Abstract
Post-chemotherapy treated cancer patients frequently report cognitive difficulties. The biology of this phenomenon is poorly understood, with uncertainty about possible direct toxic effects on the brain, secondary effects from systemic inflammation, host factors/genetic predisposition to cognitive complaints, or hormonal changes influencing cognitive function. To elucidate possible mechanisms associated with post-treatment cognitive dysfunction among breast cancer survivors, in 2007 we established a prospective, longitudinal, observational cohort study of early stage breast cancer patients, recruited at the end of initial treatments (primary treatment exposure included surgery, ± radiation, ± chemotherapy), and prior to the initiation of adjuvant endocrine therapy. We assessed cognitive complaints, neuropsychological (NP) test performance, markers of inflammation, and brain imaging at baseline, 6 months and 12 months after enrollment. In this analysis of data from the first 93 patients enrolled in the cohort study, we focus on the relationship of circulating levels of proinflammatory cytokines to cerebral functioning and chemotherapy exposure. Among the proinflammatory cytokines tested (IL-1ra, sTNF-RII, CRP, and IL-6) at baseline, only sTNF-RII was increased among chemotherapy exposed patients, with a significant decline in the year after treatment (p=0.003). Higher baseline sTNF-RII was significantly associated with increased memory complaints. In chemotherapy exposed patients, the longitudinal decline in sTNF-RII was significantly correlated with fewer memory complaints over 12 months (r=-0.34, p=0.04). Higher baseline sTNF-RII was also associated with relatively diminished brain metabolism in the inferior frontal cortex (r=-0.55, p=0.02), as well as relatively increased inferior frontal metabolism after one year, in chemotherapy-exposed subjects. These preliminary findings suggest that post-chemotherapy increases in TNF-α may be playing an important role in the manifestations of cognitive complaints in breast cancer survivors.
Keywords: breast cancer, cognitive complaints, chemotherapy, proinflammatory cytokines, TNF-α, neuropsychological testing, brain imaging
1. Introduction
With the growing number of cancer survivors, there has been increased interest in the risk factors for and mechanisms by which certain post-treatment symptoms occur and persist (Castellon et al. 2004;Ahles and Saykin,2007;Donovan et al. 2004;Stein et al. 2000;Bower et al. 2000;Bower, 2007;Bower, 2008;Collado-Hidalgo et al. 2008;Miller et al. 2008). Cognitive dysfunction after cancer treatments has been among the most feared post-treatment concern in cancer survivors, and this problem has been particularly associated with cranial radiation, intrathecal chemotherapy, as well as systemic chemotherapy and biotherapy (e.g. interleukin-2, interferon-α) (Meyers ,2000;Butler et al. 1994;Meyers, 2008;Wefel et al. 2008;Capuron et al. 2004). Breast cancer patients and survivors have been among the most extensively studied with regard to cognitive dysfunction among adult cancer survivors. Early studies were primarily cross-sectional and conducted in long term survivors (Schagen et al. 1999;van Dam et al. 1998;Ahles et al. 2002;Castellon et al. 2004), and suggested that chemotherapy exposure had an adverse effect on neuropsychological (NP) test performance. Neuroimaging studies also identified abnormalities in brain functioning, with MRI and PET scanning suggesting that chemotherapy exposure alters functional and metabolic activity in the frontal and cerebellar portions of the brain (Ferguson et al. 2007;McDonald et al. 2010;Silverman et al. 2007). A series of prospective longitudinal studies have been conducted to examine cognitive changes before chemotherapy administration and then after treatment (Wefel et al. 2004;Jenkins et al. 2006;Ahles et al. 2010). These studies have had mixed results, and have not consistently found NP impairment after chemotherapy exposure, with some studies showing NP test performance abnormalities prior to the administration of chemotherapy (Wefel et al. 2004;Ahles et al. 2008).
In 2007 we began a prospective, longitudinal, observational cohort study of early stage, newly-diagnosed, breast cancer patients, who were recruited immediately after the completion of primary treatment (surgery, adjuvant chemotherapy, radiation therapy), and prior to the initiation of adjuvant endocrine therapy if indicated (the UCLA Mind Body Study-[MBS]). By design, not all patients entering the MBS cohort had received chemotherapy, and the planned analyses focused on differences between these two groups (chemotherapy vs. no chemotherapy), before and after the initiation of adjuvant endocrine therapy, as well as an examination of how reproductive factors might modify the initial treatment exposures. At the time our study was designed, many longitudinal prospective studies were underway to examine pre- chemotherapy treatment cognitive function in the setting of breast cancer adjuvant therapy (Jenkins et al. 2006;Ahles et al. 2008;Ahles et al. 2010;Wefel et al. 2010;Schagen et al. 2006), with post-treatment follow-up that was often short term, as well as small samples that were often confounded by concomitant use of endocrine therapy (tamoxifen or aromatase inhibitors) in the comparison groups and in patients exposed to chemotherapy. As clinical observations suggested that changes in menopause status associated with chemotherapy could be contributing to post-treatment cognitive complaints, our cohort study focused on recovery post-primary adjuvant therapy, and the interaction with menstrual status and targeted endocrine therapies in the follow-up year.
The MBS also evaluated self-reported cognitive complaints, as there is an emerging literature supporting the ability of individuals to subjectively detect changes in cognitive function long before they are documented with more objective tests (Saykin et al. 2006). In breast cancer patients, this is supported by two recent studies demonstrating that patients who have received chemotherapy report cognitive complaints (memory and executive function) that align with relevant NP test domains and abnormalities in related anatomic regions on brain imaging (Deprez et al. 2012;Kesler et al. 2011).
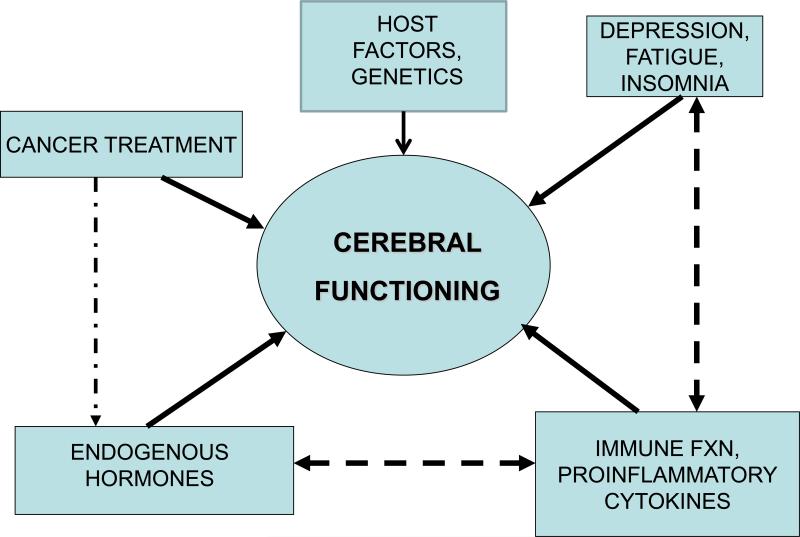
Little is known about the potential mechanisms that might contribute to changes in cognitive function associated with cancer treatments (Ahles and Saykin ,2007;Wefel et al. 2008). In line with recommendations from two international workshops on this topic (Tannock et al. 2004;Vardy et al. 2008), our research program's conceptual model included robust assessments of potential mechanisms by which cancer treatments (chemotherapy, radiation, hormonal treatments) could affect cerebral functioning, including the role of behavioral symptoms (fatigue, depressive symptoms, insomnia), and immune alterations (proinflammatory cytokines), in addition to endogenous endocrine exposures (estrogen, cortisol) (See figure 1). In this context, for cerebral functioning, we included measures of self-reported cognitive function, results of formal NP testing, and brain metabolism as assessed by positron emission tomography (PET) scanning (the latter in a substudy population). Although all of the factors in our conceptual model may affect cerebral functioning, they often interact among each other, e.g., chemotherapy leads to premature menopause (change in estradiol level), as well as having a potentially independent effect on cerebral functioning.
