Abstract
Objectives/hypotheses
Regenerative properties of age-associated changes in the intrinsic laryngeal muscles following injury are unclear. The purpose of this study was to investigate the regenerative properties of the thyroarytenoid muscle (TA) in an aging rat model. The hypothesis was that, following myotoxic injury, old animals would exhibit a decrease in mitotic activities of muscle satellite cells when compared with younger rats, suggesting reduced regenerative potential in the aging rat TA.
Study Design
Animal group comparison.
Method
Regeneration responses following injury to the TA were examined in 18 young adult, middle-aged, and old Fischer 344/Brown Norway rats. TA muscle fiber cross sectional area (CSA), satellite cell mitosis (number/fiber), and regeneration index (CSA injured side/CSA non-injured side) were measured and compared across age groups.
Results
Young animals had a significantly higher regeneration index than the middle-aged and old groups. Within the lateral region of the TA (LTA), the regeneration index was significantly higher in the young animals than in the middle-aged and old animals. The regeneration index of the medial TA (MTA) was significantly higher than the LTA across all age groups.
Conclusions
The regenerative capacity of the TA muscle is impaired with increasing age.
Evidence
N/A
Keywords: voice/dysphonia, larynx, aging, thyroarytenoid muscle, satellite cells, regeneration, mitosis, rat
Introduction
The population of elderly people continues to grow rapidly. In 2010, the United States population aged 65 years and older was estimated at over 40 million, representing 13% of the population (http://www.census.gov). By 2050, it is projected that over 88.5 million people will be 65 years and older, based on US Census projections released in 2008, representing 20% of the total US population (Census 2000 data; http://www.census.gov/). Manifestations of physical degeneration, such as fatigue and weakness negatively affect the lives of elderly people.1 Despite evidence that aging affects physical functions, mechanisms that account for those changes have not been well defined.2–4 Accordingly, an increased understanding of physiologic changes involved in the aging process is warranted. Of particular interest in this work was physiologic changes with aging that underlie deficits in vocalization, swallowing and airway patency.
A primary problem associated with aging may lie in the degeneration of the musculoskeletal system. Muscles of aged animals are more susceptible to contraction-induced injuries, which may have a role in muscle atrophy and the decrease in muscle function seen with age.5 In fact, contraction-induced injuries may be the most common mechanism underlying skeletal muscle damage associated with daily activities.6, 7 The repair of damaged skeletal muscles is critical for muscular force production and resulting movements. It has been shown in limb muscles that the capacity for such muscle repair may be less efficient or less complete in older individuals.8–15 It is unclear if the same loss of efficiency with muscle repair applies to the muscles of the larynx.
Previous studies of the aging larynx have reported alterations in laryngeal structure and function including decrease in total mass of laryngeal muscles, decrease in number of fibers in laryngeal muscles, alterations in myosin heavy chain (MHC) isoforms, alterations in neuromuscular junctions morphology, and decreased magnitude of electromyographic activity.16–20 In contrast to limb skeletal muscle, little is known about the role of satellite cells in the aging thyroarytenoid muscle (TA), the muscle comprising the bulk of the vocal folds. Satellite cells are a dynamic population of muscle-specific stem cells located between the sarcolemma of the myofiber and the basal membrane.21–23 Normally quiescent in adult muscle, satellite cells are activated in response to exercise, myofiber injury, and denervation.24, 25 Upon activation, satellite cells enter the cell cycle, and begin the processes of proliferation, differentiation, and self-renewal.26 Thus, satellite cells are a mediator for replacement or repair of lost or damaged muscle fibers and self-renewal in mature animals.27, 28 A sufficient pool of satellite cells with proliferative potential is necessary for successful repair of damaged muscle fibers. A number of investigations have reported that both the frequency and proliferative ability of satellite cells are diminished in an age-dependent manner in limb muscles in both humans and mammals.29–35 Age-related changes within the satellite cell microenvironment, greatly affect proliferation and function, including changes in myofibers, basal lamina, vascular, neural, interstitial, and systemic factors (for review, please see Gopinath and Rando, 2008).36 As such, intrinsic age-associated changes in the satellite cell population and extrinsic environmental factors may be major determinants contributing to the decline in the regeneration capacity of aged skeletal muscles.
