SUMMARY
Changes in food availability alter the output of hypothalamic nuclei that underlie energy homeostasis. Here, we asked whether food deprivation impacts the ability of GABA synapses in the dorsomedial hypothalamus (DMH), an important integrator of satiety signals, to undergo activity-dependent changes. GABA synapses in DMH slices from satiated rats exhibit endocannabinoid-mediated long-term depression (LTDGABA) in response to high-frequency stimulation of afferents. When CB1Rs are blocked, however, the same stimulation elicits long-term potentiation (LTPGABA), which manifests presynaptically and requires heterosynaptic recruitment of NMDARs and nitric oxide (NO). Interestingly, NO signaling is required for eCB-mediated LTDGABA. Twenty-four hour food deprivation results in a CORT-mediated loss of CB1R signaling and, consequently, GABA synapses only exhibit LTPGABA. These observations indicate that CB1R signaling promotes LTDGABA and gates LTPGABA. Furthermore, the satiety state of an animal, through regulation of eCB signaling, determines the polarity of activity-dependent plasticity at GABA synapses in the DMH.
INTRODUCTION
Abrupt changes in an organism’s environment precipitate requisite and rapid adaptive changes in neural circuits. In particular, synapses in hypothalamic nuclei that form the neural network underlying energy balance and food intake are remarkably susceptible to variations in the availability of food. The dearth of food is of such importance to an organism that it triggers both direct changes in food-related signals and the immediate activation of the stress response that increases circulating corticosteroids (CORT) (Bligh et al., 1990; Dallman et al., 1999; McGhee et al., 2009). The dorsomedial nucleus of the hypothalamus (DMH) regulates food intake and serves as a center for the integration of food and stress signals (Bellinger and Bernardis, 2002; DiMicco et al., 2002). More recently, the DMH has also been implicated as being the key food entrainable oscillator in the brain that exhibits synchronous activity in response to food deprivation (Gooley et al., 2006; Mieda et al., 2006). Although both of these roles are key to an organism’s survival, surprisingly little is known about synaptic processing in the DMH and even less is known about the effects of food deprivation on synaptic function and plasticity in this nucleus.
The synapses connecting key nuclei in circuits regulating energy homeostasis are sensitive to a number of chemical mediators, but no signal is as ubiquitously expressed as endocannabinoids (eCBs). These lipophilic molecules are produced in response to increases in postsynaptic Ca2+ and act as retrograde signals to quench both glutamate and GABA release at nerve terminals (Wilson and Nicoll, 2002). Although there is widespread support for the hypothesis that eCBs are orexigenic signals and that targeting the eCB system is beneficial for the treatment of eating disorders (Di Marzo and Matias, 2005; Gaetani et al., 2008), emerging evidence suggests the relationship between eCBs and energy homeostasis is more complex. Using a genetic and pharmacological approach, recent work has revealed that eCBs have divergent actions on food intake. eCB-mediated hyperphagic actions appear to be the result of actions at CB1Rs located on glutamate terminals. By contrast, eCB actions at GABA terminals suppress food intake (Bellocchio et al., 2010).
Nitric oxide (NO), like the eCBs, is a retrograde signal that is produced in response to a rise in intracellular Ca2+. Unlike eCBs, however, NO has stimulatory effects on GABA release (Bains and Ferguson, 1997; Di et al., 2009; Horn et al., 1994; Nugent et al., 2007; Stern and Ludwig, 2001). Although these retrograde transmitters have opposing actions at GABA synapses, accumulating evidence hints at a more nuanced interaction between eCBs and NO in mediating changes in synaptic strength. Specifically in some conditions, NO appears to be necessary for the induction of eCB-mediated plasticity (Kyriakatos and El Manira, 2007; Makara et al., 2007; Safo and Regehr, 2005), although the exact mechanism is unclear. We therefore asked how the control of GABAergic transmission in feeding circuits is regulated by eCBs and NO under conditions of satiety and food deprivation.
Because food deprivation increases circulating CORT, which, in other systems, downregulates CB1Rs (Hill et al., 2008; Mailleux and Vanderhaeghen, 1993; Rossi et al., 2008; Wamsteeker et al., 2010), we hypothesized that the absence of food, through associated changes in eCB signaling, would play a deterministic role in the ability of GABA synapses in the DMH to undergo activity-dependent plasticity. DMH neurons receive abundant GABAergic input from various hypothalamic nuclei, including the arcuate nucleus (Thompson and Swanson, 1998), and primarily send glutamatergic projections to the para-ventricular nucleus of the hypothalamus (PVN) (Boudaba et al., 1997; Ulrich-Lai et al., 2011), where they play a role in the integration of satiety and stress signals. Our results indicate that in satiated animals, plasticity at GABA synapses relies on the combined effects of eCBs and NO and is biased, particularly during prolonged, repetitive recruitment of afferents, toward long-term depression (LTDGABA). Following food deprivation, however, CORT-induced impairment of eCB signaling converts this system to one that only exhibits NO-dependent potentiation of GABA synapses (LTPGABA).
RESULTS
In addition to being innervated by axons immunoreactive for CB1Rs, neurons in the DMH contain the machinery required for retrograde NO signaling including the Ca2+-dependent enzyme NO synthase (Yamada et al., 1996), soluble guanylate cyclase (sGC; the NO receptor), and the cGMP-dependent protein kinase 1α (El-Husseini et al., 1999). We first examined the capacity of GABA synapses onto DMH neurons in slices obtained from satiated male Sprague-Dawley rats (postnatal days 21–30) to undergo activity-dependent plasticity in response to high-frequency stimulation (HFS) of synaptic inputs.
Blockade of CB1Rs Shifts Plasticity of GABA Synapses from LTD to LTP
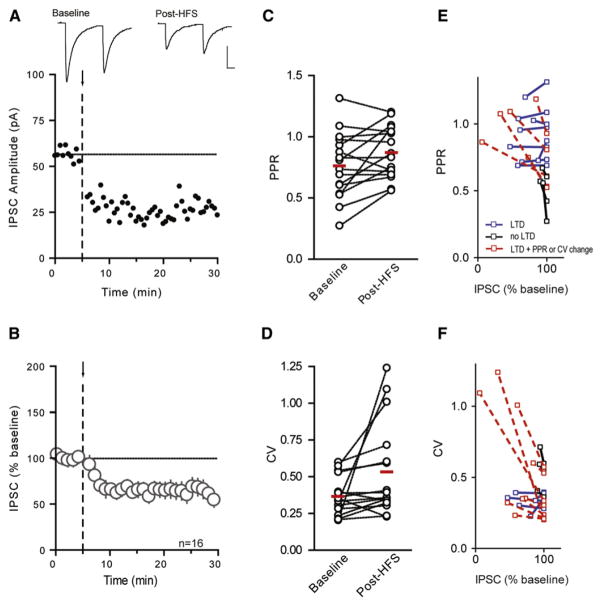
Although eCBs and NO have opposing effects on GABA release, they are both produced following bursts of afferent activity that release glutamate on to postsynaptic glutamate receptors and increase intracellular Ca2+. HFS (100 Hz for 4 s × 2, 0.05 Hz interval) elicited LTDGABA, as assessed by examining the amplitude of evoked inhibitory postsynaptic currents (IPSCs; 65% ± 7.7% of baseline, n = 16, p = 0.0004, Figures 1A and 1B).
Figure 1. HFS Induces LTDGABA in DMH Neurons.
Representative neuron depicts effects of HFS (arrow and dashed line) on IPSCs (A). Sample traces of averaged IPSCs before and after HFS are shown above. Data from multiple cells are summarized in (B). HFS does not alter PPR (C) or CV (D). Changes in PPR (E) and CV (F) were examined before and after HFS-induced LTDGABA in individual cells. Cells that displayed ≥10% LTDGABA and ≥10% PPR/CV increase are shown in red. Cells that displayed ≥10% LTDGABA but <10% PPR/CV increase are shown in blue. Cells with <10% LTDGABA are shown in black regardless of PPR/CV increase. PPR and CV changes were variable and were not observed in all cells displaying LTDGABA. Traces in all figures depict IPSCs averaged from the 5 min period immediately before (baseline) and 10–15 min after (post-HFS) HFS. Scale bars in all figures represent 25 pA and 10 ms.
