Abstract
Objectives
To assess whether heart rate (HR) reduction following an exercise stress test (ExStrT), an easily quantifiable marker of vagal reflexes, might identify high and low risk long QT syndrome (LQTS) type 1 (LQT1) patients.
Background
Identification of LQTS patients more likely to be symptomatic remains elusive. We have previously shown that depressed baroreflex sensitivity (BRS), an established marker of reduced vagal reflexes, predicts low probability of symptoms among LQT1.
Methods
We studied 169 LQTS genotype-positive patients below age 50 who performed an ExStrT with the same protocol, on and off β-blockers including 47 South African LQT1 patients all harboring the KCNQ1-A341V mutation and 122 Italian LQTS patients with impaired (IKs−, LQT1, n=66) or normal (IKs+, 50 LQT2 and 6 LQT3) IKs current.
Results
Despite similar maximal HR and workload, by the first minute after cessation of exercise the symptomatic patients in both IKs− groups had a greater HR reduction compared to the asymptomatic (19±7 vs 13±5 and 27±10 vs 20±8 bpm, both p=0.009). By contrast, there was no difference between the IKs+ symptomatic and asymptomatic patients (23±9 vs 26±9 bpm, p=0.47). LQT1 patients in the upper tertile for HR reduction had a higher risk of being symptomatic (OR 3.28, 95% CI 1.3–8.3, p=0.012).
Conclusions
HR reduction following exercise identifies LQT1 patients at high or low arrhythmic risk, independently of β-blocker therapy, and contributes to risk stratification. Exercise training, which potentiates vagal reflexes, should be avoided by LQT1 patients.
Keywords: autonomic nervous system, exercise testing, genetics, long QT syndrome, sudden death
INTRODUCTION
During the last 40 years significant progress has been made in the understanding and management of the long QT syndrome (LQTS), the best known among arrhythmogenic channelopathies1–3. Its prevalence has been defined4, effective therapies are available2,3, a growing number of disease-causing genes has been identified3, and complex genotypephenotype correlations are being elucidated5,6. However, it remains challenging to assess the probability of an asymptomatic patient to suffer cardiac events, such as syncope or cardiac arrest.
Recently, some common genetic variants have been associated with increased risk for life-threatening arrhythmias7–9; however, their role in clinical practice is uncertain. Similarly, markers of electrical instability such as the presence of T wave alternans10 or notches on the T wave11 or specific echocardiographic abnormalities12–14 are often confined to patients with a QTc > 500 ms, an established risk factor15.
Given the differential arrhythmic risk associated with different mutations, based on their intragenic location and on their specific functional effect on ionic currents16, large populations are usually needed to draw meaningful conclusions for risk stratification but the confounding effect of individual mutations is difficult to assess. Founder populations17, characterized by the presence of the same mutation in a relatively large number of individuals, offer the unique opportunity to identify factors, other than the primary mutation, able to influence phenotypic differences.
The availability of a well characterized South African (SA) LQT1 founder population segregating the malignant KCNQ1-A341V mutation18,19, allowed us to demonstrate that the autonomic nervous system could act as an arrhythmia risk modifier in LQTS20. Specifically, we have shown that patients with relatively lower values of baroreflex sensitivity (BRS), measured by the phenylephrine method, were also at lower risk for life-threatening arrhythmias20. This suggests that when heart rate (HR) changes occur too rapidly, due to strong autonomic reflexes, there is a higher probability of being symptomatic. Unfortunately, the phenylephrine method to assess BRS is cumbersome21 and this has prevented its widespread use for risk stratification in clinical practice.
We thought that a simpler parameter providing information similar to that of BRS might be equally useful in the risk stratification for LQT1 patients. The extent of the HR reduction at the end of an exercise stress test (ExStrT) is an easily quantifiable marker of reflex vagal activation22–25 and here we have tested our hypothesis that it might perform as well as BRS in the identification of those LQT1 patients more likely to be at risk for life-threatening arrhythmias.
METHODS
Study Population
The present study involved 169 LQTS genotype-positive patients from two distinct populations. One served as a discovery cohort and included 47 patients belonging to a large SA kindred harboring an identical LQT1-causing mutation in KCNQ1 (A341V)18–20. The second (n=122) originated from our data base in Pavia, served as validation cohort, and included 66 LQT1, 50 LQT2 and 6 LQT3 patients. These latter patients (referred to as non- SA) were selected on the basis of having an available ExStrT performed in the same standardized conditions as those of the SA population to allow a proper comparison; accordingly, they had to be below age 50 and to have performed the ExStrT off β-blocker therapy. To avoid any selection bias, not a single subject with these characteristics was excluded. Our primary analysis focused on patients exercised off β-blocker therapy. Because of the clinical relevance of β-blocker therapy, most patients were studied also on therapy.
Clinical and genetic data were recorded on specifically designed forms including demographic information, personal and family history of disease, symptoms and therapy. Cardiac events were syncope or aborted cardiac arrest. Mutation carriers (MCs) were classified as either symptomatic or asymptomatic. Symptomatic MCs had experienced at least one cardiac event irrespective of therapy, whereas asymptomatic MCs had to be at least 15- years-old and without previous cardiac events in the absence of therapy.
From the SA population we included 44 MCs (34 with symptoms and 10 without) who had an available ExStrT test off β-blocker therapy and who were below 50 years; 3 patients were tested only on β-blocker therapy. The reason for excluding MCs above age 50, which corresponds to the 75th percentile of the age distribution, is due to the negative correlation existing between age and: 1) the maximal HR reached during ExStrT at the same workload, 2) vagal reflexes, and 3) BRS21. In this way we did control for age as a potentially confounding factor.
All subjects in the study were genetically confirmed LQTS patients; those with multiple independent, mutations26,27 were excluded. All probands and family members provided written informed consent for clinical and genetic evaluations, as approved by the Ethical Review Boards of the Stellenbosch, Vanderbilt and Pavia University.
Basal ECG Evaluation
A baseline ECG in the absence of β-blocker therapy was recorded for 160 (44 SA and 116 non-SA) patients. Baseline HR, duration of the QT and RR intervals were measured in leads II and V3 from resting 12-lead ECGs and the mean between the two values was considered. The QT interval was corrected for HR by the Bazett’s formula.
ExStrT Evaluation
A multistage fatigue-limited ExStrT was performed on a bicycle ergometer in the upright position. The initial workload was 25 watt, with subsequent stepwise increments of 25 watt every 2 minutes at a pedaling rate of 60 rpm; peak workload was followed by at least a 5-minute cool-down period. Standard 12-lead ECG and blood pressure were recorded in pre-test condition (when there is already sympathetic activation), every minute during exercise, at peak exercise, and every minute during recovery. During the ExStrT, HR increases were measured at 1 (HR1’ExStrT) and 2 (HR2’ExStrT) minutes after the beginning and at the peak exercise (HRMAX). HR decreases during the recovery (“Rec”) phase were calculated as the difference (Δ) in HR between the values recorded at the peak exercise (HRMAX) and those recorded 1 (HR1’rec) and 2 (HR2’rec) minutes after termination of exercise.
