Abstract
Background
Few marijuana smokers in treatment achieve sustained abstinence, yet factors contributing to high relapse rates are unknown.
Study 1: Methods
Data from five inpatient laboratory studies assessing marijuana intoxication, withdrawal and relapse were combined to assess factors predicting the likelihood and severity of relapse. Daily, nontreatment-seeking marijuana smokers (n=51; 10 ± 5 marijuana cigarettes/day) were enrolled.
Results
49% of participants relapsed the first day active marijuana became available. Tobacco cigarette smokers (75%), who were not abstaining from cigarettes, were far more likely to relapse than non-cigarette smokers (OR=19, p<0.01). Individuals experiencing more positive subjective effects (i.e. feeling “high”) after marijuana administration and those with more negative affect and sleep disruption during marijuana withdrawal were more likely to have severe relapse episodes (p<0.05).
Study 2: Methods
To isolate the effects of cigarette smoking, marijuana intoxication, withdrawal and relapse were assessed in daily marijuana and cigarette smokers (n=15) under two within-subject, counter-balanced conditions: while smoking tobacco cigarettes as usual (SAU) and after at least 5 days without cigarettes (Quit).
Results
Most participants (87%) relapsed to marijuana whether in the SAU or Quit phase. Tobacco cigarette smoking did not significantly influence relapse, nor did it affect marijuana intoxication or most symptoms of withdrawal relative to tobacco cessation.
Conclusions
Daily marijuana smokers who also smoke cigarettes have high rates of marijuana relapse and cigarette smoking versus recent abstinence does not directly influence this association. These data indicate that current cigarette smoking is a clinically important marker for increased risk of marijuana relapse.
Keywords: withdrawal, cannabis, tobacco, treatment, cannabinoids, self-administration
Marijuana-use disorders are ubiquitous worldwide (1), and an increasing number of marijuana smokers are seeking treatment (2, 3, 4, 5). In the U.S., 6.9 million individuals smoke near-daily (>20 days per month; 6), and approximately 16% of patients entering treatment have a diagnosis of primary marijuana abuse (7). Treatment outcome among these patients is poor. Clinical trials using psychological (8, 9, 10), behavioral (11, 12) and pharmacological (13) interventions report only 15–37% of patients achieve continued abstinence. Given these poor outcomes, a clear understanding of the factors associated with high relapse rates is crucial for improving marijuana treatment outcomes.
One factor presumed to contribute to marijuana relapse rates is a withdrawal syndrome. Controlled, human laboratory studies have shown that replacing active marijuana with placebo marijuana for several days produces a time-dependent increase in irritability, anxiety, sleep disruption and decreased appetite (14, 15, 16, 17, 18, 19). Replacement with Δ9-tetrahydrocannabinol (THC; dronabinol) under double-blind conditions selectively reduces withdrawal symptoms in laboratory (16, 20) and clinical (13) settings, demonstrating the pharmacological specificity of marijuana withdrawal.
In an effort to improve treatment options, we have conducted placebo-controlled studies testing the effects of a range of medications on marijuana intoxication, withdrawal, and relapse. Because marijuana is administered, only individuals explicitly not seeking treatment for their marijuana use are enrolled. Thus, relapse is operationally defined as marijuana self-administration, at a financial cost, after a period of abstinence (17, 21, 22, 23): Participants purchase self-administered marijuana using their study earnings. Although the motivation to relapse or abstain may differ from that of a patient in treatment, laboratory measures of self-administration predict clinical outcome (13, 21, 24, 25), and thus contribute controlled and clinically-relevant data on factors affecting marijuana use.
After combining data from the placebo medication phases of five studies, a pattern emerged: Despite closely similar marijuana-use and demographic profiles, only half of the participants relapsed, i.e., chose to pay the high financial cost to resume marijuana smoking after several days of abstinence. Given the detailed data collected on intoxication, withdrawal, and demographic variables, the objective of Study 1 was to evaluate what predicted (1) the odds an abstinent marijuana smoker would relapse to active marijuana use, and (2) the severity of relapse, i.e., how much marijuana was self-administered among those who relapsed. The results from Study 1 showed that tobacco cigarette-smoking status robustly predicted marijuana relapse. Thus, Study 2 evaluated the direct effects of tobacco cigarette smoking on marijuana relapse compared to tobacco cessation.
Study 1: Methods and Materials
Data from 5 studies (n=51 participants) using a similar model of marijuana relapse were analyzed. Four medication studies (lofexidine, dronabinol, quetiapine, mirtazapine, nabilone; 17, 22, 23, 26) enrolled participants in up to 4 inpatient phases, each testing a different medication dose in counter-balanced order, and allowing time between phases for medication clearance. Only the placebo condition was analyzed. One non-medication study (n=9) tested the effects of marijuana-paired cues and marijuana exposure on relapse; only the condition in which neither cues nor marijuana preceded the decision to relapse was analyzed herein.
Participants
Healthy marijuana smokers solicited through advertisements in New York, NY were enrolled from August 2006 to September 2011. Inclusion criteria included: 21–50 years of age and current marijuana use (minimum 2 marijuana cigarettes/day, 5 days/week). No participant could: (1) regularly use any other illicit drug or be dependent on alcohol, (2) meet criteria for a current Axis I disorder requiring medical intervention, (3) be taking medication, or (4) be seeking marijuana treatment. Assessments of health included physical examination, psychiatric evaluation, electrocardiogram, urinalysis, and blood chemistry. Participants signed a consent form approved by The New York State Psychiatric Institute (NYSPI) Institutional Review Board, which described the study, outlined possible risks, and indicated that two different strength marijuana cigarettes would be tested. Volunteers were compensated for participation.