To investigate the locus of LTDGABA, we examined the paired pulse ratio (PPR), the coefficient of variation (CV), and the frequency and amplitude of spontaneous IPSCs (sIPSCs). HFS did not significantly affect the PPR (baseline: 0.763 ± 0.067, post-HFS: 0.872 ± 0.054; p = 0.221, Figure 1C) or the coefficient of variation (baseline: 0.366 ± 0.033, post-HFS: 0.533 ± 0.08; p = 0.127, Figure 1D). Analysis of sIPSCs also indicated that HFS had no effect on either the frequency (88% ± 13.5% of baseline, p = 0.404) or the amplitude (99% ± 5.3% of baseline, p = 0.853). We did note, however, that changes in these parameters, regardless of the magnitude of LTD, were highly variable across different cells (Figures 1E and 1F).
In an effort to examine this more closely, we conducted a more systematic analysis of individual cells following HFS. There appear to be two types of neurons in the DMH with distinct electrophysiological fingerprints. Some neurons display a low-threshold spike in response to depolarizing pulses when held at hyperpolarized potentials (6 of 16 cells; Figure S1A), whereas others show continuous firing (10 of 16 cells; see Figure S1A available online). We examined the magnitude of depression in these two groups and observed no difference in the ability of HFS to induce plasticity (Figure S1B). Therefore, both cell types were pooled for the remainder of the experimental analyses. The variability in the PPR and CV did not correlate with the postsynaptic cell types and were evenly distributed in the cells with a low-threshold spike (40% and 80% for ≥10% PPR and CV increase, respectively) and continuously firing cells (38% and 63% for PPR and CV, respectively).
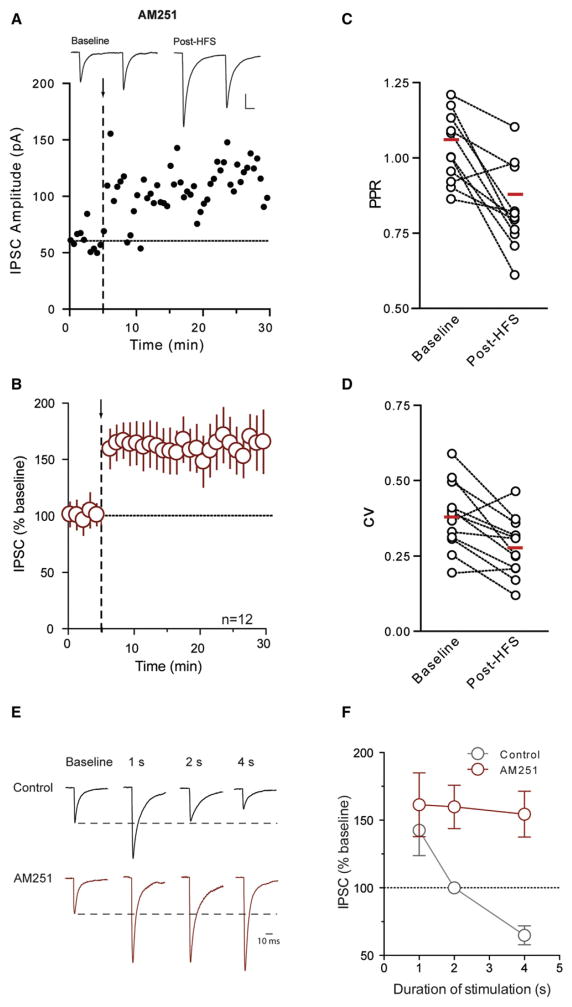
In other systems, eCBs, acting either at CB1Rs (Alger, 2009; Safo et al., 2006) or TRPV channels (Chávez et al., 2010; Grueter et al., 2010) have been implicated in similar activity-dependent LTD. We therefore tested whether eCBs were necessary for LTDGABA in the DMH. Surprisingly, inclusion of the CB1R antagonist, AM251 (5 μM), rather than blocking LTDGABA, unmasked a robust, long-term potentiation of GABA synapses (154% ± 16.9% of baseline, n = 12, p = 0.005; Figures 2A and 2B). LTPGABA was accompanied by a decrease in PPR (baseline: 1.061 ± 0.045; post-HFS: 0.879 ± 0.065; p = 0.003; Figure 2C) and CV (baseline: 0.379 ± 0.032; post-HFS: 0.278 ± 0.028; p = 0.004; Figure 2D), and an increase in the frequency of sIPSCs (177% ± 32.9% of baseline, p = 0.048), with no change in sIPSC amplitude (97% ± 12.0% of baseline, p = 0.793). In agreement with an essential role for CB1Rs in gating LTPGABA, HFS also elicited LTPGABA in CB1−/− mice (138% ± 6.9% of baseline, n = 5, p = 0.039; Figure S2). These results suggest that eCBs produced during HFS act as a retrograde signal to induce LTDGABA through their actions at presynaptic CB1Rs. In the absence of CB1Rs, LTD does not manifest and the same stimulus induces LTP.
Figure 2. Blockade of CB1Rs Shifts Plasticity of DMH Neurons from LTDGABA to LTPGABA.
(A and B) Representative neuron depicts effects of HFS (arrow and dashed line) on IPSCs in the presence of the CB1R antagonist AM251 (5 μM). Sample traces of averaged IPSCs are shown above. Data from multiple cells are summarized.
(C and D) HFS-induced changes in PPR and CV in the presence of AM251. HFS duration impacts plasticity differently in control versus AM251.
(E) Post-HFS traces in control and AM251 are scaled to the peak of the average baseline trace. Baseline traces for each HFS duration (1, 2, and 4 s) are normalized. Only one representative baseline trace is shown for clarity.
(F) Summary data showing the effect of different HFS durations on the ability of GABA synapses to undergo plasticity in control and AM251.
We next asked what impact the duration of stimulation had on the ability of these synapses to undergo plasticity. We examined the effects of recruiting fibers at the same frequency but in shorter stimulation epochs (1 and 2 s) in control and AM251. Following stimulation with 1s epochs (100 Hz for 1 s × 2, 0.05 Hz interval), GABA synapses exhibited heterogeneous responses that were biased toward LTPGABA in control conditions (142% ± 18.7% of base-line, n = 5, p = 0.085). Synaptic potentiation was more reliable in the presence of AM251 (161% ± 23.6% of baseline, n = 7, p = 0.041, Figures 2E and 2F). When the duration of each stimulus epoch was increased to 2 s, we failed to observe any reliable changes in synaptic strength (100% ± 4.0% of baseline, n = 5, p = 0.960; Figures 2E and 2F). Once again, in the presence of AM251, we observed a robust potentiation (160% ± 16.0% of baseline, n = 5, p = 0.024, Figures 2E and 2F). At 4 s, there is clear evidence of LTDGABA that shifts to LTPGABA in the presence of AM251. Overall, these data indicate that increasing the duration of the presynaptic burst shifts GABA synapses from those that are unreliable, but favor potentiation, to ones that exhibit reliable depression. In the absence of CB1R signaling, stable LTPGABA is observed regardless of burst duration, suggesting that CB1Rs cause LTDGABA and gate LTPGABA at these synapses. To delve more deeply into the mechanisms responsible for LTDGABA versus LTPGABA, the remaining experiments were all conducted using 4 s stimulus epochs.
