Abstract
This paper reviews the published literature on the hyperscanning methodologies using hemodynamic or neuro-electric modalities. In particular, we describe how different brain recording devices have been employed in different experimental paradigms to gain information about the subtle nature of human interactions. This review also included papers based on single-subject recordings in which a correlation was found between the activities of different (non-simultaneously recorded) participants in the experiment. The descriptions begin with the methodological issues related to the simultaneous measurements and the descriptions of the results generated by such approaches will follow. Finally, a discussion of the possible future uses of such new approaches to explore human social interactions will be presented.
Keywords: HYPERSCANNING, SOCIAL NEUROSCIENCE, EEG, fMRI, NIRS
1. Introduction
More than 2300 years ago Aristotle wrote in his work “The Politics” that the human being is a “political animal” (ζωον πολιτικόν); and that, in particular, humans are “more of a political animal than bees or any other gregarious animals”. In fact, “it is a characteristic of man, that he alone has any sense of good and evil, of just and unjust, and the like, and the association of living beings who have this sense makes a family and a state” (Aristotle, 1998). Therefore, the idea that an important trait of being “humans” consists of our relationship with others is deeply rooted in ancient culture. This concept is not limited to Classical culture, as shown by the African word Ubuntu, which means that “a person becomes a person only through other people” (Hari and Kujala, 2009).
Although the social nature of humans has been evidenced for thousands of years, the field of neuroscience has only started to investigate brain activity during social interactions in the last decades. Social cognition includes all of the cognitive processes necessary to properly understand and store personal information as well as information from other people, including the rules at the basis of interactions with other humans. In recent years, neuroscientists have started to investigate the cerebral structures supporting the processes involved in the social cognition abilities of humans, starting with experimental evidence drawn from brain lesion studies (Wood et al., 2005) and autism (Frith and Frith, 2001; Baron-Cohen, 2006; Williams, 2008). Hundreds of studies performed using normal subjects have elucidated the role of particular brain regions in social cognition tasks. Such studies are reviewed in papers using meta-analysis related to different aspects of social cognition (Hari and Kujala, 2009; van Overwalle, 2009; van Overwalle & Baetens, 2009; van Overwalle, 2011).
From these studies it appears that specific cerebral regions are involved in tasks that require the processing of information relevant for social cognition. In particular, the temporo-parietal junction (TPJ) was described as being consistently activated during tasks involving the short-time estimate of intentions, desires and goals related to other people. Interestingly, the TPJ activation persists also when there is a negative judgment about such goals and intentions (van Overwalle, 2009). The activity of the TPJ is connected to the consistent activity of the medial prefrontal cortex (mPFC) when the tasks performed need the encoding of more stable and durable information regarding the behavior of people under multiple circumstances, and recognize a common goal in this behavior. In one particular model, proposed after a review of more than 200 fMRI studies, it was hypothesized that the TPJ could be mainly responsible for transient mental inferences about other people, such as their goals or beliefs, while the mPFC supports the processes that enrich such observations with more durable traits and qualities about both others and the self (van Overwalle, 2009). Thus, it has been suggested that the union of the TPJ and mPFC structures could constitute the “mentalizing” system in humans, which enables the extraction and understanding of the goals of other people by using the capability to properly decode their intentions (Amodio & Frith, 2006; van Overwalle, 2009). Although the role of the mPFC has been consistently observed in tasks that involve cognitive reasoning, including relational processing of objects (Legrand & Ruby, 2009), a meta-analysis of the literature has shown that it is more likely that cognitive reasoning activates the mPFC because inferences about social agency and the mind are involved in the tasks proposed (van Overwalle, 2011).
Another cerebral system that has been identified in the last decade and hypothesized to be able to decode actions performed by body parts of other people, such as arms, hands, fingers and limbs, irrespective of the sensory or verbal format of the input, is the so-called Mirror Neuron System (MNS) (Iacoboni et al., 1999; Rizzolatti et al., 2001; Gallese et al., 2004). The MNS, consisting of cerebral structures located in the anterior intraparietal sulcus and in the premotor cortex, allows other people’s goals to be rapidly sensed on the basis of low-level behavioral inputs, although this understanding may be limited to familiar executed actions (Cross et al., 2006; van Overwalle & Baetens, 2009). Since we often make an estimation of the beliefs and attitude of the others on the basis of their overt actions, it could be hypothesized that the MNS and the mentalizing system work together in the decoding of the other people’s mental states (Amodio & Frith, 2006; Frith & Frith, 2006). However, such a statement was not supported by a recent meta-analysis of the literature, which suggested that the MNS and the mentalizing system can be complementary, but that none of the systems are subservient to the other (van Overwalle & Baetens, 2009). On the other hand, evidence of the cooperation of the two systems has been recently reported (Schippers et al., 2010). A possible synthesis of these debates could lie in the recent suggestion, provided by a meta-analysis of fMRI literature, which suggests that the MNS could extend beyond the cerebral regions typically attributed to it (Molenberghs et al., 2012). This could be consistent with the idea that the vicarious brain activity made possible by mirror neurons extends beyond actions to include the sharing of emotions and the sensations of others as well (Keysers and Gazzola, 2009).
All of these considerations of the existence of different neural systems supporting the recognition in our brains of relevant movements or the behavioral attributes of others mainly arose from experimental paradigms in which one subject was monitored during their interaction with an external partner (either human or computer). However, it is well known that humans behave differently if they are aware that they are interacting with computers instead of with other people (Rilling et al., 2008, 2011). Moreover, the reaction to another person’s behavior is possibly linked to a kind of relationship arising between the subject and the specific person that they are interacting with, which is not simply described by behavioral data. This requires a direct observation of the “interaction” emerging between the brains of different subjects, which is a possibility that can be only be obtained by measuring the brain activity of the participants simultaneously during the proposed tasks. In addition, the laboratory and technical limitations of brain scanning devices often offer poorly ecological settings for the execution of the experiments, which seriously affects the kind of social behavior that can be analyzed. To reach a deeper comprehension of the mechanisms involved in social interactions during “normal” life situations with our peers it is necessary to generate experimental paradigms that are as “natural” as possible. As noted in a recent review by Hari and Kujala (2009) “much of the fleeting, moment-to-moment information of social interaction remains beyond the reach of studies involving limited stimuli and tasks. The current challenge for brain imaging is to bring everyday human interaction, occurring in a complex natural environment between two or more subjects, into the laboratory”.
A natural answer to this research need is the collection of brain activities of all of the subjects involved in the investigated “transaction” or “interaction”. This led to the idea of performing simultaneous functional Magnetic Resonance Imaging (fMRI) scans of cerebral activity during simple interactions between humans, as shown by the group of Montague in 2002 (Montague et al., 2002). In this seminal publication, two subjects were scanned using two different fMRI devices during a simple interaction game. The simultaneous acquisition of the cerebral data from two subjects was named “hyperscanning” (Montague et al., 2002). After this publication, about 80% of the studies in the area of social cognition have been performed by fMRI to date. Although Montague’s paper was the first to report the possibility of performing cerebral recordings by two synchronized fMRI devices, it must be noted that it was not the first time that two subjects were recorded simultaneously to investigate their brain activities. In fact, forty years before Montague’s paper, a report appeared in Science describing the execution of multiple electroencephalograph (EEG) recordings in a series of twin pairs, during an experiment attempting to prove the existence of “extrasensory” communication between them (Duane & Behrendt, 1965). While this paper was largely criticized for the poor statistical protection employed in the data analysis, this was indeed the first case in which the idea of using multiple EEG brain recordings was introduced. However, since the EEG suffered from many problems at that time related to insufficient spatial sampling and insufficient spatial resolution, the idea of “EEG hyperscanning” was rapidly forgotten in the scientific community, and remained so for about 40 years. Fostered by the dramatic increase of the spatial resolution of EEGs that are now possible with modern recording and signal processing techniques (as reviewed in Michel & Murray, 2012), EEG hyperscanning has been recently re-introduced to investigate the brain activity of different individuals during their motor and cognitive interactions.
The necessity and potentiality of hyperscanning studies to address open questions in the study of the social brain were recently highlighted in a number of reviews (Hasson et al., 2012; Dumas et al., 2012; Sanger et al., 2011). This paper will review the published literature on the hyperscanning methodologies based on both hemodynamic and neuroelectric modalities. In particular, we will describe how different brain recording devices have been employed in different experimental paradigms to gain information about the subtle nature of human interactions and will address the main methodological problems arising in this new approach. In addition, the main solutions provided so far in the literature according to the different modalities will be discussed. This review will also include papers based on single-subject recordings in which a correlation was found between the activities of different (non-simultaneously recorded) participants to the experiment.
The description will begin with the methodological issues related to simultaneous measurements and will be followed with the description of the results by such approaches. Finally, a discussion regarding possible future uses of this new approach to explore human social interactions will be presented.
2. Synchronization and calibration of different devices
The aim of hyperscanning is to provide simultaneous recordings of brain activities in two or more subjects that are interacting during a particular motor or cognitive task. These multi-subject recordings are challenged by several technical difficulties, related to the availability and the synchronization of the acquisition devices to be employed, as well as to the removal of movement-related artifacts from brain data arising from “ecologic” experimental designs.
2.1 Multi-subject EEG recordings
All of the published EEG hyperscanning experiments were performed using different EEG devices located in the same laboratory, solving the issue of the synchronization of the different acquisition machines in a straightforward way. Given the short distance between the subjects, often the EEG data are synchronized by an external trigger that reaches all of the acquisition machines, or by feeding the data into a unique device. With the same sampling rate of 200–500Hz employed (Babiloni et al., 2006; 2011; Dumas et al., 2010) the EEG data are usually also synchronized to avoid the possible jitter introduced by the Local Area Networks (LAN). When the analyses are performed in the same time domain, a higher sampling rate is employed (up to 5Khz), making the direct interconnection of the acquisition devices the preferred option, by attaching the same trigger to all of the machines (Lindenberger et al., 2009). The issue of different sensitivities between the EEG systems can be adjusted by attaching all of them to a trigger signal with a fixed amplitude, which allows the calibration of all of these devices.
In the last decade several electrooculogram (EOG) and electromyogram (EMG) filtering techniques have been introduced in the EEG field to remove or filter the effects of eye-movements and muscle artifacts in the EEG data. In particular, the ability to easily collect the EOG and the EMG data from different subjects alleviates and facilitates the removal of such influences from the EEG data (Babiloni et al., 2004).
A typical example of an EEG hyperscanning device employed in the acquisition of data from four subjects simultaneously is provided in Fig 1A (from Babiloni et al., 2011), while a more straightforward architecture for the recording of two subjects is provided in Fig. 1B (from Dumas et al., 2010).
Figure 1. Different hyperscanning recording architectures.
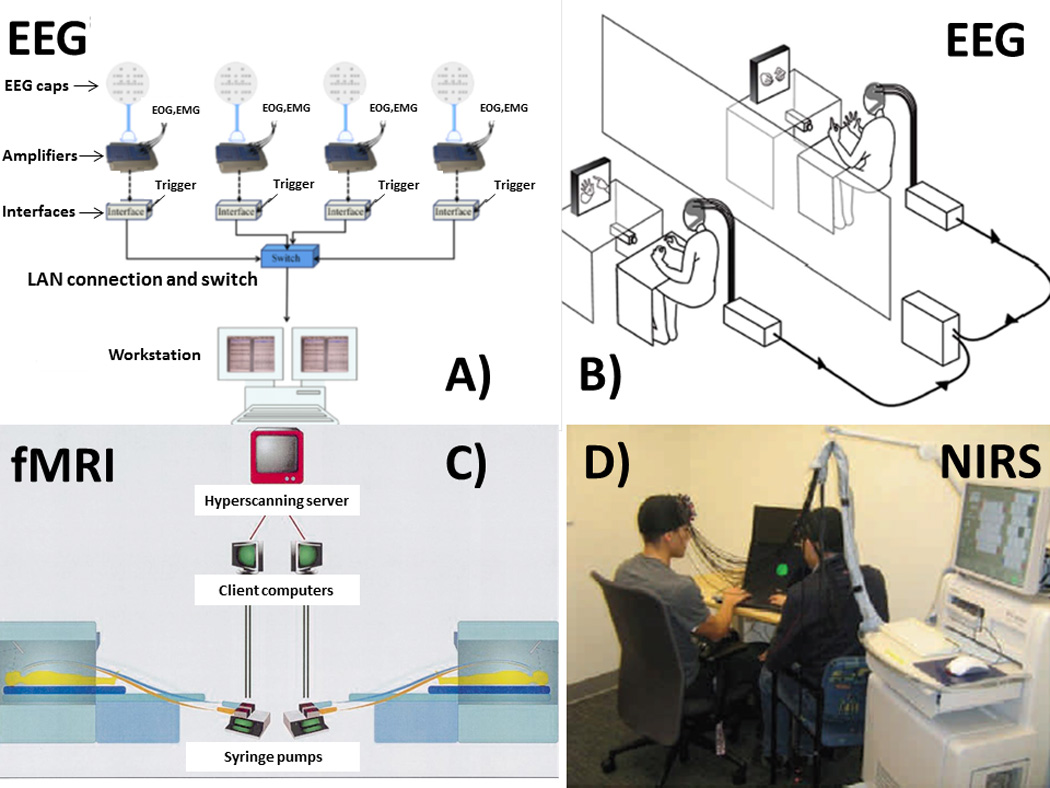
Different hyperscanning architectures employed for EEG recordings (A and B), fMRI recordings C) and NIRS recordings (from Montague et al., 2002, Dumas et al., 2010, Cui et al., 2012, Babiloni et al., 2011)
2.2 Multi-subject fMRI recordings
In the case of fMRI hyperscanning recordings, the main synchronization problems are related to the fact that the different acquisition devices are seldom available in the same location using the same LAN. It has been pointed out that some characteristics of the different fMRI machines (including different gradient strengths, head coil sensitivities and different principal strengths of the magnetic fields) could generate a significant inter-site variance (Montague et al., 2002). A possible procedure that can be used to prevent this fMRI inter-site variance involves the use of signal processing techniques that are not based on the amplitudes of the signals. These techniques highlight correlations between the hemodynamic activities of the two brains being analyzed. In addition, the use of a well-characterized MR phantom for all the recording fMRI sites can be used to get information regarding the variances between the sites. The synchronization problem could be solved by using a computer server that is responsible for the generation of the time of the acquisition for all of the fMRI devices (Montague et al., 2002, King-Casas et al., 2005). Figure 1C shows the original setup employed in the first published fMRI hyperscanning experiment (Montague et al., 2002).