Figure 1.
Conceptual Model for the Study, which focuses on the multiple factors influencing cerebral functioning (as measured by self-report, neuropsychological testing, brain imaging). Cancer treatment includes surgery, radiation therapy, chemotherapy and targeted endocrine therapy (tamoxifen, aromatase inhibitors) and endogenous hormones include estrogen and cortisol. All of these factors may influence cerebral functioning, but can also interact with each other, for example, inflammatory cytokines may have direct effects on the brain, but can also influence cerebral functioning via their causal role in fatigue and other behavioral symptoms.
The main purpose of this examination of the MBS cohort study was to determine whether or not there was a significant relationship between recent chemotherapy exposure in women with early stage breast cancer and proinflammatory cytokines, and how these markers of inflammation might interact with various aspects of cerebral function and other behavioral symptoms. Based on our prior work examining persistent fatigue in breast cancer survivors (Bower et al. 2002;Bower et al. 2007;Bower et al. 2009;Collado-Hidalgo et al. 2006), we anticipated finding increases in proinflammatory cytokines in these post-treatment patients, but did not know whether this would affect cognitive complaints or neuropsychological performance, as we had not assessed these outcomes previously in this patient population. Therefore, to assess whether or not the laboratory studies should be continued across the entire longitudinal cohort study, we conducted this analysis that focuses on inflammation and cognitive dysfunction related to chemotherapy treatment exposure as the main effect.
In this report, we present data on the relationship between chemotherapy treatment exposure and cognitive complaints, behavioral symptoms, markers of inflammation, and NP performance at baseline prior to the start of endocrine therapy (cross-sectional evaluation). In longitudinal analyses, we focus on the trajectory of inflammatory markers over time, according to chemotherapy exposure, and their relationship to cognitive complaints as well as cerebral metabolism in the initial group of participants in the PET scan substudy (longitudinal evaluation). While successful recovery after breast cancer treatments has been described by our group previously in an earlier cohort study (Ganz et al. 2011b), the longitudinal relationship of chemotherapy exposure to post-treatment inflammation and recovery of symptoms has not been explored previously, to the best of our knowledge.
1. METHODS
1.1 Study participants and recruitment
This was an observational cohort study that recruited women with early stage breast cancer from the Los Angeles community, with the goal of studying the effects of endocrine therapy for breast cancer on cognitive function. To that end, women entering the cohort may have either had chemotherapy or not prior to study enrollment. Eligibility for this study included: 1) women aged 21-65 years; 2) newly diagnosed with Stage 0, I, II, IIIA breast cancer; 3) completion of primary treatment (surgery, radiation, and/or chemotherapy) within the past 3 months; 4) prior to start of endocrine targeted therapy if planned; 5) geographically accessible for follow-up in one year; 6) English language proficient; 7) able to provide informed consent. Exclusions and ineligibility were 1) evidence of current or past disorder/disease of the central nervous system or any medical condition that might be expected to impact cognitive functioning (e.g. multiple sclerosis, thyroid dysfunction); 2) history of head trauma with loss of consciousness greater than 30 minutes; 3) epilepsy, dementia, or severe learning disability; 4) current psychotic-spectrum disorder (e.g. schizophrenia, bipolar disorder; major depressive disorder) or current substance abuse or dependence; 5) history of whole brain irradiation or surgery; 6) history of past cancer treatment with chemotherapy; 7) active diagnosis of autoimmune and/or inflammatory disorder (e.g., systemic lupus erythematosis, rheumatoid arthritis, vasculitis) or disorders that may influence inflammatory processes (e.g., insulin-dependent diabetes; uncontrolled allergic condition or asthma); 8) chronic use of oral steroid medication; 9) hormone therapy (estrogen, progestin compounds) other than vaginal estrogen. An upper age-limit was used in this study due to the known age-related changes in cognitive function and brain volume beyond age 65, as well as the desire to limit confounding with treatment exposure (i.e. chemotherapy, which is less frequently given to older women based on tumor characteristics and lack of benefit in this age group). Medical conditions that were excluded were chosen for their known potential impact on cognitive function or inflammation.
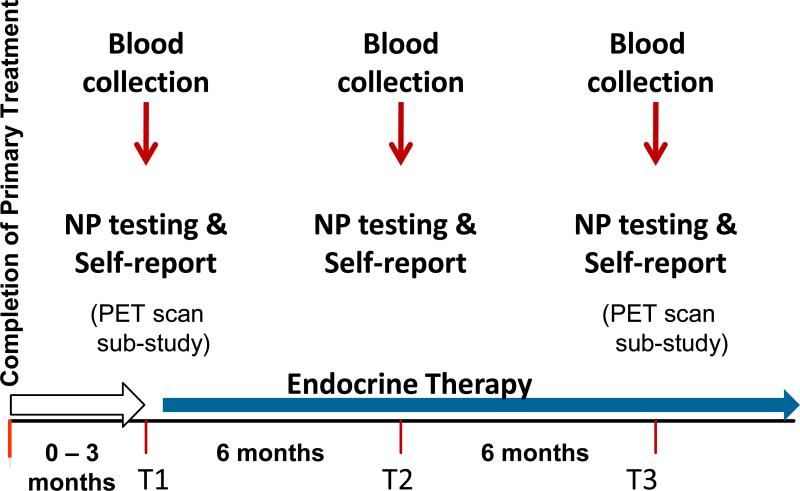
Initial study recruitment occurred through practices of physicians treating breast cancer patients. When this yielded few participants, we used the Los Angeles County SEER registry for rapid case ascertainment of stage eligible patients from selected hospitals where the collaborating physicians practiced. After mailed notification to physicians of our intent to contact the patient (with physician advice if contact was contraindicated), we mailed the patient a study invitation letter and brochure. This was the major source of study participants. Patients contacted us for more information, and were screened by telephone to determine eligibility. Interested and eligible women were scheduled for an in-person appointment where they had anthropometric assessments (weight, height, waist circumference), and underwent neuropsychological (NP) testing, collection of biological specimens, and completion of psychosocial questionnaires. Assessments were conducted in the morning (before 11am) after a two hour fast. Women rested for 20 minutes before the blood draw. (Note: a CONSORT recruitment figure will be provided when the full cohort is reported). Figure 2 shows the overall study design, including PET scan brain imaging that was done in a sub-study. Some preliminary results from the baseline sample of patients examining the relationship between markers of inflammation and common treatment associated symptoms (e.g., fatigue, sleep disturbance, depressive symptoms) have been reported elsewhere (Bower et al. 2011). The research was approved by the UCLA institutional review board and all participants provided informed consent.
Figure 2.
Study Design. Patients entered the study within 3 months of completing primary breast cancer treatment but before initiating breast cancer targeted endocrine therapy. A comprehensive assessment occurred at study entry (baseline-T1), 6 months later (T2) and 12 months later (T3). A substudy focused on brain imaging with FDG PET scans at T1 and T3 only.
