Abstract
Objectives
Postoperative chemoradiation (CRT) has been shown to be more effective than postoperative radiotherapy (RT) alone in high risk HNSCC patients. Multimodality therapy is associated with more treatment related-toxicity. In this study, we assessed cervical lymph node histological characteristics to detect prognostic and predictive value differences to help guide therapeutic decision making.
Study Design
Retrospective analysis of Cancer Registry data
Methods
HNSCC surgical patients who had tumor resection and neck dissection at our institution from 1980 to 2008 were identified (n=1510). Multivariable Cox proportional hazards regression models were developed to identify significant predictors of 3 outcomes: overall survival (OS), disease-specific survival (DSS) and neck disease recurrence (NDR). Hazard ratios were estimated for the number of cervical nodal metastases and presence of extracapsular spread (ECS) by adjuvant treatment after controlling for significant covariates.
Results
Increasing number of positive nodes was significantly associated with poorer outcomes in OS, DSS, and NDR models (p<0.0001, p<0.0001, p=0.0002, respectively). OS and DSS associated with adjuvant treatment (none, RT or CRT) were modified by number of positive nodes, ECS status and cancer site. The presence of ECS was associated with reduced OS and DSS (p=0.077, p=0.001 respectively), but not significantly associated with NDR (p=0.179). Nodal positive patients benefited from adjuvant therapy regardless of ECS status. CRT consistently conferred a survival advantage over RT across all nodal categories, although the difference was not statistically significant.
Conclusion
We observed a consistent survival advantage with CRT over RT for patients with positive cervical nodal metastasis, although the difference was not statistically significant.
Keywords: head and neck cancer, extracapsular spread, lymph node metastasis, chemoradiotherapy
Introduction
Head and neck cancer, which includes cancers of the mouth, pharynx, and larynx, is the sixth most common cancer worldwide, accounting for 650,000 new cancer cases and 350,000 cancer deaths each year [1]. Approximately 90% of head and neck cancers are squamous cell carcinomas, and about two-thirds of the patients with Head and Neck Squamous Cell Carcinoma (HNSCC) present with advanced stage disease, commonly involving regional lymph nodes [1]. Cervical lymph node metastasis and presence of extracapsular spread of the lymph nodes (ECS) have both been shown to be associated with worse prognosis [2–4]. The addition of postoperative radiotherapy (RT) to definitive surgical therapy has long been considered the standard of care for patients with advanced locoregional disease. Between 1985 and 1989, surgery with postoperative RT accounted for 24.1% of all treatments for head and neck cancer. Since the addition of chemotherapy to enhance treatment efficacy of head and neck cancer, chemoradiation therapy (CRT) in conjunction with surgery has gained increasing popularity [5]. From 1990 to 2004, the use of CRT with surgery in all head and neck cancer has nearly doubled, from 2.1% to 4% [6]. However, with the increased use of CRT come significant toxicities associated with the multimodality treatment. Histological factors that identify patients most likely to benefit from chemotherapy in addition to radiation therapy have not yet been clearly established. We undertook this extended retrospective review in an effort to further define guidelines for use of CRT based upon histologic findings of the cervical lymph nodes.
Materials and Methods
Study Subjects
A retrospective database review from University of Pittsburgh Medical Center (UPMC) Head and Neck Oncology Registry of all patients who were treated for HNSCC at this institution from January1980 through June 2008 was conducted (n=3019). Patients who underwent both surgical resection of the primary tumor and neck dissection with curative intent including histological evaluation of the neck lymph node status as part of primary treatment were included in the study. Because this study examined how control of the primary tumor and neck may affect prognosis, those patients who had positive margins after the surgical resection were considered to have inadequate control of the primary tumor and were excluded from the study. Other exclusion criteria include those with survival of 30 days or less following surgery. 1510 patients were defined as eligible by these inclusion/exclusion criteria (Fig. 1). Demographic and clinicopathologic parameters, including age, sex, race, anatomical site, tumor grade, tumor pathological stage (T stage), node pathological stage (N stage), number of positive lymph nodes, presence of extracapsular spread of the lymph nodes, post-surgery adjuvant radiotherapy, post-surgery adjuvant chemotherapy, date of surgery and clinical outcomes data were obtained from the UPMC Head and Neck registry.
Figure 1.
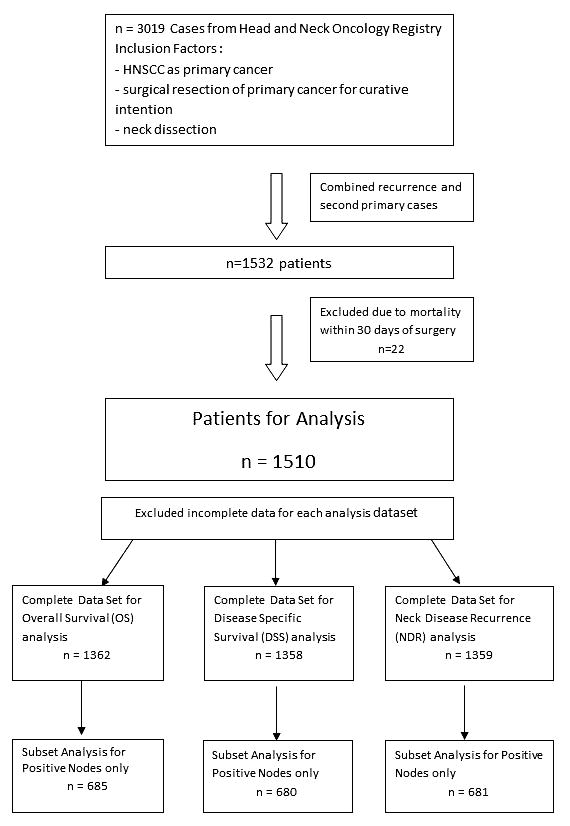
Overall schematic of patient numbers for Overall Survival (OS), Disease-Specific Survival (DSS) and Neck Disease Recurrence (NDR) analyses.
Statistical Methods
In this retrospective study, three main outcomes, overall survival (OS), disease-specific survival (DSS) and neck disease recurrence (NDR), were examined. OS was defined as the time from the date of surgery until the date of death from any cause. DSS was defined as time from the date of surgery until the earliest date of locoregional recurrence, distant metastasis, second primary or death from cancer. Patients without a date of death were censored on their last known follow-up date for DSS and OS models. NDR was defined as the time from the date of the surgery to date of neck recurrence. Patients without a recurrence or who had a recurrence at a site other than the neck were censored on their last known follow-up date for the NDR model.