2.3 Multi-subject NIRS recordings
The Near Infrared Spectroscopy (NIRS) technique has been employed as a brain imaging method in different research areas, such as those related to the measurement of BOLD responses (Emir et al., 2008; Huppert et al., 2009), brain computer interfaces (Power et al., 2010; Sitaram et al., 2007), and in the analysis of resting states (Lu et al., 2010; White et al., 2009; Zhang et al., 2010). Published works approach the problems of the synchronization of different devices with solutions very similar to those adopted in the case of EEG hyperscanning (Funane et al., 2011). However, in this case the issue of different sensitivities of the devices required a calibration process. A solution to this problem was proposed in a recent study, where a single NIRS device was split into two, in order to simultaneously record two subjects, using half of the channels for each patient (Cui et al., 2012). In this case, the use of a single device solved the synchronization problem. Fig. 1D shows the NIRS hyperscanning setting with a single acquisition device (Cui et al., 2012).
3. Methodological approaches
The availability of data simultaneously recorded from multiple subjects opens the way not only for the analysis of how the activity in the brain of each subject is related to their specific behavior, but also for the analysis of how this is related to the activity in the brain of the interacting, concurrently recorded partner engaged in the social task. This analysis is linked to the problem of the estimation of the functional connectivity (i.e. the existence of a functional relation, or causality, between the activities in different brain sites, which is not necessarily based on the existence of a direct physical link between the two sites). All of the methods that already exist for the estimation of brain connectivity are based on the assumption that the time series representing the cerebral activity has been generated by the same system, and, therefore, by the same brain. For this reason, a new methodological approach must be defined to deal with data coming from different brains. The choice of the correct estimator must be based on the properties of the multi-subject data, the domain of interest for the analysis, and the kind of relationship that one wants to describe.
For a time domain analysis, the correlation or coherence (King-Casas et al., 2005; Cui et al., 2012; Funane et al., 2011) or Granger-based correlation (Schippers et al., 2010) have been employed. In the frequency domains, different estimators, such as the Principal Locking Value (Dumas et al., 2010), the Partial Directed Coherence (Babiloni et al., 2006, 2007; Astolfi et al., 2010a, 2010b, 2010c, 2011) or the Estimator Phase Shift (phi1 and phi2, Tognoli et al., 2007) have been used. Usually, the frequency-based connectivity estimators are more suitable for neuroelectrical hyperscanning, while the temporal correlation or Granger-based causality is used on hemodynamic data (i.e. obtained with fMRI and NIRS scanners), due to the peculiar properties of the different signals. However, it is important to underline that the hemodynamic response is not equal across brain regions, and that this regional variability could cause problems for Granger causality analyses (David et al., 2008; de Marco et al., 2009; Roebroeck et al., 2005; Astolfi et al., 2005, 2006; Friston, 2009; Chang et al., 2008). On the one hand, it is feared that spurious Granger causality findings could be reported as a difference in hemodynamic response, which might introduce temporal relationships where there are none. On the other hand, a difference in hemodynamic response might invert the reported direction of Granger causality (Schippers et al, 2011). The study of Deshpande et al. (2010) showed how the sensitivity of Granger causality is affected by variability in hemodynamic response at the level of the single subject. To overcome this limitation, using the modulation of connectivity between different conditions was suggested, rather than within one condition (Roerbroeck et al, 2005). Schippers and colleagues (2011) investigated whether differences in the hemodynamic response have an effect on the group Granger causality results. Evaluating differential Granger causality across a group of participants was shown to provide a valid measure of underlying effective connectivity (Schippers et al, 2011).
3.1 The meaning of the estimated hyperlinks
As described before, the estimation of functional connectivity between brain signals recorded in a dyad of patients performing a “social interaction” task can be obtained by a variety of methodologies, in both time and frequency domains. Fig 2 describes some of the links estimated between the different brain regions from a series of papers employing such “hyperscanning” technology. In particular, Fig. 2A shows the time correlation between different hemodynamic signals deriving from an fMRI hyperscanning experiment related to neuroeconomy in two subjects with different roles in the experiment (the “investor” is on the left, and the “trustee” is on the right) (King-Casas et al., 2005). Fig.2B shows the pattern of inter-brain coherence during simultaneous music production between two guitarists during an EEG hyperscanning experiment (Lindenberger et al., 2009). Fig. 2C shows the functional connections estimated in the two brains of participants during an EEG hyperscanning experiment involving motor recognition and coordination (Dumas et al., 2010). The different colors of the connectivity estimates are relative to the different frequency bands employed. Fig. 2D shows the statistically significant Granger-based correlation in the frequency domains between different brain regions for a group of dyads involved in a Prisoner’s Dilemma EEG hyperscanning task. The arrows indicate the direction of the interaction (Astolfi et al., 2011). Once such statistical connectivity links have been estimated, it is necessary to interpret the meaning of the “hyperconnectivity”. Of course, the existence of statistically significant correlations or covariances between different brain signals does not mean that a physical “communication channel” exists between the two brains, which was erroneously concluded by Duane & Behrendt (1965). Instead, it is an indication of an indirect chain of events that starts from the particular cerebral regions of the first subject and ends in the cerebral processes elicited in the brain of the second subject. Hence, the computational links that have been estimated in many hyperscanning papers described here are a form of spatio-temporal map of the cerebral regions involved in the generation of the social task investigated in each analyzed experiment.
Figure 2. Different measures of interbrain synchronization or causal interactions in fMRI and EEG hyperscanning experiments.
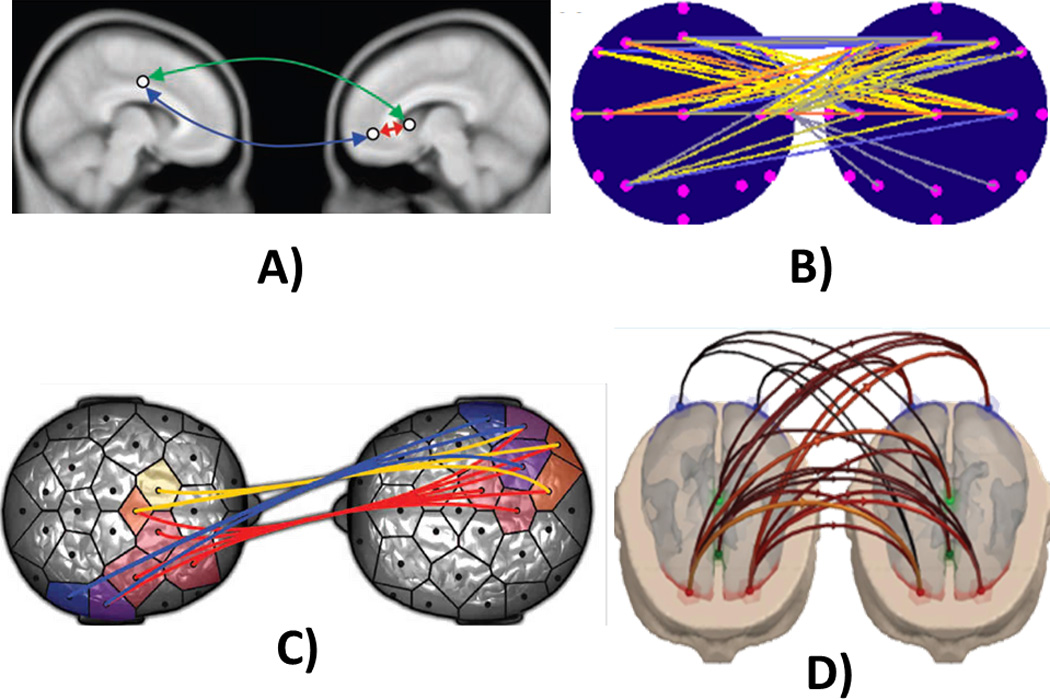
The figure shows different modalities to estimate interbrain correlations from hyperscanning recordings A) Time correlations between different hemodynamic signals (King-Casas et al., 2005) in two groups of subjects in a neuroeconomy game (“investor” on the left, “trustee” on the right). B) Patterns of interbrain coherence during simultaneous music production between two guitarists in an EEG hyperscanning experiment (head seen from above, nose up) (Lindenberger et al., 2009). C) Functional connections estimated between two brains of participants in an EEG hyperscanning experiment involving motor recognition and coordination (Dumas et al., 2010). Statistically significant Partial directed coherence (PDC) correlation in the frequency domains between different brain regions of a group of dyads involved in a Prisoner’s Dilemma EEG hyperscanning task. The arrows indicate the direction of the interaction (Astolfi et al., 2011)
4. Hyperscanning studies across different experimental paradigms
In the last decade, several hyperscanning studies involving different brain imaging devices and paradigms have been performed. In the following section, we would like to summarize the outcome of these studies and their contribution to the particular field investigated. Table I summarizes the series of studies that are reviewed in this paper. The papers can be obtained from the PUBMED and ISI databases by performing a search using the terms “hyperscanning” and “multiple persons” as primary keywords, with other ancillary keywords such as “multiple scanning” and “simultaneous EEG/fMRI/NIRS recordings”. The contents of all of the selected papers have been reviewed in order to select those relevant to the hyperscanning methodology.
Table I.
