Abstract
Manganese (Mn2+)-enhanced MRI (MEMRI) provides the potential for the in vivo evaluation of calcium (Ca2+) uptake in the heart. Recent studies have also suggested the role of the sodium–calcium (Na+–Ca2+) exchanger (NCX) in Mn2+ retention, which may have an impact on MEMRI signals. In this study, we investigated whether MEMRI with fast T1 mapping allowed the sensitive detection of changes in NCX activity. We quantified the dynamics of the Mn2+-induced T1 changes in isolated perfused rat hearts in response to SEA0400, an NCX inhibitor. The experimental protocol comprised 30 min of Mn2+ perfusion (wash-in), followed by a 30-min wash-out period. There were three experimental groups: 1, NCX inhibition by 1 μM SEA0400 during Mn2+ wash-in only (SEAin, n = 6); 2, NCX inhibition by 1 μM SEA0400 during Mn2+ wash-out only (SEAout, n = 6); 3, no NCX inhibition during both wash-in and wash-out to serve as the control group (CNTL, n = 5). Rapid T1 mapping at a temporal resolution of 3 min was performed throughout the perfusion protocol using a triggered saturation–recovery Look–Locker sequence. Our results showed that NCX inhibition during Mn2+ wash-in caused a significant increase in relaxation rate (R1) at the end of Mn2+ perfusion. During the wash-out period, NCX inhibition led to less reduction in R1. Further analysis of Mn2+ content in myocardium with flame atomic absorption spectroscopy was consistent with the MRI findings. These results suggest that Mn2+ accumulation and retention in rat hearts are, in part, dependent on NCX activity. Hence, MEMRI may provide an imaging method that is also sensitive to changes in NCX activity.
Keywords: manganese-enhanced MRI, calcium uptake, sodium–calcium exchanger, T1 mapping
INTRODUCTION
Cardiac excitation–contraction (EC) coupling, the process that converts an electrical stimulus to muscle contraction, is fundamental to ventricular function. Calcium (Ca2+), the ubiquitous second messenger that directly activates myofilaments, is of key importance in EC coupling (1). During a cardiac action potential, Ca2+ influx, mostly via the voltage-sensitive L-type Ca2+ channels, triggers Ca2+ release from the sarcoplasmic reticulum (SR), leading to a transient increase in cytosolic Ca2+ concentration during systole. During diastole, Ca2+ is removed from the cytosol, mostly through the Ca2+-ATPase located in the SR and the sarcolemmal sodium–calcium (Na+–Ca2+) exchanger (NCX) (2,3). Myocardial contractility is dependent on the total Ca2+ concentration which must be supplied to and removed from the cytosol during each heart beat. Abnormal Ca2+ cycling has been implicated in contractile dysfunction (4).
The current investigation of Ca2+ cycling largely relies on the characterization of isolated cells or ex vivo hearts using fluorescent dyes or electrophysiological methods (5–7). Recently, manganese (Mn2+)-enhanced MRI (MEMRI) has been proposed for the in vivo evaluation of Ca2+ cycling in the heart (8). Mn2+ is a potent T1-shortening agent (9). Unlike a gadolinium (Gd)-based contrast agent that is confined to the extracellular space, Mn2+ enters the cell through L-type Ca2+ channels (10,11) and perhaps NCX (12). Thus, it offers the unique opportunity for the in vivo delineation of an important cellular process that initiates EC coupling. Several studies have shown that the MEMRI signal reflects changes in Ca2+ uptake in the myocardium in vivo (13,14). More recently, Waghorn et al. (15,16) have also observed T1 changes associated with Mn2+ efflux via the NCX 1 h after the withdrawal of Mn2+. Their observation suggests that MEMRI may also be used for the evaluation of NCX activity, another important determinant of Ca2+ cycling. However, significantly higher temporal resolution and sensitivity are required to evaluate Mn2+ transport via NCX without prolonged imaging times.
In the current study, we evaluated the potential of MEMRI for the sensitive delineation of NCX activity via fast T1 mapping. We hypothesized that Mn2+ accumulation and retention in cardiomyocytes were dependent on Mn2+ efflux via the NCX, which can be calculated from Mn2+-induced T1 changes. Using a fast T1 mapping method, we quantified the dynamics of Mn2+-induced T1 changes in isolated perfused rat hearts in response to SEA0400, an NCX inhibitor (17), during Mn2+ perfusion (wash-in) and wash-out. Myocardial Mn2+ content was also measured by flame atomic absorption spectrophotometry to validate the findings by MRI. In addition, the cardiotoxicity of Mn2+ was evaluated in isolated cardiac myocytes exposed to 50–500 μM Mn2+. These results will contribute to our understanding of the cellular processes that may have an impact on MEMRI measurements.