BRS Measurements
As previously reported20, BRS was determined by the phenylephrine method, relating a transient increase in blood pressure (20–30 mmHg) induced by bolus injections of phenylephrine (2–3 µg/kg) to the resultant lengthening of the RR interval21. The slope of a best-fit regression line defined BRS. Beat-to-beat RR interval and blood pressure were continuously recorded (FINAPRES, Ohmeda) and then digitally converted. Given the significant negative correlation between BRS and age, we focused our analysis on the second and third age quartiles (age 26 to 47 years)20 in order to reliably assess BRS while controlling for the effect of age. BRS was determined only in the SA population because of the difficulty in obtaining phenylephrine in Italy at the time of the study.
Statistical analysis
Continuous variables are presented as mean ± SD and compared by Student’s t test or one-way ANOVA for independent samples, with Bonferroni correction for multiple comparisons. Changes in ExStrT parameters mean values measured on β-blockers compared to wash out were evaluated by paired t test. Categorical variables were expressed as absolute and relative frequencies and compared by the Fisher’s exact test. To determine the association of ΔHRmax-1’ recovery with the occurrence of cardiac events in the population under study, this variable was dichotomized at the upper tertile of its distribution, and unadjusted ORs with 95%CI were estimated by logistic regression. The relationship of BRS with the reduced HR during the first minute of recovery after termination of exercise (ΔHRmax-1’ recovery) was analyzed by non-parametric Spearman correlations. Receiver-operating characteristics (ROC) curves were constructed and the area under the curves (AUC) were used to determine the performance of both BRS and ΔHRmax – HR1’rec tests in discriminating between MCs with and without cardiac events. A 2-sided p-value <0.05 was considered statistically significant. All analyses were performed with SPSS software (version 19).
RESULTS
Study Populations
Table 1 shows the study populations with their demographic and baseline ECG characteristics. Table 2 presents the individual mutations identified in the non-SA population, whereas all patients in the founder SA population share the same mutation (KCNQ1-A341V). In the non-SA population not a single mutation is over represented and carries therefore undue weight.
Table 1.
Baseline clinical and electrocardiographic features in the entire study population.
| LQT1 -A341V SA IKs− (n=47) |
LQT1 non-SA IKs− (n=66) |
LQT2/3 IKs+ (n=56) |
p-value | |
|---|---|---|---|---|
| Female gender, n(%) | 31(66) | 41(62) | 32(57) | 0.65 |
| Age,yrs (mean±SD) | 30±10 | 29±11 | 33±10 | 0.12 |
| QTc, ms (mean±SD) | 487±43* | 455±48 | 460±54 | 0.003 |
| Basal heart rate (bpm), (mean ±SD) | 69±11 | 69±13 | 66±13 | 0.35 |
p <0.05 vs both IKs− and IKs+ groups from post-hoc Bonferroni test for multiple comparisons.
Table 2.
Individual mutations identified in the non-SA population
| GENE | REGIO N |
NUCLEOTID E CHANGE |
MUTATIO N |
MUTATIO NTYPE |
LOCATIO N |
No. of Patient s |
No. of Familie s |
|---|---|---|---|---|---|---|---|
| KCNQ1 | Exon 1 | 172G>C | A58P | Missense | N-term | 2 | 1 |
| KCNQ1 | Exon 1 | 319C>T | Q107X | Nonsense | N-term | 1 | 1 |
| KCNQ1 | Exon 1 | 332A>G | Y111C | Missense | N-term | 2 | 2 |
| KCNQ1 | Exon 2 | 409C>T | L137F | Missense | S1 | 2 | 2 |
| KCNQ1 | Exon 3 | 444T>G | Y148X | Nonsense | S2 | 2 | 1 |
| KCNQ1 | Exon 3 | 569G>A | R190Q | Missense | S2-S3 | 2 | 1 |
| KCNQ1 | Exon 3 | 568C>T | R190W | Missense | S2-S3 | 6 | 5 |
| KCNQ1 | Exon 4 | 612C>G | I204M | Missense | S3 | 1 | 1 |
| KCNQ1 | Exon 5 | 691C>T | R231C | Missense | S4 | 6 | 2 |
| KCNQ1 | Exon 5 | 760G>C | V254L | Missense | S4-S5 | 1 | 1 |
| KCNQ1 | Exon 5 | 760G>A | V254M | Missense | S4–S5 | 1 | 1 |
| KCNQ1 | Exon 5 | 775C>T | R259C | Missense | S4–S5 | 2 | 2 |
| KCNQ1 | Intron 5 | G781-2G | IVS5-2A/G | Splicing | S5-pore | 4 | 3 |
| KCNQ1 | Intron 5 | G781-1A | IVS5-1G/A | Splicing | S5-pore | 2 | 1 |
| KCNQ1 | Exon 6 | 839T>A | V280E | Missense | S5 | 1 | 1 |
| KCNQ1 | Exon 6 | 904G>A | A302T | Missense | Pore | 1 | 1 |
| KCNQ1 | Exon 6 | 914G>C | W305S | Missense | Pore | 3 | 2 |
| KCNQ1 | Exon 7 | 940G>A | G314S | Missense | Pore | 4 | 1 |
| KCNQ1 | Exon 7 | 943T>A | Y315N | Missense | Pore | 2 | 1 |
