Summary
Epithelial-Mesenchymal Transition (EMT) is implicated in converting stationary epithelial tumor cells into motile mesenchymal cells during metastasis. However, the involvement of EMT in metastasis is still controversial due to the lack of a mesenchymal phenotype in human carcinoma metastases. Using a spontaneous squamous cell carcinoma mouse model, we show that activation of the EMT-inducing transcription factor Twist1 is sufficient to promote carcinoma cells to undergo EMT and disseminate into blood circulation. Importantly, in distant sites, turning off Twist1 to allow reversion of EMT is essential for disseminated tumor cells to proliferate and form metastases. Our study demonstrates in vivo the requirement of “reversible EMT” in tumor metastasis and may resolve the controversy on the importance of EMT in carcinoma metastasis.
Introduction
During metastasis, epithelial tumor cells invade surrounding extracellular matrix (ECM), disseminate into the systemic circulation, and then establish secondary tumors in distant sites. A developmental program termed Epithelial-Mesenchymal Transition (EMT) has been implicated in giving rise to the dissemination of single carcinoma cells. During EMT, stationary epithelial cells lose their epithelial characteristics, including adherent junctions and apical-basal polarity, and acquire a mesenchymal morphology and the ability to migrate and invade (Hay, 1995). Biochemically, cells switch off the expression of epithelial markers such as adherens junction proteins E-cadherin and catenins, and turn on mesenchymal markers including vimentin and fibronectin. Studies using cell culture and tumor xenograft models show that activation of EMT promotes carcinoma cells to dissociate from each other and metastasize to distant organs (Hay, 1995; Kalluri and Weinberg, 2009; Thiery, 2002; Thiery et al., 2009).
However, the involvement of EMT in tumor metastasis in vivo is still hotly debated (Garber, 2008; Ledford, 2011; Tarin et al., 2005; Thompson et al., 2005). In human carcinoma, although primary tumors show many morphological and molecular features of EMT in subpopulations of invasive cells, distant metastases present an epithelial morphology (Peinado et al., 2007). This phenomenon contradicts the assumption that activation of EMT in tumor cells should result in metastases with a mesenchymal phenotype, therefore casting doubts on the occurrence of EMT during metastasis. This discrepancy could be due to the interpretation of the EMT program as a permanent non-reversible course during tumor metastasis. A reversible EMT model has been proposed to explain this apparent paradox: carcinoma cells undergo EMT to invade and disseminate from the primary tumor; once reaching distant sites, tumor cells need to revert to an epithelial identity to form macrometastases (Thiery, 2002). However, this hypothesis has not been attested in vivo.
The EMT program is orchestrated through a network of transcription factors, including Twist1 (Yang et al., 2004), Snail1/2 (Batlle et al., 2000; Cano et al., 2000; Hajra et al., 2002), Zeb1/2 (Comijn et al., 2001; Eger et al., 2005), and FOXC2 (Mani et al., 2007). Our previous study found that Twist1 is a potent inducer of EMT and invadopodia-mediated ECM degradation (Eckert et al., 2011; Yang et al., 2004). In mouse and human breast tumor xenograft models, Twist1 expression can promote tumor metastasis (Yang et al., 2004). Clinical studies have also associated expression of Twist1 in primary tumors with disease aggressiveness and poor survival in many types of human cancers, such as squamous cell carcinoma, breast cancer, prostate cancer, and gastric cancer (Eckert et al., 2011; Kallergi et al., 2011; Peinado et al., 2007; Watson et al., 2007).
Unlike human carcinoma metastases, most established metastatic tumor cell lines present a permanent mesenchymal phenotype (Blick et al., 2008) and cannot be used to address the dynamic EMT process during tumor metastasis in vivo. Recent elegant studies using autochthonous mouse tumor models observed the occurrence of EMT in primary carcinoma, but how EMT spatiotemporally regulates metastasis has not been investigated in these models (Husemann et al., 2008; Rhim et al., 2012). The chemical carcinogenesis mouse skin model has been shown to recapitulate the multi-step process of human carcinoma progression, including initiation, growth, invasion, and metastasis (Kemp, 2005; Perez-Losada and Balmain, 2003). At the molecular and genetic levels, the skin carcinogenesis model shares strong similarities with a number of carcinoma in humans, including activating mutations in Ras family members, activation of PI3K- and Stat3-mediated signaling pathways, elevated expression of transforming growth factor β1 (TGFβ1) and activation of the TGFβ/Smad signaling pathways, and, at later stages, Trp53 mutations (DiGiovanni, 1992; Kemp, 2005). Importantly, like human squamous cell carcinoma, this model develops distant metastases with an epithelial morphology in lymph nodes and lungs (Han et al., 2005), making it a suitable model to study the involvement of EMT in vivo. Furthermore, extensive studies have shown that expression of Twist1 in primary human squamous cell carcinoma, including esophageal cancer (Sasaki et al., 2009; Xie et al., 2009; Yuen et al., 2007) and head and neck cancer (Ou et al., 2008; Wushou et al., 2012), correlates with distant metastasis and poor prognosis. In this study, we investigate the importance of the dynamic EMT process in metastasis in vivo using the skin carcinogenesis model.