MATERIALS AND METHODS
Heart perfusion protocol
Male Sprague–Dawley rats (8–10 weeks) were heparinized (1000 units/kg, intraperitoneally) and anesthetized by sodium pentobarbital (85 mg/kg, intraperitoneally). The heart was excised, cannulated and perfused with Krebs–Henseleit (KH) buffer containing (in mM): NaCl, 118.5; KCl, 4.7; MgSO4, 1.2; KH2PO4, 1.2; CaCl2, 1.5; glucose, 11.1; NaHCO3, 25. The perfusate was maintained at 37 °C and equilibrated with 95% O2–5% CO2. The perfusion pressure was maintained at a constant level (100 cmH2O). A water-filled latex balloon was inserted into the left ventricle and connected to a pressure transducer to record the left ventricular end-diastolic pressure (LVEDP), left ventricular systolic pressure (LVSP) and heart rate (HR). The left ventricular developed pressure (LVDP) was calculated as the difference between LVSP and LVEDP. The rate–pressure product (RPP), i.e. the product of LVDP and HR, was calculated as an index of the workload.
Hearts were paced at 360 beats/min using a Grass stimulator (Grass Technologies, West Warwick, RI, USA). Once the heart rate and pressure were stabilized, the perfusate was switched to modified KH buffer containing 30 μM MnCl2 for 30 min (the wash-in period), followed by a 30-min wash-out period with Mn2+-free buffer. During Mn2+ perfusion, phosphate and sulfate were replaced with chloride in the modified KH buffer to prevent Mn2+ precipitation. There were three experimental groups: 1, NCX inhibition by 1 μM SEA0400 during Mn2+ wash-in only (SEAin, n = 6); 2, NCX inhibition by 1 μM SEA0400 during Mn2+ wash-out only (SEAout, n = 6); 3, no NCX inhibition during both wash-in and wash-out to serve as the control group (CNTL, n = 5).
Image acquisition
The perfusion column was placed inside a 9.4-T vertical-bore spectrometer (Bruker Biospin Co., Billerica, MA, USA). Image acquisition used a 20-mm volume coil. A 1-mm-thick short-axis slice at the midventricular level was prescribed for imaging. A triggered saturation–recovery Look–Locker sequence was used for rapid T1 mapping (18). Pacing signals were used to trigger the image acquisition. Prior to Mn2+ perfusion, two baseline T1 maps were acquired. To delineate the kinetics of Mn2+-induced contrast enhancement, sequential T1 maps were acquired at a temporal resolution of 3 min during the Mn2+ wash-in and wash-out periods. Imaging parameters were as follows: TE, 2 ms; TR, trigger interval, 166 ms; flip angle, 10°; field of view, 2.5 × 2.5 cm2; matrix size, 128 × 64. At the end of imaging acquisition, hearts were frozen in liquid nitrogen for the quantification of Mn2+ content by flame atomic absorption spectrophotometry. To quantify the Mn2+ content at the end of Mn2+ wash-in, an additional set of hearts was frozen at the end of the wash-in period.
Image analysis
Image analysis used in-house-developed MATLAB-based software described in detail previously (18). T1 maps of the whole heart were generated by performing pixel-wise curve fitting. The myocardial free wall was selected as the region of interest to quantify the changes in the T1 relaxation time during the imaging protocol.
Mn2+ quantification by flame atomic absorption spectrophotometry
Frozen ventricular tissues were burned in a furnace at 600 °C for 2 h. The ashes were dissolved in 20% nitric acid. The Mn2+ content was measured by a flame atomic absorption spectrophotometer (Buck Scientific, Norwalk, CT, USA).
