Abstract
This article is the presentation I gave at the D'Arsonval Award Ceremony on June 14, 2011 at the Bioelectromagnetics Society Annual Meeting in Halifax, Nova Scotia. It summarizes my research activities in acoustic and electromagnetic millimeter waves over the past 47 years. My earliest research involved acoustic millimeter waves, with a special interest in diagnostic ultrasound imaging and its safety. For the last 21 years my research expanded to include electromagnetic millimeter waves, with a special interest in the mechanisms underlying millimeter wave therapy. Millimeter wave therapy has been widely used in the former Soviet Union with great reported success for many diseases, but is virtually unknown to Western physicians. I and the very capable members of my laboratory were able to demonstrate that the local exposure of skin to low intensity millimeter waves caused the release of endogenous opioids, and the transport of these agents by blood flow to all parts of the body resulted in pain relief and other beneficial effects.
Keywords: ultrasound, diagnostic imaging, therapy, safety
The following is a presentation I gave on June 14, 2011 at the D'Arsonval Award Ceremony of the Bioelectromagnetics Society (BEMS) Annual Meeting. I think nothing better characterizes the significance of the D'Arsonval Award than the list of the twelve former honorees (Table 1). For this award I am truly honored and very grateful to the BEMS Award Committee, the BEMS Officers and the BEMS Board of Directors for choosing me. I thank those who wrote letters in support of my nomination, but most of all, I thank the members of my lab, for without their scientific collaboration I would not be getting this award.
Table 1.
Former D'Arsonval Award Recipients
| 1985 | Herman P. Schwan, Ph.D. | University of Pennsylvania, Philadelphia, PA |
| 1987 | Arthur W. Guy, Ph.D. | University of Washington, Seattle, WA |
| 1989 | W. Ross Adey, M.D. | Veterans Administration Hospital, Loma Linda, CA |
| 1991 | C.A.L. Bassett, M.D. | Columbia University, New York, NY |
| 1993 | Carl Durney, Ph.D. | University of Utah, Salt Lake City, UT |
| 1995 | Om P. Gandhi, Ph.D. | University of Utah, Salt Lake City, UT |
| 1999 | Nancy Wertheimer, Ph.D. | University of Colorado, Boulder, CO |
| 2001 | Thomas S. Tenforde, Ph.D. | Pacific Northwest National Laboratory, Richland, WA |
| 2003 | James C. Lin, Ph.D. | University of Illinois, Chicago, IL |
| 2006 | C. K. Chou, Ph.D. | Motorola Research Laboratory, Plantation, FL |
| 2007 | Eleanor R. Adair, Ph.D. | Yale University, New Haven, CT |
| 2010 | Shoogo Ueno, Ph.D. | Tokyo University, Yokyo, Japan |
This award is named after Jacques-Arsene d'Arsonval, a French physician and biophysicist who lived from 1851 to 1940. He invented the moving-coil galvanometer, the thermocouple ammeter, and was an important contributor to the emerging field of electrophysiology. He found that an alternating current having a frequency greater than 10 kHz produces no muscular contractions and does not excite sensory nerves, a phenomenon named after him.
ACOUSTIC AND ELECTROMAGNETIC MILLIMETER WAVES
Millimeter waves (MMWs) possess wavelengths from 1 mm to 10 mm. They can be acoustic or electromagnetic in nature. Acoustic waves occur as the propagated mechanical vibration of particles in a medium. They cannot travel in a vacuum. Electromagnetic waves consist of an alternating electric and magnetic field that propagates throughout space, including a vacuum. Both acoustic and electromagnetic MMWs are used in medicine. While acoustic MMWs are used in both diagnosis and therapy, electromagnetic MMWs currently are used solely in therapy.
The spectrum of acoustic frequencies is divided into three regions, depending on whether the frequency can be sensed by the human ear. They are, in order of increasing frequency: infrasonic, audible, and ultrasonic. The audible spectrum ranges from 20 Hz to 20 kHz. Acoustic MMWs lie entirely in the ultrasonic range, and from this point on will be referred to as ultrasound waves to more easily distinguish them from electromagnetic MMWs, which will be referred to as simply MMWs. Most of the medical use of ultrasound involves frequencies in the low megahertz range. The wavelength of a 1 MHz acoustic wave in average soft tissue is 1.5 mm. Most diagnostic applications utilize frequencies between 1 and 10 MHz.
Early Research in Ultrasound
I have been actively involved in ultrasound research for the past 46 years. In 1965, as a Research Associate in Diagnostic Ultrasound at Hahnemann Medical College in Philadelphia, Pennsylvania, I pioneered the establishment of two-dimensional ultrasonography as a valuable diagnostic modality. At that time, diagnostic ultrasound was in its infancy, and image quality was very limited. In fact, the bi-stable images1 were so poor that it was difficult convincing my clinical colleagues that the anatomical structures they were looking at were not pictures of craters on the moon. Nevertheless, this new form of imaging drew much attention. In September 1965, a photograph of my lab appeared on the cover of LIFE magazine (the most popular magazine in the USA at that time) showing the ultrasonic image of a fetal head in the uterus of a pregnant wife of a medical student (Fig. 1). Since that time, the technology of ultrasound in medicine has continually improved throughout the years. Today, the imaging of anatomical detail is exquisite and diagnostic ultrasound has become indispensible to medical practice.
Figure 1.
Cover of LIFE magazine showing the fetal head during an ultrasound examination of a pregnant woman.
While still at Hahnemann, using a combined radiographic and through-transmission ultrasonic technique, I identified the source of an echo giving rise to false-positive results in the ultrasonic diagnosis of pericardial effusion [Ziskin, 1968]. It was the anterior surface of a thoracic vertebral body.
In 1968, after a two-year tour of duty in the US Air Force Aerospace Medical Research Laboratory in Dayton, Ohio, I returned to my alma mater, Temple University Medical School (Philadelphia, PA), where I have remained up to the present day. My earliest studies here involved investigating the physiological meaningfulness of the Doppler signal. I developed a procedure for the detection of carotid artery stenosis using Doppler ultrasound, and was the first to use ultrasound to detect the cavitation that occurs at catheter tips during rapid intravenous injections [Bove et al., 1969; Ziskin, 1969, 1971]. While performing blood flow studies in dogs, I noted a dramatic amplification of the Doppler signal several seconds following intravenous injections of fluids at distant sites. This led to a detailed study of cavitation developed at catheter tips, and ultimately, to the development of contrast agents for clinical diagnostic ultrasonography [Ziskin et al., 1972].