LTPGABA Is Mediated by Heterosynaptic NMDAR Activation and Retrograde NO Signaling
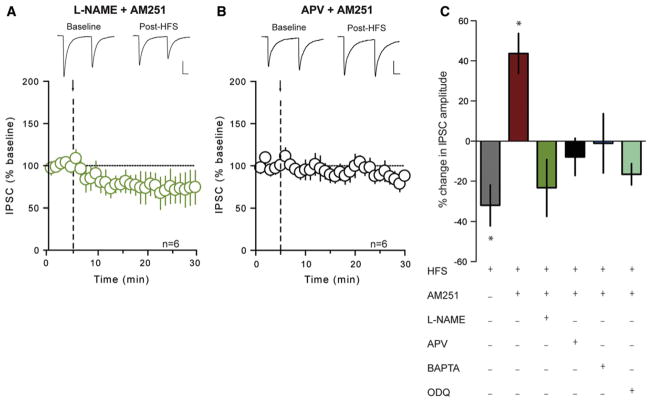
The LTPGABA observed here is reminiscent of NO-dependent LTPGABA described in the ventral tegmental area (Nugent et al., 2007). To test the hypothesis that retrograde NO signaling mediates LTPGABA, we first blocked NO production with the NO synthase inhibitor, Nω-nitro-L-arginine methyl ester (L-NAME; 200 μM) and repeated the HFS in the presence of AM251. This abolished LTPGABA (77% ± 14.1% of baseline, n = 6, p = 0.175; Figure 3A) and prevented the change in PPR (baseline: 0.884 ± 0.131; post-HFS: 0.856 ± 0.103; p = 0.928) and CV (baseline: 0.133 ± 0.023; post-HFS: 0.150 ± 0.032; p = 0.691). Next, to determine whether activity-dependent production of NO in DMH neurons relies on an increase in intracellular Ca2+, which is often secondary to the activation of NMDARs (Bains and Ferguson, 1997; Nugent et al., 2007; Szabadits et al., 2011), we conducted two independent experiments. First, we delivered HFS in the presence of AM251 and the NMDAR antagonist APV (50 μM). Under these conditions, HFS failed to elicit LTPGABA (94% ± 10.7% of baseline, n = 7, p = 0.436; Figure 3B). In the second experiment, the postsynaptic cell was loaded with the Ca2+ chelator BAPTA (10 mM), and HFS was delivered. This manipulation also completely abolished LTPGABA in the presence of AM251 (99% ± 14.8% of baseline, n = 5, p = 0.944; Figure 3C), indicating that a rise in postsynaptic Ca2+ is necessary for NO-mediated potentiation of GABA synapses.
Figure 3. LTPGABA is Mediated by Heterosynaptic NMDAR Activation and Retrograde NO Signaling.
(A) Summary data showing no activity-dependent plasticity following HFS (arrow and dashed line) in the presence of AM251 (5 μM) and L-NAME (200 μM). Sample traces of averaged IPSCs before and after HFS are shown above.
(B) Summary data showing no activity-dependent plasticity following HFS in the presence of AM251 and APV (50 μM). Sample traces of averaged IPSCs before and after HFS are shown above.
(C) Summary data showing percent change in IPSC amplitude after HFS under different conditions as shown in graph. “+” indicates the presence of the drug. All values are mean ± SEM; *p < 0.05.
The effects of NO on GABA release require the activation of presynaptic soluble guanylate cyclase (sGC), with a subsequent rise in cyclic GMP (cGMP) (Nugent et al., 2007). Consistent with these observations, we failed to elicit LTPGABA (87% ± 6.7% of baseline, n = 6, p = 0.053; Figure 3C) in the presence of both AM251 and the sGC inhibitor, ODQ (10 μM). When taken together, these findings are consistent with the hypothesis that GABA synapses are potentiated by NO recruited in a heterosynaptic fashion by the activation of NMDARs.
Actions of CB1R Ligand and NO Donor on GABA Transmission in the DMH
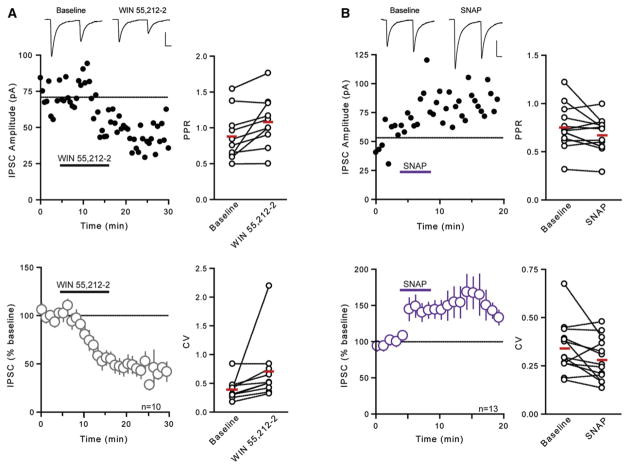
We next examined whether these synapses were sensitive to pharmacological manipulations using exogenous ligands that either activate CB1Rs or liberate NO directly. Bath application of the CB1R agonist WIN 55,212-2 (5 μM) elicited a robust depression in evoked IPSC amplitude (51% ± 7.0% of baseline, n = 10, p = 0.0001; Figure 4A). This was accompanied by an increase in the PPR (baseline: 0.887 ± 0.110; post-drug: 1.087 ± 0.112; p = 0.020; Figure 4A) and CV (baseline: 0.394 ± 0.058; post-drug: 0.707 ± 0.174; p = 0.001; Figure 4A), suggesting that these effects are localized at the presynaptic terminal. This is consistent with its action elsewhere in the hypothalamus (Hirasawa et al., 2004; Huang et al., 2007; Oliet et al., 2007; Wamsteeker et al., 2010) and throughout the brain (Kano et al., 2009). To determine whether the NO donor SNAP (200 μM) modulates GABA release in the DMH, we assessed its effects on evoked IPSCs. SNAP caused a rapid increase in IPSC amplitude (145% ± 14.7% of baseline, n = 13, p = 0.0003, Figure 4B) and a decrease in PPR (baseline: 0.757 ± 0.074; post-drug: 0.655 ± 0.056; p = 0.042; Figure 4B) and CV (baseline: 0.343 ± 0.037; post-drug: 0.284 ± 0.031; p = 0.039; Figure 4B). This is also consistent with previous reports that NO increases GABA release in the CNS (Bains and Ferguson, 1997; Di et al., 2009; Horn et al., 1994; Nugent et al., 2007; Stern and Ludwig, 2001). Together, our findings confirm that GABA synapses in the DMH are sensitive to manipulations that directly activate CB1Rs or deliver NO to the tissue.
Figure 4. Actions of CB1R Ligand and NO Donor on GABA Transmission in the DMH.
(A) Representative neuron depicts effects of WIN 55,212-2 (5 μM) on IPSCs. Sample traces of averaged IPSCs before and after WIN 55,212-2 are shown above. Data from multiple cells are summarized below. Panels on the right summarize WIN 55,212-2-induced changes in PPR and CV.
(B) Representative neuron depicts effects of SNAP (200 μM) on IPSCs. Sample traces of averaged IPSCs before and after SNAP are shown above. Data from multiple cells are summarized below. Panels on the right summarize SNAP-induced changes in PPR and CV.