Multivariable analyses were performed using Cox proportional hazards regression models in order to assess the association of the number of positive lymph nodes with OS, DSS and NDR after adjusting for significant candidate variables. The following candidate variables were considered for entry into each of the Cox models: age at diagnosis, sex, race, cancer site, tumor grade, pathologic T stage, pathological N stage, post-surgery adjuvant treatment (none, radiotherapy (RT) or chemoradiotherapy (CRT)), and treatment era (defined as date of surgery 1980–1990, 1991–2000, 2001–2008). Chemotherapy included conventional chemotherapeutics such as platinum-based treatments and microtubule stabilizing/destabilizing agents. Targeted therapies were not considered chemotherapies. N stage was not entered into the model because it was highly correlated with number of positive nodes. Because Extracapsular Spread (ECS) was also a predictor of interest, additional models were developed that contained the variable ECS. The multivariable Cox proportional hazards models evaluating ECS were modified from OS, DSS and NDR models by the inclusion of an ECS variable and were restricted to patients with positive nodes because only patients with positive nodes could have ECS. Any observation with an unknown or missing value for one of the covariates in the model or outcome was automatically removed from the analysis.
For model development, candidate variables were first entered into a model one at a time and the log likelihood ratio test was performed whereby the −2 log L value was compared to that of the null model to determine which variables on their own significantly reduced the value of this statistic. The variables that were significant in the univariate models were assessed in a joint model. Each variable was omitted from the set one at a time and those that lead to a significant increase in −2 log L were retained in the model. Variables that were not statistically significant on their own were then added to the model one at a time; any variable that reduced −2 log L significantly was retained in the model. A final check was made to ensure that no term in the model could be omitted without significantly increasing the value of −2 log L. Significance was defined as log likelihood ratio test associated p<0.05.
Results
Patient Demographics
A total of 1510 patients met the inclusion criteria (Table 1). The majority of the patients were white (92.7%) and male (70.7%). The median age at diagnosis was 61. There was a relatively even distribution of T1, T2, T3 and T4 disease (21.1%, 29.4%, 20.8%, and 21.6% respectively). No nodal involvement was found in 42.2% of the patients. Among patients with positive nodes, 50.1% had ECS positive nodes, 40.3% had ECS negative nodes and 9.5% had unknown ECS status. 44.0% of the patients received no adjuvant therapy, 36.2% of the patients received postoperative radiation alone (RT), 17.5% of the patients received chemoradiation (CRT) and 3.7% of the patients had unknown chemotherapy or radiation status. One patient received chemotherapy (CT) without RT. This patient had been previously treated with RT for a prior lymphoma and refused RT for the HNSCC event in our study. Patients with adjuvant treatment defined as none, RT or CRT were included in models evaluating the association of adjuvant treatment with survival.
Table 1.
Summary of Patient Characteristics by Analysis
| Characteristic | Eligible Patients (n=1510) | OS Model Patients (n=1362) | DSS Model Patients (n=1358) | NDR Model Patients (n=1359) | ||||
|---|---|---|---|---|---|---|---|---|
| Age, median (range) | 61 (18–92) | 61 (18–92) | 61 (18–92) | 61 (18–92) | ||||
| Sex, N % | ||||||||
| Male | 1067 | 70.7% | 957 | 70.3% | 956 | 70.4% | 956 | 70.3% |
| Female | 443 | 29.3% | 405 | 29.7% | 402 | 29.6% | 403 | 29.7% |
| Race, N % | ||||||||
| White | 1400 | 92.7% | 1274 | 93.5% | 1265 | 93.2% | 1266 | 93.2% |
| Non-white | 96 | 6.4% | 88 | 6.5% | 84 | 6.2% | 84 | 6.2% |
| Unknown | 14 | 0.9% | 0 | 0.0% | 9 | 0.7% | 9 | 0.7% |
| Cancer Site, N % | ||||||||
| Oral cavity | 549 | 36.4% | 513 | 37.7% | 510 | 37.6% | 510 | 37.5% |
| Oropharynx | 337 | 22.3% | 293 | 21.5% | 291 | 21.4% | 291 | 21.4% |
| Hypopharynx | 124 | 8.2% | 114 | 8.4% | 113 | 8.3% | 114 | 8.4% |
| Larynx | 394 | 26.1% | 364 | 26.7% | 366 | 27.0% | 366 | 26.9% |
| Other | 106 | 7.0% | 78 | 5.7% | 78 | 5.7% | 78 | 5.7% |
| Tumor Grade, N % | ||||||||
| Poor or undifferentiated | 272 | 18.0% | 248 | 18.2% | 249 | 18.3% | 249 | 18.3% |
| Moderate | 967 | 64.0% | 893 | 65.6% | 891 | 65.6% | 892 | 65.6% |
| Well | 179 | 11.9% | 168 | 12.3% | 165 | 12.2% | 165 | 12.1% |
| Unknown | 92 | 6.1% | 53 | 3.9% | 53 | 3.9% | 53 | 3.9% |
| T stage (pathological), N % | ||||||||
| T0/TX | 104 | 7.2% | 69 | 5.1% | 67 | 4.9% | 68 | 5.0% |
| T1 | 306 | 21.1% | 288 | 21.1% | 288 | 21.2% | 288 | 21.2% |
| T2 | 427 | 29.4% | 408 | 30.0% | 409 | 30.1% | 409 | 30.1% |
| T3 | 302 | 20.8% | 296 | 21.7% | 294 | 21.6% | 294 | 21.6% |
| T4 | 313 | 21.6% | 301 | 22.1% | 300 | 22.1% | 300 | 22.1% |
| Unknown | 58 | 4.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| N stage (pathological), N % | ||||||||