Procedures
Participants, in groups of 3 or 4, lived in a residential laboratory for 9–11 days, with four private rooms, two bathrooms and a recreational area; video- and audio-monitoring systems allowed for continuous observation of participants (14). Before the inpatient stay, participants sampled an active marijuana cigarette (labeled “Dose A”) and a placebo marijuana cigarette (labeled “Dose B”) using procedures described below. They were told that the strength of Dose A and Dose B would not change, and that they should pay attention to how each dose made them feel as they would later make decisions regarding self-administration.
On each inpatient day, participants completed one sleep scale, eight mood scales (17) and six 30-min psychomotor/cognitive task batteries (27) between 0815 and 2330. A variety of food to be consumed ad libitum was available. The recreation area was available at lunchtime and from 1700–2200. At 2330, participants were given faux money representing a portion of their earnings that could be used to purchase marijuana on self-administration days or exchanged for cash upon study completion. Lights were turned off by 2400.
Marijuana
Marijuana was administered using a cued-smoking procedure, where duration of inhalation, holding smoke in the lungs, and inter-puff interval was controlled (28). Marijuana was either experimenter-administered at no cost or was available to purchase for self-administration; participants were informed of that day’s condition at 0950 each morning.
During experimenter-administered days (first day of each inpatient phase), participants smoked 3 puffs of active marijuana [3.3–6.2% THC (depending on study); Dose A] six times throughout the day. The purpose of this day was to standardize marijuana exposure prior to abstinence. On the subsequent 2–3 inpatient days, Dose B (placebo marijuana) was available for self-administration [withdrawal], followed by 1–4 days when Dose A was available for self-administration [relapse]. During self-administration days, participants had six opportunities to purchase up to 3 puffs of the available dose. The cost was $9-10 for the first puff of the day, and $2-3 for all subsequent puffs, depending on the study; prices were derived from pilot data showing that marijuana self-administration following abstinence varies as a function of cost (22). Participants paid for marijuana puffs using money representing their earnings. They smoked self-administered marijuana in private to keep other participants blind to their choice.
Sleep
Subjective ratings of the previous night’s sleep were obtained using a 7-item VAS sleep questionnaire. For objective sleep measurement, participants wore the Nightcap® (n=8) or the Actiwatch® Activity Monitoring System (n=43; Respironics, Bend OR).
Subjective-Effects Battery
A 44-item subjective-effects questionnaire, comprising a series of 100-mm visual analog scales (VAS) anchored with "Not at all" (0 mm) and "Extremely" (100 mm), included mood, physical symptom and drug effect descriptors; participants rated the extent to which each descriptor applied to them at that moment. Based on a cluster analyses, we employed arithmetic means of individual item scores to produce seven subscales: miserable (example items: “miserable”, “irritable,”); anxious (e.g. “anxious,” “on edge”); bad effect (e.g.,“muscle pain,” “chills”); sedated (e.g., “sleepy,” “tired”); social (e.g., “friendly,” “talkative”); high (e.g., “high,” “good effect”); confused (e.g., “forgetful,” “confused”). We also analyzed ratings of drug craving using VAS ratings of “I Want Marijuana,” “I Want Alcohol,” and “I Want Cigarettes.”
Tobacco cigarette smoking
The number of tobacco cigarettes smoked was recorded by counting cigarette butts in each participant’s ashtray each evening.
Data Analysis
The primary outcome measure was the number of marijuana puffs (0–18) purchased on the first day active marijuana was available after abstinence. Of the 51 participants, 26 (51%) bought zero puffs of marijuana and 25 participants (49%) bought at least 1 marijuana puff. Puffs of marijuana were analyzed using a Poisson hurdle regression model using PROC NLMIXED in SAS 9.2. The Poisson hurdle, or two-part model, models whether the number puffs of marijuana is zero or non-zero using logistic regression, and models the number of puffs of marijuana purchased using a truncated-at-zero Poisson regression, conditional on non-zero outcomes. This model reflects the two-stage decision-making process present: deciding to self-administer the first puff of marijuana, and then deciding how many marijuana puffs to self-administer. Table 1 lists the covariates examined in both parts of the model; those included in the final model had a p-value <0.05. The logistic regression component utilized a logit link function, and predicted the odds of abstinence, i.e., buying zero marijuana puffs. The factors included in the logistic portion of the final model were 1) whether participants were tobacco cigarette smokers and 2) age of onset of daily marijuana use. The truncated Poisson regression part utilized a log link function, and predicted the conditional incidence rates of marijuana puffs purchased. The factors included in the Poisson portion of the model were 1) peak ratings of ‘high’ following experimenter-administered marijuana, 2) peak cluster ratings of miserable during marijuana withdrawal, and 3) sleep latency during marijuana withdrawal. Akaike information criterion was used to assess goodness of fit.
TABLE 1.
Factors included in logistic regression equation predicting likelihood of relapse and truncated Poisson regression equation predicting severity of relapse".
| Demographic Variables: |
| Age |
| Age of daily marijuana use |
| Years smoking marijuana regularly |
| Number of marijuana cigarettes smoked/day |
| Cigarette smoker (yes/no) |
| Cigarettes/day (#) |
| Alcohol drinks/week |
| Acute Marijuana Effects |
| Peak ratings of “High” |
| Marijuana Withdrawal Symptoms |
| Peak ratings of marijuana craving |
| Peak cluster ratings of “Miserable” |
| Peak cluster ratings of “Anxious” |
| Peak ratings of Fell Asleep Early, Sleep Satisfaction, Woke Early |
| Sleep Latency, Percent Time Asleep |
Study 1: Results
Participant Characteristics
Table 2 portrays demographic data on the participants (n=51) included in the analysis.
TABLE 2.