CB1R Activation Impairs NO-Mediated Increase in GABA Release
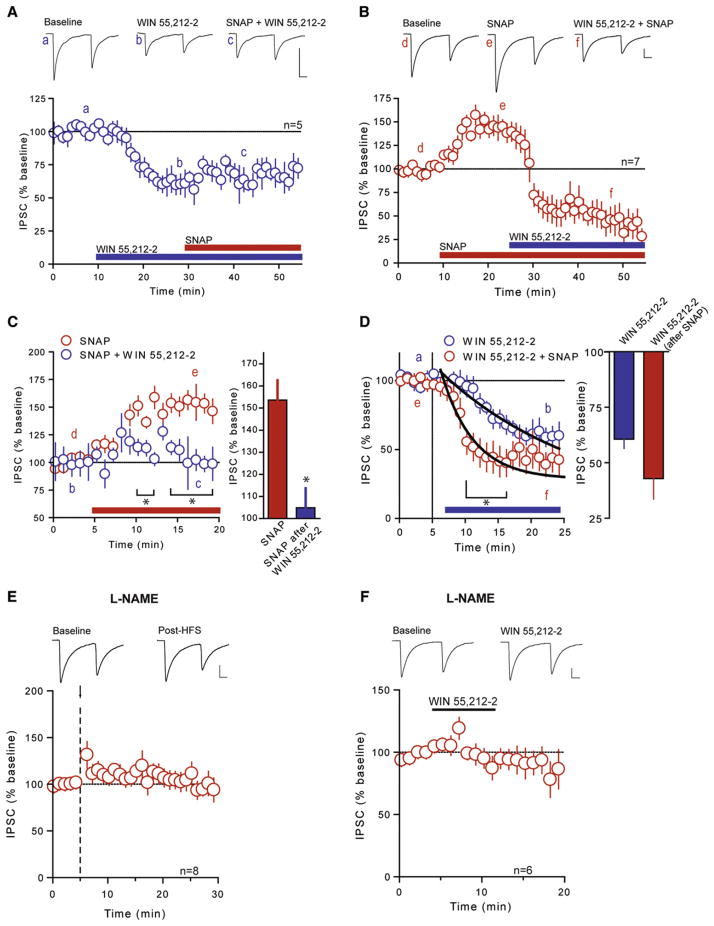
Our data demonstrate that NO-mediated LTPGABA is more prevalent in the absence of CB1R signaling. This raises the question as to how these signaling pathways interact at DMH synapses. If CB1R activation at the presynaptic terminal precludes the effects of NO to enhance GABA release, then the application of a CB1R agonist should block the potentiation of GABA transmission by the NO donor, SNAP. Consistent with this idea, SNAP failed to increase evoked IPSC amplitude when applied to slices that were continuously perfused with WIN 55,212-2 (104% ± 12.6% of WIN 55,212-2, n = 5, p = 0.646; Figures 5A and 5C). Similarly, it did not affect PPR (baseline: 0.961 ± 0.119; post-drug: 0.883 ± 0.178; p = 0.544) or CV (baseline: 0.502 ± 0.071; post-drug: 0.500 ± 0.045; p = 0.962). Conversely, WIN 55,212-2 still effectively depressed IPSCs that were first potentiated by SNAP (36% ± 12.0% of SNAP, n = 7; Figures 5B and 5D). This change was accompanied by an increase in PPR (baseline: 0.663 ± 0.109; post-drug: 0.950 ± 0.099; p = 0.048) and CV (baseline: 0.332 ± 0.084; post-drug: 0.593 ± 0.117; p = 0.049), consistent with the effect of WIN 55,212-2 in the absence of SNAP. Interestingly, the onset of the WIN 55,212-2–induced depression was accelerated in the presence of SNAP when compared with WIN 55,212-2 alone, as evidenced by a decrease in the decay constant of the depression after drug application by approximately 80% (from 13.0 ± 2.8 min to 2.5 ± 0.7 min; Figure 5D). These data suggest that activation of CB1Rs attenuates the NO-induced increase in GABA release, whereas NO itself enhances the effects of a CB1R ligand.
Figure 5. CB1R Signaling Precludes NO-mediated Potentiation but NO Signaling Is Required for CB1R-Mediated Depression of GABA Synapses.
(A) Summary data showing that SNAP (200 μM) fails to potentiate IPSCs in the presence of WIN 55,212-2 (5 μM). Sample traces of averaged IPSCs before WIN 55,212-2 (a), during WIN 55,212-2 (b), and during WIN 55,212-2 + SNAP (c) are shown above.
(B) Summary data showing WIN 55,212-2–induced depression in IPSCs in the presence of SNAP. Sample traces of averaged IPSCs before SNAP (d), during SNAP (e), and during SNAP + WIN 55,212-2 (f).
(C) Summary data showing the effect of SNAP alone and in the presence of WIN 55,212-2 (left) and summary bar graph showing percent of baseline in IPSC amplitude with SNAP alone and SNAP in the presence of WIN 55,212-2 (right).
(D) Summary data showing the effect of WIN 55,212-2 alone and in the presence of SNAP (left) and summary bar graph showing percent of baseline in IPSC amplitude with WIN 55,212-2 alone and WIN 55,212-2 in the presence of SNAP (right). A single exponential fit of each time course is shown and is used to calculate the time constant for the depression.
(E) Summary data showing L-NAME (200 μM) prevents eCB-mediated LTDGABA. Sample traces of averaged IPSCs before and after HFS (arrow and dashed line) are shown above.
(F) Summary data showing that WIN 55,212-2 fails to depress IPSCs in the presence of L-NAME. Sample traces of averaged IPSCs before and during incubation of slices in WIN 55,212-2 with continuous incubation in L-NAME are shown above. All values are mean ± SEM; *p < 0.05.
NO Is Required for eCB-Induced Depression of GABA Signaling
Next, we conducted experiments to determine the consequences of NO production on eCB-mediated LTDGABA. When NO synthesis was inhibited by L-NAME, HFS (100 Hz for 4 s × 2, 0.05 Hz interval) failed to elicit LTDGABA (111% ± 11.3% of base-line, n = 8, p = 0.350; Figure 5E), the changes in PPR (baseline: 0.860 ± 0.086; post-HFS: 0.826 ± 0.102; p = 0.369), or the changes in CV (baseline: 0.311 ± 0.028; post-HFS: 0.336 ± 0.07; p = 0.452). This suggests that NO signaling is required either for eCB production or CB1R signaling. Consistent with the latter idea, direct activation of CB1Rs by WIN 55,212-2 in the presence of L-NAME failed to significantly depress evoked IPSC amplitude (88% ± 10.8% of baseline, n = 6, p = 0.375; Figure 5F), PPR (baseline: 0.903 ± 0.129; post-drug: 0.889 ± 0.092; p = 0.850), or CV (baseline: 0.362 ± 0.067; post-drug: 0.410 ± 0.094; p = 0.168). Overall, these data point to an inherent complexity in the signaling of the retrograde transmitters eCBs and NO in the DMH. Specifically, they argue that eCB signaling prevents NO-mediated potentiation of GABA synapses but that NO signaling is required for eCB-induced depression of GABA signaling.
CORT-Mediated Downregulation of CB1Rs following Acute Food Deprivation Unmasks LTPGABA
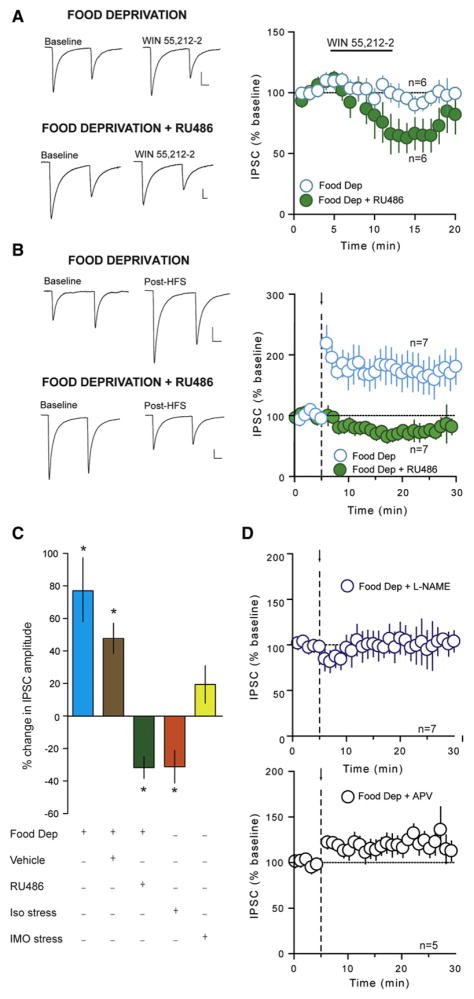
We have demonstrated thus far that CB1R signaling precludes NO-mediated LTPGABA in the DMH. Here, we hypothesized that a physiological state in which CB1R signaling is compromised should favor the induction of LTPGABA. There is compelling evidence that CORT reduces the expression of CB1Rs in the brain (Hill et al., 2008; Mailleux and Vanderhaeghen, 1993; Rossi et al., 2008; Wamsteeker et al., 2010) and that acute food deprivation results in significant elevations in circulating CORT (Bligh et al., 1990; Dallman et al., 1999; McGhee et al., 2009). We first examined the impact of food deprivation on CB1R function in DMH neurons by testing the ability of WIN 55,212-2 to depress GABA synapses. Animals were food-deprived for 24 hr prior to slice preparation. Unlike naïve animals (Figure 4A), WIN 55,212-2 had no effect on the amplitude of evoked IPSCs (99% ± 6.6% of baseline, n = 6, p = 0.370, Figure 6A), PPR (baseline: 0.938 ± 0.062; post-drug: 0.967 ± 0.114; p = 0.460), or CV (baseline: 0.103 ± 0.015; post-drug: 0.137 ± 0.052; p = 0.234) in food-deprived animals. To determine whether elevated levels of CORT were responsible for the loss of CB1R signaling, we administered the genomic glucocorticoid receptor antagonist, RU486 (25 mg/kg, subcutaneous) at 12 hr intervals during the 24 hr food deprivation period. In slices obtained from animals receiving RU486, CB1R agonist-mediated depression was recovered (64% ± 12.3% of baseline, n = 6, p = 0.037; Figure 6A). We next asked whether food deprivation unmasked LTPGABA. Indeed, in neurons from food-deprived animals, HFS elicited a robust LTPGABA (177% ± 26.9% of baseline, n = 7, p = 0.029; Figure 6B). This was accompanied by a decrease in PPR (baseline: 