| N0/NX | 669 | 44.3% | 610 | 44.8% | 613 | 45.1% | 613 | 45.1% |
| N1 | 320 | 21.2% | 311 | 22.8% | 310 | 22.8% | 310 | 22.8% |
| N2 | 439 | 29.1% | 417 | 30.6% | 411 | 30.3% | 412 | 30.3% |
| N3 | 26 | 1.7% | 24 | 1.8% | 24 | 1.8% | 24 | 1.8% |
| Unknown | 56 | 3.7% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Number Positive Nodes, N % | ||||||||
| No positive nodes | 637 | 42.2% | 612 | 44.9% | 615 | 45.3% | 615 | 45.3% |
| 1 positive node | 273 | 18.1% | 259 | 19.0% | 258 | 19.0% | 258 | 19.0% |
| 2 positive nodes | 183 | 12.1% | 172 | 12.6% | 170 | 12.5% | 171 | 12.6% |
| 3 positive nodes | 116 | 7.7% | 107 | 7.9% | 107 | 7.9% | 107 | 7.9% |
| 4 positive nodes | 66 | 4.4% | 57 | 4.2% | 55 | 4.1% | 55 | 4.0% |
| 5+ positive nodes | 170 | 11.3% | 155 | 11.4% | 153 | 11.3% | 153 | 11.3% |
| Unknown | 65 | 4.3% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| ECS (patients with positive nodes), N % | ||||||||
| ECS positive | 405 | 50.1% | 376 | 50.1% | 369 | 49.7% | 370 | 49.7% |
| ECS negative | 326 | 40.3% | 312 | 41.6% | 314 | 42.3% | 314 | 42.2% |
| Unknown | 77 | 9.5% | 62 | 8.3% | 60 | 8.1% | 60 | 8.1% |
| Adjuvant Treatment, N % | ||||||||
| No | 664 | 44.0% | 633 | 46.5% | 631 | 46.5% | 632 | 46.5% |
| RT | 546 | 36.2% | 515 | 37.8% | 516 | 38.0% | 516 | 38.0% |
| CRT | 264 | 17.5% | 214 | 15.7% | 211 | 15.5% | 211 | 15.5% |
| CT | 1 | 0.1% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Unknown | 35 | 2.3% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Treatment Era, N % | ||||||||
| 1980–1989 | 359 | 23.8% | 310 | 22.8% | 305 | 22.5% | 305 | 22.4% |
| 1990–2000 | 626 | 41.5% | 588 | 43.2% | 584 | 43.0% | 585 | 43.0% |
| 2001–2008 | 525 | 34.8% | 464 | 34.1% | 469 | 34.5% | 469 | 34.5% |
| HNSCC Disease Status, N % | ||||||||
| Alive no evidence of HNSCC | 490 | 32.5% | 448 | 32.9% | 455 | 33.5% | 455 | 33.5% |
| Alive with evidence of HNSCC | 12 | 0.8% | 10 | 0.7% | 9 | 0.7% | 9 | 0.7% |
| Died from HNSCC | 418 | 27.7% | 371 | 27.2% | 362 | 26.7% | 363 | 26.7% |
| Died from other cause | 341 | 22.6% | 311 | 22.8% | 309 | 22.8% | 309 | 22.7% |
| Died unknown cause | 249 | 16.5% | 222 | 16.3% | 223 | 16.4% | 223 | 16.4% |
| Overall Survival | ||||||||
| Deceased, N % | 1008 | 66.8% | 904 | 66.4% | 894 | 65.8% | 895 | 65.9% |
| Months, median (range) | 32.0 (1.0–320.5) | 34.0 (1.0–320.5) | 34.0 (1.0–320.5) | 34.0 (1.0–320.5) | ||||
| Alive, N % | 502 | 33.2% | 458 | 33.6% | 464 | 34.2% | 464 | 34.1% |
| Months, median (range) | 72.1 (1.0–309.3) | 72.1 (1.9–309.3) | 72.1 (1.9–309.3) | 72.1 (1.9–309.3) | ||||
| Disease Specific Survival | ||||||||
| Disease Progression, N % | 618 | 40.9% | 541 | 39.7% | 543 | 40.0% | 543 | 40.0% |
| Months, median (range) | 12.9 (0.9–268.3) | 12.0 (0.9–223.3) | 12.0 (0.9–223.3) | 12.0 (0.9–223.3) | ||||
| No Disease Progression, N % | 876 | 58.0% | 808 | 59.3% | 815 | 60.0% | 815 | 60.0% |
| Months, median (range) | 64.6 (1.0–320.5) | 66.0 (1.0–320.5) | 66.0 (1.0–320.5) | 66.0 (1.0–320.5) | ||||
| Unknown | 16 | 1.1% | 13 | 1.0% | 0 | 0.0% | 1 | 0.1% |
| Neck Disease Recurrence | ||||||||
| Neck recurrent disease, N % | 231 | 15.3% | 198 | 14.5% | 199 | 14.7% | 199 | 14.6% |
| Months, median (range) | 9.1 (0.9–206.3) | 9.0 (0.9–206.3) | 9.0 (0.9–206.3) | 9.0 (0.9–206.3) | ||||
| No Neck recurrent disease, N % | 1264 | 83.7% | 1152 | 84.6% | 1159 | 85.3% | 1160 | 85.4% |
| Months, median (range) | 55.1 (1.0–320.5) | 57.1 (1.0–320.5) | 57.1 (1.0–320.5) | 57.1 (1.0–320.5) | ||||
| Unknown | 15 | 1.0% | 11 | 0.8% | 0 | 0.0% | 0 | 0.0% |
Overall Survival (OS); Disease-Specific Survival (DSS); Neck Disease Recurrence (NDR), Extracapsular spread status (ECS); Radiation therapy (RT); Chemoradiation therapy (CRT), Chemotherapy (CT)
Patient subsets and significant variables for three outcome models evaluating lymph node positive and negative patients
The OS analysis of all patients included 1362 patients with complete data (Table 1). The variables that were included in the final OS model were age at diagnosis (p<0.0001), race (p=0.026), cancer site (p<0.0001), T stage (p<0.0001), number of positive nodes (p<0.0001) and adjuvant therapy (p=0.0006). Oral cavity tumors and increasing patient age, T stage and number of positive nodes were associated with worse OS. CRT was associated with significantly improved OS as was non-white race. Patient sex and treatment era were not significantly associated with OS (p=0.177 and p=0.062, respectively) in independent models adjusted for age, race, cancer site, T stage, number of positive nodes and adjuvant therapy.
The DSS analysis of all patients included 1358 patients with complete data (Table 1). The final DSS model contained cancer site (p<0.0001), T-stage (p=0.004), number of nodes (p<0.0001) and adjuvant therapy (p=0.015). Oral cavity tumors and increasing number of positive nodes and T stage were associated with worse DSS. RT and CRT were associated with improved DSS compared to no adjuvant treatment. Patient age, sex, race and era of treatment were not significantly associated with DSS (p=0.054, p=0.541, p=0.278 and p=0.943, respectively) in independent models adjusted for cancer site, T stage, number of positive nodes and adjuvant therapy.