Demographic characteristics of participants
| Study 1 | Study 2 | |
|---|---|---|
| Number of participants | 51 (46M; 5F) | 15 (13M; 2F) |
| Race (Black/White/Mixed/Pacific Islander) | 41/7/2/1 | 9/2/4 |
| Ethnicity (Hispanic/non-Hispanic) | 11/40 | 10/5 |
| Age (years) | 28 ± 7 | 30 ± 6 |
| Marijuana use (#days/wk) | 6.9 ± 0.4 | 6.9 ± 0.3 |
| Marijuana cigarettes/day | 9.9 ± 5.6 | 8.9 ± 5.7 |
| Age beginning daily marijuana use | 17.9 ± 5.8 | 16.1 ± 4.5 |
| Cigarette Smokers (#) | 38 | 15 |
| Cigarettes/day | 9.0 ± 4.8 | 10.3 ± 3.8 |
| Alcohol Drinkers (#)* | 29 | 7 |
| Alcohol: Drinks/week | 4.4 ± 3.5 | 12.3 ± 8.0 |
| Education (years) | 12.6 ± 2.4 | 12.5 ± 1.3 |
Note: Data are presented as means (± standard deviation) or as frequency.
Only included those reporting at least 1 drink/week.
Odds of Marijuana Relapse
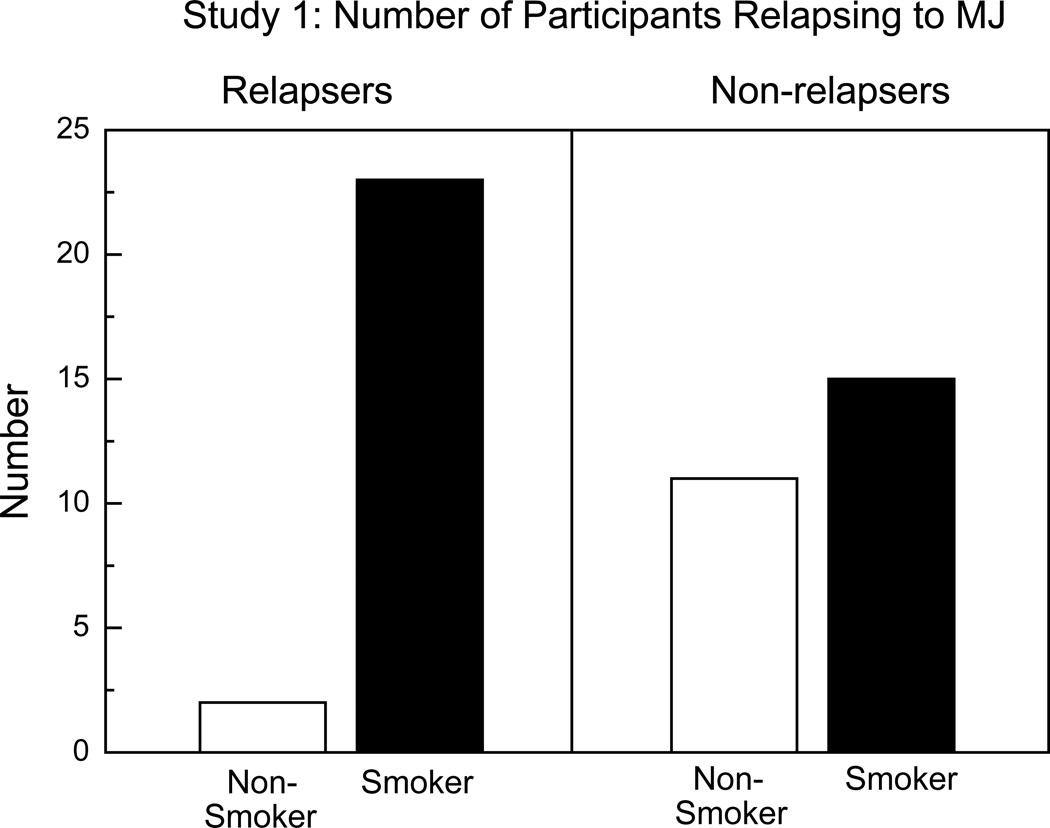
Non-cigarette smokers had about 19 times the odds of not relapsing to marijuana (OR=19.02; 95%CI:=2.18, 165.95, t = 2.73, p = 0.009), with age at daily use held constant. This effect is portrayed in Figure 1, which shows the number of daily marijuana smokers who relapsed as a function of their tobacco cigarette use. As shown in Table 2, 75% of participants smoked tobacco cigarettes (range: 1–18 cigarettes/day) and continued to do so while inpatient.
Figure 1.
Total number of participants in Study 1 (n=51) relapsing to marijuana as a function of tobacco cigarette smoking status. ‘Relapsers’ (left panel) purchased at least 1 puff of active marijuana on the first day it became available. ‘Smokers’ smoked at least 1 tobacco cigarette/day.
The only other significant predictor was the age when daily marijuana use was initiated, with those who started daily marijuana use later in life being more likely to relapse. The odds of not purchasing any marijuana were estimated as 87.3% of what it would be compared to starting marijuana use one year older (OR=0.87; 95% CI = 0.77, 0.99, t = −2.15, p = 0.036), cigarette smoking held fixed. Table 2 shows that on average, participants were 18 years old when they started daily marijuana use (range: 9–40 years). Everyone in the non-relapsing group was under the age of 25 when they started smoking marijuana everyday, while the relapse group included 5 individuals who were older when commencing daily marijuana use (28–40 years).
Severity of Marijuana Relapse
Among the group who relapsed, three factors predicted relapse severity, i.e., the number of marijuana puffs self-administered following a period of abstinence. All other factors were held fixed when investigating each of these factors. The first factor was peak cluster ratings of “High” following experimenter-administered marijuana (range: 0–100 mm). For every 10 mm increase in peak ratings of “High,” the estimated number of puffs purchased during the relapse increased by 12.4% [Incidence Rate Ratio (IRR) =1.12; 95%CI = 1.05, 1.20, t =3.50, p = 0.001]. The second predictive factor was an objective sleep measure during marijuana withdrawal. For every 10-minute increase in sleep onset latency, the estimated number of puffs purchased increased by 8.1% (IRR = 1.08; 95% CI = 1.03, 1.13, t=3.31, p = 0.002). The third predictive factor was cluster ratings of “Miserable” during the withdrawal phase. For every 10 mm increase in “Miserable” ratings, the estimated number of puffs purchased following a period of marijuana abstinence increased by 4.3% (IRR = 1.04; 95% CI = 1.002, 1.09, t=2.13, p = 0.038).