2.2 Demographic, Clinical, and Behavioral Measures
Demographic and clinical information was obtained from self-report and medical record abstraction. Fatigue was assessed using the Fatigue Symptom Inventory (FSI), a valid and reliable 14-item measure specifically designed to assess fatigue in cancer populations (Hann et al. 1998;Donovan et al. 2008;Donovan ,2010). The Beck Depression Inventory-II (BDI-II), a 21-item measure with excellent reliability and validity, was used to assess symptoms of depression during the past two weeks (Beck et al. 1996). Subjective sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI), a 19-item questionnaire with high internal consistency, test-retest reliability, and diagnostic validity with insomnia diagnosis.(Buysse et al. 1991). For the FSI, BDI-II, and PSQI, higher scores indicate worse symptoms. The Squire Memory Questionnaire (SMQ) (Squire et al. 1979), a validated 18-item self-report measure, was used to provide an assessment of memory complaints, where lower scores indicate greater severity of memory complaints.
2.3 Neuropsychological (NP) Assessments
NP testing was conducted by a trained technician, closely supervised by a licensed clinical neuropsychologist. Review of NP testing data occurred throughout the study via case presentations and problem-focused discussions. The test battery used validated, reliable, and commonly used NP tests (see Appendix A), and took approximately 120 minutes to administer. All of the NP test scores were standardized to z- scores where positive scores indicated outcomes better than the age-matched normative means and negative scores worse than the normative means (normative references found in Appendix A). These scores were then used to create NP test domains based upon prior factor analytic studies of larger NP data sets and groupings used in other studies with this population (Tulsky and Price, 2003). We identified a priori variables most salient for the cognitive domain being studied. An estimate of full-scale IQ (the Wechsler Test of Adult Reading (WTAR), see Appendix A) was administered at baseline (T1) and provided a covariate in all NP related analyses.
2.4 Biological Specimens
Blood samples for circulating inflammatory markers were collected by venipuncture into EDTA tubes, placed on ice, centrifuged for acquisition of plasma, and stored at -80°C for subsequent batch testing. We evaluated four inflammatory markers that have been examined in association with cancer-related fatigue in previous research: IL-1 receptor antagonist (IL-1ra) and soluble TNF receptor type II (sTNF-RII), surrogate markers for IL-1 and TNF-α activity, respectively, as well as IL-6 and C reactive protein (CRP)(Bower et al. 2002;Bower et al. 2009;Collado-Hidalgo et al. 2006;Orre et al. 2009;Alexander et al. 2009;Bower et al. 2007; Wang et al., 2010, 2012). Plasma levels of IL-1ra and sTNF-RII were determined by regular sensitivity ELISA (lower limit of detection of 31 and 234 pg/ml, respectively), and IL-6 by high sensitivity ELISA (lower limit 0.2 pg/ml) (R&D Systems, Minneapolis, MN) according to the manufacturer's protocols. CRP levels were determined by a high sensitivity ELISA (Immundiagnostik, ALPCO Immunoassays, Salem, NH) according to the manufacturer's protocol, but with an extended standard curve to a lower limit of detection of 0.2 mg/L. All samples were run in duplicate, and assays were repeated on two separate assay days for sTNF-RII and IL-1ra; the intra- and inter-assay precision of all tests was less than or equal to 10%.
2.5 PET Scan Imaging
Acquisition and analysis of PET scans were performed as we have detailed previously (Silverman et al. 2007), with modifications as described here. We present the entire PET scan protocol, although only data from the resting FDG studies were examined in this interim analysis. The PET scan protocol is as follows: First, the radiotracer [O-15] water was used to assess acute changes in cerebral blood flow associated with performance of control and memory tasks. In a standard word-pair association cognitive tasking protocol, cue words were projected from a computer onto each subject's bilateral visual fields, through the use of electronic goggles worn by subjects during scanning. These words were presented 10 min (short-term recall task) or 1 day (long-term recall task) after having been presented paired with other words. Tasks were presented twice each, in counterbalanced fashion, i.e. a control read-repeat task, short-term read-recall task, long-term read-recall task, long-term read-recall task, short-term read-recall task and control read-repeat task. All subjects doing the O-15 activation studies performed both short-term and long-term recall tasks. PET was performed with 3D acquisitions, using a 64-slice PET/CT scanner (Siemens). Low-dose CT scans were used for attenuation correction. Six PET images using [O-15]water were acquired at 14-min intervals. For each scan, after administration of 555 MBq [O-15]water, PET data were obtained in list mode, and acquired data were framed in six five-s frames followed by nine 10-s frames, then summed for all frames after appearance of tracer into the brain (beginning approximately 25 s after injection). Following the conclusion of the activation study, FDG was used to assess regional cerebral metabolism during mental rest. Subjects were scanned in the supine position, 40 min following injection of 185 MBq FDG in a dimly lit room having low ambient noise, with eyes and ears unoccluded. Effects on resting metabolism were evaluated by both standardized volume of interest (VOI) and statistical parametric methods. In sVOI analyses, mean activities in 47 standardized VOIs were quantified for each scan, and normalized to mean global activity, using a commercially available display-and-analysis software package (NeuroQ; Syntermed, Inc.). Activation studies with [O-15]water PET, as well as metabolism studies with FDG PET, were also examined with statistical parametric mapping (SPM) software by methods previously described (Rasgon et al. 2005). Briefly, images were coregistered and reoriented into a standardized coordinate system (Talairach and Tournoux, 1988), using the nonlinear spatial transformation package in SPM8 (Friston et al. 2007), smoothed three-dimensionally at a full-width half-maximum of 8 mm, and normalized to mean global activity. Pooled data were then statistically assessed to identify the voxels which significantly differed between cognitive tasks (activation [O-15]water scans) and/or between treatment groups, or within treatment groups at different points of time, or which significantly correlated with a specified neuropsychologic parameter or peripheral cytokine measure. All results were reported in terms of locations of the most significant effects (regionally and/or in x,y,z Talairachstyle millimeter coordinates; Z and P values) and, when statistical strength of peak voxels or of regional volumes was high enough to survive standard statistical correction for multiple comparisons at P < 0.05, also by those corrected values. Results of the cerebral blood flow activation studies will be reported separately.
2.6 Statistical Methods
Patient demographic and medical characteristics were summarized for the entire study sample and stratified by chemotherapy exposure status. Chi-squared and t-tests were used to test for significant differences between the two treatment groups. Baseline (T1) mean scores on behavioral symptom measures, NP test domains and inflammatory markers were also compared with t-tests. For the bivariate relationships of chemotherapy and self-reported symptoms, to adjust for multiple comparisons, P-values ≤ 0.0125 are significant. 100% of plasma samples had detectable IL-1ra, sTNF-RII, and IL-6; 12% of samples were undetectable (<0.2 mg/L) for CRP. Undetectable CRP samples were assigned a value of 0.1 mg/L for analytical purposes. sTNF-RII was examined with both mean and log-transformed scores in various analyses, with similar findings. For ease of interpretation mean values are presented. NP test domains were adjusted for estimated IQ and inflammatory marker means were adjusted for patient age, BMI and radiation. For comparison of NP test domains by chemotherapy status, due to multiple comparisons, a P-value ≤ 0.0063 is significant. Repeated measures ANOVA controlling for age, BMI, radiation, and time since completion of chemotherapy, were conducted to compare sTNF-RII by chemotherapy status at each of the three assessments. The association between the Squire Memory scale with sTNF-RII was examined at baseline with a partial correlation, after controlling for age, BMI, radiation, time since last chemotherapy treatment among chemotherapy patients, depression, with and without the addition of fatigue to the model. To assess the association of changes in sTNF-RII with changes in Squire Memory over the 12-month study period, additional partial correlations were conducted for changes from T1 to T3.
