
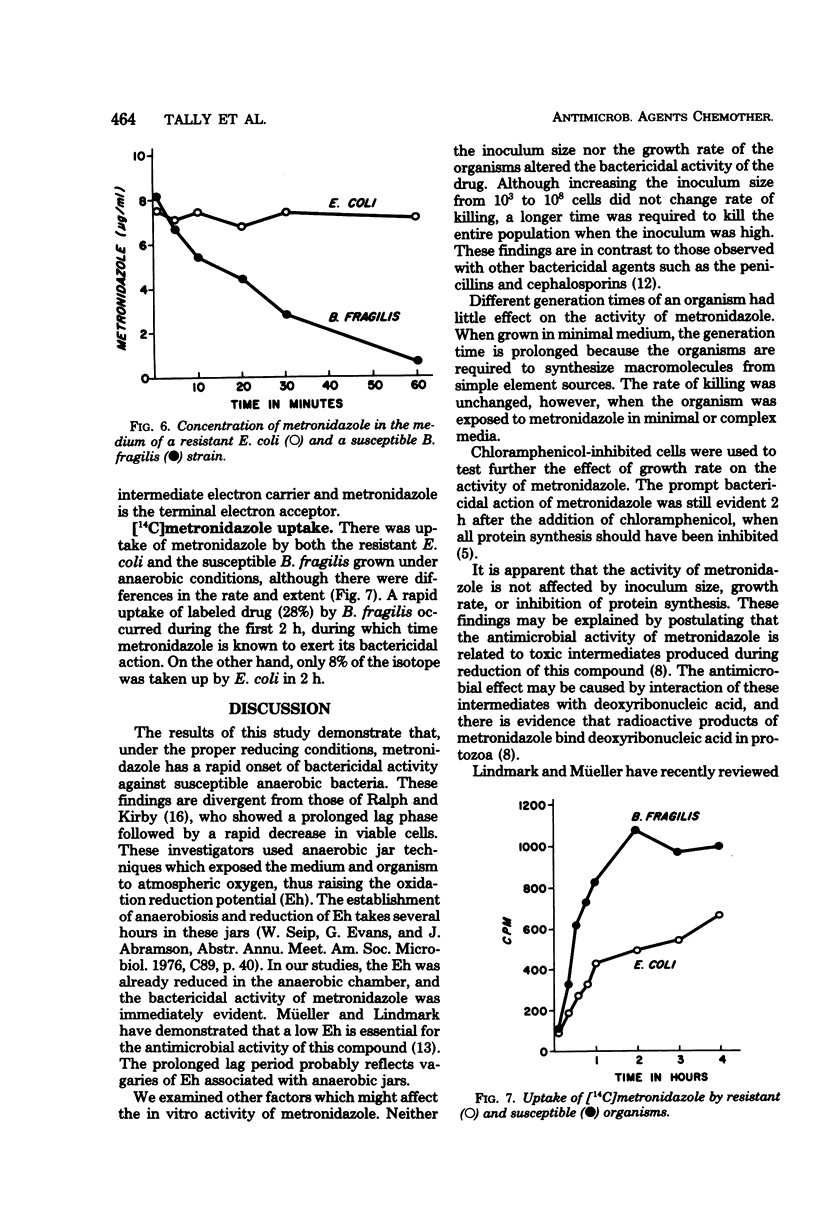
The antimicrobial activity of metronidazole was investigated in anaerobic bacteria by use of time-viability studies. This antimicrobial agent has a rapid onset of bactericidal activity under proper reducing conditions. The bactericidal rates were not affected by inoculum size or nutritional requirements, nor by inhibition of growth and protein synthesis by chloramphenicol. Using supernatant fractions of actively growing cultures of susceptible organisms, we observed a disappearance of metronidazole and a loss of biological activity, but there was no significant change in preparations from resistant bacteria. The decrease in drug concentration with susceptible cells occurred during the time that its bactericidal action was being exerted. Extracts from susceptible organisms rapidly reduced the concentration of metronidazole, confirming previous observations which suggest that the drug acts as a terminal electron acceptor. Radioisotope experiments with [14C]metronidazole revealed that the compound was taken up by both resistant and susceptible bacteria, although there was a difference in rate and extent of accumulation. These studies demonstrate that metronidazole's antimicrobial activity against anaerobic bacteria is bactericidal and independent of growth rate, and that it involves the uptake and metabolism of the compound.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSAR C., JULOU L. Activité de l'(hydroxy-2'éthyl)-1 méthyl-2 nitro-5 imidazole (8.823 R. P.) vis-à-vis des infections expérimentales à Trichomonas vaginalis. Ann Inst Pasteur (Paris) 1959 Feb;96(2):238–241. [PubMed] [Google Scholar]
- Edwards D. I., Mathison G. E. The mode of action of metronidazole against Trichomonas vaginalis. J Gen Microbiol. 1970 Nov;63(3):297–302. doi: 10.1099/00221287-63-3-297. [DOI] [PubMed] [Google Scholar]
- Everett E. D. Metronidazole and amebiasis. Am J Dig Dis. 1974 Jul;19(7):626–636. doi: 10.1007/BF01073018. [DOI] [PubMed] [Google Scholar]
- Goldring J., McNaught W., Scott A., Gillespie G. Prophylactic oral antimicrobial agents in elective colonic surgery. A controlled trial. Lancet. 1975 Nov 22;2(7943):997–1000. doi: 10.1016/s0140-6736(75)90289-5. [DOI] [PubMed] [Google Scholar]
