Abstract
Objective
Tailoring to psychological constructs (e.g. self-efficacy, readiness) motivates behavior change, but whether knowledge tailoring alone changes healthcare preferences - a precursor of behavior change in some studies - is unknown. We examined this issue in secondary analyses from a randomized controlled trial of a tailored colorectal cancer (CRC) screening intervention, stratified by ethnicity/language subgroups (Hispanic/Spanish, Hispanic/English, non-Hispanic/English).
Methods
Logistic regressions compared effects of a CRC screening knowledge-tailored intervention versus a non-tailored control on preferences for specific test options (fecal occult blood or colonoscopy), in the entire sample (N = 1164) and the three ethnicity/language subgroups.
Results
Pre-intervention, preferences for specific tests did not differ significantly between study groups (experimental, 64.5%; control 62.6%). Post-intervention, more experimental participants (78.6%) than control participants (67.7%) preferred specific tests (P <0.001). Adjusting for pre-intervention preferences, more experimental group participants than control group participants preferred specific tests post-intervention [average marginal effect (AME) = 9.5%, 95% CI 5.3-13.6; P <0.001]. AMEs were similar across ethnicity/language subgroups.
Conclusion
Knowledge tailoring increased preferences for specific CRC screening tests across ethnic and language groups.
Practice Implications
If the observed preference changes are found to translate into behavior changes, then knowledge tailoring alone may enhance healthy behaviors.
1. Introduction
Colorectal cancer (CRC) screening reduces CRC mortality,[1] but screening is under-utilized.[2] CRC screening disparities also exist: Hispanic persons are particularly under-screened, mostly attributable to language barriers.[2, 3] Thus, developing effective ways to motivate more individuals to undergo CRC screening and mitigating ethnic screening disparities are important public health priorities.[4]
Patient preference has been shown to be a precursor of various health behaviors, including CRC screening behavior.[5-9] Helping patients formulate preferences may be particularly important in the context of CRC screening, since several evidence-based test options are available.[1, 5] Evidence also suggests tailored interventions are more effective than non-tailored interventions in encouraging adoption of health behaviors, including CRC screening.[10-21] However, whether simply providing information tailored to patient knowledge regarding CRC screening is sufficient to increase expressed preference for a specific test option is unknown. Health behavior theories underpinning tailored interventions suggest that tailoring to constructs beyond knowledge - such as social influence, self-efficacy, perceived barriers, and stage of readiness - may be necessary to influence patient preferences.[22] However, no empirical studies have addressed this question. Tailoring to knowledge is simpler conceptually and logistically than tailoring to such behavioral constructs.[23] Thus, if knowledge tailoring alone were sufficient to influence patient CRC screening preferences (and, in turn, screening behavior), it could have important implications for increasing CRC screening uptake, and for the design and expanded use of tailored interventions.
Also, based on health behavior and communication theory, tailored interventions may have the potential to mitigate socio-demographic disparities in health behaviors.[24] However, prior studies were not designed to address whether tailoring effects are comparable across ethnic and language groups. This is an important question, given disparities in CRC screening and other health behaviors and outcomes affecting ethnic minorities in the U.S.[2, 3, 25]
To address these issues, we employed data from an ongoing multicenter, randomized controlled trial (RCT) of an experimental interactive multimedia computer program (IMCP) tailored to patients’ knowledge regarding behaviorally-relevant CRC screening issues: evidence-based test options, potential harms, and common inconveniences. Randomization was stratified by ethnicity (Hispanic versus non-Hispanic) and language (Spanish versus English), and the trial was powered to examine ethnicity and language effects. We hypothesized that the experimental knowledge-tailored intervention would be more effective than the control non-tailored intervention in encouraging formulation of a preference for a specific CRC screening test (either fecal occult blood testing [FOBT] or colonoscopy). We also hypothesized that the effects of knowledge tailoring on test preference would not differ by ethnicity or language.
2. Methods
Study activities were conducted from February 1, 2010 through October 31, 2011. The Institutional Review Boards at all participating sites approved the study. The study was registered on ClinicalTrials.gov (Identifier: NCT00786747).
2.1. Study setting, sample recruitment, and randomization
A convenience sample of patients aged 50-75 years who were not up to date for CRC screening was recruited from primary care offices at five centers: greater Sacramento area, California (10 offices, 9 within a university-affiliated network); the Bronx, New York (1 federally qualified health-center [FQHC]); Rochester, New York (2 FQHCs and 1 hospital-based practice); San Antonio, Texas (2 FQHCs and 2 private practices); and Colorado (1 FQHC near Denver, and 7 offices within a FQHC system in the rural San Luis Valley, 200 miles south of Denver). Consistent with evidence-based test option recommendations,[1] and prevailing expert opinion regarding testing intervals,[26] patients were considered up to date for CRC screening (and ineligible for the study) if they had one or more of the following documented in their records: FOBT within one year; flexible sigmoidoscopy (FS) within five years; or colonoscopy within 10 years. At all but one of the study sites (see below) determination of initial eligibility was based on review of electronic or paper medical records.
Persons meeting initial eligibility criteria were contacted by study personnel fluent in both English and Spanish, primarily via telephone, to solicit their participation. Mailed study announcements in both languages, including a phone number to contact recruitment staff, were employed as a secondary recruitment method. During phone calls patients were asked whether they had received FOBT, FS, and/or colonoscopy within the previously specified time intervals to help detect testing not documented in medical records (e.g. occurring in another health system). Those reporting such testing were excluded from study participation. Patients reporting no such testing were evaluated for the following additional eligibility criteria: ability to speak and read English or Spanish; and adequate eyesight, hearing, and hand function to use a touch screen computer program. Eligible patients agreeing to participate were asked to arrive one hour before an upcoming appointment they had scheduled with their primary care provider for any reason (i.e. not a special study appointment). The early arrival was necessary to allow completion of informed consent and the pre-visit portion of the study interventions.
At the FQHC in the Bronx, advance medical record review and phone calls were not feasible. Recruitment personnel at this office approached patients in the waiting area, prior to their appointments, and determined study eligibility based on patient self-report.