Mn2+ toxicity on isolated myocytes
To evaluate the cardiotoxicity of Mn2+, myocyte shortening and Ca2+ transients were measured in isolated mouse myocytes. Briefly, mice were anesthetized with pentobarbital (85 mg/kg) and heparin (1000 units/kg). The heart was excised, cannulated and perfused with Ca2+-free Tyrode solution containing 0.8 mg/mL collagenase type II (Worthington Biochemical Co., Lakewood, NJ, USA) for 5 min. The original Tyrode solution contained (in mM): NaCl, 136; KCl, 5.4; MgCl2, 1.0; HEPES, 10; NaH2PO4, 1.2; glucose, 5.6; L-glutamine, 2; taurine, 5. The heart was then removed from the perfusion column. The ventricles were minced, gently agitated and rinsed. Isolated myocytes were collected and incubated in Media 199 (GIBCO, Grand Island, NY, USA) containing 1.8 mM Ca2+ and 0, 50, 100 and 500 μM of MnCl2, respectively, for 1 h. The temperature and pH were maintained at 37°C and pH 7.2–7.4, respectively.
Myocytes were placed in a glass-bottomed Petri dish on the stage of an Olympus IX71 inverted fluorescence microscope (Olympus America, Center Valley, PA, USA). Myocyte contractility was evaluated by calculating the maximum fractional shortening from changes in cell length during 1-Hz stimulation using a Grass stimulator (Grass Technologies). To measure Ca2+ transients, myocytes were incubated at 37 °C with 1 μM fura-2-acetoxymethyl ester for 15 min, and the extra dye was washed out. The dye was excited at 340 and 380 nm using a xenon arc lamp through a computer-controlled high-speed random access monochromator (Photon Technology International, Birmingham, NJ, USA). The fluorescent signals were detected at 510 nm by an analog/photon counting photomultiplier detector (Photon Technology International). Ca2+ transients were calculated as the ratio of the detected fluorescence in response to 340 and 380-nm excitation wavelengths (F340/F380), respectively, using in-house-developed MATLAB software.
Statistical analysis
All the data are expressed as the mean ± standard deviation. Mean values in the CNTL, SEAin and SEAout groups were compared by one-way analysis of variance. If there were statistical differences, multiple pairwise comparisons were performed using Tukey’s test. P < 0.05 was considered to be statistically significant.
RESULTS
Impact of Mn2+ on myocyte shortening and Ca2+ transients
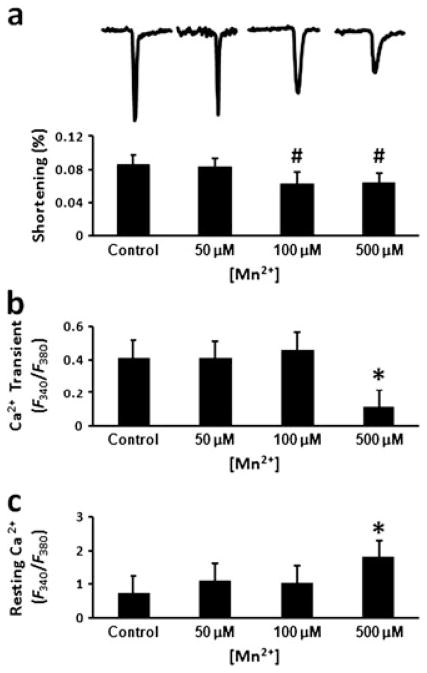
At a concentration of 50 μM, Mn2+ had no obvious impact on myocyte contractility and Ca2+ handling (Fig. 1). Both myocyte shortening and Ca2+ transients were similar to those of the control myocytes [p = not significant]. At 100 μM Mn2+ concentration, peak Ca2+ transients remained normal. However, myocyte shortening was decreased significantly from 8.65 ± 1.15% without Mn2+ to 6.21 ± 1.43% (p < 0.005). At 500 μM Mn2+ concentration, both myocyte shortening and peak Ca2+ transients were significantly reduced (p < 0.001). In addition, the baseline fluorescence ratio increased significantly (p < 0.001).
Figure 1.
Manganese (Mn2+) toxicity on isolated myocytes. (a) Representative recordings of changes in myocyte length during electrical stimulation and the calculated fractional shortening. (b) Peak calcium (Ca2+) transients. (c) Resting intracellular Ca2+ concentration. #p < 0.005 compared with the control; *p <0.0001 compared with the other groups.
