Abstract
Non-negative matrix factorization is a useful tool for reducing the dimension of large datasets. This work considers simultaneous non-negative matrix factorization of multiple sources of data. In particular, we perform the first study that involves more than two datasets. We discuss the algorithmic issues required to convert the approach into a practical computational tool and apply the technique to new gene expression data quantifying the molecular changes in four tissue types due to different dosages of an experimental panPPAR agonist in mouse. This study is of interest in toxicology because, whilst PPARs form potential therapeutic targets for diabetes, it is known that they can induce serious side-effects. Our results show that the practical simultaneous non-negative matrix factorization developed here can add value to the data analysis. In particular, we find that factorizing the data as a single object allows us to distinguish between the four tissue types, but does not correctly reproduce the known dosage level groups. Applying our new approach, which treats the four tissue types as providing distinct, but related, datasets, we find that the dosage level groups are respected. The new algorithm then provides separate gene list orderings that can be studied for each tissue type, and compared with the ordering arising from the single factorization. We find that many of our conclusions can be corroborated with known biological behaviour, and others offer new insights into the toxicological effects. Overall, the algorithm shows promise for early detection of toxicity in the drug discovery process.
Introduction
The aim of this work is to highlight the usefulness of a recently proposed extension to the technique of non-negative matrix factorization (NMF) by demonstrating its promise for early detection of toxicity in the drug discovery process. In particular, we (a) show that any number of related datasets can be treated simultaneously with this approach, (b) deal with practical issues that arise when the algorithm is applied to real datasets, (c) demonstrate its use with a new large scale microrray dataset, and (d) interpret the results from a biological perspective.
Computational Background
NMF seeks to represent a large complex dataset in terms of smaller factors. The name covers many algorithms. Each approximates a non-negative matrix as the product of two or more smaller non-negative matrices, by attempting to minimise some objective function. Lee and Seung [1] showed that when applying multiplicative non-negative factorization to images of faces, each row/column pair of the factors expresses a recognisable facial feature. These techniques have since been used in many settings to learn parts of the data as well as to factorize and cluster datasets. For example, when applied to text data in [1] the algorithm can differentiate multiple meanings of the same word by context. On microarray data, NMF has been used to find patterns in genes or samples, typically bi-clustering both groups in a similar manner to two-way hierachical clustering [2]–[7]. The review article [8] shows how NMF has also been successful in other areas of computational biology, including molecular pattern discovery, class comparison and biomedical informatics. The new challenge that we address in this work is to apply the NMF methodology to multiple, related, large scale, data sets simultaneously. We use the work of Badea [9], [10], who considered an extension of NMF that deals with two data matrices. Simultaneous NMF is used in [9] to study pancreatic cancer microarray data alongside extra information concerning transcription regulatory factors. In [10] microarray datasets for pancreatic ductal adenocarcinoma and sporadic colon adenocarcinoma are sumiltaneously factorized in order to discover expression patterns common to both data sets. This simultaneous NMF approach readily extends to the case of an arbitrary number of data matrices and here, for what we believe to be the first time, we implement and evaluate the method on more than two. We also consider various practical issues that must be tackled in order to produce a useful computational tool. To minimize the number of algorithmic parameters, make the results straightforward to interpret, and exploit the natural sparsity in the algorithm [9, section 3], we focus on hard clustering. The interesting issue of allowing clusters to overlap in this context is therefore left as future work.
Biological Background
We analyse gene expression data describing the molecular changes in four tissue types due to different dosages of an experimental pan-peroxisome proliferator-activated receptor (pan-PPAR) agonist PPM-201, provided by Plexxikon. PPARs have attracted great interest as potential therapeutic targets for diabetes [11], but major concerns have arisen due to clinically observed side-effects [12]. Hence, there are compelling reasons for toxicological studies at the gene expression level.
The material is organised as follows. In Section we describe the simultaneous NMF algorithm and outline our approach for using the output to order and cluster a dataset. Section describes the mouse microarray data, and the NMF results that arise when we treat it as a single dataset are given in Section . This is followed in Section by the analysis of the data split into four datasets corresponding to the known tissue types; liver, kidney, heart and skeletal muscle. In Section we compare the gene clusters from Sections and , and Section discusses the results. Conclusions are given in Section .
Methods
Algorithms
Given  non-negative data matrices
non-negative data matrices 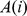 of size
of size 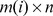 for
for 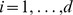 , our aim is to simultaneously factorize all matrices so that
, our aim is to simultaneously factorize all matrices so that
with the additional constraints that 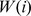 is a non-negative matrix of size
is a non-negative matrix of size 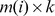 for
for 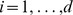 , and
, and 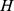 is a non-negative matrix of size
is a non-negative matrix of size 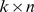 . Generalising naturally from the
. Generalising naturally from the 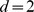 case in [9], we seek to minimise the objective function
case in [9], we seek to minimise the objective function
| (1) |
where 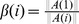 . Here
. Here  denotes the Frobenius norm. As in [9] the
denotes the Frobenius norm. As in [9] the 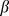 coefficients are designed to give equal weight to the different error terms. Based on the multiplicative update rules developed in [13], an iterative algorithm that attempts to solve the optimisation problem can be derived using a gradient descent method
coefficients are designed to give equal weight to the different error terms. Based on the multiplicative update rules developed in [13], an iterative algorithm that attempts to solve the optimisation problem can be derived using a gradient descent method 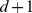 times. This gives us the following sequence of approximations for
times. This gives us the following sequence of approximations for 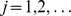 , given initial choices
, given initial choices 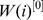 and
and 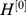 ,
,
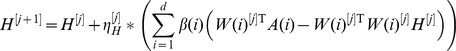 |
for some small positive matrices 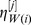 , and
, and 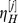 , with
, with  representing element-wise multiplication. The iteration may be motivated through the intuition that when
representing element-wise multiplication. The iteration may be motivated through the intuition that when 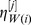 and
and 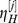 are sufficiently small and positive each of these equations should reduce the objective function. This allows us to set
are sufficiently small and positive each of these equations should reduce the objective function. This allows us to set
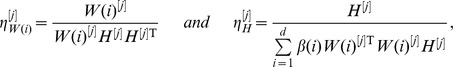 |
again with the division being performed element-wise. Hence the overall iteration has the form
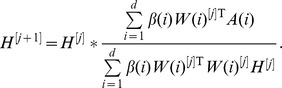 |
The values in 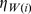 and
and 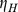 are non-negative due to the constraints on the matrices, however they are not necessarily small. The iteration decreases the objective function (1), so this leads to a locally optimum solution, but we cannot guarantee convergence to a global optimum. In particular, different initial conditions can lead to different factorizations of different quality.
are non-negative due to the constraints on the matrices, however they are not necessarily small. The iteration decreases the objective function (1), so this leads to a locally optimum solution, but we cannot guarantee convergence to a global optimum. In particular, different initial conditions can lead to different factorizations of different quality.
Having iterated up to some stopping criterion and produced the factorizations, we use them to bi-cluster the data. Each sample is assigned to the cluster for which it has the largest value in the gene cluster and vice versa. In reordering the data for easy visualisation we organise the rows and columns by cluster number (assigned arbitrarily) and sort the elements within each cluster from the appropriate sample/gene set, with the largest value at the bottom/right of that cluster. Given that the second factor is common to all the factorizations, it produces a matching ordering of the columns of the data.
Because the result depends on the choice of initial condition, and because the choice of 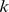 is not automatic, further information is needed in order to specify a practical algorithm. To deal with the lack of uniqueness, we try several initial conditions and pick a realisation that minimises the objective function (1). We then continue until further runs do not significantly alter the results. The objective function value is also one of the criteria we use in order to decide which rank/clustering is the most “appropriate” for the data. By regarding the objective function as a function of
is not automatic, further information is needed in order to specify a practical algorithm. To deal with the lack of uniqueness, we try several initial conditions and pick a realisation that minimises the objective function (1). We then continue until further runs do not significantly alter the results. The objective function value is also one of the criteria we use in order to decide which rank/clustering is the most “appropriate” for the data. By regarding the objective function as a function of 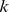 , we identify values of