Patients were provided with touch screen notebook computers for use before and after their index primary care office visits. Prior to the visit, research assistants logged the patients into the study software program via a unique study identifier, and showed them how to navigate the program using the touch screen. After answering initial program questions regarding their ethnicity (Hispanic or non-Hispanic) and preferred language for software use (English or Spanish), patients were randomly assigned by the software to receive either the tailored experimental or non-tailored control software program in their preferred language. Randomization was stratified by ethnicity and software use language, and implemented in blocks of 10 within each stratum, using a random number generation program. The software program did not notify patients of their randomly assigned study group, and in-office research assistants were also unaware. Subjects received a $20 gift card or $20 in cash for participating.
2.2. Study interventions
2.2.1. IMCP programming and implementation
Experimental and control IMCPs were created collaboratively by the study investigators and experienced programmers with input from the study project managers. Traditional software engineering principles guided development, including phases for requirements, design, prototyping, implementation, integration, component and system testing, and maintenance.[27]
The tailored and non-tailored IMCPs employed a layered architecture based on Lenovo (Lenovo, Morrisville, NC) touch screen laptops, running the Windows XP or Windows 7 operating system (Microsoft Corporation, Renton, WA). Three unmodified open-source software packages were added to the stock operating system: the Apache 2 web server (Apache Software Foundation), PHP 5 web programming language (PHP Group), and MySQL 5 database server (Oracle Corporation, Redwood Shores, CA). Experimental and control IMCP questionnaires were developed in LimeSurvey 1.85 (Black Duck Software, Burlington, MA), with customization to support background computerized block randomization and create administrative (e.g. data export) functions for use by research staff.
The experimental and control IMCPs operated within the Google Chrome web browser (Google Incorporated, Mountain View, CA). Subjects navigated through both programs using a touch screen interface on the dedicated study laptops provided. In both programs users had the option of listening to audio narration of on-screen text, accessed by touching icons next to the text. Patients’ questionnaire responses were automatically stored in a MySQL database.
English and Spanish text and audio narration within the program was written as simply as possible, with most passages having a Flesch-Kincaid 6th grade reading level.[28] Initially the study investigators created English language versions of the experimental and control program materials. These materials were reviewed by plain language specialists (Transcend Translations, Davis, CA) who provided suggestions to improve their readability for lay audiences. After incorporating these suggestions into the English materials, initial Spanish translations were developed by certified health translators, and then edited by a certified linguist and a literacy specialist, to ensure language matched the desired reading grade level and typical conversational word choices. The resulting translations were reviewed by several members of the Sacramento area Latino community, to ensure they would meet the needs of individuals without a medical background. After incorporating feedback from the community reviewers, initial versions of the Spanish IMCPs were programmed and pilot tested with a small convenience sample of patients at each study center. Patient feedback was incorporated in the finalized Spanish language IMCPs.
2.2.2. Experimental (tailored) intervention
Development of the algorithm for tailoring to CRC screening knowledge in the experimental IMCP was guided by a previously described approach.[29] The experimental IMCP allowed patients to “self-tailor” the amounts of CRC screening knowledge information they received, in accordance with research demonstrating differences among individuals in the amount of health information desired, and in the cognitive and emotional impact of varying amounts of information.[30, 31] The program content focused on FOBT and colonoscopy, since other tests were not routinely offered at the participating offices.
Experimental group patients first answered a question regarding their CRC screening preference. Next, the program presented a brief tailored message acknowledging the preference as reasonable, while encouraging the patient to remain open to information about other options.
After answering questions regarding other baseline characteristics, patients were presented with a block of questions assessing their knowledge of recommended (i.e. evidence-based) CRC screening tests. The software then provided a brief feedback message individually tailored to participant answers, reinforcing correct responses and gently clarifying incorrect responses. Within the message patients were invited to touch hyperlinked text to view detailed optional information if they desired. Opening the optional information section automatically created an entry in a background event log, invisible to users. The optional information also addressed the potential harms and inconveniences of screening and of the individual tests. Participants then answered a second block of knowledge questions dealing with the potential harms and common inconveniences of screening, followed again by a brief feedback message tailored to their answers with the option to view more detailed information regarding these issues. After completing the second block of knowledge questions and receiving tailored feedback, patients again answered the CRC screening preference question.
2.2.3. Control (non-tailored “electronic leaflet”) intervention
Patients randomly assigned to the control IMCP answered the same CRC screening preference and knowledge questions as those in the tailored arm, but received no feedback regarding their answers. After the knowledge questions, control patients were presented with general CRC screening information developed by the National Cancer Institute: http://www.cancer.gov/cancertopics/factsheet/detection/colorectal-screening. After viewing this information control patients again answered the CRC screening preference question.
2.3. Measures
2.3.1. CRC screening preference
CRC screening preference was assessed pre- and post-intervention with a single item.[21] Response options were: “I do not want to be screened for colon cancer;” “I prefer stool blood testing;” “I prefer colonoscopy;” “I prefer to have colon cancer screening with a test not listed here;” and “I want to be screened for colon cancer but have no preference regarding which test is used.”
2.3.2. Other measures
Knowledge regarding recommended CRC screening test options was measured with three items, and knowledge regarding potential harms and inconveniences of screening with six items.[21] As noted previously, in the experimental group the latter six items were answered after mandatory tailored and optional non-tailored information had been presented. Example items asked whether digital rectal examination is a recommended test option (false); whether one risk of screening is a false finding, leading to unnecessary testing (true); and whether a common inconvenience of colonoscopy is the need for someone to drive you home after the test (true). Response options for all knowledge items were “true” (1 point) or “false” or “don’t know” (zero points).
The remaining measures were included to verify that the intervention groups were well-matched on characteristics that could influence CRC screening preference.
Socio-demographic characteristics. In addition to ethnicity (Hispanic or non-Hispanic) and software use language (Spanish or English), other characteristics included: age; gender; race (White, Black, or Other); income (<$10,000, $10,000 to <$15,000, $15,000 to <$25,000, $25,000 to <$50,000, or >$50,000); and education level (less than high school, some high school, high school graduate, some college, or college graduate.