| KCNQ1 | Exon 7 | 973 G>C | G325R | Missense | pore-S 6 | 1 | 1 |
| KCNQ1 | Exon 7 | 1022C>T | A341V | Missense | S6 | 47 | 1 Founder |
| KCNQ1 | Exon 7 | 1032G>A | A344 | Splicing | S6 | 1 | 1 |
| KCNQ1 | Exon 8 | 1075_1086del | 359-362delQRQ K | Deletion | C-term | 1 | 1 |
| KCNQ1 | Exon 8 | 1097G>A | R366Q | Missense | C-term | 2 | 2 |
| KCNQ1 | Exon 8 | 1101G>T | Q367H | Missense | C-term | 1 | 1 |
| KCNQ1 | Exon 8 | 1115C>A | A372D | Missense | C-term | 3 | 2 |
| KCNQ1 | Exon 12 | 1541T>C | I514T | Missense | C-term | 1 | 1 |
| KCNQ1 | Exon 13 | 1615G>T | R539W | Deletion | C-term | 1 | 1 |
| KCNQ1 | Exon 14 | 1709C>T | P570L | Missense | C-term | 1 | 1 |
| KCNQ1 | Exon 14 | 1717T>C | F573L | Missense | C-term | 1 | 1 |
| KCNQ1 | Exon 14 | 1725_1728del | S575+15X | Deletion | C-term | 2 | 1 |
| KCNQ1 | Exon 15 | 1772G>T | R591L | Missense | C-term | 3 | 1 |
| KCNQ1 | Exon 15 | 1781G>A | R594Q | Missense | C-term | 1 | 1 |
| KCNQ1 | Exon 16 | 1799C>T | T600M | Missense | C-term | 1 | 1 |
| KCNQ1 | Exon 16 | 1893insC | P631+19X | Insertion | C-term | 1 | 1 |
| KCNH2 | Exon 1 | 65T>A | F22Y | Missense | N-term | 1 | 1 |
| KCNH2 | Exon 2 | 148G>T | E50X | Nonsense | PAS | 1 | 1 |
| KCNH2 | Exon 2 | 174G>C | E58D | Missense | PAS | 1 | 1 |
| KCNH2 | Exon 2 | 215C>G | P72R | Missense | N-term | 1 | 1 |
| KCNH2 | Exon 3 | 442C>T | R148W | Missense | N-term | 1 | 1 |
| KCNH2 | Exon4 | 526C>T | R176W | Missense | N-term | 4 | 2 |
| KCNH2 | Exon 5 | 1096C>T | R366X | Nonsense | N-term | 3 | 1 |
| KCNH2 | Exon 6 | 1283delC | Y427+5X | Frame shift | S1–S2 | 4 | 1 |
| KCNH2 | Exon 6 | 1283C>A | S428X | Nonsense | S1-S2 | 1 | 1 |
| KCNH2 | Exon 6 | 1468G>C | A490P | Missense | S2–S3 | 3 | 1 |
| KCNH2 | Exon 6 | 1490G>T | W497L | Missense | S3 | 1 | 1 |
| KCNH2 | Exon 7 | 1700T>C | I567T | Missense | S5 | 1 | 1 |
| KCNH2 | Exon 7 | 1747A>G | I583V | Missense | S5-pore | 3 | 1 |
| KCNH2 | Exon 7 | 1810G>A | G604S | Missense | S5-pore | 1 | 1 |
| KCNH2 | Exon 7 | 1877G>C | G626A | Missense | Pore | 1 | 1 |
| KCNH2 | Exon 7 | 1898A>G | N633S | Missense | pore-S6 | 1 | 1 |
| KCNH2 | Exon 7 | 1912_1914del | 638delK | In-frame del | pore-S6 | 4 | 1 |
| KCNH2 | Exon 8 | 1979C>T | S660L | Missense | C-term | 1 | 1 |
| KCNH2 | Intron 9 | 2399-28G | IVS9-28A/G | Splicing | C-term | 2 | 1 |
| KCNH2 | Exon 9 | 223 0C>T | R744X | Nonsense | C-term | 1 | 1 |
| KCNH2 | Exon 10 | 2453C>T | S818L | Missense | C-term | 1 | 1 |
| KCNH2 | Exon 10 | 2467C>T | R823W | Missense | C-term | 1 | 1 |
| KCNH2 | Exon 10 | 2521G>C | V841L | Missense | C-term | 1 | 1 |
| KCNH2 | Exon 11 | 2616delC | P872+4X | Frame shift | C-term | 1 | 1 |
| KCNH2 | Exon 12 | 2775_2776ins G | G925+13X | Frame shift | C-term | 1 | 1 |
| KCNH2 | Exon 12 | 2780G>A | W927X | Nonsense | C-term | 3 | 2 |
| KCNH2 | Exon 12 | 2932G>T | E978X | Nonsense | C-term | 1 | 1 |
| KCNH2 | Exon 13 | 3100delC | P1034+22X | Frame shift | C-term | 1 | 1 |
| KCNH2 | Exon 13 | 3128A>G | D1043G | Nonsense | C-term | 1 | 1 |
| KCNH2 | Exon 13 | 3139C>T | R1047C | Missense | C-term | 1 | 1 |
| KCNH2 | Exon 14 | 3254_3255del | P1084+32X | Frame shift | C-term | 1 | 1 |
| KCNH2 | Exon 15 | 3347C>T | A1116V | Missense | C-term | 1 | 1 |
| SCN5A | Exon 6 | 647C>T | S216L | Missense | DI-S3/S4 | 1 | 1 |
| SCN5A | Exon 10 | 1231G>A | V411M | Missense | DI-S6 | 1 | 1 |
| SCN5A | Exon 26 | 4501C>G | L1501V | Missense | DIII-DIV | 2 | 1 |
| SCN5A | Exon 28 | 5272A>G | I1758V | Missense | DIV-S6 | 1 | 1 |
| SCN5A | Exon 28 | 5350G>A | E1784K | Missense | C-term | 1 | 1 |
The SA and non-SA populations were comparable for gender and age at ExStrT, whereas significant differences were present in baseline QTc interval prolongation, with an expected longer QTc observed in the SA A341V group consistent with the established severity of this mutation18,19.
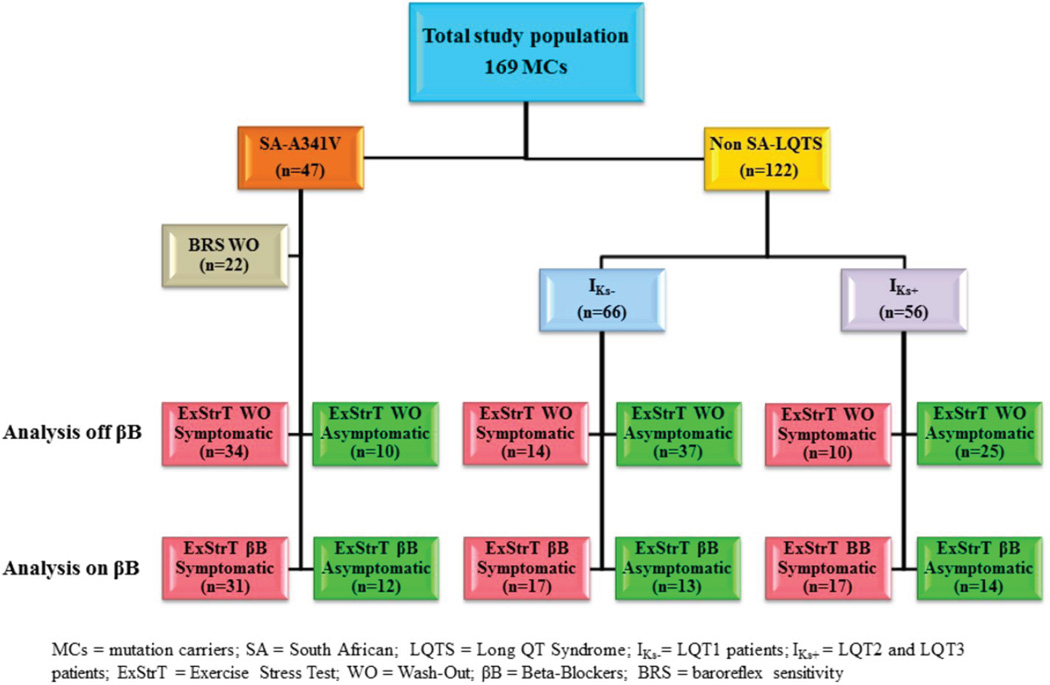
We did assess the effect of β-blocker therapy on the ExStrT measurements because very often when LQTS patients are referred for consultation and risk stratification they are already on therapy. Accordingly, we did this by analyzing 104 LQTS patients (43 SA, 30 LQT1, 29 LQT2 and 2 LQT3) with an ExStrT performed on β-blockers. Fig. 1 illustrates how different numbers of patients, belonging to the various groups, were tested on and offβ-blockers.
Figure 1. Study Population.
Outline of the study population.