, we identify values of 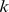 where the decay in the objective function begins to diminish. In addition we also form a consensus matrix as in [3], [14] for the clustering of the objects. This is the average of the connectivity matrices
where the decay in the objective function begins to diminish. In addition we also form a consensus matrix as in [3], [14] for the clustering of the objects. This is the average of the connectivity matrices  where for each initialisation
where for each initialisation 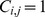 if objects
if objects  and
and 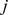 are clustered together and
are clustered together and  otherwise. So the consensus matrix contains values between
otherwise. So the consensus matrix contains values between  and
and  with the
with the 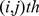 element being the likelihood that objects
element being the likelihood that objects  and
and 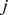 cluster together. The cumulative density of these values is constructed, by summing the appropriate probabilities, and the area under this curve is the second measure we look at when considering choices for
cluster together. The cumulative density of these values is constructed, by summing the appropriate probabilities, and the area under this curve is the second measure we look at when considering choices for 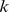 . The third measure is the Pearson correlation of the cophenetic distances, as explained in [3].
. The third measure is the Pearson correlation of the cophenetic distances, as explained in [3].
Mouse data
We apply these techniques to mouse gene expression data quantifying changes in four different tissue types following administration of different dosages (vehicle, therapeutic and toxic) of an experimental pan-PPAR agonist. The study design and clinical chemistry results are summarised in Table 1. ALT and AST are known markers in rodents for liver toxicity [15] and from this criterion mouse E may be showing a toxic response to PPM201, despite it being administered at a supposedly therapeutic dose level. This conditions our expectation of the gene-expression pattern for mouse E and suggests that it may be similar to the toxic level group III for liver.
Table 1. Blood clinical chemistry analysis for each mouse.
| Group | Mouse | Dose | ALT | AST | Creatinine | BUN | LDH | CK |
| ID | ID | (mg/kg b.wt) | (U/L) | (U/L) | (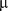 mol/L) mol/L) |
(U/L) | (U/L) | (U/L) |
| I | A | Vehicle | 42 | 188 | 12 | 4 | 348 | 484 |
| I | B | Vehicle | 41 | 92 | 9 | 6 | 364 | 258 |
| I | C | Vehicle | 29 | 75 | 9 | 6 | 278 | 166 |
| II | D | 6 | 95 | 441 | 11 | 8 | 1218 | 4930 |
| II | E | 6 | 692 | 981 | 8 | 7 | 2126 | 1130 |
| II | F | 6 | 52 | 83 | 9 | 8 | 294 | 152 |
| III | G | 20 | 312 | 1300 | 6 | 8 | 3172 | 2544 |
| III | H | 20 | 462 | 937 | 8 | 6 | 1760 | 1182 |
| III | I | 20 | 698 | 1090 | 6 | 7 | 2616 | 1592 |
The mice were randomly divided into three groups and treated with either Vehicle or two concentrations of PPM201 (6 or 20 mg/kg body weight). The response to the “therapeutic dose”, 6 mg/kg, was found to vary widely for ALT (alanine aminotransferase), AST (aspartate aminotransferase), LDH (lactate dehydrogenase) and CK (creatine kinase). AST is raised in PPM201 treated animals, with mouse E (6 mg/kg) seeming to be especially raised; AST is known to be variable between animals, but mouse E also shows a higher level of ALT, indicating that there may be a shared mechanism for the two enzymes. Creatinine is decreased in liver and possibly kidney disease; the contrasts observed here are inconclusive. BUN (Blood, Urea and Nitrogen) is raised in kidney disease; results are again inconclusive. Following cardiac infarction LDH is increased after 12 hours, possibly also caused by liver toxicity; mouse E is markedly lower than the other PPM201 treated animals and it may be that its heart muscle profile might be more similar to the untreated mice. CK is, like LDH, increased in myocardial infarction and this supports the LDH findings for mouse E.
Nine wild type mice (strain: C57BL/6J) were randomly divided into three groups; - Group-I, II and III. PPM-201 in the vehicle base was administered daily for 14 days at 6 mg/kg body weight dose rate to each mouse in Group-II and at 20 mg/kg body weight dose rate to each mouse in Group-III while the mice in Group-I received only the vehicle base. On 15th day, the mice were sacrificed to harvest blood, heart, skeletal muscle, liver and kidney tissues for clinical chemistry, microarray and histopathology analysis. In the clinical chemistry analysis, alanine aminotransferase (ALT, U/L), aspartate aminotransferase (AST, U/L), creatinine kinase (CK, U/L), blood urea nitrogen (BUN, mmol/L), creatinine (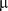 mol/L) and lactate dehydrogenase (LDH, U/L) were measured from the blood of each mouse. Two sections of liver, two sections of kidney, one or two sections of skeletal muscle, and one section of heart were prepared from each mouse, stained with hematoxylin and eosin (H&E), and examined by a veterinary pathologist. Total RNA was isolated from murine tissues using Qiazol-based homogenization and subsequent column-based purification (Qiagen) with on-column DNAse-treatment. DNAse-free RNA was assessed for quality using Agilent Bioanalyser electrophoresis and acceptance criteria of RNA Integrity Number (RIN) greater than seven. 50 ng of total RNA was subsequently utilized as input to cDNA-based amplification and biotin-labelling using single-primer isothermal amplification according to the manufacturer's instructions (Ovation System, NuGEN Technologies). Unlabelled and biotin-labelled cDNA was qualitatively assessed by Agilent Bioanalyser electrophoresis to ensure identical size distributions of all samples pre- and post-fragmentation. Fragmented, biotin-labelled cDNA were hybridized to MOE430 2.0 GeneChip arrays (Affymetrix) with subsequent scanning and feature extraction according to the manufacturer's instructions.
mol/L) and lactate dehydrogenase (LDH, U/L) were measured from the blood of each mouse. Two sections of liver, two sections of kidney, one or two sections of skeletal muscle, and one section of heart were prepared from each mouse, stained with hematoxylin and eosin (H&E), and examined by a veterinary pathologist. Total RNA was isolated from murine tissues using Qiazol-based homogenization and subsequent column-based purification (Qiagen) with on-column DNAse-treatment. DNAse-free RNA was assessed for quality using Agilent Bioanalyser electrophoresis and acceptance criteria of RNA Integrity Number (RIN) greater than seven. 50 ng of total RNA was subsequently utilized as input to cDNA-based amplification and biotin-labelling using single-primer isothermal amplification according to the manufacturer's instructions (Ovation System, NuGEN Technologies). Unlabelled and biotin-labelled cDNA was qualitatively assessed by Agilent Bioanalyser electrophoresis to ensure identical size distributions of all samples pre- and post-fragmentation. Fragmented, biotin-labelled cDNA were hybridized to MOE430 2.0 GeneChip arrays (Affymetrix) with subsequent scanning and feature extraction according to the manufacturer's instructions.
The dataset has been approved by the GEO curators and assigned the accession number GSE31561.
Ethics Statement
The in vivo procedures undertaken during the course of this study (Ref: CXR0631) were subject to the provisions of the United Kingdom Animals (Scientific Procedures) Act 1986. The study was approved by the CXR Biosciences Local Ethics Committee and complied with all applicable sections of the Act and the associated Codes of Practice for the Housing and Care of Animals used in Scientific Procedures and the Humane Killing of Animals under Schedule 1 to the Act, issued under section of the Act.
Results
Single dataset
First, the samples are treated as a single dataset, with thirty six samples and 45037 genes, hence the data matrix 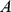 is
is 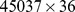 . This corresponds to the case where
. This corresponds to the case where 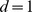 in Section . The factorizations were performed twenty times for each
in Section . The factorizations were performed twenty times for each 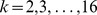 , with a consensus matrix formed from the clustering of the samples. All gene clusters associated with this analysis are labelled with a subscript 1, e.g.,
, with a consensus matrix formed from the clustering of the samples. All gene clusters associated with this analysis are labelled with a subscript 1, e.g., 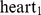 .
.
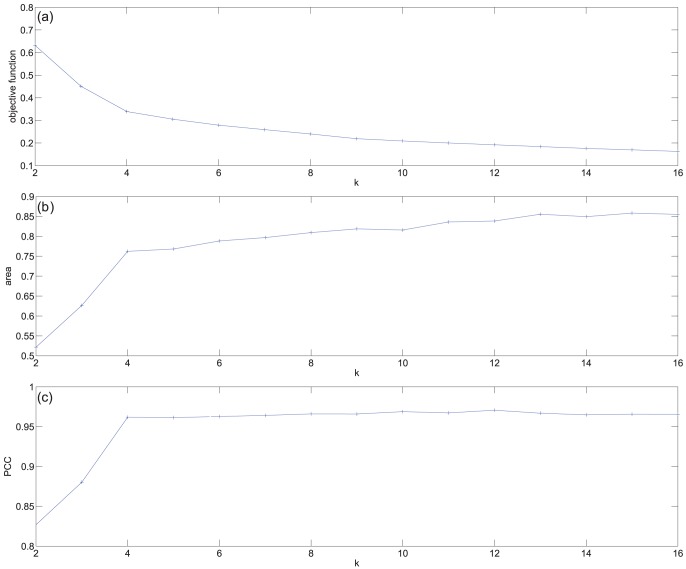
Figure 1(a) shows the minimum size of the objective function that we observed for each value of 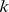 . We see that this value decreases monotonically, with a slower rate starting at around
. We see that this value decreases monotonically, with a slower rate starting at around 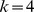 . Figure 1(b) shows the area under the cumulative density curves for the same values of
. Figure 1(b) shows the area under the cumulative density curves for the same values of 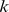 . This subfigure clearly points to
. This subfigure clearly points to 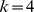 , as does subfigure (c) showing the cophenetic correlation.
, as does subfigure (c) showing the cophenetic correlation.
Figure 1. Three measures of the performance versus specified cluster size,
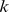 , when the data set is factorised as a single entity. (a) The value of the objective function for
, when the data set is factorised as a single entity. (a) The value of the objective function for 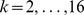 . (b) The area under consensus cumulative density, [3], [14]. (c) The cophenetic correlation coefficient, [3].
. (b) The area under consensus cumulative density, [3], [14]. (c) The cophenetic correlation coefficient, [3].
Based on Figure 1, we conclude that when the data is factorized as a single entity, 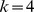 clusters is the most appropriate choice. Reordering the dataset using the ordering for
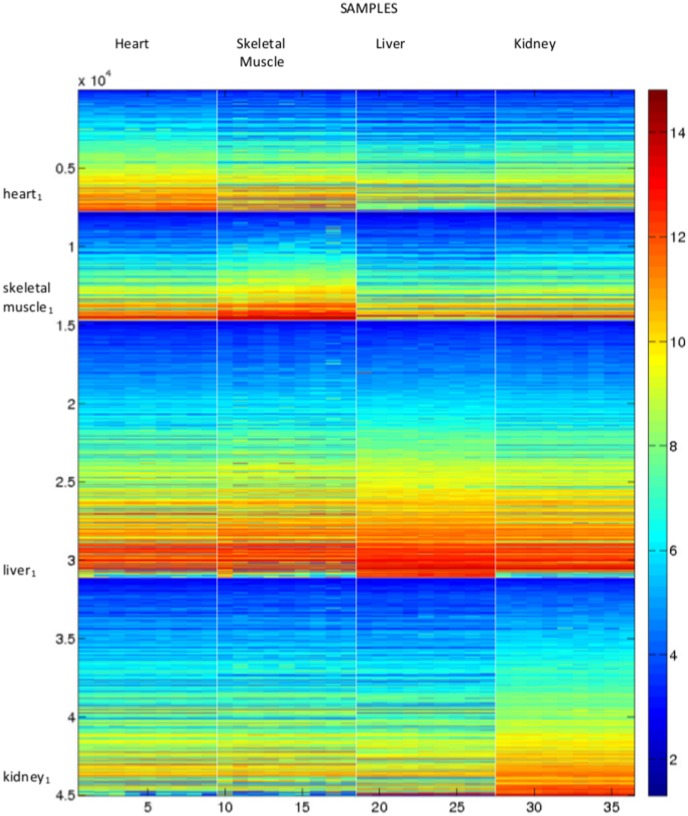
clusters is the most appropriate choice. Reordering the dataset using the ordering for 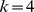 in the manner described in Section gives the images shown in Figure 2. This figure shows the samples in the columns with cluster one at the top. To aid visualisation, the sample clusters are split by white lines, as are the gene clusters. This reordered data matrix shows a distinctive “ramp” effect in the blocks on the diagonal, placing genes that are most influential in identifying each tissue type to the bottom of the block. This figure also shows some of the differences in expression behaviour between the tissue types, particularly for the most influential genes.