Health-related characteristics. Health status was measured with the SF-12 Mental Component Summary and Physical Component Summary scores (range 0-100, higher scores = better health).[32] Health literacy was assessed using a single item: “How often do you need to have someone help you when you read instructions, pamphlets, or other written material from your doctor or pharmacy?” (five response options, 1 = never to 5 = always).[33] Two items assessed whether a health care provider had ever recommended CRC screening via FOBT or colonoscopy (“yes” versus “no” or “don’t know”). Duration of the current primary care provider-patient relationship was also assessed (< 1 year, 1-2 years, 3-5 years, > 5 years, or unsure).
Behavioral characteristics. Patients indicated whether they had ever undergone FOBT or colonoscopy (“yes” versus “no” or “don’t know”). Patient activation was assessed using the validated 13-item Patient Activation Measure.[34, 35] Respondents indicated the degree to which they agreed or disagreed with each of 13 statements. Items were summed and averaged, yielding a mean score of 1-4 (higher scores = greater activation; Cronbach’s α in this sample = 0.89). Five Factor Model personality factors were measured using the Big Five Inventory.[36-38] Respondents indicated their degree of disagreement or agreement with a series of 44 statements. Items related to each personality factor were summed and averaged, yielding a mean factor score of 1-5 (higher scores = higher standing). Cronbach’s alphas in this study were: Agreeableness, 0.74; Conscientiousness, 0.73; Extraversion, 0.70; Neuroticism, 0.75; Openness, 0.70. Patient satisfaction with randomly assigned study software was assessed using a five item scale (score range 1-5, higher scores = greater satisfaction).
2.4. Data analysis
Data were analyzed using Stata version 12.1 (Stata Corporation, College Station, TX). Descriptive comparisons used chi squared tests (categorical variables) and t-tests (continuous variables). The primary analyses used logistic regression to examine the effects of the key predictor (experimental versus control intervention) on post-intervention CRC screening preference, the dependent variable, dichotomized as preference for a specific test (FOBT or colonoscopy) versus no specific test preference. The base regression model adjusted only for pre-intervention test preference. A second model added ethnicity/language subgroups as well as pre-intervention specific preference*ethnicity/language and experimental intervention*ethnicity language interaction terms to the base model. None of the regressions were adjusted for other variables, since the intervention groups were well-matched on other characteristics (Table 1). Secondary analyses examined the effects of viewing versus not viewing the optional information. This information concerned test options, harms, and inconveniences and was available only to participants viewing the IMCP, following the first block of knowledge questions.
Table 1.
Characteristics of participants by study intervention group
| Study intervention group | P valuea | ||
|---|---|---|---|
| Control N = 569 |
Experimental N = 595 |
||
| Patient enrollment by site, % | 0.99 | ||
| Denver, Colorado | 15.6 | 16.0 | |
| Bronx, New York | 24.0 | 24.1 | |
| Rochester, New York | 20.9 | 21.0 | |
| Sacramento, California | 21.6 | 22.2 | |
| San Antonio, Texas | 17.9 | 16.7 | |
| Socio-demographics | |||
| Age, mean (SD) | 57.1 (6.2) | 57.0 (6.1) | 0.90 |
| Female, % | 65.8 | 65.0 | 0.77 |
| Spanish language version of software, % | 23.5 | 23.4 | 0.96 |
| Ethnicity/race category, % | 0.87 | ||
| Hispanic (any race) | 51.5 | 49.7 | |
| Non-Hispanic | |||
| White | 20.7 | 21.0 | |
| Black | 23.0 | 24.9 | |
| Other race | 4.7 | 4.4 | |
| Education level, % | 0.36 | ||
| Less than high school | 15.8 | 19.0 | |
| Some high school | 21.5 | 18.1 | |
| High school graduate | 25.1 | 23.8 | |
| Some college | 18.5 | 20.9 | |
| College graduate | 19.0 | 18.3 | |
| Income level, % | 0.69 | ||
| <$10,000 | 33.2 | 35.0 | |
| $10,000 to <$15,000 | 18.9 | 17.8 | |
| $15,000 to <$25,000 | 17.8 | 14.7 | |
| $25,000 to <$50,000 | 14.5 | 15.3 | |
| >$50,000 | 15.6 | 17.2 | |
| Health-related characteristics | |||
| Health status scores, mean (SD) | |||
| SF-12 Physical Component Summary | 42.9 (11.1) | 42.1 (11.7) | 0.31 |
| SF-12 Mental Component Summary | 45.4 (11.4) | 45.5 (11.3) | 0.88 |
| Health literacy score (range 1-5), mean (SD) | 2.0 (1.2) | 2.1 (1.3) | 0.21 |
| Provider recommendation for FOBT or colonoscopy,% | 43.0 | 45.3 | 0.43 |
| Duration of current primary provider relationship, % | 0.31 | ||
| <1 year | 26.3 | 24.6 | |
| 1-2 years | 18.9 | 16.8 | |
| 3-5 years | 15.6 | 19.9 | |
| >5 years | 36.1 | 36.5 | |
| Unsure/unknown | 3.0 | 2.2 | |
| Behavioral characteristics | |||
| Prior CRC screening, % | |||
| FOBT | 26.5 | 26.9 | 0.88 |
| Colonoscopy | 13.2 | 15.9 | 0.20 |
| Patient activation score (range 1-4), mean (SD) | 3.2 (0.4) | 3.2 (0.4) | 1.0 |
| Personality factor scores (range 1-5), mean (SD) | |||
| Agreeableness | 3.9 (0.5) | 3.9 (0.5) | 0.64 |
| Conscientiousness | 3.7 (0.6) | 3.7 (0.5) | 0.48 |
| Extraversion | 3.3 (0.6) | 3.3 (0.6) | 0.46 |
| Neuroticism | 2.7 (0.7) | 2.7 (0.7) | 0.68 |
| Openness | 3.6 (0.5) | 3.6 (0.5) | 0.23 |
| Satisfaction with study software (range 1-5), mean(SD) | 4.2 (0.5) | 4.3 (0.5) | 0.12 |
| CRC screening knowledge b | |||
| Screening methods (range 0-3), mean (SD) | 1.6 (0.8) | 1.7 (0.8) | 0.42 |
| Harms and inconveniences (range 0-6), mean (SD) | 3.7 (2.1) | 4.3 (2.1) | <0.001 |
Notes: CRC = colorectal cancer; FOBT = fecal occult blood test; SD = standard deviation.