For all the analyses involving the non SA-LQTS subgroup, as the 6 LQT3 patients had mean values and patterns very similar to those of the 50 LQT2 patients and as both these groups have a well preserved IKs current, we considered them together. Thus, we compared two groups identified as IKs+ (i.e. preserved IKs), which included the LQT2 and LQT3 patients, and as IKs− (i.e. impaired IKs) which included all the LQT1 patients, SA and non-SA.
HR during the ExStrT and arrhythmic risk
Table 3 compares the symptomatic and asymptomatic subjects for the relevant parameters measured at pre-specified times during ExStrT off β-blockers in 130 MCs aged <50 years. We present first the results off- and then the results on β-blocker therapy.
Table 3.
Absolute HR and HR Changes (Δ) during ExStrT in washout in the entire study population, according to genetic and clinical status
| Symptomatic MCs (n=58) |
Asymptomatic MCs (n=72) |
p-value | |
|---|---|---|---|
| HR Pre-Test | |||
| SA | 78±13 | 77±11 | 0.78 |
| IKs− | 73±11 | 76±14 | 0.60 |
| IKs+ | 72±15 | 71±12 | 0.96 |
| HR max | |||
| SA | 124±15 | 128±18 | 0.55 |
| IKs− | 141±18 | 148±18 | 0.27 |
| IKs+ | 147±24 | 157±18 | 0.18 |
| HR1’rec | |||
| SA | 105±17 | 115±20 | 0.14 |
| IKs− | 114±21 | 128±18 | 0.023 |
| IKs+ | 123±24 | 131±16 | 0.28 |
| HR2’rec | |||
| SA | 91±15 | 98±19 | 0.29 |
| IKs− | 100±16 | 110±17 | 0.08 |
| IKs+ | 113±21 | 113±18 | 0.94 |
| Δ HRmax–HR1’rec | |||
| SA | 19±7 | 13±5 | 0.009 |
| IKs− | 27±10 | 20±8 | 0.009 |
| IKs+ | 23±9 | 26±9 | 0.47 |
| Δ HRmax–HR2’rec | |||
| SA | 33±11 | 30±13 | 0.50 |
| IKs− | 41±9 | 37±10 | 0.23 |
| IKs+ | 37±14 | 42±13 | 0.37 |
| Maximum workload (watt) | |||
| SA | 129±42 | 117±26 | 0.41 |
| IKs− | 141±41 | 147±49 | 0.70 |
| IKs+ | 105±26 | 167±56 | 0.002 |
All HR are expresses as beats per minute (bpm)
Off β-blockers- SA population
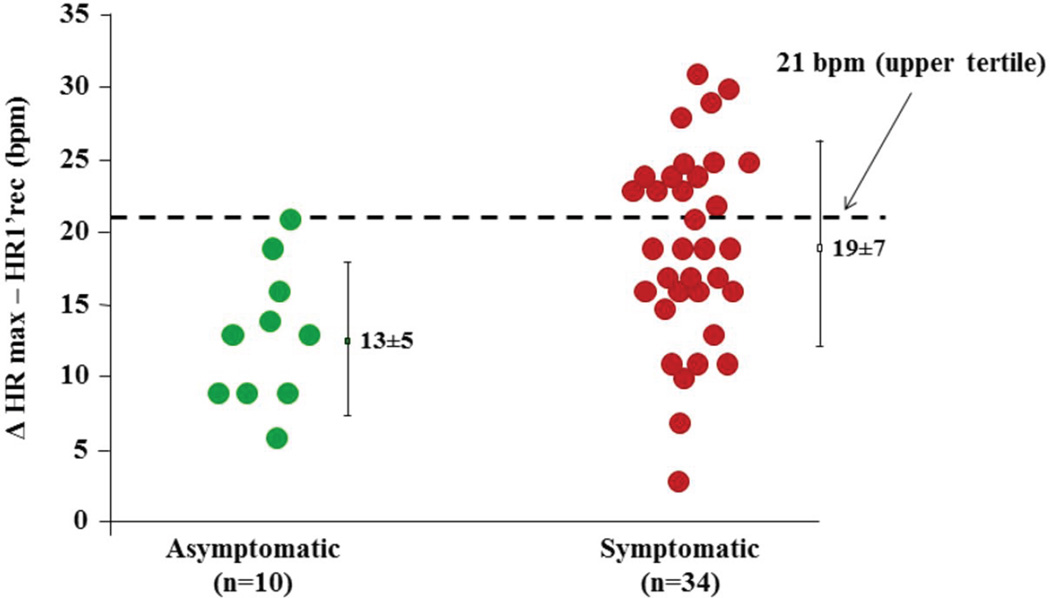
In the A341V SA population, HRmax (124±15 vs 128±18 bpm, p =0.55) and the maximum workload reached during ExStrT (129±42 vs 117±26 watt, p =0.41) were similar between symptomatic and asymptomatic MCs. However, a significant difference was observed in the HR decrease during the first minute of recovery from peak of exercise (ΔHRmax–HR1’rec). Between these two time points of the ExStrT, the symptomatic MCs reduced their HR significantly more than the asymptomatic MCs (19±7 vs 13±5 bpm, p=0.009). Furthermore, when dichotomized at 21 bpm, representing the upper tertile of its distribution, ΔHRmax–HR1’rec carried a differential arrhythmic risk among the MCs (Fig. 2 ). Indeed, all patients with a reduction in HR > 21 bpm had previously suffered from cardiac events. Conversely, all 10 asymptomatic SA MCs had ΔHRmax–HR1’rec values below this threshold (p=0.02).
Figure 2. Post-exercise HR changes in the SA-LQT1 patients.
HR reduction from peak exercise to the first minute after cessation of exercise in the 44 SA patients off beta-blockers. The horizontal line at 21 bpm represents the upper tertile for the entire SA population (n=44), and values above this cut-off are associated with an increased risk for cardiac events.
To assess the potential importance and mechanistic significance of the rapidity of the HR reduction, the analyses were repeated focusing on the decrease in HR from peak exercise to the 2nd minute of recovery. There were no significant differences between symptomatic and asymptomatic MCs in ΔHRmax–HR2’rec (33±11 vs 30±13 bpm, p=0.50). This suggests that the difference between symptomatic and asymptomatic depends on the rapidity of HR decrease, which is the direct consequence of the vagal activation occurring at the cessation of exercise25.
Off β-blockers - Non-SA LQTS population
The same analyses were performed in 86 mutation-confirmed LQTS patients, aged < 50 years and off-β-blockers therapy (Table 3). While the ΔHRmax – HR1’rec was not significantly different (23±9 vs 26±9 bpm, p=0.47) between symptomatic and asymptomatic LQT2/LQT3 patients (the IKs+ group), among the LQT1 patients (the IKs− group), this parameter showed the same pattern observed in the SA MCs. Indeed, the LQT1 patients with cardiac events had a significantly greater decrease in HR during the first minute of recovery from peak exercise than the asymptomatic MCs (27±10 vs 20±8 bpm, p=0.009), once again without significant differences in HRmax (141±18 vs 148±18 bpm, p=0.27) and in the maximum workload reached during the ExStrT (141±41 vs 147±49 watt, p=0.70). The validation in this independent cohort of 51 LQT1 non-A341V MCs of the finding observed in the SA A341V MCs clearly strengthens the clinical relevance of the ΔHRmax–HR1’rec parameter in differentiating between symptomatic and asymptomatic LQT1 patients independently of their specific disease-causing mutation.