The laryngeal muscles, unlike limb skeletal muscles, retain an activated population of satellite cells.37 Proliferating satellite cells in the aged human thyroarytenoid may be related to age-related increases in muscle fiber damage or death.38 In addition, aged TA muscle may undergo constant myonuclear addition with continual satellite cell proliferation and myofiber fusion in the uninjured state, suggesting continual fiber remodeling or turnover of myonuclei.39 It has been shown that there is a concurrent reduction of the absolute number of satellite cells in aging TA muscle, suggesting an age-related loss in the regeneration potential.40 Conclusions, however, cannot be made solely based on this observation because the proliferative potential of satellite cells, one of the major determinants of muscle regeneration, is unclear in the TA muscle.
The purpose of this study was to quantify the mitotic behavior of muscle satellite cells and muscle repair response after injury in the TA muscle in young, middle-aged, and old rats. Our hypothesis was that with age the thyroarytenoid muscle would exhibit decreased regeneration following myotoxic injury.
Materials and Methods
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Madison (Madison, WI) and were performed in accordance with the United States Public Health Service Policy on Humane Care and Use of laboratory Animals, the National Institute of Health Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.)
Eighteen male Fischer 344/Brown Norway rats were used in this study. Six animals were studied in each of 3 age groups: 6 months old (young adult), 24 months old (middle-aged), and 32 months old (old). All animals were purchased from National Institute of Aging (NIA) animal colony and housed at the University of Wisconsin-Madison School of Medicine and Public Health (UWSMPH) animal care unit. Animals were transferred to the UWSMPH at least 2 weeks before the experiment began. Animals were placed in a 12-hour dark and light cycle and food and water were provided ad libitum. Rats were anesthetized via intraperitoneal injection of a mixture of ketamine (90mg/kg) with 1% xylazine (9mg/kg). Laryngeal movement was visualized during quiet breathing via microlaryngoscopic technique41, 42 to ensure proper vocal fold motion and to exclude the presence of any pathological abnormalities. To induce acute muscle fiber degeneration, the myotoxic agent, bupivacaine hydrochloride (BPVC, 30 μL of 0.75%, Astra USA, Westborough, MA) was injected unilaterally into the mid-belly of the left TA muscle of each rat. BPVC is a myotoxic agent that induces acute muscle fiber degeneration, presumably by binding to sarcolemma and affecting Ca++ uptake.16, 43, 44 The muscle injury caused by BPVC is transient and followed by rapid, near-complete recovery of the muscle tissue.45, 46 The satellite cell population, basal lamina, peripheral nerves and blood supply are preserved, all of which are essential for successful muscle regeneration.43, 44,47 Myotoxin muscle injury was confirmed by observation of paralysis of the vocal fold under microlaryngoscopy. In most of the rats studied, unilateral vocal fold paralysis on the injected side became apparent either immediately or within 3 or 4 seconds. Complete vocal fold paralysis on the injured side and reduced vocal fold motion on the contralateral side were observed in one rat.
Immediately after the BPVC injection, a BrdU-filled mini-osmotic pump (Alzet model 2ML1, Durect Corporation, Cupertino, CA) releasing approximately 10 μL/hr, 10 μg/μL BrDu, (Sigma, MO) (at a concentration of 125 μg/g of body weight) was implanted subcutaneously in the inter scapular area to identify the division history of muscle cells for 7 days.48
Seven days post-injury, the pump was removed and the surgical opening was re-closed with skin clips. Ten days post-injury, laryngeal motion was visualized and recorded as described previously. With the exception of 2 rats that died under anesthesia, complete recovery of function was observed at the time of euthanasia. Symmetrical or near-symmetrical bilateral vocal fold motion was observed in all rats. Rats were then euthanized, the larynx was harvested, rinsed in physiological saline, snap-frozen in liquid nitrogen precooled in 2-methylbutane, and cryosectioned. Eight μm thickness serial coronal sections of the entire larynx were made starting from the most anterior aspect of the thyroid cartilage using a cryostat (Leica 2135, Boston, Massachusetts) at −22°C and mounted on glass slides (Fisher Scientific, Pittsburgh, PA). Indirect immunohistochemical labeling of laryngeal sections