List of the analyzed studies performed with hyperscanning methodologies
| Research area | Authors, Journal, Year |
Task Instructions | Number of subjects |
Subjects × Method |
Results |
|---|---|---|---|---|---|
| Neuroeconomy | Montague et al., 2002, Neuroimage | Handy-Dandy: the sender see one of two color on the screen and sent one of two colors to the receiver. The receiver have to say if the color sent by the sender correspond to the color the sender saw before or not. | 6 | 2xfMRI | A cluster of activity is identified in the region of the supplementary motor area, but this is stronger in the Sender than in the Receiver |
| Neuroeconomy | King-Casas et al., 2005, Science | Trust game | 96 | 2xfMRI | Results suggest that the head of the caudate nucleus receives or computes information about (i) the fairness of a social partner’s decision and (ii) the intention to repay that decision with trust |
| Neuroeconomy | Tomlin et al., 2006, Science | Trust Game | 200 | 2xfMRI | (i) agent-specific response types localized on the medial bank of cingulate cortex, (ii) a systematic spatial variation of each response type across the anterior-posterior axis of cingulate cortex, and (iii) a dependence of both signals on the presence of a responding agent. |
| Neuroeconomy | Chiu et al., 2008, Neuron | Trust Game | 30 | 2xfMRI | showed that high-functioning males with autism spectrum disorder exhibit a severely diminished cingulate self-response when playing the game with a human partner. |
| Neuroeconomy | Fliessbach et al., 2007, Science. | Simple performance lead to a monetary reward that were compared on-line by both subjects scanned | 33 (38 recorded) | 2xfMRI | This study shows a relationship between relative income and hemodynamic responses in the ventral striatum. Receiving less than another subject was associated with a reduced BOLD signal in this area |
| Neuroeconomy | Babiloni et al., 2007, IEEE Conf. Proc. Eng. Med. Biol. | Prisoner’s Dilemma | 14 | 2xEEG | Results generated from EEG hyperscanning are related to the increased activity in the dorsolateral prefrontal and orbitofrontal areas during the different task phases when compared to the rest state. These cortical activities are specifically larger during the Defect conditions than in the other experimental situations. |
| Neuroeconomy | Astolfi et al., 2009,2010a IEEE Conf. Proc. Eng. Med. Biol. | Prisoner’s Dilemma | 36 | 2xEEG | Statistically significant links between homologous cortical areas in the two couples of subjects performing the PD game have been observed in the prefrontal areas of both subjects during the Cooperation condition whereas they are almost absent during the Defect condition |
| Neuroeconomy | De Vico Fallani et al., 2010, Plos One | Prisoner’s Dilemma | 52 | 2xEEG | It is possible to make predictions at 91% accuracy of the outcome of the decisions of couple of players in the PD game by using indexes estimated on the inter-brains EEG causal relations estimated during the 4 seconds preceding the decision of the dyads |
| Neuroeconomy | Astolfi et al., 2010b, IEEE Conf. Proc. Eng. Med. Biol. | Chicken’s Game | 38 | 2xEEG | A large involvement of the prefrontal regions during the Defect condition is observed when compared to the other conditions |
| Neuroeconomy | Astolfi et al., 2011, IEEE Intelligent Systems | Prisoner’s Dilemma | 52 | 2xEEG | Estimated interbrain connectivity by using Partial Directed Coherence, suggested an important role of the prefrontal and fronto-orbital regions of both hemispheres in all the experimental conditions examined |
| Decision-making | Babiloni et al., 2006, 2007 IEEE Conf. Proc. Eng. Med. Biol. | “Bridge-like” card game | 8 | 4xEEG | Results reveal larger activity in prefrontal and anterior cingulated cortex in different frequency bands for the player that start the game when compared to other player |
| Decision-making | Astolfi et al., 2010c, Brain Topography | “Bridge-like” card game | 14 | 4xEEG | Results presented suggested the existence of Granger-sense causal relations between the EEG activity estimated in the prefrontal areas 8 and 9/46 of one player with the EEG activity estimated in the ACC of their companion. |
| Temporal synchronization | Cui et al., 2012, Neuroimage | Button press minimizing time difference (cooperation) | 22 | 2xNIRS | Right superior frontal cortices activity increased during cooperation, but not during competition |
| Temporal synchronization | Funane et al., 2011, J. Biomed. Optics | Button press minimizing time difference | 12 | 2xNIRS | Increased between brain covariance of prefrontal cortices during cooperation |
| Music production, temporal aspects | Lindenberger et al., 2009, BMC Neuroscience | Music production synchronously with the aid of a metronome | 16 | 2xEEG | Coordinated actions for music production are preceded and accompanied by between-brain oscillatory couplings in the theta frequency band in scalp locations consistent with prefrontal cortices |
| Music production, emotional aspects | Babiloni et al., 2011a,b, Neuroimage, Cortex | Execution of a musical quartet piece, successive observation of such performance, rest condition | 12 | 4xEEG | Increased brain activity in alpha band in right ventral-lateral frontal gyrus (BA 44/45) correlated with the increase of empathy as revealed by psychometric test |
| Recognition of gesture in engaged couples | Schippers et al., 2010, Neuroimage | Participants in turns have to gesticulate during fMRI acquisition to inform the partner about a particular object or action. Partners successively have to guess (from video recording) the object or action mimicked by the partner | 18 | 1xfMRI | The activity in the dorsal and ventral premotor, somatosensory cortex, anterior inferior parietal lobule, and midtemporal gyrus (putative Mirror Neural System) and the activity in the ventromedial prefrontal cortex (vmPFC) including the anterior cingulate and paracingulate gyrus of the guesser is Granger-caused by fluctuations in activity in the pMNS of the gesture |
| Recognition of emotional faces in engaged couples | Anders et al., 2011, Neuroimage | Generation of particular faces associated to a precise emotional feeling | 12 | 2xfMRI | Emotional face communication elicited arousal response in couple. Measured brain activity elicited the same cerebral network in the couple of subjects, in anterior temporal, insular and somato-motor brain regions |
| Recognition of eye gaze direction | Saito et al., 2010, Frontiers in Integrative Neuroscience | Follow the other gaze or sustain mutual eye-contact | 38 | 2xfMRI | Right inferior frontal gyrus was significantly active in the couple of subjects during shared intentional state through eye-contact |
| Recognition and imitation of hand gestures | Dumas et al., 2010, PlosOne | Follow the other’s hand movements or propose the own hand movement to the other partner | 18 | 2xEEG | States of interactional synchrony correlate with the emergence of an interbrain synchronizing network in the alpha-mu (7–12 Hz) frequency band between the right centroparietal scalp regions. |
| Finger movement synchronization | Tognoli et al., 2007, Proc. Nat. Acad. Sci. | Follow the other’s finger movements or propose the own finger movement to the other partner | 16 | 2xEEG | A pair of oscillatory components (named phi(1) and phi(2)) located above right centro-parietal cortex distinguished effective from ineffective coordination: increase of phi(1) favored independent behavior and increase of phi(2) favored coordinated behavior |
| Finger movement synchronization | Naeem et al., 2011, Neuroimage | Follow the other’s finger movements or propose the own finger movement to the other partner | 12 | 2xEEG | A right sided cerebral network in the 10–12Hz range appears to be involved in integrating the mutual information among the members of a dyad that enables the dynamics of social interaction to unfold in time |
| Gestual interactions (no hyperscanning) | Redcay et al., 2010, Neuroimage | Interact with a partner outside the scanner in live or video-recorded situation | Two experiments with 16 and 13 subjects | 1xfMRI | During the "Live" interaction, as compared to the Recorded conditions, greater activation was seen in brain regions including the right temporoparietal junction (rTPJ), anterior cingulate cortex (ACC), right superior temporal sulcus (rSTS), ventral striatum, and amygdala |
| Speech comprehension (no hyperscanning) | Wilson et al., 2008, Cerebral Cortex | Observation and comprehension of a story telling observed in a videotape perfomed by an actor | 24 | 1xfMRI | Anterior cingulate and adjacent medial frontal cortex, as well as the posterior cingulate and adjacent precuneus were modulated by the time-varying profile of the audiovisual input being largely deactivated relative to rest condition. Comprehension of the audiovisual inputs involved the activation of a network of bilateral inferior frontal and premotor regions |
| Movie observation | Hasson et al.,2004,Science | Subjects were scanned while they are watching a movie | 5 | 1xfMRI | significant inter-subject correlations of hemodynamic waveforms was revealed in sensory specific cortices, the fusiform gyrus, and the limbic system |
| Observation and memorization of a movie | Hasson et al., 2008, Neuron | Subjects were scanned when they are watching a movie and 3 weeks later when they remembered it | 8 | 1xfMRI | brain regions whose BOLD response is significantly more correlated across subjects during portions of the movie that are successfully as compared to unsuccessfully encoded. These regions include the parahippocampal gyrus, superior temporal gyrus, anterior temporal poles, and the temporal-parietal junction. |
| Movie observation | Jääskeläinen et al., 2008 | Subjects that were scanned while they are watching a movie | 12 | 1xfMRI | Significant frontal-cortical inter-subject correlations between pairs of subjects was obtained in addition those observed in sensory and association areas |
| Movie observation | Kauppi et al., 2010, Frontiers Neuroinform. | Analysis of data from Subjects that were scanned while they are watching a movie | 12 | 1xfMRI | several regions within the frontal and temporal lobes show inter-subject correlation predominantly at low frequency bands, whereas visual cortical areas exhibit such correlation also at higher frequencies. |
4.1 Temporal synchronization of subject pairs during very simple motor acts (i.e. button pressing)
The question of what happens in the brain of subjects forced to synchronize their button pressing during a “cooperation task” is important. Results provided recently by two research teams suggest that a robust synchronization of the brain activities in the prefrontal cortices of two subjects occurs during the temporal synchronization of the button press (Cui et al., 2012; Funane et al., 2011). Interestingly, both groups used the NIRS hyperscanning technology to investigate such events, with the results generated showing overlap. In particular, Cui and colleagues (2012) tested two experimental situations in which the subjects were forced to synchronize their button press in order to gain points (cooperation) or instead to answer before the other (competition). They measured the inter-brain coherence of the hemodynamic time waveforms collected during the two tasks for 22 subjects. The results suggest that the coherence between the NIRS signals generated by the right superior frontal cortices in participants increased during cooperation, but not during competition. Increased coherence was also associated with a better cooperation performance. However, the most interesting aspect of this study was the fact that the individual time series analysis did not reveal any task-specific patterns of brain activity. Instead, the inter-brain coherence analysis clearly revealed a task-specific pattern, which showed an increase in coherence during task blocks. This suggests that the simultaneous collection and analysis of brain activity from multiple interacting subjects can reveal an additional layer of information regarding the study of social cognition.
A button press after a countdown was the task executed by the 12 subjects investigated using the NIRS hyperscanning method by another team of researchers (Funane et al., 2011). The participants were told to count 10s in their mind after an auditory cue and press a button. They were also told to adjust the timing of their button presses to make them as synchronized as possible. As in Cui et al. (2012), some information was fed back to the participants by a beeping sound after each trial, which indicated the interval between the two button presses of each participant pair and which of the participants was the fastest. Funane and colleagues (2011) described how the spatial and temporal covariance computed for the brain signals gathered from prefrontal cortices in the dyads increased when the interval between the button-presses was shorter. They concluded that such results suggest that the synchronized activation patterns of the two participants' brains are associated with their performance when they interact in a cooperative task.
It must be noted that both of the NIRS hyperscanning studies were performed by sampling the brain activity with emitters and detectors located only over the prefrontal cortices. Therefore, it is not currently possible to state if other cortical areas of both subjects could be involved in this synchronization task.
4.2 Temporal and emotional synchronization of subjects during music production
It is well known that the production of music in a duo or an ensemble requires a precise understanding of time from the musicians, in order to appropriately execute parts involving all musicians, as well as for the generation of solo segments. However, the precise time synchronization between players is not the only ingredient of a good musical performance, since the “emotional” feeling between musicians often determines the outcome of the music production. In recent years, a series of studies involving EEG hyperscanning techniques related to both the temporal and emotional aspects of music production have been published (Lindenberger et al., 2009; Babiloni et al., 2011a, 2011b).
In the study performed by Lindenberger et al. (2009), the brains of eight pairs of guitarists playing a short melody together were simultaneously recorded by EEG to explore the extent and the functional significance of synchronized cortical activities during the course of interpersonally coordinated actions. In particular, the guitarists were exposed to metronome sounds, which indicate the correct tempo required to generate both solo and coordinated musical performances. The estimation of the coupling of the recorded brain signals was computed using the Phase Locking Index (PLI) and the Inter-Brain Phase Coherence (IPC). The results of this study showed that interpersonally coordinated actions are preceded by and accompanied by interbrain oscillatory couplings in frequency bands below 20Hz. In particular, it appeared that synchronized theta (4–7 Hz) oscillations, both within and between the brains, were most pronounced when the musicians listened to the metronome to set their tempo, as well as when they started playing a short melody together. This activity occurred in the frontal and central electrodes, which are located over the prefrontal cortices. Synchronization patterns during guitar playing were assessed in terms of phase alignment after the onset of playing, and were also related to behavioral play onset asynchrony. Thus, patterns of interbrain synchronization reflect the temporal dynamics of interpersonal coordination. Synchronization at central electrode sites may indicate the coordinated firing of neuronal assemblies located in the motor and somatosensory cortices, which control and coordinate motor activity and are activated during music production. Interbrain synchronization occurs between the prefrontal cortices of the two players. Fig. 2B describes the interbrain connections occurring in a pair of subjects during the experiments, in which different intensities of the coherence are coded by a color scale. However, some caution is needed in the interpretation of these results due to the fact that both dyads of guitarists are exposed to the same metronome sensory inputs during the recordings, which could trigger similar processes in the brain.