Study 2: Methods and Materials
Study 1 demonstrated that the factor having the largest impact on marijuana relapse was cigarette-smoking status. Participants were free to smoke tobacco cigarettes, so marijuana relapse did not reflect an effort to attenuate nicotine withdrawal (29). One possibility is that nicotine directly enhances marijuana’s effects and that repeatedly pairing tobacco cigarettes and marijuana results in one drug cueing use of the other (30, 31). There is close overlap in the distribution of nicotinic and cannabinoid receptors (32), and pretreatment with a nicotine patch (21 mg) has been shown to increase marijuana ‘high’ (33). Further, some individuals report smoking tobacco cigarettes immediately after marijuana, purportedly to produce greater intoxication (34).
Thus, the purpose of Study 2 was to test the direct effects of tobacco cigarette smoking on marijuana relapse. We used the same model of marijuana intoxication, withdrawal and relapse described in Study 1 to compare daily marijuana and cigarette smokers across two inpatient phases: when they were smoking cigarettes as usual (SAU) and after they had undergone a period without smoking cigarettes (Quit). The order of Quit and SAU phases was counter-balanced.
Participants
Screening procedures and inclusion criteria were identical to Study 1 except that eligible volunteers smoked ≥5 cigarettes/day and could not be seeking tobacco cessation treatment.
Procedures
Each 8-day, inpatient SAU or Quit phase was preceded by at least 7 outpatient days to allow time to either quit or resume smoking tobacco cigarettes. For the Quit phase, our objective was to have participants quit smoking tobacco cigarettes at least 1 week prior to study onset to reduce the impact of peak nicotine withdrawal on measures of marijuana’s effects, although some symptoms would persist (35). Participants underwent modified contingency management procedures to foster tobacco cessation. They were given a date to quit tobacco cigarettes and then visited the lab every 2–3 days to contribute a urinary sample for cotinine measurement (NicAlert; Global Business Support Systems, San Diego CA), a breath sample for CO measurement, and to complete ratings of nicotine withdrawal. Participants were given $50 for each outpatient day in which they achieved low cotinine levels (<100 ng/ml; 36). They were told to avoid nicotine replacement products and smoking marijuana in blunts (cigar tobacco leaves), which can increase urinary cotinine levels (37). For ethical reasons, participants who had the Quit phase first were not instructed to resume SAU; they were told that they would be re-enrolled regardless of tobacco smoking status. However, all resumed SAU.
As in Study 1, participants smoked experimenter-administered, active marijuana (5.5% THC) on the first inpatient day. For the next 3 days, inactive marijuana (0.0% THC) was available for self-administration (withdrawal), followed by 4 days when 5.5% THC was available for self-administration (relapse).
Data Analysis
Repeated measures analyses of variance (ANOVA) with planned comparisons were used to determine the effect of tobacco cigarette smoking on marijuana’s direct effects, withdrawal and relapse. An advantage of using repeated-measures, within-subjects designs is that they are well powered due to substantial correlations between levels. Behavioral outcomes included: the number of marijuana puffs purchased, peak subjective effects, drug craving, task performance, number of tobacco cigarettes smoked, and objective and subjective sleep measures. There were two within-group factors: tobacco smoking condition (SAU, Quit) and marijuana condition. Planned comparisons were conducted to determine if the Quit vs SAU condition affected marijuana: (1) intoxication when active marijuana was experimenter-administered, (2) withdrawal (mean peak values on days 2 and 3 of abstinence), and (3) relapse (number of marijuana puffs purchased on the first day of active marijuana availability). Results were considered statistically significant at p values < 0.05. Huynh-Feldt corrections were used, when appropriate.
Study 2: Results
Participant Characteristics
Table 2 portrays demographic data on the 15 participants who completed the study. During the SAU phase, participants averaged 10–12 tobacco cigarettes/day, regardless of marijuana condition. At the onset of the Quit phase, 13 of the 15 participants met criteria for tobacco cessation (CO: ≤4 ppm and/or cotinine <100 ng/ml); 2 participants showed a reduction in cigarette use but did not achieve either criteria prior to move-in.
Marijuana Intoxication
Tobacco cigarette smoking condition did not significantly affect peak ratings of “High” when active marijuana was experimenter-administered [SAU: 67 ± 7 mm; Quit: 68 ± 6 mm].