In 1972, because of concerns about the safety of clinical ultrasound, I conducted an international survey of clinical users. I reported that no adverse effects attributed to examination by ultrasound had been identified by any of the 68 respondents to the survey in over 121,000 patient examinations. The report represented a combined total of 292 institute years of experience in the clinical use of diagnostic ultrasound [Ziskin, 1972].
The safety of ophthalmologic ultrasonography was investigated using the rabbit. Continuous wave ultrasound at an intensity of 33.7 mW/cm2 was directed to the left eye for durations of 1 and 4 h. This intensity is within the range of most diagnostic ultrasound instruments used clinically. The right retina served as a control. No damage was observed from any of the exposures as determined with meticulous microscopic examination by an ophthalmic pathologist [Ziskin et al., 1974].
In 1977, I was a visiting scientist for 6 months at the Acoustics Laboratory in Sydney, Australia. There, working with M.J. Edwards, Ph.D., at the University of Sydney, I exposed pregnant guinea pigs on the 21st day of gestation to 1 MHz continuous wave ultrasound at intensities ranging from 50–1100 mW/cm2 for a period of 1 h. Internal body temperature was monitored with a thermocouple inserted through the rectum to the level of the uterus. Results showed a reduction in the brain/body weight ratio between control animals and those in which the internal temperature rose greater than 2 °C. This affirmed that a reduction in the brain weight of a newborn is the most sensitive indicator of biological damage known to result from gestational hyperthermia. Furthermore, I showed that the temperature elevation achieved internally was the most appropriate measure of the exposure dosage because intestinal gas situated between the pregnant uterus and the skin altered ultrasonic transmission to the uterus in an unpredictable way [Ziskin et al., 1978].
From February 1979 to January 1982, I was a co-investigator with Dr. Wesley Nyborg at the University of Vermont (Burlington, VT) on a National Institutes of Health (NIH) grant entitled “Low Intensity Ultrasonic Effects in Mammalian Tissue”. By subjecting various intact animals to slow pressurization and rapid decompression, we were able to conclude that micro-bubbles (approximately 1 μm in diameter) exist within mammalian tissues, probably in small crevices between cells. We demonstrated several blood flow disturbances when bubbles in the blood were of “resonance size” to the pulse repetition frequency of the insonating beam [Nyborg and Ziskin, 1985]. For a constant valued incident ultrasound beam, the resonance size of a bubble is the diameter for which the amplitude of oscillation is maximum. Also during this time, multicellular tumor spheroids were developed as an experimental model to test the ability of an ultrasound beam to dislodge cells from a tumor and induce metastasis. We showed that this would not occur if the spatial-peak temporal-average intensity was less than 1 W/cm2 [Conger et al., 1981; Conger and Ziskin, 1983]. This intensity, which is higher than that used in diagnosis, is commonly used in therapeutic applications of ultrasound for soft tissue injuries.
In 1982, I investigated the nature of image artifacts appearing in clinical ultrasonograms. I showed that an artifact with a tapering appearance seen distal to a highly reflecting object was due to ultrasonic reverberations between the transducer and object. I coined the term “comet tail artifact”, which is a commonly used term in clinical ultrasonography today [Ziskin et al., 1982].
Over the next several years, I was involved in developing the concept of the Thermal Index and the Mechanical Index. These indexes are displayed today on clinical ultrasound scanners to provide sonographers with information concerning the risk of harm to the patient at all times during an ultrasound examination. The Thermal Index provides a non-dimensional number proportional to the likelihood of an adverse effect caused by excessive temperature elevation during the ultrasound examination. The Mechanical Index is proportional to the likelihood of an adverse effect due to a mechanical mechanism such as radiation force or cavitation. By keeping the values of these indexes as low as possible during clinical examinations, consistent with obtaining the necessary diagnostic information, the sonographer can best insure patient safety [NCRP, 1992, 2002; AIUM, 2009].
Later Research in Ultrasound
In 1989, because of my concern that tissue heating might pose a risk for obstetrical ultrasound, I systematically analyzed the world's literature on fetal developmental abnormalities resulting from fetal hyperthermia. Congenital abnormalities included a wide range of conditions including microcephaly, hydrocephalus, cleft palate, microphthalmia, ear defects, and skeletal defects (Fig. 2). The results were summarized in a paper entitled “Biological Consequences of Hyperthermia” [Miller and Ziskin, 1989].
Figure 2.
Thermal thresholds for various fetal developmental abnormalities. Solid lines link the same abnormality occurring at different temperature-duration combinations. Dashed line denotes a boundary on the temperature-duration graph below which no hyperthermia-induced abnormalities have been observed.
Each data point in Figure 2 represents the temperature-duration threshold for a given abnormality. In those cases where an investigator performed the exposure at different temperatures, it was found that the higher the temperature, the shorter the time necessary to cause the same congenital abnormality. Each straight line in Figure 2 shows the set of temperature-duration thresholds for a single abnormality. Seeing that each of these lines were essentially parallel allowed me to establish the relationship between temperature and exposure duration for producing an adverse biological effect in fetuses. That is:
| (1) |
where t43 is the time required (in min), at a temperature of 43 °C, to produce the same congenital abnormality as that produced by an exposure of t min at temperature T. The value of R is 0.25 for T<43 °C, and 0.50 for T>43 °C. This relationship is used today by national and international organizations for setting safety standards for clinical ultrasound [Ziskin, 1990; Abramowicz et al., 2008; AIUM, 2009]. More recently, this relationship has been utilized in developing safety standards for radiofrequency electromagnetic radiation [Ziskin, 2010; Ziskin and Morrissey, 2011].
In 1993, I co-edited a book entitled “Ultrasonic Exposimetry” that included my chapter on Measurement Uncertainty in Ultrasound Exposimetry [Ziskin and Lewin, 1993]. The material contained in this chapter has been adopted as the current standard on expressing uncertainty in ultrasound measurements by the American Institute of Ultrasound in Medicine (AIUM, Laurel, MD), the National Electrical Manufacturers Association (NEMA, Rosslyn, VA), and the Federal Drug Administration (FDA, Silver Spring, MD) [Ziskin, 2003].