P values are for comparisons between groups (chi-squared test for categorical variables, t-test for continuous variables)
In the experimental group, screening methods questions were answered before any tailored information was presented; harms and inconveniences questions were answered after presentation of some tailored information
We report adjusted odds ratios in the Results text and Table 3. To further facilitate study interpretation, in Table 4, we report adjusted average marginal effects (AMEs) of the intervention;[39] subtracting the control group AME from the experimental group AME yields the absolute percentage point difference in outcome between groups.
Table 3.
Adjusted odds of having a specific CRC screening test preference post-intervention by pre-intervention preference, ethnicity/language group, study intervention, and interactions among these variables
| Logistic regression model variables | Adjusteda OR (95% CI) | P value |
|---|---|---|
| Pre-intervention preference for FOBT or colonoscopy | 15.74 (9.66, 25.62) | <0.001 |
| Ethnicity/language group (ref = Non-Hispanic/English) | ||
| Hispanic/English | 0.89 (0.48, 1.64) | 0.70 |
| Hispanic/Spanish | 1.09 (0.53, 2.25) | 0.81 |
| Ethnicity/language group*pre-intervention preference interaction (ref = Non-Hispanic/English, no specific test preference) | ||
| Hispanic/English, prefer FOBT or colonoscopy | 1.85 (0.79, 4.37) | 0.16 |
| Hispanic/Spanish, prefer FOBT or colonoscopy | 0.75 (0.32, 1.75) | 0.51 |
| Experimental intervention (versus control) | 2.32 (1.49, 3.61) | <0.001 |
| Experimental intervention*ethnicity/language group interaction (ref = Non-Hispanic/English, control intervention) | ||
| Experimental, Hispanic/English | 0.67 (0.30, 1.49) | 0.33 |
| Experimental, Hispanic/Spanish | 0.79 (0.35, 1.79) | 0.58 |
Notes: CI = confidence interval; FOBT = fecal occult blood testing; OR = odds ratio; ref = analytic reference comparison
Table 4.
Adjusted average marginal effects of the study interventions on colorectal cancer screening preference overall, and by ethnicity/language
| Post-intervention proportion preferring FOBT or colonoscopy Adjusteda AME (95% CI) | ||
|---|---|---|
| Control N = 569 |
Experimental N = 595 |
|
| Entire study sample | 0.69 (0.65, 0.72) | 0.78 (0.75, 0.81) |
| Ethnicity/language groups | ||
| Non-Hispanic | 0.68 (0.64, 0.73) | 0.80 (0.76, 0.84) |
| Hispanic/English | 0.70 (0.64, 0.76) | 0.76 (0.70, 0.82) |
| Hispanic/Spanish | 0.67 (0.60, 0.74) | 0.76 (0.69, 0.83) |
Notes: AME = average marginal effect (probability of specific preference); CI = confidence interval; FOBT = fecal occult blood testing
Adjusted for pre-intervention preference
3. Results
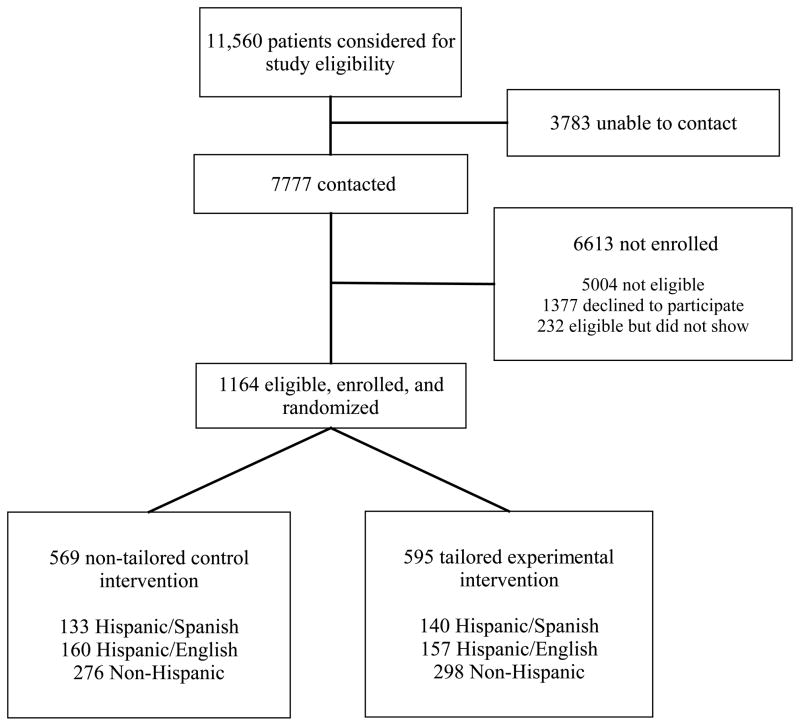
The Figure shows the flow of subjects through the RCT. Table 1 provides a summary of patient characteristics by study group. We enrolled 1164 subjects, 49.3% non-Hispanic, 27.2% Hispanic/English, and 23.4% Hispanic/Spanish. There were no significant study group differences on baseline (pre-intervention) characteristics, or in satisfaction with study software.
Figure.
Flow of patients through the study
Knowledge of CRC screening methods (questions asked before any tailoring) did not differ by intervention group (Table 1). Knowledge of potential harms and inconveniences of CRC screening (assessed following the delivery of some knowledge-tailored information in the experimental group) were significantly higher in the experimental than control groups (Table 1). Within the experimental group, 31.0% viewed the optional information on test options, harms, and inconveniences following the first block of knowledge questions.
Pre- and post-intervention CRC screening preferences are shown by study group in Table 2. Pre-intervention preference for a specific test option (FOBT or colonoscopy) was not statistically different between groups (64.5% experimental and 62.6% control). Post-intervention, 78.6% of experimental group and 67.7% of control group patients preferred a specific test (P <0.001 for group comparison).
Table 2.