Marijuana Withdrawal
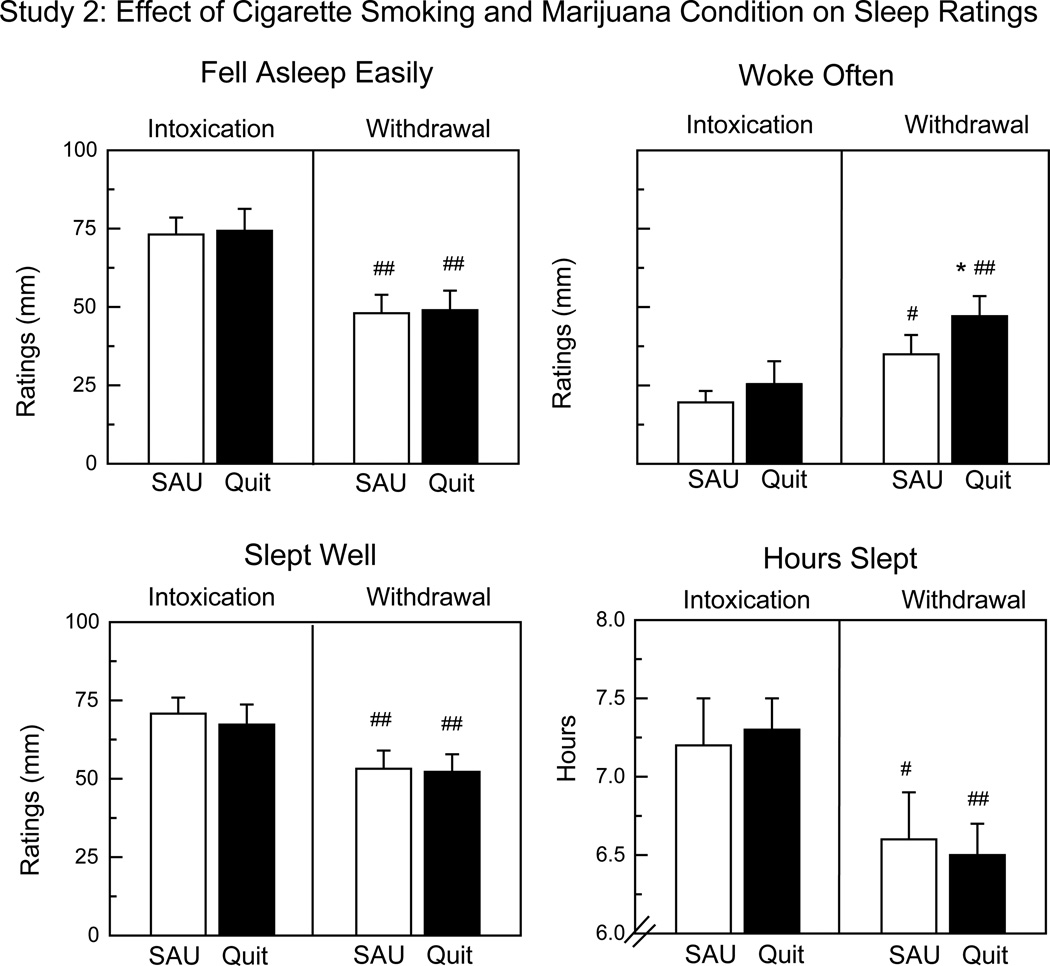
Craving for tobacco cigarettes was significantly higher during the Quit phase (55 ± 6 mm) relative to SAU [45 ± 8 mm: F(1,98) = 5.53, p < 0.03] during marijuana withdrawal, but cigarette smoking condition did not significantly affect marijuana craving or the mood symptoms characterizing marijuana withdrawal. Figure 2, which portrays sleep ratings as a function of marijuana and tobacco cigarette condition, shows that marijuana withdrawal significantly worsened sleep ratings relative to intoxication. Tobacco cigarette smoking had few effects on sleep, although participants reported waking more often during the Quit phase than SAU during marijuana withdrawal.
Figure 2.
Sleep ratings in Study 2 as a function of marijuana condition (Intoxication vs Withdrawal) and tobacco cigarette smoking condition (SAU vs Quit). SAU: smoking tobacco cigarettes as usual. Quit: tobacco cessation. Intoxication: Mean peak ratings following repeated marijuana administration (5.5%). Withdrawal: Mean peak ratings on the second and third day of marijuana abstinence. Asterisks indicate a significant difference between SAU and Quit condition (* p < 0.05). Number signs indicate a significant difference between Intoxication and Withdrawal condition (# p < 0.05; ## p < 0.01). Each bar represents 15 participants.
Marijuana Relapse
Tobacco cigarette smoking status did not significantly influence marijuana relapse. On the first day of active marijuana availability, 93% of participants purchased at least 1 puff of marijuana in the SAU condition compared to 87% in the Quit condition; the number of puffs purchased was identical (8 ± 1) in the two conditions. By contrast, placebo marijuana self-administration was significantly influenced by tobacco cigarette condition. During abstinence from active marijuana, participants in the Quit condition purchased significantly more placebo marijuana puffs (3 ± 1) compared to SAU [1 ± 1; F(1,84)=3.97, p<0.05].
Overall Discussion
Study 1 evaluated the factors predicting whether daily marijuana users who have gone through several days of marijuana abstinence will relapse, defined in this laboratory model as paying a financial cost to reinitiate active marijuana smoking on the first day of its availability. The results show that the factor best predicting marijuana relapse was tobacco cigarette-smoking status. Withdrawal symptoms did not predict the likelihood of relapse. However, the intensity of mood and sleep disruption during withdrawal predicted more severe relapse, measured in the amount of marijuana self-administered after an abstinence period. Another factor predicting the severity of relapse was ratings of ‘high’ following active marijuana smoking: greater intoxication predicted more marijuana self-administration after abstinence. These findings, which parallel tobacco cessation data (38), suggest that both the positive and negative reinforcing effects of marijuana influence the severity of relapse.
To follow-up, Study 2 assessed the direct impact of tobacco cigarette smoking by comparing marijuana withdrawal and relapse in the same individuals when they smoked cigarettes ad libitum and following a period of tobacco cessation. Consistent with findings from Study 1, the vast majority of participants in Study 2 (>87%), all cigarette smokers, relapsed to marijuana whether they were smoking tobacco cigarettes as usual or were recently abstinent from cigarettes. These findings rule out acute nicotine exposure or the cueing effects of tobacco cigarettes as an explanation for the high marijuana relapse rates observed among cigarette smokers. Tobacco cigarette smoking, which was consistent across marijuana conditions, had little influence on the direct or indirect effects of marijuana. In addition to not affecting marijuana relapse rates, tobacco cigarette smoking did not alter marijuana intoxication or most symptoms of marijuana withdrawal relative to tobacco cessation.