As mentioned before, an important aspect when different musicians are grouped together is the “emotional” feeling between them, both before and during the execution of musical performances. In a couple of related studies (Babiloni et al., 2011a, 2011b), researchers hypothesized a relationship between an individual empathy trait in musicians playing in an ensemble and the cortical activation modeled in the ventral-lateral and ventromedial frontal areas supposed to serve the “emotional” and “cognitive” empathic abilities, respectively. These researchers simultaneously recorded the brain activity of three quartets of professional saxophonists subjected to different experimental situations using EEG hyperscanning. The first condition studied involved the saxophonists playing music in an ensemble (EXECUTION), the second was when they observed videos of their own music performance (OBSERVATION), the third was a control task (CONTROL), and the fourth condition was when they remained quietly rested in a relaxed but awake state (RESTING). Their empathy trait was measured using a psychometric questionnaire (Empathy Quotient Test; EQT), and the cortical activation was indexed by the analysis of EEG power in the alpha band (7–12 Hz), called “desynchronization”, using the RESTING condition as a baseline. Results showed that the condition of OBSERVATION, but not EXECUTION or CONTROL, induced a statistically significant correlation between the EQT score and the alpha desynchronization in the right ventral-lateral frontal gyrus (BA 44/45). The higher the EQT score, the higher the cortical activation, as revealed by alpha desynchronization. During the OBSERVATION condition, there was also a slight correlation trend between the EQT score and the alpha desynchronization in ventromedial bilateral frontal gyrus (BA 10/11). These results, which were not observed in non-musician control subjects, were site and function-specific, and the correlations were found neither in the control primary sensory/motor areas nor in the CONTROL conditions. Keeping in mind these data, it can be speculated that the empathy trait predicts the cortical activation of a right ventral-lateral frontal region in expert musicians observing their music performance, which is supposed to sub-serve “emotional” empathy. It must be noted that no attempt was made to describe the possible synchronization between recorded brain activities in players in this study with particular coherence or covariance estimators. Rather, a source imaging approach was performed and the activities of particular brain regions were correlated with the outcome of psychological tests, in an aim to describe the “affective dimension” of the musician.
4.3 Transmitting gestural words in couples
It is common to see people gesticulate in order to better convey their speech. This habit varies across different regions of the world. However, it is clear that hand movements can help to substitute words, regardless of the geographical locations of the persons. In gestural communication, a person who gesticulates in order to convey information is defined as the “sender”, and the person who has to understand such movements is the “receiver”. The recognition of the hand movements and the decoding of the information related to the mimed word or action is an activity that involves the “internal” representation of the hand movements from the “receiver”, who also has to guess the “internal” state of the “sender”. There is a lot of evidence that the “internal” representation of movements observed in others could evoke activity in the MNS as described before. In contrast, the “mentalizing” cerebral network is responsible for guessing the intention of others. The scientific question at the basis of the study performed by Schippers and colleagues (2010) is related to the extent of the hypothesized cooperation between the MNS and the “mentalizing” cerebral network during the decoding of intentional movements from the external world by the subjects. In particular, the aim is to understand if the cerebral systems are related to the action required to encode and decode gestures in couples who have a romantic relationship. All of the analyzed couples had already developed a particular attitude to decode the movements of their partner, so that they were able to understand the partner’s actions. For the game of charades, partners went in turn into the fMRI scanner, alternating the roles of gesturing and guessing. Words were either objects, such as a nutcracker, watch, or pencil sharpener, or actions, such as painting, knitting, and shaving. In this experiment no simultaneous acquisition of cerebral activity in the interacting subjects was performed due to the lack of available fMRI devices. However, researchers recorded the brain activity of each “sender” on a videotape and presented these videos to the “receiver” during the acquisition of their brain activity. The signal processing of the hemodynamic information was performed by an elaborate analysis involving the estimation of the Granger-causality between the hemodynamic waveforms of the “sender” and those gathered in the “receiver” while they were watching the videotape. Inter-brain Granger causality was computed above the level of chance if there was a significant “causal relationship” between the hemodynamic activity in the cerebral areas of the sender and those of the receiver. The results showed that the activity in the dorsal and ventral premotor cortex, the somatosensory cortex, the anterior inferior parietal lobule, and the midtemporal gyrus (termed the putative Mirror Neural System; pMNS) and the activity in the ventromedial prefrontal cortex (vmPFC) of the guesser was caused by fluctuations in the activity of the pMNS of the gesturer, for each of the 9 couples analyzed. This conclusion supports the idea that the pMNS and the “mentalizing” networks cooperate during the decoding of movements performed by others, and that this cooperation was “similar and caused” by the activity observed in the pMNS of the sender.
Although the experiment was not performed using the fMRI hyperscanning methodology, it did use the concept to “estimate” causal relationships between the brain activities gathered during the experiment in a couple of subjects. The particular nature of the experiment allows conclusions to be drawn, even if the simultaneous recording of the brain activities of the participants was not performed.
4.4 Transmitting emotions by facial expression in couples
It is our understanding that couples engaged in a romantic relationship develop a “special” sense to understand immediately the change of mood of the partner on the basis of subtle facial expressions. As a natural extension of the previous study on the transmission of gestural words between couples, researchers have investigated what happens when the transmission of information between partners is related to the affective state through the encoding of facial expressions (Anders et al., 2011). In order to investigate if such facial communications elicited some synchronized flow of emotions that were served by synchronized or delayed brain activity, the group of Anders and colleagues (2011) used a true fMRI hyperscanning setup, involving six couples engaged in a romantic relationship, with each couple scanned simultaneously in two fMRI machines. As in the experiment by Schippers et al. (2010), which was previously described, in each couple there was a “sender” and a “receiver”. The sender was instructed about the emotion they had to communicate to their partner through a screen projected within the scanner. Instructions were to “feel” such emotion, and a video camera recorded the facial expression and transmitted it to the partner (“the receiver”). The receiver had to guess the emotion generated by the sender. In each couple the sender was female, since it was hypothesized that females are more capable of generating and transmitting emotions through their faces. Interestingly, the researchers also monitored the Skin Conductance Response (SCR), which is a measure of the activity of the autonomous nervous system that regulates the excitation of the exocrine glands on the hands. Results suggested that the transmission of affective emotions increased the arousal level in both partners, which was detected by an increase of SCR levels from the baseline. For all of the brain regions involved in the transmission and in the decoding of the affective facial expression, researchers found that the level of neural activity within a distributed network of the perceiver's brain could be successfully predicted from the neural activity in the same network in the sender's brain, depending on the emotion that was being communicated. The cortical regions involved in this network are located in the anterior temporal, insular and somato-motor brain regions, which some researchers have previously associated with the observation of emotions and first-hand emotional experience (Hennenlotter et al., 2005; van der Gaag et al., 2007). Furthermore, there was a temporal succession in the flow of affective information from the sender's brain to the perceiver's brain, with information in the perceiver's brain being significantly delayed with respect to information in the sender's brain. This delay decreased over time, possibly reflecting some “tuning in” of the perceiver with the sender. Although the observed delay was higher than expected by the standard hemodynamic delay of about 8 seconds, this finding could be explained by the fact that human emotions have different components that unfold over time (Anders et al., 2009; Leventhal and Scherer, 1987).
The novelty of this study was in the fact that the group of Anders and colleagues was able to describe the same cerebral network activated in both partners and also detect a time-delayed flow of information from one network of the “sender” to the network of the “receiver” thanks to the hyperscanning design. In addition to the previous study of Schippers et al. (2010), the described study of Anders and coworkers (2011) showed that information about the specific content of communication (in this case the sender's affective state) from the sender's brain is subsequently reflected in the perceiver's brain.
4.5 Interacting through eye contact
In humans, eye contact is regarded as sharing the attention directed towards another. It is a strong sign of the interaction between subjects and can convey important emotional information, as demonstrated by the observation of mutual gaze interactions between children and adults. Thus, it is not surprising that researchers are trying to take advantage of this, using the feature of the hyperscanning methodology, to investigate, in an “ecologic” situation, if specific eye contact during a physiologically shared state is represented by the inter-subject correlation of intrinsic brain activity. In a study of Saito and coworkers (2010) they employed fMRI hyperscanning of two subjects while they were engaged in joint attention tasks with eye contact as the baseline. They defined the joint attention of the two subjects within the fMRI scanners when one partner followed the direction of the other’s gaze towards an object in space (Materna et al., 2008). Saito and collaborators performed fMRI analysis on 38 subjects (19 couples) in a complex experimental paradigm in which the subjects could recognize the gaze of the other partner on a screen on which there was also depicted other target objects. By comparing the pair-specific correlations of intrinsic brain activity during eye contact with that of non-paired subjects who were not in eye contact, Saito and coworkers were able to depict the neural substrates of the shared intentional state over and above that of stimulus-driven effects. In particular, the analysis performed suggested that the right inferior frontal gyrus (IFG) was significantly active in couples during such shared intentional states. This sharing might create a context that enhances the detection of communicative intent emitted by eye movement (Frith and Frith, 2006), making collaborative activities with shared goals possible, i.e. looking at the same objects.
4.6 Interaction by synchronizing hand movements
It is a matter of fact that in a “real-life interactions” between two subjects, such as a discussion, they are involved in a continuous change of behavior, with each person modifying their own actions in response to the continuously changing actions of their partner (Oullier et al., 2008, Oullier and Kelso, 2009). These “ecologic” situations are difficult to recreate in laboratory settings, where the “roles” assigned to the people involved in the experiment are often rigidly coded, e.g. one for the “sender” and another for the “receiver” as in the previously described works of Schippers et al. (2010) or Anders et al. (2011).
The attempt to closely follow the continuous exchange of roles between the “sender” and the “receiver” in an ecologically valid interaction has been performed by the group of Dumas and coworkers (Dumas et al., 2010; comments in Dumas, 2011). In particular, they attempted to characterize to what extent oscillatory synchronization could emerge between two brains during such kinds of social interaction, in which there is a continuous mutual adaptation of the partner to the action of the other and vice versa. The EEG hyperscanning experiment designed in this study aimed to follow the motor interactions and the related brain activity of couples during a time period in which they were free to move their hands while they were one in front of each other (through a video screen). Eighteen participants paired as 9 dyads were recorded with dual-video and dual-EEG setups while they were engaged in the spontaneous imitation of hand movements. Appropriate offline software recognizes the videotape the synchrony moments in the experiment as the moments in which the movements were performed together by the participants. In addition, such software is able to label the “sender” and the “receiver” according to the sequence of the video action recorded. By using a measure of interactional synchrony between the gathered EEG signals in the brains of the dyads during the interactions, the group of Dumas and coworkers observed that the states of synchrony of EEG waveforms correlated with the emergence of a cerebral network that involved the two interacting brains, in the right centroparietal scalp regions in the alpha frequency band (7–12 Hz). Fig. 2C shows the synthesis of the EEG data in a graphical fashion. Each color line describes a statistically significant synchronicity of the EEG waveforms in a particular frequency band. It is possible to note that such synchronization between brains remains symmetrical up to the higher frequency bands. According to the authors, this could be due to the top-down modulation of the roles of the model and imitator in the ongoing interaction. It is worth to note that right centroparietal scalp regions roughly overlap with the right TPJ, which has been advocated to play a crucial role in social interactions (Decety & Lamm al., 2007).
In this context, such inter-brain synchronizations could occur in the right TPJ of both of the “sender” and “receiver”, which had already been shown by the experiments reported by Schippers et al. (2010) using a more rigid protocol. In such experiments, the putative MNS of both subjects, which are roughly located at the right centro-parietal scalp regions, are simultaneously activated during the exchange of gestures.
4.7 Interaction by synchronizing finger movements in couples
In this review, we have often said that imitation is at the base of social interactions. In this particular case the issue is to investigate what the neural signatures are (if any) of sustained synchronized behavior between couples that are instructed to move their fingers freely one in front to the other. A transparent screen is interposed between fingers that can be obscured from the observer. Subjects are free to move their fingers in whatever directions they want, and hence are free to synchronize or not synchronize their movements. It is interesting to note that such a paradigm recalls, in part, what was proposed through the hand gestures by Dumas et al. (2010). The experiment was run by Tognoli and coworkers using EEG hyperscanning in eight couples of subjects (Tognoli et al., 2007). A social coordination phase in the behavior of the two subjects was defined when the relative phase of the finger movements entered a stable phase-locked state shortly after visual contact (less than 2 s) that persisted over the entire period of visual contact. Transient synchronization was defined when brief episodes of phase-locking were observed during the period of visual contact but were not maintained throughout the period. Unsynchronized behavior was defined by the persistent absence of phase-locking across the entire period of visual contact. The high-resolution spectral analysis of electrical brain activity before and during visually-mediated social coordination revealed a marked depression in occipital alpha and rolandic mu rhythms during social interaction, which was independent of whether the behavior was coordinated or not. In contrast, a pair of oscillatory components (named by the Authors as phi(1) and phi(2)) were located above the right centro-parietal cortex, which distinguished effective from ineffective coordination: the increase of phi(1) favored independent behavior and the increase of phi(2) favored coordinated behavior. The authors suggested that the topography of the phi complex was consistent with neuroanatomical structures within the human MNS. A plausible mechanism is that the phi complex reflects the influence of the other on a person's ongoing behavior, with phi(1) expressing the inhibition of the human mirror neuron system and phi(2) expressing its enhancement. While the phi complex could be indicated as the “neuromarker” relative to the presence of social coordination, it must be noted that such localization was obtained separately in the scalp areas of the participants, without the estimation of possible inter-brain connectivity. To this respect, such an approach seems less powerful than the approach proposed by Dumas and coworkers, which has also derived signatures of inter-brain synchronicity that are lacking in Tognoli’s analysis, even though they elicited responses in almost the same cortical areas.
