Abstract
Pseudomonas aeruginosa, a significant cause of human morbidity and mortality, uses a type 3 secretion system (T3SS) to inject effector toxins into host cells. We previously reported that P. aeruginosa uses ADP-ribosyltransferase (ADPr) activity of the T3SS effector ExoS for intracellular replication. T3SS translocon (ΔpopB)-mutants, which can export, but not translocate effectors across host membranes, retained intracellular replication. We hypothesized that secreted effectors mediate translocon-independent intracellular replication. Translocon mutants of PAO1 lacking one or more of its three known effectors (ExoS, ExoT and ExoY) were used. All translocon mutants, irrespective of effectors expressed, localized to intracellular vacuoles. Translocon-effector null mutants and translocon-exoS mutants showed defective intracellular replication. Mutants in exoT, exoY or both replicated as efficiently as translocon mutants expressing all effectors. Complementation of translocon-effector null mutants with native exoS or a membrane localization domain mutant of exoS, but not the ADPr mutant exoS (pUCPexoSE381D), restored intracellular replication, correlating with increased bacteria per vacuole. Thus, P. aeruginosa is capable of intravacuolar replication that requires ExoS ADPr activity, but not the translocon. These data suggest that T3SS effectors can participate in pathogenesis without translocon-mediated translocation across host membranes, and that intracellular bacteria can contribute to P. aeruginosa pathogenesis within epithelial cells.
Keywords: Pseudomonas aeruginosa, intracellular replication, ExoS, ADPr domain, translocon, vacuole, epithelial cells
1. Introduction
Pseudomonas aeruginosa is a leading cause of hospital-acquired respiratory pneumonia, chronic lung infection in cystic fibrosis patients, septicemia, wound infections, and corneal ulcers [1–4]. The P. aeruginosa genome encodes numerous virulence factors, environmental sensor-regulator systems, and mechanisms for antimicrobial resistance, making it a versatile and persistent pathogen [5, 6]. In recent years, many studies have shown the importance of the ExsA-regulated type 3 secretion system (T3SS) in the pathogenesis of P. aeruginosa infections [7–11]. T3SSs are used by many gram-negative bacteria to deliver effector proteins directly from the bacterial cytoplasm into the cytoplasm of host cells. This process involves a needle complex (psc genes for P. aeruginosa) required for effectors to be secreted out of the bacterium, and a translocon apparatus (pcrV, popB/popD genes for P. aeruginosa) used for pore formation in the host cell membrane such that effectors can be delivered into the host cell. There are four known P. aeruginosa T3SS effectors; ExoS, ExoU, ExoT and ExoY, each with one or more enzymatic activities that modulate host cell function and contribute to virulence [10–16].
Clinical isolates of P. aeruginosa encode different combinations of the four T3SS effectors. Almost all P. aeruginosa isolates encode ExoT, the majority encode ExoY, while genes encoding ExoU and ExoS tend to be mutually exclusive [17, 18]. Since this variability in effector expression impacts how P. aeruginosa interacts with cells, isolates have been broadly divided into two categories; invasive or cytotoxic strains. Classical invasive strains (encoding ExoS, ExoT, and often ExoY), enter, survive and replicate within epithelial cells. Cytotoxic strains (encoding ExoU, ExoT, and often ExoY) quickly kill host cells from an extracellular location since ExoU is a potent phospholipase cytotoxin [9]. Both invasive and cytotoxic strains are virulent in vertebrate and non-vertebrate models of P. aeruginosa infection [10, 11, 19–21].
Recently we reported that the T3SS system of P. aeruginosa modulated the fate of intracellular bacteria after they are internalized by epithelial cells. Wild-type invasive strains of P. aeruginosa and exoU mutants of cytotoxic strains, both localized to a novel intracellular niche which involved the formation of, and trafficking to, plasma membrane blebs [22]. In contrast, mutants lacking either the T3SS needle or T3SS effectors, lacked the capacity to form membrane bleb niches, and instead trafficked to perinuclear vacuoles that label with the late endosomal/lysosomal marker LAMP3. These differences in intracellular trafficking correlated with reduced intracellular survival/replication. Unexpectedly, the data also showed that T3SS translocon (ΔpopB) mutants retained the capacity to replicate intracellularly, even though were unable to induce and traffic to membrane blebs. These mutants were found localized to vacuoles that did not label with LAMP3. In subsequent studies using translocon competent P. aeruginosa, we found that either the ADP-ribosyltransferase (ADPr) of ExoS or the adenylate activity of ExoY could mediate membrane bleb niche formation, but that only the former enabled intracellular replication [23, 24].
The aim of the present study was to determine why translocon mutants, in constrast to needle mutants, replicate intracellularly, when neither mutant traffics to membrane blebs. Because translocon mutants, but not needle mutants, can secrete effectors into their extracellular environment, we tested the hypothesis that effector secretion enables intracellular replication by translocon mutants. The results show that ExoS, and specifically its ADPr activity, is required. The implication of these findings is that ExoS has the capacity to impact host cell function without having to be translocated across host cell membranes. The data also provide evidence that intracellular bacteria can participate in P. aeruginosa pathogenesis via local production of T3SS effectors.
2. Materials and Methods
2.1 Bacterial strains
All experiments were done using invasive P. aeruginosa strain PAO1 (Serotype O5 [25]). Mutants used are listed in Table 1. Bacteria were grown on trypticase soy agar (TSA) at 37°C overnight. For plasmid-complemented strains, TSA was supplemented with carbenicillin (500 μg/ml). For use in experiments, bacteria were suspended in tissue-culture media (KGM-2) without antibiotics to an OD650 of ~0.1 (~108 cfu/mL), and diluted in fresh KGM-2 to achieve inocula of 106 or 107 cfu/mL for intracellular replication assays or microscopy respectively.