representing the mid-belly of the TA muscle was performed, using antibodies directed against BrdU and laminin. Sections from the mid-belly of the larynx were air-dried for 30 minutes, washed in phosphate buffered saline (PBS), and fixed with 1% paraformaldehyde for 10 minutes. To inhibit endogenous peroxidase activation sections were incubated with 3% H2O2 in methanol for 5 minutes. Sections were then treated with 0.1% triton in PBS for 15 minutes and then with 1% triton in PBS and in 4N Hydrochloric Acid (HCl) for 30 minutes to permeabilize of the membrane and to denature the DNA, respectively. Following wash in PBS, sections were blocked for 2 hours in 5% goat serum in PBS, then washed in PBS. Anti-BrdU mouse monoclonal antibody (ICN Biomedicals, Irvine, CA) was applied to sections at a concentration of 1: 20 in 0.1 % tween (Sigma, St. Louis, MO) in PBS overnight at 4° C. Slides for negative control contained the diluent only, omitting the anti-BrdU antibody. No reaction to BrdU was detected in the slides for negative control. A secondary goat anti-mouse IgG peroxidased antibody (Jackson ImmunoResearch, West Grove, PA) was applied at 1: 500 in PBS for 1 1/2 hour at room temperature. Subsequently, after sections were washed in PBS 5 minutes 3 times, 3'-diaminobenzidine substrate (Sigma, St. Louis, MO) was applied to the sections. Laminin antibody staining was used to demarcate muscle fiber cross-sections, thus allowing for identification of the muscle fibers and measurement of cross-sectional area. For the laminin double staining, sections were incubated with Glycine-HCl for 1 hour, fixed with 1% PAF 10 min to stabilize the initial staining and incubated with rabbit anti-laminin polyclonal antibody (Sigma, St. Louis, MO). After washed in PBS for 5 minutes 3 times, Cy3-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA) in a concentration of 1: 200 in PBS was applied to the sections for 1 hour, sections were then counterstained with 0.4% Methyl Green in PBS (Sigma, St. Louis, MO) for 4 minutes to stain all cell nuclei. Slides for negative control contained the diluent only, omitting the anti-BrdU antibody. No reaction to BrdU was detected in the slides for negative control.
After immunohistochemical staining, micrographs were collected from 80 slides using a camera-assisted microscope. One image from each stained section was captured at both 20× and 40× using a Nikon Eclipse E600 microscope (Nikon, Melville, NY), a Pixera color camera (Pixera, Los Gatos, CA) and Metamorph Image Analysis Software (Universal Imaging, West Chester, PA). The two different levels of magnification were alternately used for verification and manual counting of mitotic satellite cells, as described below.
Mitotic satellite cells involved in muscle fiber repair were identified as cells that stained positive for both BrdU and methyl green (Figure 1)49, 50 and located on the periphery of the muscle fibers immediately adjacent to laminin staining (Figure 2). This enumeration method ensured that only those BrdU-stained nuclei fused to muscle fibers were counted. Satellite cell mitosis was expressed as the number of labeled nuclei per muscle fiber. To evaluate TA muscle regeneration, areas of intense degeneration and regeneration of the injured side were selected and measured with Image J (NIH, Bethesda, MD). The areas of degeneration and regeneration were determined by the presence of multiple BrdU-stained cells. The cross-sectional areas of muscle fibers (CSA) were selected as a recovery parameter in this study because muscle fiber CSA is directly related to measures of muscle strength.51 Further, increases in muscle fiber CSA following strength training are associated with increases in satellite cell number.52 Using laminin staining as a marker for cell boundary, muscle fiber CSAs were measured from the areas of intense degeneration and regeneration on the injured side and from the corresponding areas on the contralateral uninjured side. The area of injury and subsequent repair was variable among individual animals. As a result, the number of fibers included for analysis varied for each animal, and were normalized for measures of regeneration (mitosis=cell number per fiber, regeneration index=CSA injured side/CSA uninjured side). The Regeneration Index (RI) was expressed as a ratio between the mean muscle fiber CSA of the injured side to the mean muscle fiber CSA of the contralateral uninjured side.14 The extent of mitosis and regeneration were determined for the whole TA (MTA + LTA), the medial TA (MTA), and the lateral TA (LTA).
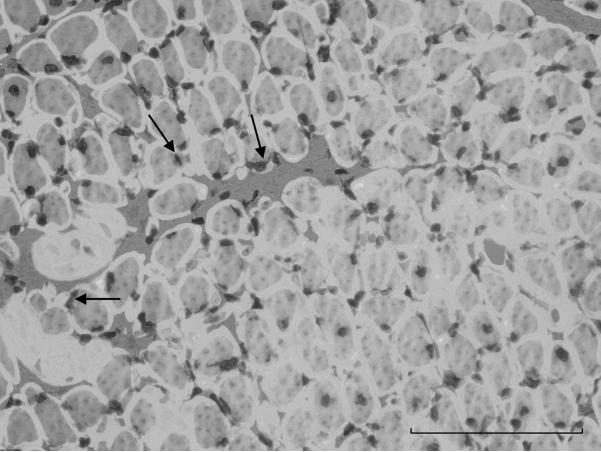
Figure 1.