3.0 Results
3.1 Patient Characteristics at Baseline (T1)
Study entry began in May 2007, and we conducted batched evaluations of inflammatory markers in February and November 2010 for the 98 patients who had entered the study. Among these patients, 3 were excluded from this analysis because they did not have any follow-up data after the baseline, and 2 were excluded because they had clinical infections at the time of sampling, leaving 93 patients with serial longitudinal data (e.g., 87 with complete case data and 6 with T1 (baseline) and either T2 (6 months after baseline) or T3 (12 months after baseline), who are the subject of this report. Forty-nine patients had received chemotherapy prior to study entry at T1, and 44 had not received chemotherapy. Table 1 presents the demographic and medical characteristics of the patients at T1. There were few differences between the patients who received chemotherapy compared with those who did not. Specifically, there was no significant difference in the subsequent use of endocrine targeted therapy (e.g., tamoxifen, aromatase inhibitors) of breast cancer during the following 12 months. (The examination of endocrine effects will be the focus of the main study results for the full cohort; the four cells examining endocrine therapy with or without chemotherapy, no chemotherapy or endocrine therapy, and chemotherapy alone were too small, with insufficient power for examination in this analysis).
Table 1.
Patient demographic and medical characteristics by chemotherapy status.
| Total (n=93) | Chemo (n=49) | No chemo (n=44) | |||||
|---|---|---|---|---|---|---|---|
| % | n | % | n | % | n | p-value | |
| Age at Baseline, mean (SD) | 51.3 (7.8) | 49.9 (8.5) | 52.8 (6.7) | 0.0685 | |||
| Months from diagnosis to T1, mean (SD) | 7.0 (2.8) | 8.7 (2.4) | 5.0 (1.6) | <0.0001 | |||
| Race | |||||||
| White | 85% | 79 | 84% | 41 | 86% | 38 | 0.7172 |
| Marital status | |||||||
| Married | 71% | 65 | 77% | 37 | 64% | 28 | 0.1571 |
| Education | |||||||
| Post college | 49% | 46 | 49% | 24 | 50% | 22 | 0.9471 |
| College | 33% | 31 | 35% | 17 | 32% | 14 | |
| Post HS | 17% | 16 | 16% | 8 | 18% | 8 | |
| WTAR, mean (SD) | 113.8 (9.2) | 113.8 (9.0) | 113.9 (9.5) | 0.9710 | |||
| Employment | |||||||
| Full or part-time | 61% | 56 | 54% | 26 | 68% | 30 | 0.1688 |
| Household Income | |||||||
| >$100K | 70% | 62 | 60% | 28 | 81% | 34 | 0.0285 |
| BMI, mean (SD) | 24.7 (4.5) | 24.3 (4.6) | 25.1 (4.4) | 0.3922 | |||
| Stage at Diagnosis | |||||||
| 0 | 17% | 16 | 0% | 0 | 37% | 16 | <0.0001 |
| 1 | 45% | 41 | 35% | 17 | 56% | 24 | |
| 2 | 34% | 57% | 28 | 7% | 3 | ||
| 3 | 4% | 8% | 4 | 0% | 0 | ||
| Surgery | |||||||
| Mastectomy | 28% | 26 | 33% | 16 | 23% | 10 | 0.2869 |
| Lumpectomy | 72% | 67 | 67% | 33 | 77% | 34 | |
| Radiation | |||||||
| Yes | 76% | 71 | 80% | 39 | 73% | 32 | 0.4367 |
| Hormone Replacement | |||||||
| Therapy | |||||||
| Yes | 27% | 24 | 21% | 10 | 33% | 14 | 0.1810 |
| Change in period | |||||||
| Post-menopausal | 48% | 45 | 45% | 22 | 52% | 23 | <0.0001 |
| No change | 18% | 17 | 4% | 2 | 34% | 15 | |
| Became irregular | 5% | 5 | 0% | 0 | 11% | 5 | |
| Stop but resumed | 3% | 3 | 4% | 2 | 2% | 1 | |
| Amenorrhea | 25% | 23 | 47% | 23 | 0% | 0 | |
| Chemotherapy regimen: | |||||||
| Anthracycline | |||||||
| Yes | ---- | ---- | 29% | 14 | ---- | ---- | ---- |
| Targeted Endocrine | |||||||
| Therapy at 6-month | |||||||
| Yes | 72% | 67 | 78% | 38 | 66% | 29 | 0.2117 |
| If Yes, type: | |||||||
| Tamoxifen | 54% | 36 | 45% | 17 | 66% | 19 | 0.0910 |
| Aromatase Inhibitor | 46% | 31 | 55% | 21 | 34% | 10 | |
WTAR= Wechsler Test of Adult Reading; BMI=Body Mass Index
The chemotherapy treated patients entered the study at a longer time after diagnosis due to this additional therapy (8.7 months vs. 5.0 months, p <0.0001). The study participants were largely white, married, highly educated, and employed, with high household income. There were no significant differences in cancer treatments (type of surgery, radiation receipt, or subsequent endocrine therapy use); however, as expected, patients receiving chemotherapy had more advanced stage disease (p <0.0001), with 37% of the no chemotherapy patients being stage 0. Among the chemotherapy treated patients, 29% (n=14) received an anthracycline containing regimen (doxorubicin, cyclophosphamide, and a taxane, n=12; or fluorouracil, epirubicin, cyclophosphamide, n=2). The distribution of regimens in the remaining 35 patients were as follows: docetaxel and cyclophosphamide, n=25; a taxane and carboplatin, n=9; gemcitabine with cisplatin , n=1. These regimens reflect standard patterns of contemporary adjuvant chemotherapy treatment. As expected, chemotherapy-induced amenorrhea occurred in 47% of those treated; however, the number of women who were postmenopausal in each group before and after treatment were similar (see Table 1).
3.2 Relationship of Self-reported Behavioral Symptoms to Chemotherapy Treatment Exposure
Table 2 provides the scores on the self-reported behavioral symptoms at baseline, after treatment with surgery, and/or radiation, and/or chemotherapy and prior to the initiation of endocrine therapy (if planned). Patients exposed to chemotherapy had significantly greater fatigue severity (p=0.003) and memory complaints (p<0.0001). Sleep problems and depressive symptoms were marginally significant at the Bonferroni-corrected P=0.0125. Fatigue, memory complaints, and depressive symptoms are all highly correlated with each other in these patients: FSI severity with SMQ: r = -0.51601, p < 0.0001; FSI severity with BDI-II: r = 0.50254, p < 0.0001; SMQ with BDI-II: r = -0.54698, p < 0.0001, suggesting the possibility of a common underlying biological process mediating these symptoms.
Table 2.
Self-reported Behavioral Symptoms at baseline by chemotherapy status.
| Total (n=93) | Chemo (n=49) | No chemo (n=44) | |||||
|---|---|---|---|---|---|---|---|
| mean | STD | mean | STD | mean | STD | p-value | |
| FSI Severity | 3.88 | 2.12 | 4.49 | 2.26 | 3.20 | 1.74 | 0.0030 |
| SMQ | -12.34 | 15.64 | -19.29 | 17.41 | -4.75 | 8.58 | <0.0001 |
| PSQI | 8.15 | 4.02 | 9.16 | 3.88 | 7.11 | 3.94 | 0.0157 |
| BDI-II | 9.14 | 7.36 | 10.92 | 6.73 | 7.20 | 7.59 | 0.0148 |
FSI=Fatigue Symptom Inventory; SMQ=Squire Memory Questionnaire; PSQI=Pittsburgh Sleep Quality Index; BDI-II= Beck Depression Inventory-II.