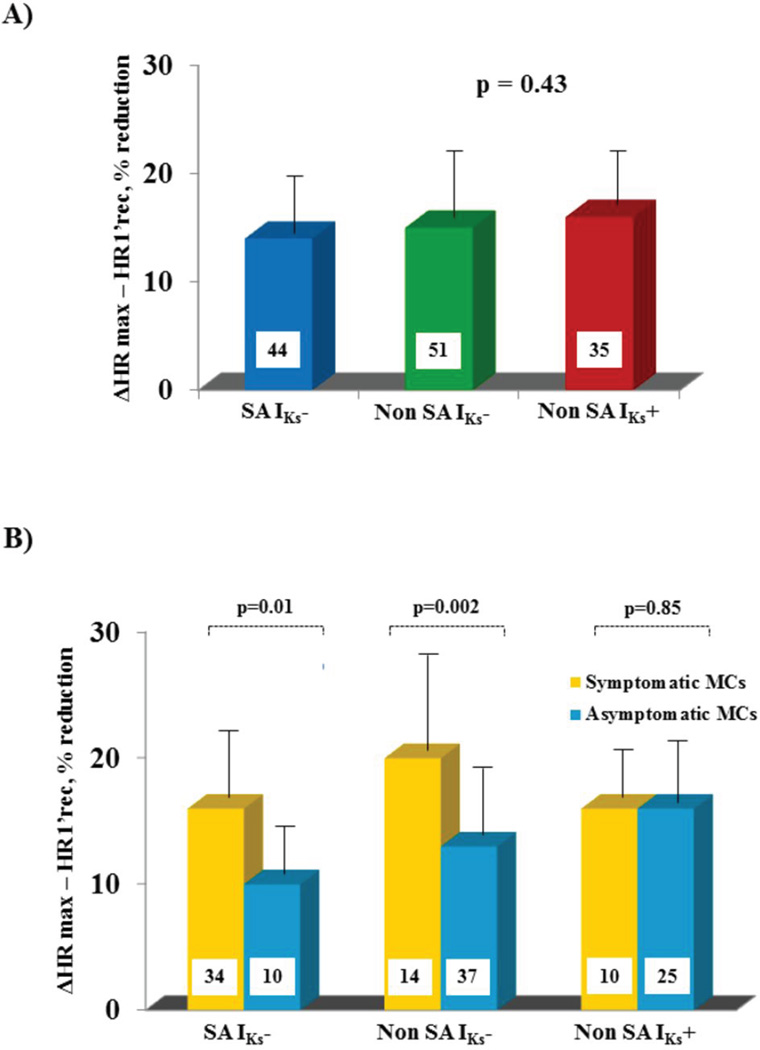
When the decrement in HR during the first minute of recovery was calculated as percent reduction from HR at peak exercise {[(ΔHRmax–HR1’rec)/HRmax]*100}, it was found to be similar (14±6%, 15±7%, and 16±6%, p=0.43) (Fig. 3A) in the SA, IKs− and IKs+ groups, respectively. However, when this decrease was related to the clinical status the differences, i.e. larger values among symptomatic than asymptomatic MCs, were fully confirmed in both discovery and validation cohorts of LQT1 patients (20±8 vs 13±5 %, p=0.002) but not at all among the patients with preserved IKs current (LQT2/3, 16±6 vs 16±6% )(Fig. 3B).
Figure 3. Post-exercise percent HR changes in the three study subgroups.
HR reduction from peak exercise to the first minute after cessation of exercise. Figure 3A shows this reduction in the three groups (SA LQT1, Non-SA LQT1, and Non-SA LQT2 and LQT3) irrespective of cardiac symptoms. Figure 3B shows this reduction within the symptomatic and the asymptomatic patients. Note the lack of difference among the patients with preserved IKs current.
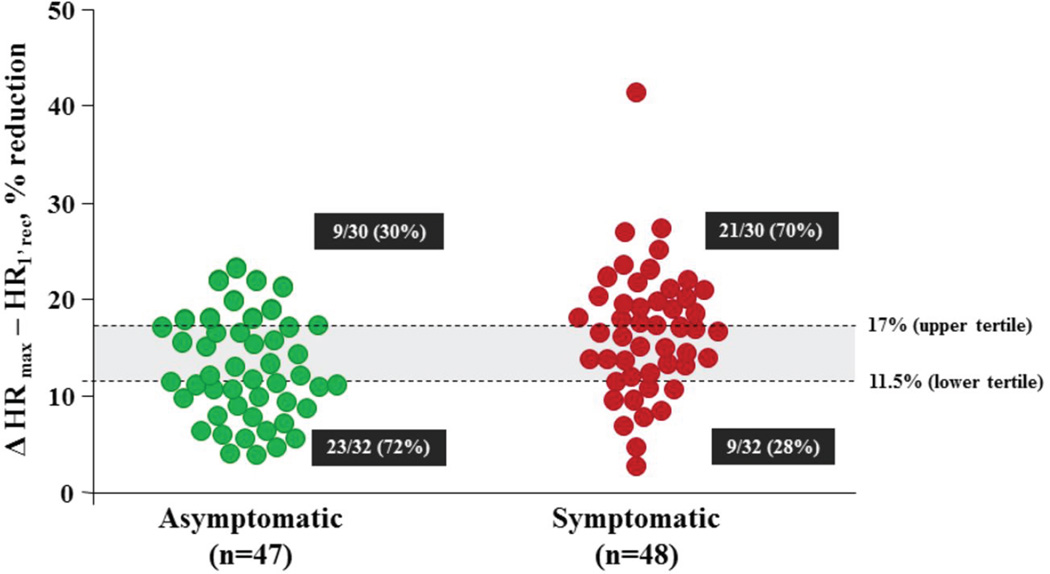
Based on the very similar behavior of the SA and non-SA LQT1 patients, they were merged in one group (IKs−) to facilitate comparisons. Given the differences in absolute HR levels between the SA and the non-SA populations, we focused on the percent change in HR. A cutoff value of 17%, corresponding to the upper tertile of the % ΔHRmax–HR1’rec distribution, usefully differentiated between symptomatic and asymptomatic MCs of both genetic subgroups. Indeed, SA and non-SA IKs− MCs (LQT1) with a HR reduction during the 1’ rec >17% of the HRmax had a significantly higher probability of having suffered cardiac events compared to those with a lower percent decrement (OR 3.28, 95% CI 1.3–8.3, p=0.012, Fig. 4). At the opposite end, HR reduction below the first tertile (11.5%) identified a significantly higher proportion (72%) of SA and non-SA LQT1 patients very likely to remain asymptomatic. The probability of having experienced cardiac events for MCs with a % ΔHRmax–HR1’rec below 11.5% vs all those with a greater decrement corresponded to an OR of 0.24 (95% CI 0.09–0.61, p=0.003).
Figure 4. Post-exercise HR changes in symptomatic and asymptomatic LQTS patients.