Animal characteristics and contractile function
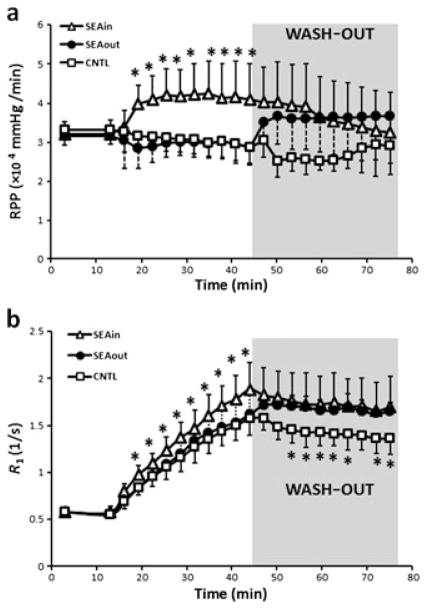
The age, body weight and heart weight of the animals are listed in Table 1. There were no significant differences among the three groups. Figure 2a shows the time course of RPP changes during the imaging protocol. Ventricular pressure and RPP before Mn2+ perfusion (baseline), as well as during Mn2+ wash-in and wash-out, are listed in Table 2. Mn2+ perfusion showed no impact on ventricular function. However, NCX inhibition with SEA0400 induced a significant increase in both LVSP and LVEDP, with LVSP increased to a greater extent (p < 0.001). As a result, both LVDP and RPP increased significantly in the SEAin and SEAout groups when compared with the controls (p < 0.001).
Table 1.
Animal characteristics
| Age (weeks) | Body weight (g) | Heart weight (g) | |
|---|---|---|---|
| Control (n = 5) | 8.89 ± 0.91 | 317.20 ± 22.13 | 1.54 ± 0.12 |
| SEA0400 wash-in (n = 6) | 9.40 ± 0.76 | 334.01 ± 16.87 | 1.58 ± 0.27 |
| SEA0400 wash-out (n = 6) | 9.86 ± 0.62 | 332.33 ± 26.55 | 1.66 ± 0.27 |
Figure 2.
Time courses of rate–pressure product (RPP) (a) and relaxation rate (R1) (b) during imaging protocol. Shaded areas indicate the wash-out period. Sodium–calcium (Na+–Ca2+) exchanger (NCX) inhibition was induced by 1 μM SEA0400 during either manganese (Mn2+) perfusion (SEAin) or wash-out (SEAout). *p < 0.05 compared with control (CNTL).
Table 2.
Ventricular function during the imaging protocol
| Baseline | Wash-in | Wash-out | ||
|---|---|---|---|---|
| Control (n = 5) | LVSP (mmHg) | 90.58 ± 5.85 | 84.16 ± 10.81 | 74.95 ± 8.30b |
| LVEDP (mmHg) | 1.25 ± 1.68 | 3.04 ± 1.83 | 3.45 ± 1.30 | |
| LVDP (mmHg) | 88.40 ± 6.97 | 81.96 ± 10.61 | 71.40 ± 8.70b | |
| RPP (×104 mmHg/min) | 3.32 ± 0.20 | 3.06 ± 0.39 | 2.60 ± 0.32b | |
| SEA0400 wash-in (n = 6) | LVSP (mmHg) | 90.94 ± 8.25 | 113.20 ± 19.10a | 102.09 ± 26.45a |
| LVEDP (mmHg) | 2.92 ± 0.79 | 1.73 ± 1.44a | 1.50 ± 1.61a | |
| LVDP (mmHg) | 87.97 ± 9.02 | 111.47 ± 19.72a | 100.59 ± 26.84a | |
| RPP (×104 mmHg/min) | 3.21 ± 0.33 | 4.07 ± 0.72a | 3.67 ± 0.98a | |
| SEA0400 wash-out (n = 6) | LVSP (mmHg) | 88.68 ± 5.25 | 84.94 ± 10.83 | 101.51 ± 22.07a |
| LVEDP (mmHg) | 1.19 ± 2.07 | 2.78 ± 1.71 | 1.18 ± 2.38a | |
| LVDP (mmHg) | 86.58 ± 5.75 | 81.12 ± 11.62 | 99.31 ± 23.39a | |
| RPP (×104 mmHg/min) | 3.16 ± 0.21 | 2.96 ± 0.42 | 3.61 ± 0.86a |
LVDP, left ventricular developed pressure; LVEDP, left ventricular end-diastolic pressure; LVSP, left ventricular systolic pressure; RPP, rate–pressure product.
p < 0.001 compared with the control group at the same time point.
p < 0.05 compared with baseline in the same group.