3.3 NP Test Results at Baseline by Chemotherapy Exposure
We examined the relationship between the 8 domains of NP functioning and chemotherapy exposure at baseline, adjusting for the WTAR score. As can be seen in Table 3, the chemotherapy exposed patients had somewhat lower, but non-significant differences in performance in the psychomotor domain (p=0.07). There were no differences in the other domains examined. Also, it is worth noting that both of the treatment groups performed above average in reference to available age-matched normative data. This is not particularly surprising, given the above average estimated IQ for the participants in this study, and their high educational attainment (see Table 1). We also examined the relationship between each of the NP domains with each of the four proinflammatory cytokines, controlling for age, BMI, WTAR, radiation, and chemotherapy, and there were no significant relationships found (data not shown).
Table 3.
NP test domains* at baseline by chemotherapy status adjusted for IQ (n=93).
| Chemo (n=49) | No chemo (n=44) | ||||
|---|---|---|---|---|---|
| mean | SE | mean | SE | p-value | |
| Psychomotor | 0.37 | 0.10 | 0.63 | 0.10 | 0.0728 |
| Executive function | 0.29 | 0.09 | 0.49 | 0.10 | 0.1419 |
| Verbal learning | 0.42 | 0.10 | 0.59 | 0.10 | 0.2299 |
| Verbal memory | 0.68 | 0.09 | 0.74 | 0.09 | 0.6066 |
| Visual learning | 0.10 | 0.09 | 0.24 | 0.10 | 0.2789 |
| Visual memory | 0.03 | 0.11 | 0.16 | 0.12 | 0.4622 |
| Visuo-spatial | 0.46 | 0.10 | 0.59 | 0.11 | 0.3947 |
| Motor speed | -0.41 | 0.16 | -0.30 | 0.17 | 0.6396 |
Detailed description of NP test domains is included in the appendix
3.4 Proinflammatory Cytokine Results and Their Relationship to Cognitive Complaints
After controlling for patient age, BMI and radiation, the mean plasma level of sTNF-RII was significantly higher among chemotherapy-treated patients at baseline (T1) compared to those patients who did not receive chemotherapy (2629 vs. 2149 pg/ml, p=0.0015) (See Table 4). There were no differences in mean IL-1ra, IL-6 or CRP between the two groups at baseline (Table 4), and none of these three markers changed significantly over the following 12 months (data not shown). To determine whether greater sTNF-RII levels were associated with greater cognitive complaints, we also examined the correlations between sTNF-RII at baseline and self-reported memory complaints, and found that higher levels of sTNF-RII were significantly correlated with greater memory complaints after controlling for age, BMI, radiation and depression, and time since last chemotherapy treatment among chemotherapy patients (SMQ r=-0.21, p=.05). To determine whether the same variance in sTNF-RII levels might contribute to both cognitive complaints and previously observed elevations in fatigue, we controlled for the sTNF-RII relationship to fatigue (FSI severity) and found that associations between sTNF-RII and memory complaints were rendered nonsignificant, although still positively correlated (r=-0.17, p=0.13).
Table 4.
Inflammatory cytokines at baseline by chemotherapy status adjusted for age and BMI.
| Chemo (n=49) | No chemo (n=44) | ||||
|---|---|---|---|---|---|
| mean | SE | mean | SE | p-value | |
| Il-lra, pg/mL | 261 | 51 | 331 | 53 | 0.3484 |
| IL-6, pg/mL | l.6 | 0.1 | 1.6 | 0.2 | 0.8303 |
| CRP, mg/L | 1.99 | 0.43 | 2.30 | 0.45 | 0.6230 |
| sTNF-RII, pg/mL | 2580 | 100 | 2113 | 104 | 0.0019 |
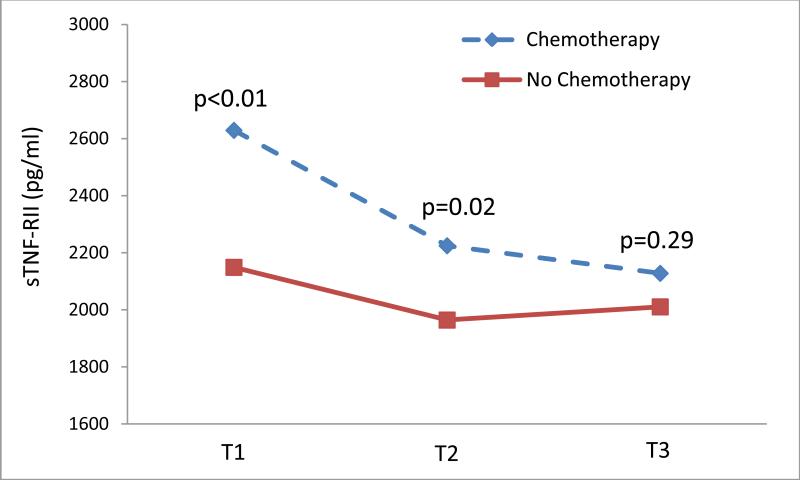
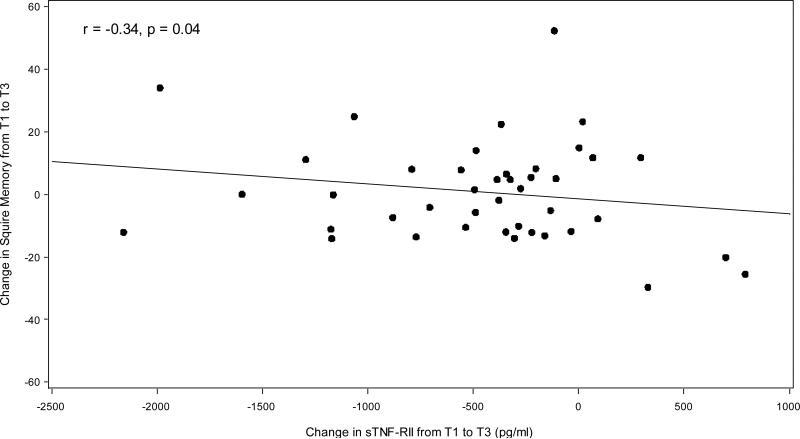
The level of sTNF-RII declined significantly in the chemotherapy treated patients from T1 to T3 (p=0.003), but remained unchanged in those who were not treated with chemotherapy (see Figure 3). Examination of the longitudinal change over time in sTNF-RII by chemotherapy exposure status revealed significantly higher levels in the chemotherapy patients at baseline and 6 months, but no significant difference at 12 months (see Figure 3). Finally, in the chemotherapy exposed patients, we examined the relationship between the change sTNF-RII between T1 and T3 (a decline), and the change in SMQ, adjusting for age, BMI, radiation, depressive symptoms, and time since last chemotherapy treatment among chemotherapy patients (see Figure 4). The two were significantly correlated (r=-0.34, p=0.04) suggesting that the decline in sTNF-RII was significantly associated with improvements in self-reported memory. There was no significant correlation found among those patients who did not received chemotherapy.
Figure 3.
Mean plasma sTNF-RII levels over 12 months by chemotherapy exposure status. Comparisons between chemotherapy exposed (dotted line) and no chemotherapy (solid line) treatment groups, controlling for age, BMI, and radiation, showed significantly higher levels of sTNF-RII in the chemotherapy treated group at T1 and T2.
Figure 4.
Scatter plot of change in Squire Memory Questionnaire (SMQ) vs. change in plasma sTNF-RII from baseline (T1) to 12 months (T3) among chemotherapy patients (n=43). Correlation adjusted for age, BMI, radiation, depressive symptoms, and time since last chemotherapy treatment. Result: r=-0.34, p=0.04.