As a member of the National Council on Radiation Protection and Measurements (NCRP) Scientific Committee 66, under the Chairmanship of Dr. Nyborg, I participated in the writing of a three-volume treatise entitled “Biological Effects of Ultrasound: Mechanisms, Clinical Implications, and Exposure Criteria Based on All Known Mechanisms” [NCRP, 1983, 1992, 2002]. This opus provides the most comprehensive and authoritative presentation of what is currently known about ultrasound bioeffects.
I have been a member of the AIUM Bioeffects Committee and the AIUM Technical Standards Committee since 1973, and involved in the writing of many safety-related and technical documents. I was a member of the committee that wrote “Acoustic Output Labeling Standard for Diagnostic Ultrasound Equipment: A Standard for How Manufacturers Should Specify Acoustic Output Data” [AIUM, 2004]. I chaired the committee that wrote the AIUM Recommended Terminology, which is currently in its third edition [AIUM, 1980, 1997, 2008]. I also chaired the committee that wrote the manual entitled “Medical Ultrasound Safety, Second Edition”, which is currently required by the FDA to accompany all diagnostic ultrasound scanners sold in the United States [AIUM, 2009]. From 1982 to 1984 I served as President of the AIUM, and from 2003 to 2006 I served as President of the World Federation of Ultrasound in Medicine and Biology (WFUMB), an international organization with over 50,000 members.
MILLIMETER WAVES
Whereas the acoustic spectrum has only three divisions, the electromagnetic spectrum has many more divisions, with the millimeter range lying between the microwave and infrared regions. Although there are medical applications utilizing all parts of the electromagnetic spectrum, I will concentrate only on the millimeter range–30 GHz to 300 GHz.
Early Research in Millimeter Waves
In 1991 I was invited by Richard J. Fox, Chairman of the Board of Trustees of Temple University, to accompany him to the former Soviet Union to visit laboratories and clinics using low-intensity millimeter waves (MMWs) to treat a large variety of medical conditions.
Convinced that millimeter wave therapy (MMW therapy) had great potential, and that it would be a significant plus for Temple University, Mr. Fox created the Center for Biomedical Physics with very generous financial support. I accepted the appointment as Director of the center, on the condition that I had scientific autonomy and that if it turned out that MMW therapy was unsuccessful as a therapeutic modality, I would report it as such.
The proposed center research plan was designed to establish the scientific basis of MMW therapy, to determine its mechanism(s) of action, and to quantitatively evaluate its effect on certain selected diseases. My approach to understanding MMW therapy has been broad, including studying the fundamental interactions of MMWs with tissue, studying the local and systemic biological effects of MMW exposure, and evaluating its safety for clinical usage.
MMW THERAPY
MMW therapy is the application of low-intensity, millimeter-wavelength electromagnetic waves as an alternative treatment for a variety of diseases. Importantly for medical applications, MMWs do not possess sufficient photonic energy to break chemical bonds or cause ionization. Thus, they are incapable of producing chromosomal mutations and do not cause cancer.
MMWs are administered onto a localized area of the skin, at a sufficiently low intensity that there is no perceptible heating. Strikingly high success rates have been reported by Soviet physicians in the treatment of cardiovascular diseases, diabetes, dermatitis, gastrointestinal disorders, wound healing, pain relief, and the reduction of toxic side effects of chemotherapy and radiotherapy in cancer patients. MMW therapy is a non-invasive, painless, relatively inexpensive modality with exceedingly rare and minor side effects [Rojavin and Ziskin, 1997, 1998]. Although MMW therapy has been and continues to be used extensively throughout the former Soviet Union, it is virtually unknown to Western physicians
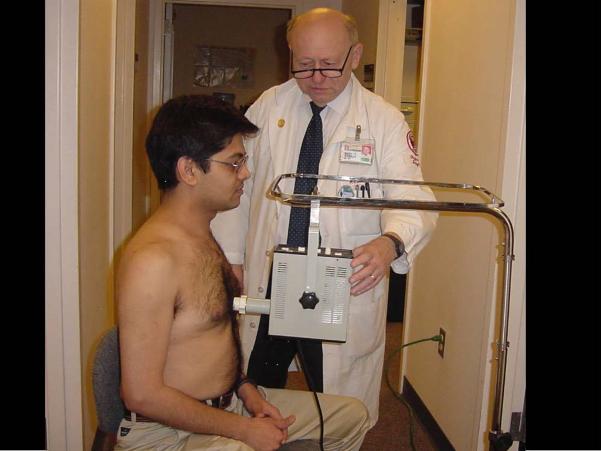
The usual MMW regimen consists of 15 to 30 min of daily treatment for 5 to 15 days. The MMW device is typically a book-sized instrument that is brought in close contact with the skin surface. The site of application varies with the disease being treated. Surface wounds and skin diseases are usually treated at the site of the lesion. In treating arthritis, the site of application is at the affected joint. In treating internal diseases, the recommended site of application may be at any one of a number of anatomic or acupuncture points. A common site of application is the lower end of the sternum, as pictured in Figure 3.
Figure 3.
Typical application of millimeter wave (MMW) treatment. The MMW device is brought in contact with the lower end of the sternum and the MMWs are applied for 20 min. The patient perceives no pain or temperature elevation.
Currently available MMW devices are made in Russia and other countries of the former Soviet Union. One of the most reliable and well designed among these is the YAV-1 MMW applicator, manufactured by ISTOK, a major military electronic research and development center located in Fryazino, Russia. Furthermore, this applicator has a plastic stand-off that is placed between the antenna and the skin to provide an optimum distance for producing a nearly Gaussian distribution of MMW energy.
The wide use of MMW therapy in the former Soviet Union appears to have been motivated by two synergistic factors: available technological capability, and lack of drugs. MMW technology was highly developed within the Soviet military establishment, especially for short-range radar. As political conditions between Russia and the United States improved, there was less need for military uses of MMW technology. Medical applications became an attractive spinoff. A major stimulus for applying MMW devices for medical conditions was the general lack of drugs available to physicians. Physicians eagerly accepted the MMW devices and used them in the treatment of their patients.