T1 changes during wash-in and wash-out
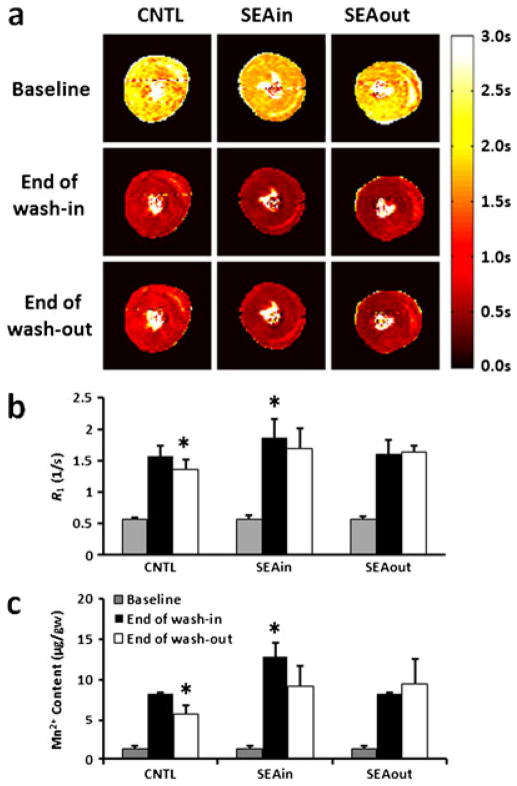
Figure 3a shows representative T1 maps at baseline, at the end of Mn2+ wash-in and at the end of the wash-out period. The time courses of R1 changes during the imaging protocol are shown in Fig. 2b. All three groups showed a progressive increase in R1 during the wash-in period. However, the SEAin group exhibited an accelerated increase in R1. After 3 min of Mn2+ perfusion, R1 in the SEAin group was significantly higher than that in the other two groups (p < 0.05). At the end of Mn2+ wash-in, the R1 values were 1.61 ± 0.18, 1.88 ± 0.28 and 1.62 ± 0.21 s−1 for the CNTL, SEAin and SEAout groups, respectively (Fig. 3b).
Figure 3.
Longitudinal relaxation time (T1), rate (R1) and manganese (Mn2+) content. (a) Representative T1 maps before Mn2+ perfusion (baseline), at the end of Mn2+ wash-in and at the end of wash-out. Relaxation rate (R1) (b) and Mn2+ content (c) at the corresponding time points. Sodium–calcium (Na+–Ca2+) exchanger (NCX) inhibition was induced by 1 μM SEA0400 during either Mn2+ perfusion (SEAin) or wash-out (SEAout). *p < 0.05 compared with the other two groups at the same time points. CNTL, control.
All three groups showed a slight decrease in R1 during the wash-out period (Fig. 2b). The R1 decrease was smallest in the SEAout group. At the end of wash-out, R1 in both the SEAout and SEAin groups was significantly higher than that in the CNTL group (Fig. 3b, p <0.05). The R1 values at the end of wash-out were 1.36 ± 0.17, 1.70 ± 0.32 and 1.65 ± 0.10 s−1 for CNTL, SEAin and SEAout groups, respectively.
The wash-out curves were fitted to two exponential functions, i.e. y = A e−t/B and y = A e−t/B + C, respectively. The fitted parameters are listed in Table 3. Using a simple exponential curve fitting (y = A e−t/B), the mean half-times for R1 reduction were 3.18, 4.25 and 6.66 h for the CNTL, SEAin and SEAout groups, respectively. Using an expoenntial function with a constant term (y = A e−t/B + C), the fitted time constant was reduced significantly for all three groups. The C/A ratio ranged from 5.87 to 10.71.
Table 3.
Time constants of R1 decrease during the wash-out period
|
y = A e−t/B
|
y = A e−t/B + C
|
||||||
|---|---|---|---|---|---|---|---|
| Control | SEA0400 wash-in | SEA0400 wash-out | Control | SEA0400 wash-in | SEA0400 wash-out | ||
| A | 1.63 | 1.80 | 1.72 | A | 0.24 | 0.16 | 0.23 |
| B (h) | 4.57 | 6.09 | 9.62 | B (h) | 0.42 | 0.22 | 1.07 |
| C | 1.40 | 1.67 | 1.49 | ||||
| C/A | 5.87 | 10.71 | 6.44 | ||||
| R2 | 0.94 | 0.82 | 0.74 | R2 | 0.96 | 0.88 | 0.74 |
Myocardial Mn2+ content
Consistent with MRI findings, NCX inhibition increased significantly the Mn2+ content in SEAin hearts (12.9 ± 1.6 μg/g wet weight) at the end of wash-in when compared with controls (8.2 ± 0.2 μg/g wet weight) (Fig. 3c, p <0.001). Perfusion with Mn2+-free buffer caused a significant decrease in myocardial Mn2+ content at the end of wash-out in CNTL hearts (6.3 ± 0.9 μg/g wet weight, p < 0.005). NCX inhibition led to increased Mn2+ retention in the SEAout group (9.5 ± 3.0 μg/g wet weight) when compared with the CNTL group (Fig. 3c, p < 0.05). As a result of increased Mn2+ accumulation during the wash-in period, SEAin hearts also showed elevated Mn2+ content at the end of wash-out (9.1 ± 2.5 μg/g wet weight) when compared with the CNTL group (Fig. 3c, p < 0.05).