3.5 Relationship of Plasma sTNF-RII with Regional Distribution of Cerebral Metabolism
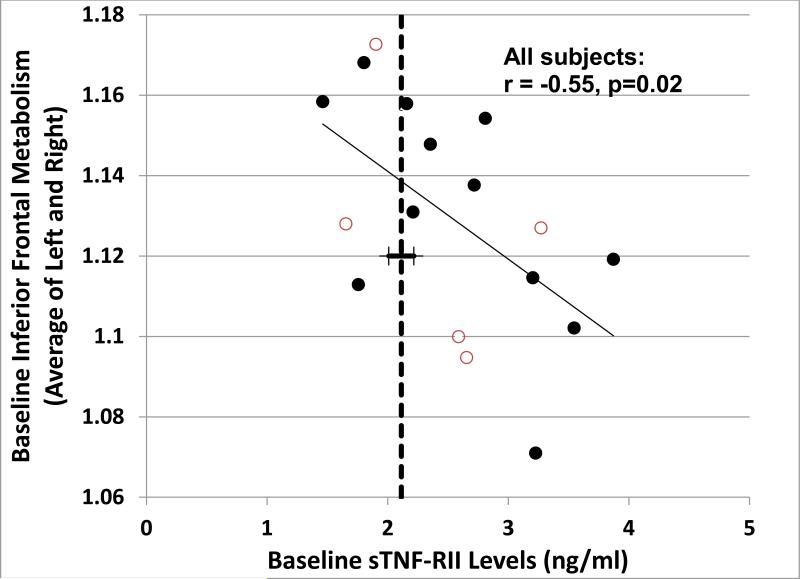
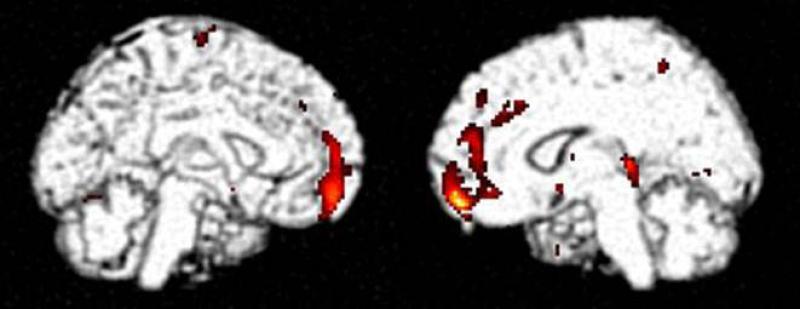
During the MBS recruitment and follow-up period included in this analysis, we enrolled 17 women at baseline (12 chemotherapy and 5 no chemotherapy) and 16 women had completed follow-up scans at T3 (11 chemotherapy and 5 no chemotherapy). We used this sample to explore the potential impact of proinflammatory cytokines on cerebral function as reflected by alterations in brain metabolism. This was accomplished through assessment of relationships between peripheral cytokine markers and the distribution of FDG measured using brain PET, focusing for the purposes of the present analysis particularly upon sTNF-RII. We previously identified the region of the brain having diminished resting metabolism bearing the strongest correlation with relatively diminished cognitive function in adjuvant chemotherapy-exposed patients as being located in the inferior frontal gyrus, in the vicinity of Broca's area (Silverman et al., 2007); this region thus served as the basis for an a priori hypothesis about where to look for a negative correlation with the relatively elevated sTNF-RII marker identified in the current adjuvant chemotherapy-exposed cohort. Within the group undergoing PET, 9 of the 12 chemotherapy-exposed subjects demonstrated baseline plasma sTNF-RII above the mean level found for all non-chemotherapy exposed subjects; across these subjects, as well as across all subjects undergoing PET, a significant negative baseline correlation (r = -0.55; p = 0.02) was in fact observed between plasma sTNF-RII and mean inferior frontal metabolism (Figure 5). Statistical parametric mapping analysis (see section 2.5) further revealed a relationship between plasma sTNF-RII and cerebral metabolism of the chemotherapy-exposed subjects that spanned the one-year longitudinal time-frame of the present study. The single most significant, and concomitantly most extensive, brain region demonstrating metabolism at one year positively correlating with baseline plasma sTNF-RII levels was found in inferior frontal cortex, along the anteromedial aspects of the bilateral frontal lobes (Figure 6).
Figure 5.
Relationship of plasma sTNF-RII to inferior frontal metabolism, both measured at baseline (r=-.55, p=0.02) in 17 subjects who participated in the PET scan substudy, 12 chemotherapy exposed (filled circles) and 5 without chemotherapy (open circles). The mean baseline value of sTNF-RII for all non-chemotherapy treated patients in the full sample (n=44) is included as a reference (bold dashed line). Regional metabolism values reflect the mean of standardized volumes of interest assessing the left and right inferior frontal gyri, as described in the text.
Figure 6.
Statistical parametric map of areas in brains of chemotherapy-exposed subjects having metabolism after one year longitudinal follow-up (n=11 chemotherapy exposed patients) that is significantly correlated with baseline plasma sTNF-RII . Colorscale reflects location of significantly correlated voxels, mapped upon co-registered grayscale structural template for anatomical reference; peak voxel p<0.0005, is located at (14, 56, -14) mm, and is part of a cluster of 501 contiguous voxels having p<0.01.
4.0 Discussion
Cognitive dysfunction after cancer treatment is common, and the causal mechanisms are uncertain (Ahles and Saykin ,2007). This dysfunction can be manifested by self-reported increased cognitive complaints after treatment (most often memory and executive functioning problems), or with documented declines in NP test performance in certain domains, or with changes in brain function through various imaging strategies (i.e., MRI, PET). Relatively few studies have examined all of these components in the same study population (Kesler et al. 2011;Deprez et al. 2012). The MBS focused, in part, on the impact of treatment associated inflammation as a potential risk factor for cognitive dysfunction after primary adjuvant therapy. The final assembled cohort for the MBS is 190 women entered after the end of primary treatment, recruited over a 4 year period. Rather than delaying all analyses until the entire cohort completed the 12 month assessment, we performed this evaluation of the inflammatory markers to determine whether or not the hypothesized relationships between post-chemotherapy treatment, inflammation and symptoms were on target. As we have reported elsewhere, plasma sTNF-RII was significantly correlated with fatigue at the baseline assessment, but not sleep or depressive symptoms (Bower et al. 2011). In this report, we extend these initial findings to examine the relationship of the inflammatory markers to cognitive dysfunction, examining results from self-reported cognitive complaints, formal NP testing, and brain metabolism. We examined both baseline (cross-sectional) and longitudinal changes in inflammatory markers after exposure to chemotherapy and other primary breast cancer treatments. We identified significant correlations between self-reported memory complaints and plasma sTNF-RII levels at baseline and longitudinally in patients treated with chemotherapy. In addition to demonstrating an association between TNF dynamics and self-reported memory complaints, these analyses suggest that both memory complaints and fatigue might share a common underlying source in pro-inflammatory cytokine signaling, in that controlling for covariance between sTNF-RII and fatigue reduced associations between sTNF-RII and memory complaints to nonsignificant levels. Thus both symptom components appear to share a common underlying contribution from TNF signaling.
Significant correlations were also noted for plasma sTNF-RII and cerebral metabolic changes in the hypothesized regions of the brain on FDG scans. However, no significant relationships were seen between NP test performance and either chemotherapy exposure or the inflammatory markers. The other markers of inflammation (CRP, IL-6, and IL1ra) were not significantly associated with chemotherapy exposure in this sample at baseline or longitudinally. It is possible that inflammatory cytokines, although correlated, may have distinct associations with the central nervous system (CNS) and physical health. For example, sTNF-RII, but not IL-6, was correlated with stress-induced changes in the CNS (Slavich et al, 2010), and sTNF-RII predicted coronary heart disease independent of CRP (Shai et al., 2005). Taken together, these findings suggest that elevations in TNF-α after adjuvant chemotherapy exposure in breast cancer patients may play an important role in cognitive dysfunction. These effects may be subtle, as manifested through self-report and brain imaging studies, and not be routinely detected in standardized NP tests in this high functioning population.