Results
Induction of Twist1 promotes invasive carcinoma conversion
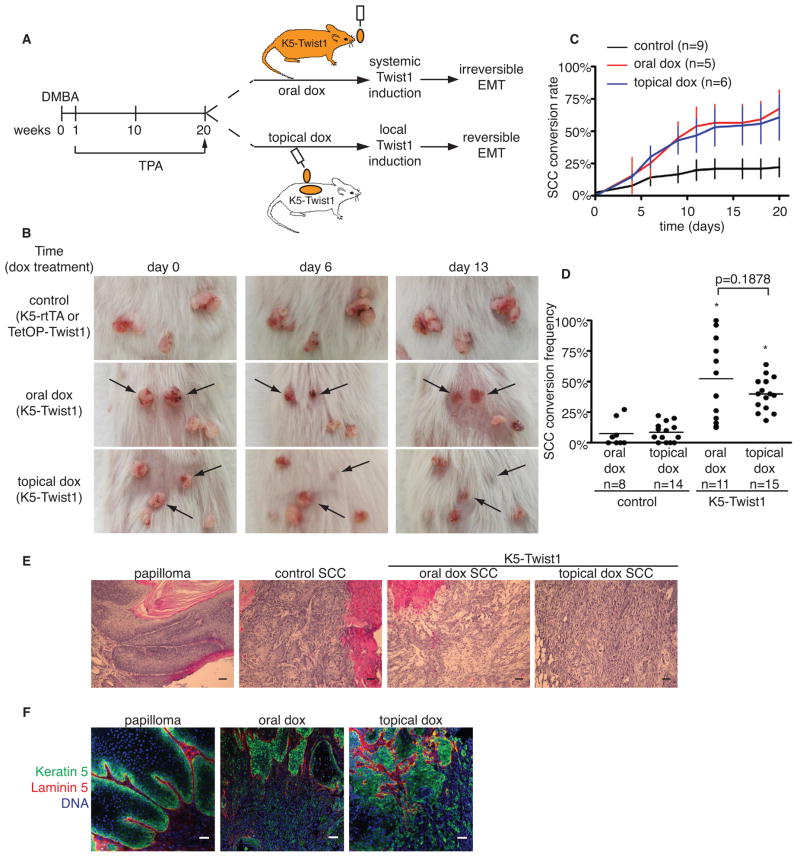
Previous studies have demonstrated the necessary role of Twist1 as an inducer of EMT. To understand the contribution of Twist1 in metastatic carcinoma, we analyzed 99 primary human carcinomas with patient-matched lymph node metastases for Twist1 expression. Of the 20 cases with high Twist1 expression in the primary tumor, we found 16 cases with over 50% drop in Twist1 levels in the lymph node metastases (Figure S1A and S1B), suggesting Twist1 is activated in the primary tumor but not distant metastases. To study how dynamic activation of Twist1 directly impacts carcinoma progression, we generated skin-specific Twist1 Tet-on inducible mice by crossing transgenic mice carrying a single copy of a TetOP-Twist1 transgene with Keratin 5 promoter-driven reverse tetracycline-controlled transactivator mice (K5-rtTA) (Diamond et al., 2000). Bitransgenic mice (referred to as K5-Twist1 mice) showed specific expression of Twist1 protein in the basal epidermal layer upon doxycycline (dox) treatment (Figure S1C). Long-term induction of Twist1 alone in K5-Twist1 mice did not result in visible skin abnormalities (data not shown). To generate squamous cell carcinoma (SCC), K5-Twist1 mice and control single transgene littermates were treated with a single dose of DMBA, followed by weekly applications of TPA for 20 weeks to allow skin tumor development (Abel et al., 2009; Kemp, 2005; Sun et al., 2007) (Figure 1A). At the end of TPA treatment when all mice have developed multiple papillomas, we randomly divided these mice into two groups. One group of mice received doxycycline in the drinking water to allow continuous Twist1 expression in K5-positive tumor cells even if tumor cells have migrated out of the skin and disseminated throughout the body. We used this systemic Twist1 induction group as the model for “irreversible EMT”. The second group of mice received doxycycline topically on the dorsal skin area containing papillomas to induce Twist1 only at the primary tumor site, such that tumor cells would lose Twist1 expression once they have disseminated from the skin. This local induction of Twist1 was used as the model for “reversible EMT” (Figures 1A and S1D).
Figure 1. Induction of Twist1 promotes invasive carcinoma conversion.
(A) A schematic of the DMBA/TPA skin tumor model and two doxycycline (dox) induction approaches in K5-Twist1 mice.
(B) Representative images of tumor lesions in control and doxycycline-treated K5-Twist1 mice over time. Control mice are single transgene littermates that received oral or topical doxycycline.
(C) Graph of conversion rates from papillomas to SCCs over time for a representative cohort ± standard error of mean (SEM) at each time point.
(D) Scatter plot of SCC conversion frequency in control and doxycycline-treated K5-Twist1 mice. Each dot represents one mouse, and the bar represents the mean of each group. * p<0.0001 compared to control group, Student’s t test.
(E) Histologic sections of tumors stained with hematoxylin and eosin (H&E). Papillomas have well-defined cellular organization, whereas control SCCs are well- to moderately-differentiated. In contrast, doxycycline-treated tumors are disorganized and poorly-differentiated. Bar=50μm.
(F) Frozen tumor sections were co-stained for tumor cells (K5, green), basement membrane (laminin 5, red), and nuclei stain (blue) to examine the breachment of basement membrane by tumor cells. Bar=50μm.
See also Figure S1
Within 7 days of doxycycline treatment through either oral or topical routes, papillomas on the K5-Twist1 mice began to invaginate into the skin and converted to SCCs at similar rates in both groups (Figures 1B and 1C). By three weeks, both groups of K5-Twist1 mice presented over 3-fold higher conversion frequencies than their control littermates (Figure 1C). Importantly, induction of Twist1 by oral or topical doxycycline resulted in similar conversion rates and frequencies of papillomas to SCCs (52% for oral treatment versus 40% for topical treatment (Figure 1C and 1D)), demonstrating similar efficacy of Twist1 induction at the primary site using both doxycycline delivery methods. Histological analysis confirmed that papillomas have converted to poorly-differentiated SCCs with many regions presenting a spindle-cell phenotype in both groups of K5-Twist1 mice, while the naturally converted SCCs in the control group showed a well- to moderately-differentiated epithelial morphology (Figure 1E). In Twist1-induced SCCs, tumor cells invaded through the underlying basement membrane, demonstrating a role of Twist1 in matrix degradation (Figure 1F). Together these data indicate that Twist1 is sufficient to promote invasive carcinoma progression in vivo.
Reversible induction of Twist1 promotes carcinoma metastasis
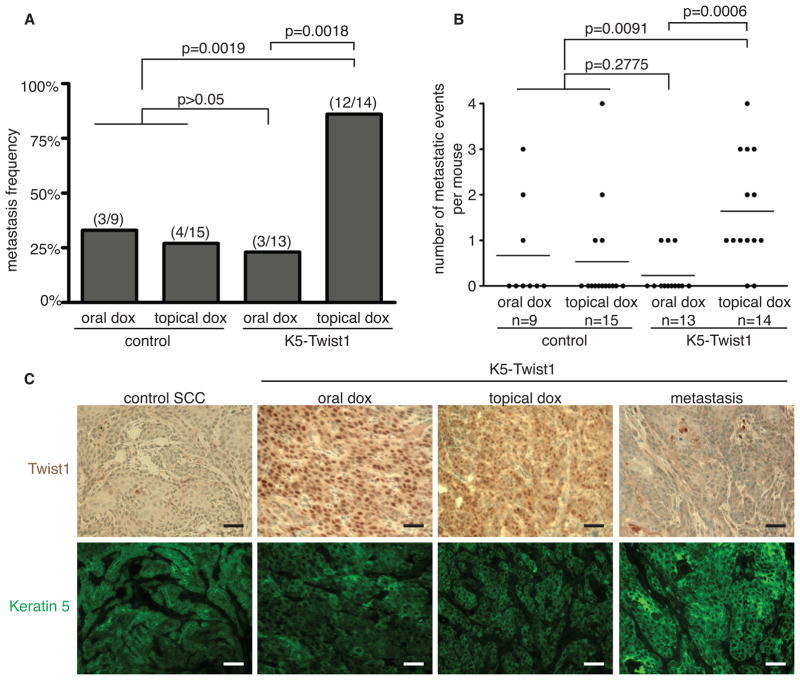
To understand how irreversible versus reversible induction of Twist1 impacts metastasis, we examined individual mice for distant metastases by macroscopic and histological analysis. Starting at 5 weeks after doxycycline induction, mice with heavy metastasis burden were sacrificed together with mice in the comparison groups and all mice were terminated by 8 weeks. Consistent with published data, 27–33% of control SCC-bearing mice developed distant metastases in the lymph node and/or the lung (Abel et al., 2009; Kemp, 2005). Strikingly, 12 out of 14 K5-Twist1 mice (86%) receiving topical doxycycline developed distant metastases. In contrast, only 3 out of 13 K5-Twist1 mice (23%) receiving oral doxycycline developed distant metastases (Figure 2A). K5-Twist1 mice receiving topical doxycycline also developed significantly more metastatic lesions per mouse than mice receiving oral doxycycline, highlighting the drastic difference in metastasis incidences between these two groups (Figure 2B). It is also important to note that this difference is not due to non-specific effects of doxycycline since control mice receiving oral or topical doxycycline presented similar metastasis frequencies (Figures 2A and 2B).