Photomicrograph of a cross-section of injured side of the TA. Taken at 40× magnification. Arrows point to examples of double-stained cells in reaction to BrdU and methyl green. Scale bar represents 100 μm.
Figure 2.
Composed image of cross-section of injured TA. Taken at 40× magnification. Arrows point to examples of muscle satellite cells. Scale bar represents 100 μm.
Analysis of variance (ANOVA) with Fisher's protected least significant difference tests was used to compare muscle satellite cell mitosis and regeneration across the young, middle-aged, and old animal groups. Both means and standard deviation (SD) were obtained and reported for each measure for all groups. A criterion α level of less than 0.05 was used to determine statistical significance based on two-tailed tests. All analyses were performed using SAS statistical software (SAS Institute Inc., Cary, NC). All measurements, including those for reliability were performed on data masked for group identifying characteristic by a single observer. Intra-rater reliability of measurement was assessed by replicating measurement on 10% of the data randomly selected from the total data set, and subjecting these data to a paired t-test. The difference between the initial measurement and the repeated measurement did not reach significance indicating intra-rater reliability was acceptable (p=0.16).
RESULTS
BPVC treatment to the TA successfully injured muscle fibers as demonstrated by an increase in BrdU-positive cells (Figures 3(A), 3(B)) and, qualitatively, greater variability in muscle fiber size, including many with a smaller diameter than those found in the contralateral uninjured side (Figures 4(A), 4(B)). The area of injury and subsequent repair was variable across individual animals (Figure 5(A), 5(B)). The number of fibers measured in each animal ranged from 153 to 742. There was not a significant difference in the number of fibers analyzed across age groups (p> .05).
Figure 3.
Photomicrographs of the (A) injured side and the (B) uninjured contralateral side of the TA. Taken at 4× magnification 10 days post injury. TA=thyroarytenoid muscle. On the injured side (A), there are numerous BrdU-stained cells visible, suggesting a recent cycle of degeneration-regeneration, which is in direct contrast to the contralateral side (B) where few BrdU-stained cells are observed. Scale bar represents 400 μm.
Figure 4.
Photomicrographs of cross-sections of the injured side (A) and contralateral side (B) of the TA. Taken at 20× magnification 10 days post injury. On the injured side (A), muscle fibers are smaller and varied more in size and shape than those of the non-injured, contralateral side (B). Scale bar represents 200 μm.
Figure 5.
Photomicrographs of the injured side of the TA from two different rats. In (A), the area of regeneration is more localized than that of (B) in which more diffuse area of regeneration is observed. Taken at 4× magnification. TA=thyroarytenoid muscle. Scale bar represents 400 μm.
The relatively short time period selected to analyze the regenerative capacity of the TA focused on the early events of satellite cell activation and proliferation. Continuous BrdU infusion over 7 days following injury provided a cumulative history of all satellite cell mitotic divisions by showing the location of activated satellite cells and the myonuclei they produced. Overall, there were no age-associated differences in the extent of muscle cell mitotsis during repair following myotoxic injury in the TA (F2,15=2.76; p=.10).
The regeneration index (RI) indicated the level of recovery of the formed fibers following myotoxic injury (for review of measurement, see Methods). Significant differences in RI were observed between age groups (F2,15=2, 15 = 7.96, p=0.004). Six-month-old animals demonstrated a significantly higher RI (mean RI= 0.78 [SD= 0.08]) than the 24-month-old (mean RI = 0.60 [SD = 0.1]; LSD p = .002), and 32-month-old animals (mean RI = 0.64 [SD = 0.05]; LSD p = .01) animals, indicating faster recovery in the young adult group.
The medial (MTA) and lateral (LTA) portions of the TA muscle were examined. No significant differences were found for the MTA regeneration indices between any age groups (mean RI 6 mo = 0.94 [SD=.06]; mean RI 24 mo =0.80 [SD=0.28]; mean RI 32 mo = 0.90 [SD=.06]; F2,9=1.36, p=.31). However, the regeneration index was significantly higher in the LTA of 6-month-old animals (mean RI = 0.68 [SD=0.12]) than the 24-month-old (mean RI= 0.54 [SD=0.11] and the 32-month-old animals (mean RI= 0.55 [SD=0.07]; F2,15=3.57, p=.05; LSD paired comparisons 6 mo vs. 24 mo; p= .03; 6 mo vs. 32 mo, p= .04). These findings indicate that the actual regeneration responses of the LTA, as measured with mean fiber CSA, were age-associated (Figures 6(A), 6(B)). Additionally, the MTA regeneration index (mean regeneration index of 0.89[SD=0.12]) was significantly higher than in the LTA (mean regeneration index of 0.57[SD=0.11]; t11=7.9; p<0.0001), suggesting greater recovery of muscle fiber CSA in the MTA than in the LTA following the injury.