TABLE 1.
Bacterial strains and mutants used in this study.
| Strain or Plasmid | Relevant Description | Source or Reference |
|---|---|---|
| PAO1ΔpopB* | Translocon Mutant. Expresses ExoS, ExoT, ExoY |
[11] |
| PAO1ΔpopBΔexoSΔexoTΔexoY | Translocon Mutant. Deficient in ExoS, ExoT, ExoY. No Known Effectors. |
This study |
| PAO1ΔpopBΔexoTΔexoY | Translocon Mutant. Deficient in ExoT and ExoY. Expresses ExoS. |
This Study |
| PAO1ΔpopBΔexoSΔ exoY | Translocon Mutant. Deficient in ExoS and ExoY. Expresses ExoT. |
This Study |
| PAO1ΔpopBΔexoSΔexoT | Translocon mutant. Deficient in ExoS and ExoT. Expresses ExoY. |
This Study |
| PAO1ΔpopBΔexoT | Translocon mutant. Deficient in ExoT. Expresses ExoS and ExoY. |
This Study |
| PAO1ΔpopBΔexoY | Translocon mutant. Deficient in ExoY. Expresses ExoS and ExoT. |
This study |
| PAO1ΔpopBΔexoS | Translocon mutant. Deficient in ExoS. Expresses ExoT and ExoY. |
This Study |
| pUCPexoS | Plasmid bearing exoS gene. | [42] |
| pUCPexoSE381D | Plasmid bearing exoS gene with mutation in ADPr domain. | [42] |
| pUCP18 | Vector control for complementation. | |
| PAO1ΔpopBΔexoSΔexoTΔexoY + pUCPexoS | Translocon and triple effector mutant complemented with ExoS | This Study |
| PAO1ΔpopBΔexoSΔexoTΔexoY + pUCPexoSE381D | Translocon and triple effector mutant complemented with ADPr domain mutant of ExoS. | This Study |
| PAO1ΔpopBΔexoSΔexoTΔexoY + pUCP18 | Translocon and triple effector mutant complemented with plasmid control. | This Study |
| pUCPexoSΔ51-77 | Plasmid bearing exoS gene with mutation in membrane localization domain. | [13] |
| PAO1ΔpopBΔexoSΔexoTΔexoY + pUCPexoSΔ51-77 | Translocon and triple effector mutant complemented with exoS with mutation in membrane localization domain. | This Study |
This mutant, and the type 3 secretion effector mutants without translocon mutation, were kindly provided by Dr. Arne Rietsch, Case Western Reserve University. Translocon (ΔpopB) mutations were then introduced into the effector mutants as part of the present study (see Materials and Methods).
2.2 Deletion of popB in type three secretion mutants
Chromosomal deletion of popB, which encodes a major translocon protein, was introduced into a panel of T3SS effector mutants of P. aeruginosa strain PAO1 using E. coli SM10/pEX-popB as previously described [26]. Briefly, recipient P. aeruginosa type three secretion mutants were inoculated onto Luria-Bertani (LB) agar plates and incubated overnight at 42 °C. The donor (E. coli SM10/pEX-popB) was grown on LB agar plates with gentamicin (15 μg/ml), and incubated overnight at 37 °C. Mating was performed on a LB plate for 1 h at 37 °C, and co-integrates selected by plating the mating mixture on LB agar plates supplemented with gentamicin (30 μg/mL) and triclosan (5 μg/mL), and incubation overnight at 37 °C. Single colonies were re-plated and grown again on the same selective media under the same conditions. Single colonies were then inoculated into LB broth with 5% sucrose without sodium chloride, grown for 2 h at 37 °C with shaking, then plated on LB agar plates with 5 % sucrose and incubated overnight at 30 °C. Colonies were patched on LB-gentamicin-triclosan plates. Colonies that grew on the sucrose agar, but not on the LB-gentamicin-triclosan agar, were saved and frozen at −80 °C. Deletion of popB gene was confirmed by polymerase chain reaction (PCR) using primers designed to amplify popB gene [17], and deletion re-confirmed in triplicate for each mutant used (data not shown). Western immunoblot confirmed the absence of the PopB protein in popB mutants grown under T3SS-inducing conditions (data not shown).
2.3 Cell culture
Human telomerase-immortalized corneal epithelial cells (hTCEpi) were grown in 75 mm tissue culture flasks with vented caps at 37 °C with 5% CO2 [27]. Every two days, cells were washed with PBS (Sigma-Aldrich, MO) and replenished with fresh keratinocyte growth media (KGM-2) supplemented with antibiotics (gentamicin, penicillin, streptomycin, fungizone) (UCSF Cell Culture Facility, CA). Before each experiment, cells were seeded onto 12-well culture-treated plates (intracellular replication assay) or 22-mm glass coverslips (microscopy), the latter placed in 6 well of non-tissue culture treated plates (Becton Dickinson, NJ). Cells were grown to ~80% confluence. Two days before an experiment, cells were fed with KGM-2 supplemented with only gentamicin. Before and during experiments, hTCEpi were grown at 37 °C with 5% CO2.