In a successive study of the same research group (Naeem et al., 2012) the relationship between patterns of activation and deactivation of mu activity suggests that the localized neural circuitry in the right central-parietal regions mediates how individuals interpret the movements of others in the context of their own actions. A right sided mechanism in the 10–12Hz range appears to be involved in integrating mutual information among the members of a dyad, which enables the dynamics of social interaction to unfold over time.
Taken together, the EEG hyperscanning studies related to the synchronization of the finger movements (Tognoli et al., 2007; Naeem et al., 2011), suggest that the right centro-parietal scalp areas could be the location of a cerebral network able to change its activity during the appreciation and decoding of the other movements. This also serves the change of behavior in order to better synchronize with others.
These observations form a coherent view in agreement with the previously described papers that used different hyperscanning paradigms involving fMRI and EEG (Schippers et al., 2010; Anders et al., 2011). In all of these motor and affective tasks, two main cerebral networks were found to support the temporal, affective and motor coordination between couples. The first one is located on the prefrontal cortices (Babiloni et al., 2011; Cui et al., 2012; Funane et al., 2011; Schippers et al., 2010) and the other is located primarily on the right hemisphere, either over the TPJ or in the right IFG, as shown by analysis with fMRI hyperscanning (Schippers et al., 2010; Anders et al., 2011; Saito et al., 2010), or in the right centro-parietal scalp areas, as shown by EEG hyperscanning (Dumas et al., 2010; Tognoli et al., 2007; Naeem et al., 2012).
4.8 Interactions between subjects in game theory contexts
Humans usually interact to exchange information that is valuable for their life, and in this review we have already seen which cerebral structures are involved in the understanding of others’ actions, as well as in the decoding of their intentions. However, besides the observation of the movement of others in order to guess their decisions or intentions, it is also important to study the “rational” choices that are made every day, in accordance with the others. In fact, there is a field of research in neuroscience which studies the cerebral systems supporting the generation of decisions during the interaction with other humans (or agents), in order to also include the bargain occurring with automatic computerized systems. Such decisions are usually taken in order to bring rewards (immediate or delayed) to the agents. However, social decisions can be different from non-social ones. In fact, in social decisions, the value associated with the action of one agent depends critically on the changing actions (and to the mental states) of other social agents. In such social dilemmas, strategic decisions must be tailored and updated to the particular mental state of another (Kable and Glimcher, 2009). One particular way to code social dilemmas is through the use of game theory experiments, which involves a body of experimental paradigms useful to model situations in which a reciprocal exchange is expected.
The study of social decisions has also been tackled using the hyperscanning methodology since the publication of the paper by Montague and colleagues in 2002 (Montague et al., 2002). In this paper they investigated the common brain activity elicited during a simple deception game in which two players were involved. The scheme included one sender and one receiver. One of two colors (i.e. black and white) was presented on the screen of the sender, who could decide which color to transmit to the receiver (black or white). In return, the receiver had to state if the color transmitted by the sender was correct or not (i.e. whether the sender was telling the truth). If the receiver guessed the correct color they won, otherwise the sender won. Since the sample size was not large (only 6 subjects were investigated) the conclusions were based more on the methodological side than on the neuroscience side. However, a common activity was observed in the supplementary motor areas of both the sender and the receiver. Nevertheless, this paper represents the first example of an application of hyperscanning recording with fMRI devices.
4.9 Interactions between subjects in a game theory context: the Trust Game
In every interaction with other people, trust has a special importance. Whenever you purchase a used car or decide to make a financial investment, the trust in the other person will condition your final decision. In order to describe the neural correlate of trust in humans, King-Casas and coworkers employed an fMRI hyperscanning experiment involving the Trust Game (King-Casas et al., 2005). In this experimental paradigm, derived from game theory, a player must decide how much of an endowment to invest with a partner (the Trustee). Once transferred, this money is multiplied by some factor with the Trustee, who is then given the opportunity to return all, some, or none of the amount back to the Investor. If the Trustee honors the trust, and returns money to the Investor, both players can end up with a higher monetary payoff than was originally obtained. However, if the Trustee abuses the trust and keeps the entire amount, the Investor ends up with a loss. King-Casas employed a dual fMRI device to record the brain activity of 48 dyads involved in this game. In this case, the experimental paradigms coded by the Trust Game are rather rigid regarding the roles that each person has to interpret (Trustee and Investor), without the possibility of change roles during the experiment. Nevertheless, the results were impressive. Such results suggest that the head of the caudate nucleus receives or elaborates information about (i) the fairness of a social partner’s decision and (ii) the intention to repay that decision with trust. In particular, the analyses within and between brains revealed two signals: one encoded by response magnitude, and the other by response timing. Response magnitude correlated with the “intention to trust” on the next play of the game, and the peak of these “intention to trust” responses shifted its time of occurrence by 14 seconds as player reputations developed. In other words, in the early rounds of the game, the “intention to trust” was evident only after an investment was revealed. With experience, this signal shifted to a time preceding the revelation of the investment. Such results describe the interaction between the brain activities of players as only the hyperscanning design is capable of doing, given the particularity of the interaction between the subjects.
However, we know that trust in another person could be better built on a previous personal knowledge of such a person. In such a case, does the neural substrate of trust change? In an fMRI hyperscanning study performed one year later by the same research group (Tomlin et al., 2006) the brain responses during a Trust Game were tested to see whether they differed when the participants knew each other before the experiment. In particular, they employed two groups of people. The first group involved subjects who met before the task, were instructed together, saw a picture of their partner during each round of the game, and met their partner afterwards, when they were paid in front of each other (n = 102). The second group was composed of subjects that had never met, had no chance of a subsequent encounter, and received no information about one another (n = 96). The results of the two groups were compared in order to understand if the outcome could be biased by previous social interactions. Results surprisingly showed that the submission of one’s own decision elicited maximal activation in the middle cingulate regions, whereas viewing the outcome of a partner’s decision yielded maximal activation in the anterior and posterior cingulate. However, the spatial effects on the activation of the cingulate gyrus were not affected by previous social interactions that had occurred. In other words, the two groups present the same pattern of activity, mainly located at the level of the cingulate area. In addition to that, another important result showed that the activation of the cingulate gyrus could be obtained only in the presence of a “living” partner and when one of the two persons was removed from the scanner. Thus, one of the main results of the fMRI hyperscanning study by Tomlin and coworkers (2006) was that the cingulate gyrus was activated during a Trust Game in a different spatial fashion when the own decision was generated or when the other’s decision was generated. Under these conditions there was a clear difference in the neural activity related to the distinction between the own and the other. It was therefore hypothesized that in a particular class of patients in which this distinction is problematic (i.e. in the case of autism) the outcome of such games could elicit different brain responses.
In order to test such a scenario, an fMRI hyperscanning experiment involving the Trust Game was performed by Chiu and coworkers with two groups of subjects: one being composed of patients affected by Autism Spectrum Disorders (ASD) and the other of normal controls (Chiu et al., 2008). Results showed that during the multi-round trust game, high-functioning males with ASD lacked a neural activation pattern in cingulate regions that had been previously shown to encode robust self-specific responses (Tomlin et al., 2006). In contrast with the missing self-response, the ASD cingulate cortex responded normally when shown a social partner’s decision. This suggests that in the context of the iterated Trust Game, individuals with ASD may be impaired in their capacity to represent the social intent of their own behaviors, yet remain able to represent the actions of others. That is, the capacity to represent simple social actions of others may exist despite impoverished models of their own intentions. It is important to note that such conclusions were made due to the particular hyperscanning setup that was able to capture the essence of the interaction between ASD patients.
4.10 Interactions between subjects in game theory context: the Prisoner’s Dilemma
Previously, several hyperscanning studies involving the Trust Game have been described, all of which are derived from game theory. Another well studied paradigm often applied to the investigation of cerebral processes of the decision-making is known as the Prisoner’s Dilemma (PD). Such a paradigm is similar to the Trust Game, except for the fact that both players simultaneously choose whether or not to trust each other without knowledge of their partner’s choice. The PD paradigm was employed using EEG hyperscanning by the research group of Babiloni and coworkers (Babiloni et al 2007; Astolfi et al., 2009, 2010b, 2011; De Vico Fallani et al., 2010).
In this series of papers the cerebral activity of 52 subjects involved in the PD game was investigated. In the iterated PD, each player faces two possible choices: to cooperate with or defect the opponent. The choice is blind, meaning that each player has to decide their behavior without knowing what the other player is going to do. The outcome is not fixed, but depends on the combination of choices. If both players cooperate, they both win some money (Pure Cooperation condition), if only one player cooperates the advantage is given to the defector, and if both players defect, they both lose (Pure Defect condition). This so-called Tit-for-Tat is a situation in which each player adopts a certain behavior, not to maximize his or her gain, but to react to his or her opponent’s behavior in the previous run of the game, by iterating the opponent’s past choice in the next move (Tit-for-Tat condition). The aim of the game is to reach the highest possible score.
Results of the statistically significant spectral activity obtained from the EEG hyperscanning analysis performed in the 52 subjects showed an increased activity in the dorsolateral, prefrontal and orbitofrontal areas during the different task phases when compared to the resting state. In particular, these cortical activities are specifically larger in the Defect condition than in the other situations, characterizing the social interaction between the subjects investigated (i.e. Cooperation, Tit-for-Tat or mixed behavior). Successively, directed inter-brain communication was estimated through Partial Directed Coherence, and was computed for the 26 couples of players. Statistically significant links were observed between the prefrontal areas of both subjects during the Cooperation condition, yet they are almost absent during the Defect condition. Fig. 2D shows typical patterns of hyperconnectivity between subjects. Also, parietal hyperlinks connected with the prefrontal areas have been noted. These patterns of interbrain activity are consistent with the activity of both the pMNS and prefrontal areas already observed in couples involved in social interactions, as revealed by the other described paradigms visualized with fMRI hyperscanning (Schippers et al., 2010; Anders et al., 2011). In addition, the same research group (Babiloni et al) underlined how it is possible to predict the outcome of the joint decisions of the couples playing the PD game on the basis of the EEG traces analyzed in the four seconds preceding the decision making (De Vico Fallani et al., 2010). This result was obtained by applying advanced graph theory measurements to the hyperconnectivity derived from the EEG hyperscanning data recorded. Prediction was achieved with an accuracy of up to 91% in the theta band.
4.11 Interactions between subjects in game theory context: the Chicken’s, the Public Good and the Ultimatum Games
In a study involving 52 subjects, Astolfi and colleagues investigated the pattern of cortical activity from high resolution EEG hyperscanning recording during the Chicken’s Game (CG), which is a variation of the PD game with more severe fees for the simultaneous deception (Astolfi et al., 2010a)..
Results obtained from statistical spectral mapping showed that Defect and Tit-for-Tat conditions elicited a significant cortical activity in the beta frequency band, when compared to the Cooperation condition. It can be hypothesized that this increase in the power spectra activity reflects the major penalty and risky conditions in the generation of such conditions by the subjects when compared to the Cooperation decisions. In fact, each time a player decides to defect, they run the risk of incurring a “crash” with the other player, with a big penalty for both. It could be also hypothesized that a large involvement of the prefrontal regions during the Defect condition is generated by the effort of the “mentalizing” system, which is in agreement with all of the previous observations reported in this review. The interbrain connectivity results, estimated using Partial Directed Coherence, suggested an important role for the prefrontal and fronto-orbital regions of both hemispheres, which is again in agreement with previous observations related to the activity of the prefrontal areas in this decision-making context.
Little information derived only from conference proceedings are available from the published works of another group of researchers who investigated the brain activity of couples involved in game theory experiments using EEG hyperscanning measurements (Chung et al., 2008; Yun et al., 2010). EEG hyperscanning was performed on 26 subjects during the execution of a Public Good Game, which was an experimental setup during which they were asked to choose to either cooperate by paying their promissory money to the group or to defect (i.e. free-ride) by keeping the money. Every player in the group received a bonus if more than three people cooperated in each trial. EEG hyperscanning results provided evidence for activity in the prefrontal regions for cooperators before a decision was taken. Such results appear compatible with the prefrontal activity observed in EEG hyperscanning studies of the PD experimental paradigms described before (Astolfi et al., 2009, 2010b, 2011; De Vico Fallani et al., 2010). In conference papers, still using a decision-making task (the Ultimatum Game), Yun and coworkers (Yun et al., 2008, 2010) observed an increase of activity in the fronto-central scalp areas for high frequency bands, together with the estimation of an interbrain activity between the proposer and the responder in the Ultimatum game.