In spite of the large number of patients treated, and the very high success rates attributed to MMW therapy by hospitals and clinics in the former Soviet Union, there have been just a handful of publications in peer-reviewed scientific journals with sufficient details to satisfy Western physicians and scientists. Because of this lack of detail and quantization, the interpretation and evaluation of the results have been difficult and unreliable [Rojavin and Ziskin, 1998; Pakhomov et al., 1998]. Therefore, it is necessary to independently test the validity of the Soviet claims before MMW therapy can become an accepted alternative modality for clinical applications in the United States or other Western countries.
With the wide use of MMW therapy in the former Soviet Union, there have emerged three established general effects: (1) anti-inflammatory and repair-stimulating actions, (2) immune system stimulation, and (3) sedative and analgesic effects. Furthermore, undesirable side effects are extremely rare and mild [Rojavin and Ziskin, 1998].
FUNDAMENTAL PHYSICAL INTERACTIONS OF MMWs WITH THE SKIN
MMWs are rapidly absorbed by the skin. Penetration depths are only a few tenths of a millimeter [Alekseev and Ziskin, 2003]. Consequently, any biological response to MMW irradiation must be initiated within the skin. Gaining an understanding of the mechanisms by which MMWs can have a therapeutic effect begins with learning how they interact with the skin and its structures.
The high absorption of MMWs in the skin results mostly from the MMW interaction with water [Alekseev and Ziskin, 2001, 2003]. Water, which constitutes 70–80% of the tissues and components of the skin, has the greatest absorption coefficient. The applied MMW electromagnetic field induces rotational movement of water molecules by interaction with their permanent dipoles. The water of hydration associated with proteins and other organic molecules exhibits little absorption in the MMW frequency range due to the strong restriction of their motion. Thus, the amount of water contributing to MMW absorption in tissues equals the total water content minus the fraction of “bound” or physically restricted water.
Because the structure and free water content of each skin layer are different, internal reflections between skin layers can change the total reflection from the skin. The MMW absorption in some layers may increase due to multiple reflections within these layers [Alekseev and Ziskin, 2000]. In this case, the reflection or absorption may exhibit sharp frequency dependences.
Skin structure
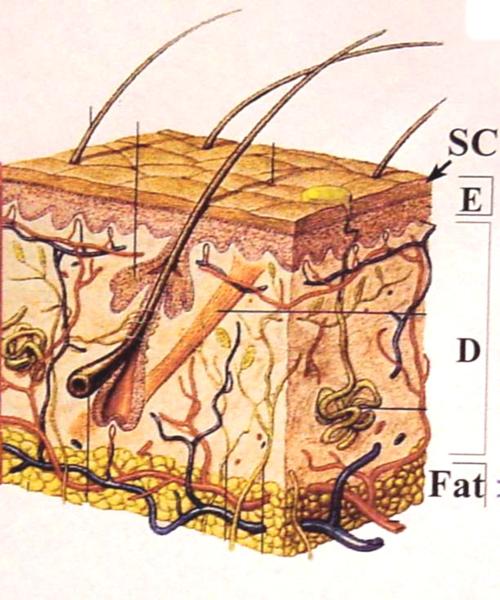
The physical properties of skin and its appendages greatly affect the amount and distribution of MMWs absorbed. A diagram of human skin is provided in Figure 4. The thickness of the human epidermis and dermis varies in the range of 40–150 μm and 1.1–2.8 mm, respectively. The stratum corneum has low water content (15–40%), and the total water concentration in the rest of the epidermis and dermis is 70–80%. Thus, MMW energy penetrates the stratum corneum fairly easily but is rapidly absorbed within the deeper epithelium and dermis.
Figure 4.
Diagram of human skin. The skin is divided into three major layers: epidermis (E), dermis (D), and subcutaneous fat. The outermost layer of the epidermis is the stratum corneum (SC). Also shown are hair follicles, sweat glands, nerves, and blood vessels.
MMW heating of the skin
MMW irradiation has a certain number of physical features – one of which is that heating is a major mechanism for bioeffects; also, most of the energy is absorbed within a few tenths of one millimeter. In addition, wavelengths are compatible with the dimensions of biological structures. In therapy, irradiation is frequently applied in the near field.
Exposure of human skin with MMWs at incident power densities (IPD) ≥ 10 mW/cm2, frequently used in MMW therapy and laboratory experiments, can lead to localized heating of the skin. Rapid temperature increase during irradiation, even if only by a small total increment, can cause certain biological effects [Alekseev et al., 1997]. Therefore, determination of the heating rates and temperature rise distributions within the skin during exposure with various radiators is important in evaluating thermal mechanisms of MMW action and in assessing dosimetry of the skin [Alekseev and Ziskin, 2001, 2003].
The presence of cutaneous blood vessels affects MMW-induced heating of the skin. The orientation of the exposed vessels to the E-field of the incident MMW energy has a significant effect. The greatest temperature rise occurs with vessels oriented parallel to the direction of the E-field [Alekseev and Ziskin, 2009a, 2011]. Blood flow greatly affects the temperature rise resulting from MMW exposure. The temperature elevation was well modeled using a modified form of the Pennes' bioheat equation, in which the thermal conduction coefficient was varied depending on the magnitude of blood flow [Alekseev and Ziskin, 2009b].
Dosimetry
The absorption of microwave electromagnetic fields in biological tissue is characterized by the specific absorption rate (SAR) [NCRP, 1981]:
| (1) |
where σ is the conductivity of tissue, E is the internal electric field, and ρ is the mass density. In those cases where the experimental determination of E is difficult, the SAR can be obtained from measuring the initial temperature rise rate dT/dt|t=0 [IEEE, 2002]:
| (2) |
where C is the specific heat of tissue. The SAR within tissue can be also determined from the incident power density (IPD) measurements [Gandhi and Riazi, 1986]:
| (3) |
where x is the depth into tissue, R is the power reflection from the skin, I is the incident power density, and δ is the depth at which the amplitude of the MMW falls to e−1 of its value (the power drops by e−2).