2.4 Intracellular Replication Assays
Intracellular replication assays were performed as described previously [23]. Briefly, hTCEpi cultured in 12 well tissue culture plates were washed twice with sterile PBS, then inoculated with ~1 × 106 cfu bacteria in 1 mL of media. Bacteria and epithelial cells were incubated at 37 °C for 3 h. After removing the bacterial suspension, the epithelial cells were washed three times with sterile PBS, followed by 1 h or 4 h of gentamicin treatment (KGM-2 supplemented with 200 μg/ml gentamicin) to kill remaining extracellular bacteria. Data were expressed as a mean ± SD % increase in viable intracellular bacteria over 3 h (i.e. 1 h versus 4 h gentamicin treatments) over three independent experiments.
2.5 Quantification of Bacterially Occupied Vacuoles
The hTCEpi were grown on glass coverslips, washed three times with PBS, and infected with ~2 × 107 cfu bacteria in 2 mL of media. After a 3 h incubation, coverslips were washed three times with PBS, then KGM-2 supplemented with 200 μg/ml gentamicin added for a further 4 h. For microscopy, coverslips in gentamicin-KGM-2 media were placed in Attofluor Cell Chambers (Molecular Probes, OR) and maintained at 37 °C for up to 1 h. Phase-contrast microscopy was performed at 1000 × magnification, and still images captured for analysis using a computer with Volocity™ image analysis software. Bacteria-occupied vacuoles and the number of intravacuolar bacteria per vacuole were quantified by randomly selecting 20 fields per sample, each field with 8 or more epithelial cells. A total of ~ 65 bacteria-occupied vacuoles were quantified per sample per experiment. For each sample, data were expressed as the number of bacteria per vacuole, and observed frequency of the event, as a percentage of the total number of occupied vacuoles. Data were pooled from 3 independent experiments (~195 bacteria occupied vacuoles per sample).
2.6 Statistical analysis
For statistical analysis of intracellular replication assays, ANOVA was used with Fisher PLSD for post-hoc analysis. For quantification of intracellular occupied vacuoles, Student’s t-test was used. In both types of analysis, P values < 0.05 were considered significant.
3. Results
3.1 Role of effectors in translocon-independent intracellular replication
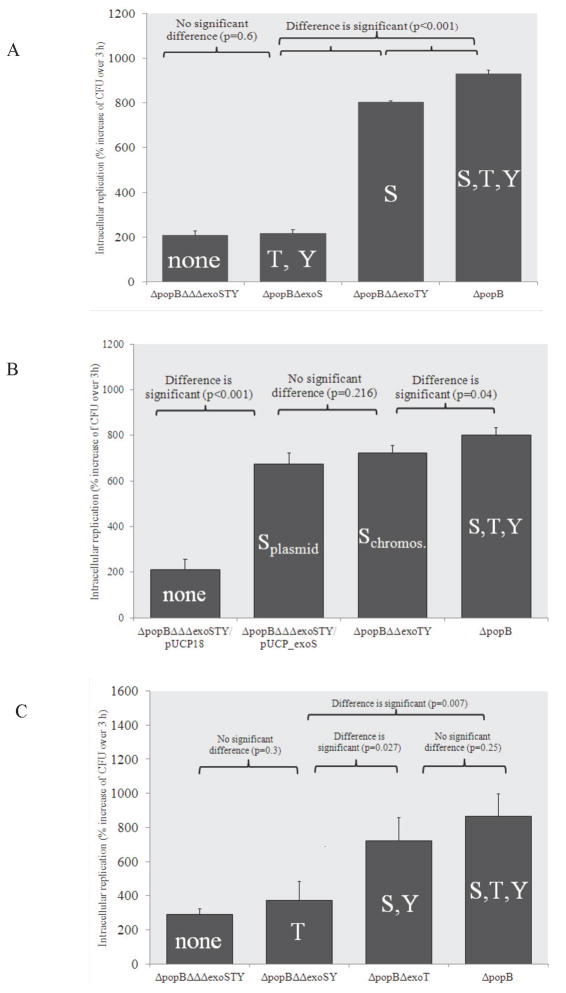
To determine if T3SS effectors were involved in translocon independent intracellular replication, a translocon mutant lacking all three known effectors (ΔpopBΔexoSΔexoTΔexoU) was compared to a translocon mutant with functional effector genes (ΔpopB mutant) in intracellular survival assays. As expected [22], translocon mutants expressing all three effectors of PAO1 (ExoS, ExoT and ExoY) replicated within corneal epithelial cells (Fig. 1A). In contrast, translocon-effector null mutants were significantly reduced in intracellular replication (Fig. 1A). This showed that effector expression is required for intracellular replication in the absence of the translocon.
Figure 1.
Intracellular replication of translocon (ΔpopB) mutants of P. aeruginosa strain PAO1 combined with single, double or triple mutations in known type three secreted effectors. (A) Expression of ExoS alone in a translocon-null background was sufficient to maintain intracellular replication similar to the translocon-null wild-type (ΔpopB). Combined expression of ExoT and ExoY did not confer intracellular replication. (B) Complementation of a translocon and triple effector mutant (PAO1ΔpopBΔΔΔexoSTY) with pUCPexoS was sufficient for intracellular replication. (C) Combined expression of ExoS and ExoY also maintained intracellular replication, but expression of ExoT alone did not. Epithelial cells were inoculated with ~106 cfu bacteria, incubated for 3 h, followed by 1 h or 4 h of gentamicin exposure (see methods) to kill extracellular bacteria. Data are expressed as the mean ± SD % increase in viable intracellular bacteria over 3 h (1 h versus 4 h gentamicin treatments) for each mutant over 3 independent experiments.