4.12 Interactions between subjects related to the comparisons of obtained rewards
It is a common understanding that if we and our peers performed the same job we expect to receive the same reward for the performance. Until a few years ago, previous economic theory paradigms predicted the differential activation of brain structures only in response to changes in one’s own rewards, but not to changes in the rewards of others. In fact, it was known that the brain regions engaged in the prediction and registration of rewards include the midbrain-striatal and midbrain-prefrontal dopaminergic projections (Rilling et al., 2008). Activity in these brain regions is influenced by both primary rewards, such as food delivery, and more abstract forms of rewards, such as monetary incentives (Rilling & Sanfey 2011). However, until the 2007, the activity in brain structures was thought to be determined by the absolute level of a reward without being influenced by the simultaneous reward of others for the same job. This situation was challenged by the fMRI hyperscanning study of Fliessbach and colleagues (Fliessbach et al, 2007), which compared the brain activity of 19 couples that were involved in a simple decision task that could bring a monetary revenue for them after each trial. In this experimental paradigm, both of the subjects had access to the income of their peers for the same kind of performance that they did. Computer programs generated a reward according to different situations, so that the subjects could appreciate getting a different monetary reward (sometime less, sometime more) from their peers for the same job performed. The study showed a relationship between relative income and hemodynamic responses in the ventral striatum. Receiving less than another subject was associated with a reduced BOLD signal in such areas. This result is neurophysiological evidence for the importance of social comparisons of reward processing in the human brain, and it was reached using the simultaneous recording of the brain activity of both subjects involved in the evaluation game.
4.13 Interactions between subjects involved in different games
Playing with others is one of the first social activities of childhood, but playing with peers also represents an important moment of social life and interaction for adults. Thus, it is not strange that one of the first applications of EEG hyperscanning was in the analysis of the brain activity during a card game (Babiloni et al., 2006, 2007; Astolfi et al., 2010c). In particular, the activity of 14 subjects involved, in couples, in an Italian card game similar to the international game of “Bridge” was recorded. Interestingly, each game involved 4 subjects (2 teams of 2 players each), which resulted in an EEG hyperscanning of 4 subjects simultaneously being obtained. Fig. 3A shows the experimental setup involving 4 players. The experimental hypothesis was that the cerebral activity of the winning team generates neuroelectrical markers different from those derived from the defeated team. The interbrain links were estimated by Partial Directed Coherence in the frequency domain. Results highlighted the existence of statistically significant functional connectivity between the anterior cingulate cortex (ACC) in one player and the prefrontal Brodmann areas 8 and 9/46 of their companion before the successful card was chosen. It appeared that the successful couple in the card game presented a more marked neural signature in the mentalizing system (i.e. roughly in the prefrontal areas) than the defeated couple. This suggested that a winning team was composed of persons who are more able to “imagine” their companion’s cards, and this “imagination” elicits higher levels of neural activity in prefrontal brain areas.
Figure 3. Hyperscanning recordings related to games.
This picture presents two EEG hyperscanning procedures, related to the execution of a card game (A) and of a ping-pong game (B). In this last application the cursors are moved by the modulation of the EEG activity of the two subjects. From Astolfi et al., 2010c and Babiloni et al., 2009.
In 2009, the same group employed hyperscanning during interactions involving the popular and old fashioned “ping-pong” computer game (Babiloni et al., 2009). Fig. 3B shows a picture of the interaction between two persons playing “brain-pong”. Although the EEG activity was taken just to be decoded on-line by the computer in order to generate the cursor displacements on the screen, this is an example of EEG hyperscanning applied to the field of Brain Computer Interface (BCI). It must be noted that before publication, R. Goebel and coworkers reported an application of hyperscanning with two fMRI devices in which people were able to play ping-pong. Today, playing ping-pong through a BCI is a general feature of several commercially available software packages for BCI (i.e. that of Guger Technologies; G-tec).
5. Studies of the interaction between subjects without the use of simultaneous recordings
In this section we will describe a series of studies that do not use the simultaneous recording of the subjects involved in the experiment, but compute off-line measurements of synchronicity between the brain activity waveforms instead. We believe that offering a partial review of such studies could increase the scenario that has until now been described solely by the hyperscanning investigations.
5.1 Off-line estimated interactions between subjects using manual gestures
One question at the base of many hyperscanning experiments is whether the brain activity is similar when the subject within the scanner interacts with an external human in a “live” interaction, or with a video representing a human, in a “recorded” interaction. In fact, although the performance of the scanned subjects could be similar or even identical in the two experimental situations, the issue of what changes are undergone in the brain in such conditions is important. In a study performed by Redcay and coworkers (Redcay et al., 2010) functional MRI data were collected while participants interacted with a human experimenter face-to-face via a live video feed as they engaged in simple cooperative games. In one experimental condition, participants engaged in a live interaction with the experimenter ("Live") or watched a video of the same interaction ("Recorded"). During the "Live" interaction a greater activation was seen in the brain regions involved in social cognition and reward when as compared to the “Recorded” conditions, including the right temporoparietal junction (rTPJ), the anterior cingulate cortex (ACC), the right superior temporal sulcus (rSTS), the ventral striatum, and the amygdala. The interesting point here is the demonstration that “Live” interactions generate cortical activities different from those elicited by the “Recorded” interactions. This means that ecologically valid situations which test social interactions are necessary, in order to obtain more information regarding the neural basis of such interactions.
5.2 Off-line estimated interactions between subjects by the observation of movies
Several times during this review it has been suggested that the usual conventional research protocols in social neuroscience can suffer from a lack of ecological validity, as they are sometimes too simplified and highly controlled. Conversely, naturalistic stimuli could be responsible for a large variance in the gathered brain signals due the complexity of the multidimensional nature of such stimuli. However, in the course of the last decade it was repeatedly demonstrated that the human brain activity can be highly reliable under naturalistic stimulus conditions (free viewing of movies, or listing speeches or dialogues; Hasson et al., 2004, 2008, 2010; Wilson et al., 2008; Stephens et al., 2010). All of these studies used fMRI devices to measure brain activity, and employed a computational methodology to estimate the similarity between the hemodynamic waveforms across the subjects, which was called inter-subject correlation. This methodology was used to report that as much as 45 per cent of the cortex showed a high (and statistically significant) intra- and inter-subject correlation during movie watching. These cortical areas include the auditory cortex in the temporal lobe, the visual cortex in the occipital lobes, and the multisensory and language areas in the temporal and parietal lobes (Hasson et al., 2004). These observations were confirmed several years later by the same research group, who looked for inter-subject correlations of hemodynamic signals when subjects watched a movie and then again three weeks later when they were asked to recall it from memory (Hasson et al., 2008). To increase the ecological validity of the study, participants were not informed of a pending memory questionnaire and were not asked to explicitly memorize the movie content. Interestingly, the analysis revealed a set of brain areas whose response time courses were significantly more correlated across subjects during the portions of the movie that were successfully encoded into memory. These regions included the parahippocampal gyrus, the superior temporal sulcus, the anterior temporal poles and the temporal parietal junction. Recently, other research groups have replicated these findings by assessing an inter-subject correlation in the prefrontal areas, in addition to the primary sensory and auditory cortices, as previously observed (Hasson et al., 2004, 2008). Interestingly, these prefrontal inter-subject correlations were found both in the time (Jääskeläinen et al., 2008) and the frequency domains (Kauppi et al., 2010). Taken together, all of these results suggested that the methodology of intra-subject correlation could be employed in assessing inter-brain synchronizations in ecological stimulation paradigms, even when a unique scanning device is available.
5.3 Off-line estimated interactions between subjects by auditory modality
It is well known that the comprehension of movements and gestures of the others does not exhaust our modalities of interaction with them. One essential capability of humans is the ability to properly understand the main contents and ideas of other people’s speech in real time. Aspects of the interaction between brains during speech production and comprehension have been investigated in several studies involving the use of a particular inter-subject correlational analysis introduced previously by Hasson and coworkers (Hasson et al., 2004). Wilson and coworkers (Wilson et al., 2008) performed a study on 24 subjects by providing auditory or audiovisual narratives as sensory stimuli. They used the model-free inter-subject correlational analyses to identify cortical areas which were systematically modulated by the linguistic input and the processing it entailed. The results obtained revealed an extended network of brain areas that were modulated in a consistent way across subjects during the narratives. In fact, the authors observed that the anterior cingulate and adjacent medial frontal cortex, as well as the posterior cingulate and adjacent precuneus, were modulated by the time-varying profile of the audiovisual input provided, despite being largely deactivated relative to rest conditions. Comprehension of the audiovisual inputs involved the activation of a network of bilateral inferior frontal and premotor regions. This network of regions beyond the superior temporal cortex was important for higher-level linguistic processes, and interfaced with extralinguistic cognitive, affective, and interpersonal systems.
In a successive study using fMRI by the research group of Stephens and coworkers (Stephens et al., 2010), brain activity was recorded in a group of 14 subjects during a story telling involving a long, unrehearsed story. Subsequently, brain activity was measured in the same group who already heard the story. By looking at the interbrain coupling of speaker-listener hemodynamic activities, using inter-subject correlational analysis, it was demonstrated that the speaker’s activity was spatially and temporally coupled with the listener’s activity. Interestingly, such interbrain coupling vanishes when participants fail to communicate. This suggests that it is possible to “measure” the efficacy of the communication from a cerebral point of view. In addition to these results, it was also demonstrated that the listener’s brain activity mirrored the speaker’s activity with a delay. The striatum and medial and dorsolateral prefrontal regions of the listener’s brains exhibited predictive anticipatory responses. It was suggested that such an anticipatory process could be linked to a compensation for problems with noisy or ambiguous input (Hasson et al., 2012; Garrod and Pickering, 2004).
6. An integrated summary and a view on the future trends in hyperscanning methodology
Since the re-introduction of hyperscanning recordings in literature by Red Montague in 2002, many studies have been published, and almost all of them have been reviewed here. Thus, at the end of this overview we would like to end with a series of questions, depicted in the following, that will be of help in determining the importance of this methodological approach to the study of human “social” functions. In particular, we provide in the following answers to the following questions:
What are the specific contributions of these hyperscanning studies to the comprehension of neural processes in humans during simple “social” interactions?
Are there any concrete applications that can be expected from this research area?
6.1 Contribution of hyperscanning studies to the comprehension of neural processes during “social” interactions
The “unique” contribution of hyperscanning studies to the comprehension of neural processes during social interactions is related to the simultaneous measurement of brain activities in all of the subjects involved in the interaction. This capability can return information about the temporization of the neural processes involved in the two brains during the interaction. In fact, it is known that the brain reacts differently to the external stimulus provided by the surrounding environment if the source of this stimulus is an agent that is perceived to be similar to us (Keysers and Gazzola, 2009). To date, two kinds of experimental setups have been used in hyperscanning studies.
The first one involved scanning individuals to generate an action perceived by another individual, i.e. an experimental setup in which there is a clear “sender” and a clear “receiver” of the action. Such an action can be, for example, an overt verbalization of a story (Stephens et al., 2010), a series of gestures (Schippers et al., 2010), a facial expression related to internal emotions (Anders et al., 2011), or information about an inner “intention” of the sender in a game (King-Casas et al., 2005; Tomlin et al., 2006; Astolfi et al., 2010). Reported evidence supports the notion that in both the “sender “ and “receiver“ the proposed interaction generates a pattern of brain activity that is functionally interconnected on the basis of a simple temporal delay (positive or negative) or asserted on the basis of the use of causal estimations between such brain activities. In addition, some of the studies suggested that the sequenced and structured interaction between the subjects generates brain signals that maintain the temporal or causal structure of the external stimuli. In particular cases (as for example for the speech) it has been suggested that a “resonance” can occur between the measured oscillatory brain activity and the oscillatory pattern of the speech prosody (Hasson et al., 2012). In the case of simple proposition of gestures between subjects, the brain activity of the subject who performs the movement “causes” (in the Granger sense) part of the brain activity of the subject who saw the movement. Such “caused” brain activity in the observer is generated in cortical areas similar to those activated by the subject who actually performs the gesture. All of these facts cannot be hypothesized and verified in the absence of the brain recordings performed on both of the subjects involved in the structured social interaction. Hyperscanning recordings can thus ensure the simultaneous gathering of brain activity data that could be successively used in relation to simple or complex mathematical measurements of correlation or causality.
In the second kind of scheme used in the reviewed studies, both of the subjects performed an action simultaneously to reach a common goal. Such an action can be a movement of simple synchronization, like pressing a button, playing music together or moving limbs together (Fukane et al., 2011; Cui et al., 2011; Lindenberger et al., 2009; Dumas et al., 2011; Babiloni et al., 2011), or a decision-making task (Astolfi et al., 2011). A common tract of these tasks is related to the hypothesis that synchronous brain oscillations could sustain synchronous or coordinated behavior between the participants of the task. In addition, results from these experimental paradigms suggest an important role of the prefrontal cortices of both subjects. Such regions are consistently activated across different specific paradigms, not only in the isolated brain of the participants but also in the estimated functional links between subjects. The sustained presence of the activation of the prefrontal cortices in all of the tasks related to this second experimental scheme suggests that the activity in the associative cortex sub-serves the social interaction.