These laboratory findings parallel marijuana treatment data. In a large sample of adolescents, 69% of marijuana smokers who smoked cigarettes relapsed to marijuana 1 year later, compared to 54% of non-smokers (39). Among adults in marijuana treatment (n=174), marijuana and cigarette smokers provided about half as many cannabis-negative urines and had fewer weeks of abstinence than former smokers, despite no differences in treatment retention or marijuana dependence severity (30). Tobacco cigarette smoking also predicts treatment outcome for other drugs (40, 41, 42). A meta-analysis of 19 randomized clinical trials evaluating tobacco treatment interventions during drug treatment found that smoking cessation was associated with a 25% increased likelihood of long-term abstinence from alcohol and illicit drugs (43, 44). Thus, there is a clustering of quitting behavior, where stopping use of one drug increases the likelihood that other drug use will stop (45).
Although clinical data show that quitting cigarettes predicts better treatment outcome than continued smoking, short-term tobacco cessation did not decrease marijuana relapse in Study 2. Yet, it is worth noting that tobacco cessation was brief and a desire to quit cigarette smoking was an exclusion criterion. Participants (except for 2) complied with the requirements to not smoke cigarettes prior to admission, but none maintained this abstinence when not reinforced for doing so. Given that the absence of nicotine did not reduce marijuana relapse, perhaps it is the demonstrated ability to quit smoking cigarettes that best predicts the ability to remain abstinent from other drugs (30). Anti-smoking initiatives are increasing, particularly in New York City where cigarette packs cost over $12, leading to a 35% drop in adult cigarette smoking prevalence from 2002 to 2010 (46). Perhaps those who persist in smoking cigarettes despite mounting societal prohibitions are particularly recalcitrant smokers, unable to maintain abstinence from cigarettes (47) or from drug use overall.
Impulsivity may contribute to this recalcitrance. Delayed discounting, or the devaluing of a reward obtained in the future relative to one that is immediate, is one measure of impulsivity (48). Drug-dependent individuals tend to discount distant rewards more than controls, consistent with their choice for immediately-available drug intoxication or withdrawal relief over deferred rewards of higher long-term value, e.g., healthy lungs, job opportunities. Among cigarette smokers, those with higher rates of delay discounting chose more cigarette puffs over money (49). Similarly, although all of the current participants were daily drug users (marijuana), those who also smoked tobacco cigarettes were more likely to choose the immediate reward of marijuana smoking over a more delayed reward (higher study earnings), regardless of tobacco cigarette availability.
Shared risk factors may also explain the current findings (50). Adolescents who smoke tobacco cigarettes are over 9× more likely to smoke marijuana than nonsmokers (51, 52), and rates of tobacco cigarette use among marijuana smokers (47–78%; 7, 30, 53) are over twice the national average (23%; 54). In terms of mechanism, preclinical data show that nicotine produces epigenetic effects that enhance drug reward. In mice, repeated nicotine administration produced selective changes in striatal gene expression that enhanced the subsequent rewarding effects of cocaine (55). Preclinical (56, 57, 58) and clinical (59, but see 60, 61) studies show that cannabinoids also elevate striatal dopamine levels, albeit more modestly than cocaine. Thus, it is intriguing to consider that one explanation for the current finding is that cigarette smoking prior to the onset of marijuana use resulted in more intractable marijuana use patterns.
Among those who relapsed to marijuana, two factors were associated with the severity of relapse: marijuana withdrawal symptoms and intoxication. Again, there are interesting parallels to the tobacco literature. Among cigarette smokers, breath-holding duration, which measures the ability to tolerate discomfort, predicted relapse to cigarettes during early abstinence, while the severity of nicotine withdrawal did not (62). Perhaps the intensity of marijuana withdrawal is less predictive of relapse than the ability to tolerate the discomfort of withdrawal.
The present findings suggest several future studies to pursue: Given that clinical data support treatment for tobacco dependence in substance-dependent participants (63), it is important to determine whether patients seeking marijuana and tobacco cessation treatment who successfully quit smoking cigarettes reduce their marijuana use relative to patients who failed to quit cigarettes. In addition, participants in Study 1 began smoking marijuana every day at about 18 years of age. Studies are needed to clarify why individuals who started daily marijuana use when they were older were more likely to relapse than those who started at a younger age. This finding appears counter-intuitive, but perhaps those with a longer history of daily marijuana use have had more experience attempting to moderate this behavior.
There are several issues to consider with the present design: First, it may be that marijuana withdrawal severity did not predict relapse because the abstinence phase was too short to capture the full range of symptoms (23). Second, the sample was homogenous, comprised primarily of black men, potentially limiting our ability to generalize the findings to women or racially-mixed populations. Finally, our design was laboratory-based and used nontreatment-seeking volunteers. However, although it may appear that such an approach is not relevant to patients, the validity of the model is supported by clinical evidence that cigarette smoking predicts marijuana relapse. Further, medication effects on drug self-administration in this model are consistent with clinical outcome for marijuana (13, 16, 21, 22), as well as for treatment of other drugs (24, 25). Thus, human laboratory models of drug self-administration offer a controlled yet clinically-relevant method to study factors underlying problematic drug use.
In sum, these data indicate that daily marijuana smokers have considerable difficulty maintaining abstinence from marijuana, and the factor best predicting this failure to maintain abstinence is tobacco cigarette-smoking status. Whether individuals are currently smoking tobacco cigarettes or have been recently abstinent does not alter these high relapse rates, suggesting that cigarette smoking does not directly or indirectly influence marijuana relapse. Rather, cigarette smoking in concert with daily marijuana use may reflect intrinsic factors, such as greater impulsivity or distress intolerance, that render these individuals susceptible to relapse. Overall, the data suggest that current cigarette smoking is a clinical marker for a greater risk of drug relapse (41), including marijuana relapse.
Acknowledgments
The U.S. National Institute on Drug Abuse (NIDA) supported this research (DA19239, DA09236, DA031005) and supplied the marijuana cigarettes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures:
The authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.UNODC, World Drug Report. 2010 (United Nations Publication, Sales No. E.10.XI.13).
- 2. http://www.oas.samhsa.gov.