Since ExoS is critical for intracellular replication when the translocon is functional, its role in translocon-independent intracellular replication was examined. This involved comparing translocon mutant replication with and without effectors to translocon-exoS mutants (express both ExoY and ExoT) and translocon-exoTexoY mutants (express only ExoS). The translocon mutant expressing only ExoS replicated as efficiently as the translocon mutant expressing all three effectors, while the translocon mutant lacking ExoS that expresses ExoT and ExoY was as defective in replication as a translocon mutant lacking all three effectors (Fig. 1A). Thus, expression of ExoS is sufficient for translocon independent intracellular replication.
Complementation of the translocon efffector mutant with pUCPexoS restored intracellular replication to that of the translocon mutant expressing all known effectors (Fig. 1B). These data show that exoS is sufficient for P. aeruginosa to replicate intracellularly in the absence of the popB translocon gene, i.e. exoT and exoY were not required for intracellular replication under these conditions.
Since ExoT is functionally similar to ExoS in that it also has both an ADPr domain and a GAP domain, its capacity to confer intracellular replication was also explored. Intracellular replication by translocon-exoT mutants (express ExoS and ExoY) was not significantly different from translocon mutants expressing all three effectors, while a translocon-exoSexoY mutant (expresses only ExoT) was similar to translocon mutants lacking all three effectors of PAO1 (Fig. 1C). Thus, ExoT, in contrast to ExoS, cannot enable translocon independent intracellular replication.
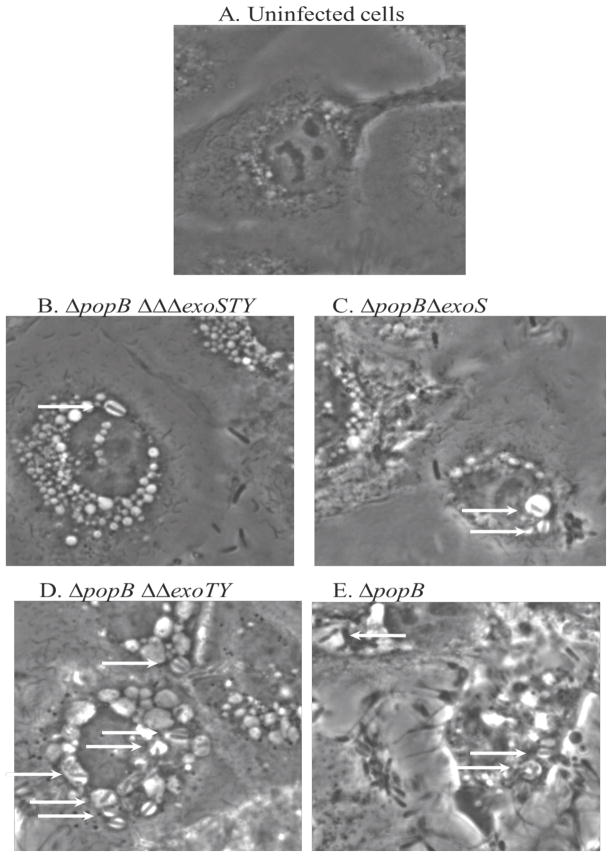
3.2 Translocon mutant localization and impact on epithelial cell morphology
As expected, all of the translocon mutants, irrespective of effector expression, localized to intracellular vacuoles, and membrane bleb formation (which requires the translocon) was not observed (Fig 2). Surprisingly, epithelial cells infected with the translocon mutants showed signs of general stress, as evidenced by extensive cytosolic vacuolization (Fig. 2). Indeed, even the translocon mutants lacking all known effectors induced vacuolization (Fig. 2B). The capacity to express ExoT and ExoY did not worsen the effect, as demonstrated by the very similar impact of a translocon/exoS mutant (Fig. 2C). In contrast, the capacity to secrete ExoS (translocon/exoT/exoY mutants) induced the formation of more and larger vacuoles, and there appeared to be a greater number of bacteria within them (Fig. 2D). Altered morphology by translocon mutants was even more marked in cells infected with mutants expressing all three effectors (ExoS, ExoT, and ExoY), with cell rounding and retraction also occurring (Fig. 2E). This suggests that while ExoT and ExoY are not sufficient to impact cell morphology in the translocon mutant background, they can synergize with ExoS to enhance its effects.
Figure 2.
For phase-contrast microscopy of human corneal epithelial cells after incubation with ~2 × 107 cfu translocon/effector mutants of P. aeruginosa strain PAO1 for 3 h, followed by 4 h of gentamicin treatment (see methods). (A) Uninfected cells appeared healthy. (B, C) Without all known effectors, or ExoS alone, single bacterial cells were located within vacuoles (arrows) and epithelial cells showed increased numbers of vacuoles, but showed normal surface attachment, (D) With ExoS alone, many vacuoles contained single or multiple bacteria, but the epithelial cells remained attached, (E) With all known effectors present, vacuoles also contained multiple bacteria (arrows) but the epithelial cells also showed surface retraction.
3.3 The role of ExoS ADPr activity, the membrane localization domain (MLD) and GAP activity in translocon-independent intracellular replication
ExoS has two known enzymatic activities, provided by an ADP-ribosyltransferase (ADPr) domain and a Rho GTPase-activating protein (GAP) domain. ADPr activity of ExoS requires binding to factor activating ExoS (FAS) protein, a member of 14-3-3 protein family, and it acts on (ADP-ribosylates) numerous host targets including; Ras small GTPases, Rab proteins, Hsp27, vimentin, and ERM (erzin/radixin/moesin) proteins of the host mammalian cell to modify intracellular signaling, trafficking and cytoskeleton function [14, 28–33]. RhoGAP activity of ExoS, like that of ExoT, targets RhoA, Rac1, Cdc42 of the host mammalian cell to also interfere with cytoskeleton function [34–36]. The membrane localization domain targets ExoS to the host cell plasma membrane and membranes of perinuclear endosomes. It has also been shown to be important for RhoGAP specificity, and ADPr activity of ExoS in cell-based assays, but not for T3SS secretion [13, 37, 38].