Percent reduction in HR from peak exercise to the first minute after cessation of exercise in the SA and Non-SA LQT1 patients (IKs−) according to presence or absence of symptoms. The horizontal lines at 17% and 11.5% represent the upper and the lower tertiles of the entire LQT1 population (n=95). The odds ratios for the risk of cardiac events are respectively 3.28 and 0.24.
On β-blockers - SA population
Forty-three SA MCs (31 symptomatic and 12 asymptomatic) had an available ExStrT on β-blocker therapy. As expected, HR both at peak exercise and during the 1st minute of recovery was lower compared to that measured off βB (HRmax, from 125±16 to 110±16 bpm; HR1’rec from 108±17 to 88±16 bpm, p<0.001 for both tests). Nonetheless, the HR decrease during the first minute of recovery from peak exercise among SA patients on therapy preserved the meaningful pattern just reported in the off therapy condition and was significantly greater in symptomatic than in asymptomatic MCs (24±8 vs 17±5 bpm, respectively, p= 0.01).
On β-blockers- Non-SA LQTS population
As for the SA population, in 30 LQT1 patients (17 symptomatic and 13 asymptomatic) with an ExStrT on β-blocker therapy, the HR decrease during the first minute 11 of recovery from peak exercise was greater in symptomatic than in asymptomatic MCs (23±9 vs 16±6 bpm, respectively, p=0.03). By contrast, in the IKs+ subset (29 LQT2, 2 LQT3), the ΔHRmax–HR1’rec on β-blocker therapy showed a totally different pattern, being greater in asymptomatic than in symptomatic subjects (24±7 bpm vs 19±6 bpm, p=0.03), thus confirming that the presence of a normal IKs prevents the phenomenon that we have observed for LQT1 patients.
Correlation between BRS and HR decrease during the first minute of recovery
We have previously shown that symptomatic LQT1 patients with the KCNQ1-A341V mutation have higher BRS values20. Here, we have demonstrated that they also have a greater HR reduction in the first minute of a recovery from exercise compared to the asymptomatic MCs. As both phenomena are due to increased vagal reflexes it seemed likely that they were correlated and that one could predict the other. To test this reasonable hypothesis we evaluated the correlation between BRS and HR decrease during the first minute of recovery from exercise.
This analysis was limited to SA MCs with BRS measurements available off ΒB therapy and we focused on the same MCs reported in the previous study20 because of the need of controlling for the effect of age on BRS21. Accordingly, we plotted the BRS values of the 22 MCs aged 26–47 years against the ΔHRmax–HR1’rec during the ExStrT and observed a strong positive correlation (r=0.64, p=0.001). A similar correlation was found also between BRS values and the percent HR reduction (r 0.69, p<0.001).
When the discriminatory power, i.e. the ability of a test to discriminate between patients with and without cardiac events, of both BRS and ΔHRmax–HR1’rec were evaluated by the use of ROC curves, the Area Under the Curve (AUC) of both tests were very similar (0.77; 95% CI 0.57–0.97 vs.0.80; 95% CI 0.61–0.99). This analysis also showed that both parameters performed moderately well (AUC >0.70) for the identification of those MCs at risk of life-threatening arrhythmias, thus implying that one could substitute for the other.
DISCUSSION
The present study provides the novel evidence that vagal reflexes, assessed by the extent of HR reduction in the first minute after cessation of an ExStrT, can contribute to identify patients at high or low risk for life-threatening arrhythmias. It also demonstrates that this relationship is gene-specific because it is valid only for LQT1 but not for LQT2 and LQT3 patients. Furthermore, and clinically relevant, the risk stratification value of HR reduction and its correlation with cardiac events is not affected by β-blocker therapy.
The underlying mechanism that explains why this is a gene-specific phenomenon is the presence or absence of a fully preserved IKs current, respectively characteristic of LQT2/LQT3 and of LQT1 patients. The possibility of using the HR changes produced by a tool as simple, inexpensive and easily available such as an ExStrT carries significant clinical implications, especially for risk stratification but also in the direction of previously unsuspected recommendation for gene-specific life-style management. It also helps to clarify the apparently complex relationship between the autonomic nervous system, mutations affecting or not affecting the IKs current, and propensity to potentially lethal arrhythmias.
Role of the ExStrT in LQTS
There has always been interest for the ExStrT in LQTS but, with few exceptions, most of the results in terms of risk stratification have been frustrating. Initially, it was performed to elicit arrhythmias as in real life with the idea of being then able to test different interventions to assess their therapeutic value, as it is routinely done for catecholaminergic polymorphic ventricular tachycardia (CPVT)28. However, at variance with CPVT, LQTS patients almost never develop arrhythmias during an ExStrT. Our interpretation has always been that probably LQTS patients with arrhythmias during exercise, and this feature is almost exclusively limited to LQT1 patients6, do so because of a combination of physical exercise with some degree of “mental activation” or psychological stress as it occurs while running with fear or in a competitive setting. By contrast, an ExStrT performed in the reassuring hospital setting, does not elicit this compounded adrenergic activation.
The focus then shifted to ECG changes during or at cessation of an ExStrT. We called attention to the diagnostic value of certain striking repolarization changes occurring within the first few minutes after cessation of exercise11, such as the appearance of notched or biphasic T waves, and we still use these changes to increase our clinical suspicion of LQTS. However, most of the investigators focused on QT interval changes at the end of an ExStrT, as we have recently reviewed29. The differences in the degree of QT prolongation after cessation of exercise seem to help differentiating between LQT1 and LQT2/330 but a relationship with a differential susceptibility to cardiac events has not been demonstrated. Recently, the interesting concept has been proposed that sudden standing from the supine position may produce differential QT changes between LQTS patients and unaffected individuals31. Even though actual exercise is not involved, this maneuver calls into question brisk autonomic changes as it happens with an ExStrT.
The role of the autonomic nervous system
Among cardiologists there is a rather widespread tendency to overlook the fact that the autonomic nervous system has two components, sympathetic and vagal, and to think primarily in terms of the sympathetic nervous system. The knowledge that sympathetic activation often plays a critical role in initiating life-threatening arrhythmias in LQTS goes back to the initial reports of 50 years ago and is nothing new. What was new was the realization that sympathetic activation has different effects according to genes involved5,6. This important and apparently puzzling phenomenon is largely, but not entirely, explained by the critical role of IKs in shortening the QT interval during HR increases as a direct result of sympathetic activation32,33. Patients with mutations affecting IKs, i.e. the LQT1 patients (IKs−, in this manuscript), being unable to appropriately shorten their QT interval when HR increases, are at high arrhythmic risk whenever sympathetic activity increases. Conversely, for the specific condition of exercise which involves progressive increase in sympathetic activity, this is not the case for the LQT2 and LQT3 patients who have a well preserved IKs (IKs+, in this manuscript). What had almost completely escaped attention was the potential impact of vagal activation in LQTS patients, not to mention a gene-specific effect. The only suggestion in this regard came by our own 2008 study20 in which we proposed that lowerthan- normal vagal reflexes might have been protective for LQT1 patients, at variance of what happens among post-myocardial infarction patients34. The present study demonstrates the previously unforeseen value for risk stratification of vagal reflexes determined by the HR changes occurring in the first minute following cessation of exercise. This prognostic information is gene-specific, as it applies only to LQT1 but not to LQT2 and LQT3 patients. These results may also contribute to explain the puzzling observation that swimming is the main trigger for cardiac events in LQT1 patients6. Indeed, swimming in cold water implies the synergistic combination of vagal activation superimposed on a condition of adrenergic activation, and in patients with an impaired IKs powerful vagal reflexes are more likely to elicit EADs and life-threatening arrhythmias.