The use of an infrared camera provides an excellent way of determining the dosage of MMWs because of its ability to remotely record the temperature with great sensitivity (< 50 mK). Since the radiation is within the near field, the resulting heating pattern is very non-uniform. SAR values in hot-spot areas can exceed one thousand watts per kilogram with an incident power density of 10 mW/cm2 [Khizhnyak and Ziskin, 1994, 2000; Alekseev and Ziskin, 2003]
Mechanism of Action of MMWs
The mechanisms by which MMWs are able to produce systemic whole-body effects from local exposures where the penetration is very shallow are not well understood. However, two major mechanisms seem to be involved: 1) stimulation of the nervous system, and 2) stimulation of the immune system [Ziskin, 2005]. In either case, the initial interaction with the MMWs occurs within the skin. Free nerve endings extending into the epidermis can be stimulated directly. Also, immunocompetent cells such as Langerhans cells and keratinocytes within the skin epidermis can also be stimulated directly. The neurons in the skin are thus either stimulated directly or indirectly by the release of cytokines from stimulated dermal cells. The resulting “millimeter wave signal” is transmitted through the cutaneous nerve through the dorsal root ganglion into the spinal cord [Radzievsky et al., 2001]. At the first synapse in the spinal cord, there is a release of endogenous opioids.
The release of endogenous opioids occurs in at least two other spots in the brain. The subsequent release of endogenous opioids into the blood stream spreads these chemicals throughout the body, and certainly is adequate for explaining why pain relief can result from MMW exposures. The involvement of endogenous opioids in MMW therapy is verified by the fact that the beneficial effect of MMW therapy is completely abolished upon the administration of naloxone, a general opioid inhibitor [Radzievsky et al., 2000, 2008]. Opioids are also known to have wide-ranging effects on various systems in the body including the immune system. The transmission of the MMW signal through the cutaneous nerve is verified by the fact that the beneficial effect of MMW therapy is completely abolished by severing the nerve leading to the spinal cord. Furthermore, it was found that the greatest effect of MMW therapy occurred when the site of exposure had the greatest neural density [Radzievsky et al., 2000].
Direct Stimulation of Neurons by MMWs
Laboratory demonstration of MMW stimulation of neurons can be seen in experiments using pond snails [Alekseev et al., 2000]. The special neural ganglia in the neck region of these mollusks are well known biologically. They act as pacemaker neurons for the entire organism. The neurons' firings can be detected by microelectrodes and recorded [Alexeev et al., 1997, 2000; Alekseev and Ziskin, 1999]. The displayed traces show a pattern of regular spike firing. However, with increasing intensities of MMWs, the frequency of the firing rate slows. The slowing is dose dependent on the SAR level. At sufficiently high SAR levels there is a complete cessation of firing until the MMW exposure is stopped. At moderate SAR levels there is an initial slowing of the firing rate followed by a later compensatory increase in the firing rate. This can be explained on a thermal basis in that there are two opposing thermally sensitive mechanisms involved. The most rapid mechanism is thermal stimulation of the sodium pump, which increases the membrane potential and causes the slowing of neuron firing. The slower mechanism is the thermally increased permeability of the neuron membrane, which allows the transfer of ions that will decrease the membrane potential and ultimately cause an increase in the firing range. It was found that the rate of rise in temperature, even though the absolute temperature rise is not high, is the significant factor [Alekseev et al., 1997]. The threshold of the firing of these neurons is 0.0025 °C per s. This is very similar to what is found in the literature for human warmth receptors and human cold receptors.
In summary, MMW irradiation causes a biphasic change in the firing range of neurons. The rate of temperature rise plays an important role in development of the neural response. Therefore, MMW irradiation used in therapy is capable of activating thermoreceptors and other thermosensitive nerve endings in the upper layers of the skin.
Effects on Keratinocytes
Extensive histological and histochemical studies of skin reveal that MMWs at therapeutic levels cause no damage to the skin [Szabo et al., 2003]. However, at higher intensities it is possible to see that apoptosis is induced in keratinocytes if the temperature exceeds 43 °C. Significantly, apoptosis does not reveal itself until 24 h following the exposure. Therefore, studies looking for small effects should include observation lasting 24 h after the exposure [Szabo et al., 2003]. Most of the results of our studies on the effects of MMWs at therapeutic levels on the cell cultures of human keratinocytes were negative. However, we did find an increased release of the cytokine IL-1β in one study [Szabo et al., 2001].
Delayed-Type Hypersensitivity
MMWs have been found to produce a number of changes influencing the immune system. A particularly interesting one involves delayed-type hypersensitivity (DTH). DTH is a memory-dependent immune response; it is T-cell-mediated. Common examples are poison ivy and poison oak dermatitis. There is a two-stage process involved. The first is sensitization, in which a topical application of an allergen induces an immunological memory for the allergen but little to no reaction. The second stage is the challenge stage, in which exposure at a later time to the same allergen, even in very small doses, evokes a significant inflammatory skin reaction.
Our study involved sensitization with dinitrochlorobenzene (DNCB) on the right ears of mice at days 5, 6 and 7, following exposure to MMWs for 30 min on their backs [Logani and Ziskin, 1999]. On day 7, a small amount of DNCB was placed on the left ear. This induced a generalized immune response in the mice. Measurement of the effect was accomplished by measuring the thickness of the ear, as the ear provides a convenient spot to measure the overall tissue edema resulting from the immune reaction. In hairless mice, which have a somewhat compromised immune system, we saw a significant increase in the ear thickness that appeared to be dose dependent and greater than in sham controls. When immunocompetent mice such as BALB/c were tested, we found no significant response to MMWs. However, when the BALB/c mouse was pretreated with a chemotherapeutic agent such as cyclophosphamide (CPA), we found that the therapeutic effect of MMWs was observed. This is consistent with reports from Russia, that MMW therapy has no effect on normal functioning conditions but will have a positive effect on altered states of the body.
In summary, MMWs enhance the DTH reaction in hairless mutant mice. MMWs do not affect the DTH reaction in BALB/c mice. However, MMWs enhance the DTH reaction in BALB/c mice pretreated with cyclophosphamide (CPA). These findings support the hypothesis that MMWs normalize disturbed immune functions.
Effect on Cutaneous Melanoma
An experimental model that has a proved quite useful in our experience has been the effect of MMWs on the growth of cutaneous B16 melanoma in mice [Radzievsky et al., 2004; Szabo et al., 2004]. B16 melanoma is a fast-growing tumor in mice that mimics malignant cutaneous melanoma in humans. Because of their black color, melanomas are readily visible 24–48 h following the subcutaneous injection of B16 melanoma cells. Thus, tumor growth can be easily monitored. Without MMW treatment, melanomas continue to enlarge. However, we were able to show that exposure to MMWs significantly decreased the rate of growth of these tumors. Furthermore, in tumor challenge studies, we were able to show that a cytokine released from a tumor that had been treated with MMWs significantly suppressed the growth of tumors in other parts of the body. Further investigation showed that TNF-alpha concentrations in the solid tumors in groups of MMW-treated mice were significantly increased over sham controls. This has potential clinical benefits in the treatment of cutaneous melanomas and in suppressing their metastasis [Szabo et al., 2004; Logani et al., 2006].