Figure 2. Reversible induction of Twist1 promotes carcinoma metastasis.
(A) A histogram showing metastasis frequencies in control and K5-Twist1 mice group receiving oral or topical doxycycline. The fraction of mice developing metastases in individual groups is represented above each bar. Fisher’s exact test analysis was performed to determine statistical significance.
(B) An event was defined as a tumor nodule in an individual lymph node and/or the presence of at least a single nodule in the lung tissue. Each dot represents a single mouse. Student’s t test statistical analysis was performed to compare average events per group.
(C) Representative images of tumor sections co-stained for Twist1 (brown) and K5 (green). Paraffin-embedded tumor sections were stained for Twist1 using immunohistochemistry (IHC, brown) followed by immunofluorescent (IF) staining for keratin 5 (K5, green) on the same section to identify tumor cells. Bar=50μm.
We next examined the expression of Keratin 5 and Twist1 in the primary tumors and metastatic nodules. We found that all skin tumor cells express Keratin 5 both with and without Twist1 induction, suggesting that Keratin 5 can be used to specifically mark skin tumor cells in this model. Importantly, we detected robust nuclear Twist1 expression in the primary tumors following both oral and topical doxycycline treatment; in contrast, all distant metastatic lesions showed no Twist1 expression (Figure 2C). Together, these results indicate that only reversible, but not irreversible induction of Twist1 significantly promotes distant metastasis.
Twist1 regulates EMT in a reversible fashion during metastasis in vivo
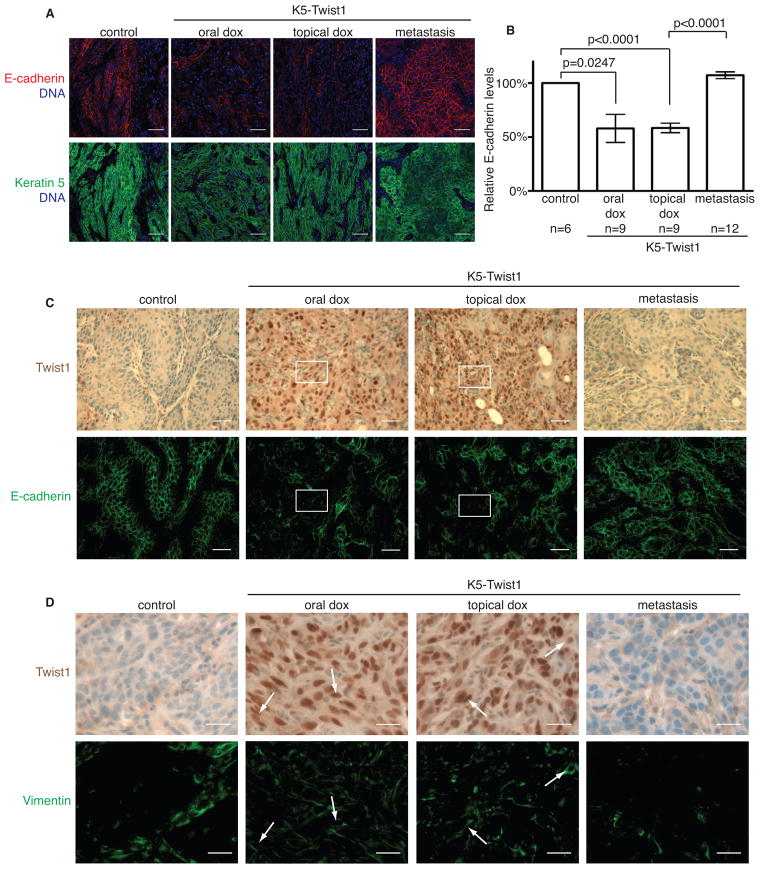
To understand whether Twist1 indeed regulates EMT in a reversible manner during metastasis, we examined both primary tumors and metastatic nodules for the expression of Twist1 and key EMT markers. In control mice, the naturally converted SCCs showed strong expression of epithelial markers, including E-cadherin, β-catenin, and γ-catenin (Figures 3A–3C and S2A) and no expression of mesenchymal marker vimentin in the tumor cells (Figure 3D). Primary tumors from K5-Twist1 mice receiving either oral or topical doxycycline, presented diminished epithelial markers and strong vimentin expression, indicating that Twist1 can effectively induce EMT in primary tumors (Figures 3, S2A and S2B). In contrast, all corresponding distant metastases in the topical induction group present an epithelial morphology with no vimentin expression and strong E-cadherin staining (Figures 3C, 3D, and S2C). The fact that topical induction of Twist1 drastically increased metastasis incidence and that distant metastases presented an epithelial phenotype indicates that “reversible EMT” can effectively promote tumor metastasis. To our surprise, while oral doxycycline induction of Twist1 reduced E-cadherin expression and induced EMT in primary tumors in all 13 mice (Figure 3), the rare metastatic nodules developed in three mice also presented an epithelial morphology (Figure S2D). Immunostaining analyses of these metastases showed strong E-cadherin along with weak Twist1 expression in the tumor cells (Figure S2E), suggesting that these rare metastases are likely due to additional selective genetic and/or epigenetic changes that circumvent Twist1-induced EMT to allow the formation of epithelial metastases. Together, these results strongly support a requirement for the reversion of EMT in forming distant metastases in vivo.
Figure 3. Twist1 regulates EMT in a reversible fashion during metastasis.
(A) Primary and metastatic tumor samples were co-stained for E-cadherin (red) and K5 (green) to identify tumor cells undergoing EMT. Bar=50μm.
(B) The relative E-cadherin levels in K5+ tumor cells were quantified in individual tumor samples from (A). Values were normalized to control samples and plotted on a histogram ± SEM. Student’s t test statistical analysis was performed.
(C) Representative images of tumor sections from control and K5-Twist1 mice co-stained for Twist1 (brown) and E-cadherin (green) expression. Boxed regions highlight areas of Twist1-positive tumor cells with disrupted or absent E-cadherin expression. Bar=50μm.
(D) Representative images of tumor sections from control and K5-Twist1 mice co-stained for Twist1 (brown) and vimentin (green) expression. Arrows indicate Twist1-positive tumor cells that express vimentin. Bar=25μm.