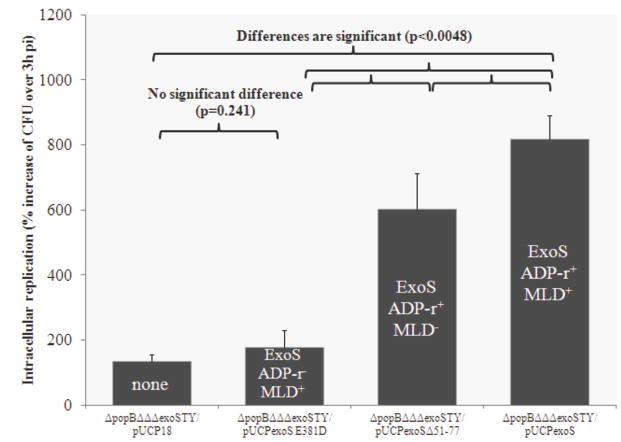
We previously showed that intracellular replication in the presence of a functional translocon requires the ADPr activity of ExoS, but not the GAP activity or the MLD. Thus, we hypothesized that the ADPr activity also confers the capacity for intracellular replication in the absence of the translocon. To test this, translocon-effector null mutants were complemented with functional and mutant forms of exoS, which were then compared in intracellular replication assays. They included native exoS (pUCPexoS), ADPr inactive exoS (pUCPexoSE381D), exoS with a deletion in the MLD (pUCPexoSΔ51-77), and a plasmid control (pUCP18).
Complementation with ADPr active exoS, but not ADPr inactive exoS rescued intracellular replication (Fig. 3), showing that the ADPr activity of ExoS is indeed required for translocon-independent intracellular replication. Bacteria containing the MLD mutant form of exoS (ADPr activity remains active) were able to replicate intracellularly, but not to the level of bacteria with native exoS (Fig. 3). There was no difference in intracellular survival rates between bacteria containing a plasmid vector control and those containing a mutant form of exoS expressing only GAP activity (Fig. 3), suggesting that ExoS GAP activity alone did not affect intracellular replication.
Figure 3.
Intracellular replication assay showing complementation of a translocon/triple effector mutant (PAO1ΔpopBΔΔΔexoSTY) with an inactive membrane-localization domain form of ExoS (pUCPexoSΔ51-77), caused a small decrease in intracellular replication compared to bacteria complemented with ExoS (pUCPexoS). However, both of those complemented bacteria showed significantly greater intracellular replication than translocon/triple effector mutants complemented with pUCPexoSE381D (ADPr inactive) which was not different from plasmid control. Thus, the MLD was not required for translocon-independent intracellular replication mediated by ExoS ADPr activity. Epithelial cells were inoculated with ~106 cfu bacteria, incubated for 3 h, followed by 1 h or 4 h of gentamicin exposure (see methods) to kill extracellular bacteria. Data are expressed as the mean ± SD % increase in viable intracellular bacteria over 3 h (1 h versus 4 h gentamicin treatments) for each mutant over 3 independent experiments.
3.4 Translocon mutants expressing ExoS ADPr activity show increased numbers of bacteria per vacuole
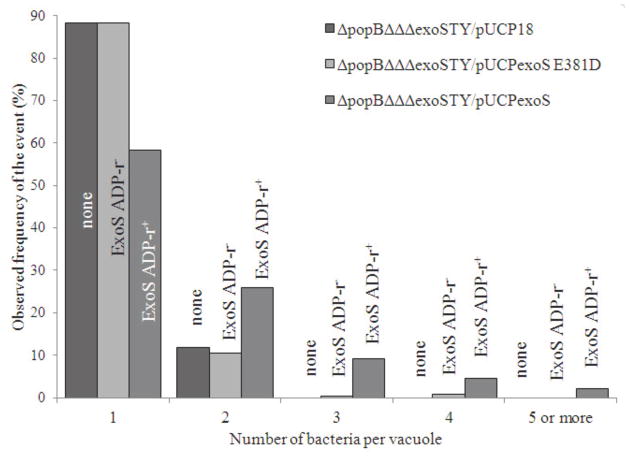
Since the ADPr activity of ExoS was found to confer intracellular replication by translocon mutants, and since translocon mutants localize to vacuoles, we hypothesized that translocon mutants with ExoS ADPr activity would show increased numbers of bacteria per vacuole. Phase contrast imaging of infected cells had revealed that the majority of translocon-effector mutants lacking exoS, or those possessing only ADPr-inactive exoS, resulted in single bacterial occupancy of vacuoles (Fig. 2). In contrast, cells infected with translocon mutants possesing ADPr-active exoS showed multiple bacteria within individual vacuoles (Fig. 2). Thus, in another experiment, the number of bacteria within vacuoles was quantified and subject to statistical analysis for three bacterial populations; translocon-effector null mutants complemented with functional exoS (pUCPexoS), those complemented with ADPr-inactive exoS (pUCPexoSE381D), or plasmid control (pUCP18) (Fig. 4). Statistically significant differences (increased) numbers of bacteria per vacuole were found with ADPr-active (pUCPexoS) versus ADPr-inactive (pUCPexoSE381D) bacteria (p < 0.001), and between ADPr-active and cotnrol plasmid populations (p < 0.001). There was no statistically significant difference found between ADPr-inactive mutants and the plasmid control complemented bacteria (p = 0.2). These data suggest that intracellular replication of translocon mutants conferred by ExoS occurs within vacuoles.