Suppression of Pain
Numerous studies have been performed in our lab demonstrating the ability of MMWs to suppress various types of pain, including acute, chronic non-neuropathic, and chronic neuropathic pain [Radzievsky et al., 1999, 2000, 2008; Rojavin et al., 2000]. The best experimental model for acute pain is the hot water tail-flick test; the best for chronic non-neuropathic pain is the cold water tail-flick test; and the best for chronic neuropathic pain is the wire surface test following chronic constriction injury of the sciatic nerve. MMW irradiation to 61.22 GHz at 15 mW/cm2 has been applied to the nasal region of mice to determine the hypoalgesic effect of MMWs. MMWs significantly increased the duration in which the mice could withstand the hot water. It was found that naloxone, a general inhibitor of opioids, was effective and completely blocked the effects of MMWs on reducing acute pain. This substantiated the concept that the effect of MMWs was mediated by endogenous opioids. This is similar in respect to other forms of localized stimulation such as acupuncture. A double-blind, prospective, human volunteer study showed that MMW exposure was able to suppress pain sensation when the subjects' hands were placed in ice water [Radzievsky et al., 1999].
Non-suppression of the gastrointestinal tract
Patients with postoperative pain frequently require morphine for relief. However, a common side effect of morphine is the suppression of gastrointestinal (GI) mobility. We conducted studies to see if MMW therapy could relieve pain but not suppress the GI tract [Radzievsky et al., 2002]. By comparing the time required by mice to eject a small pellet placed 2 cm in their colon, we were able to assess the degree of GI tract suppression. We found that while morphine caused significant suppression, MMW therapy produced no greater suppression than controls.
Studies using specific opioid blockers revealed that MMWs act primarily through delta and kappa opioid receptors and not mu opioid receptors [Radzievsky et al., 2008]. This is important because morphine acts primarily through mu opioid receptors, and it is the mu opioid system that is responsible for the GI tract suppression. Consequently, MMW therapy is recommended for postoperative pain to either replace morphine or reduce the amount of morphine required.
Concluding Remarks
Although MMW therapy has been shown to be effective in numerous experiments in animals, its use in the treatment of human illness still remains anecdotal in nature and awaits scientific confirmation by means of well-controlled clinical trials.
Space does not permit the listing of all of the research performed in my laboratory. I have attempted to present some of the more important highlights. It is obvious that the research on MMWs described in this article was performed by a number of individuals in my laboratory. Without their help, this work would have been impossible. It is therefore fitting that I acknowledge, with gratitude and affection, those who made significant contributions. They are listed in alphabetical order in Table 2.
Table 2.
Contributors to the Center for Biomedical Physics
| SCIENTIST | SPECIALTY |
|---|---|
| Stanislav I. Alexeev, Ph.D. | Biophysicist |
| Alan Cowan, Ph.D. | Pharmacologist |
| Stephane Egot-Lemaire, Ph.D. | Bioengineer |
| Oleg V. Gordiienko, M.D. | Neuroscientist |
| Eugene P. Khizhnyak, Ph.D. | Biophysicist |
| Mahendra K. Logani, Ph.D. | Biochemist / Immunologist |
| Vera Makar, Ph.D. | Immunologist |
| Michael R. Manning, M.S. | Biomedical Engineer |
| Alexander A. Radzievsky, M.D., Ph.D. | Neuroscientist |
| Michael A. Rojavin, Ph.D. | Microbiologist |
| William S. Slovinsky, M.S. | Biomechanical Engineer |
| Imre Szabo, M.D., Ph.D. | Dermatologist |
| Maxim Zhadobov, Ph.D. | Bioengineer |
Acknowledgements
I thank the Richard J. Fox Foundation for the creation and significant initial support of the center. I also thank the NIH Center for Complementary and Alternative Medicine (NCCAM) for a number of R01 grants, as well as a specialized center of research excellence grant (Grant number: P01AT002025) entitled “Mechanisms Underlying Millimeter Wave Therapy”.
Footnotes
A bistable image is one in which each pixel is either intense black or intense white. There are no shades of gray.
REFERENCES
- Abramowicz JS, Barnett SB, Duck FA, Edmonds PD, Hynynen KH, Ziskin MC. Fetal thermal effects of diagnostic ultrasound. J Ultrasound Med. 2008;27:541–559. doi: 10.7863/jum.2008.27.4.541. [DOI] [PubMed] [Google Scholar]
- AlUM . Recommended nomenclature, physics and engineering. American Institute of Ultrasound in Medicine; Laurel, MD: 1980. [Google Scholar]
- AIUM . Recommended ultrasound terminology. Second Edition American Institute for Ultrasound in Medicine; Laurel, MD: 1997. [Google Scholar]
- AIUM . Acoustic output measurement standard for diagnostic ultrasound equipment. American Institute for Ultrasound in Medicine; Laurel, MD: 2004. [Google Scholar]
- AIUM . Recommended ultrasound terminology. Third Edition American Ultrasound Institute of Ultrasound in Medicine; Laurel, MD: 2008. [Google Scholar]
- AIUM . Medical ultrasound safety. Second Edition American Institute of Ultrasound in Medicine; Laurel, MD: 2009. [Google Scholar]
- Alekseev SI, Gordiienko OV, Ziskin MC. Reflection and penetration depth of millimeter waves in murine skin. Bioelectromagnetics. 2008;29:340–344. doi: 10.1002/bem.20401. [DOI] [PubMed] [Google Scholar]