For the NDR model of all patients, which included 1359 patients (Table 1), cancer site (p<0.0001), T stage (p=0.006), node number (p=0.0002) and adjuvant therapy (p<0.0001) were retained in the final model. Oral cavity was associated with worse NDR while the larynx was associated with improved NDR. Stage T4 tumors and increasing number of positive lymph nodes were associated with poorer NDR. RT and CRT were associated with improved NDR compared to no adjuvant therapy. Patient age, sex, race and era of treatment were not significantly associated with NDR (p=0.638, p=0.442, p=0.197 and p=0.442, respectively), in independent models adjusted for cancer site, T stage, node number and adjuvant therapy. Summaries of patient and disease characteristics and survival outcomes for the three models are presented in Table 1.
Histological factors: Effectiveness of adjuvant therapy was modified by number of positive lymph nodes
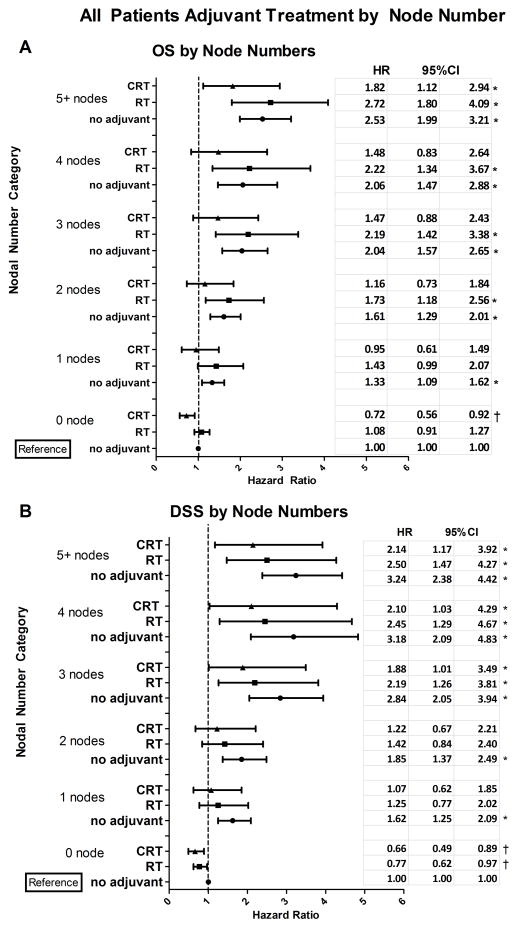
In the OS analysis that included patients with both node negative and positive disease, patients with no positive nodes had significantly better OS than those with 1 positive node (p=0.007). When the number of positive nodes category was evaluated further by adjuvant therapy, patients with positive nodes who received no adjuvant therapy had significantly worse OS compared to the reference group, which was defined as patients with 0 positive nodes and no adjuvant therapy (Fig. 2A). In the group with 0 positive nodes, CRT adjuvant treatment was associated with significantly improved OS compared to the reference group while no significant reduction in OS was associated with RT in this subset of patients. In the 1 positive node group, no adjuvant therapy was associated with significantly poorer OS compared to the reference group, while RT or CRT treatment of patients with 1 positive node had OS similar to the reference group with 0 positive nodes. Furthermore, in patients with 2, 3, or 4 positive nodes, no adjuvant therapy or RT was associated with significantly worse OS compared to the reference group. In this same subset of patients, CRT was associated with OS that was similar to patients with 0 nodes without adjuvant treatment (the reference group). Neither RT nor CRT improved OS in patients with 5 or more positive nodes to levels comparable to 0 positive lymph nodes without adjuvant treatment. However, while hazard ratio (HR) point estimates were similar for no adjuvant and RT treatment in this patient population (2.53 and 2.72, respectively), the HR point estimate for CRT treatment in this patient population was reduced to 1.82, though not statistically significantly. While there was no significant difference in OS between RT and CRT, for each nodal group category, CRT was associated with an estimated 33% reduction in the HR associated with RT.
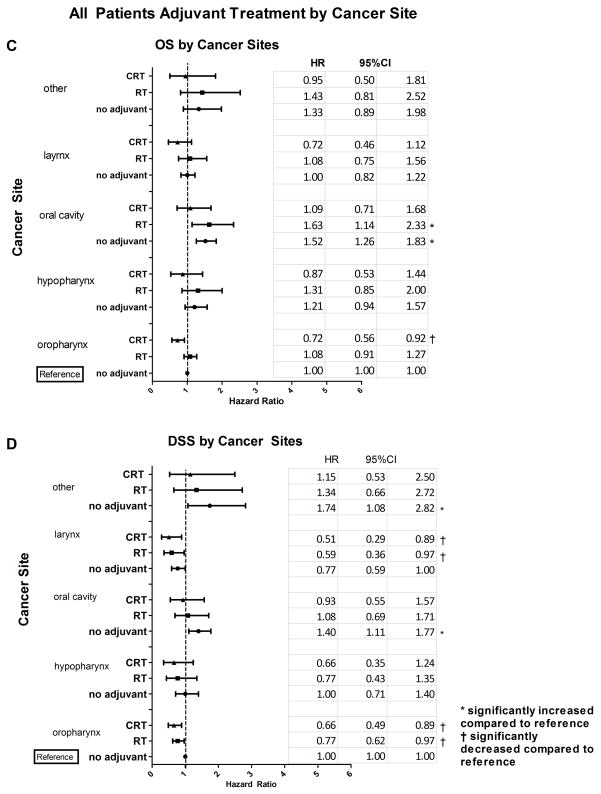
Figure 2.
Overall Survival (OS) and Disease-Specific Survival (DSS) associated with adjuvant therapies were modified by number of positive lymph nodes and tumor anatomic site. (A) Estimates of adjusted OS hazards ratio (HR) and 95% confidence interval (CI) associated with each adjuvant therapy (no, RT or CRT) are presented by number of positive nodes. OS HR estimates have been adjusted for age, race, T stage and cancer site. (B) Estimates of adjusted HR and 95% CI for DSS by number of positive nodes. DSS HR estimates have been adjusted for cancer site and T stage. (C) OS HR estimates and 95% CI for adjuvant therapies by cancer site adjusted for age, race, T stage and number of positive nodes. (D) DSS HR estimates and 95% CI for adjuvant therapies by cancer site adjusted for cancer site and T stage. Node negative disease without adjuvant treatment constituted the reference group for (A) and (B). Oropharyngeal tumors without adjuvant treatment constituted the reference group for (C) and (D). These analyses included patients with node negative or node positive disease.