See also Figure S2
Activation of EMT in primary tumors promotes intravasation
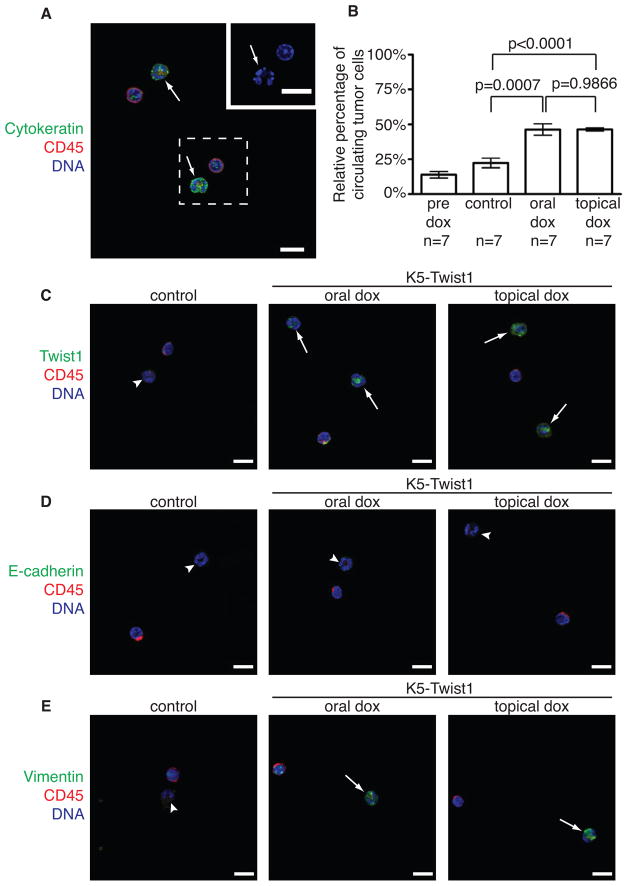
To successfully metastasize, carcinoma cells need to complete distinct steps, including invasion, intravasation, extravasation, and growth at distant sites. To investigate how activation of EMT impacts tumor cell intravasation into the blood circulation, we isolated circulating tumor cells (CTCs) from peripheral blood of K5-Twist1 and control mice. CTCs are defined as cells that are CD45− and pan-cytokeratin (CK)+ and present irregular nuclear shape (Figure 4A). The percentages of CTCs in the blood increased over 2-fold in K5-Twist1 mice following both oral or topical doxycycline treatment, compared to samples from control mice or from K5-Twist1 mice prior to doxycycline treatment (Figure 4B). Importantly, upon Twist1 induction, these CTCs were positive for Twist1 and mesenchymal marker vimentin, but negative for epithelial markers E-cadherin and β-catenin (Figures 4C–4E and S3), indicating an EMT phenotype in the CTCs. This result is consistent with studies showing that CTCs from human cancer patients present many features of EMT (Hou et al., 2011; Kallergi et al., 2011; Min et al., 2009) and that the presence of CTCs in human squamous cell carcinoma cancer patients is associated with distant metastasis and poor survival (Jatana et al., 2010; Pajonk et al., 2001; Winter et al., 2009). Our data show that both reversible and irreversible activation of EMT are equally effective in promoting tumor cell intravasation, and therefore the ability to disseminate is not the cause of different metastasis rates.
Figure 4. Activation of EMT promotes tumor cell intravasation.
(A) Representative image of circulating tumor cells (CTCs). CTCs are defined as cells that are CD45− (red) and CK+ (green) and present irregular nuclear shape (arrows). Inset shows magnified irregular nucleus in CTC. Bar=10μm.
(B) Quantification of CTCs in K5-Twist1 mice prior to doxycycline treatment (pre dox), in control mice and K5-Twist1 mice receiving oral and topical doxycycline. The percentages of CTCs among all nucleated cells were plotted on a histogram ± SEM. Student’s t test statistical analysis was performed.
(C) CTCs from control and doxycycline-treated K5-Twist1 mice were examined for Twist1 expression. Representative images of CTCs co-stained for Twist1 (green), CD45 (red), and nuclei (blue). Arrows represent CTCs that are Twist1-positive, arrowheads represent CTCs that are Twist1-negative. Bar=10μm.
(D) Representative images of CTCs co-stained for E-cadherin (green), CD45 (red), and nuclei (blue). Arrowheads represent CTCs that are E-cadherin-negative. All CTCs show no E-cadherin expression. Bar=10μm.
(E) Representative images of CTCs stained for vimentin (green), CD45 (red), and nuclei (blue). Arrows represent CTCs that are vimentin-positive, arrowheads represent CTCs in the control littermates that are vimentin-negative. Bar=10μm.
See also Figure S3
Activation of EMT promotes tumor cell extravasation
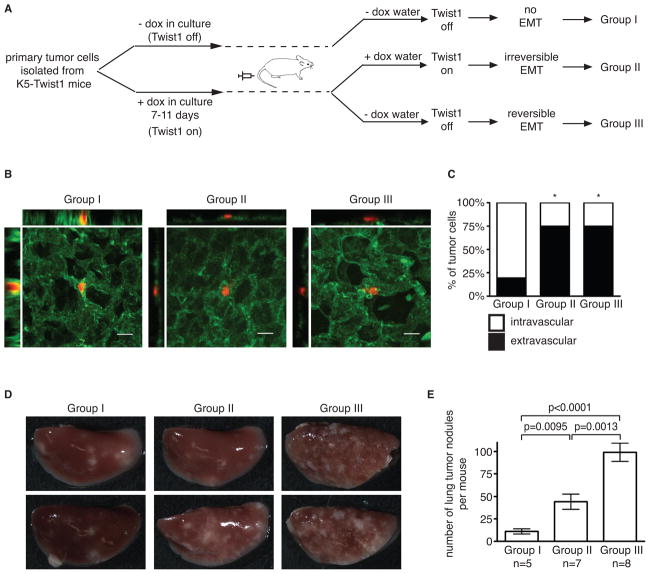
To examine the impact of EMT on tumor cell extravasation in distant organs, we isolated primary tumor cells from a K5-Twist1 mouse and treated them in culture with doxycycline for 7–11 days to induce Twist1 and EMT (Figures 5A and S4). These cells were then labeled with fluorescent cell tracker and injected via tail vein into wild-type mice receiving doxycycline or no doxycycline in drinking water, mimicking “irreversible” versus “reversible” EMT respectively. The parental primary tumor cells were also injected as the “no EMT” control (Figure 5A). At 36 hours after injection, we quantified the number of tumor cells extravasated from the lung vasculature. While only 20% “no EMT” control cells (Group I) extravasated out of the lung vasculature, induction of Twist1 promoted 75% of tumor cells to extravasate under both “irreversible” and “reversible” conditions (Group II and III) (Figures 5B and 5C). Supporting these results, four weeks after tail vein injection, the “no EMT” group (Group I) resulted in very few lung metastases compared to the other two groups (Group II and III) (Figure 5D and 5E). This strongly indicates that activation of Twist1 and EMT is critical to promote extravasation. Furthermore, consistent with previous studies (Cameron et al., 2000; Mendoza et al., 2010), tumor cells in circulation can extravasate from the blood within 1–2 days, much shorter than the time required for EMT reversion (~ 5 days). Therefore, these data also show that reversible activation of EMT in tumor cells can persist long enough to allow effective extravasation from the vasculature into distant organs before EMT reversion.
Figure 5. Activation of EMT promotes tumor cell extravasation.
(A) A schematic of the experimental lung metastasis design.
(B) Confocal images of tumor cell (red) extravasation from lung vasculature (green). Bar=20μm.
(C) Quantification of tumor cell extravasation at 36 hours post tail vein injection. The number of tumor cells inside or outside of the vasculature was counted and then divided by the total number of cells assayed (n=28–32 cells per group). The percentage of tumor cells inside (intravascular) or outside (extravascular) of the vessel was plotted on a stacked bar graph. * p<0.0001 as determined by Fisher’s exact test, compared to control group I.