in the manner described in Section gives the images shown in Figure 2. This figure shows the samples in the columns with cluster one at the top. To aid visualisation, the sample clusters are split by white lines, as are the gene clusters. This reordered data matrix shows a distinctive “ramp” effect in the blocks on the diagonal, placing genes that are most influential in identifying each tissue type to the bottom of the block. This figure also shows some of the differences in expression behaviour between the tissue types, particularly for the most influential genes.
Figure 2. Factorising as a single dataset; reordering using the NMF for .
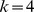 . The columns show the samples and the rows the gene expression for each of the 45037 genes. Genes and samples are organised by cluster number. Elements within each cluster are ordered, with the largest value at the bottom/right. Each tissue is characterised by a group of highly expressed genes; from the top left to bottom right these are heart, skeletal muscle, liver and kidney. For comparison purposes, the characteristic 100 “best” genes in the four columns are names
. The columns show the samples and the rows the gene expression for each of the 45037 genes. Genes and samples are organised by cluster number. Elements within each cluster are ordered, with the largest value at the bottom/right. Each tissue is characterised by a group of highly expressed genes; from the top left to bottom right these are heart, skeletal muscle, liver and kidney. For comparison purposes, the characteristic 100 “best” genes in the four columns are names 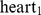 ,
, 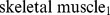 ,
, 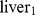 and
and 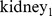 .
.
Because we know the origin of the samples, we can confirm that the algorithm has put the heart samples in cluster one, the skeletal muscle samples in cluster two, the liver samples in cluster three, and the kidney samples in cluster four. The exact ordering of the samples is shown in Table 2. This table also shows the mouse identification information for each sample, and we see that the mice are not ordered in the same way within each cluster. It is the liver and skeletal muscle samples that most closely respect the dosage levels within the clusters. Both these clusters only have one sample mis-ordered.
Table 2. Ordering of the tissue samples after single factorization of rank 4 of the entire dataset.
| Cluster | Tissue type | Mouse | Dosage |
| 1 | Heart | D | 6 mg/kg |
| 1 | Heart | B | Vehicle |
| 1 | Heart | C | Vehicle |
| 1 | Heart | I | 20 mg/kg |
| 1 | Heart | H | 20 mg/kg |
| 1 | Heart | A | Vehicle |
| 1 | Heart | G | 20 mg/kg |
| 1 | Heart | E | 6 mg/kg |
| 1 | Heart | F | 6 mg/kg |
| 2 | Skeletal Muscle | H | 20 mg/kg |
| 2 | Skeletal Muscle | D | 6 mg/kg |
| 2 | Skeletal Muscle | I | 20 mg/kg |
| 2 | Skeletal Muscle | G | 20 mg/kg |
| 2 | Skeletal Muscle | F | 6 mg/kg |
| 2 | Skeletal Muscle | E | 6 mg/kg |
| 2 | Skeletal Muscle | C | Vehicle |
| 2 | Skeletal Muscle | A | Vehicle |
| 2 | Skeletal Muscle | B | Vehicle |
| 3 | Liver | I | 20 mg/kg |
| 3 | Liver | H | 20 mg/kg |
| 3 | Liver | G | 20 mg/kg |
| 3 | Liver | E | 6 mg/kg |
| 3 | Liver | A | Vehicle |
| 3 | Liver | F | 6 mg/kg |
| 3 | Liver | D | 6 mg/kg |
| 3 | Liver | C | Vehicle |
| 3 | Liver | B | Vehicle |
| 4 | Kidney | G | 20 mg/kg |
| 4 | Kidney | I | 20 mg/kg |
| 4 | Kidney | H | 20 mg/kg |
| 4 | Kidney | E | 6 mg/kg |
| 4 | Kidney | A | Vehicle |
| 4 | Kidney | C | Vehicle |
| 4 | Kidney | F | 6 mg/kg |
| 4 | Kidney | B | Vehicle |
| 4 | Kidney | D | 6 mg/kg |
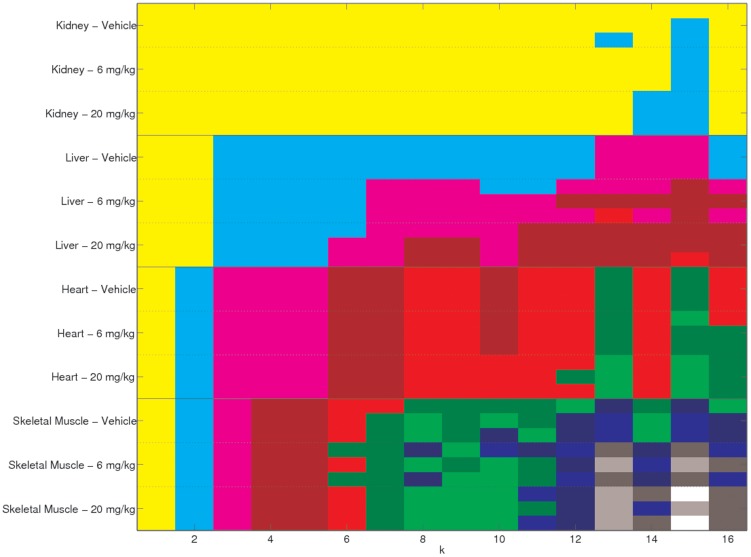
Given that the factorization has been performed for 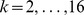 we know what the clustering would be from all these rank factorizations. This information is displayed in Figure 3. Here the rows representing the samples are ordered in tissue then dosage subgroups. For each rank
we know what the clustering would be from all these rank factorizations. This information is displayed in Figure 3. Here the rows representing the samples are ordered in tissue then dosage subgroups. For each rank 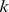 , samples with the same colour are assigned to the same cluster. As we have seen before, for
, samples with the same colour are assigned to the same cluster. As we have seen before, for 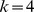 the samples are split into tissue types. The figure shows that this split persists at
the samples are split into tissue types. The figure shows that this split persists at 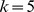 with an empty cluster forming. In fact, for this range of
with an empty cluster forming. In fact, for this range of 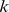 there are at most twelve clusters of samples. We also see from this figure that for no value of
there are at most twelve clusters of samples. We also see from this figure that for no value of 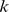 are the twelve tissue/dosage subgroups found.
are the twelve tissue/dosage subgroups found.
Figure 3. Factorising as a single dataset.
The clustering of the mouse samples for 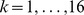 . Within each column the samples in the same colour are clustered together. No value of
. Within each column the samples in the same colour are clustered together. No value of 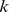 reveals the known tissue/dosage subgroups, or places different tissues in the same cluster.
reveals the known tissue/dosage subgroups, or places different tissues in the same cluster.
Multiple datasets
The test in Section indicates that the basic NMF factorization approach can deliver biologically meaningful results—separating the twelve samples by tissue type. But the failure to order correctly within tissue type according to dosage motivates the use of the multiple dataset generalization introduced in Section , where the four tissue types are treated as separate sources of information across a common set of mice. Intuitively, we would expect to add value to the data analysis by building known biology into the algorithm in this way. In this section, we therefore factorize the four new datasets simultaneously. This is similar to the test in Section in the sense that it produces a single ordering for the mice, but it has the potential to add extra information by providing four different, tissue-level, gene orderings. We thus have 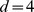 matrices of size
matrices of size 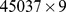 . We again performed 20 factorizations, this time for
. We again performed 20 factorizations, this time for 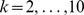 and these have been used to generate a consensus for clustering the mice.
and these have been used to generate a consensus for clustering the mice.
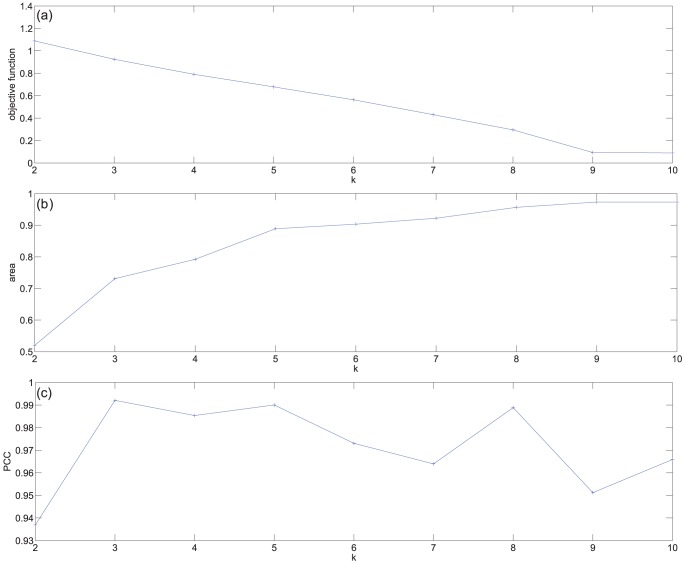
The objective function and the consensus measurements are shown in Figure 4. The objective function in subfigure (a) does not show much decrease in convergence rate until we get to nine clusters. This is the point where each mouse is put into a cluster on its own. The area under the cumulative density curve in Figure 4(b) suggests using either rank 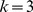 , or
, or 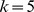 factorizations for the clustering. The correlation coefficients shown in subfigure (c) give the same two values as peaks, as well as
factorizations for the clustering. The correlation coefficients shown in subfigure (c) give the same two values as peaks, as well as 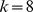 , though the
, though the 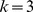 peak is the highest.
peak is the highest.
Figure 4. Three measures of the performance versus specified cluster size,
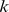 , when the four tissue types are factorised separately. (a) The value of the objective function for
, when the four tissue types are factorised separately. (a) The value of the objective function for 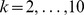 . (b) The area under consensus cumulative density function for
. (b) The area under consensus cumulative density function for 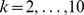 , [3], [14]. (c) The cophenetic correlation coefficient, [3].
, [3], [14]. (c) The cophenetic correlation coefficient, [3].
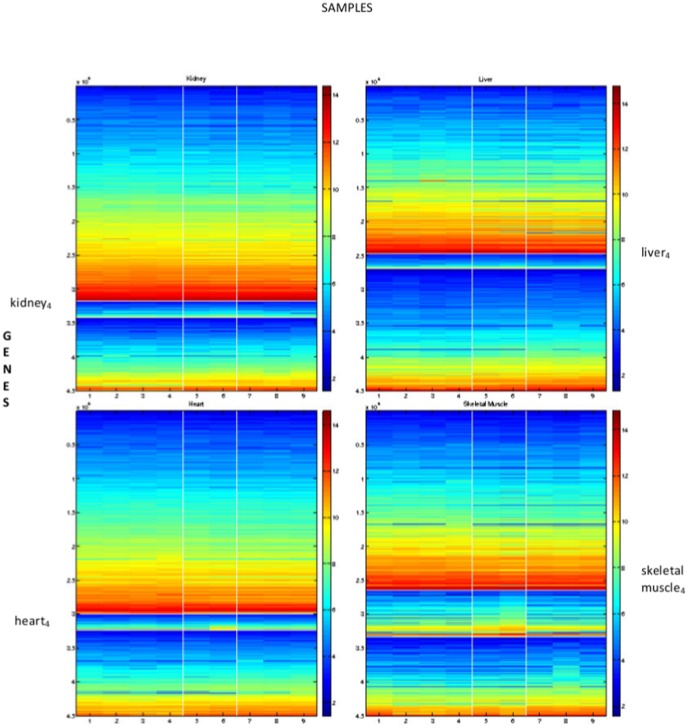
Given these measurements we consider the four-way simultaneous factorization for 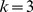 in Figure 5. The reordered datasets are shown separately with the kidney dataset in the top left, the liver dataset in the top right, the heart dataset in the bottom left and the skeletal muscle in the bottom right. The mouse ordering and mouse clusters that arise are shown in Table 3. The four subfigures in Figure 5 also illustrate that the gene clusters are different for each dataset. The three clusters for each tissue in this 4-way factorization are subsequently refered to in the form “
in Figure 5. The reordered datasets are shown separately with the kidney dataset in the top left, the liver dataset in the top right, the heart dataset in the bottom left and the skeletal muscle in the bottom right. The mouse ordering and mouse clusters that arise are shown in Table 3. The four subfigures in Figure 5 also illustrate that the gene clusters are different for each dataset. The three clusters for each tissue in this 4-way factorization are subsequently refered to in the form “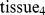 , cluster 1,2 or 3. ” Table 3 shows that the simultaneous NMF approach has recovered the known mouse treatments except for one misplacement. Figure 6 shows the clustering for the four-way simultaneous factorizations for
, cluster 1,2 or 3. ” Table 3 shows that the simultaneous NMF approach has recovered the known mouse treatments except for one misplacement. Figure 6 shows the clustering for the four-way simultaneous factorizations for 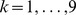 . This indicates that this mouse does not cluster with all those of the same dosage for any rank of factorization greater than two, instead it associates with the higher more toxic dosage. This is borne out by the known blood chemistry, as summarised in Table 1; the mouse that is mis-classified exhibits a toxic response and is therefore classified with the mice that received the higher dose.