- 3.Hall W. The mental health risks of adolescent cannabis use. PLoS Medicine. 2006;3(2):159–162. doi: 10.1371/journal.pmed.0030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhana A, Parry CD, Myers B, Pluddemann A, Morojele NK, Flisher AJ. The South African Community Epidemiology Network on Drug Use (SACENDU) project, phases 1-8-cannabis and Mandrax. S Afr Med J. 2002;92:542–547. [PubMed] [Google Scholar]
- 5.EMCDDA. Annual Report on the State of the Drugs Problem. European Monitoring Centre for Drugs and Drug Addiction, Lisbon. 2009 [Google Scholar]
- 6.SAMHSA (Substance Abuse and Mental Health Services Administration, Office of Applied Studies.) Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. [Google Scholar]
- 7.Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychol Med. 2006;36:1447–1460. doi: 10.1017/S0033291706008361. [DOI] [PubMed] [Google Scholar]
- 8.Stephens RS, Roffman RA, Curtin L. Extended versus brief treatment for marijuana use. J Consult Clin Psychol. 2000;68:898–908. [PubMed] [Google Scholar]
- 9.Copeland J, Swift W, Roffman R, Stephens R. A randomized controlled trial of brief cognitive-behavioral interventions for cannabis use disorder. J Subst Abuse Treat. 2001;21:55–64. doi: 10.1016/s0740-5472(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 10.MTPRG. Brief treatments for cannabis dependence: Findings from a randomized multisite trial. J Consult Clin Psychol. 2004;72:455–466. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- 11.Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J Consult Clin Psychol. 2006;74:307–316. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- 12.Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addict Behav. 2007;32:1220–1236. doi: 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes E. Dronabinol for the Treatment of Cannabis Dependence: A Randomized, Double-Blind, Placebo-Controlled Trial. Drug Alcohol Depend. 2011;116(1–3):142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacol. 1999;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- 15.Haney M, Hart CL, Ward AS, Foltin RW. Nefazodone decreases anxiety during marijuana withdrawal in humans. Psychopharmacol. 2003;165:157–165. doi: 10.1007/s00213-002-1210-3. [DOI] [PubMed] [Google Scholar]
- 16.Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacol. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- 17.Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Cooper ZD, et al. Effects of baclofen and mirtazapine on laboratory model of marijuana withdrawal and relapse. Psychopharmacology. 2010;211:233–244. doi: 10.1007/s00213-010-1888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart C, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral delta(9)-tetrahydrocannabinol in humans. Psychopharmacol. 2002;164:407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- 19.Schierenbeck T, Riemann D, Berger M, Hornyak M. Effect of illicit recreational drugs upon sleep: cocaine, ecstasy, and marijuana. Sleep Med Rev. 2008;12:381–389. doi: 10.1016/j.smrv.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9 tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86:22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Haney M. Self-administration of cocaine, cannabis and heroin in the human laboratory: benefits and pitfalls. Addict Biol. 2009;14(1):9–21. doi: 10.1111/j.1369-1600.2008.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacol. 2008;197:157–168. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper ZD, Foltin RW, Hart CL, Vosburg SK, Comer SD, Haney M. A controlled human laboratory study investigating the effects of quetiapine on marijuana withdrawal and relapse in daily marijuana smokers. Addiction Biology. doi: 10.1111/j.1369-1600.2012.00461.x. (in press). in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haney M, Spealman R. Controversies in translational research: Drug self-administration. Psychopharmacol. 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL. The role of human drug self-administration procedures in the development of medications. Drug and Alcohol Dependence. 2008;96:1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haney M, Bedi G, Cooper ZD, Vosburg SK, Comer SD, Foltin RW. Nabilone decreases marijuana withdrawal and relapse in the human laboratory. doi: 10.1038/npp.2013.54. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foltin RW, Haney M, Comer SD, Fischman MW. Effects of fenfluramine in food intake, mood, and performance of humans living in a residential laboratory. Physiol Behav. 1996;59:295–305. doi: 10.1016/0031-9384(95)02098-5. [DOI] [PubMed] [Google Scholar]
- 28.Foltin RW, Fischman MW, Pedroso JJ, Pearlson GD. Marijuana and cocaine interactions in humans: cardiovascular consequences. Pharmacol Biochem Behav. 1987;28:459–464. doi: 10.1016/0091-3057(87)90506-5. [DOI] [PubMed] [Google Scholar]
- 29.Balerio GN, Aso E, Berrendero F, Murtra P, Maldonado R. Δ9-tetrahydrocannabinol decreases somatic and motivational manifestations of nicotine withdrawal in mice. Eur J Neurosci. 2004;20:2737–2748. doi: 10.1111/j.1460-9568.2004.03714.x. [DOI] [PubMed] [Google Scholar]
- 30.Moore BA, Budney AJ. Tobacco smoking in marijuana-dependent outpatients. J Substance Abuse. 2001;13:583–596. doi: 10.1016/s0899-3289(01)00093-1. [DOI] [PubMed] [Google Scholar]
- 31.Vandrey RG, Budney AJ, Hughs JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug and Alcohol Dependence. 2008;92:48–54. doi: 10.1016/j.drugalcdep.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viveros MP, Marco EM, File SE. Nicotine and cannabinoids: Parallels, contrasts and interactions. Neuroscience and Biobehavioral Review. 2006;30:1161–1181. doi: 10.1016/j.neubiorev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Penetar DM, Kouri EM, Gross MM, McCarthy EM, Rhee CK, Peters EN, et al. Transdermal nicotine alters some of marijuana’s effects in male and female volunteers. Drug and Alcohol Dependence. 2005;79:211–223. doi: 10.1016/j.drugalcdep.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Ream GL, Benoit E, Johnson BD, Dunlap E. Smoking tobacco along with marijuana increases symptoms of cannabis dependence. Drug and Alcohol Dependence. 2008;95:199–208. doi: 10.1016/j.drugalcdep.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine & Tobacco Research. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- 36.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 37.Murray A, Levin FL, Bisaga A, Glass A, Brooks D. Higher cotinine levels among non-cigarette smoking blunt users versus joint users. In preparation. [Google Scholar]