Pre- and post-intervention colorectal cancer screening preference by study intervention group
| Colorectal cancer screening preference | Pre-interventiona | Post-interventionb | ||
|---|---|---|---|---|
| Control | Experimental | Control | Experimental | |
| No screening | 8.5 | 7.8 | 7.2 | 6.3 |
| FOBT | 32.6 | 34.3 | 35.4 | 39.5 |
| Colonoscopy | 30.0 | 30.2 | 32.3 | 39.0 |
| Specific test besides FOBT or colonoscopy | 3.2 | 2.2 | 4.2 | 2.5 |
| No specific test preferred | 25.7 | 25.5 | 20.9 | 12.7 |
Notes: FOBT = fecal occult blood testing
P = 0.82 for between group difference pre-intervention, chi-squared test
P <0.001 for between group difference post-intervention, chi-squared test
In a base logistic regression, both pre-intervention preference for FOBT or colonoscopy [adjusted odds ratio [AOR] 16.55, 95% confidence interval [CI] 11.84-23.13; P <0.001] and exposure to the experimental intervention (AOR 2.04, 95% CI 1.48-2.81; P <0.001) were significant independent predictors of post-intervention preference for FOBT or colonoscopy. Table 3 shows the results of a second logistic regression, adding ethnicity/language subgroups as well as pre-intervention specific preference*ethnicity/language and experimental intervention*ethnicity language interaction terms to the base model. In this analysis, significant main effects of pre-intervention preference for a specific test and experimental intervention exposure persisted, while none of the ethnicity/language subgroup or interaction terms were significant.
Table 4, showing the adjusted average marginal effects of the study interventions, reveals the intervention increased the probability of a preference for FOBT or colonoscopy relative to the control group by an absolute 9.5% (95% CI 5.3-13.6; P <0.001).
Secondary analyses revealed that the effect of the experimental intervention was greater in those viewing the optional material (AOR 4.3, 95% CI = 2.6 – 7.0; P < 0.001) than those not viewing the material (AOR 1.5, 95% CI = 1.1 – 2.2; P < 0.001); both AORs use the control group as reference.
Pre-intervention, 77.9% of Hispanic persons using the Spanish software, 65.1% of Hispanic persons using the English software, and 56.0% of non-Hispanic persons preferred FOBT or colonoscopy. Post-intervention, these preferences increased to 82.7%, 78.8%, and 76.4%, respectively. As shown in Table 4, the marginal effects (adjusted for pre-intervention preference) of the experimental intervention were similar among the three ethnicity/language groups. In analyses exploring intervention interaction effects with language and ethnicity on post-intervention preference for FOBT and colonoscopy, the interaction terms were not significant (p all > 0.33; detailed data available upon request).
4. Discussion and conclusion
4.1. Discussion
An IMCP tailored simply to practical knowledge regarding CRC screening was significantly more successful than a non-tailored control IMCP in helping patients to formulate a preference for a specific screening test option (FOBT or colonoscopy), with the effect strongest among (but not limited to) participants viewing optional additional information. The findings suggest that both key elements of the experimental intervention - the personal tailoring of the brief information presented to all experimental group participants, and the optional additional information (“self-tailoring”) – contributed to the effect of the intervention on screening preference. The intervention was similarly effective among Hispanic and non-Hispanic persons, and, within the Hispanic group, among both Spanish and English language software users, despite differences in pre-intervention screening preferences among these groups.
We also found the mean score for the second block of CRC screening knowledge questions in the IMCPs, concerning potential harms and inconveniences of screening, was significantly greater in the tailored intervention group. Echoing its effects on preference, the experimental IMCP likely enhanced knowledge acquisition both via personal tailoring of information and by allowing participants to choose whether to view additional information.[40] A chance group imbalance in this aspect of CRC screening knowledge would seem unlikely to explain the difference in knowledge scores, given that randomization resulted in the study groups being well-matched on all of the pre-intervention characteristics (Table 1). Additionally, there was no difference in scores between the two groups for the first block of knowledge questions, which participants answered before receiving any tailored information or being offered optional additional material.
Prior intervention studies, tailoring to constructs such as self-efficacy, perceived barriers, and stage of readiness, typically addressed several of these constructs simultaneously. Several of these studies found significant positive effects on behavior or behaviorally-relevant outcomes.[10-14] Regarding studies of tailored interventions focused specifically on enhancing CRC screening behavioral mediators or uptake, some showed benefits,[15-21] while others did not.[41-46] No prior studies have addressed whether knowledge tailoring alone is sufficient to influence patient preference for health care (in this case, CRC screening test option). Thus, our findings add to the literature on tailored interventions.
Patient preferences regarding certain behaviors (e.g. treatments, preventive interventions) relevant to specific health issues predict the likelihood of pursuing those behaviors, including CRC screening, across socio-demographic groups.[5-9] Thus, our findings suggest that knowledge-tailored IMCPs may have the potential to increase CRC screening, including in English- and Spanish-speaking Hispanics.[2, 3] However, analyses examining effects of our tailored IMCP on actual CRC screening uptake at one year follow-up are required to test this hypothesis.
Prevailing health behavior theories hold knowledge itself to be a relatively weak correlate of preferences and behaviors.[22] Some investigators have delineated a more nuanced view in which the influence of knowledge on behavior may hinge on whether the knowledge is personally relevant and “actionable;” these investigators still question whether non-tailored enhancement of such knowledge alone would be sufficient to influence behavior-relevant outcomes in the absence of material tailored to address constructs such as social support and self-efficacy.[47] If our tailored IMCP is found to increase CRC screening in future analyses, it would suggest the need to reconsider the role of knowledge in influencing healthcare preferences and health behaviors. It may also be that by providing patients with control over knowledge acquisition, knowledge tailored IMCPs could enhance self-efficacy as well as preferences.[48] Experimental studies exploring this possibility are needed.
Even if the benefits of tailored information are found in our future analyses to extend to screening behavior, it would be unclear whether knowledge tailoring alone is sufficient to influence other health behaviors - particularly those that, unlike CRC screening, must be sustained over time, and for which population level knowledge tends to be high and behavioral mediator (e.g. self-efficacy) status unfavorable. Additional empirical studies also will be required to address this question.