(D and E) Images of lung tissues and quantification of average lung nodules per mouse ± SEM 4 weeks after tail vein injection. Student’s t test statistical analysis was performed.
See also Figure S4
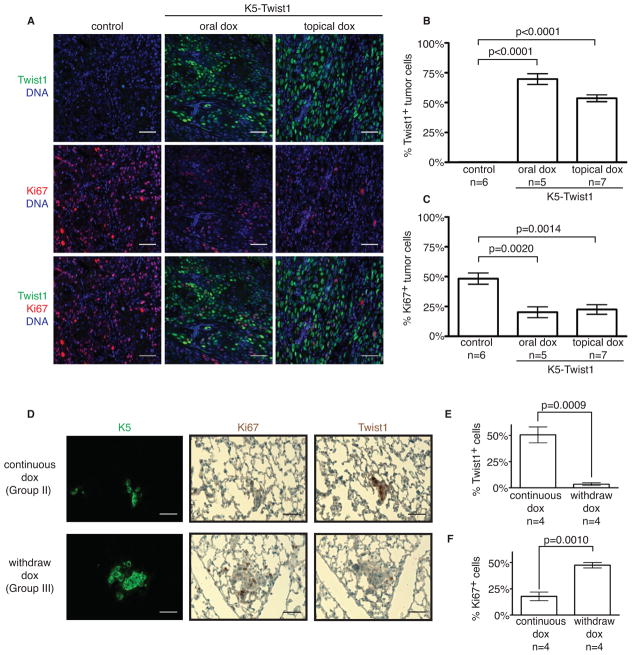
Reversion of EMT promotes colonization in distant sites
Since reversible and irreversible activation of EMT can both effectively promote local invasion, intravasation, and extravasation, the ability to grow in distant organs is likely to be the critical step regulated by the reversion of EMT. Since cell proliferation is essential for establishing macrometastases and EMT-inducing factors are shown to reduce cell proliferation (Evdokimova et al., 2009; Vega et al., 2004), we analyzed the effect of Twist1 on tumor cell proliferation. Indeed, individual primary tumor cells expressing Twist1 showed very low to non-detectable expression of the proliferation marker Ki67 (Figure 6A). Tumor cell proliferation appeared to be negatively correlated with Twist1 expression (Figures 6B and 6C). To demonstrate that reversion of EMT to promote cell proliferation at distant sites is the essential step in establishing early metastatic colonies, we performed experimental lung metastasis studies as described in figure 5A and examined cell proliferation and Twist1 expression in early metastatic lesions in the lung (7 days post-injection). Remarkably, early metastatic colonies showed strong positive Ki67 expression and low Twist1 expression under “reversible” EMT condition, while “irreversible” EMT resulted in colonies with high Twist1 expression and low Ki67 (Figures 6D–6F). This demonstrates that reversion of EMT promotes proliferation and establishment of early metastatic colonies in distant sites. Consistent with this result, four weeks after tail injection, mice in the reversible EMT group (Group III) developed significantly more metastatic lung nodules than the irreversible group (Group II) (Figures 5D and 5E). Combined with our results above, these data show that disseminated tumor cells need to turn off Twist1 to reverse the EMT program, thus allowing proliferation to facilitate colonization in distant sites.
Figure 6. Reversion of EMT promotes colonization in distant sites.
(A) Representative images of tumor sections co-stained with Twist1 (green), Ki67 (red), and nuclear stain (blue). Tumor sections from control and doxycycline-treated K5-Twist1 mice were co-stained for Twist1 and Ki67 to identify proliferation tumor cells. Bar=50μm.
(B and C) Relative levels of Twist1 and Ki67 expression in primary tumors from control and doxycycline-treated K5-Twist1 mice. Values were plotted on a histogram ± SEM. Student’s t test statistical analysis was performed. n=tumor samples per group.
(D–F) Representative images of lung sections co-stained for K5 (green), Twist1 (brown) or Ki67 (brown) and quantification of Twist1 and Ki67 expression at 7 days post tail vein injection. Values were plotted on a histogram ± SEM. Student’s t test statistical analysis was performed. n=mice per group.
Discussion
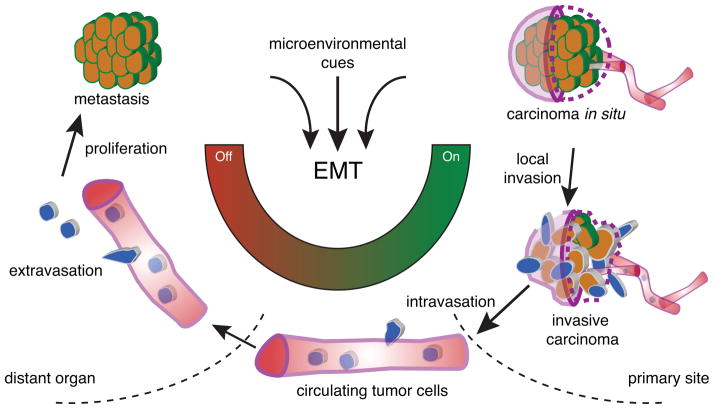
The in vivo role of EMT in tumor metastasis has been under intense debate due to conflicting observations in human primary carcinoma and their corresponding distant metastases. In a spontaneous squamous cell carcinoma mouse model, we demonstrate the dynamic requirement of EMT in tumor metastasis: activation of EMT promotes local tumor invasion, intravasation and extravasation of the systemic circulation; while reversion of EMT is essential to establish macrometastases (Figure 7). This mouse model mimics many genetic, molecular, and cellular features of human carcinoma. Our study, together with other clinical studies in breast, ovarian, and prostate cancers (Chao et al., 2010; Hudson et al., 2008; Hugo et al., 2007), indicates that EMT is activated in many types of primary human carcinoma, but not in their distant metastases. Therefore, the reversible EMT model demonstrated in the mouse squamous cell carcinoma is likely a general principle applicable to human carcinoma metastasis.
Figure 7. Reversible EMT model for tumor metastasis.
During tumor progression, local microenvironmental cues in the primary tumor activate the EMT program. This triggers local tumor cell invasion and intravasation into the blood vessels. Circulating tumor cells maintain an EMT phenotype and travel to a distant site, after which the cells extravasate into the tissue parenchyma. The loss of EMT activating signals is essential for tumor cells to reverse phenotype and proliferate to form macrometastases.
The “reversible” EMT model implies a level of cellular plasticity in the tumor cells. In other words, it is rather unlikely that genes involved in the EMT program will be permanently altered on the genome level, thus being unable to revert in distant sites during metastasis. This is supported by the fact that key EMT-inducing transcription factors and other key genes involved in the EMT pathway have not been reported to be prime targets for genomic deletion or mutation in various human cancer genome sequencing and mouse tumor model studies. Instead, the EMT program is largely controlled at the transcriptional and translational level in response to various pro-invasion signals in the local tumor microenvironment, such as hypoxia, inflammation, and nutrient conditions. TGFβ1, one such EMT-inducing signal from tumor stroma, has been examined for its role in promoting invasion and metastasis in the skin carcinogenesis model. Interestingly, the inducible TGFβ1 mice used in the study required topical induction to activate the TGFβ1 transgene. In these mice, topical activation of TGFβ1 signaling promoted invasive SCCs with spindle cell morphology and resulted in distant metastases with epithelial characteristics (Han et al., 2005; Weeks et al., 2001). These results could also be due to tumor cells undergoing a reversible EMT to form epithelial metastases, as demonstrated in our Twist1 mouse model.