. This indicates that this mouse does not cluster with all those of the same dosage for any rank of factorization greater than two, instead it associates with the higher more toxic dosage. This is borne out by the known blood chemistry, as summarised in Table 1; the mouse that is mis-classified exhibits a toxic response and is therefore classified with the mice that received the higher dose.
Figure 5. Factorisation of the four separate tissue types using simultaneous NMF with .
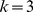 . Top left, kidney; top right, liver; lower left, heart; lower right, skeletal muscle. The four tissue types are treated as separate sources of information across a common set of mice. Genes are therefore ordered differently in each of the four tissues, but the mice ordering is global. The resulting mouse ordering and mouse clusters are detailed in Table 3.
. Top left, kidney; top right, liver; lower left, heart; lower right, skeletal muscle. The four tissue types are treated as separate sources of information across a common set of mice. Genes are therefore ordered differently in each of the four tissues, but the mice ordering is global. The resulting mouse ordering and mouse clusters are detailed in Table 3.
Table 3. Ordering of the tissue samples after a four-way factorization of rank 3.
| Cluster | Mouse | Dosage |
| 1 | E | 6 mg/kg |
| 1 | G | 20 mg/kg |
| 1 | I | 20 mg/kg |
| 1 | H | 20 mg/kg |
| 2 | F | 6 mg/kg |
| 2 | D | 6 mg/kg |
| 3 | B | Vehicle |
| 3 | A | Vehicle |
| 3 | C | Vehicle |
The mouse clusters when split by tissue type and reordered using the 4-way simultaneous factorization for 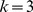 .
.
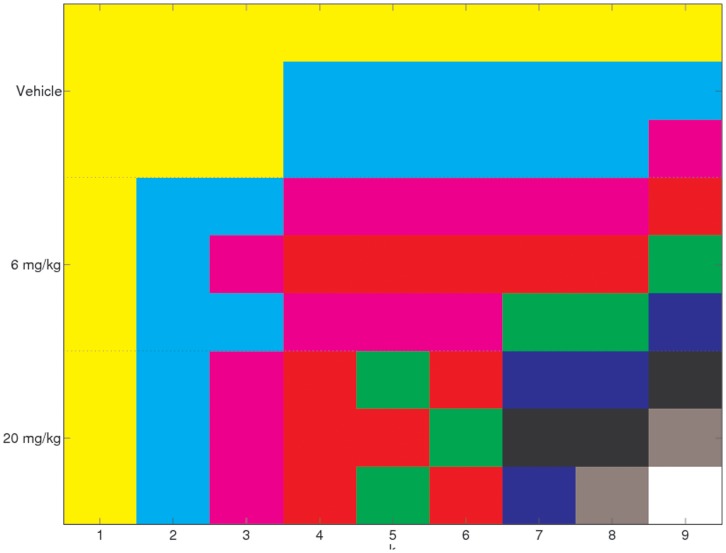
Figure 6. Factorisation of the four separate tissue types simultaneously.
The clustering of the mice for 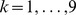 ; colour indicates cluster number. One “misclassification” is found for several values of
; colour indicates cluster number. One “misclassification” is found for several values of 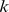 . This involves the mouse showing a toxic response to the lower (6 mg/kg) dose of PPAR agonist, as discussed in section .
. This involves the mouse showing a toxic response to the lower (6 mg/kg) dose of PPAR agonist, as discussed in section .
Comparing Gene clusters
Our aim now is to test the results from the novel multi-way NMF algorithm used in Section in order to see whether they (a) show consistency and (b) add value to the results in Section from standard NMF. We know that the four simultaneously factorized datasets correspond to the four clusters of samples that were discovered in an unsupervised manner from the single factorization of the full dataset. It could therefore be conjectured that the most influential genes in the first factorization will appear as influential genes in the four-way simultaneous factorization for that dataset, but less so for the other datasets.
Our comparisons involve four reference sets. For illustration, we chose an arbitrary threshold of one hundred; that is, we consider the top one hundred most influential genes from the four clusters in the first factorization shown in Figure 2. For easy reference these sets are referred to using the known tissue type. This means that the genes from cluster one are the 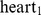 genes, those from cluster two are the
genes, those from cluster two are the 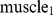 genes, those from cluster three are the
genes, those from cluster three are the 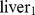 genes and those from cluster four are the
genes and those from cluster four are the 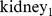 genes. The 4-way factorization shown in Figure 5 identifies differently ordered gene clusters for each tissue, which we will refer to as “
genes. The 4-way factorization shown in Figure 5 identifies differently ordered gene clusters for each tissue, which we will refer to as “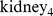 , cluster 1,2 or 3, etc. ” Table 4 shows the total number of co-incident genes between the top 100 lists arising from the one-way and four-way factorisations. The table also shows the probability of the two lists having that number of genes in common if the second list were randomly selected; hence these values come from the hypergeometric distribution. We see that the important genes for each tissue type appear significantly highly in the clusters from that tissue's data type. In addition, all the tissue type genes also appear significantly within the reordering of the heart dataset. This link is reciprocated, with the heart genes appearing significantly frequently within the skeletal muscle dataset. Surprisingly, the greatest overlap arose between
, cluster 1,2 or 3, etc. ” Table 4 shows the total number of co-incident genes between the top 100 lists arising from the one-way and four-way factorisations. The table also shows the probability of the two lists having that number of genes in common if the second list were randomly selected; hence these values come from the hypergeometric distribution. We see that the important genes for each tissue type appear significantly highly in the clusters from that tissue's data type. In addition, all the tissue type genes also appear significantly within the reordering of the heart dataset. This link is reciprocated, with the heart genes appearing significantly frequently within the skeletal muscle dataset. Surprisingly, the greatest overlap arose between 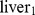 and
and 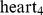 cluster 2. One of these genes, Apoliprotein A1, is being considered as a marker for cardiac toxicity [16].
cluster 2. One of these genes, Apoliprotein A1, is being considered as a marker for cardiac toxicity [16].
Table 4. Gene cluster comparison for indivdual tissues in the single matrix, “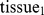 ,” with the four separate tissue matrices “
,” with the four separate tissue matrices “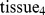 .”.
.”.
| H1 | SM1 | L1 | K1 | ||||||
| Cluster | No. | Probability | No. | Probability | No. | Probability | No. | Probability | |
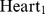
|
Clust.1 | 22 | 2.2188e-38 | 0 | 0.8005 | 0 | 0.8005 | 0 | 0.8005 |
| Clust.2 | 1 | 0.1785 | 2 | 0.0195 | 49 | 5.444e-108 | 0 | 0.8005 | |
| Clust.3 | 11 | 4.3469e-16 | 7 | 2.8360e-09 | 1 | 0.1785 | 5 | 3.0037e-06 | |
| total | 34 | 5.4969e-67 | 9 | 1.4338e-12 | 50 | 6.309e-111 | 5 | 3.0037e-06 | |
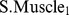
|
Clust.1 | 4 | 7.3075e-05 | 15 | 1.1260e-12 | 8 | 6.8371e-11 | 0 | 0.8005 |
| Clust.2 | 0 | 0.8005 | 0 | 0.8005 | 0 | 0.8005 | 0 | 0.8005 | |
| Clust.3 | 4 | 7.3075e-05 | 14 | 1.0243e-21 | 0 | 0.8005 | 0 | 0.8005 | |
| total | 8 | 6.8371e-11 | 29 | 1.3672e-54 | 8 | 6.8371e-11 | 0 | 0.8005 | |
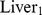
|
Clust.1 | 1 | 0.1785 | 0 | 0.8005 | 13 | 8.4974e-11 | 1 | 0.1785 |
| Clust.2 | 0 | 0.8005 | 0 | 0.8005 | 0 | 0.8005 | 0 | 0.8005 | |
| Clust.3 | 1 | 0.1785 | 2 | 0.0195 | 16 | 1.1336e-25 | 2 | 0.0195 | |
| total | 2 | 0.0195 | 2 | 0.0195 | 29 | 1.3672e-54 | 3 | 0.0014 | |
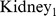
|
Clust.1 | 0 | 0.8005 | 0 | 0.8005 | 1 | 0.1785 | 0 | 0.8005 |
| Clust.2 | 2 | 0.0195 | 1 | 0.1785 | 1 | 0.1785 | 0 | 0.8005 | |
| Clust.3 | 0 | 0.8005 | 0 | 0.8005 | 2 | 0.0195 | 18 | 8.9507e-30 | |
| total | 2 | 0.0195 | 1 | 0.1785 | 4 | 7.3075e-05 | 18 | 8.9507e-30 | |
H1, SM1, L1 and K1 are the gene clusters most characteristic for the heart, skeletal muscle, liver and kidney, respectively, in the single (combined) data set, as in Figure 2. Clust.1, 2, or 3 denotes the 100 genes most securely placed within the clusters of the diferently ordered genes in the 4-way factorization shown in Figure 5. The order of the clusters is 1–3, from the top of the figire, for each tissue. We refer to these clusters as “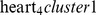 ,” etc. The overlap of the
,” etc. The overlap of the 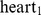 from the one-way factorization to
from the one-way factorization to 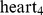 is referred to as
is referred to as 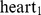
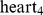 cluster 1.
cluster 1.
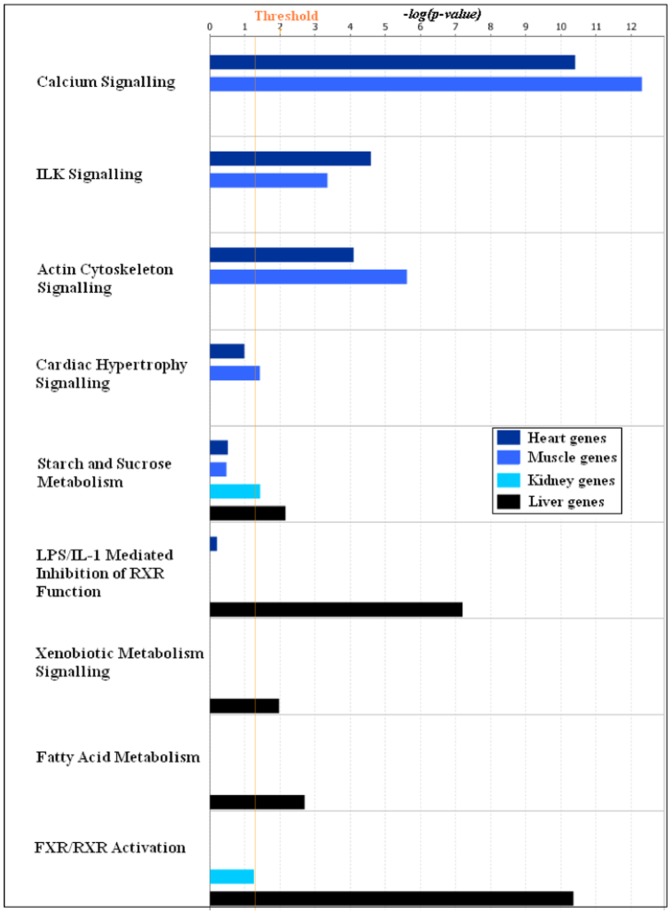
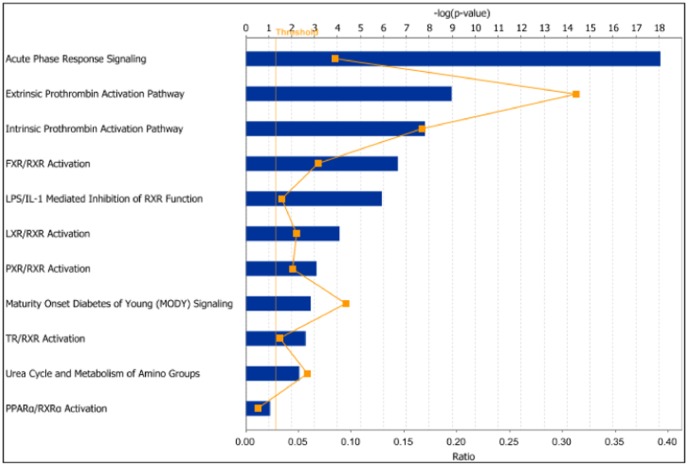
We would like to demonstrate the utility of the factorization method by using the gene clusters obtained in our analysis to understand tissue specific effects of the experimental drug, PPM-201. Of course, we are not claiming that this is an exhaustive analysis of the effects of PPM-201. We analysed the gene clusters for pathways enrichment and gene ontology enrichment using DAVID [17] and Ingenuity Pathways Analysis (IPA) [18] tools. Table 5 shows the comparison of KEGG pathways enriched in the four tissue specific top one hundred most influential probe-sets obtained in the first factorization. Pathways enriched in these clusters differ according to the tissue types and can be considered as the pathways that are most perturbed by PPM-201. For example, arrhythmogenic right ventricular cardiomyopathy, hypertrophic cardiomyopathy and dilated cardiomyopathy are enriched in heart, whereas starch and sucrose metabolism, drug metabolism and PPAR signalling pathway are enriched in liver. Similarly, Figure 7 shows the enrichment of canonical pathways in the four tissue specific clusters analysed using IPA. It also shows the tissue specific enrichment of pathways—calcium signalling, integrin linked kinase (ILK) signalling and cardiac hypertrophy signalling are enriched in 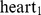 and
and 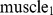 clusters, whereas fatty acid metabolism and farnesoid X receptor (FXR)/retinoid X receptor (RXR) activation are enriched in the
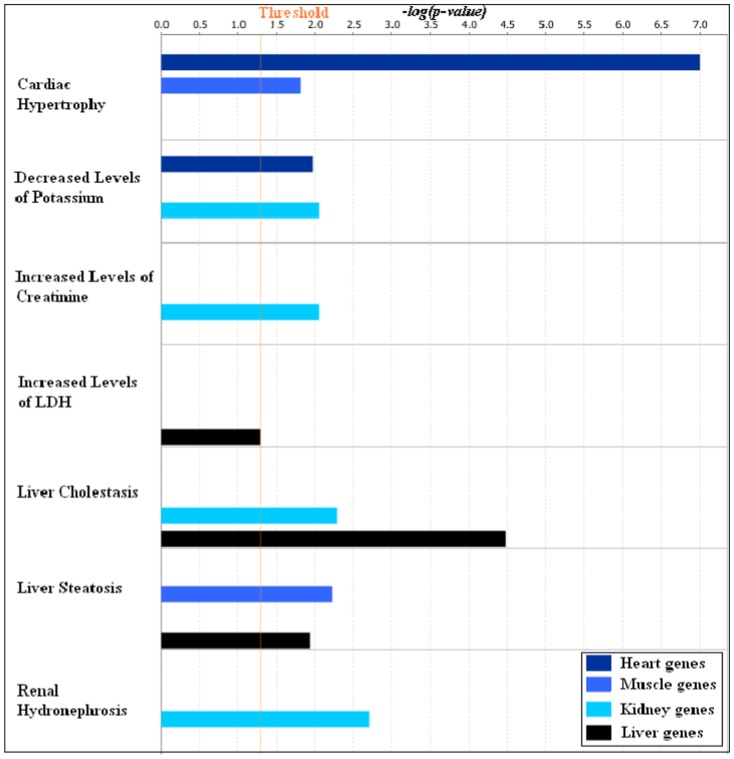
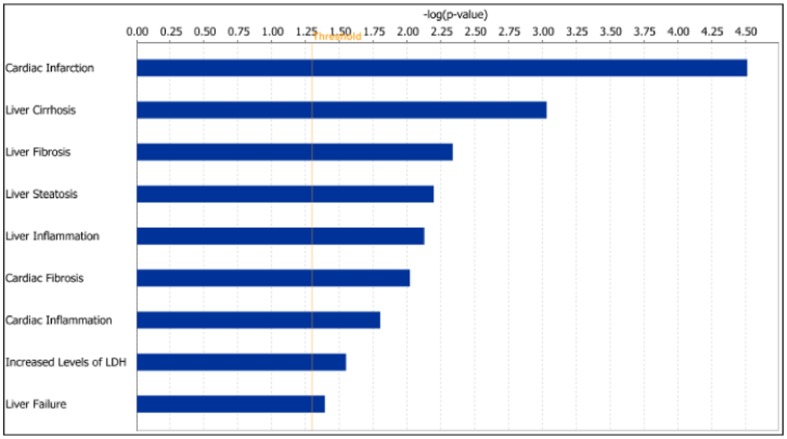
clusters, whereas fatty acid metabolism and farnesoid X receptor (FXR)/retinoid X receptor (RXR) activation are enriched in the 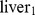 cluster. Analysis of the same sets of genes for enrichment of toxicity functions in the IPA shows, in Figure 8, cardiac hypertrophy in
cluster. Analysis of the same sets of genes for enrichment of toxicity functions in the IPA shows, in Figure 8, cardiac hypertrophy in 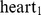 genes, increased level of creatinine and hydronephrosis in
genes, increased level of creatinine and hydronephrosis in 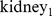 genes, and increased levels of lactate dehydrogenase (LDH) and steatosis in
genes, and increased levels of lactate dehydrogenase (LDH) and steatosis in 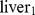 genes.
genes.
Table 5. Enrichment of KEGG pathways in the four tissue specific gene clusters.
| Kegg Pathways | Heart | Muscle | Kidney | Liver |
| 1 mmu05412: Arrhythmogenic right ventricular cardiomyopathy |