However, if knowledge tailoring alone is found to improve CRC screening and some other health behaviors, it would greatly simplify programming of IMCPs addressing those behaviors. It is likely to be easier and cheaper to create modules tailored to patient knowledge –which can consist simply of text, with or without narration - than to create materials aimed at enhancing the status of behavioral constructs such as self-efficacy. The latter often involve developing computer programs capable of generating many message variants tailored to gradations in patient standing on multiple behavioral constructs,[23] and may also involve the production of persuasive multimedia elements (e.g. patient testimonial videos).[21] The cost entailed is considerable,[49] as is the incremental cost of implementing such interventions.[45] Lower creation and implementation costs associated with simple knowledge tailoring could stimulate the development of tailored IMCPs to address health behaviors responsive to tailored knowledge alone.
The effect of the tailored intervention on preference among patients who did not view the optional information following the first block of knowledge items suggests benefits of the mandatory tailored information provided to all tailored IMCP patients. The larger intervention effect among those viewing the optional information could reflect reinforcement of test preferences formulated in response to the mandatory tailored information. This explanation would support maintaining optional content in tailored IMCPs. Alternatively, the optional information may have determined test preference among those viewing the material, suggesting that making some of the optional information mandatory viewing could increase intervention effectiveness. Conversely, increasing the amount of mandatory information could overwhelm some patients, reducing their ability to formulate a specific preference.[30, 31, 50] Exploring these hypotheses in future studies could better inform the design of tailored IMCPs.
Limitations of our study include uncertain generalizability. In addition to unclear applicability of our findings to other health behaviors, our patient sample differed from the general population on some characteristics associated with health behavior. For example, compared with the general population of comparably aged persons,[51] our sample had a somewhat lower mean score for the personality trait Neuroticism, which suggests a tendency toward higher adherence to salutary health behaviors.[52-55] Mean scores on patient activation, a more mutable behavioral characteristic associated with adherence to healthy behaviors,[56, 57] were relatively high.[58] Also, while our RCT population was large and diverse, with strong representation of English- and Spanish-speaking Hispanic persons as well as Black persons, it is unclear whether our findings are applicable to other racial/ethnic (e.g. Asian American) and language groups. Finally, the CRC screening preference measure we employed did not assess the strength of patients’ preferences, a potential moderator of the effect of the intervention.
4.2. Conclusion
An experimental IMCP tailored to patient knowledge regarding CRC screening was more effective than a non-tailored control IMCP in helping patients to form a preference for a specific test option (either FOBT or colonoscopy). Furthermore, the experimental tailored IMCP effect was observed in both Hispanic and non-Hispanic patients and in those using both the English-and Spanish-language versions.
4.3. Practice implications
Interventions tailored to knowledge alone may have promise as tools for increasing CRC screening across ethnic and language groups. Analyses examining effects of knowledge tailoring on screening behavior will be required to further explore this promise.
Acknowledgments
Role of funding
This work was funded in part by the National Cancer Institute grant R01CA131386. The funder had no role in the study design; the collection, analysis and interpretation of data; the writing of the report; or the decision to submit the paper for publication.
We are grateful to the following individuals, who coordinated or facilitated recruitment and participation of patients in the study: Christina Slee, MPH, Dionne Evans-Dean, MHA, Dustin Gottfeld, BS, and Lizette Macias, BS (Sacramento); Mechelle R. Sanders, BA and Leticia E. Serrano, AAS (Rochester); Sandra Monroy, MA (New York City); and Brandon Tutt, MA (Colorado). We also wish to thank Robert Burnett, MA, for his programming contributions to the tailored software program. Finally, we are indebted to all of the primary care offices and patients who participated.
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anthony Jerant, Email: afjerant@ucdavis.edu, Department of Family and Community Medicine, Center for Healthcare Policy and Research, University of California Davis School of Medicine 4860 Y Street, Suite 2300, Sacramento, CA 95817, USA.
Richard L. Kravitz, Department of Internal Medicine, Division of General Medicine, Center for Healthcare Policy and Research, University of California Davis School of Medicine, Sacramento, CA, USA.
Kevin Fiscella, Department of Family Medicine and Community and Preventive Medicine, Center to Improve Communication in Health Care, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA.
Nancy Sohler, Department of Community Health and Social Medicine, Sophie Davis School of Biomedical Education of The City College of New York, New York, NY, USA.
Raquel Lozano Romero, Department of Family and Community Medicine, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA.
Bennett Parnes, InnovAge Greater Colorado PACE, Department of Family Medicine, University of Colorado Health Sciences Center, Aurora, CO, USA.
Sergio Aguilar-Gaxiola, Department of Internal Medicine, Center for Reducing Health Disparities, University of California Davis School of Medicine, Sacramento, CA, USA.
Charles Turner, IET-Academic Technology Services, University of California Davis, Sacramento, CA, USA.
Simon Dvorak, IET-Academic Technology Services, University of California Davis, Sacramento, CA, USA.
Peter Franks, Department of Family and Community Medicine, Center for Healthcare Policy and Research, University of California Davis School of Medicine, Sacramento, CA, USA.