Figure 4.
Quantification of numbers of bacteria per vacuole within epithelial cells using phase-contrast microcsopy 8 h after infection with a translocon/triple effector mutant of P. aeruginosa strain PAO1 complemented with exoS (pUCPexoS) or ADPr inactive exoS (pUCPexoSE381D). Complementation with ADPr-inactive exoS resulted similar numbers of bacteria per vacuole as the plasmid control (p = 0.2, t-Test), i.e. one bacterial cell per vacuole represented the majority of observations. Complementation with pUCPexoS was associated with more bacteria (up to 5) per vacuole (p < 0.001, t-Test versus pUCPexoSE381D). Data from 20 randomly selected fields per sample, each field with 8 or more epithelial cells. Over 3 independent experiments, ~195 bacteria occupied vacuoles were quantified per sample (see Materials and Methods).
4. Discussion
We previously reported that the ADPr activity of ExoS enables P. aeruginosa to replicate within epithelial cells. The data presented in this study show that this function of ExoS does not require the T3SS translocon or the MLD of ExoS, and that ExoS ADPr activity is associated with increased bacterial numbers within epithelial cell vacuoles.
It is generally thought that T3SS effectors such as ExoS which are secreted via the type 3 secretion needle apparatus, need to be translocated into the host cell cytoplasm via the translocon pore so that they can exert their biochemical effects on cytoplasmic proteins [7]. The data presented in this report show that effector translocation is not required for exoS-mediated intracellular replication of P. aeruginosa.
Host cell contact triggers ExoS secretion [39], but does not do so in translocon mutants [26]. Thus, ADP-ribosylation of host proteins outside of host cells, e.g. serum IgG3 and apolipoprotein A1 [40], is unlikely to explain ExoS-mediated intracellular replication by translocon mutants. Instead, it is likely that ExoS is expressed after bacterial internalization, and within the vacuoles in which these translocon mutant bacteria are trapped. A likely trigger for T3SS would be the low calcium environment that exists within cells, low calcium being a known inducer of the T3SS in P. aeruginosa. How ExoS produced in a vacuole exerts it effects without the translocon is not clear. One possibility is that ExoS modifies the intra-vacuolar environment to allow bacterial survival and replication. Such a mechanism would be consistent with our finding that intracellular replication does not need the MLD of ExoS for either wild-type or translocon mutants [23]. An alternative would be if ExoS exits the intracellular vacuoles into the cytoplasm without the assistance of the translocon. There, it could ADP-ribosylate known cytosolic targets, such as Rab5 and Rab9 which can each modulate endosomal trafficking [41]. The morphological changes found to occur in epithelial cells infected with exoS-expressing translocon mutants compared to translocon mutants that can’t express ExoS, show that there is indeed ExoS mediated intoxication. Further research will be needed to determine exactly how this occurs.
Surprisingly, cells infected with P. aeruginosa mutants lacking the translocon and all known T3SS effectors showed extensive vacuolization compared to control uninfected cells. This could be a consequence of the T3SS needle or other residual T3SS components, or the mechanism might be independent of the T3SS, since P. aeruginosa produces other virulence factors, including proteases, phosholipases, and other toxins. Alternatively, vacuolization could be a deliberate defense response of the host cell in response to bacterial invasion for the purpose of degrading internalized bacteria. Indeed, these translocon/ effector null mutants did not replicate within the epithelial cells studied.
In cell-based assays, Barbieri and colleagues showed that the ADPr activity of exoS towards Ras, and other small-molecular-weight proteins, was diminished in the absence of the MLD, but not other ExoS cytotoxic effects [13]. Here, we showed that the MLD was not required for translocon-independent intracellular replication conferred by the ADPr activity of ExoS. The latter finding is probably not an artifact associated with the use of a translocon mutant; in an earlier study we found that MLD was not required for ExoS ADPr effects using bacteria that were not translocon mutants [23]. There are several differences between this and the previous study including different P. aeruginosa strains (PAO1 versus PA103) and different host cell types (corneal epithelial cells versus CHO cells). Indeed, an exoUexoT double mutant of strain PA103 (its wild-type is cytotoxic to host cells) can also replicate intracellularly and form membrane bleb-niches, but does not encode ExoS [22]. Further studies will be needed to elucidate molecular mechanisms for ExoS ADPr-mediated intracellular survival, with or without the translocon, and the lack of requirement for the MLD in this regard.
In conclusion, the data presented in this study show that the role of ExoS ADPr activity in P. aeruginosa intracellular survival does not require the T3SS translocon. This differs from membrane bleb-niche formation, another phenomenon dependent on the ADPr activity of ExoS, which utilizes the translocon. Translocon-independent intracellular replication conferred by ExoS involved an increase in bacterial numbers per vacuole suggesting that it occurs within the vacuoles. That would be interesting because previously described targets of the ADPr activity of ExoS are all cytosolic, and since the translocon is the only known mechanism by which T3SS effectors cross host cell membranes, this suggests intravacuolar targets, or that ExoS can cross host cell membranes without the T3SS translocon to access known cytosolic targets. Further research will be required to establish if either is the case. Whatever the mechanism involved, the data presented in this report provide evidence that intracellular bacteria can contribute directly to cellular pathogenesis by P. aeruginosa despite the fact that it is commonly considered an extracellular pathogen.