- Ings R. M., McFadzean J. A., Ormerod W. E. The mode of action of metronidazole in Trichomonas vaginalis and other micro-organisms. Biochem Pharmacol. 1974 May 1;23(9):1421–1429. doi: 10.1016/0006-2952(74)90362-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levison M. E. Microbiological agar diffusion assay for metronidazole concentrations in serum. Antimicrob Agents Chemother. 1974 May;5(5):466–468. doi: 10.1128/aac.5.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmark D. G., Müller M. Antitrichomonad action, mutagenicity, and reduction of metronidazole and other nitroimidazoles. Antimicrob Agents Chemother. 1976 Sep;10(3):476–482. doi: 10.1128/aac.10.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. E., Jr, Garner C., Wilcox C., Finland M. Antibiotic susceptibility of gram-negative bacilli isolated from blood cultures. Results of tests with 35 agents and strains from 169 patients at Boston City Hospital during 1972. Am J Med. 1974 Aug;57(2):225–238. doi: 10.1016/0002-9343(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Müller M., Lindmark D. G. Uptake of metronidazole and its effect on viability in trichomonads and Entamoeba invadens under anaerobic and aerobic conditions. Antimicrob Agents Chemother. 1976 Apr;9(4):696–700. doi: 10.1128/aac.9.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastro L. J., Finegold S. M. Bactericidal activity of five antimicrobial agents against Bacteroides fragilis. J Infect Dis. 1972 Jul;126(1):104–107. doi: 10.1093/infdis/126.1.104. [DOI] [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Effect of metronidazole on hydrogen production by Clostridium acetobutylicum. Arch Mikrobiol. 1972;84(3):225–233. doi: 10.1007/BF00425200. [DOI] [PubMed] [Google Scholar]
- Ralph E. D., Kirby W. M. Unique bactericidal action of metronidazole against Bacteroides fragilis and Clostridium perfringens. Antimicrob Agents Chemother. 1975 Oct;8(4):409–414. doi: 10.1128/aac.8.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Jacobus N. V., Bartlett J. G., Gorbach S. L. Susceptibility of anaerobes to cefoxitin and other cephalosporins. Antimicrob Agents Chemother. 1975 Feb;7(2):128–132. doi: 10.1128/aac.7.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Sutter V. L., Finegold S. M. Treatment of anaerobic infections with metronidazole. Antimicrob Agents Chemother. 1975 May;7(5):672–675. doi: 10.1128/aac.7.5.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz H. B. In vitro studies on the differential toxicity of metronidazole in protozoa and mammalian cells. Ann Trop Med Parasitol. 1975 Mar;69(1):19–28. doi: 10.1080/00034983.1975.11686980. [DOI] [PubMed] [Google Scholar]
- Varel V. H., Bryant M. P. Nutritional features of Bacteroides fragilis subsp. fragilis. Appl Microbiol. 1974 Aug;28(2):251–257. doi: 10.1128/am.28.2.251-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe M. S. Giardiasis. JAMA. 1975 Sep 29;233(13):1362–1365. [PubMed] [Google Scholar]