References
- 1.Screening for colorectal cancer: U. S Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 2.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20:1611–21. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jerant AF, Fenton JJ, Franks P. Determinants of racial/ethnic colorectal cancer screening disparities. Arch Intern Med. 2008;168:1317–24. doi: 10.1001/archinte.168.12.1317. [DOI] [PubMed] [Google Scholar]
- 4.Jerant A. Increasing uptake of colorectal cancer screening. BMJ. 2009;338:a2658. doi: 10.1136/bmj.a2658. [DOI] [PubMed] [Google Scholar]
- 5.Hawley ST, McQueen A, Bartholomew LK, Greisinger AJ, Coan SP, Myers R, Vernon SW. Preferences for colorectal cancer screening tests and screening test use in a large multispecialty primary care practice. Cancer. 2011 Sep 21; doi: 10.1002/cncr.26551. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McQueen A, Vernon SW, Myers RE, Watts BG, Lee ES, Tilley BC. Correlates and predictors of colorectal cancer screening among male automotive workers. Cancer Epidemiol Biomarkers Prev. 2007;16:500–9. doi: 10.1158/1055-9965.EPI-06-0757. [DOI] [PubMed] [Google Scholar]
- 7.Wiggers LC, Stalmeier PF, Oort FJ, Smets EM, Legemate DA, de Haes JC. Do patients’ preferences predict smoking cessation? Prev Med. 2005;41:667–75. doi: 10.1016/j.ypmed.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Clark NM, Janz NK, Dodge JA, Mosca L, Lin X, Long Q, Little RJ, Wheeler JR, Keteyian S, Liang J. The effect of patient choice of intervention on health outcomes. Contemp Clin Trials. 2008;29:679–86. doi: 10.1016/j.cct.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swift JK, Callahan JL. The impact of client treatment preferences on outcome: a meta-analysis. J Clin Psychol. 2009;65:368–81. doi: 10.1002/jclp.20553. [DOI] [PubMed] [Google Scholar]
- 10.Basch CE, Wolf RL, Brouse CH, Shmukler C, Neugut A, DeCarlo LT, Shea S. Telephone outreach to increase colorectal cancer screening in an urban minority population. Am J Public Health. 2006;96:2246–53. doi: 10.2105/AJPH.2005.067223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short-message service. Am J Prev Med. 2009;36:165–73. doi: 10.1016/j.amepre.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 12.Krebs P, Prochaska JO, Rossi JS. A meta-analysis of computer-tailored interventions for health behavior change. Prev Med. 2010;51:214–21. doi: 10.1016/j.ypmed.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noar SM, Benac CN, Harris MS. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol Bull. 2007;133:673–93. doi: 10.1037/0033-2909.133.4.673. [DOI] [PubMed] [Google Scholar]
- 14.Wanyonyi KL, Themessl-Huber M, Humphris G, Freeman R. A systematic review and meta-analysis of face-to-face communication of tailored health messages: implications for practice. Patient Educ Couns. 2011;85:348–55. doi: 10.1016/j.pec.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Dietrich AJ, Tobin JN, Cassells A, Robinson CM, Greene MA, Sox CH, Beach ML, DuHamel KN, Younge RG. Telephone care management to improve cancer screening among low-income women: a randomized, controlled trial. Ann Intern Med. 2006;144:563–71. doi: 10.7326/0003-4819-144-8-200604180-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiscella K, Humiston S, Hendren S, Winters P, Idris A, Li SX, Ford P, Specht R, Marcus S. A multimodal intervention to promote mammography and colorectal cancer screening in a safety-net practice. J Natl Med Assoc. 2011;103:762–8. doi: 10.1016/s0027-9684(15)30417-x. [DOI] [PubMed] [Google Scholar]
- 17.Manne SL, Coups EJ, Markowitz A, Meropol NJ, Haller D, Jacobsen PB, Jandorf L, Peterson SK, Lesko S, Pilipshen S, Winkel G. A randomized trial of generic versus tailored interventions to increase colorectal cancer screening among intermediate risk siblings. Ann Behav Med. 2009;37:207–17. doi: 10.1007/s12160-009-9103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcus AC, Mason M, Wolfe P, Rimer BK, Lipkus I, Strecher V, Warneke R, Morra ME, Allen AR, Davis SW, Gaier A, Graves C, Julesberg K, Nguyen L, Perocchia R, Speyer JB, Wagner D, Thomsen C, Bright MA. The efficacy of tailored print materials in promoting colorectal cancer screening: results from a randomized trial involving callers to the National Cancer Institute’s Cancer Information Service. J Health Commun. 2005;10 (Suppl 1):83–104. doi: 10.1080/10810730500257754. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqui AA, Sifri R, Hyslop T, Andrel J, Rosenthal M, Vernon SW, Cocroft J, Myers RE. Race and response to colon cancer screening interventions. Prev Med. 2011;52:262–4. doi: 10.1016/j.ypmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Walsh JM, Salazar R, Nguyen TT, Kaplan C, Nguyen LK, Hwang J, McPhee SJ, Pasick RJ. Healthy colon, healthy life: a novel colorectal cancer screening intervention. Am J Prev Med. 2010;39:1–14. doi: 10.1016/j.amepre.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerant A, Kravitz RL, Rooney M, Amerson S, Kreuter M, Franks P. Effects of a tailored interactive multimedia computer program on determinants of colorectal cancer screening: a randomized controlled pilot study in physician offices. Patient Educ Couns. 2007;66:67–74. doi: 10.1016/j.pec.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Glanz K, Rimer BK, Lewis FM, editors. Health behavior and health education. Theory, research, and practice. 3. San Francisco, CA: Jossey-Bass; 2002. Part two: models of health behavior. [Google Scholar]
- 23.Schumann A, John U, Ulbricht S, Ruge J, Bischof G, Meyer C. Computer-generated tailored feedback letters for smoking cessation: theoretical and empirical variability of tailoring. Int J Med Inform. 2008;77:715–22. doi: 10.1016/j.ijmedinf.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Jerant A, Sohler N, Fiscella K, Franks B, Franks P. Tailored interactive multimedia computer programs to reduce health disparities: opportunities and challenges. Patient Educ Couns. 2011;85:323–30. doi: 10.1016/j.pec.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC health disparities and inequalities report - United States, 2011. Morbidity and Mortality Weekly Report. 2011;60(Suppl):1–113. [PubMed] [Google Scholar]
- 26. [[assessed on 03.03.12].];American Cancer Society guidelines for the early detection of cancer. Available at: http://www.cancer.org/Healthy/FindCancerEarly/CancerScreeningGuidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer.