Figure 6.
Regeneration index (mean muscle fiber cross-sectional area on the injured side/contralateral side) of the LTA. In (A), values are mean ± SD with error bars representing standard deviations. In (B), individual data points are plotted per each age group. CSA=cross-sectional area. Significant differences were observed between group 1 (6-month-old rats) and group 2 (24-month-old rats) and between group 1 and group 3 (32-month-old rats) (p=0.03, p=0.04, respectively). N=6 in each age group.
Discussion
The purpose of this study was to compare thyroarytenoid muscle repair between young adult (6 month), middle-aged (24 month), and old (32 month) rats. We hypothesized the aged TA muscle would exhibit decreased regeneration capabilities following myotoxic injury. Our results demonstrated an age-associated reduction in muscle fiber recovery after injury. TA muscles, particularly the LTA, from young rats exhibited a significantly higher regeneration index than the middle-aged and old adult rats. The differences we observed in regenerative capacity between the LTA and MTA may be explained in part by their anatomic and functional differences and this should be explored further. In this study, data suggested that the LTA may be more sensitive to aging based on the regeneration index.
Because satellite cells function to replace damaged muscle fibers, these results suggest that there may be a decrease in the satellite cell regenerative potential with aging within the TA muscle that contributes to reduced muscle regeneration in old animals. However, we did not find a significant age effect when examining early stages of satellite cell mitosis. This finding may be due to our small sample size, the sensitivity of our measure of mitosis, or our short time period of investigation. Examination of formed myofibers in the aged environment beyond 10 days following injury would allow examination of how extrinsic factors associated with neural input and vascular supply support the regeneration outcome and should be considered in future research.
Few studies have examined the effects of aging on the early cellular events of muscle regeneration in the TA muscle, namely activation and proliferation of satellite cells on the TA muscle. Numerous investigations in human limb and animal hindlimb suggest that the aging process affects satellite cell number and the proliferative potential, 29, 30, 35, 53,54–57 particularly when motor innervation to the muscle was impaired.58, 59 However, most studies examined the satellite cell populations in a static state.29, 30, 34, 35, 56 To investigate cellular activation and proliferation for regeneration, the satellite cells must be activated with a myotoxin or other mechanism, as was done in the present study,in the TA muscle. Disparity in results of this study to prior work in the hindlimb may be due to differential effects of aging between the limb and cranial muscles,60 the increased proliferative and regenerative capacity of laryngeal satellite cells over limb muscle satellite cells61 or other differences between satellite cells in limb and laryngeal muscles.62
There are several age-related changes within the TA muscle that also occur within limb muscles, while some alterations appear specific to the TA (For review, see Thomas et al., 2008).63 One important consideration in evaluating age-related influences on satellite cell activation and proliferation in different muscles is muscle fiber type. An age-related decline was found in slowly contracting Type I fibers of the human TA in the ratio of the satellite cells to myonuclei, but this was not found in rapidly-contracting Type II muscle fibers.40, 64 Therefore, alterations in muscle fiber type that occur with aging must be carefully considered. Because muscle fibers within the larynx may transform to a slower contracting fiber type with age,65 an age-related decline in satellite cell function within slowly-contracting muscle fibers may be one factor associated with reduced TA muscle regeneration, as we found in the regeneration index in this study.
Human adult TA muscles exhibit spontaneous fiber regeneration associated with age-related muscle fiber loss.18, 38 In addition, continuous repair and remodeling of muscle fibers has been documented in the uninjured TA of adult rabbits. Specifically, the TA of mature rabbits exhibited continuous addition of new myonuclei to the existing muscle fibers, suggesting the existence of on-going low-level injury in the TA.39 In this study, age-matched control groups that did not receive myotoxic injury to the TA were not included and thus we were not able to investigate the level of activated satellite cells retained in the TA in an uninjured state. These studies will be performed in future research. Processes that result in spontaneous fiber regeneration would put constant proliferative demand on the satellite cell population. With aging, a decline in satellite cell function would negatively impact the regenerative response, as suggested by the results of the present study.