An alternative model of EMT in tumor metastasis proposes that epithelial tumor cells can seed metastasis without undergoing EMT in the presence of mesenchymal tumor cells that have undergone EMT (Celià-Terrassa et al., 2012; Tsuji et al., 2008). Our results showing that circulating tumor cells express no E-cadherin (Figure 4D) would argue that these epithelial cells might have undergone a transient EMT, perhaps in response to an inducing signal from co-existing mesenchymal tumor cells in primary tumors, to metastasize.
The transient nature of EMT requires a delicate balance between the maintenance and loss of epithelial traits to promote efficient metastasis in vivo. In culture, epithelial cells undergoing a complete EMT lose epithelial markers including cytokeratin expression. Our data suggests carcinoma cells in vivo may only need to undergo a partial EMT for dissemination, as evident by detectable cytokeratin expression in the CTCs (Figure 4). This is supported by observations that human cytokeratin-positive CTCs also present an EMT signature (Hou et al., 2011; Kallergi et al., 2011; Min et al., 2009; Rhim et al., 2012). A partial EMT would be sufficient to promote tumor cell dissemination, but also facilitate disseminated mesenchymal tumor cells to quickly revert to an epithelial phenotype for proliferation and colonization in distant organs.
The ability to proliferate at distant sites is essential for the establishment of early metastatic lesions. Previous studies have shown that EMT-inducing factors can reduce cell proliferation in various tumor cells (Bierie and Moses, 2006; Evdokimova et al., 2009; Vega et al., 2004). Independent studies also found that invasive tumor cells also present a gene signature that implicates decreased cellular proliferation and increased motility (Goswami et al., 2004; Wang et al., 2004). Our study demonstrates that Twist1 expression decreased cell proliferation in vivo and turning off Twist1 at distant sites promoted metastatic growth, therefore suggesting that tumor cells need to toggle proliferation and migration to achieve efficient metastasis. However, given that colonization, a rate-limiting step in metastasis, has been shown to require numerous cellular and molecular events to accomplish (Chambers et al., 2002; Luzzi et al., 1998; Sugarbaker, 1993; Weiss, 1990), it is evident that reversion of EMT to increase proliferation in distant sites alone is not sufficient for colonization. Indeed, the results from our spontaneous skin tumor model show that the number of metastatic lesions in individual mice is still much lower (average two lesions per mouse) compared to the abundant circulating tumor cells detected in the blood upon Twist1 induction. Therefore, future studies are needed to identify additional molecular events that contribute to colonization in this tumor model.
Cancer patients can develop metastases from dormant tumor cells years after primary tumor resection (Chambers et al., 2002; Goss and Chambers, 2010; Meng et al., 2004). Although it is technically challenging to detect single dormant tumor cells in distant organs in our spontaneous tumor model and in cancer patients, both our study and several clinical studies found that circulating tumor cells in the blood present many molecular features of EMT (Hou et al., 2011; Kallergi et al., 2011; Min et al., 2009; Rhim et al., 2012). Therefore, our study raises the possibility that dormant tumor cells are in an EMT state and need to revert EMT to regain proliferation. Therapeutic agents that inhibit EMT have been proposed as a treatment option against tumor metastasis (Garber, 2008). The transient nature of EMT in carcinoma metastasis cautions that such approach alone could be counter-productive and promote metastatic colonization when patients already present circulating tumor cells. Instead, inhibiting the reversion of EMT could be a logical approach to prevent resurrection of dormant tumor cells.
Experimental Procedures
Generation of inducible Twist1 mice and tumor model
All animal care and experiments were approved by the Institutional Animal Care and Use Committee of the University of California, San Diego. TetOP-Twist1 mice were generated using a site-specific single copy integration strategy (Beard et al., 2006). Mice were backcrossed over nine generations onto the FVB/N strain. Skin-specific inducible Twist1 mice were generated by crossing TetOP-Twist1 mice with K5-rtTA mice (Diamond et al., 2000) (Kindly provided by Dr. Stuart Yuspa, NCI, Bethesda, MD). DMBA/TPA multi-stage chemical carcinogenesis model was performed as previously described (Abel et al., 2009; Sun et al., 2007). Briefly, 20μg of DMBA was applied topically on the dorsal skin of transgenic mice. Mice were then treated with 12.5μg of TPA twice a week for 20 weeks. Papilloma-bearing mice were then randomly divided to receive doxycycline (2mg/ml) in the drinking water or topically on the dorsal skin.
The conversion rate from papilloma to squamous cell carcinoma (SCC) was calculated by dividing the number of ulcerated tumors by the total number of papillomas plus ulcerated tumors per mouse. Ulcerated tumors were defined as nodules that were previously papillomas and have invaginated into the dorsal skin. Mice with heavy metastasis burden were sacrificed together with mice in the comparison groups and all mice were examined for macrometastases. A metastatic event was defined as a tumor nodule in an individual lymph node and/or the presence of at least a single nodule in the lung tissue.
Biochemistry and immunohistological staining and analysis
Paraffin embedded tumor sections were stained with a mouse anti-Twist1 antibody (Santa Cruz Biotech, Santa Cruz, CA), rabbit anti-Keratin 5 antibody (K5, Covance, Princeton, NJ), rabbit anti-pan-cytokeratin antibody (pan-CK, Abcam, Cambridge, MA), rabbit anti-E-cadherin (Abcam), mouse anti-β-catenin (BD Biosciences, San Diego, CA), mouse anti-γ-catenin (BD Biosciences), rabbit anti-vimentin (GeneTex, Irvine, CA), or rabbit anti-Ki67 antibody (Abcam). Endogenous mouse antigen was blocked using Mouse on Mouse blocking agent (Vector labs, Burlingame, CA). Immunohistochemistry was performed using the ABC kit (Vector labs) and developed with DAB chromogen (Vector labs). Frozen tumor sections were stained with a chicken anti-K5 antibody (gift from Dr. Colin Jamora) and rabbit anti-laminin 5 antibody (gift from Dr. Monique Aumailley) to identify K5 tumor cells and basement membrane. Alexafluor dyes (Invitrogen, Carlsbad, CA) conjugated to the appropriate species were used as secondary antibodies. Hoechst 33258 dye or 4′6-diamidino-2-phnylindole (DAPI) were used for nuclear stain. Western blot analysis for Twist1, E-cadherin, β-catenin, and GAPDH protein expression was performed as previously described (Eckert et al., 2011).
Images were collected using an Olympus FV-1000 confocal microscope or Nikon E600 upright microscope. For quantification of Twist1 and Ki67 expression, at least 3 fields were collected for each tumor and at least 5 tumors from each group were examined. Twist1 positive cells, Ki67 positive cells, and total number of cells (nuclear stain positive) from each field were counted using Volocity software (PerkinElmer, Waltham, MA). For quantification of relative E-cadherin levels, images were analyzed for E-cadherin expression by measuring threshold level of staining using Image J software (National Institutes of Health), then divided by area of positive K5 staining to calculate relative E-cadherin levels in tumor regions. Values were normalized to E-cadherin levels in control tumors.