Results from the DSS analysis in lymph node positive and negative patients were similar to the OS analysis results. In patients with 0 positive nodes, RT and CRT treatments were associated with significantly improved DSS compared to the reference group, defined as patients with 0 positive nodes and no adjuvant therapy (Fig. 2B). Even a single positive lymph node was associated with reduced DSS compared to 0 positive nodes (p<0.001). When nodal disease category DSS was evaluated by adjuvant therapy, positive nodal groups with no adjuvant therapy were consistently associated with significantly worse DSS compared to the reference group (patients with 0 positive nodes and no adjuvant therapy) (Fig. 2B). RT and CRT treatments of patients with 1 or 2 positive nodes were associated with improved DSS comparable to the reference group. However, DSS was not significantly improved with CRT or RT treatment in patients with 3 or more positive nodes. CRT was associated with a 14% reduction in the hazard ratio compared to RT across the node categories, but the difference was not statistically significant.
For NDR, similar to DSS, RT and CRT in the 0 node positive patients were associated with improved NDR compared to patients with 0 positive nodes without adjuvant treatment, which constituted the reference group. Similar to OS and DSS, no adjuvant treatment in node positive cancers was associated with significantly worse NDR compared to the reference group (data not show). Also similar to DSS, NDR was improved by the addition of RT or CRT to patients with 1 or 2 positive nodes (data not shown). In contrast to DSS, this improvement was observed in all node positive categories (data not shown).
Cancer site modified OS, DSS and NDR associated with adjuvant treatment in lymph node positive and negative disease
We interrogated our models to evaluate OS, DSS and NDR associated with specific tumor sites and adjuvant treatments in lymph node positive and negative patients adjusted for number of positive lymph nodes, pathological T stage and identified significant covariates as described above. For the OS analysis, oral cavity was associated with significantly poorer OS compared to the oropharynx (p<0.001), which was the reference for cancer site analyses. Hypopharynx and larynx did not differ from oropharynx (p=0.122 and p=0.95, respectively). Evaluating cancer site by adjuvant therapy, patients with oral cavity cancer who received either no adjuvant therapy or RT alone had significantly worse OS than the reference (patients with oropharynx cancer with no adjuvant therapy), but CRT treatment for oral cavity tumors was associated with OS that was similar to the reference group (Fig. 2C). In the oropharynx group, CRT had significantly improved OS compared to the reference group. OS for all other tumor sites and treatments did not differ significantly from oropharyngeal tumors without adjuvant treatment (Fig. 2C).
Similar to the OS results, oral cavity was associated with significantly worse DSS than the oropharynx (p=0.003). This association was also observed with NDR (p=0.009). Again, no significant difference in DSS between oropharynx and hypopharynx (p=0.93) was observed. Laryngeal tumors tended to have improved DSS compared to oropharyngeal tumors, but this did not reach statistical significance (p=0.051). When cancer sites were further evaluated by adjuvant treatment, oral cavity cancer with no adjuvant therapy was associated with significantly worse DSS compared to the reference group (patients with oropharynx cancer and no adjuvant therapy); RT and CRT adjuvant treatment of oral cavity tumors were associated with DSS that was similar to the level of the reference group (Fig. 2D). Differing somewhat from the OS results, there was a significant improvement in DSS with both RT and CRT in larynx and oropharynx cancer sites, compared to the reference group (patients with oropharynx cancer and no adjuvant therapy) (Fig. 2D). In the NDR analysis, RT and CRT treatments were associated with improved NDR for tumors of the oropharynx, hypopharynx, and larynx relative to the reference group (data not shown). No adjuvant treatment for tumors of the oral cavity was associated with worse NDR compared to the reference group of oropharyngeal tumors without adjuvant treatment (data not shown).
While the importance of HPV status as a prognostic factor for patient survival has become widely accepted, with HPV positivity being associated with improved outcomes, tumor HPV status was not available for tumors in this study. Because HPV positive tumors are concentrated in the oropharynx, we utilized the oropharynx as an imperfect surrogate for HPV in order to evaluate whether the improved survival associated with HPV positive HNSCC could be detected in our dataset. We expected to observe improved OS and DSS in oropharynx, which has the highest proportion of HPV positive tumors, compared to the oral cavity, larynx and hypopharynx, which have predominantly HPV negative tumors. In multivariable OS models adjusted for significant covariates as described above, tumors of the larynx, hypopharynx and oral cavity were associated with reduced survival compared to tumors of the oropharynx (HR=1.26; 95% CI=1.07–1.49). A similar difference in DSS was not observed (HR=1.10; 95% CI=0.89–1.37). Because the prevalence of HPV positive oropharyngeal tumors has increased over the timeframe of this retrospective study, we hypothesized that OS and DSS for patients with cancer of the oropharynx would improve over the three treatment eras, reflecting the increasing prevalence of HPV positive oropharyngeal tumors and their associated improved survival. However, the interaction between cancer site (oropharynx vs. other sites) and treatment era were tested in OS and DSS models and found to be not significantly associated with either (p=0.248 and p=0.903, respectively). Therefore, we observed a cancer site-specific difference in overall survival between tumors that are typically HPV negative versus oropharyngeal tumors, which are enriched for HPV positive tumors relative to other sites. However, we do not observe expected changes in survival across the three treatment eras for oropharyngeal tumors. These results necessitate that we restrict our discussion and interpretation of our findings to cancer site rather than speculate about HPV status and its possible impacts on our results.
ECS status and number of positive lymph nodes modified survival associated with adjuvant treatment in lymph node positive patients
In the subset analysis restricted to patients with positive nodal disease, those with 1 positive node had similar OS compared to patients with 2 positive nodes (p=0.538). However, compared to patients with 1 positive node, OS was reduced for 3 positive nodes (p=0.029), 4 positive nodes (p=0.152) and 5 or more positive nodes (p<0.001), though the difference between 1 and 4 positive nodes was not statistically significant. Similar results were observed in the DSS model. Patients with 2 positive nodes had similar DSS to patients with 1 positive node (p=0.866). However, patients with 3, 4, or 5 or more positive nodes had significantly worse DSS compared to patients with 1 positive node (p=0.021, p=0.024 and p<0.001, respectively).