|

|
||
| 2 mmu04020: Calcium signalling pathway |

|

|
||
| 3 mmu04260: Cardiac muscle contraction |

|

|
||
| 4 mmu05414: Dilated cardiomyopathy |

|

|
||
| 5 mmu05410: Hypertrophic cardiomyopathy (HCM) |

|

|
||
| 6 mmu04530: Tight junction |

|

|
||
| 7 mmu00590: Arachidonic acid metabolism |

|
|||
| 8 mmu00983: Drug metabolism |

|
|||
| 9 mmu04610: Complement and coagulation cascades |

|
|||
| 10 mmu00980: Metabolism of xenobiotics by cytochrome P450 |

|
|||
| 11 mmu03320: PPAR signalling pathway |

|
|||
| 12 mmu00830: Retinol metabolism |

|
|||
| 13 mmu00040: Pentose and glucuronate interconversions |

|
|||
| 14 mmu00591: Linoleic acid metabolism |

|
|||
| 15 mmu00053: Ascorbate and aldarate metabolism |

|
|||
| 16 mmu00860: Porphyrin and chlorophyll metabolism |

|
|||
| 17 mmu00500: Starch and sucrose metabolism |

|
|||
| 18 mmu00150: Androgen and estrogen metabolism |

|
|||
| 19 mmu00140: Steroid hormone biosynthesis |

|
The top one hundred most influential probesets in the four tissue specific gene clusters were analysed using DAVID functional annotation tool. This table shows the comparison of KEGG pathways enriched in the four tissue specific gene clusters. The  icon indicates a p-value
icon indicates a p-value 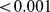 and the
and the  a
a 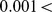 p-value
p-value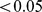 showing the significance of the enrichment.
showing the significance of the enrichment.
Figure 7. Enrichment of canonical pathways in the four tissue specific gene clusters.
The top one hundred most influential probe-sets in the four tissue specific gene clusters obtained in the first factorization were subjected to signalling and metabolic pathways analysis in the IPA software. This graph shows the comparison of canonical pathways enriched in the four tissue specific gene clusters, 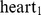 ,
, 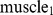 ,
, 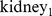 and
and 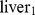 . The coloured bars show the significance of the enrichment for a particular pathway in the cluster computed by Fisher's exact test.
. The coloured bars show the significance of the enrichment for a particular pathway in the cluster computed by Fisher's exact test.
Figure 8. Enrichment of toxicity functions in the four tissue specific gene clusters.
The top one hundred most influential probe-sets in the four tissue specific gene clusters obtained in the first factorization were subjected to IPA-Tox analysis in the IPA software. This graph shows the comparison of toxicity functions enriched in the four tissue specific gene clusters. The coloured bars show the significance of the enrichment for a particular toxicity functions in the cluster computed by Fisher's exact test.
The common genes between the top one hundred most influential probe-sets in the four tissue specific clusters and the top one hundred most influential probe-sets in the clusters formed by 4-way simultaneous factorization of the split dataset were also analysed for enrichment of pathways, gene ontology and toxicity functions using DAVID and IPA. Tables 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 summarise the results of this analysis, which are discussed further in the next section.
Table 6. Enrichment of KEGG pathways in the common genes between the clusters found by the two ways of factorization.
| Kegg Pathways | Heart1 | Heart1 | Muscle1 | Liver1 | Liver1 | Liver1 | Liver1 |
| Heart4 | Muscle4 | Muscle4 | Liver4 | Liver4 | Heart4 | Muscle4 | |
| clust. 1 | clust. 3 | clust. 1 | clust. 1 | clust. 3 | clust. 2 | clust. 1 | |
| 1 mmu04020: Calcium signalling pathway |