1.276 ± 0.113; post-HFS: 0.833 ± 0.064; p = 0.006) and CV (baseline: 0.376 ± 0.061; post-HFS: 0.240 ± 0.026; p = 0.035), and an increase in the frequency of sIPSCs (269% ± 46.6% of baseline, p = 0.049), but a decrease in sIPSC amplitude (79% ± 4.4% of baseline, p = 0.006), suggesting an increase in the probability of GABA release from the presynaptic terminal. These observations indicate that acute food deprivation converts LTDGABA to LTPGABA in DMH neurons. RU486 treatment in food-deprived animals completely abolished LTPGABA and unmasked an activity-dependent depression (68% ± 6.6% of baseline, n = 7, p = 0.018; Figure 6B). In food-deprived animals receiving vehicle, HFS potentiated GABA synapses (148% ± 9.4% of baseline, n = 8, p = 0.0020; Figure 6C), confirming the specificity of the effect of RU486. These experiments provide direct evidence that elevations in CORT accompanying food deprivation are necessary for these synapses to undergo LTPGABA. Similar to LTPGABA in slices from naïve animals following CB1R blockade or from CB1R−/− animals, this synaptic potentiation was completely abolished in the presence of either L-NAME (102% ± 14.7% of baseline, n = 7, p = 0.921; Figure 6D) or APV (117% ± 10.3% of baseline, n = 5, p = 0.157; Figure 6D), indicating that it is mediated by NO produced by heterosynaptic activation of NMDARs. To determine whether these changes are specific to the prolonged stress of food deprivation, we conducted two additional experiments. First, animals were subjected to 30 min of immobilization, a stressor that transiently increases CORT (Romeo et al., 2006) prior to experimentation. This manipulation failed to elicit LTPGABA in response to HFS (119% ± 11.5% of baseline, n = 8, p = 0.284; Figure 6C). These data suggest that prolonged activation of the HPA axis (~24 hr) is required to shift synapses from a depressing to a potentiating state. One additional prolonged stressor may result from animals being housed individually during the food deprivation period. To rule out the possibility that social isolation alone (as a mild stressor) is sufficient to unmask LTPGABA, we investigated whether HFS would elicit LTPGABA in animals housed alone but given ad libitum access to food for 24 hr prior to slice preparation. Under these conditions, synapses did not exhibit LTPGABA, but instead underwent an activity-dependent depression in GABA transmission (71% ± 12.2% of baseline, n = 5, p = 0.032; Figure 6C), indicating that social isolation is not sufficient to shift the polarity of the plasticity.
Figure 6. CORT-Mediated Downregulation of CB1Rs following Acute Food Deprivation Unmasks LTPGABA.
(A) Summary data showing the effect of WIN 55,212-2 (5 μM) in animals experiencing 24 hr of food deprivation prior to experimentation and food-deprived animals receiving the genomic glucocorticoid receptor antagonist RU486 (25 mg/kg; 2 s.c. injections, 12 hr apart during the food deprivation period). Sample traces of averaged IPSCs before and during incubation of slices in WIN 55,212-2 in food-deprived animals and food-deprived animals receiving RU486 are shown to the left.
(B) Summary data showing LTPGABA in food-deprived animals, which is blocked with RU486. Sample traces of averaged IPSCs before and after HFS (arrow and dashed line) in food-deprived animals and in food-deprived animals receiving RU486 are shown to the left.
(C) Summary data showing percent change in IPSC amplitude following HFS under different conditions as shown in graph.
(D) Summary data showing L-NAME (200 μM; top) and APV (50 μM; bottom) prevent LTPGABA in food-deprived animals. All values are mean ± SEM; *p < 0.05.
Finally, we asked whether these synaptic changes could be reversed by the re-introduction of food. Following food deprivation, animals were given unlimited access to food for 24 hr and then slices containing the DMH were prepared for electrophysiology. Refeeding following food deprivation restores circulating CORT to basal levels within 6 hr of food presentation (Jahng et al., 2005). Following refeeding, HFS did not elicit LTPGABA (88% ± 12.7% of baseline, n = 5, p = 0.919; Figure 6C), potentially suggesting partial functional recovery of CB1Rs. In agreement with this finding, WIN 55,212-2–induced depression of GABA synapses was restored after 24 hr of refeeding (63% ± 11.0% of baseline, n = 4, p = 0.021). Taken together, these results indicate that a food deprivation–induced rise in CORT leads to a downregulation of CB1Rs, thus creating a permissive state that favors the induction of LTPGABA (Figure 7).
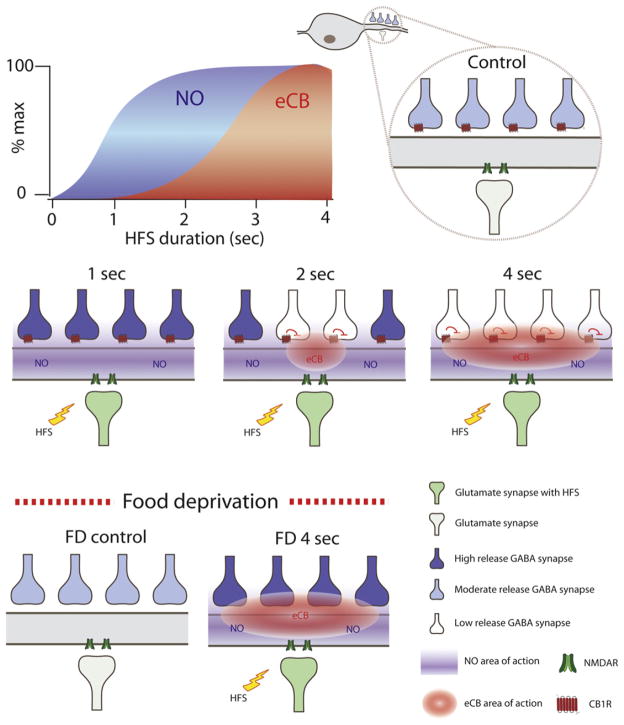
Figure 7. Schematic Diagram of the Effect of NO and eCBs on GABA Synapses following Different Durations of Stimulation and Food Deprivation.
Both eCBs and NO are produced in postsynaptic cells following a burst of activity in afferent inputs (high-frequency stimulation; HFS) but exhibit different thresholds for production. A brief synaptic burst (1 s) generates NO but not eCBs. Longer bursts (2 and 4 s) generate increasing amounts of eCBs in addition to NO. The different thresholds for NO or eCB production creates local environments that are exposed to only NO or both NO and eCBs. NO stimulates GABA release while eCBs through actions at presynaptic CB1Rs inhibit GABA release. In satiated animals, eCBs gate the ability of NO to induce LTP. Food deprivation inactivates CB1Rs via an increase in the circulating levels of CORT. In the absence of eCB signaling, HFS results in a uniform NO-mediated potentiation.
DISCUSSION
The data presented here demonstrate that the feeding state of an animal determines the polarity of plasticity exhibited by GABA synapses in the DMH in response to repetitive synaptic stimuli. In satiated animals, GABA synapses undergo eCB-mediated LTD that requires NO. Following acute food deprivation, however, only LTP is evident. LTPGABA, which requires the heterosynaptic activation of NMDARs, is constrained in satiated animals by eCBs. Blockade of CB1Rs or their down-regulation during food deprivation by circulating CORT biases the synapses toward LTP. These findings provide, to the best of our knowledge, the first demonstration of state-dependent plasticity of a feeding circuit in response to acute food deprivation, and highlight a complex interaction between retrograde signals in which NO is necessary for LTD, and eCBs gate LTPGABA.