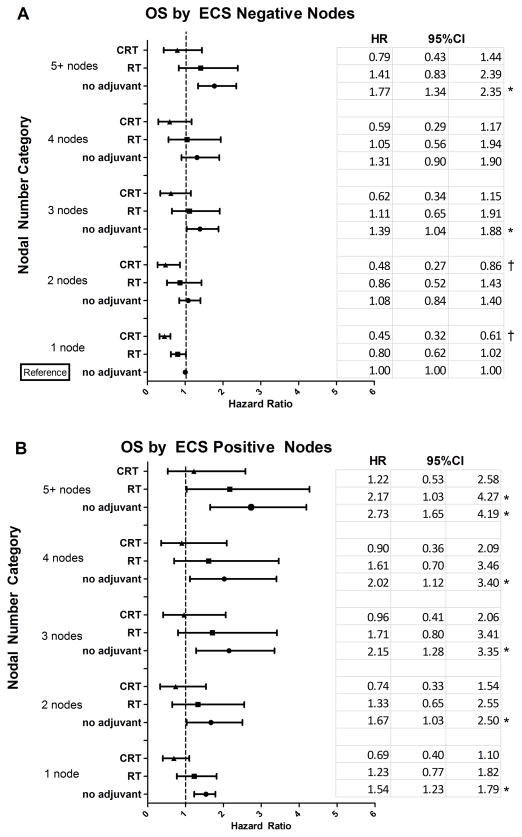
Among patients with positive nodal disease, ECS positivity was associated with significantly worse OS (p<0.001). Evaluating OS among node positive patients by adjuvant therapy, patients with ECS positive nodes who received no adjuvant therapy had significantly worse OS than the reference group (patients with 1 positive node, ECS negative, and no adjuvant therapy) (Fig. 3A and 3B). In the ECS negative group, patients with 1 or 2 positive nodes who received CRT had significantly improved OS compared to the reference group, while no such improvements were seen with the RT groups. For patients with 3 or more ECS negative nodes, both CRT and RT were associated with OS that was similar to the reference group while those with no adjuvant therapy in each of these nodal groups was associated with worse OS.
Figure 3.
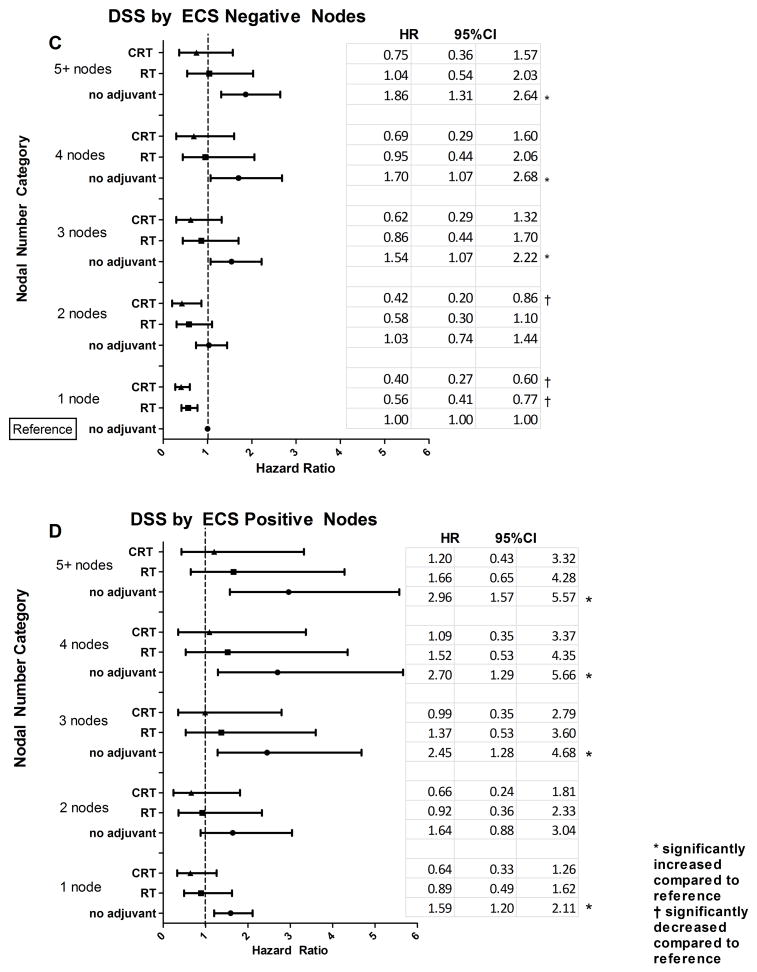
Overall Survival (OS) and Disease-Specific Survival (DSS) associated with adjuvant therapies for lymph node positive disease were modified by number of positive lymph nodes and extracapsular spread (ECS) status. (A) Estimates of adjusted OS HR and 95% CI for adjuvant therapies by number of positive lymph nodes for ECS negative disease. (B) Adjusted OS HR estimates and 95% CI for adjuvant therapies by number of positive lymph nodes for ECS positive disease. OS models presented in (A) and (B) were adjusted for age, race, T stage and cancer site. (C) Estimates of adjusted DSS HR and 95% CI for adjuvant therapies by number of positive lymph nodes for ECS negative disease. (D) Adjusted DSS HR and 95% CI for adjuvant therapies by number of positive lymph nodes for ECS positive disease. DSS models presented in (C) and (D) were adjusted for cancer site and T stage. These analyses included only patients with lymph node positive disease.
For the ECS positive group with 1, 2, 3, or 4 positive nodes, both CRT and RT were associated with OS that was similar to the reference group (1 positive node, ECS negative, no adjuvant treatment), while no adjuvant treatment groups were associated with significantly poorer OS in across all ECS positive node categories (Fig. 3B). In patients with 5 or more nodes and ECS positivity, only CRT adjuvant treatment had OS comparable to the reference group. In each nodal category, the CRT group generally had improved OS compared to the respective RT group (HR reduced by 44%), but the difference was not statistically significant.
Similar to the OS analysis, ECS positive nodes were associated with worse DSS (p=0.001). When ECS status and positive node number were evaluated together with adjuvant treatment, ECS positive nodes and no adjuvant therapy were generally associated with significantly worse DSS compared to the reference group (patients with 1 positive node, ECS negative, and no adjuvant therapy) (Fig. 3C and 3D). For those patients with 1 ECS negative node, both CRT and RT were associated with DSS comparable to the reference group. For patients with 2 ECS negative nodes, only CRT improved DSS to be comparable to the reference group. For patients with 3 or more ECS negative nodes, both CRT and RT had hazard ratios similar to the baseline. In patients with ECS positive nodes, no adjuvant treatment was generally associated with poorer DSS across all positive lymph node categories (Fig. 3D), while treatment with either CRT or RT was associated with hazard ratios that did not differ statistically from the reference group. We did note that the hazard ratios associated with CRT were lower than those associated with RT in each nodal category by 27%, but the differences were not statistically significant.