Circulating tumor cell staining and analysis
Peripheral blood was obtained from tumor bearing mice via submandibular bleeding or intracardiac puncture at the termination of the experiment. Red blood cells (RBCs) were removed by incubating whole blood in RBC lysis solution. Remaining cells were spun down and fixed in 4% paraformaldehyde. Cells were then spun onto slides using a cytospin and stained with a rat anti-CD45 (BD Biosciences) and rabbit anti-pan-CK (Abcam) antibodies, followed by DAPI nuclear stain. Circulating tumor cells (CTCs) were identified as irregularly shaped nucleated cells that were CD45 negative CK positive cells. All cells in at least 5 high-powered fields (hpf) were counted, and the relative percentage of CTCs were calculated and plotted on a histogram.
Experimental lung metastasis assay
Primary inducible Twist1 skin tumor cells were isolated from a tumor-bearing K5-Twist1 mouse according to manufacturer’s protocol for Defined Keratinocyte Serum-Free Media protocol (Invitrogen). Briefly, tumors were removed and incubated in PBS with 2X antibiotic cocktail solution (Invitrogen) for 1–2 hours at 4°C. Tumors were transferred to dispase solution supplemented with 2X antibiotic cocktail solution and incubated at 4°C overnight. Tumors were then minced in 0.5% Trypsin/ETDA solution and incubated at 37°C for about 15 minutes. Soybean trypsin inhibitor (Invitrogen) was used to stop the trypsinization. Cells were maintained in serum-free keratinocyte media. To induce Twist1 in culture, doxycycline (1μg/ml) was added into the media. After 7–11 days, 1–1.5×106 cells were injected via tail vein injection into mice receiving no doxycycline water or 2mg/ml doxycycline water. Mice were monitored and euthanized when breathing appeared difficult. Lung tissue was perfused with and fixed in 4% paraformaldehyde. Tumor nodules on the surface of every lung lobe were counted and the numbers were plotted on a histogram. For tumor cell proliferation analysis, mice were euthanized 7 days post-injection of cells. Lung tissue was perfused and embedded in paraffin. Tissue sections were stained for K5, Twist1, and Ki67 as previously mentioned.
For tumor cell extravasation analysis, cells were labeled with CellTracker-Red (Invitrogen) according to manufacturer’s recommendation. 1–1.5×106cells were injected into mice via tail vein and mice were euthanized after 36 hours. To label lung vasculature, mice were injected with Fluorescein labeled Lycopersicon Esculentum Lectin (Vector Labs) 30 minutes prior to euthanasia. Thick lung tissue sections were obtained by manually slicing the tissue and were then mounted on slides for viewing. Confocal z-stack images were obtained using an Olympus FV-1000 microscope and analyzed using FluoView (Olympus) and Image J software.
Human breast cancer tissue microarray (TMA)
TMA of 99 human breast carcinoma and matched metastases were purchased from US Biomax Inc. The TMA contained human tissues obtained with informed consent according to US federal law and are exempt from Institutional Review Board review by the University of California, San Diego Human Research Protections Program. Staining for Twist1 and cytokeratin was performed as described above. All samples were analyzed for Twist1 expression, and patient samples were considered positive for Twist1 expression only if >10% of tumor cells in the primary tumor stained positive for nuclear Twist1. Out of 99 matched samples, only 20 samples met our criteria for being positive for Twist1. Cells were counted using the cell counter function in Image J software.
Statistical analysis
Statistical analysis was performed using GraphPad Software (La Jolla, CA). Student’s t test was applied for comparisons between two groups. The Fisher’s exact test was applied to analyze the metastasis frequency and tumor cell extravasation rate using a contingency table. The one-tailed exact binomial test was performed for statistical analysis of Twist1 expression in human breast cancer TMA.
Supplementary Material
Highlights.
Twist1 is sufficient to promote EMT and invasive tumor progression in vivo.
Twist1 promotes carcinoma cell intravasation and extravasation in vivo.
Induction of Twist1 and EMT reduces tumor cell proliferation.
Reversion of EMT is essential for establishing macrometastases.
Significance.
EMT features are frequently observed in many types of primary human carcinoma, but not their corresponding metastases. Our findings indicate that reversible EMT likely represents a key driving force in human carcinoma metastasis. Delayed onset of metastasis following primary tumor removal is thought to be due to resurrection of latent carcinoma cells in distant organs. Our study raises the possibility that tumor dormancy could be due to the inability of disseminated tumor cells to revert EMT and proliferate. The dynamic involvement of EMT in metastasis cautions that therapies inhibiting EMT could be counterproductive in preventing distant metastases when patients already present circulating tumor cells. Instead, blocking EMT reversion may prevent dormant tumor cells from establishing metastases.
Acknowledgments
We thank Konrad Hochedlinger, Colin Jamora, Caroline Beard, Edward Vizcarra, Ferenc Reinhardt, Esmeralda Casas, Naoto Yoshizuka, and Monique Aumailley for reagents and invaluable technical help. We thank Robert Weinberg for his initial support on this work, members of the Yang lab for helpful discussions, and Sylvia Evans and Ittai Ben-Porath for critically reading the manuscript. We thank the Shared Microscope Facility and UCSD Cancer Center Specialized Support Grant P30 CA23100. This work was supported by grants from American Cancer Society (RSG-09-282-01-CSM), NIH (DP2 OD002420-01), Sidney Kimmel Foundation for Cancer Research, and University of California Cancer Research Coordinating Committee to J. Y. J.H.T. was supported by NIH (T32CA121938) and California Breast Cancer Program postdoctoral fellowship (16FB-0009).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 2009;4:1350–1362. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Beard C, Hochedlinger K, Plath K, Wutz A, Jaenisch R. Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis. 2006;44:23–28. doi: 10.1002/gene.20180. [DOI] [PubMed] [Google Scholar]
- Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME, Neve RM, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25:629–642. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- Cameron MD, Schmidt EE, Kerkvliet N, Nadkarni KV, Morris VL, Groom AC, Chambers AF, MacDonald IC. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 2000;60:2541–2546. [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Celià-Terrassa T, Meca-Cortés Ó, Mateo F, de Paz AM, Rubio N, Arnal-Estapé A, Ell BJ, Bermudo R, Díaz A, Guerra-Rebollo M, et al. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Invest. 2012;122:1849–1868. doi: 10.1172/JCI59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- Chao YL, Shepard CR, Wells A. Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer. 2010;9:179. doi: 10.1186/1476-4598-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–794. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- Evdokimova V, Tognon C, Ng T, Ruzanov P, Melnyk N, Fink D, Sorokin A, Ovchinnikov LP, Davicioni E, Triche TJ, Sorensen PH. Translational activation of snail1 and other developmentally regulated transcription factors by YB-1 promotes an epithelial-mesenchymal transition. Cancer Cell. 2009;15:402–415. doi: 10.1016/j.ccr.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Garber K. Epithelial-to-mesenchymal transition is important to metastasis, but questions remain. J Natl Cancer Inst. 2008;100:232–233. 239. doi: 10.1093/jnci/djn032. [DOI] [PubMed] [Google Scholar]