The propensity for higher or lower vagal reflexes is largely determined at genetic level35, even though this remains so far a mostly uncharted territory. As this aspect of genetic control is totally independent of LQTS-related mutations one would expect similar HR decreases at cessation of exercise for the 3 genotypes under study here. Indeed, Fig. 3A shows that the percent reduction in HR is almost identical between IKs− and IKs+ patients, taken all together independently of their symptoms. Obviously, each of these groups comprises several different individuals and each of them has his/her individual HR response, which will reflect the combination of genetic and non-genetic factors such as physical training. Thus, the overall HR reductions will be similar among different LQTS groups as expected but, within patients with an impaired IKs, they may cluster differently according to the risk for cardiac arrhythmias. As a matter of fact, Fig. 3B shows very clearly that IKs− patients with cardiac events, never mind whether SA carriers of the A341V mutation or Italian LQT1 patients with all sorts of different mutations, have significantly greater HR reductions markers of enhanced vagal reflexes.
The significance of this finding is now clear. For patients with a preserved IKs function, such as LQT2 and LQT3, whatever happens in terms of HR changes at cessation of exercise does not matter in terms of their arrhythmic risk. By striking contrast, for patients with an impaired IKs function, the LQT1 patients, the association between their disease-causing mutation and the propensity toward powerful vagal reflexes may have life-threatening consequences.
Clinical Implications
The present results carry two precise and distinct sets of clinical implications. One concerns risk stratification, the other suggests novel recommendations for gene-specific management.
An ExStrT should be performed in every LQTS patient. In LQT2 and LQT3 patients attention will focus primarily on T wave and QT interval changes11,29,30, whereas in LQT1 patients the careful clinician will also quantify the HR reduction at 1 minute after cessation of exercise. According to the value observed, he/she will know whether the patient is at high- or low-risk for life-threatening arrhythmias and will tailor therapy accordingly, more or less aggressively.
The present data also imply the necessity of new considerations to be made when dealing with an LQT1 patient. So far, one simply had to proscribe competitive sports, using an extra word of caution for swimming3,36. We need to do more now, as the physiology underlying the present results cannot be ignored. As we have shown that powerful vagal reflexes are detrimental for LQT1 patients, action should follow. Genetic propensity cannot be altered but vagal reflexes are also modulated by specific behaviors. It is common knowledge that exercise training increases vagal activity and potentiates vagal reflexes. Given the evidence just presented of a strong correlation between the heart decrease at the first minute after cessation of exercise and BRS, it is highly relevant here the fact we have previously demonstrated, experimentally and clinically, that exercise training increases BRS and thereby potentiates vagal reflexes37,38. It follows that LQT1 patients who do not participate in competitive sports but continue to perform significant exercise on a regular basis are unconsciously increasing their vagal reflexes and making more likely the occurrence of dangerous arrhythmias. While pleasant play and occasional non-competitive sport activity should always be allowed, also because of its positive psychological counterpart, more serious and regular activity resulting in exercise training should be discouraged on the basis of the present study.
Limitations
The present data suggest, but do not prove, that the analysis of heart rate reduction immediately post-exercise can identify among asymptomatic subjects those more likely to develop cardiac symptoms in absence of therapy. This will require a rather large prospective study.
The limited sample size, direct consequence of the need to avoid the influence of age on autonomic responses, has precluded the analysis of potentially confounding variables.
Conclusion
Powerful vagal reflexes, assessed by the degree of HR reduction in the first minute after cessation of exercise, are associated with increased risk for arrhythmic cardiac events for the LQTS patients with mutations affecting the IKs current (LQT1). This phenomenon is not true for patients with a preserved IKs (LQT2 and LQT3), thus indicating its gene-specific nature. Its quantification during the performance of an ExStrT allows to refine risk stratification for LQT1 patients. Another important practical implication is that exercise training, which potentiates vagal reflexes, is contraindicated for LQT1 patients.
The association between genetically-mediated propensity for powerful vagal reflexes and the presence of mutations causing LQTS is a random event which would have probably no consequences for LQT2 and LQT3 patients whereas it could significantly increase the risk of potentially lethal arrhythmias for LQT1 patients, and represent the play of chance.
ACKNOWLEDGEMENTS
The Authors are grateful to Sara Bacchini for having contributed to the initial studies on the South African population, and to Pinuccia De Tomasi for expert editorial support.
Source of Funding: NIH grant HL68880; Telethon Italia grant GGP07016; Italian Ministry of Foreign Affairs grant 2010. Dr. Carla Spazzolini research activity’s is partially supported by an educational grant of the Fondazione IRCCS Policlinico S. Matteo, Pavia, Italy. There are no relationship with industry to be disclosed.