Discussion
Cervical lymph node histological characteristics have been evaluated in previous studies for prognostic significance. These previous reports have generally characterized lymph node positive versus negative disease or have evaluated node numbers for a specific HNSCC anatomical site. Specifically, the patients with regional metastatic disease that have been reported tend to be at higher risk for developing distant metastasis and thus reduced survival [7]. The presence of cervical lymph node metastasis has been reported to decrease 5-year survival by approximately 50% [2] and to double the risk for distant metastatic disease [8]. Greenberg et al. in 2003 reported that for patients with oral tongue cancer, 2 or more positive nodes was significantly associated with worse disease-specific and overall survival compared to patients with a single positive node [9]. In our multivariable analysis of a large retrospective population, we sought to further define the relationship between lymph node histological characteristics and adjuvant therapy as they relate to patient survival.
Consistent with previous reports, we found that the presence of positive nodes was an independent predictor of worse OS, DSS, and NDR. Furthermore, in the subset of patients with positive nodes, OS and DSS did not differ for patients with 1 or 2 positive nodes. However, patients with 3 or more positive nodes generally had worse OS and DSS suggesting that having limited nodal involvement (1 or 2 positive nodes) was associated with better prognosis than having 3 or more positive nodes. However, the presence of positive nodes, even if limited in number, was associated with worse prognosis compared to no nodal involvement.
The presence of ECS has been established as one of the most important independent predictors of survival [3–4] and has been reported to increase the incidence of distant metastases and recurrence by as high as 3- to 9-fold [8, 10, 11]. Our study confirms these findings but suggests that while ECS was associated with worse OS and DSS, it was not a significant predictor of neck disease recurrence.
Our study demonstrated that oral cavity tumors were associated with worse OS and DSS and reduced time to NDR compared to tumors of the oropharynx. The oropharynx was associated with survival outcomes that were generally comparable to the hypopharynx and larynx. Our data suggest that while no adjuvant therapy and RT alone in oral cavity cancers significantly decreased OS, DSS, and time to NDR, the addition of CRT was actually associated with OS, DSS and time to NDR that were similar to oropharyngeal tumors treated without adjuvant therapy, which constituted the reference group. This suggests that CRT may be especially warranted for oral cavity tumors.
Postoperative RT with definitive surgical therapy has long been considered the standard of care for patients who are at high risk of local and regional failure. Major risk factors have been identified to include pathologically assessed positive margins or the presence of ECS. Minor risk factors have been identified to be perineural, lymphatic, and vascular invasion as well as multiple positive lymph nodes [12]. Pignon et al. in 2000 and 2009 reported a small but significant survival benefit of 6.5% with the use of concomitant chemotherapy [13–14]. Subsequently, two randomized trials (EORTC trial #22931 and RTOG trial #9501) both found improved locoregional control and disease-free survival in high risk patients who received adjuvant CRT compared to postoperative RT alone [15–16]. Since then, concomitant use of chemotherapy with postoperative radiotherapy has gained wide acceptance as standard of care for patient with high risk pathologic features [17].
While the benefit of postoperative CRT or RT have been demonstrated in a select group of patients, both have been associated with acute and long term toxicities. Common acute toxicities from radiotherapy include mucositis, radiation dermatitis, xerostomia, dysphagia, weight loss, and hoarseness. Severe grade 3–4 mucositis rates in HNSCC patients who received radiotherapy have been reported to be 34% and 43% in patients who received chemoradiotherapy [18]. Long term effects including dry mouth and dysphagia were found in 70% and 50% of the patients treated with CRT, respectively [19]. Because of these toxicities, it would be beneficial to more clearly define patients who would most benefit from adjuvant therapy based on nodal histological assessment.
Despite the consensus that CRT has superior survival benefits over RT alone or without adjuvant therapy in high-risk patients with advanced loco-regional disease, the exact criteria for high-risk remain inconsistently defined. The definitions of high risk used by past studies differ somewhat from each other. For example, while both EORTC and RTOG trials define positive margin and ECS involvement to be high risk, their exact degree of lymph node involvement differs. Additionally, the EORTC trial also defines perineural disease and/or vascular embolism as high risk while RTOG does not. Other studies have identified two or more positive lymph nodes as a high-risk factor that may benefit from postoperative RT or CRT [20–21]. National Comprehensive Cancer Network (NCCN) guidelines state that for patients with ECS positive status and or positive surgical margins, CRT is the preferred adjuvant treatment, but for other high risk factors, CRT may be recommended but no specific guidelines are given [17].
In our study, we have found the benefits associated with adjuvant therapy to be nuanced for both OS and DSS. Considering all nodal positive and negative patients, those patients with positive nodes and no adjuvant therapy have significantly worse OS and DSS compared to those patients with no positive nodes and no adjuvant therapy. The addition of RT or CRT generally increased the survival of node positive patients to a level that was similar to the reference group (no positive nodes and no adjuvant therapy). Specifically, in patients with 1 positive node, the addition of either CRT or RT appeared to improve OS and DSS to a level that was comparable to patients with 0 nodes and no adjuvant therapy. In patients with 2 or more positive nodes, only the addition of CRT but not RT was associated with OS comparable to the reference group while either CRT or RT adjuvant treatment resulted in reference group-comparable DSS. We found no statistical difference in the direct comparison between CRT and RT within each nodal group, but we did observe a consistently greater reduction in the hazard ratio estimates for CRT than RT compared to no adjuvant treatment within each nodal number category for both OS and DSS. This difference between CRT and RT was greater for OS than for DSS. Interestingly, even in patients with 0 positive nodes, there was significant improvement in OS with the addition of CRT and improvement in DSS with the addition of CRT or RT. This suggests that even in patients who appear to have less advanced disease, the addition of adjuvant therapy may confer survival benefit.