- Goss PE, Chambers AF. Does tumour dormancy offer a therapeutic target? Nat Rev Cancer. 2010;10:871–877. doi: 10.1038/nrc2933. [DOI] [PubMed] [Google Scholar]
- Goswami S, Wang W, Wyckoff JB, Condeelis JS. Breast cancer cells isolated by chemotaxis from primary tumors show increased survival and resistance to chemotherapy. Cancer Res. 2004;64:7664–7667. doi: 10.1158/0008-5472.CAN-04-2027. [DOI] [PubMed] [Google Scholar]
- Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62:1613–1618. [PubMed] [Google Scholar]
- Han G, Lu SL, Li AG, He W, Corless CL, Kulesz-Martin M, Wang XJ. Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. J Clin Invest. 2005;115:1714–1723. doi: 10.1172/JCI24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Hou JM, Krebs M, Ward T, Sloane R, Priest L, Hughes A, Clack G, Ranson M, Blackhall F, Dive C. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol. 2011;178:989–996. doi: 10.1016/j.ajpath.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson LG, Zeineldin R, Stack MS. Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clin Exp Metastasis. 2008;25:643–655. doi: 10.1007/s10585-008-9171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmuller G, Klein CA. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Jatana KR, Balasubramanian P, Lang JC, Yang L, Jatana CA, White E, Agrawal A, Ozer E, Schuller DE, Teknos TN, Chalmers JJ. Significance of circulating tumor cells in patients with squamous cell carcinoma of the head and neck: initial results. Arch Otolaryngol Head Neck Surg. 2010;136:1274–1279. doi: 10.1001/archoto.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallergi G, Papadaki MA, Politaki E, Mavroudis D, Georgoulias V, Agelaki S. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011;13:R59. doi: 10.1186/bcr2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp CJ. Multistep skin cancer in mice as a model to study the evolution of cancer cells. Semin Cancer Biol. 2005;15:460–473. doi: 10.1016/j.semcancer.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Ledford H. Cancer theory faces doubts. Nature. 2011;472:273. doi: 10.1038/472273a. [DOI] [PubMed] [Google Scholar]
- Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, Groom AC. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153:865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, Kutok JL, Hartwell K, Richardson AL, Weinberg RA. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci U S A. 2007;104:10069–10074. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza A, Hong SH, Osborne T, Khan MA, Campbell K, Briggs J, Eleswarapu A, Buquo L, Ren L, Hewitt SM, et al. Modeling metastasis biology and therapy in real time in the mouse lung. J Clin Invest. 2010;120:2979–2988. doi: 10.1172/JCI40252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, Beitsch PD, Leitch M, Hoover S, Euhus D, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10:8152–8162. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- Min AL, Choi JY, Woo HY, Kim JD, Kwon JH, Bae SH, Yoon SK, Shin SH, Chung YJ, Jung CK. High expression of Snail mRNA in blood from hepatocellular carcinoma patients with extra-hepatic metastasis. Clin Exp Metastasis. 2009;26:759–767. doi: 10.1007/s10585-009-9275-6. [DOI] [PubMed] [Google Scholar]
- Ou DL, Chien HF, Chen CL, Lin TC, Lin LI. Role of Twist in head and neck carcinoma with lymph node metastasis. Anticancer Res. 2008;28:1355–1359. [PubMed] [Google Scholar]
- Pajonk F, Schlessmann S, Guttenberger R, Henke M. Epithelial cells in the peripheral blood of patients with cancer of the head and neck: incidence, detection and possible clinical significance. Radiother Oncol. 2001;59:213–217. doi: 10.1016/s0167-8140(00)00315-7. [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- Perez-Losada J, Balmain A. Stem-cell hierarchy in skin cancer. Nat Rev Cancer. 2003;3:434–443. doi: 10.1038/nrc1095. [DOI] [PubMed] [Google Scholar]
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Natsugoe S, Ishigami S, Matsumoto M, Okumura H, Setoyama T, Uchikado Y, Kita Y, Tamotsu K, Sakamoto A, et al. Significance of Twist expression and its association with E-cadherin in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2009;28:158. doi: 10.1186/1756-9966-28-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarbaker PH. Metastatic inefficiency: the scientific basis for resection of liver metastases from colorectal cancer. J Surg Oncol Suppl. 1993;3:158–160. doi: 10.1002/jso.2930530541. [DOI] [PubMed] [Google Scholar]
- Sun P, Yoshizuka N, New L, Moser BA, Li Y, Liao R, Xie C, Chen J, Deng Q, Yamout M, et al. PRAK is essential for ras-induced senescence and tumor suppression. Cell. 2007;128:295–308. doi: 10.1016/j.cell.2006.11.050. [DOI] [PubMed] [Google Scholar]
- Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005;65:5996–6000. doi: 10.1158/0008-5472.CAN-05-0699. discussion 6000–5991. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Thompson EW, Newgreen DF, Tarin D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 2005;65:5991–5995. doi: 10.1158/0008-5472.CAN-05-0616. discussion 5995. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, Hu GF. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res. 2008;68:10377–10386. doi: 10.1158/0008-5472.CAN-08-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18:1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res. 2004;64:8585–8594. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- Watson MA, Ylagan LR, Trinkaus KM, Gillanders WE, Naughton MJ, Weilbaecher KN, Fleming TP, Aft RL. Isolation and molecular profiling of bone marrow micrometastases identifies TWIST1 as a marker of early tumor relapse in breast cancer patients. Clin Cancer Res. 2007;13:5001–5009. doi: 10.1158/1078-0432.CCR-07-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks BH, He W, Olson KL, Wang XJ. Inducible expression of transforming growth factor beta1 in papillomas causes rapid metastasis. Cancer Res. 2001;61:7435–7443. [PubMed] [Google Scholar]
- Weiss L. Metastatic inefficiency. Adv Cancer Res. 1990;54:159–211. doi: 10.1016/s0065-230x(08)60811-8. [DOI] [PubMed] [Google Scholar]
- Winter SC, Stephenson SA, Subramaniam SK, Paleri V, Ha K, Marnane C, Krishnan S, Rees G. Long term survival following the detection of circulating tumour cells in head and neck squamous cell carcinoma. BMC Cancer. 2009;9:424. doi: 10.1186/1471-2407-9-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wushou A, Pan HY, Liu W, Tian Z, Wang LZ, Shali S, Zhang ZY. Correlation of increased twist with lymph node metastasis in patients with oral squamous cell carcinoma. J Oral Maxillofac Surg. 2012;70:1473–1479. doi: 10.1016/j.joms.2011.06.212. [DOI] [PubMed] [Google Scholar]
- Xie F, Li K, Ouyang X. Twist, an independent prognostic marker for predicting distant metastasis and survival rates of esophageal squamous cell carcinoma patients. Clin Exp Metastasis. 2009;26:1025–1032. doi: 10.1007/s10585-009-9292-5. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yuen HF, Chan YP, Wong ML, Kwok WK, Chan KK, Lee PY, Srivastava G, Law SY, Wong YC, Wang X, Chan KW. Upregulation of Twist in oesophageal squamous cell carcinoma is associated with neoplastic transformation and distant metastasis. J Clin Pathol. 2007;60:510–514. doi: 10.1136/jcp.2006.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.