|
||||||
| 2 mmu04260: Cardiac muscle contraction |

|

|
|||||
| 3 mmu04610: Complement and coagulation cascades |

|
||||||
| 4 mmu05414: Dilated cardiomyopathy |

|

|
|||||
| 5 mmu00983: Drug metabolism |

|
||||||
| 6 mmu05410: Hypertrophic cardiomyopathy (HCM) |

|

|
|||||
| 7 mmu03320: PPAR signalling pathway |

|

|

|
||||
| 8 mmu04530: Tight junction |

|

|
The probesets common to clusters formed by the 4-way simultaneous factorization and the top one hundred most influential probesets in the four tissue specific clusters were analysed for enrichment of KEGG pathways using DAVID functional annotation tool. Fishers' exact test p-values for pathway enrichment in the clusters are shown graphically in this table. The  icon indicates a p-value
icon indicates a p-value 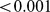 and the
and the  a
a 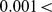 p-value
p-value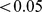 .
.
Table 7. Muscle genes present in the calcium signalling pathway.
| Sr. | Probeset ID | Gene Symbol | Entrez Gene ID | Entrez Gene Name |
| 1 | 1427735 a at | ACTA1 | 11459 | Actin, alpha 1, skeletal muscle |
| 2 | 1419312 at | ATP2A1 | 11937 | ATPase, Ca++ transporting, cardiac muscle, fast twitch 1 |
| 3 | 1422598 at | CASQ1 | 12372 | Calsequestrin 1 (fast-twitch, skeletal muscle) |
| 4 | 1427520 a at | MYH1 | 17879 | Myosin, heavy chain 1, skeletal muscle, adult |
| 5 | 1425153 at | MYH2 | 17882 | Myosin, heavy chain 2, skeletal muscle, adult |
| 6 | 1458368 at | MYH4 | 17884 | Myosin, heavy chain 4, skeletal muscle |
| 7 | 1452651 a at | MYL1 | 17901 | Myosin, light chain 1, alkali; skeletal, fast |
| 8 | 1457347 at | RYR1 | 20190 | Ryanodine receptor 1 (skeletal) |
| 9 | 1440962 at | SLC8A3 | 110893 | Solute carrier family 8, member 3 |
| 10 | 1417464 at | TNNC2 | 21925 | Troponin C type 2 (fast) |
| 11 | 1416889 at | TNNI2 | 21953 | Troponin I type 2 (skeletal, fast) |
| 12 | 1450118 a at | TNNT3 | 21957 | Troponin T type 3 (skeletal, fast) |
| 13 | 1419739 at | TPM2 | 22004 | Tropomyosin 2 (beta) |
| 14 | 1426144 x at | TRDN | 76757 | Triadin |
Table shows the probe-sets enriched for calcium signalling among the top 100 probe-sets from the  gene cluster.
gene cluster.
Table 8. Heart genes present in the calcium signalling pathway.
| Sr. | Probeset ID | Gene Symbol | Entrez Gene ID | Entrez Gene Name |
| 1 | 1415927 at | ACTC1 | 11464 | Actin, alpha, cardiac muscle 1 |
| 2 | 1416551 at | ATP2A2 | 11938 | ATPase, Ca++ transporting, cardiac muscle, slow twitch 2 |
| 3 | 1422529 s at | CASQ2 | 12373 | Calsequestrin 2 (cardiac muscle) |
| 4 | 1448827 s at | MYH6 | 17888 | Myosin, heavy chain 6, cardiac muscle, alpha |
| 5 | 1448394 at | MYL2 | 17906 | Myosin, light chain 2, regulatory, cardiac, slow |
| 1427769 x at | MYL3 | 17897 | Myosin, light chain 3, alkali; ventricular, skeletal, slow | |
| 7 | 1421126 at | RYR2 | 20191 | Ryanodine receptor 2 (cardiac) |
| 8 | 1418370 at | TNNC1 | 21924 | Troponin C type 1 (slow) |
| 9 | 1422536 at | TNNI3 | 21954 | Troponin I type 3 (cardiac) |
| 10 | 1440424 at | TNNT2 | 21956 | Troponin T type 2 (cardiac) |
| 11 | 1423049 a at | TPM1 | 22003 | Tropomyosin 1 (alpha) |
| 12 | 1451940 x at | TRDN | 76757 | Triadin |
Table shows the probe-sets enriched for calcium signalling among the top 100 probe-sets from the 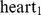 gene cluster.
gene cluster.
Table 9. Liver genes present in the calcium signalling pathway.
| Sr. | Probeset ID | Gene Symbol | Entrez Gene ID | Entrez Gene Name |
| 1 | 1449817 at | ABCB11 | 27413 | ATP-binding cassette, sub-family B (MDR/TAP), member 11 |
| 2 | 1419393 at | ABCG5 | 27409 | ATP-binding cassette, sub-family G (WHITE), member 5 |
| 3 | 1419232 a at | APOA1 | 11806 | Apolipoprotein A-I |
| 4 | 1418278 at | APOC3 | 11814 | Apolipoprotein C-III |
| 5 | 1449309 at | CYP8B1 | 13124 | Cytochrome P450, family 8, subfamily B, polypeptide 1 |
| 6 | 1418190 at | PON1 | 18979 | Paraoxonase 1 |
| 7 | 1450261 a at | SLC10A1 | 20493 | Solute carrier family 10, member 1 |
| 8 | 1449112 at | SLC27A5 | 26459 | Solute carrier family 27, member 5 |
| 9 | 1449394 at | SLCO1B3 | 28253 | Solute carrier organic anion transporter family, member 1B3 |
| 10 | 1424934 at | UGT2B4 | 71773 | UDP glucuronosyltransferase 2 family, polypeptide B4 |
Table shows the probe-sets enriched for calcium signalling among the top 100 probe-sets from the 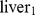 gene cluster.
gene cluster.
Table 10.
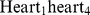 cluster 1. Common probesets between the top one hundred most influential probesets in the
cluster 1. Common probesets between the top one hundred most influential probesets in the 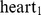 cluster and 20 mg/kg dosage cluster (cluster 1) of the
cluster and 20 mg/kg dosage cluster (cluster 1) of the 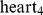 dataset.
dataset.
| Sr. | Probeset ID | Gene Symbol | Entrez Gene Name |
| 1 | 1415927 at | ACTC1 | actin, alpha, cardiac muscle 1 |
| 2 | 1416551 at | ATP2A2 | ATPase, Ca++ transporting, cardiac muscle, slow twitch 2 |
| 3 | 1452363 a at | ATP2A2 | ATPase, Ca++ transporting, cardiac muscle, slow twitch 2 |
| 4 | 1417607 at | COX6A2 | cytochrome c oxidase subunit VIa polypeptide 2 |
| 5 | 1460318 at | CSRP3 | cysteine and glycine-rich protein 3 (cardiac LIM protein) |
| 6 | 1416023 at | FABP3 | fatty acid binding protein 3, muscle and heart (mammary-derived growth inhibitor) |
| 7 | 1453628 s at | LRRC2 | leucine rich repeat containing 2 |
| 8 | 1451203 at | MB | myoglobin |
| 9 | 1418551 at | MYBPC3 | myosin binding protein C, cardiac |
| 10 | 1448554 s at | MYH6 | myosin, heavy chain 6, cardiac muscle, alpha |
| 11 | 1448826 at | MYH6 | myosin, heavy chain 6, cardiac muscle, alpha |
| 12 | 1448394 at | MYL2 | myosin, light chain 2, regulatory, cardiac, slow |
| 13 | 1427768 s at | MYL3 | myosin, light chain 3, alkali; ventricular, skeletal, slow |
| 14 | 1428266 at | MYL3 | myosin, light chain 3, alkali; ventricular, skeletal, slow |
| 15 | 1418769 at | MYOZ2 | myozenin 2 |
| 16 | 1450952 at | PLN | phospholamban |
| 17 | 1423859 a at | PTGDS | prostaglandin D2 synthase 21 kDa (brain) |
| 18 | 1418370 at | TNNC1 | troponin C type 1 (slow) |
| 19 | 1422536 at | TNNI3 | troponin I type 3 (cardiac) |
| 20 | 1418726 a at | TNNT2 | troponin T type 2 (cardiac) |
| 21 | 1424967 x at | TNNT2 | troponin T type 2 (cardiac) |
| 22 | 1423049 a at | TPM1 | tropomyosin 1 (alpha) |
Table 11.
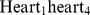 cluster 3.
cluster 3.
| Sr. | Probeset ID | Gene Symbol | Entrez Gene Name |
| 1 | 1422529 s at | CASQ2 | calsequestrin 2 (cardiac muscle) |
| 2 | 1444429 at | LRTM1 | leucine-rich repeats and transmembrane domains 1 |
| 3 | 1439101 at | MYLK3 | myosin light chain kinase 3 |
| 4 | 1426615 s at | NDRG4 | NDRG family member 4 |
| 5 | 1436188 a at | NDRG4 | NDRG family member 4 |
| 6 | 1438452 at | NEBL | nebulette |
| 7 | 1437442 at | PCDH7 | protocadherin 7 |
| 8 | 1436277 at | RNF207 | ring finger protein 207 |
| 9 | 1423145 a at | TCAP | titin-cap (telethonin) |
| 10 | 1436833 x at | TTLL1 | tubulin tyrosine ligase-like family, member 1 |
| 11 | 1444638 at | TTN | titin |
Common probesets between the top one hundred most influential probesets in the 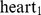 cluster and vehicle dose cluster (cluster 3) of the
cluster and vehicle dose cluster (cluster 3) of the 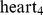 dataset.
dataset.
Table 12.
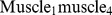 cluster 1.
cluster 1.
| Sr. | Probeset ID | Gene Symbol | Entrez Gene Name |
| 1 | 1427735 a at | ACTA1 | actin, alpha 1, skeletal muscle |
| 2 | 1418677 at | ACTN3 | actinin, alpha 3 |
| 3 | 1419312 at | ATP2A1 | ATPase, Ca++ transporting, cardiac muscle, fast twitch 1 |
| 4 | 1417614 at | CKM | creatine kinase, muscle |
| 5 | 1438059 at | CTXN3 (includes EG:629147) | cortexin 3 |
| 6 | 1455736 at | MYBPC2 | myosin binding protein C, fast type |
| 7 | 1427868 x at | MYH1 | myosin, heavy chain 1, skeletal muscle, adult |
| 8 | 1427026 at | MYH4 | myosin, heavy chain 4, skeletal muscle |
| 9 | 1448371 at | MYLPF | myosin light chain, phosphorylatable, fast skeletal muscle |
| 10 | 1418155 at | MYOT | myotilin |
| 11 | 1427306 at | RYR1 | ryanodine receptor 1 (skeletal) |
| 12 | 1417464 at | TNNC2 | troponin C type 2 (fast) |
| 13 | 1416889 at | TNNI2 | troponin I type 2 (skeletal, fast) |
| 14 | 1450118 a at | TNNT3 | troponin T type 3 (skeletal, fast) |
| 15 | 1426142 a at | TRDN | triadin |
Common probesets between the top one hundred most influential probesets in the 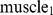 cluster and 20 mg/kg dosage cluster (cluster 1) of the
cluster and 20 mg/kg dosage cluster (cluster 1) of the 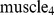 dataset.
dataset.
Table 13.
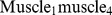 cluster 3.
cluster 3.
| Sr. | Probeset ID | Gene Symbol | Entrez Gene Name |
| 1 | 1453657 at | 2310065F04RIK | RIKEN cDNA 2310065F04 gene |
| 2 | 1434722 at | AMPD1 | adenosine monophosphate deaminase 1 |
| 3 | 1460256 at | CA3 | carbonic anhydrase III, muscle specific |
| 4 | 1422598 at | CASQ1 | calsequestrin 1 (fast-twitch, skeletal muscle) |
| 5 | 1439332 at | DDIT4L | DNA-damage-inducible transcript 4-like |
| 6 | 1427400 at | LBX1 | ladybird homeobox 1 |
| 7 | 1419487 at | MYBPH | myosin binding protein H |
| 8 | 1458368 at | MYH4 | myosin, heavy chain 4, skeletal muscle |
| 9 | 1441111 at | MYLK4 | myosin light chain kinase family, member 4 |
| 10 | 1418373 at | PGAM2 | phosphoglycerate mutase 2 (muscle) |
| 11 | 1444480 at | PRKAG3 | protein kinase, AMP-activated, gamma 3 non-catalytic subunit |
| 12 | 1417653 at | PVALB | parvalbumin |
| 13 | 1422644 at | SH3BGR | SH3 domain binding glutamic acid-rich protein |
| 14 | 1449206 at | SYPL2 | synaptophysin-like 2 |
Common probesets between the top one hundred most influential probesets in the 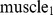 cluster and vehicle dose cluster (cluster 3) of the
cluster and vehicle dose cluster (cluster 3) of the 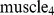 dataset.
dataset.
Table 14.
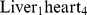 cluster 2.
cluster 2.
| Sr. | Probeset ID | Gene Symbol | Entrez Gene Name |
| 1 | 1449817 at | ABCB11 | ATP-binding cassette, sub-family B (MDR/TAP), member 11 |
| 2 | 1425260 at | ALB | albumin |
| 3 | 1416649 at | AMBP | alpha-1-microglobulin/bikunin precursor |
| 4 | 1419233 x at | APOA1 | apolipoprotein A-I |
| 5 | 1438840 x at | APOA1 | apolipoprotein A-I |
| 6 | 1455201 x at | APOA1 | apolipoprotein A-I |
| 7 | 1419232 a at | APOA1 | apolipoprotein A-I |
| 8 | 1417950 a at | APOA2 | apolipoprotein A-II |
| 9 | 1417610 at | APOA5 | apolipoprotein A-V |
| 10 | 1417561 at | APOC1 | apolipoprotein C-I |
| 11 | 1418278 at | APOC3 | apolipoprotein C-III |
| 12 | 1418708 at | APOC4 | apolipoprotein C-IV |
| 13 | 1416677 at | APOH | apolipoprotein H (beta-2-glycoprotein I) |
| 14 | 1424011 at | AQP9 | aquaporin 9 |
| 15 | 1419549 at | ARG1 | arginase, liver |
| 16 | 1421944 a at | ASGR1 | asialoglycoprotein receptor 1 |
| 17 | 1450624 at | BHMT | betaine–homocysteine S-methyltransferase |
| 18 | 1451600 s at | CES3 | carboxylesterase 3 |
| 19 | 1455540 at | CPS1 | carbamoyl-phosphate synthase 1, mitochondrial |
| 20 | 1418113 at | CYP2D10 | cytochrome P450, family 2, subfamily d, polypeptide 10 |
| 21 | 1416913 at | ES1 | (includes EG:13884) esterase 1 |
| 22 | 1418897 at | F2 | coagulation factor II (thrombin) |
| 23 | 1417556 at | FABP1 | fatty acid binding protein 1, liver |
| 24 | 1418438 at | FABP2 | fatty acid binding protein 2, intestinal |
| 25 | 1424279 at | FGA | fibrinogen alpha chain |
| 26 | 1428079 at | FGB | fibrinogen beta chain |
| 27 | 1416025 at | FGG | fibrinogen gamma chain |
| 28 | 1426547 at | GC | group-specific component (vitamin D binding protein) |
| 29 | 1419196 at | HAMP | hepcidin antimicrobial peptide |
| 30 | 1419197 x at | HAMP | hepcidin antimicrobial peptide |
| 31 | 1436643 x at | HAMP | hepcidin antimicrobial peptide |
| 32 | 1425137 a at | HLA-A | major histocompatibility complex, class I, A |
| 33 | 1448881 at | HP | haptoglobin |
| 34 | 1423944 at | HPX | hemopexin |
| 35 | 1434110 x at | LOC100129193 | major urinary protein pseudogene |
| 36 | 1428005 at | MOSC1 | MOCO sulphurase C-terminal domain containing 1 |
| 37 | 1417835 at | MUG1 | murinoglobulin 1 |
| 38 | 1451054 at | ORM1 | orosomucoid 1 |
| 39 | 1418190 at | PON1 | paraoxonase 1 |
| 40 | 1417246 at | PZP | pregnancy-zone protein |
| 41 | 1426225 at | RBP4 | retinol binding protein 4, plasma |
| 42 | 1451513 x at | SERPINA1 | serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 1 |
| 43 | 1418282 x at | SERPINA1 | serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 1 |
| 44 | 1423866 at | SERPINA3K | serine (or cysteine) peptidase inhibitor, clade A, member 3K |
| 45 | 1417909 at | SERPINC1 | serpin peptidase inhibitor, clade C (antithrombin), member 1 |
| 46 | 1449112 at | SLC27A5 | solute carrier family 27 (fatty acid transporter), member 5 |
| 47 | 1449394 at | SLCO1B3 | solute carrier organic anion transporter family, member 1B3 |
| 48 | 1419093 at | TDO2 | tryptophan 2,3-dioxygenase |
| 49 | 1422604 at | UOX | urate oxidase, pseudogene |
Common probesets between the top one hundred most influential probesets in the 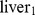 cluster and 6 mg/kg dosage cluster (cluster 2) of the
cluster and 6 mg/kg dosage cluster (cluster 2) of the 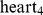 dataset'.
dataset'.
Table 15.
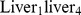 cluster 1.
cluster 1.
| Sr. | Probeset ID | Gene Symbol | Entrez Gene Name |
| 1 | 1425260 at | ALB | albumin |
| 2 | 1419059 at | APCS | amyloid P component, serum |
| 3 | 1419232 a at | APOA1 | apolipoprotein A-I |
| 4 | 1419233 x at | APOA1 | apolipoprotein A-I |
| 5 | 1438840 x at | APOA1 | apolipoprotein A-I |
| 6 | 1455201 x at | APOA1 | apolipoprotein A-I |