In young rats, acute food deprivation results in a plethora of metabolic changes including a considerable reduction in body weight, an increase in circulating CORT levels (Dallman et al., 1999) and robust changes in eCB and NO levels in the hypothalamus (Di Marzo et al., 2001; Kirkham et al., 2002; Squadrito et al., 1994). While we investigate GABA signaling in the DMH following food deprivation, other studies have reported an increase in neuronal activity in the DMH, as assessed by changes in Fos expression, in response to refeeding following food deprivation (Johnstone et al., 2006; Renner et al., 2010). Our finding that food deprivation produces an increase in GABA drive to DMH neurons is in agreement with these studies and suggests that enhanced inhibition of these neurons is a mechanism to cope with the lack of food. These neurons are then activated upon re-feeding following a period of food deprivation. Although we report LTPGABA of evoked synaptic responses following HFS in food-deprived animals, this is accompanied by a small decrease in the amplitude of spontaneous IPSCs. Under these conditions, in which CB1Rs are compromised, we surmise that HFS will still produce eCBs, but they have no presynaptic binding partner available. Thus, the eCBs may preferentially bind to postsynaptic TRPV channels and promote a postsynaptic LTDGABA. This putative postsynaptic LTD may rely on a mechanism similar to that reported at glutamate synapses in other brain regions (Chávez et al., 2010; Grueter et al., 2010) and may explain why we observe a slight depression following HFS when both the CB1R and NO signaling pathways are blocked.
Food deprivation is one of the most fundamental stressors to an organism, with elevated CORT levels observed within just 4 hr following removal of food from young rats (Dallman et al., 1999). In this study, we demonstrate that CORT, through actions at genomic glucocorticoid receptors, is essential for shifting the plasticity from LTDGABA to LTPGABA in the DMH following acute food deprivation. Accumulating evidence suggests that CORT can interfere with CB1R expression and signaling. In the PVN, we have recently reported a downregulation of CB1Rs following repeated stress that is mediated by activation of genomic glucocorticoid receptors (Wamsteeker et al., 2010). Prolonged CORT treatment also decreases the density of CB1Rs in the hippocampus (Hill et al., 2008) and impairs CB1R-mediated control of GABA transmission in the striatum (Rossi et al., 2008). Importantly, it appears that the increase in CORT must be robust and prolonged because challenges that cause either prolonged but small changes in CORT (social isolation) or robust but transient increases (30 min immobilization) failed to shift the balance of plasticity toward LTP. It is important to note that CORT can also induce eCB biosynthesis and release (Di et al., 2003; Hill et al., 2005; Malcher-Lopes et al., 2006), and that elevated levels of eCBs can result in desensitization and synaptic exclusion of CB1Rs (Mikasova et al., 2008). Unlike the current data, however, the rapid effects of CORT on eCB synthesis are mediated by the putative membrane-bound glucocorticoid receptor and not the classical genomic receptor that is sensitive to RU486 (Di et al., 2005).
We provide evidence that eCBs, through actions at CB1Rs, gate LTP at GABA synapses. In addition, our study also reveals two interesting interactions between the NO and eCB systems in regulating GABA transmission in the DMH. First, eCB signaling impairs NO-mediated potentiation of GABA synapses. This is evident following a prolonged burst of afferent activity where eCB-mediated LTDGABA is favored over NO-mediated potentiation. With shorter durations of stimulation, however, we observed a shift from LTDGABA to LTPGABA. It is likely that shorter bursts of afferent activity favor the production of NO over eCBs. Although both retrograde signals are produced following a rise in intracellular Ca2+, it is possible that NO may be synthesized at a faster rate because of coupling of NO synthase to the NMDA receptor (Bredt and Snyder, 1989; Garthwaite et al., 1989). With longer stimulation, both NO and eCBs are present and eCB signaling impairs NO-mediated LTPGABA. Figure 7 summarizes our current hypothesis regarding the activity-dependent production and action of NO and eCBs in regulating GABA transmission in satiated and food-deprived conditions.
The mechanism of the eCB-mediated blockade of NO action is not known, but our observation that the NO donor SNAP fails to potentiate GABA synapses in the presence of WIN 55,212-2 suggests that CB1R activation impedes NO signaling in the DMH. eCB-mediated LTD requires inhibition of protein kinase A (PKA). Thus, one possibility is that there may be an interaction between the cAMP-PKA and cGMP-PKG signaling pathways (Barman et al., 2003; Nugent et al., 2009) such that inhibition of PKA interferes with PKG.
We also show that NO signaling is necessary for eCB-mediated LTD of GABA synapses. When NO production is blocked, GABA synapses do not depress in response to HFS-induced eCB production or application of a CB1R agonist. Conversely, when NO signaling is augmented, CB1R-induced depression of GABA synapses is even more effective. These findings are consistent with evidence indicating that the induction of eCB-mediated plasticity in other brain areas is blocked by disrupting NO signaling (Daniel et al., 1993; Kyriakatos and El Manira, 2007; Makara et al., 2007; Safo and Regehr, 2005; Shibuki and Okada, 1991). The exact mechanism of this blockade is not known (Alger, 2005), but several potential mechanisms have been proposed. NO appears to be acting downstream of CB1R activation to mediate LTD in the cerebellum (Safo and Regehr, 2005) and striatum (Chepkova et al., 2009), whereas in the hippocampus, under certain conditions, eCB-mediated plasticity requires NO actions upstream of the CB1R (Makara et al., 2007). Alternatively, NO may act directly at the CB1R to enhance eCB signaling. There is evidence that NO prevents agonist-induced desensitization and internalization of G protein–coupled receptors (GPCRs). This is accomplished by nitrosylation (and therefore inactivation) of the GPCR kinases that target the receptors for internalization (Kokkola et al., 2005; Whalen et al., 2007).
Although the production of eCBs and NO is similarly triggered by a rise in intracellular Ca2+ in the postsynaptic cell, these retrograde signals have opposing actions on GABA release (reviewed in Feil and Kleppisch, 2008; Wilson and Nicoll, 2002). In this study, we demonstrate that a loss in CB1R signaling is a necessary prerequisite for NO-mediated LTPGABA following acute food deprivation. This finding, that abolition of CB1R signaling is associated with a drive to eat, appears to be at odds with the overwhelming support for an orexigenic role of the eCB system. For example, following acute food deprivation, most evidence points to an increase in hypothalamic eCB levels (Di Marzo et al., 2001; Kirkham et al., 2002), and CB1−/− mice exhibit hypophagia compared with their wild-type littermates (Bellocchio et al., 2010). Recent work, however, provides clear evidence that activation of CB1Rs on GABA terminals is associated with a reduction in feeding. In the ventral striatum, CB1R-mediated inhibition of GABAergic transmission is associated with a hypophagic action of eCBs (Bellocchio et al., 2010). Thus, it is possible that a loss of CB1Rs at GABAergic terminals in the DMH in response to food deprivation is akin to removing the brake from the brain’s feeding circuitry.
Our findings provide a demonstration of state-dependent plasticity in the DMH, a hypothalamic nucleus that plays a vital role in integrating information related to caloric status and stress. Evidence suggests that DMH neurons send glutamatergic projections to the PVN (Thompson et al., 1996), an autonomic nucleus that plays a critical role in regulating food intake (Cowley et al., 1999). In agreement with this, our unpublished observations indicate that the majority of DMH neurons from which we record demonstrate antidromic activation from the PVN. An increase in GABAergic drive in the DMH would therefore suppress glutamatergic signaling to PVN neurons that suppress food intake. This signaling would ultimately result in an enhanced drive to eat in the face of minimal food availability. Although stress-induced increases in CORT down-regulate CB1Rs and therefore promote the increase in GABA drive, signaling at the genomic glucocorticoid receptor has no effect on food intake or body weight (unpublished observations). Overall, these data demonstrate a potentially new form of state-dependent plasticity in a feeding circuit that may ensure satiety-sensing neurons in DMH neurons are not recruited when food is not available.