The decline in the regenerative capability of laryngeal muscles with aging may be an underlying mechanism contributing to changes observed in laryngeal anatomy and physiology. Previous investigations showed that aged had altered laryngeal-respiratory kinematics, changes in TA neuromuscular connections, and differences in myosin heavy chain composition in several intrinsic laryngeal muscles when compared with young adult rats.19, 42, 65–68 Furthermore, significant age-related vascular changes within the microcirculation of the TA muscle have been found in rats 28–30 months old compared with 9-month-old rats.69 These observations, in addition to the findings of the present study, suggest that structural and functional alterations occur with aging and become evident by the age of 24 months in a rat model. Vascular and neural factors contribute not only to age-related functional changes in laryngeal-respiratory kinematics in normal muscles of aged animals but also to the reduced regeneration of myofibers and their subsequent development.
Muscle denervation may contribute to age-related changes in muscle mass (for review, see Doherty)70 and denervation-like changes have been reported in aged rat TA muscles.19, 67, 71, 72 Although satellite cell activation still occurs in denervated laryngeal muscles there is a decrease in the satellite cell population resulting in either regenerative myogenesis through compensatory mechanisms or the deterioration of regenerative capacity.73 Thus, denervation or denervation-like changes in muscle with aging may affect regenerative capacity and should be examined directly in future studies.
A potential clinical implication of prior research, together with the results of the present study, is that an age-associated decline in the TA muscle regenerative capacity may contribute to the TA muscle fiber loss observed in elderly people.18 While the TA muscle appears to maintain regeneration capabilities throughout adulthood to compensate for fiber loss associated with on-going injury or disease, the repair response of the TA appears to be age-dependent, exhibiting a lower level of regeneration with aging. Given that the muscles of older animals are more prone to injury,7 one might postulate that the age-associated decline in the regeneration capacity of the TA may be detrimental to key functions of the larynx, including airway protection, voice production and respiration, in elderly people.
Acknowledgments
The authors gratefully acknowledge the contributions of Glen Leverson, PhD, for statistical analyses..
This work was funded by grants from the NIDCD (R01DC005935, R01DC008149). No other financial or material support were was provided and there are no conflicts to report.
References
- 1.Knapowski J, Wieczorowska-Tobis K, Witowski J. Pathophysiology of ageing. J Physiol Pharmacol. 2002;53(2):135–146. [PubMed] [Google Scholar]
- 2.Shigemoto K, Kubo S, Mori S, Yamada S, Akiyoshi T, Miyazaki T. Muscle weakness and neuromuscular junctions in aging and disease. Geriatr Gerontol Int. 2010;10(Suppl 1):S137–S147. doi: 10.1111/j.1447-0594.2010.00608.x. [DOI] [PubMed] [Google Scholar]
- 3.Boirie Y. Physiopathological mechanism of sarcopenia. J Nutr Health Aging. 2009;13(8):717–723. doi: 10.1007/s12603-009-0203-x. [DOI] [PubMed] [Google Scholar]
- 4.Vinciguerra M, Musaro A, Rosenthal N. Regulation of muscle atrophy in aging and disease. Adv Exp Med Biol. 2010;694:211–233. doi: 10.1007/978-1-4419-7002-2_15. [DOI] [PubMed] [Google Scholar]
- 5.Faulkner JA, Brooks SV, Zerba E. Muscle atrophy and weakness with aging: contraction-induced injury as an underlying mechanism. J Gerontol A Biol Sci Med Sci. 1995;50(Spec):124–129. doi: 10.1093/gerona/50a.special_issue.124. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. Journal of Applied Physiology. 1983;54(1):80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- 7.Zerba E, Komorowski TE, Faulkner JA. Free radical injury to skeletal muscles of young, adult, and old mice. American Journal of Physiology. 1990;258(3 PT 1):C429–C435. doi: 10.1152/ajpcell.1990.258.3.C429. [DOI] [PubMed] [Google Scholar]