| 7 | 1417950 a at | APOA2 | apolipoprotein A-II |
| 8 | 1416677 at | APOH | apolipoprotein H (beta-2-glycoprotein I) |
| 9 | 1419549 at | ARG1 | arginase, liver |
| 10 | 1417556 at | FABP1 | fatty acid binding protein 1, liver |
| 11 | 1428079 at | FGB | fibrinogen beta chain |
| 12 | 1426547 at | GC | group-specific component (vitamin D binding protein) |
| 13 | 1448881 at | HP | haptoglobin |
Common probesets between the top one hundred most influential probesets in the 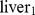 cluster cluster and 20 mg/kg dosage cluster (cluster 1) of the
cluster cluster and 20 mg/kg dosage cluster (cluster 1) of the 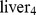 dataset.
dataset.
Table 16.
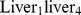 cluster 3.
cluster 3.
| Sr. | Probeset ID | Gene Symbol | Entrez Gene Name |
| 1 | 1428981 at | 2810007J24RIK | RIKEN cDNA 2810007J24 gene |
| 2 | 1449817 at | ABCB11 | ATP-binding cassette, sub-family B (MDR/TAP), member 11 |
| 3 | 1417085 at | AKR1C4 | aldo-keto reductase family 1, member C4 (chlordecone reductase; 3-alpha hydroxysteroid dehydrogenase, type I; dihydrodiol dehydrogenase 4) |
| 4 | 1451600 s at | CES3 | carboxylesterase 3 |
| 5 | 1449242 s at | HRG | histidine-rich glycoprotein |
| 6 | 1431808 a at | ITIH4 | inter-alpha (globulin) inhibitor H4 (plasma Kallikrein-sensitive glycoprotein) |
| 7 | 1434110 x at | LOC100129193 | major urinary protein pseudogene |
| 8 | 1420465 s at | LOC100129193 | major urinary protein pseudogene |
| 9 | 1426154 s at | LOC100129193 | major urinary protein pseudogene |
| 10 | 1420525 a at | OTC | ornithine carbamoyltransferase |
| 11 | 1436615 a at | OTC | ornithine carbamoyltransferase |
| 12 | 1448680 at | SERPINA1 | serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 1 |
| 13 | 1448506 at | SERPINA6 | serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 6 |
| 14 | 1449394 at | SLCO1B3 | solute carrier organic anion transporter family, member 1B3 |
| 15 | 1424934 at | UGT2B4 | UDP glucuronosyltransferase 2 family, polypeptide B4 |
| 16 | 1422604 at | UOX | urate oxidase, pseudogene |
Common probesets between the top one hundred most influential probesets in the 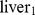 cluster and vehicle dose cluster (cluster 3) of the
cluster and vehicle dose cluster (cluster 3) of the 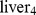 dataset.
dataset.
Table 17.
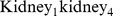 cluster 3.
cluster 3.
| Sr. | Probeset ID | Gene Symbol | Entrez Gene Name |
| 1 | 1456190 a at | ACSM2A | acyl-CoA synthetase medium-chain family member 2A |
| 2 | 1427223 a at | ACSM2A | acyl-CoA synthetase medium-chain family member 2A |
| 3 | 1425207 at | BC026439 | cDNA sequence BC026439 |
| 4 | 1424713 at | CALML4 | calmodulin-like 4 |
| 5 | 1424592 a at | DNASE1 | deoxyribonuclease I |
| 6 | 1448485 at | GGT1 | gamma-glutamyltransferase 1 |
| 7 | 1460233 at | GUCA2B | guanylate cyclase activator 2B (uroguanylin) |
| 8 | 1415969 s at | KAP | kidney androgen regulated protein |
| 9 | 1415968 a at | KAP | kidney androgen regulated protein |
| 10 | 1435094 at | KCNJ16 | potassium inwardly-rectifying channel, subfamily J, member 16 |
| 11 | 1450719 at | MEP1A | meprin A, alpha (PABA peptide hydrolase) |
| 12 | 1418923 at | SLC17A3 | solute carrier family 17 (sodium phosphate), member 3 |
| 13 | 1417072 at | SLC22A6 | solute carrier family 22 (organic anion transporter), member 6 |
| 14 | 1423279 at | SLC34A1 | solute carrier family 34 (sodium phosphate), member 1 |
| 15 | 1425606 at | SLC5A8 | solute carrier family 5 (iodide transporter), member 8 |
| 16 | 1449301 at | SLC7A13 | solute carrier family 7, (cationic amino acid transporter, y+ system) member 13 |
| 17 | 1435064 a at | TMEM27 | transmembrane protein 27 |
| 18 | 1423397 at | UGT2B17 | UDP glucuronosyltransferase 2 family, polypeptide B17 |
Common probesets between the top one hundred most influential probesets in the 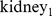 cluster and vehicle dose cluster (cluster 3) of the
cluster and vehicle dose cluster (cluster 3) of the 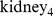 dataset.
dataset.
Discussion
The factorization and reordering of the dataset as a whole set (Figure 2 and Table 2) successfully clustered samples from the same tissue and further investigation showed that it simultaneously identified genes with a known relevance to those tissues. It was therefore reasonable to study the genes that were responsible for this differentiation. In the one-way clustering, the top 100 probe-sets from each of the four tissue specific clusters show remarkable coherence for tissue specific pathways. The calcium signalling pathway is highly enriched in both 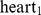 and
and 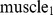 clusters; these genes are linked to muscle contraction function. Muscle contraction is the prime function of cardiac and skeletal muscles. A deeper look at the probe-sets (Tables 7 and 8) from the heart and skeletal muscle clusters shows a successful identification of differences in the tissue types for this pathway; see Figure 9. MYH1, MYH2, MYH4 and MYL1 of the myosin family, which are specific to skeletal muscle, are found in the
clusters; these genes are linked to muscle contraction function. Muscle contraction is the prime function of cardiac and skeletal muscles. A deeper look at the probe-sets (Tables 7 and 8) from the heart and skeletal muscle clusters shows a successful identification of differences in the tissue types for this pathway; see Figure 9. MYH1, MYH2, MYH4 and MYL1 of the myosin family, which are specific to skeletal muscle, are found in the 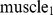 cluster while cardiac muscle specific myosin family members MYH6, MYL2 and MYL3 are found in the
cluster while cardiac muscle specific myosin family members MYH6, MYL2 and MYL3 are found in the 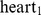 cluster [19]. This pattern is also true for troponin, calsequestrin, ryanodine and actin family members [20]–[25] (Tables 7 and 8). FXR/RXR activation pathway genes are significantly enriched in
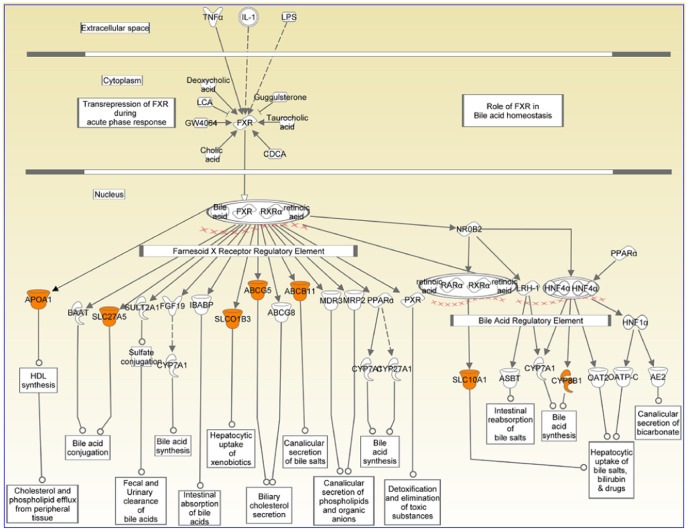
cluster [19]. This pattern is also true for troponin, calsequestrin, ryanodine and actin family members [20]–[25] (Tables 7 and 8). FXR/RXR activation pathway genes are significantly enriched in 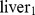 cluster (Figure 7) with most of the enriched genes present in the bile acid synthesis and regulation (Figure 10) pathway, which is one of the core functions of liver [26]–[28]. FXR/RXR activation is also found in the
cluster (Figure 7) with most of the enriched genes present in the bile acid synthesis and regulation (Figure 10) pathway, which is one of the core functions of liver [26]–[28]. FXR/RXR activation is also found in the 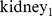 cluster, albeit with moderate significance; FBP1 and HNF4A are the two genes present in this pathway and they may be involved in gluconeogenesis in kidney [29].
cluster, albeit with moderate significance; FBP1 and HNF4A are the two genes present in this pathway and they may be involved in gluconeogenesis in kidney [29].
Figure 9. Heart and muscle genes enriched in calcium signalling – muscle contraction pathway.
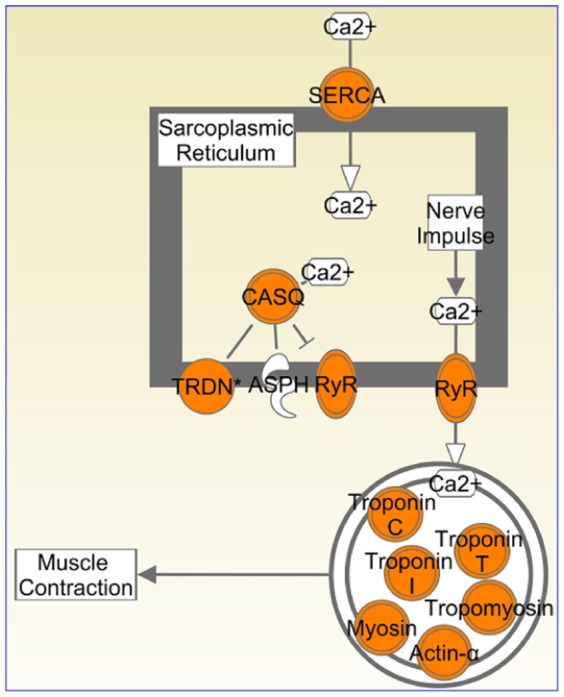
IPA analysis of the top 100 probe-sets from heart and muscle gene clusters (Figure 7) showed the enrichment of calcium signalling pathway. In this figure, we have highlighted the genes present in this pathway in orange. Though this pathway is generalised for skeletal muscle contraction and cardiac muscle contraction, they differ in the members of the same gene family. The heart and muscle genes present in this pathway are given in Tables 7 and 8. Pathway diagram was drawn using Path Designer function of IPA [18].
Figure 10. Liver genes enriched in FXR/RXR activation pathway IPA analysis of the top 100 probe-sets from the .
 cluster (
Figure 7
) showed the enrichment of FXR/RXR activation pathway. The genes present in this pathway are highlighted in orange. The liver genes present in the pathway are given in Table 9. Pathway diagram was drawn using Path Designer function of IPA [18].
cluster (
Figure 7
) showed the enrichment of FXR/RXR activation pathway. The genes present in this pathway are highlighted in orange. The liver genes present in the pathway are given in Table 9. Pathway diagram was drawn using Path Designer function of IPA [18].
Splitting the dataset into four on the basis of tissue types and simultaneous non-negative factorization of them gave us the added reassurance of clustering the samples according to the dosage groups (Figure 5 and Table 3). The clustering of one mouse (Mouse E) from the lower dosage group (Group-II) with the higher dosage group (Group-III) can be explained by the higher PPM201 drug sensitivity of that mouse, indicated by the elevated levels of the toxocology markers ALT, AST, LDH and CK, compared with the rest of its group (Table 1). Comparisons of top probe-sets in tissue specific clusters with dosage specific clusters also show very high overlap of tissue specific genes in the four tissue types.  cluster1 has 22 probe-sets that are common between the top 100 probe-sets of
cluster1 has 22 probe-sets that are common between the top 100 probe-sets of  cluster and 20 mg/kg dosage cluster of
cluster and 20 mg/kg dosage cluster of 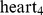 dataset, and are highly enriched for cardiac muscle contraction and hypertrophic cardiomyopathy pathways (Table 6). ACTC1, ATP2A2, MYH6, MYL2, MYL3, TNNC1, TNNI3, TNNT2 and TPM1 are the genes enriched for these two pathways and shared between these two clusters. However,
dataset, and are highly enriched for cardiac muscle contraction and hypertrophic cardiomyopathy pathways (Table 6). ACTC1, ATP2A2, MYH6, MYL2, MYL3, TNNC1, TNNI3, TNNT2 and TPM1 are the genes enriched for these two pathways and shared between these two clusters. However, 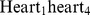 cluster 3, with 11 probe-sets in common between the top 100 probe-sets of
cluster 3, with 11 probe-sets in common between the top 100 probe-sets of 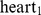 cluster and vehicle dose cluster of
cluster and vehicle dose cluster of 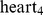 dataset, does not show enrichment for cardiac muscle contraction and hypertrophic cardiomyopathy pathways. From this we may assume that perturbation of cardiac muscle contraction and hypertrophic cardiomyopathy pathways by 20 mg/kg dosage may indicate toxic responses. We also see a similar pattern in skeletal muscle. Between the top 100 probe-sets of
dataset, does not show enrichment for cardiac muscle contraction and hypertrophic cardiomyopathy pathways. From this we may assume that perturbation of cardiac muscle contraction and hypertrophic cardiomyopathy pathways by 20 mg/kg dosage may indicate toxic responses. We also see a similar pattern in skeletal muscle. Between the top 100 probe-sets of 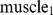 cluster and 20 mg/kg dosage cluster of
cluster and 20 mg/kg dosage cluster of 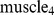 , and between the top 100 probe-sets of
, and between the top 100 probe-sets of 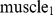 and vehicle dose cluster of
and vehicle dose cluster of 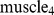 , 15 and 14 probe-sets were in common and are named as
, 15 and 14 probe-sets were in common and are named as 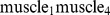 cluster 1 and
cluster 1 and 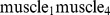 cluster 3, respectively. The calcium signalling–skeletal muscle contraction pathway is enriched in
cluster 3, respectively. The calcium signalling–skeletal muscle contraction pathway is enriched in 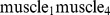 cluster 1 with the presence of ACTA1, ATP2A1, MYH1, MYH4, RYR1, TNNC2, TNNI2, TNNT3 and TRDN genes, whereas
cluster 1 with the presence of ACTA1, ATP2A1, MYH1, MYH4, RYR1, TNNC2, TNNI2, TNNT3 and TRDN genes, whereas 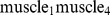 cluster 3 does not show any significant enrichment for signalling or metabolic pathways.
cluster 3 does not show any significant enrichment for signalling or metabolic pathways.
Interestingly, 49 probe-sets in the 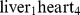 cluster 2 are common between the top 100 probe-sets of
cluster 2 are common between the top 100 probe-sets of 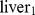 cluster cluster and 6 mg/kg dosage cluster of
cluster cluster and 6 mg/kg dosage cluster of 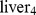 and highly enriched for acute phase response signalling, prothrombin activation and FXR/RXR activation pathways with the presence of ALB, ABCB11, AMBP, APOA1, APOA2, APOC3, APOH, F2, FGA, FGB, FGG, HAMP, HP, HPX, ORM1, PON1, RBP4, SERPINA1, SERPINC1, SLC27A5 and SLCO1B3 genes (Figure 11). This suggests alterations in lipid metabolism in liver along with tissue injury in heart induced by PPM-201 at 6 mg/kg dosage [30]–[33], which becomes more plausible when we look at the genes in
and highly enriched for acute phase response signalling, prothrombin activation and FXR/RXR activation pathways with the presence of ALB, ABCB11, AMBP, APOA1, APOA2, APOC3, APOH, F2, FGA, FGB, FGG, HAMP, HP, HPX, ORM1, PON1, RBP4, SERPINA1, SERPINC1, SLC27A5 and SLCO1B3 genes (Figure 11). This suggests alterations in lipid metabolism in liver along with tissue injury in heart induced by PPM-201 at 6 mg/kg dosage [30]–[33], which becomes more plausible when we look at the genes in 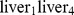 cluster 1 that are common between the top
cluster 1 that are common between the top 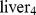 genes and 20 mg/kg dosage cluster of
genes and 20 mg/kg dosage cluster of 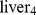 dataset. Enrichment of toxicity functions in