DISCUSSION
The major findings of the present study were that, in perfused rat hearts, the Mn2+ content and T1 mapping with MEMRI were dependent on SEA0400 and thus, presumably, on NCX activity. Previously, Waghorn et al. (15,16) have investigated Mn2+ retention in an in vivo mouse study. They observed that treatment with SEA0400 reduced significantly the rate of decrease in ΔR1 hours after Mn2+ infusion was withdrawn. In the current study, we used a fast T1 mapping method to follow the dynamic changes in R1 during Mn2+ perfusion and wash-out. Our results suggest that altered Mn2+ efflux via NCX is reflected in R1 changes during both wash-in and wash-out.
Physiologically, Ca2+ entry into myocytes via L-type Ca2+ channels is balanced by its efflux through NCX (1). Although Mn2+ also enters the myocytes through L-type Ca2+ channels, its efflux via NCX has been considered to be negligible because of the long Mn2+ retention time. However, recent studies by Waghorn et al. (15,16) have shown that differences in R1 changes induced by NCX inhibition can be observed 4 h after Mn2+ withdrawal. With NCX inhibition, these authors observed an increase in the half-time of ΔR1 reduction from 3.4 h (without SEA0400) to 5.6 h (with SEA0400) in mouse hearts. In the current study, the estimated half-time for R1 reduction increased from 3.18 h with-out SEA0400 to 6.66 h in the presence of SEA0400, which was similar to that reported by Waghorn et al. The average half-time for R1 reduction in the SEAin group (4.25 h) was also higher than that in the controls because of the incomplete elimination of the inhibitor during the wash-out period (19). Consistent with the MRI findings, the Mn2+ content at the end of the wash-out period was also significantly higher in hearts perfused with SEA0400 than in the control group.
It is interesting to note that, when the wash-out curves were fitted to an exponential function with a constant term, the fitted time constant was significantly reduced by an order of magnitude (Table 3). Moreover, the ratio of the constant term to the exponential term (C/A) was more than five-fold in all three groups, which may suggest the existence of a large Mn2+ pool that is washed away very slowly.
With NCX inhibition by SEA0400, the R1 values at the end of the wash-in period increased in the SEAin group when compared with hearts without the inhibitor (SEAout and CNTL groups), suggesting increased Mn2+ accumulation in the presence of the NCX inhibitor during the wash-in period. Atomic absorption spectroscopy analysis of hearts at the end of the wash-in period also showed that the Mn2+ content in the SEAin group increased by 57% compared with the other two groups. As SEA0400 does not enhance Ca2+ channel activity under normal physiological conditions (20), this increased Mn2+ accumulation presumably reflects reduced Mn2+ efflux via NCX. These data suggest that MEMRI with fast T1 mapping may provide a tool for the evaluation of NCX activity.
The contribution of NCX to Ca2+ extrusion from myocytes is species dependent (21,22). Sham et al. (21) evaluated the rates of Ca2+ removal in isolated myocytes from rat, guinea-pig, hamster ventricles and human atria. Their study suggests that NCX activity in the rat ventricle is lower than that in hamsters, guinea-pigs and humans. As such, larger animals and humans may manifest a greater Mn2+ efflux rate than that observed in rats in the current study. However, careful further investigations are necessary to evaluate whether this difference in NCX contribution to Ca2+ extrusion will render MEMRI more sensitive to alterations in NCX activity in large animals and humans.
The inhibition of NCX also induced a small increase in LVDP. This result is consistent with previous studies on perfused rat hearts (23,24). Previously, Acsai et al. (20) have reported an increase in myocyte shortening in isolated rat myocytes incubated with SEA0400. In addition, they observed an increase in Ca2+ transients and a trend of increase in diastolic Ca2+ level. However, this increase in cytosolic Ca2+ was not accompanied by an increase in the L-type Ca2+ current. Therefore, the observed increase in Ca2+ transients is probably caused by the blockage of Ca2+ efflux via NCX, leading to increased ventricular contractility.