- Alekseev SI, Ziskin MC. Effects of millimeter waves on ionic currents of lymnaea neurons. Bioelectromagnetics. 1999;20:24–33. [PubMed] [Google Scholar]
- Alekseev SI, Ziskin MC. Reflection and absorption of millimeter waves by thin absorbing films. Bioelectromagnetics. 2000;21:264–271. doi: 10.1002/(sici)1521-186x(200005)21:4<264::aid-bem3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Alekseev SI, Ziskin MC. Millimeter wave power density in aqueous biological samples. Bioelectromagnetics. 2001;22:288–291. doi: 10.1002/bem.52. [DOI] [PubMed] [Google Scholar]
- Alekseev SI, Ziskin MC. Local heating of human skin by millimeter waves: A kinetics study. Bioelectromagnetics. 2003;24:571–581. doi: 10.1002/bem.10137. [DOI] [PubMed] [Google Scholar]
- Alekseev SI, Ziskin MC. Millimeter-wave absorption by cutaneous blood vessels: A computational study. IEEE Transactions on Biomedical Engineering. 2009a;56:2380–2388. doi: 10.1109/TBME.2009.2024692. [DOI] [PubMed] [Google Scholar]
- Alekseev SI, Ziskin MC. Influence of blood flow and millimeter wave exposure on skin temperature in different thermal models. Bioelectromagnetics. 2009b;30:52–58. doi: 10.1002/bem.20444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseev SI, Ziskin MC. Enhanced absorption of millimeter wave energy in murine subcutaneous blood vessels. Bioelectromagnetics. 2011;32:423–433. doi: 10.1002/bem.20658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseev SI, Ziskin MC, Kochetkova NV. Effects of millimeter wavelength radiation on neurons: Electrophysiological study. Critical Reviews in Biomedical Engineering. 2000;28:52–59. doi: 10.1615/critrevbiomedeng.v28.i56.80. [DOI] [PubMed] [Google Scholar]
- Alekseev SI, Ziskin MC, Kochetkova NV, Bolshakov MA. Millimeter waves thermally alter the firing rate of the lymnaea pacemaker neuron. Bioelectromagnetics. 1997;18:89–98. doi: 10.1002/(sici)1521-186x(1997)18:2<89::aid-bem1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Bove AA, Ziskin MC, Mulchin WL. Ultrasonic detection of in-vivo cavitation and pressure effects of high speed injections through catheters. Investigative Radiology. 1969;4:236–240. [PubMed] [Google Scholar]
- Conger AD, Ziskin MC. Growth of mammalian multicellular tumor spheroids. Cancer Research. 1983;43:556–560. [PubMed] [Google Scholar]
- Conger AD, Ziskin MC, Wittels H. Ultrasonic effects on mammalian multicellular tumor spheroids. J of Clin Ultrasound. 1981;9:167–174. doi: 10.1002/jcu.1870090405. [DOI] [PubMed] [Google Scholar]
- Gandhi OP, Riazi A. Absorption of millimeter waves by human beings and its biological implications. IEEE Trans Microwave Theory Tech. 1986;34:228–235. [Google Scholar]
- IEEE . Std C95.3: IEEE Recommended practice for measurement and computations of radiofrequency electromagnetic fields with respect to human exposure to such fields, 100 kHz – 300 GHz. Institute of Electrical and Electronics Engineers; Piscataway, NJ: 2002. [Google Scholar]
- Khizhnyak EP, Ziskin MC. Heating patterns in biological tissue phantoms caused by millimeter wave electromagnetic irradiation. IEEE Trans Biomed Engineering. 1994;9:865–873. doi: 10.1109/10.312094. [DOI] [PubMed] [Google Scholar]
- Khizhnyak EP, Ziskin MC. Infrared thermography in experimental dosimetry of radio frequency and millimeter wavelength radiation exposure. In: Klauenberg BJ, Miklavcic D, editors. Radio Frequency Radiation Dosimetry. Kluwer Academic Publishers; Norwell, MA: 2000. pp. 199–206. [Google Scholar]
- Logani MK, Szabo I, Makar V, Bhanushali A, Alekseev S, Ziskin MC. Effect of millimeter wave irradiation on tumor metastasis. Bioelectromagnetics. 2006;27:258–264. doi: 10.1002/bem.20208. [DOI] [PubMed] [Google Scholar]
- Logani MK, Yi L, Ziskin MC. Millimeter waves enhance delayed-type hypersensitivity in mouse skin. Electro- and Magnetobiology. 1999;18:165–176. [Google Scholar]
- Miller MW, Ziskin MC. Biological consequences of hyperthermia. Ultrasound in Med and Biol. 1989;15:707–722. doi: 10.1016/0301-5629(89)90111-7. [DOI] [PubMed] [Google Scholar]
- NCRP . Report No. 67. Radiofrequency electromagnetic fields: Properties, quantities and units, biophysical interaction, and measurements. National Council on Radiation Protection and Measurements; Bethesda, MD: 1981. [Google Scholar]
- NCRP . Report No. 74. Biological effects of ultrasound: Mechanisms and clinical applications. National Council on Radiation Protection and Measurements; Bethesda, MD: 1983. [Google Scholar]
- NCRP . Report No. 113. Exposure criteria for medical diagnostic ultrasound: I. Criteria based on thermal mechanisms. National Council on Radiation Protection and Measurements; Bethesda, MD: 1992. [Google Scholar]
- NCRP . Report No. 140. Exposure criteria for medical diagnostic ultrasound: II. Criteria based on all known mechanisms. National Council on Radiation Protection and Measurements; Bethesda, MD: 2002. [Google Scholar]
- Nyborg WL, Ziskin MC. Biological effects of ultrasound. Churchill Livingstone; New York: 1985. [Google Scholar]
- Pakhomov AG, Akyel Y, Pakhomova ON, Stuck BE, Murphy MR. Current state and implications of research on biological effects of millimeter waves: A review of the literature. Bioelectromagnetics. 1998;19:393–413. doi: 10.1002/(sici)1521-186x(1998)19:7<393::aid-bem1>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Radzievsky AA, Cowan A, Byrd C, Radzievsky AA, Jr, Ziskin MC. Single millimeter wave treatment does not impair gastrointestinal transit in mice. Life Sciences. 2002;71:1763–1770. doi: 10.1016/s0024-3205(02)01944-6. [DOI] [PubMed] [Google Scholar]