dataset. Enrichment of toxicity functions in 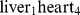 cluster 2 using IPA shows increased level of LDH as one of the toxicity functions (Figure 12) which has been validated with the increased level of LDH in the clinical chemistry results.
cluster 2 using IPA shows increased level of LDH as one of the toxicity functions (Figure 12) which has been validated with the increased level of LDH in the clinical chemistry results.
Figure 11. Enrichment of canonical pathways in the liver heart gene cluster no. 2.
This gene cluster has 49 common probe-sets between the top one hundred most influential probe-sets in the liver gene cluster and top one hundred probe-sets in cluster number 2 (6 mg/kg dose rate) of the heart dataset reordered by 4-way simultaneous factorization. Canonical pathways enrichment for these 49 probe-sets analysed using the IPA software is shown in this figure. The length of the bars shows the Fisher's exact test p-value for enrichment for a particular pathway in the cluster.
Figure 12. Enrichment of toxicity functions in .
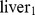
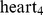 cluster 2. This gene cluster has 49 common probe-sets between the top one hundred most influential probe-sets in
cluster 2. This gene cluster has 49 common probe-sets between the top one hundred most influential probe-sets in 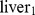
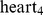 cluster 2 (6 mg/kg dose rate). Toxicity functions enrichment for these 49 probe-sets analysed using the IPA software is shown in this figure. The length of the bars shows the Fisher's exact test p-value for enrichment for a particular pathway in the cluster.
cluster 2 (6 mg/kg dose rate). Toxicity functions enrichment for these 49 probe-sets analysed using the IPA software is shown in this figure. The length of the bars shows the Fisher's exact test p-value for enrichment for a particular pathway in the cluster.
Conclusions
We have demonstrated that multi-way simultaneous nonnegative matrix factorization can be usefully applied to the case of multiple datasets—here, for what we believe to be the first time, more than two large scale matrices were treated. The results were shown to be consistent with, and to add value to, standard nonnegative matrix factorization of the whole dataset.
In summarizing our biological findings, we first note that the roles of the three different isoforms of PPARs - PPAR- , PPAR-
, PPAR-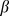 (also known as PPAR-
(also known as PPAR- ) and PPAR-
) and PPAR- in metabolism and their difference in expression in different tissues and different species are well known [34]–[36]. In mouse, PPAR-
in metabolism and their difference in expression in different tissues and different species are well known [34]–[36]. In mouse, PPAR- is highly expressed in liver and to a lesser degree in kidney, heart and skeletal muscle; PPAR-
is highly expressed in liver and to a lesser degree in kidney, heart and skeletal muscle; PPAR-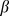 is expressed in many tissues but peaks in kidney, heart and intestine whereas PPAR-
is expressed in many tissues but peaks in kidney, heart and intestine whereas PPAR- is mostly expressed in adipose tissue [34], [37]. Pan-PPAR agonists activate two or all of the pan-PPAR isoforms and differ in their pharmacological actions. Factorisation of the dataset after splitting it on the tissue basis appears to be beneficial in identifying tissue specific and dosage effects of the experimental pan-PPAR agonist PPM-201 in this study. This approach could be useful in understanding molecular mechanisms and identifying potential tissue specific toxicological effects before they are apparent in histopathology studies. In this study, histopathology examination of heart did not show any defect though our method of gene expression analysis could identify enrichment of acute phase response signalling genes in heart that may point towards building up of toxic responses in heart. Given the fact that many PPAR agonist drugs have been shown to cause cardiac toxicity on prolonged usage and FDA's requirement of one year toxicity study for PPAR agonist drugs, our results show promising early detection of toxicity in the drug discovery process.
is mostly expressed in adipose tissue [34], [37]. Pan-PPAR agonists activate two or all of the pan-PPAR isoforms and differ in their pharmacological actions. Factorisation of the dataset after splitting it on the tissue basis appears to be beneficial in identifying tissue specific and dosage effects of the experimental pan-PPAR agonist PPM-201 in this study. This approach could be useful in understanding molecular mechanisms and identifying potential tissue specific toxicological effects before they are apparent in histopathology studies. In this study, histopathology examination of heart did not show any defect though our method of gene expression analysis could identify enrichment of acute phase response signalling genes in heart that may point towards building up of toxic responses in heart. Given the fact that many PPAR agonist drugs have been shown to cause cardiac toxicity on prolonged usage and FDA's requirement of one year toxicity study for PPAR agonist drugs, our results show promising early detection of toxicity in the drug discovery process.
Overall, our aim here is to establish a proof of principle for the approach of simultaneously analysing multiple, related large datasets. We therefore focused on a dataset where clear-cut validation is possible. However, we note that the technique is very general, and therefore opens up many new opportunities in data-driven computational biology. In particular, it can be applied to heterogeneous sources of data; for example, generated by different laboratories or experimental methodologies. We are currently pursuing this approach in the study of colon cancer.
Acknowledgments
The computational work reported here made extensive use of the High Performance Computer Facilities of the Faculty of Engineering and Institute of Complex Systems at the University of Strathclyde. The authors also acknowledge Plexxikon for use of the compound.
Funding Statement
This work was supported by the Translational Medicine Research Collaboration—a consortium made up of the Universities of Aberdeen, Dundee, Edinburgh and Glasgow, the four associated NHS Health Boards (Grampian, Tayside, Lothian and Greater Glasgow & Clyde), Scottish Enterprise and Pfizer, by EPSRC Grant EP/E49370/1, by the Knowledge Transfer Account of the University of Strathclyde and by the 2007 DTI grant “New serum Biomarkers for Preclinical and Clinical Drug Safety Assessment”. CML and DJH were supported by the Engineering and Physical Sciences Research Council of the UK, under their Fundamentals of Complexity Science call. DJH was funded by a Fellowship from the Leverhulme Trust. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lee DD, Seung HS (1999) Learning the parts of objects by non-negative matrix factorization. Nature 401: 788–791. [DOI] [PubMed] [Google Scholar]
- 2. Carmona-Saez P, Pascual-Marqui R, Tirado F, Carazo J, Pascual-Montano A (2006) Biclustering of gene expression data by non-smooth non-negative matrix factorization. BMC Bioinformatics 7: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brunet JP, Tamayo P, Golub TR, Mesirov JP (2004) Metagenes and molecular pattern discovery using matrix factorisation. Proc Nat Acad Sci 101: 4164–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fogel P, Young SS, Hawkins DM, Ledirac N (2007) Inferential, robust non-negative matrix factorization analysis of microarray data. Bioinformatics 23: 44–49. [DOI] [PubMed] [Google Scholar]
- 5. Kim H, Park H (2007) Sparse non-negative matrix factorizations via alternating non-negativityconstrained least squares for microarray data analysis. Bioinformatics 23: 1495–1502. [DOI] [PubMed] [Google Scholar]
- 6. Pascual-Montano AD, Carmona-Saez P, Chagoyen M, Tirado F, Carazo JM, et al. (2006) bioNMF: a versatile tool for non-negative matrix factorization in biology. BMC Bioinformatics 7: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao Y, Church G (2005) Improving molecular cancer class discovery through sparse non-negative matrix factorization. Bioinformatics 21: 3970–3975. [DOI] [PubMed] [Google Scholar]
- 8. Devarajan K (2008) Nonnegative matrix factorization: An analytical and interpretive tool in computational biology. PLoS Comput Biol 4: e1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badea L (2007) Combining gene expression and transcription factor regulation data using simultaneous nonnegative matrix factorization. In: Proc. BIOCOMP-2007. pp. 127–131.
- 10.Badea L (2008) Extracting gene expression profiles common to colon and pancreatic adenocarcinoma using simultaneous nonnegative matrix factorization. In: Proc. Pacific Symposium on Biocomputing PSB-2008. pp. 267–278. [PubMed]
- 11. Artis DR, Lin JJ, Zhang C, WangW,Mehra U, et al. (2009) Scaffold-based discovery of indeglitazar, a ppar pan-active anti -diabetic agent. Proceedings of the National Academy of Sciences 106: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones D (2010) Potential remains for PPAR-targeted drugs. Nature Reviews Drug Discovery 9: 668–669. [DOI] [PubMed] [Google Scholar]
- 13. Lee DD, Seung HS (2001) Algorithms for non-negative matrix factorization. Adv Neural Info Proc Syst 13: 556–562. [Google Scholar]
- 14. Monit S, Tamayo P, Mesirov J, Golub T (2003) Consensus clustering: a resampling-basedmethod for class discovery and visualization of gene expression microarray data. Machine Learning 52: 91–118. [Google Scholar]
- 15.Hall R (2001) Principles of clinical pathology for toxicology studies. In Principles and methods of toxicology. Taylor and Francis, Philadelphia, 4th edition.
- 16. Florvall G, Basu S, Larsson A (2006) Apolipoprotein A1 is a stronger prognostic marker than are HDL and LDL cholesterol for cardiovascular disease and mortality in elderly men. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences 61: 1262–1266. [DOI] [PubMed] [Google Scholar]
- 17. Huang DW, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protoc 4: 44–57. [DOI] [PubMed] [Google Scholar]
- 18.Redwood City CISI (2011). Ingenuity: Ingenuity pathways analysis; version 8.8.
- 19. Weiss A, McDonough D, Wertman B, Acakpo-Satchivi L, Montgomery K, et al. (1999) Organization of human and mouse skeletal myosin heavy chain gene clusters is highly conserved. Proc Natl Acad Sci USA 96: 2958–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR (1997) Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem 23389–97. [DOI] [PubMed] [Google Scholar]
- 21. Jin J, Zhang Z, Bautista J (2008) Isoform diversity, regulation, and functional adaptation of troponin and calponin. Crit Rev Eukaryot Gene Expr 18: 93–124. [DOI] [PubMed] [Google Scholar]
- 22. Parmacek M, Bengur A, Vora A, Leiden J (1990) The structure and regulation of expression of the murine fast skeletal troponin c gene. identification of a developmentally regulated, muscle-specific transcriptional enhancer. J Biol Chem 265: 15970–6. [PubMed] [Google Scholar]
- 23. Farah C, Reinach F (1995) The troponin complex and regulation of muscle contraction. FASEB J 9: 755–67. [DOI] [PubMed] [Google Scholar]
- 24. Perry S (2003) What is the role of tropomyosin in the regulation of muscle contraction? J Muscle Res Cell Motil 24: 593–6. [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi T, Solaro R (2005) Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol 67: 39–67. [DOI] [PubMed] [Google Scholar]
- 26. Matsukuma K, Bennett M, Huang J, Wang L, Gil G, et al. (2006) Coordinated control of bile acids and lipogenesis through fxr-dependent regulation of fatty acid synthase. J Lipid Res 47: 2754–61. [DOI] [PubMed] [Google Scholar]
- 27. Scotti E, Gilardi F, Godio C, Gers E, Krneta J, et al. (2007) Bile acids and their signaling pathways: eclectic regulators of diverse cellular functions. Cell Mol Life Sci 64: 2477–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gadaleta R, van Mil S, Oldenburg B, Siersema P, Klomp L, et al. (2010) Bile acids and their nuclear receptor fxr: Relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta 1801: 683–92. [DOI] [PubMed] [Google Scholar]
- 29. Rhee J, Inoue Y, Yoon J, Puigserver P, Fan M, et al. (2003) Regulation of hepatic fasting response by ppargamma coactivator - 1alpha (pgc-1): Requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc Natl Acad Sci USA 100: 4012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gervois P, Vu-Dac N, Kleemann R, Kockx M, Dubois G, et al. (2001) Negative regulation of human fibrinogen gene expression by peroxisome proliferator-activated receptor alpha agonists via inhibition of CCAAT box/enhancer-binding protein beta. J Biol Chem 276: 33471–7. [DOI] [PubMed] [Google Scholar]
- 31. Oldgren J, Wallentin L, Grip L, R L, Nørgaard BL, et al. (2003) Myocardial damage, inflammation and thrombin inhibition in unstable coronary artery disease. EurHeart J 24: 86–93. [DOI] [PubMed] [Google Scholar]
- 32. Baumann H, Gauldie J (1994) The acute phase response. Immunol Today 15: 74–80. [DOI] [PubMed] [Google Scholar]
- 33. T M (2010) Nuclear receptors in the regulation of lipid metabolism. Curr Cardio Risk Rep 4: 142–149. [Google Scholar]
- 34. Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, et al. (1994) Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci, U S A 91: 7355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, et al. (1992) Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell 68: 879–87. [DOI] [PubMed] [Google Scholar]
- 36. Guo Y, Jolly R, Halstead B, Baker T, Stutz J, et al. (2007) Underlying mechanisms of pharmacology and toxicity of a novel ppar agonist revealed using rodent and canine hepatocytes. Toxicol Sci 96: 294–309. [DOI] [PubMed] [Google Scholar]
- 37. Kersten S, Desvergne B, Wahli W (2000) Roles of PPARs in health and disease. Nature 405: 421–4. [DOI] [PubMed] [Google Scholar]