- 38.Perkins KA, Broge M, Gerlach D, Sanders M, Grobe JE, Cherry C. Acute Nicotine Reinforcement, but Not Chronice Tolerance, Predicts Withdrawal and Relapse After Quitting Smoking. Health Psychology. 2002;21(4):332–339. doi: 10.1037//0278-6133.21.4.332. [DOI] [PubMed] [Google Scholar]
- 39.de Dios MA, Vaughan EL, Stanton CA, Niaura R. Adolescent tobacco use and substance abuse treatment outcomes. J Substance Abuse Treatment. 2009;37:17–24. doi: 10.1016/j.jsat.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frosch DL, Shoptaw S, Nahom D, Jarvik ME. Associations between tobacco smoking and illicit drug use among methadone-maintained opiate-dependent individuals. Experimental and Clinical Psychopharmacology. 2000;8(1):97–103. doi: 10.1037//1064-1297.8.1.97. [DOI] [PubMed] [Google Scholar]
- 41.Kohn CS, Tsoh JY, Weisner CM. Changes in smoking status among substance abusers: baseline characteristics and abstinence from alcohol and drugs at 12-month follow-up. Drug and Alcohol Dependence. 2003;69:61–71. doi: 10.1016/s0376-8716(02)00256-9. [DOI] [PubMed] [Google Scholar]
- 42.Shoptaw S, Jarvik ME, Ling W, Rawson RA. Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addictive Behaviors. 1996;21(3):409–412. doi: 10.1016/0306-4603(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 43.Prochaska JJ, Delucchi K, Hall SM. A Meta-Analysis of Smoking Cessation Interventions With Individuals in Substance Abuse Treatment or Recovery. J Consulting and Clinical Psychology. 2004;72(6):1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- 44.Gulliver SB, Kamholz BW, Helstrom AW. Smoking Cessation and Alcohol Abstinence: What Do the Data Tell Us? Alcohol Research & Health. 2006;29:208–212. [PMC free article] [PubMed] [Google Scholar]
- 45.Metrik J, Spillane NS, Leventhal AM, Kahler CW. Marijuana use and tobacco smoking cessation among heavy alcohol drinkers. Drug and Alcohol Dependence. 2011;119:194–200. doi: 10.1016/j.drugalcdep.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. http://www.nyc.gov/html/doh/html/episrv/epidata.shtml.
- 47.Hughes JR. The hardening hypothesis: Is the ability to quit decreasing due to increasing nicotine dependence? A review and commentary. Drug and Alcohol Dependence. 2011;117:111–117. doi: 10.1016/j.drugalcdep.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- 49.Dallery J, Raiff BR. Delay discounting predicts cigarette smoking in a laboratory model of abstinence reinforcement. Psychopharmacology. 2007;190:485–496. doi: 10.1007/s00213-006-0627-5. [DOI] [PubMed] [Google Scholar]
- 50.Kendler KS, Myers J, Prescott CA. Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry. 2007;64:1313–1320. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- 51.Rigotti NA, Lee JE, Wechsler H. US college students’ use of tobacco products: results of a national survey. J American Medical Association. 2000;284:699–705. doi: 10.1001/jama.284.6.699. [DOI] [PubMed] [Google Scholar]
- 52.Mathers M, Toumbourou JW, Catalano RF, Williams J, Patton GC. Consequences of youth tobacco use: a review of prospective behavioral studies. Addiction. 2006;101:948–958. doi: 10.1111/j.1360-0443.2006.01438.x. [DOI] [PubMed] [Google Scholar]
- 53.Ford DE, Vu HT, Anthony JC. Marijuana use and cessation of tobacco smoking in adults from a community sample. Drug and Alcohol Dependence. 2002;67:243–248. doi: 10.1016/s0376-8716(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 54.SAMHSA (Substance Abuse and Mental Health Services Administration, Office of Applied Studies.) Treatment Episode Data Set (TEDS) Highlights - - 2007 National Admissions to Substance Abuse Treatment Services. Rockville, MD: 2009. OAS Series #S-45, HHS Publication No. (SMA) 09-4360. [Google Scholar]
- 55.Levine A, Huang Y, Drisaldi B, Griffin EA, Jr, Pollak DD, et al. Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci Transl Med. 2011;3(107) doi: 10.1126/scitranslmed.3003062. 107ps43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fadda P, Scherma M, Spano MS, Salis P, Melis V, et al. Cannabinoid self-administration increases dopamine release in the nucleus accumbens. Neuroreport. 2006;17(15):1629–1632. doi: 10.1097/01.wnr.0000236853.40221.8e. [DOI] [PubMed] [Google Scholar]
- 58.Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms-a review of recent preclinical data. Psychopharmacology. 2003;169:115–134. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- 59.Bossong MG, van Berckel BNM, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, et al. Δ9-Tetrahydrocannabinol Induces Dopamine Release in the Human Striatum. Neuropsych. 2009;34:759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- 60.Stokes PRA, Mehta MA, Curran HV, Breen G, Grasby PM. Can recreational doses of THC produce significant dopamine release in the human striatum? NeuroImage. 2009;48:186–190. doi: 10.1016/j.neuroimage.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 61.Barkus E, Morrison PD, Vuletic D, Dickson JC, Ell PJ, Pilowsky LS, et al. Does intravenous Δ9-tetrahydrocannabinol increase dopamine release? A SPET study. J Psychopharmacology. 2011;25(11):1462–1468. doi: 10.1177/0269881110382465. [DOI] [PubMed] [Google Scholar]
- 62.Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, et al. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine & Tobacco Research. 2009;11(5):493–502. doi: 10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prochaska JJ. Failure to treat tobacco use in mental health and addiction treatment settings: A form of harm reduction? Drug and Alcohol Dependence. 2010;110:177–182. doi: 10.1016/j.drugalcdep.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]