- Radzievsky AA, Gordiienko OV, Alekseev S, Szabo I, Cowan A, Ziskin MC. Electromagnetic millimeter wave induced hypoalgesia: Frequency dependence and involvement of endogenous opioids. Bioelectromagnetics. 2008;29:284–295. doi: 10.1002/bem.20389. [DOI] [PubMed] [Google Scholar]
- Radzievsky AA, Gordiienko OV, Szabo I, Alekseev SI, Ziskin MC. Millimeter wave-induced suppression of B16 F10 melanoma growth in mice: Involvement of endogenous opioids. Bioelectromagnetics. 2004;25:466–473. doi: 10.1002/bem.20018. [DOI] [PubMed] [Google Scholar]
- Radzievsky AA, Rojavin MA, Cowan A, Alekseev SI, Radzievsky AA, Jr, Ziskin MC. Peripheral neural system involvement in hypoalgesic effect of electromagnetic millimeter waves. Life Sciences. 2001;68:1143–1151. doi: 10.1016/s0024-3205(00)01016-x. [DOI] [PubMed] [Google Scholar]
- Radzievsky AA, Rojavin MA, Cowan A, Alekseev SI, Ziskin MC. Hypoalgesic effect of millimeter waves in mice: Dependence on the site of exposure. Life Sciences. 2000;66:2101–2111. doi: 10.1016/s0024-3205(00)00536-1. [DOI] [PubMed] [Google Scholar]
- Radzievsky AA, Rojavin MA, Cowan A, Ziskin MC. Suppression of pain sensation caused by millimeter waves: A double blinded prospective human volunteer study. Anesthesia and Analgesia. 1999;88:836–840. doi: 10.1097/00000539-199904000-00029. [DOI] [PubMed] [Google Scholar]
- Rojavin MA, Radzievsky AA, Cowan A, Ziskin MC. Pain relief caused by millimeter waves in mice: Results of cold water tail flick tests. Int J Radiat Biol. 2000;76:575–579. doi: 10.1080/095530000138592. [DOI] [PubMed] [Google Scholar]
- Rojavin MA, Ziskin MC. Therapy with millimeter radiation in Eastern Europe: treatments unknown to Western doctors. EMF Health Report. 1997;5:1–6. [Google Scholar]
- Rojavin MA, Ziskin MC. Medical applications of millimeter waves. Quarterly Journal of Medicine. 1998;91:57–66. doi: 10.1093/qjmed/91.1.57. [DOI] [PubMed] [Google Scholar]
- Szabo I, Alesxeev SI, Acs G, Radzievsky AA, Logani LK, Makar VR, Gordiienko OR, Ziskin MC. Destruction of cutaneous melanoma with millimeter wave hyperthermia in mice. IEEE Trans on Plasma Science. 2004;32:1653–1660. [Google Scholar]
- Szabo I, Maning MR, Radzievsky AA, Wetzel MA, Rogers TJ, Ziskin MC. Low power millimeter wave irradiation exerts no harmful effect on human keratinocytes in vitro. Bioelectromagnetics. 2003;24:165–173. doi: 10.1002/bem.10077. [DOI] [PubMed] [Google Scholar]
- Szabo I, Rojavin MA, Rogers TJ, Ziskin MC. Reactions of keratinocytes to in vitro millimeter wave exposure. Bioelectromagnetics. 2001;22:358–364. doi: 10.1002/bem.62. [DOI] [PubMed] [Google Scholar]
- Ziskin MC. Identification of anatomic sources of ultrasonic echoes using a combined radiographic and through-transmission ultrasonic technique. Digest of the 2nd Canadian Medical and Biological Engineering Conference; Toronto, Canada. 1968. pp. 1–3. [Google Scholar]
- Ziskin MC. Detection of carotid artery bifurcation stenosis by Doppler ultrasound, a review. Investigative Radiology. 1969;4:112. [Google Scholar]
- Ziskin MC. Ultrasonic detection of cavitation at catheter tips, a review. Invest Radiology. 1971;6:293. [Google Scholar]
- Ziskin MC. Survey of patient exposure to diagnostic ultrasound. In: Reid JM, Sikov MR, editors. Interaction of Ultrasound and Biological Tissues. DHEW Publication (FDA)73-8008; Rockville, MD: 1972. pp. 203–206. [Google Scholar]
- Ziskin MC. Update on the safety of ultrasound in obstetrics. Seminars in Roentgenology. 1990;25:294–298. doi: 10.1016/0037-198x(90)90060-h. [DOI] [PubMed] [Google Scholar]
- Ziskin MC. Measurement uncertainty in ultrasonic exposimetry, chapter 14. In: Ziskin MC, Lewin PA, editors. Ultrasonic Exposimetry. CRC Press; Boca Raton, FL: 1993. pp. 409–443. [Google Scholar]
- Ziskin MC. Specification of acoustic output level and measurement uncertainty in ultrasonic exposimetry. IEEE Trans on Ultrasonics, Ferroelectrics, and Frequency Control. 2003;50:1023–1034. doi: 10.1109/tuffc.2003.1226546. [DOI] [PubMed] [Google Scholar]
- Ziskin MC. Physiological mechanisms underlying millimeter wave therapy. In: Ayrapetyan SN, Markov MS, editors. Current Concepts in Bioelectromagnetics. Springer; The Netherlands: 2005. pp. 241–251. [Google Scholar]
- Ziskin MC. The thermal dose index. J Ultrasound Med. 2010;29:1475–1479. doi: 10.7863/jum.2010.29.10.1475. [DOI] [PubMed] [Google Scholar]
- Ziskin MC, Barnett SB, Edwards MJ. Fetal brain weight reduction following ultrasonic exposure in guinea pigs. J Acoust Soc Amer. 1978;63:528. [Google Scholar]
- Ziskin MC, Bonakdarpour A, Weinstein D, Lynch PR. Contrast agents for diagnostic ultrasound. Invest Radiol. 1972;7:500–525. doi: 10.1097/00004424-197211000-00006. [DOI] [PubMed] [Google Scholar]
- Ziskin MC, Morrissey J. Thermal thresholds for teratology, reproduction, and development. Int J Hyperthermia. 2011;27:374–387. doi: 10.3109/02656736.2011.553769. [DOI] [PubMed] [Google Scholar]
- Ziskin MC, Romayananda N, Harris K. Ophthalmologic effect of ultrasound at diagnostic intensities. Journal of Clinical Ultrasound. 1974;2:119–122. doi: 10.1002/jcu.1870020207. [DOI] [PubMed] [Google Scholar]
- Ziskin MC, Thickman DI, Goldenberg NJ, Lapayowker MS, Becker JM. The comet tail artifact. J Ultrasound in Med. 1982;1:1–7. doi: 10.7863/jum.1982.1.1.1. [DOI] [PubMed] [Google Scholar]