- 8.Jarvinen M, Aho AJ, Lehto M, Toivonen H. Age dependent repair of muscle rupture. A histological and microangiographical study in rats. Acta Orthop Scand. 1983;54(1):64–74. doi: 10.3109/17453678308992871. [DOI] [PubMed] [Google Scholar]
- 9.Sadeh M. Effects of aging on skeletal muscle regeneration. J Neurol Sci. 1988;87(1):67–74. doi: 10.1016/0022-510x(88)90055-x. [DOI] [PubMed] [Google Scholar]
- 10.Brooks SV, Faulkner JA. Contraction-induced injury: recovery of skeletal muscles in young and old mice. American Journal of Physiology. 1990;258(3) doi: 10.1152/ajpcell.1990.258.3.C436. Pt 1. [DOI] [PubMed] [Google Scholar]
- 11.McBride TA, Gorin FA, Carlsen RC. Prolonged recovery and reduced adaptation in aged rat muscle following eccentric exercise. Mech Ageing Dev. 1995;83(3):185–200. doi: 10.1016/0047-6374(95)01629-e. [DOI] [PubMed] [Google Scholar]
- 12.Marsh DR, Criswell DS, Carson JA, Booth FW. Myogenic regulatory factors during regeneration of skeletal muscle in young, adult, and old rats. Journal of Applied Physiology. 1997;83(4):1270–1275. doi: 10.1152/jappl.1997.83.4.1270. [DOI] [PubMed] [Google Scholar]
- 13.Marsh DR, Criswell DS, Hamilton MT, Booth FW. Association of insulin-like growth factor mRNA expressions with muscle regeneration in young, adult, and old rats. American Journal of Physiology. 1997;273(1 PT 2):R353–R358. doi: 10.1152/ajpregu.1997.273.1.R353. [DOI] [PubMed] [Google Scholar]
- 14.Carlson BM, Faulkner JA. Muscle regeneration in young and old rats: effects of motor nerve transection with and without marcaine treatment. J Gerontol A Biol Sci Med Sci. 1998;53(1):B52–B57. doi: 10.1093/gerona/53a.1.b52. [DOI] [PubMed] [Google Scholar]
- 15.Marsh DR, Hinds LR, Lester WS, Reinking BE, Booth FW. The force-frequency relationship is altered in regenerating and senescent rat skeletal muscle. Muscle Nerve. 1998;21(10):1265–1274. doi: 10.1002/(sici)1097-4598(199810)21:10<1265::aid-mus4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Honjo I, Isshiki N. Laryngoscopic and Voice Characteristics of Aged Persons. Archives of Otolaryngology. 1980;106:149–150. doi: 10.1001/archotol.1980.00790270013003. [DOI] [PubMed] [Google Scholar]
- 17.Sato T, Tauchi H. Age changes in human vocal muscle. Mech Ageing Dev. 1982;18(1):67–74. doi: 10.1016/0047-6374(82)90031-8. [DOI] [PubMed] [Google Scholar]
- 18.Malmgren LT, Fisher PJ, Bookman LM, Uno T. Age-related changes in muscle fiber types in the human thyroarytenoid muscle: An immunohistochemical and stereological study using confocal laser scanning microscopy. Otolaryngology. 1999;121(4):442–451. doi: 10.1016/S0194-5998(99)70235-4. [DOI] [PubMed] [Google Scholar]
- 19.Connor NP, Suzuki T, Lee K, Sewall GK, Heisey DM. Neuromuscular junction changes in aged rat thyroarytenoid muscle. Ann Otol Rhinol Laryngol. 2002;111(7 PT 1):579–586. doi: 10.1177/000348940211100703. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki T, Connor NP, Lee K, Bless DM, Ford CN, Inagi K. Age-related alterations in myosin heavy chain isoforms in rat intrinsic laryngeal muscles. Ann Otol Rhinol Laryngol. 2002;111(11):962–967. doi: 10.1177/000348940211101102. [DOI] [PubMed] [Google Scholar]
- 21.Ambrosio F, Kadi F, Lexell J, Fitzgerald GK, Boninger ML, Huard J. The effect of muscle loading on skeletal muscle regenerative potential: an update of current research findings relating to aging and neuromuscular pathology. Am J Phys Med Rehabil. 2009;88(2):145–155. doi: 10.1097/PHM.0b013e3181951fc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mauro A. Satellite cell of skeletal muscle fibers. Journal of Biophysical and Biochemical Cytology. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muir AR, Kanji AH, Allbrook D. The structure of the satellite cells in skeletal muscle. J Anat. 1965;99(PT 3):435–444. [PMC free article] [PubMed] [Google Scholar]
- 24.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54(11):1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- 25.Schultz E, Gibson MC, Champion T. Satellite cells are mitotically quiescent in mature mouse muscle: An EM and radiographic study. Journal of Experimental Zoology. 1978;206:451–456. doi: 10.1002/jez.1402060314. [DOI] [PubMed] [Google Scholar]