Acknowledgments
Supported by the National Institute of Allergy and Infectious Diseases (AI079192, SMJF), and the National Eye Institute (EY020111, VH). We would like to also express our continued thanks to Dr. Joseph Barbieri, Medical College of Wisconsin, and Dr. Arne Rietsch, Case Western Reserve University, for their helpful advice and reagents used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eber MR, Shardell M, Schweizer ML, Laxminarayan R, Perencevich EN. Seasonal and temperature-associated increases in gram-negative bacterial bloodstream infections among hospitalized patients. PLoS One. 2011;6:e25298. doi: 10.1371/journal.pone.0025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandiumenge A, Rello J. Ventilator-associated pneumonia caused by ESKAPE organisms: cause, clinical features, and management. Curr Opin Pulm Med. 2012;18:187–193. doi: 10.1097/MCP.0b013e328351f974. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton F, Carnt N. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye (Lond) 2012;26:185–193. doi: 10.1038/eye.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McColley SA, Ren CL, Schechter MS, Regelmann WE, Pasta DJ, Konstan MW. Risk factors for onset of persistent respiratory symptoms in children with cystic fibrosis. Pediatr Pulmonol. 2012 doi: 10.1002/ppul.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breidenstein EB, de la Fuente-Nunez C, Hancock RE. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 2011;19:419–426. doi: 10.1016/j.tim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Mathee K, Narasimhan G, Valdes C, Qiu X, Matewish JM, Koehrsen M, Rokas A, Yandava CN, Engels R, Zeng E, Olavarietta R, Doud M, Smith RS, Montgomery P, White JR, Godfrey PA, Kodira C, Birren B, Galagan JE, Lory S. Dynamics of Pseudomonas aeruginosa genome evolution. Proc Natl Acad Sci U S A. 2008;105:3100–3105. doi: 10.1073/pnas.0711982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol. 2009;7:654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engel J, Balachandran P. Role of Pseudomonas aeruginosa type III effectors in disease. Curr Opin Microbiol. 2009;12:61–66. doi: 10.1016/j.mib.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Fleiszig SM, Wiener-Kronish JP, Miyazaki H, Vallas V, Mostov KE, Kanada D, Sawa T, Yen TS, Frank DW. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee EJ, Cowell BA, Evans DJ, Fleiszig SM. Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Invest Ophthalmol Vis Sci. 2003;44:3892–3898. doi: 10.1167/iovs.02-1302. [DOI] [PubMed] [Google Scholar]
- 11.Vance RE, Rietsch A, Mekalanos JJ. Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect Immun. 2005;73:1706–1713. doi: 10.1128/IAI.73.3.1706-1713.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci U S A. 1998;95:13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pederson KJ, Krall R, Riese MJ, Barbieri JT. Intracellular localization modulates targeting of ExoS, a type III cytotoxin, to eukaryotic signalling proteins. Mol Microbiol. 2002;46:1381–1390. doi: 10.1046/j.1365-2958.2002.03256.x. [DOI] [PubMed] [Google Scholar]
- 14.Barbieri JT, Sun J. Pseudomonas aeruginosa ExoS and ExoT. Rev Physiol Biochem Pharmacol. 2004;152:79–92. doi: 10.1007/s10254-004-0031-7. [DOI] [PubMed] [Google Scholar]
- 15.Anderson DM, Schmalzer KM, Sato H, Casey M, Terhune SS, Haas AL, Feix JB, Frank DW. Ubiquitin and ubiquitin-modified proteins activate the Pseudomonas aeruginosa T3SS cytotoxin, ExoU. Mol Microbiol. 2011;82:1454–1467. doi: 10.1111/j.1365-2958.2011.07904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zolfaghar I, Evans DJ, Ronaghi R, Fleiszig SM. Type III secretion-dependent modulation of innate immunity as one of multiple factors regulated by Pseudomonas aeruginosa RetS. Infect Immun. 2006;74:3880–3889. doi: 10.1128/IAI.01891-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feltman H, Schulert G, Khan S, Jain M, Peterson L, Hauser AR. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology. 2001;147:2659–2669. doi: 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- 18.Zhu H, Conibear TC, Bandara R, Aliwarga Y, Stapleton F, Willcox MD. Type III secretion system-associated toxins, proteases, serotypes, and antibiotic resistance of Pseudomonas aeruginosa isolates associated with keratitis. Curr Eye Res. 2006;31:297–306. doi: 10.1080/02713680500536746. [DOI] [PubMed] [Google Scholar]