In this analysis, chemotherapy exposure at baseline was significantly associated with increased memory complaints and fatigue; insomnia and depressive symptoms were marginally significant. NP test results did not significantly differ by chemotherapy exposure, and may reflect the overall high level of function in this patient population and insensitivity of the standardized NP tests in this setting and patient population. Given their high level of NP function, the increased self-reported memory complaints may be a more sensitive indicator of subtle cognitive dysfunction associated with chemotherapy exposure. However, the observational design, and lack of a pre-post-treatment comparison is a study limitation.
While most chemotherapy agents used in contemporary breast cancer adjuvant treatments do not cross the blood brain barrier (as opposed to methotrexate and fluorouracil used in the CMF regimen popular two decades ago), recent animal model studies using the anthracycline doxorubicin suggest that this agent increases the level of systemic inflammation via reactive oxygen species, and the systemic inflammation crosses the blood brain barrier to have direct effects on microglial cells, increasing local brain inflammation, especially with TNF-α (Joshi et al. 2005;Joshi et al. 2010). Demyelination and death of progenitor cells may be a mechanism by which cognitive changes occur in association with chemotherapy exposure, as has been shown in animal studies with fluorouracil (Han et al., 2008), but more animal studies need to be conducted with contemporary chemotherapy drugs to fully understand the mechanisms by which systemic exposure leads to CNS changes (Meyers 2008). In our final study sample evaluation, we will have a sufficient number of anthracycline exposed patients to examine whether or not there are differences in cognitive function based on specific chemotherapy exposures.
The key limitation of this study is that our findings related to proinflammatory cytokines and cognitive function are correlational. We have not as yet examined NP test outcomes beyond the baseline assessment, as we will require the full sample to examine sub-groups with regard to the addition of targeted endocrine therapy—the main exposure of interest in this prospective cohort study. The MBS did not include concurrent non-cancer control subjects, and thus we need a sufficient number of non-chemotherapy/non-endocrine participants for the reference control group that will be available in the full sample. Nevertheless, our finding of an association between chemotherapy exposure, plasma sTNF-RII and cognitive complaints at baseline and in longitudinal outcomes, provides support for continued examination of post-chemotherapy inflammation as a causal factor in this syndrome. The present results implicating TNFα in chemotherapy-related cognitive complaints are consistent with recent analyses from our laboratory linking cognitive complaints to high-expressing genetic variants of the TNF-α -308 promoter SNP (Ganz et al. 2011a; Ganz et al. 2012).
The course of cognitive complaints after cancer treatments is not known, as few long-term longitudinal studies have been conducted to date (Phillips et al. 2012;Jenkins et al. 2006;Syrjala et al. 2011). Animal studies also suggest the possibility of a biphasic pattern of injury with acute and then delayed toxicities (Han et al. 2008). As with many other late effects of cancer treatments, some complications may only emerge several years after cancer treatments end, while others may be present at the time of treatment and persist. Our earlier cross-sectional study of breast cancer survivors who were greater than 5 years after diagnosis (Silverman et al. 2007) showed PET scan abnormalities in the same anatomic areas of the brain we find acutely in this study, suggesting that some cerebral function abnormalities might persist (Silverman et al. 2007). We are continuing to follow the MBS cohort of patients beyond the first post-treatment year (T3), and we hope to be able to track the course of cognitive functioning and its relationship to inflammation in this cohort over a longer period of time.
Supplementary Material
Research Highlight.
sTNF-RII is significantly associated with post-chemotherapy cognitive dysfunction in early stage breast cancer patients evaluated during the year after treatment ended.
Acknowledgements
We thank Ms. Amy Oppenheim and Ms. Barbara Kahn-Mills for their essential role in the recruitment of subjects for the study and collection of all of the data, including neuropsychological testing and evaluation. In addition, we thank Ryan Sadakane and Susanne Yoon for their technical support in performance of the laboratory assays. Finally, we are most grateful to the recently diagnosed and treated breast cancer patients who volunteered their time to participate in this study and to contribute to new knowledge about the natural history of cognitive changes after breast cancer treatments.
Funding Support: NCI R01 CA 109650; the Breast Cancer Research Foundation; an American Cancer Society Clinical Research Professorship to Dr. Ganz; the UCLA Older Americans Independence Center (OAIC) and the OAIC Inflammatory Biology Core (NIH/NIA P30-AG028748); the Norman Cousins Center for PNI. R01-AG034588; R01-AG026364; R01-CA119159; R01-HL079955; R01 HL095799; P30-AG028748; UL RR 033176 to Dr. Irwin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, Whedon MB, Bivens S, Mitchell T, Greenberg ER, Silberfarb PM. Neuropsychologic impact of standard-dose systemic chemotherapy in long- term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- Ahles T, Saykin A, McDonald B, Furstenberg C, Cole B, Hanscom B, Mulrooney T, Schwartz G, Kaufman P. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Research and Treatment. 2008;110:143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Longitudinal Assessment of Cognitive Changes Associated With Adjuvant Treatment for Breast Cancer: Impact of Age and Cognitive Reserve. J Clin Oncol. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. European Journal of Cancer. 2009;45:384–392. doi: 10.1016/j.ejca.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. BDI-II; Beck Depression Inventory. 2nd edition manual Harcourt Brace Inc; Boston: 1996. [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom.Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW. Inflammatory responses to psychological stress in fatigued breast cancer survivors: relationship to glucocorticoids. Brain Behav.Immun. 2007;21:251–258. doi: 10.1016/j.bbi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J.Clin.Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, Cole S, Aziz N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15:5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE. Cancer-related fatigue: Links with inflammation in cancer patients and survivors. Brain, Behavior, and Immunity. 2007;21:863–871. doi: 10.1016/j.bbi.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE. Behavioral Symptoms in Patients With Breast Cancer and Survivors. J Clin Oncol. 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and Behavioral Symptoms After Breast Cancer Treatment: Do Fatigue, Depression, and Sleep Disturbance Share a Common Underlying Mechanism? J Clin Oncol. 2011;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler RW, Hill JM, Steinherz PG, Meyers PA, Finlay JL. Neuropsychologic effects of cranial irradiation, intrathecal methotrexate, and systemic methotrexate in childhood cancer. J Clin Oncol. 1994;12:2621–2629. doi: 10.1200/JCO.1994.12.12.2621. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep. 1991;14:331–338. [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain, Behavior, and Immunity. 2004;18:205–213. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Castellon SA, Ganz PA, Bower JE, Petersen LA, Abraham L, Greendale GA. Neurocognitive Performance in Breast Cancer Survivors Exposed to Adjuvant Chemotherapy and Tamoxifen. Journal of Clinical and Experimental Neuropsychology. 2004;26:955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Irwin MR, Cole SW. Cytokine gene polymorphisms and fatigue in breast cancer survivors: Early findings. Brain, Behavior, and Immunity. 2008;22:1197–1200. doi: 10.1016/j.bbi.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez S, Amant F, Smeets A, Peeters R, Leemans A, Van Hecke W, Verhoeven JS, Christiaens MR, Vandenberghe J, Vandenbulcke M, Sunaert S. Longitudinal Assessment of Chemotherapy-Induced Structural Changes in Cerebral White Matter and Its Correlation With Impaired Cognitive Functioning. J Clin Oncol. 2012;30:274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- Donovan KA, Jacobsen PB. The Fatigue Symptom Inventory: a systematic review of its psychometric properties. Support.Care Cancer. 2010;19:169–185. doi: 10.1007/s00520-010-0989-4. [DOI] [PubMed] [Google Scholar]