The simultaneous recording of brain activity from multiple subjects has promoted the gathering of important information on the ongoing cerebral processes at the basis of the social interactions. Scientific results obtained by this approach are consistent with the findings obtained using conventional recording techniques, but have also added specific information about the dynamics of the social exchanges.
6.2 Possible concrete applications in this research area
Hyperscanning studies reviewed so far have underlined the importance of generating ecologically valid situations to test interactions between people (Dumas, 2011). This means that the experimental paradigms have to be similar to the complex interaction mechanisms that are usually encountered every day of our life. This is certainly a great challenge, and it represents an unexplored territory for both technological and scientific research applications.
From the point of view of the technological challenge, and in order to bring neuroscience outside the protected environment of the laboratories and into the real dynamics of our interactions in real life, new scanning devices could be designed and new experimental paradigms have to be realized. These new scanning devices could develop from existing ones, but have to be developed by taking into account the research issues raised during this first decade of hyperscanning studies. In the time of a generalized financial crisis such as those currently faced by several Western societies, often not all of the research structures could afford to buy two fMRI devices to study social interactions. However, one interesting attempt to obtain a relatively low cost fMRI hyperscanning facility has been performed by the group of Lee and coworkers (Lee et al., 2010, 2012). In fact, they have realized a novel MRI dual-head volume coil (DHVC) that allows for the acquisition of dyadic fMRI (dfMRI) signals from two subjects’ brains while the subjects are in close proximity of one another inside a single scanner. The prototype DHVC was built and integrated into a commercial MRI scanner. The research group of Leehas tested this device on more than 20 subjects, revealing the BOLD effect of the direct interaction for the first time (Lee et al., 2012). Figs. 4A and 4B present this new device explicitly designed for the fMRI hyperscanning recordings. One of the promising new scenarios opened by such a dyadic device is that the people within the scanner could even get in touch, introducing the possibility to study the somatosensory interaction between subjects in such a context. In addition, ecologically valid exchanges could also occur with audio and visual interactions, without the need to solve the critical problems related to the different sensitivities of the coils and the synchronization of the different fMRI devices that challenge the current fMRI hyperscanning studies. While the use of dual coil fMRI promises to open a new era in which fMRI hyperscanning recordings will be easy to generate, a similar attempt is performed also by the use of NIRS technology. In fact, NIRS devices could be changed to make them ready to support the multiple simultaneous subject recordings. This possibility was demonstrated by the work of Cui and colleagues (2011) in which a standard NIRS device was able to support dual subject recordings (Fig. 5A). To use a single NIRS instrument to measure two brains offers several advantages similar to those already described for an fMRI dual recording coil: there is no need to calibrate across the devices, there are no synchronization problems, and there are easy experimental designs. In order to make such an approach easy, the NIRS industry could increase the number of fibers and probes that a NIRS device can support, in order to easily realize multiple recordings without the need to purchase multiple NIRS devices. As observed previously, EEG hyperscanning is rather easy to perform, due to the flexibility and the relatively low cost of the acquisition devices. At the current stage of the technology, EEG hyperscanning is easily able to record four or even more subjects in a complex experimental paradigm. Critical developments may be the generation on the same machine of electrical ground and references that allow the same EEG device to be used for two or more people. Such an EEG device, which has not yet been developed, could overcome the need for the synchronization and calibration that is actually mandatory for all EEG hyperscanning recordings. The possibility to investigate multiple interactions of three or four subjects has been already addressed using EEG hyperscanning recordings, as shown in Fig. 5B, which shows EEG data collection during a Prisoner’s Dilemma task for three players.
Figure 4. A new device for fMRI hyperscanning.
The picture shows in the left part A) an external vision of the dual coil, while in the section B) the dual coil ready for the recording. From Lee et al., 2012.
Figure 5. Different hyperscanning devices for hemodynamic and electric modalities.
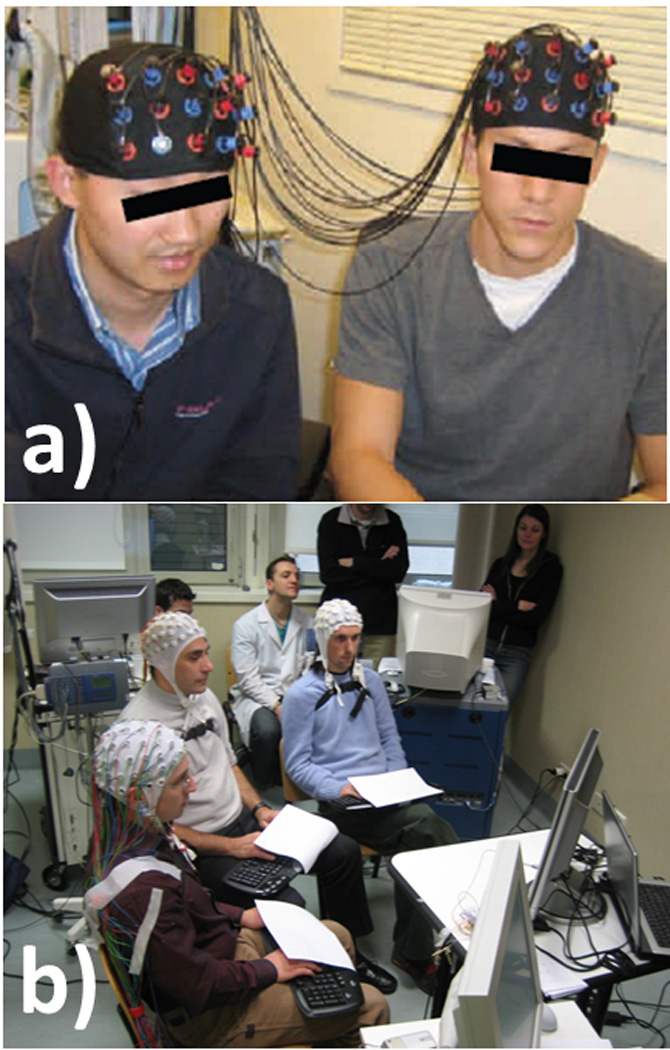
The figure shows in the upper part A) the split of the fibers to generate an NIRS hyperscanning device from one single NIRS device; in the lower part B) the EEG hyperscanning setup during an iterated Prisoner’s Dilemma task involving three subjects. Figures from Cui et al., 2011 and Astolfi et al., 2011.
From the point of view of a scientific challenge, the application of these hyperscanning methodologies could further improve the knowledge about the conditions in which social interactions are impaired in some diseases. In fact, many relevant brain pathologies concern disturbed social interactions, including depression, autism and schizophrenia. Understanding which mechanisms could be disturbed during social interactions may provide an invaluable tool for the study of such pathologies. These disruptions (if any) could be assessed using hyperscanning methodologies and simple interaction paradigms that have been described before. Sequential experimental paradigms could provide indications on where and when the cortical coupling mechanisms seen in healthy subjects are not fully performing in patients. This entirely new area of investigation could represent an important tool for the advancement of our understanding of these pathologies.
Hyperscanning is a technique that allows the simultaneous recording of brain activity of different subjects
It allows to study inter-brain correlations between cerebral activity of a group of interacting subjects as a unique system
Ecologic experimental designs have been adopted to create an interaction between subjects similar to real life social exchange
Hyperscanning is a potentially revolutionary new approach, which has not yet been used extensively in social neuroscience
It may provide an answer to many open questions in the study of the social brain
Acknowledgments
This work was supported in part by NIH RO1EB006433, by the Compagnia di San Paolo within the Project “Social and Emotive Hyperbrain”, and by the Minister of Foreign Affairs within the framework of cooperation in science and technology between Italy and Hungary.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Fabio Babiloni, Email: fabio.babiloni@uniroma1.it.
Laura Astolfi, Email: laura.astolfi@uniroma1.it.
References
- Aristotle . In: The Politics. Stalley RF, Ernest Barker, Stalley RF jr, editors. Oxford University Press; 1998. [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anders S, Eippert F, Wiens S, Birbaumer N, Lotze M, Wildgruber D. When seeing outweighs feeling: a role for prefrontal cortex in passive control of negative affect in blindsight. Brain. 2009;132(Pt 11):3021–3031. doi: 10.1093/brain/awp212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Heinzle J, Weiskopf N, Ethofer T, Haynes JD. Flow of affective information between communicating brains. Neuroimage. 2011;54(1):439–446. doi: 10.1016/j.neuroimage.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astolfi L, Cincotti F, Mattia D, De Vico Fallani F, Salinari S, Marciani MG, Wilke C, Doud A, Yuan H, He B, Babiloni F. Estimation of the cortical activity from simultaneous multi-subject recordings during the prisoner's dilemma. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:1937–1939. doi: 10.1109/IEMBS.2009.5333456. [DOI] [PubMed] [Google Scholar]
- Astolfi L, Cincotti F, Mattia D, De Vico Fallani F, Salinari S, Vecchiato G, Toppi J, Wilke C, Doud A, Yuan H, He BH, Babiloni F. Imaging the social brain: multi-subjects EEG recordings during the “Chicken’s game”; Conf Conf Proc IEEE Eng Med Biol Soc; 2010a. pp. 1734–1737. 2010a. [DOI] [PubMed] [Google Scholar]
- Astolfi L, Cincotti F, Mattia D, De Vico Fallani F, Salinari S, Vecchiato G, Toppi J, Wilke C, Doud A, Yuan H, He B, Babiloni F. Simultaneous estimation of cortical activity during social interactions by using EEG hyperscannings; Conf Proc IEEE Eng Med Biol Soc; 2010b. pp. 2814–2817. 2010, 2010b. [DOI] [PubMed] [Google Scholar]
- Astolfi L, Toppi J, De Vico Fallani F, Vecchiato G, Salinari S, Mattia D, Cincotti F, Babiloni F. Neuroelectrical hyperscanning measures simultaneous brain activity in humans. Brain Topogr. 2010c;23(3):243–256. doi: 10.1007/s10548-010-0147-9. 2010. [DOI] [PubMed] [Google Scholar]
- Astolfi L, Toppi J, De Vico Fallani F, Vecchiato G, Cincotti F, Wilke C, Han Yuan Mattia D, Salinari S, He B, Babiloni F. Imaging the Social Brain by Simultaneous Hyperscanning during Subject Interaction, 2011. IEEE Intelligent Systems. 2011;Volume 26(Issue 5):38–45. doi: 10.1109/MIS.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni F, Cincotti F, Mattia D, Mattiocco M, De Vico Fallani F, Tocci A, Bianchi L, Marciani MG, Astolfi L. Hypermethods for EEG hyperscanning. Conf Proc IEEE Eng Med Biol Soc. 2006;1:3666–3669. doi: 10.1109/IEMBS.2006.260754. [DOI] [PubMed] [Google Scholar]
- Babiloni F, Cincotti F, Mattia D, De Vico Fallani F, Tocci A, Bianchi L, Salinari S, Marciani M, Colosimo A, Astolfi L. High resolution EEG hyperscanning during a card game; Conf Proc IEEE Eng Med Biol Soc; 2007. pp. 4957–4960. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Buffo P, Vecchio F, Marzano N, Del Percio C, Spada D, Rossi S, Bruni I, Rossini PM, Perani D. Brains “in concert”: Frontal oscillatory alpha rhythms and empathy in professional musicians. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Vecchio F, Infarinato F, Buffo P, Marzano N, Spada D, Rossi S, Bruni I, Rossini PM, Perani D. Simultaneous recording of electroencephalographic data in musicians playing in ensemble. Cortex. 2012 doi: 10.1016/j.cortex.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. The hyper-systemizing, assortative mating theory of autism. Prog Neuro-Psychopharmacol Biol Psychiatry. 2006;30:865–872. doi: 10.1016/j.pnpbp.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Chang C, Thomason ME, Glover GH. Mapping and correction of vascular hemodynamic latency in the bold signal. Neuroimage. 2008;43:90–102. doi: 10.1016/j.neuroimage.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu PH, Kayali MA, Kishida KT, Tomlin D, Klinger LG, Klinger MR, Montague PR. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron. 2008;57:463–473. doi: 10.1016/j.neuron.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D, Yun K, Jeong J. Neural mechanisms of free-riding and cooperation in a public goods game: An eeg hyperscanning study. International Conference of Cognitive Science; Seoul, Korea. 2008. [Google Scholar]
- Cross ES, Hamilton AF, Grafton ST. Building a motor simulation de novo: observation of dance by dancers. NeuroImage. 2006;31:1257–1267. doi: 10.1016/j.neuroimage.2006.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Bryant DM, Reiss AL. NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. NeuroImage. 2012;59(3):2430–2437. doi: 10.1016/j.neuroimage.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David O, Guillemain I, Saillet S, Reyt S, Deransart C, Segebarth C, Depaulis A. Identifying neural drivers with functional MRI: an electrophysiological validation. PLoS Biology. 2008;6:2683–2697. doi: 10.1371/journal.pbio.0060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: How low-level computational processes contribute to metacognition. Neuroscientist. 2007;13:580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- de Marco G, Devauchelle B, Berquin P. Brain functional modeling, what do we measure with FMRI data? Neuroscience Research. 2009;64:12–19. doi: 10.1016/j.neures.2009.01.015. [DOI] [PubMed] [Google Scholar]