In the subset analysis of only patients with positive nodal disease, those with ECS positive disease who received no adjuvant therapy had significantly worse OS and DSS than the reference group (1 ECS negative node and no adjuvant therapy treatment) across all nodal categories. CRT and RT were generally associated with benefit to varying degrees in the OS and DSS models. The addition of CRT was associated with improved OS and DSS in 1 or 2 ECS negative nodes compared to patients with 1 diseased lymph node that was ECS negative without adjuvant treatment. Therefore, CRT appears to be of benefit for relatively low risk patients. For patients with 3 or more ECS negative nodes, no adjuvant treatment was associated with reduced OS and DSS while CRT or RT treatments in patients with these characteristics had OS and DSS comparable to the reference group of 1 ECS negative diseased lymph node. For ECS positive nodes, no adjuvant treatment was associated with worse OS and DSS across all node categories, while those patients with RT or CRT had reduced hazard ratios similar to that of the reference group. Although survival differences associated with CRT and RT among ECS positive patients were not significant, hazard ratio point estimates were lower for CRT compared to RT across all ECS nodal groups, with the difference, though not statistically significant, being more pronounced for OS compared to DSS.
The strengths of our study were the large sample size of the patient population analyzed and the recording of the number of positive lymph nodes for most of these patients. To our knowledge, this is the largest retrospective analysis to date from a single institution examining the role of the number of cervical lymph nodes as a prognostic indicator in HNSCC. Furthermore, our data sample spans an extensive timeframe. Interestingly, treatment era was not a significant covariate in any of the three models analyzed. This highlights the challenges of improving effective treatment for HNSCC despite medical advancement. The limitations of our study stems from the fact that our data are retrospective, and we cannot evaluate the uniformity of the decision making or patient selection processes. Furthermore, our outcome measurements were dependent upon data availability. The heterogeneous tumor sites in our data set may contribute to confounding and dilution of observed effects. Notably, in recent years HPV has been identified as an important prognostic factor, especially for oropharyngeal HNSCC. However, tumor HPV status was not available for tumors in this study.
Conclusion
Taken together, our adjuvant therapy analysis results reflect the existing problem that defining the precise risk factors that identify patients who would benefit from a specific type of adjuvant therapy continues to be challenging. Our results show that nodal positive patients benefited from adjuvant therapy regardless of ECS status. While the difference between CRT and RT were overall not statistically significant, CRT did appear to consistently confer a survival advantage over RT across all nodal categories. Interestingly, our data suggest that even those patients considered to be lower risk, such as patients with 0 diseased nodes or 1 to 2 ECS negative nodes, may benefit from CRT or RT in some instances. However, given the significant toxicities of adjuvant therapy, other clinical factors may need to be considered for these lower risk patients.
Acknowledgments
Funding: NCI/NIH K07 CA137140 (AME)
Footnotes
Financial disclosures: none
Conflict of interest: none
References
- 1.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson JT, Barnes EL, Myers EN, et al. The extracapsular spread of tumors in cervical node metastasis. Arch Otolaryngol. 1981;107:725–9. doi: 10.1001/archotol.1981.00790480001001. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JT, Myers EN, Bedetti CD, et al. Cervical lymph node metastasis-incidence and implications of extracapsular carcinoma. Arch Otolaryngol. 1985;111:534–7. doi: 10.1001/archotol.1985.00800100082012. [DOI] [PubMed] [Google Scholar]
- 4.Richard JM, Sancho-Garnier H, Micheau C, et al. Prognostic factors in cervical lymph node metastasis in upper respiratory and digestive tract carcinomas: study of 1,713 cases during a 15-year period. Laryngoscope. 1987;97:97–101. doi: 10.1288/00005537-198701000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman HT, Karnell LH, Funk GF, et al. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:951–962. doi: 10.1001/archotol.124.9.951. [DOI] [PubMed] [Google Scholar]
- 6.Cooper JS, Porter K, Mallin K, et al. National Cancer Database report on cancer of the head and neck: 10-year update. Head Neck. 2009;31:748–758. doi: 10.1002/hed.21022. [DOI] [PubMed] [Google Scholar]
- 7.Genden EM, Ferlito A, Bradley PJ, et al. Review: neck disease and distant metastases. Oral Oncology. 2003;39:207–212. doi: 10.1016/s1368-8375(02)00049-0. [DOI] [PubMed] [Google Scholar]
- 8.Leemans CR, Tiwari R, Nauta JJ, et al. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer. 1993;71:452–6. doi: 10.1002/1097-0142(19930115)71:2<452::aid-cncr2820710228>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg JS, Fowler R, Gomez J, et al. Extent of extracapsular spread: a critical prognostic indicator in oral tongue cancer. Cancer. 2003;97:1464–1470. doi: 10.1002/cncr.11202. [DOI] [PubMed] [Google Scholar]
- 10.de Carvalho MB. Quantitative analysis of the extent of extracapsular invasion and its prognostic significance: a prospective study of 170 cases of carcinoma of the larynx and hypopharynx. Head and Neck. 1998;20:16–21. doi: 10.1002/(sici)1097-0347(199801)20:1<16::aid-hed3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Oosterkamp S, de Jong JM, Van Den Ende PL, et al. Predictive value of lymph node metastases and extracapsular extension for the risk of distant metastases in laryngeal carcinoma. Laryngoscope. 2006;116:2067–2070. doi: 10.1097/01.mlg.0000240263.05198.a0. [DOI] [PubMed] [Google Scholar]
- 12.Ko C, Citrin D. Invited Medical Review: radiotherapy for management of locally advanced squamous cell carcinoma of the head and neck. Oral Diseases. 2009;15:121–132. doi: 10.1111/j.1601-0825.2008.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 14.Pignon JP, le Maitre A, Maillard E, et al. Meta analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomized trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Berneir J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 16.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 17.Pfister DG, Ang KK, Brizel D, et al. [Accessed August 10, 2011];NCCN Guidelines Version 2.2011 Head and Neck Cancers [National Comprehensive Cancer Network Website] 2011 Available at: http://www.nccn.org/clinical.asp.
- 18.Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253–262. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 19.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501) Head Neck. 2005;27:843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 20.Cooper JS, Pajak TF, Forastiere AA, et al. Precisely defining high-risk operable head and neck tumors based on RTOG #8503 and 8824: targets for postoperative radiochemotherapy? Head Neck. 1998;20:588–594. doi: 10.1002/(sici)1097-0347(199810)20:7<588::aid-hed2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 21.Ang KK, Trotti A, Brown BW, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:571–578. doi: 10.1016/s0360-3016(01)01690-x. [DOI] [PubMed] [Google Scholar]