Because both Mn2+ uptake and efflux can have an impact on the dynamics of R1 during Mn2+ infusion, care must be taken in interpreting the data. In general, the determination of both influx and efflux rates from a single wash-in curve is underdetermined unless other constraints are imposed in parameter estimation. In the current experimental settings, the measured R1 dynamics during wash-out can provide additional data to constrain curve fitting. For in vivo studies, the measurement of the Mn2+ content in blood, i.e. the arterial input function, can also provide additional constraints for curve fitting. However, such an approach requires fast and accurate T1 mapping in both myocardium and blood.
In summary, we have investigated the sensitivity of MEMRI to NCX inhibition via SEA0400. Our results show that, in rat hearts, MEMRI with fast T1 mapping is sensitive to SEA0400-dependent changes in Mn2+ accumulation and retention. Although these results suggest that MEMRI has the potential to detect altered NCX activity, further investigation is needed to assess the sensitivity of MEMRI to more subtle changes in NCX activity under disease conditions.
Acknowledgments
This work was supported by grants from the National Heart, Lung and Blood Institute (R01 HL-73315 and HL-86935 X. Yu).
Abbreviations used
- EC
excitation–contraction
- HR
heart rate
- KH
Krebs–Henseleit
- LVDP
left ventricular developed pressure
- LVEDP
left ventricular end-diastolic pressure
- LVSP
left ventricular systolic pressure
- MEMRI
manganese-enhanced MRI
- NCX
Na+–Ca2+ exchanger
- RPP
rate–pressure product
- SR
sarcoplasmic reticulum
References
- 1.Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 2.Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassani RA, Bers DM. Na–Ca exchange is required for rest-decay but not for rest-potentiation of twitches in rabbit and rat ventricular myocytes. J Mol Cell Cardiol. 1994;26:1335–1347. doi: 10.1006/jmcc.1994.1152. [DOI] [PubMed] [Google Scholar]
- 4.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium–calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 5.Guatimosim S, Guatimosim C, Song LS. Imaging calcium sparks in cardiac myocytes. Methods Mol Biol. 2011;689:205–214. doi: 10.1007/978-1-60761-950-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichols CB, Rossow CF, Navedo MF, Westenbroek RE, Catterall WA, Santana LF, McKnight GS. Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ Res. 2010;107:747–756. doi: 10.1161/CIRCRESAHA.109.216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi BR, Salama G. Simultaneous maps of optical action potentials and calcium transients in guinea-pig hearts: mechanisms underlying concordant alternans. J Physiol. 2000;529(Pt 1):171–188. doi: 10.1111/j.1469-7793.2000.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wendland MF. Applications of manganese-enhanced magnetic resonance imaging (MEMRI) to imaging of the heart. NMR Biomed. 2004;17:581–594. doi: 10.1002/nbm.943. [DOI] [PubMed] [Google Scholar]
- 9.Mendonca-Dias MH, Gaggelli E, Lauterbur PC. Paramagnetic contrast agents in nuclear magnetic resonance medical imaging. Semin Nucl Med. 1983;13:364–376. doi: 10.1016/s0001-2998(83)80048-8. [DOI] [PubMed] [Google Scholar]
- 10.Hunter DR, Haworth RA, Berkoff HA. Cellular manganese uptake by the isolated perfused rat heart: a probe for the sarcolemma calcium channel. J Mol Cell Cardiol. 1981;13:823–832. doi: 10.1016/0022-2828(81)90239-x. [DOI] [PubMed] [Google Scholar]
- 11.Brurok H, Schjott J, Berg K, Karlsson JO, Jynge P. Manganese and the heart: acute cardiodepression and myocardial accumulation of manganese. Acta Physiol Scand. 1997;159:33–40. doi: 10.1046/j.1365-201X.1997.d01-841.x. [DOI] [PubMed] [Google Scholar]