ABBREVIATIONS
- AUC
Area Under the Curve
- BRS
baroreflex sensitivity
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- ExStrT
exercise stress test
- HR
heart rate
- LQTS
long QT Syndrome
- MCs
mutation carriers
- QTc
QT interval corrected for heart rate
- SA
south African
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schwartz PJ, Periti M, Malliani A. The long Q-T syndrome. Am Heart J. 1975;89:378–390. doi: 10.1016/0002-8703(75)90089-7. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz PJ. Idiopathic long QT syndrome: Progress and questions. Am Heart J. 1985;109:399–411. doi: 10.1016/0002-8703(85)90626-x. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz PJ, Crotti L, Insolia R. Long QT syndrome: from genetics to management. Circulation Arrhyth Electrophysiol. 2012 doi: 10.1161/CIRCEP.111.962019. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz PJ, Stramba-Badiale M, Crotti L, et al. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–1767. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz PJ, Priori SG, Locati EH, et al. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation. 1995;92:3381–3386. doi: 10.1161/01.cir.92.12.3381. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 7.Crotti L, Lundquist AL, Insolia R, et al. KCNH2-K897T is a genetic modifier of latent congenital long QT syndrome. Circulation. 2005;112:1251–1258. doi: 10.1161/CIRCULATIONAHA.105.549071. [DOI] [PubMed] [Google Scholar]
- 8.Crotti L, Monti MC, Insolia R, et al. NOS1AP is a genetic modifier of the long-QT syndrome. Circulation. 2009;120:1657–1663. doi: 10.1161/CIRCULATIONAHA.109.879643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomás M, Napolitano C, De Giuli L, et al. Polymorphisms in the NOS1AP gene modulate QT interval duration and risk of arrhythmias in the long QT syndrome. J Am Coll Cardiol. 2010;55:2745–2752. doi: 10.1016/j.jacc.2009.12.065. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz PJ, Malliani A. Electrical alternation of the T wave. Clinical and experimental evidence of its relationship with the sympathetic nervous system and with the long QT syndrome. Am Heart J. 1975;89:45–50. doi: 10.1016/0002-8703(75)90008-3. [DOI] [PubMed] [Google Scholar]
- 11.Malfatto G, Beria G, Sala S, Bonazzi O, Schwartz PJ. Quantitative analysis of T wave abnormalities and their prognostic implications in the idiopathic long QT syndrome. J Am Coll Cardiol. 1994;23:296–301. doi: 10.1016/0735-1097(94)90410-3. [DOI] [PubMed] [Google Scholar]
- 12.Nador F, Beria G, De Ferrari GM, et al. Unsuspected echocardiographic abnormality in the long QT syndrome: diagnostic, prognostic, and pathogenetic implications. Circulation. 1991;84:1530–1542. doi: 10.1161/01.cir.84.4.1530. [DOI] [PubMed] [Google Scholar]
- 13.Haugaa KH, Edvardsen T, Leren TP, Gran JM, Smiseth OA, Amlie JP. Left ventricular mechanical dispersion by tissue Doppler imaging: a novel approach for identifying high-risk individuals with long QT syndrome. Eur Heart J. 2009;30:330–337. doi: 10.1093/eurheartj/ehn466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Ferrari GM, Schwartz PJ. Long QT syndrome, a purely electrical disease? Not anymore. Eur Heart J. 2009;30:253–255. doi: 10.1093/eurheartj/ehn587. [DOI] [PubMed] [Google Scholar]
- 15.Priori SG, Schwartz PJ, Napolitano C, et al. Risk stratification in the long-QT syndrome. New Engl J Med. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 16.Moss AJ, Shimizu W, Wilde AA, et al. Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circulation. 2007;115:2481–2489. doi: 10.1161/CIRCULATIONAHA.106.665406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz PJ. Sudden cardiac death, founder populations and mushrooms. What is the link with gold mines and modifier genes? Heart Rhythm. 2011;8:548–550. doi: 10.1016/j.hrthm.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 18.Brink PA, Crotti L, Corfield V, et al. Phenotypic variability and unusual clinical severity of congenital Long QT Syndrome in a founder population. Circulation. 2005;112:2602–2610. doi: 10.1161/CIRCULATIONAHA.105.572453. [DOI] [PubMed] [Google Scholar]
- 19.Crotti L, Spazzolini C, Schwartz PJ, et al. The common Long QT syndrome mutation KCNQ1/A341V causes unusually severe clinical manifestations in patients with different ethnic backgrounds: toward a mutation-specific risk stratification. Circulation. 2007;116:2366–2375. doi: 10.1161/CIRCULATIONAHA.107.726950. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz PJ, Vanoli E, Crotti L, et al. Neural control of heart rate is an arrhythmia risk modifier in long QT syndrome. J Am Coll Cardiol. 2008;51:920–929. doi: 10.1016/j.jacc.2007.09.069. [DOI] [PubMed] [Google Scholar]
- 21.La Rovere MT, Schwartz PJ. Baroreflex sensitivity. In: Zipes DP, Jalife J, editors. CARDIAC ELECTROPHYSIOLOGY. FROM CELL TO BEDSIDE. III EDITION. Philadelphia: WB Saunders Co.; 2000. pp. 771–781. [Google Scholar]
- 22.Levy MN, Schwartz PJ, editors. VAGAL CONTROL OF THE HEART: EXPERIMENTAL BASIS AND CLINICAL IMPLICATIONS. Armonk, NY: Futura Publishing Co; 1994. p. 644. [Google Scholar]
- 23.Imai K, Sato H, Hori M, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24:1529–1535. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 24.Smith LL, Kukielka M, Billman GE. Heart rate recovery after exercise: a predictor of ventricular fibrillation susceptibility after myocardial infarction. Am J Physiol Heart Circ Physiol. 2005;288:H1763–H1769. doi: 10.1152/ajpheart.00785.2004. [DOI] [PubMed] [Google Scholar]
- 25.Billman GE, Kukielka M. Effect of endurance exercise training on heart rate onset and heart rate recovery responses to submaximal exercise in animals susceptible to ventricular fibrillation. J Appl Physiol. 2007;102:231–240. doi: 10.1152/japplphysiol.00793.2006. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz PJ, Priori SG, Napolitano C. How really rare are rare diseases? The intriguing case of independent compound mutations in the long QT syndrome. J Cardiovasc Electrophysiol. 2003;14:1120–1121. doi: 10.1046/j.1540-8167.2003.03339.x. [DOI] [PubMed] [Google Scholar]
- 27.Westenskow P, Splawski I, Timothy KW, Keating MT, Sanguinetti MC. Compound mutations: a common cause of severe long-QT syndrome. Circulation. 2004;109:1834–1841. doi: 10.1161/01.CIR.0000125524.34234.13. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi M, Denjoy I, Extramiana F, et al. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–2434. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz PJ, Crotti L. QTc behavior during exercise and genetic testing for the long-QT syndrome. Circulation. 2011;124:2181–2184. doi: 10.1161/CIRCULATIONAHA.111.062182. [DOI] [PubMed] [Google Scholar]
- 30.Sy RW, van der Werf C, Chattha IS, et al. Derivation and validation of a simple exercise-based algorithm for prediction of genetic testing in relatives of LQTS probands. Circulation. 2011;124:2187–2194. doi: 10.1161/CIRCULATIONAHA.111.028258. [DOI] [PubMed] [Google Scholar]
- 31.Viskin S, Postema PG, Bhuiyan ZA, et al. The response of the QT interval to the brief tachycardia provoked by standing: a bedside test for diagnosing long QT syndrome. J Am Coll Cardiol. 2010;55:1955–1961. doi: 10.1016/j.jacc.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terrenoire C, Clancy CE, Cormier JW, Sampson KJ, Kass RS. Autonomic control of cardiac action potentials: role of potassium channel kinetics in response to sympathetic stimulation. Circ Res. 2005;96:e25–e34. doi: 10.1161/01.RES.0000160555.58046.9a. [DOI] [PubMed] [Google Scholar]
- 33.Silva J, Rudy Y. Subunit interaction determines IKs participation in cardiac repolarization and repolarization reserve. Circulation. 2005;112:1384–1391. doi: 10.1161/CIRCULATIONAHA.105.543306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ for the ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 35.Tank J, Jordan J, Diedrich A, et al. Genetic influences on baroreflex function in normal twins. Hypertension. 2001;37:907–910. doi: 10.1161/01.hyp.37.3.907. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz PJ. Management of the long QT syndrome. Nat Clin Pract Cardiovasc Med. 2005;2:346–351. doi: 10.1038/ncpcardio0239. [DOI] [PubMed] [Google Scholar]
- 37.Billman GE, Schwartz PJ, Stone HL. The effects of daily exercise on susceptibility to sudden cardiac death. Circulation. 1984;69:1182–1189. doi: 10.1161/01.cir.69.6.1182. [DOI] [PubMed] [Google Scholar]
- 38.La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106:945–949. doi: 10.1161/01.cir.0000027565.12764.e1. [DOI] [PubMed] [Google Scholar]