EXPERIMENTAL PROCEDURES
Brain Slice Preparation
All experiments were performed according to protocols approved by the University of Calgary Animal Care and Use Committee in accordance with guidelines established by the Canadian Council on Animal Care. Male Sprague-Dawley rats (postnatal day 21 [P21]–30) or CB1−/− mice maintained under specified pathogen-free conditions were anesthetized with isofluorane and decapitated. The brain was quickly removed and placed in ice-cold slicing solution containing (in mM) 87 NaCl, 2.5 KCl, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, 1.25 NaH2PO4, 25 glucose, and 75 sucrose saturated with 95%/5% O2/CO2. The brain was blocked and mounted on a vibrating slicer (Leica, Nussloch, Germany) submerged in ice-cold slicing solution. Angled horizontal slices (250 μm) containing the DMH were cut and incubated in 32.5°C artificial cerebrospinal fluid (aCSF) containing (in mM) 126 NaCl, 2.5 KCl, 26 NaHCO3, 2.5 CaCl2, 1.5 MgCl2, 1.25 NaH2PO4, and 10 glucose saturated with 95%/5% O2/CO2 for a minimum of 60 min.
Electrophysiology
Hypothalamic slices were then submerged in a recording chamber and superfused with 32.5°C aCSF at a flow rate of 1 ml/min. Whole-cell recordings were obtained from DMH neurons visualized with an Olympus upright microscope (Olympus, Center Valley, PA) fitted with infrared differential interference contrast optics. Recordings were obtained using borosilicate glass microelectrodes (tip resistance 4.5–6.5 MΩ) filled with a solution containing (in mM) 108 K gluconate, 8 Na gluconate, 2 MgCl2, 8 KCl, 1 potassium EGTA, 4 potassium ATP, 0.3 sodium GTP, and 10 HEPES, and corrected to pH 7.2 with KOH. In a subset of experiments, 10 mM BAPTA was included in the intracellular solution to chelate postsynaptic Ca2+. Recordings were accepted for analysis if changes in access resistance were <15%. Cells were voltage clamped at −80 mV and the perfusate always contained DNQX (10 μM; Tocris, Ellisville, MO) to block AMPA and kainate receptor-mediated glutamatergic transmission. GABAergic fibers were stimulated extracellularly with a patch pipette filled with aCSF and positioned within the DMH. IPSCs were evoked at a rate of 0.2 Hz and paired-pulse responses were obtained by applying a pair of synaptic stimuli 50 ms apart. For HFS, afferents were stimulated at 100 Hz for 4 s, repeated twice, 20 s apart, unless otherwise specified.
Electrophysiological signals were amplified using the Multiclamp700 B amplifier (Molecular Devices, Union City, CA), low-pass-filtered at 1 kHz, digitized at 10 kHz using the Digidata 1322 (Molecular Devices), and stored for offline analysis. Evoked currents were analyzed using Clampfit 9 (Molecular Devices). The amplitude of the synaptic current was calculated from the baseline (current before evoked response) to the peak of each evoked. For clarity, the stimulus artifacts were removed digitally from the traces depicted. Spontaneous IPSCs were analyzed using the threshold detection criteria in Minianalysis (Synaptosoft). Results are expressed as means ± SEM. In most cases, significance was determined using a one-sample or paired Student’s t test comparing the means following HFS or drug treatment to baseline with significance level of p < 0.05. The presence of unexplained outliers in the PPR and CV values in the HFS experiments in control animals necessitated the use of nonparametric statistical analysis methods and, hence, a Mann-Whitney test was used.
Food Deprivation
Twenty-four hours prior to slice preparation, animals were housed individually and food (but not water) was removed from the cages. This duration of food deprivation has been demonstrated to produce a significant reduction in body weight in young rats (Arola et al., 1984). Body weight was measured before and after food deprivation. In another set of experiments, animals were food deprived for 24 hr and then refed for another 24 hr prior to slice preparation.
Injection of RU486/Vehicle
Animals were administered 25 mg/kg RU486 suspended in canola oil (or canola oil alone as vehicle) subcutaneously two times at 12 hr intervals beginning 1 hr after lights-on when the food was removed. Animals were housed individually and food was removed for 24 hr prior to slice preparation.
Isolation and Immobilization Stress
To induce social isolation stress, animals were housed individually but were given ad libitum access to food and water for 24 hr prior to slice preparation. In a different subset of experiments, animals were placed in a Plexiglas restrainer for 30 min and then quickly anesthetized and decapitated as described above.
Supplementary Material
Acknowledgments
We thank Mio Tsutsui and Cheryl Sank for technical support. We also thank Dr. K. Sharkey for providing the CB1R−/− mice. We are grateful to members of our labs for comments on earlier drafts of the manuscript. K.M.C. is supported by an NSERC Canada Graduate Scholarship, an AI-HS Studentship, and a Hotchkiss Brain Institute Obesity Initiative Scholarship. W.I. is supported by an AI-HS Fellowship. Q.J.P. is an AI-HS Scientist and J.S.B. is an AI-HS Senior Scholar. This work was funded by operating grants to Q.J.P. and J.S.B. from the Canadian Institutes for Health Research.
Footnotes
Supplemental Information includes two figures and can be found with this article online at doi:10.1016/j.neuron.2011.06.006.
References
- Alger BE. Endocannabinoid identification in the brain: studies of breakdown lead to breakthrough, and there may be NO hope. Sci STKE. 2005;2005:pe51. doi: 10.1126/stke.3092005pe51. [DOI] [PubMed] [Google Scholar]
- Alger BE. Endocannabinoid signaling in neural plasticity. Curr Top Behav Neurosci. 2009;1:141–172. doi: 10.1007/978-3-540-88955-7_6. [DOI] [PubMed] [Google Scholar]
- Arola L, Palou A, Remesar X, Alemany M. Effects of 24-hour starvation period on metabolic parameters of 20-day-old rats. Arch Int Physiol Biochim. 1984;92:297–303. doi: 10.3109/13813458409071170. [DOI] [PubMed] [Google Scholar]
- Bains JS, Ferguson AV. Nitric oxide regulates NMDA-driven GABAergic inputs to type I neurones of the rat paraventricular nucleus. J Physiol. 1997;499:733–746. doi: 10.1113/jphysiol.1997.sp021965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman SA, Zhu S, Han G, White RE. cAMP activates BKCa channels in pulmonary arterial smooth muscle via cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1004–L1011. doi: 10.1152/ajplung.00295.2002. [DOI] [PubMed] [Google Scholar]
- Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav. 2002;76:431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- Bellocchio L, Lafenêtre P, Cannich A, Cota D, Puente N, Grandes P, Chaouloff F, Piazza PV, Marsicano G. Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci. 2010;13:281–283. doi: 10.1038/nn.2494. [DOI] [PubMed] [Google Scholar]
- Bligh ME, DeStefano MB, Kramlik SK, Douglass LW, Dubuc P, Castonguay TW. Adrenal modulation of the enhanced fat intake subsequent to fasting. Physiol Behav. 1990;48:373–381. doi: 10.1016/0031-9384(90)90331-w. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol. 1997;77:3396–3400. doi: 10.1152/jn.1997.77.6.3396. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci USA. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci. 2010;13:1511–1518. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepkova AN, Fleischer W, Kazmierczak T, Doreulee N, Haas HL, Sergeeva OA. Developmental alterations of DHPG-induced long-term depression of corticostriatal synaptic transmission: switch from NMDA receptor-dependent towards CB1 receptor-dependent plasticity. Pflugers Arch. 2009;459:131–141. doi: 10.1007/s00424-009-0714-7. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A, Horsley C, Levin N, Meijer O, Soriano LR, et al. Starvation: early signals, sensors, and sequelae. Endocrinology. 1999;140:4015–4023. doi: 10.1210/endo.140.9.7001. [DOI] [PubMed] [Google Scholar]
- Daniel H, Hemart N, Jaillard D, Crepel F. Long-term depression requires nitric oxide and guanosine 3′:5′ cyclic monophosphate production in rat cerebellar Purkinje cells. Eur J Neurosci. 1993;5:1079–1082. doi: 10.1111/j.1460-9568.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8:585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Bátkai S, Járai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG. Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and gamma-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology. 2005;146:4292–4301. doi: 10.1210/en.2005-0610. [DOI] [PubMed] [Google Scholar]