- 27.Pressman RS. Software engineering: a practitioner’s approach. 6. New York: McGraw-Hill; 2005. [Google Scholar]
- 28.Kincaid JP, Fishburne RP, Rogers RL, Chissom BS. Research Branch Report 8-75. Memphis, TN: U.S. Naval Air Station; 1975. Derivation of new readability formulas (Automated Readability Index, Fog Count and Flesch Reading Ease Formula) for Navy-enlisted personnel. [Google Scholar]
- 29.Kreuter M, Farrell D, Olevitch L, Brennan L. Tailoring health messages: customizing communication with computer technology. Mahwah, New Jersey: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- 30.Miller SM, Mangan CE. Interacting effects of information and coping style in adapting to gynecologic stress: should the doctor tell all? J Pers Soc Psychol. 1983;45:223–36. doi: 10.1037//0022-3514.45.1.223. [DOI] [PubMed] [Google Scholar]
- 31.Miller SM. Monitoring versus blunting styles of coping with cancer influence the information patients want and need about their disease. Implications for cancer screening and management. Cancer. 1995;76:167–77. doi: 10.1002/1097-0142(19950715)76:2<167::aid-cncr2820760203>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 32.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Morris NS, MacLean CD, Chew LD, Littenberg B. The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7:21. doi: 10.1186/1471-2296-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40:1918–30. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alegria M, Sribney W, Perez D, Laderman M, Keefe K. The role of patient activation on patient-provider communication and quality of care for US and foreign born Latino patients. J Gen Intern Med. 2009;24 (Suppl 3):534–41. doi: 10.1007/s11606-009-1074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benet-Martinez V, John OP. Los Cinco Grandes across cultures and ethnic groups: multitrait multimethod analyses of the Big Five in Spanish and English. J Pers Soc Psychol. 1998;75:729–50. doi: 10.1037//0022-3514.75.3.729. [DOI] [PubMed] [Google Scholar]
- 37.John OP, Donahue EM, Kentle LK. Technical report. Berkeley, CA: Institute of Personality and Social Research, University of California Berkeley; 1991. The Big Five inventory - version 4a and 54. [Google Scholar]
- 38.Soto CJ, John OP. Ten facet scales for the Big Five Inventory: Convergence with NEO PI-R facets, self-peer agreement, and discriminant validity. J Res Pers. 2009;43:84–90. [Google Scholar]
- 39.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 2004;55:652–9. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 40.Cloutier J, Neil Macrae C. The feeling of choosing: Self-involvement and the cognitive status of things past. Consciousness and cognition. 2008;17:125–35. doi: 10.1016/j.concog.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Costanza ME, Luckmann R, Stoddard AM, White MJ, Stark JR, Avrunin JS, et al. Using tailored telephone counseling to accelerate the adoption of colorectal cancer screening. Cancer Detect Prev. 2007;31:191–8. doi: 10.1016/j.cdp.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Simon SR, Zhang F, Soumerai SB, Ensroth A, Bernstein L, Fletcher RH, Ross-Degnan D. Failure of automated telephone outreach with speech recognition to improve colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2010;170:264–70. doi: 10.1001/archinternmed.2009.522. [DOI] [PubMed] [Google Scholar]
- 43.Rawl SM, Champion VL, Scott LL, Zhou H, Monahan P, Ding Y, Loehrer P, Skinner CS. A randomized trial of two print interventions to increase colon cancer screening among first-degree relatives. Patient Educ Couns. 2008;71:215–27. doi: 10.1016/j.pec.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Myers RE, Sifri R, Hyslop T, Rosenthal M, Vernon SW, Cocroft J, Wolf T, Andrel J, Wender R. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110:2083–91. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- 45.Vernon SW, Bartholomew LK, McQueen A, Bettencourt JL, Greisinger A, Coan SP, Lairson D, Chan W, Hawley ST, Myers RE. A randomized controlled trial of a tailored interactive computer-delivered intervention to promote colorectal cancer screening: sometimes more is just the same. Ann Behav Med. 2011;41:284–99. doi: 10.1007/s12160-010-9258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lairson DR, DiCarlo M, Myers RE, Wolf T, Cocroft J, Sifri R, Rosenthal M, Vernon SW, Wender R. Cost-effectiveness of targeted and tailored interventions on colorectal cancer screening use. Cancer. 2008;112:779–88. doi: 10.1002/cncr.23232. [DOI] [PubMed] [Google Scholar]
- 47.Baranowski T, Cullen KW, Nicklas T, Thompson D, Baranowski J. Are current health behavioral change models helpful in guiding prevention of weight gain efforts? Obes Res. 2003;11 (Suppl):23S–43S. doi: 10.1038/oby.2003.222. [DOI] [PubMed] [Google Scholar]
- 48.Bandura A. Self-efficacy: the exercise of control. New York, NY: Freeman; 1997. [Google Scholar]
- 49.Lairson DR, Chang YC, Bettencourt JL, Vernon SW, Greisinger A. Estimating development cost for a tailored interactive computer program to enhance colorectal cancer screening compliance. J Am Med Inform Assoc. 2006;13:476–84. doi: 10.1197/jamia.M2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill LH. Adult education for health and wellness: new directions for adult and continuing education. San Francisco, CA: Jossey-Bass; 2011. [Google Scholar]
- 51.John OP. [[assessed on 03.11.12].];The Big Five Inventory: frequently asked questions. Available at: http://www.ocf.berkeley.edu/~johnlab/bfi.htm.
- 52.Arai S, Nakaya N, Kakizaki M, Ohmori-Matsuda K, Shimazu T, Kuriyama S, Fukao A, Tsuji I. Personality and gastric cancer screening attendance: a cross-sectional analysis from the Miyagi Cohort Study. J Epidemiol. 2009;19:34–40. doi: 10.2188/jea.JE20080024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Axelsson M, Brink E, Lundgren J, Lotvall J. The influence of personality traits on reported adherence to medication in individuals with chronic disease: an epidemiological study in West Sweden. PloS One. 2011;6:e18241. doi: 10.1371/journal.pone.0018241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Emilsson M, Berndtsson I, Lotvall J, Millqvist E, Lundgren J, Johansson A, Brink E. The influence of personality traits and beliefs about medicines on adherence to asthma treatment. Primary Care Resp J. 2011;20:141–7. doi: 10.4104/pcrj.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jerant A, Chapman B, Duberstein P, Robbins J, Franks P. Personality and medication non-adherence among older adults enrolled in a six-year trial. Br J Health Psychol. 2011;16:151–69. doi: 10.1348/135910710X524219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skolasky RL, Green AF, Scharfstein D, Boult C, Reider L, Wegener ST. Psychometric properties of the patient activation measure among multimorbid older adults. Health Serv Res. 2011;46:457–78. doi: 10.1111/j.1475-6773.2010.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skolasky RL, Mackenzie EJ, Wegener ST, Riley LH., 3rd Patient activation and adherence to physical therapy in persons undergoing spine surgery. Spine. 2008;33:E784–91. doi: 10.1097/BRS.0b013e31818027f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hibbard JH, Cunningham PJ. How engaged are consumers in their health and health care, and why does it matter. [[assessed on 02.17.12].];HSC Research Brief No 8. 2008 Available at: http://hschange.org/CONTENT/1019/#ib8. [PubMed]