- 19.Shaver CM, Hauser AR. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect Immun. 2004;72:6969–6977. doi: 10.1128/IAI.72.12.6969-6977.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee VT, Smith RS, Tummler B, Lory S. Activities of Pseudomonas aeruginosa effectors secreted by the Type III secretion system in vitro and during infection. Infect Immun. 2005;73:1695–1705. doi: 10.1128/IAI.73.3.1695-1705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyata S, Casey M, Frank DW, Ausubel FM, Drenkard E. Use of the Galleria mellonella caterpillar as a model host to study the role of the type III secretion system in Pseudomonas aeruginosa pathogenesis. Infect Immun. 2003;71:2404–2413. doi: 10.1128/IAI.71.5.2404-2413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angus AA, Lee AA, Augustin DK, Lee EJ, Evans DJ, Fleiszig SM. Pseudomonas aeruginosa induces membrane blebs in epithelial cells, which are utilized as a niche for intracellular replication and motility. Infect Immun. 2008;76:1992–2001. doi: 10.1128/IAI.01221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angus AA, Evans DJ, Barbieri JT, Fleiszig SM. The ADP-ribosylation domain of Pseudomonas aeruginosa ExoS is required for membrane bleb niche formation and bacterial survival within epithelial cells. Infect Immun. 2010;78:4500–4510. doi: 10.1128/IAI.00417-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hritonenko V, Mun JJ, Tam C, Simon NC, Barbieri JT, Evans DJ, Fleiszig SM. Adenylate cyclase activity of Pseudomonas aeruginosa ExoY can mediate bleb-niche formation in epithelial cells and contributes to virulence. Microb Pathog. 2011;51:305–312. doi: 10.1016/j.micpath.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makin SA, Beveridge TJ. Pseudomonas aeruginosa PAO1 ceases to express serotype-specific lipopolysaccharide at 45 degrees C. J Bacteriol. 1996;178:3350–3352. doi: 10.1128/jb.178.11.3350-3352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cisz M, Lee PC, Rietsch A. ExoS controls the cell contact-mediated switch to effector secretion in Pseudomonas aeruginosa. J Bacteriol. 2008;190:2726–2738. doi: 10.1128/JB.01553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, Cavanagh HD, Jester JV. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2005;46:470–478. doi: 10.1167/iovs.04-0528. [DOI] [PubMed] [Google Scholar]
- 28.Masters SC, Pederson KJ, Zhang L, Barbieri JT, Fu H. Interaction of 14-3-3 with a nonphosphorylated protein ligand, exoenzyme S of Pseudomonas aeruginosa. Biochemistry. 1999;38:5216–5221. doi: 10.1021/bi982492m. [DOI] [PubMed] [Google Scholar]
- 29.Maresso AW, Deng Q, Pereckas MS, Wakim BT, Barbieri JT. Pseudomonas aeruginosa ExoS ADP-ribosyltransferase inhibits ERM phosphorylation. Cell Microbiol. 2007;9:97–105. doi: 10.1111/j.1462-5822.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 30.Ganesan AK, Frank DW, Misra RP, Schmidt G, Barbieri JT. Pseudomonas aeruginosa exoenzyme S ADP-ribosylates Ras at multiple sites. J Biol Chem. 1998;273:7332–7337. doi: 10.1074/jbc.273.13.7332. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Wang H, Masters SC, Wang B, Barbieri JT, Fu H. Residues of 14-3-3 zeta required for activation of exoenzyme S of Pseudomonas aeruginosa. Biochemistry. 1999;38:12159–12164. doi: 10.1021/bi991019l. [DOI] [PubMed] [Google Scholar]
- 32.Fraylick JE, Riese MJ, Vincent TS, Barbieri JT, Olson JC. ADP-ribosylation and functional effects of Pseudomonas exoenzyme S on cellular RalA. Biochemistry. 2002;41:9680–9687. doi: 10.1021/bi025826n. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Deng Q, Barbieri JT. Intracellular localization of type III-delivered Pseudomonas ExoS with endosome vesicles. J Biol Chem. 2007;282:13022–13032. doi: 10.1074/jbc.M606305200. [DOI] [PubMed] [Google Scholar]
- 34.Goehring UM, Schmidt G, Pederson KJ, Aktories K, Barbieri JT. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J Biol Chem. 1999;274:36369–36372. doi: 10.1074/jbc.274.51.36369. [DOI] [PubMed] [Google Scholar]
- 35.Sun J, Maresso AW, Kim JJ, Barbieri JT. How bacterial ADP-ribosylating toxins recognize substrates. Nat Struct Mol Biol. 2004;11:868–876. doi: 10.1038/nsmb818. [DOI] [PubMed] [Google Scholar]
- 36.Krall R, Sun J, Pederson KJ, Barbieri JT. In vivo rho GTPase-activating protein activity of Pseudomonas aeruginosa cytotoxin ExoS. Infect Immun. 2002;70:360–367. doi: 10.1128/IAI.70.1.360-367.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riese MJ, Barbieri JT. Membrane localization contributes to the in vivo ADP-ribosylation of Ras by Pseudomonas aeruginosa ExoS. Infect Immun. 2002;70:2230–2232. doi: 10.1128/IAI.70.4.2230-2232.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Deng Q, Porath JA, Williams CL, Pederson-Gulrud KJ, Barbieri JT. Plasma membrane localization affects the RhoGAP specificity of Pseudomonas ExoS. Cell Microbiol. 2007;9:2192–2201. doi: 10.1111/j.1462-5822.2007.00949.x. [DOI] [PubMed] [Google Scholar]
- 39.Vallis AJ, Yahr TL, Barbieri JT, Frank DW. Regulation of ExoS production and secretion by Pseudomonas aeruginosa in response to tissue culture conditions. Infect Immun. 1999;67:914–920. doi: 10.1128/iai.67.2.914-920.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knight DA, Barbieri JT. Ecto-ADP-ribosyltransferase activity of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1997;65:3304–3309. doi: 10.1128/iai.65.8.3304-3309.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng Q, Barbieri JT. Modulation of host cell endocytosis by the type III cytotoxin, Pseudomonas ExoS. Traffic. 2008;9:1948–1957. doi: 10.1111/j.1600-0854.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radke J, Pederson KJ, Barbieri JT. Pseudomonas aeruginosa exoenzyme S is a biglutamic acid ADP-ribosyltransferase. Infect Immun. 1999;67:1508–1510. doi: 10.1128/iai.67.3.1508-1510.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]