- Di S, Maxson MM, Franco A, Tasker JG. Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. J Neurosci. 2009;29:393–401. doi: 10.1523/JNEUROSCI.4546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- El-Husseini AE, Williams J, Reiner PB, Pelech S, Vincent SR. Localization of the cGMP-dependent protein kinases in relation to nitric oxide synthase in the brain. J Chem Neuroanat. 1999;17:45–55. doi: 10.1016/s0891-0618(99)00023-x. [DOI] [PubMed] [Google Scholar]
- Feil R, Kleppisch T. NO/cGMP-dependent modulation of synaptic transmission. Handb Exp Pharmacol. 2008:529–560. doi: 10.1007/978-3-540-74805-2_16. [DOI] [PubMed] [Google Scholar]
- Gaetani S, Kaye WH, Cuomo V, Piomelli D. Role of endocannabinoids and their analogues in obesity and eating disorders. Eat Weight Disord. 2008;13:e42–e48. [PubMed] [Google Scholar]
- Garthwaite J, Garthwaite G, Palmer RM, Moncada S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol. 1989;172:413–416. doi: 10.1016/0922-4106(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–1525. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Ho WS, Meier SE, Gorzalka BB, Hillard CJ. Chronic corticosterone treatment increases the endocannabinoid 2-arachido-nylglycerol in the rat amygdala. Eur J Pharmacol. 2005;528:99–102. doi: 10.1016/j.ejphar.2005.10.058. [DOI] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, Ho WS, Shi L, Patel S, Gorzalka BB, Hillard CJ. Prolonged glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus. 2008;18:221–226. doi: 10.1002/hipo.20386. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Schwab Y, Natah S, Hillard CJ, Mackie K, Sharkey KA, Pittman QJ. Dendritically released transmitters cooperate via autocrine and retrograde actions to inhibit afferent excitation in rat brain. J Physiol. 2004;559:611–624. doi: 10.1113/jphysiol.2004.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn T, Smith PM, McLaughlin BE, Bauce L, Marks GS, Pittman QJ, Ferguson AV. Nitric oxide actions in paraventricular nucleus: cardiovascular and neurochemical implications. Am J Physiol. 1994;266:R306–R313. doi: 10.1152/ajpregu.1994.266.1.R306. [DOI] [PubMed] [Google Scholar]
- Huang H, Acuna-Goycolea C, Li Y, Cheng HM, Obrietan K, van den Pol AN. Cannabinoids excite hypothalamic melanin-concentrating hormone but inhibit hypocretin/orexin neurons: implications for cannabinoid actions on food intake and cognitive arousal. J Neurosci. 2007;27:4870–4881. doi: 10.1523/JNEUROSCI.0732-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng JW, Lee JY, Yoo SB, Kim YM, Ryu V, Kang DW, Lee JH. Refeeding-induced expression of neuronal nitric oxide synthase in the rat paraventricular nucleus. Brain Res. 2005;1048:185–192. doi: 10.1016/j.brainres.2005.04.072. [DOI] [PubMed] [Google Scholar]
- Johnstone LE, Fong TM, Leng G. Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab. 2006;4:313–321. doi: 10.1016/j.cmet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkola T, Savinainen JR, Mönkkönen KS, Retamal MD, Laitinen JT. S-nitrosothiols modulate G protein-coupled receptor signaling in a reversible and highly receptor-specific manner. BMC Cell Biol. 2005;6:21. doi: 10.1186/1471-2121-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakatos A, El Manira A. Long-term plasticity of the spinal locomotor circuitry mediated by endocannabinoid and nitric oxide signaling. J Neurosci. 2007;27:12664–12674. doi: 10.1523/JNEUROSCI.3174-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Glucocorticoid regulation of cannabinoid receptor messenger RNA levels in the rat caudate-putamen. An in situ hybridization study. Neurosci Lett. 1993;156:51–53. doi: 10.1016/0304-3940(93)90437-p. [DOI] [PubMed] [Google Scholar]
- Makara JK, Katona I, Nyíri G, Németh B, Ledent C, Watanabe M, de Vente J, Freund TF, Hájos N. Involvement of nitric oxide in depolarization-induced suppression of inhibition in hippocampal pyramidal cells during activation of cholinergic receptors. J Neurosci. 2007;27:10211–10222. doi: 10.1523/JNEUROSCI.2104-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, Tasker JG. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci. 2006;26:6643–6650. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee NK, Jefferson LS, Kimball SR. Elevated corticosterone associated with food deprivation upregulates expression in rat skeletal muscle of the mTORC1 repressor, REDD1. J Nutr. 2009;139:828–834. doi: 10.3945/jn.108.099846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci USA. 2006;103:12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikasova L, Groc L, Choquet D, Manzoni OJ. Altered surface trafficking of presynaptic cannabinoid type 1 receptor in and out synaptic terminals parallels receptor desensitization. Proc Natl Acad Sci USA. 2008;105:18596–18601. doi: 10.1073/pnas.0805959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- Nugent FS, Niehaus JL, Kauer JA. PKG and PKA signaling in LTP at GABAergic synapses. Neuropsychopharmacology. 2009;34:1829–1842. doi: 10.1038/npp.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Baimoukhametova DV, Piet R, Bains JS. Retrograde regulation of GABA transmission by the tonic release of oxytocin and endocannabinoids governs postsynaptic firing. J Neurosci. 2007;27:1325–1333. doi: 10.1523/JNEUROSCI.2676-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner E, Szabó-Meltzer KI, Puskás N, Tóth ZE, Dobolyi A, Palkovits M. Activation of neurons in the hypothalamic dorsomedial nucleus via hypothalamic projections of the nucleus of the solitary tract following refeeding of fasted rats. Eur J Neurosci. 2010;31:302–314. doi: 10.1111/j.1460-9568.2009.07053.x. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Karatsoreos IN, McEwen BS. Pubertal maturation and time of day differentially affect behavioral and neuroendocrine responses following an acute stressor. Horm Behav. 2006;50:463–468. doi: 10.1016/j.yhbeh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Rossi S, De Chiara V, Musella A, Kusayanagi H, Mataluni G, Bernardi G, Usiello A, Centonze D. Chronic psychoemotional stress impairs cannabinoid-receptor-mediated control of GABA transmission in the striatum. J Neurosci. 2008;28:7284–7292. doi: 10.1523/JNEUROSCI.5346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Safo PK, Cravatt BF, Regehr WG. Retrograde endocannabinoid signaling in the cerebellar cortex. Cerebellum. 2006;5:134–145. doi: 10.1080/14734220600791477. [DOI] [PubMed] [Google Scholar]
- Shibuki K, Okada D. Endogenous nitric oxide release required for long-term synaptic depression in the cerebellum. Nature. 1991;349:326–328. doi: 10.1038/349326a0. [DOI] [PubMed] [Google Scholar]
- Squadrito F, Calapai G, Altavilla D, Cucinotta D, Zingarelli B, Campo GM, Arcoraci V, Sautebin L, Mazzaglia G, Caputi AP. Food deprivation increases brain nitric oxide synthase and depresses brain serotonin levels in rats. Neuropharmacology. 1994;33:83–86. doi: 10.1016/0028-3908(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Stern JE, Ludwig M. NO inhibits supraoptic oxytocin and vasopressin neurons via activation of GABAergic synaptic inputs. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1815–R1822. doi: 10.1152/ajpregu.2001.280.6.R1815. [DOI] [PubMed] [Google Scholar]
- Szabadits E, Cserép C, Szonyi A, Fukazawa Y, Shigemoto R, Watanabe M, Itohara S, Freund TF, Nyiri G. NMDA receptors in hippocampal GABAergic synapses and their role in nitric oxide signaling. J Neurosci. 2011;31:5893–5904. doi: 10.1523/JNEUROSCI.5938-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res Brain Res Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J Comp Neurol. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Jones KR, Ziegler DR, Cullinan WE, Herman JP. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: differential inputs to the anterior versus posterior subregions. J Comp Neurol. 2011;519:1301–1319. doi: 10.1002/cne.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsteeker JI, Kuzmiski JB, Bains JS. Repeated stress impairs endocannabinoid signaling in the paraventricular nucleus of the hypothalamus. J Neurosci. 2010;30:11188–11196. doi: 10.1523/JNEUROSCI.1046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Keys JR, Rockman HA, et al. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Yamada K, Emson P, Hökfelt T. Immunohistochemical mapping of nitric oxide synthase in the rat hypothalamus and colocalization with neuropeptides. J Chem Neuroanat. 1996;10:295–316. doi: 10.1016/0891-0618(96)00133-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.