- Donovan KA, Jacobsen PB, Andrykowski MA, Winters EM, Balducci L, Malik U, Kenady D, McGrath P. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. Journal of Pain and Symptom Management. 2004;28:373–380. doi: 10.1016/j.jpainsymman.2004.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan KA, Jacobsen PB, Small BJ, Munster PN, Andrykowski MA. Identifying Clinically Meaningful Fatigue with the Fatigue Symptom Inventory. Journal of Pain and Symptom Management. 2008;36:480–487. doi: 10.1016/j.jpainsymman.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25:3866–3870. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, Penny WD. Statistical Parametric Mapping: The Analysis of Functional Brain Images. Academic Press; Burlington, MA: 2007. [Google Scholar]
- Ganz PA, Castellon SA, Silverman DHS, Kwan L, Bower JE, Irwin MR, Cole SW. Does circulating tumor necrosis factor (TNF) play a role in post-chemotherapy cerebral dysfunction in breast cancer survivors (BCS)?. Abstract presented at Annual Meeting of the American Society of Clinical Oncology; Chicago. June 3, 2011.2011a. [Google Scholar]
- Ganz PA, Kwan L, Stanton AL, Bower JE, Belin TR. Physical and Psychosocial Recovery in the Year After Primary Treatment of Breast Cancer. J Clin Oncol. 2011b;29:1101–1109. doi: 10.1200/JCO.2010.28.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz PA, Kwan L, Castellon SA, Bower JE, Silverman DHS, Irwin MR, Cole SW. Cognitive Complaints in Breast Cancer Patients: Associations with Therapy and Cytokine Markers.. Abstract presented at the International Cognition and Cancer Task Force Conference meeting; Paris. March 16, 2012.2012. [Google Scholar]
- Han R, Yang Y, Dietrich J, Luebke A, Mayer-Proschel M, Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. Journal of Biology. 2008;7(4):12. doi: 10.1186/jbiol69. Epub Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann DM, Jacobsen PB, Azzarello LM, Martin SC, Curran SL, Fields KK, Greenberg H, Lyman G. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual.Life Res. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, Allan S, Bishop H, Hodson N, Mitra S, Sadler G, Shah E, Stein R, Whitehead S, Winstanley J. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br.J Cancer. 2006;94:828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi G, Aluise CD, Cole MP, Sultana R, Pierce WM, Vore M, St Clair DK, Butterfield DA. Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: implications for oxidative stress-mediated chemobrain. Neuroscience. 2010;166:796–807. doi: 10.1016/j.neuroscience.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi G, Sultana R, Tangpong J, Cole MP, St Clair DK, Vore M, Estus S, Butterfield DA. Free radical mediated oxidative stress and toxic side effects in brain induced by the anti cancer drug adriamycin: insight into chemobrain. Free Radical Res. 2005;39:1147–1154. doi: 10.1080/10715760500143478. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Kent JS, O'Hara R. Prefrontal Cortex and Executive Function Impairments in Primary Breast Cancer. Arch Neurol. 2011;68:1447–1453. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, Conroy S, Ahles T, West J, Saykin A. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Research and Treatment. 2010;123:819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers CA. Neurocognitive dysfunction in cancer patients. Oncology (Huntingt) 2000;14:75–79. [PubMed] [Google Scholar]
- Meyers CA. How chemotherapy damages the central nervous system. J Biol. 2008;7(4):11. doi: 10.1186/jbiol73. Epub Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-Immune Mechanisms of Behavioral Comorbidities in Patients With Cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orre IJ, Murison R, Dahl AA, Ueland T, Aukrust P, Fossσ SD. Levels of circulating interleukin-1 receptor antagonist and C-reactive protein in long-term survivors of testicular cancer with chronic cancer-related fatigue. Brain, Behavior, and Immunity. 2009;23:868–874. doi: 10.1016/j.bbi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Phillips KM, Jim HS, Small BJ, Laronga C, Andrykowski MA, Jacobsen PB. Cognitive functioning after cancer treatment. Cancer n/a. 2011 doi: 10.1002/cncr.26432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon NL, Silverman D, Siddarth P, Miller K, Ercoli LM, Elman S, Lavretsky H, Huang S-C, Phelps ME, Small GW. Estrogen use and brain metabolic change in postmenopausal women. Neurobiology of Aging. 2005;26:229–235. doi: 10.1016/j.neurobiolaging.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FS. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. J Natl Cancer Inst. 2006;98:1742–1745. doi: 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- Schagen SB, van Dam FS, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–650. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Shai I, Schulze MB, Manson JE, Rexrode KM, Stampfer MJ, et al. A prospective study of soluble tumor necrosis factor-alpha receptor II (sTNF-RII) and risk of coronary heart disease among women with type 2 diabetes. Diabetes care. 2005;28(6):1376–82. doi: 10.2337/diacare.28.6.1376. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, Waddell K, Petersen L, Phelps ME, Ganz PA. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5-10 years after chemotherapy. Breast Cancer Res Treat. 2007;103:303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc Natl Acad Sci USA. 2010;107(33):14817–22. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wetzel CD, Slater PC. Memory complaints after electroconvulsive therapy: assessment with a new self-rating instrument. Biological Psychiatry. 1979;14:791–801. [PubMed] [Google Scholar]
- Stein KD, Jacobsen PB, Hann DM, Greenberg H, Lyman G. Impact of hot flashes on quality of life among postmenopausal women being treated for breast cancer. J.Pain Symptom.Manage. 2000;19:436–445. doi: 10.1016/s0885-3924(00)00142-1. [DOI] [PubMed] [Google Scholar]
- Syrjala KL, Artherholt SB, Kurland BF, Langer SL, Roth-Roemer S, Elrod JB, Dikmen S. Prospective Neurocognitive Function Over 5 Years After Allogeneic Hematopoietic Cell Transplantation for Cancer Survivors Compared With Matched Controls at 5 Years. J Clin Oncol. 2011;29:2397–2404. doi: 10.1200/JCO.2010.33.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. 3-dimensional proportional system: An approach to cerebral imaging. Thieme; New York: 1988. [Google Scholar]
- Tannock IF, Ahles TA, Ganz PA, van Dam FS. Cognitive Impairment Associated With Chemotherapy for Cancer: Report of a Workshop. J Clin Oncol. 2004;22:2233–2239. doi: 10.1200/JCO.2004.08.094. [DOI] [PubMed] [Google Scholar]
- Tulsky DS, Price LR. The joint WAIS-III and WMS-III factor structure: Development and cross-validation of a six-factor model of cognitive functioning. Psychol Assess. 2003;15:149–162. doi: 10.1037/1040-3590.15.2.149. [DOI] [PubMed] [Google Scholar]
- van Dam FS, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, Rodenhuis S. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90:210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB. Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol. 2008;19:623–629. doi: 10.1093/annonc/mdm500. [DOI] [PubMed] [Google Scholar]
- Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain, behavior, and immunity. 2010;24(6):968–74. doi: 10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Williams LA, Krishnan S, Liao Z, Liu P, Mao Li, et al. Serum sTNF-R1, IL-6, and the development of fatigue in patients with gastrointestinal cancer undergoing chemoradiation therapy. Brain, behavior, and immunity. 2012 doi: 10.1016/j.bbi.2011.12.007. e-pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Witgert ME, Meyers CA. Neuropsychological sequelae of non-central nervous system cancer and cancer therapy. Neuropsychol.Rev. 2008;18:121–131. doi: 10.1007/s11065-008-9058-x. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.