- 12.Medina DC, Kirkland DM, Tavazoie MF, Springer CS, Jr, Anderson SE. Na+/Ca2+-exchanger-mediated Mn2+-enhanced 1H2O MRI in hypoxic, perfused rat myocardium. Contrast Media Mol Imaging. 2007;2:248–257. doi: 10.1002/cmmi.151. [DOI] [PubMed] [Google Scholar]
- 13.Hu TC, Pautler RG, MacGowan GA, Koretsky AP. Manganese-enhanced MRI of mouse heart during changes in inotropy. Magn Reson Med. 2001;46:884–890. doi: 10.1002/mrm.1273. [DOI] [PubMed] [Google Scholar]
- 14.Krombach GA, Saeed M, Higgins CB, Novikov V, Wendland MF. Contrast-enhanced MR delineation of stunned myocardium with administration of MnCl2 in rats. Radiology. 2004;230:183–190. doi: 10.1148/radiol.2301020228. [DOI] [PubMed] [Google Scholar]
- 15.Waghorn B, Yang Y, Baba A, Matsuda T, Schumacher A, Yanasak N, Hu TC. Assessing manganese efflux using SEA0400 and cardiac T1-mapping manganese-enhanced MRI in a murine model. NMR Biomed. 2009;22:874–881. doi: 10.1002/nbm.1414. [DOI] [PubMed] [Google Scholar]
- 16.Waghorn B, Schumacher A, Liu J, Jacobs S, Baba A, Matsuda T, Yanasak N, Hu TC. Indirectly probing Ca2+ handling alterations following myocardial infarction in a murine model using T1-mapping manganese-enhanced magnetic resonance imaging. Magn. Reson. Med. 2011;65:239–249. doi: 10.1002/mrm.22597. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda T, Arakawa N, Takuma K, Kishida Y, Kawasaki Y, Sakaue M, Takahashi K, Takahashi T, Suzuki T, Ota T, Hamano-Takahashi A, Onishi M, Tanaka Y, Kameo K, Baba A. SEA0400, a novel and selective inhibitor of the Na+–Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J. Pharmacol. Exp. Ther. 2001;298:249–256. [PubMed] [Google Scholar]
- 18.Li W, Griswold M, Yu X. Rapid T1 mapping of mouse myocardium with saturation recovery Look–Locker method. Magn. Reson. Med. 2010;64:1296–1303. doi: 10.1002/mrm.22544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwamoto T, Kita S, Uehara A, Imanaga I, Matsuda T, Baba A, Katsuragi T. Molecular determinants of Na+/Ca2+ exchange (NCX1) inhibition by SEA0400. J. Biol. Chem. 2004;279:7544–7553. doi: 10.1074/jbc.M310491200. [DOI] [PubMed] [Google Scholar]
- 20.Acsai K, Kun A, Farkas AS, Fulop F, Nagy N, Balazs M, Szentandrassy N, Nanasi PP, Papp JG, Varro A, Toth A. Effect of partial blockade of the Na+/Ca2+-exchanger on Ca2+ handling in isolated rat ventricular myocytes. Eur J Pharmacol. 2007;576:1–6. doi: 10.1016/j.ejphar.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 21.Sham JS, Hatem SN, Morad M. Species differences in the activity of the Na+–Ca2+ exchanger in mammalian cardiac myocytes. J Physiol. 1995;488(Pt 3):623–631. doi: 10.1113/jphysiol.1995.sp020995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka H, Namekata I, Nouchi H, Shigenobu K, Kawanishi T, Takahara A. New aspects for the treatment of cardiac diseases based on the diversity of functional controls on cardiac muscles: diversity in the excitation–contraction mechanisms of the heart. J Pharmacol Sci. 2009;109:327–333. doi: 10.1254/jphs.08r22fm. [DOI] [PubMed] [Google Scholar]
- 23.Farkas AS, Acsai K, Nagy N, Toth A, Fulop F, Seprenyi G, Birinyi P, Nanasi PP, Forster T, Csanady M, Papp JG, Varro A, Farkas A. Na+/Ca2+ exchanger inhibition exerts a positive inotropic effect in the rat heart, but fails to influence the contractility of the rabbit heart. Br J Pharmacol. 2008;154:93–104. doi: 10.1038/bjp.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szentandrassy N, Birinyi P, Szigeti G, Farkas A, Magyar J, Toth A, Csernoch L, Varro A, Nanasi PP. SEA0400 fails to alter the magnitude of intracellular Ca2+ transients and contractions in Langendorff-perfused guinea pig heart. Naunyn Schmiedebergs Arch. Pharmacol. 2008;378:65–71. doi: 10.1007/s00210-008-0296-5. [DOI] [PubMed] [Google Scholar]