- De Vico Fallani F, Nicosia V, Sinatra R, Astolfi L, Cincotti F, Mattia D, Wilke C, Doud A, Latora V, He B, Babiloni F. Defecting or Not Defecting: How to “Read” Human Behavior during Cooperative Games by EEG Measurements. PLoS ONE. 2010;5(12):e14187. doi: 10.1371/journal.pone.0014187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Sathian K, Hu X. Effect of hemodynamic variability on Granger causality analysis of fMRI. NeuroImage. 2010;52:884–896. doi: 10.1016/j.neuroimage.2009.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duane TD, Behrendt T. Extrasensory electroencephalographic induction between identical twins. Science. 1965;150(3694):367. doi: 10.1126/science.150.3694.367. [DOI] [PubMed] [Google Scholar]
- Dumas G, Nadel J, Soussignan R, Martinerie J, Garnero L. Inter-brain synchronization during social interaction. PLoS One. 2010;5(8):e12166. doi: 10.1371/journal.pone.0012166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas G. Towards a two-body neuroscience. Commun Integr Biol. 2011 May;4(3):349–352. doi: 10.4161/cib.4.3.15110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas G, Lachat F, Martinerie J, Nadel J, George N. From social behavior to brain synchronization: Review and perspectives in hyperscanning. IRBM. 2012 [Google Scholar]
- Emir UE, Ozturk C, Akin A. Multimodal investigation of fMRI and fNIRS derived breath hold BOLD signals with an expanded balloon model. Physiol. Meas. 2008;29:49–63. doi: 10.1088/0967-3334/29/1/004. [DOI] [PubMed] [Google Scholar]
- Fliessbach K, Weber B, Trautner P, Dohmen T, Sunde U, Elger CE, Falk A. Social comparison affects reward-related brain activity in the human ventral striatum. Science. 2007;318(5854):1305–1308. doi: 10.1126/science.1145876. [DOI] [PubMed] [Google Scholar]
- Friston K. Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biology. 2009;7:e33. doi: 10.1371/journal.pbio.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Frith C. The biological basis of social interaction. Curr Dir Psychol Sci. 2001:151–155. [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50(4):531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Funane T, Kiguchi M, Atsumori H, Sato H, Kubota K, Koizumi H. Synchronous activity of two people's prefrontal cortices during a cooperative task measured by simultaneous near-infrared spectroscopy. J Biomed Opt. 2011;16(7):077011. doi: 10.1117/1.3602853. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn Sci. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Garrod S, Pickering MJ. Why is conversation so easy? Trends Cogn. Sci. 2004;8:8–11. doi: 10.1016/j.tics.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Hari R, Kujala MV. Brain basis of human social interaction: from concepts to brain imaging. Physiol Rev. 2009;89(2):453–479. doi: 10.1152/physrev.00041.2007. [DOI] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303(5664):1634–1640. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- Hasson U, Furman O, Clark D, Dudai Y, Davachi L. Enhanced intersubject correlations during movie viewing correlate with successful episodic encoding. Neuron. 2008;7;57(3):452–62. doi: 10.1016/j.neuron.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Malach R, Heeger DJ. Reliability of cortical activity during natural stimulation. Trends Cogn Sci. 2010 Jan;14(1):40–48. doi: 10.1016/j.tics.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Ghazanfar A, Galantucci B, Garrod S, Keysers C. Brain-to-brain coupling: a mechanism for creating and sharing a social world. Trends in Cognitive Neuroscience, TICS-1039. 2012 doi: 10.1016/j.tics.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennenlotter A, Schroeder U, Erhard P, Castrop F, Haslinger B, Stoecker D, Lange KW, Ceballos-Baumann AO. A common neural basis for receptive and expressive communication of pleasant facial affect. Neuroimage. 2005;26(2):581–591. doi: 10.1016/j.neuroimage.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Huppert TJ, Allen MS, Diamond SG, Boas DA. Estimating cerebral oxygen metabolism from fMRI with a dynamic multicompartment Windkessel model. Hum. Brain Mapp. 2009;30:1548–1567. doi: 10.1002/hbm.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2527. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen IP, Koskentalo K, Balk MH, Autti T, Kauramäki J, Pomren C, Sams M. Inter-subject synchronization of prefrontal cortex hemodynamic activity during natural viewing. Open Neuroimag J. 2008;2:14–19. doi: 10.2174/1874440000802010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher P. The Neurobiology of Decision: Consensus and Controversy. Neuron. 2009;63:732–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppi JP, Jääskeläinen IP, Sams M, Tohka J. Inter-subject correlation of brain hemodynamic responses during watching a movie: localization in space and frequency. Front Neuroinform. 2010;19(4):5. doi: 10.3389/fninf.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Expanding the mirror: vicarious activity for actions, emotions, and sensations. Curr. Opin. Neurobiol. 2009;19:666–671. doi: 10.1016/j.conb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR. Getting to know you: reputation and trust in a two-person economic exchange. Science. 2005 Apr 1;308(5718):78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- Lee R, Dai W, Dix W. A decoupled circular-polarized volume head coil pair for studying two interacting human brains with mri. Engineering in Medicine and Biology Society (EMBC); 2010 Annual International Conference of the IEEE; 2010. pp. 6645–6648. [DOI] [PubMed] [Google Scholar]
- Lee R, Dai W, Jones J. Decoupled Circular-Polarized Dual-Head Volume Coil Pair for Studying Two Interacting Human Brains with Dyadic fMRI. Magnetic Resonance in Medicine. 2012 doi: 10.1002/mrm.23313. in press. [DOI] [PubMed] [Google Scholar]
- Legrand D, Ruby P. What is self-specific? Theoretical investigations and critical review of neuroimaging results. Psychol. Rev. 2009;116:252–282. doi: 10.1037/a0014172. [DOI] [PubMed] [Google Scholar]
- Leventhal H, Scherer KR. The relationship of emotion to cognition: a functional approach to a semantic controversy. Cogn. Emotion. 1987;1(1):3–28. [Google Scholar]
- Lindenberger U, Li S, Gruber W, Müller V. Brains swinging in concert: cortical phase synchronization while playing guitar. BMC Neurosci. 2009;10:22. doi: 10.1186/1471-2202-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CM, Zhang YJ, Biswal BB, Zang YF, Peng DL, Zhu CZ. Use of fNIRS to assess resting state functional connectivity. J. Neurosci. Methods. 2010;186:242–249. doi: 10.1016/j.jneumeth.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Materna S, Dicke PW, Their P. Dissociable roles of the superior temporal sulcus and the intraparietal sulcus in joint attention: a functional magnetic resonance imaging study. J. Cogn. Neurosci. 2008;20:1–11. doi: 10.1162/jocn.2008.20.1.108. [DOI] [PubMed] [Google Scholar]
- Michel Christoph M, Murray Micah M. Towards the utilization of EEG as a brain imaging tool. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.12.039. [DOI] [PubMed] [Google Scholar]
- Montague PR, Berns GS, Cohen JD, McClure SM, Pagnoni G, Dhamala M, Wiest MC, Karpov I, King RD, Apple N, Fisher RE. Hyperscanning: simultaneous fMRI during linked social interactions. Neuroimage. 2002;16(4):1159–1164. doi: 10.1006/nimg.2002.1150. [DOI] [PubMed] [Google Scholar]
- Molenberghs Pascal, Cunnington Ross, Jason Mattingley B. Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neuroscience & Biobehavioral Reviews. 2012;36(1):341–349. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Oullier O, de Guzman G, Jantzen K, Lagarde J, Kelso J. Social coordination dynamics: Measuring human bonding. Social neuroscience. 2008;3(2):178. doi: 10.1080/17470910701563392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oullier O, Kelso JA. Social coordination, from the perspective of coordination dynamics. Encyclopedia of complexity and systems science. 2009;19:8198–8213. [Google Scholar]
- Power SD, Falk TH, Chau T. Classification of prefrontal activity due to mental arithmetic and music imagery using hidden Markov models and frequency domain near-infrared spectroscopy. J Neural Eng. 2010;7:026002. doi: 10.1088/1741-2560/7/2/026002. [DOI] [PubMed] [Google Scholar]
- Redcay E, Dodell-Feder D, Pearrow MJ, Mavros PL, Kleiner M, Gabrieli JD, Saxe R. Live face-to-face interaction during fMRI: a new tool for social cognitive neuroscience. Neuroimage. 2010;50(4):1639–1647. doi: 10.1016/j.neuroimage.2010.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, King-Casas B, Sanfey AG. The neurobiology of social decision-making. Curr Opin Neurobiol. 2008;18(2):159–165. doi: 10.1016/j.conb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Sanfey AG. The neuroscience of social decision-making. Annu Rev Psychol. 2011;62:23–48. doi: 10.1146/annurev.psych.121208.131647. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Roebroeck A, Formisano E, Goebel R. Mapping directed influence over the brain using granger causality. Neuroimage. 2005;25:230–242. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Saito DN, Tanabe HC, Izuma K, Hayashi MJ, Morito Y, Komeda H, Uchiyama H, Kosaka H, Okazawa H, Fujibayashi Y, Sadato N. "Stay tuned": inter-individual neural synchronization during mutual gaze and joint attention. Front Integr Neurosci. 2010;5(4):127. doi: 10.3389/fnint.2010.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sänger J, Lindenberger U, Müller V. Interactive brains, social minds. Commun Integr Biol. 2011 Nov 1;4(6):655–663. doi: 10.4161/cib.17934. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers MB, Roebroeck A, Renken R, Nanetti L, Keysers C. Mapping the information flow from one brain to another during gestural communication. Proc Natl Acad Sci U S A. 2010;107(20):9388–9393. doi: 10.1073/pnas.1001791107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers MB, Renken R, Keysers C. The effect of intra- and inter-subject variability of hemodynamic responses on group level Granger causality analyses. NeuroImage. 2011 Jul 1;Volume 57(Issue 1):22–36. doi: 10.1016/j.neuroimage.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Sitaram R, Zhang HH, Guan CT, Thulasidas M, Hoshi Y, Ishikawa A, Shimizu K, Birbaumer N. Temporal classification of multichannel near-infrared spectroscopy signals of motor imagery for developing a braincomputer interface. NeuroImage. 2007;34:1416–1427. doi: 10.1016/j.neuroimage.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Stephens GJ, Silbert LJ, Hasson U. Speaker-listener neural coupling underlies successful communication. Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):14425–14430. doi: 10.1073/pnas.1008662107. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognoli E, Lagarde J, DeGuzman GC, Kelso JA. The phi complex as a neuromarker of human social coordination. Proc Natl Acad Sci U S A. 2007;104(19):8190–8195. doi: 10.1073/pnas.0611453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gaag C, Minderaa RB, Keysers C. Facial expressions: what the mirror neuron system can and cannot tell us. Soc. Neurosci. 2007;2(3–4):179–222. doi: 10.1080/17470910701376878. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Hum Brain Mapp. 2009;30(3):829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others' actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48(3):564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. A dissociation between social mentalizing and general reasoning. Neuroimage. 2011;54(2):1589–1599. doi: 10.1016/j.neuroimage.2010.09.043. [DOI] [PubMed] [Google Scholar]
- Yun K, Chung D, Jeong J. "Simultaneous EEG Hyperscanning during Linked Social Interactions of the Ultimatum Game " Organization for Human Brain Mapping, Barcelona, Spain, June 6–10, Poster Presentation.2010. [Google Scholar]
- Wood JN, Knutson KM, Grafman J. Psychological structure and neural correlates of event knowledge. Cereb Cortex. 2005;15:1155–1161. doi: 10.1093/cercor/bhh215. [DOI] [PubMed] [Google Scholar]
- Williams JH. Self-other relations in social development and autism: multiple roles for mirror neurons and other brain bases. Autism Res. 2008 Apr;1(2):73–90. doi: 10.1002/aur.15. [DOI] [PubMed] [Google Scholar]
- White BR, Snyder AZ, Cohen AL, Petersen SE, Raichle ME, Schlaggar BL, Culver JP. Resting-state functional connectivity in the human brain revealed with diffuse optical tomography. NeuroImage. 2009;47:148–156. doi: 10.1016/j.neuroimage.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhang YJ, Lu CM, Ma SY, Zang YF, Zhu CZ. Functional connectivity as revealed by independent component analysis of resting-state fNIRS measurements. NeuroImage. 2010;51:1150–1161. doi: 10.1016/j.neuroimage.2010.02.080. [DOI] [PubMed] [Google Scholar]