- 26.Ten Broek RW, Grefte S, Von den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol. 2010;224(1):7–16. doi: 10.1002/jcp.22127. [DOI] [PubMed] [Google Scholar]
- 27.Schultz E. Satellite cell behavior during skeletal muscle growth and regeneration. Med Sci Sports Exerc. 1989;21(5 SUPPL):S181–S186. [PubMed] [Google Scholar]
- 28.Schultz E, McCormick KM. Cell biology of the satellite cell. In: Partridge T, editor. Molecular and Cell Biology of Muscular Dystrophy. Champman & Hall; London: 1993. pp. 190–207. [DOI] [PubMed] [Google Scholar]
- 29.Snow MH. A quantitative ultrastructural analysis of satellite cells in denervated fast and slow muscles of the mouse. Anatomical Record. 1983;207(4):593–604. doi: 10.1002/ar.1092070407. [DOI] [PubMed] [Google Scholar]
- 30.Gibson MC, Schultz E. Age-related differences in absolute numbers of skeletal muscle satellite cells. Muscle Nerve. 1983;6(8):574–580. doi: 10.1002/mus.880060807. [DOI] [PubMed] [Google Scholar]
- 31.Monemi M, Eriksson PO, Kadi F, Butler-Browne GS, Thornell LE. Opposite changes in myosin heavy chain composition of human masseter and biceps brachii muscles during aging. J Muscle Res Cell Motil. 1999;20(4):351–361. doi: 10.1023/a:1005421604314. [DOI] [PubMed] [Google Scholar]
- 32.Nnodim JO. Satellite cell numbers in senile rat levator ani muscle. Mech Ageing Dev. 2000;112(2):99–111. doi: 10.1016/s0047-6374(99)00076-7. [DOI] [PubMed] [Google Scholar]
- 33.Renault V, Rolland E, Thornell LE, Mouly V, Butler-Browne G. Distribution of satellite cells in the human vastus lateralis muscle during aging. Exp Gerontol. 2002;37(12):1513–1514. doi: 10.1016/s0531-5565(02)00095-5. [DOI] [PubMed] [Google Scholar]
- 34.Sajko S, Kubinova L, Cvetko E, Kreft M, Wernig A, Erzen I. Frequency of M-cadherin-stained satellite cells declines in human muscles during aging. J Histochem Cytochem. 2004;52(2):179–185. doi: 10.1177/002215540405200205. [DOI] [PubMed] [Google Scholar]
- 35.Schultz E, Lipton BH. The effect of Marcaine on muscle and non-muscle cells in vitro. Anatomical Record. 1978;191(3):351–369. doi: 10.1002/ar.1091910308. [DOI] [PubMed] [Google Scholar]
- 36.Gopinath SD, Rando TA. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7(4):590–598. doi: 10.1111/j.1474-9726.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- 37.McLoon LK, Rowe J, Wirtschafter J, McCormick KM. Continuous myofiber remodeling in uninjured extraocular myofibers: myonuclear turnover and evidence for apoptosis. Muscle Nerve. 2004;29(5):707–715. doi: 10.1002/mus.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malmgren LT, Lovice DB, Kaufman MR. Age-related changes in muscle fiber regeneration in the human thyroarytenoid muscle. Arch Otolaryngol Head Neck Surg. 2000;126(7):851–856. doi: 10.1001/archotol.126.7.851. [DOI] [PubMed] [Google Scholar]
- 39.Goding GSJ, Al-Sharif KI, McLoon LK. Myonuclear addition to uninjured laryngeal myofibers in adult rabbits. Annals of Otology, Rhinology & Laryngology. 2005;114(7):552–557. doi: 10.1177/000348940511400711. [DOI] [PubMed] [Google Scholar]
- 40.Malmgren LT, Fisher PJ, Christine EJ, Bookman LM, Uno T. Numerical densities of myonuclei and satellite cells in muscle fiber types in the aging human thyroarytenoid muscle: An immunohistochemical and stereological study using confocal laser scanning microscopy. Otolaryngol Head Neck Surg. 2000;123:377–384. doi: 10.1067/mhn.2000.109487. [DOI] [PubMed] [Google Scholar]
- 41.Inagi K, Connor NP, Ford CN, et al. Physiologic assessment of botulinum toxin effects in the rat larynx. Laryngoscope. 1998;108(7):1048–1054. doi: 10.1097/00005537-199807000-00018. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki T, Connor NP, Lee K, Leverson G, Ford CN. Laryngeal-respiratory kinematics are impaired in aged rats. Ann Otol Rhinol Laryngol. 2002;111:684–689. doi: 10.1177/000348940211100805. [DOI] [PubMed] [Google Scholar]
- 43.Hall-Craggs EC. Rapid degeneration and regeneration of a whole skeletal muscle following treatment with bupivacaine (Marcain) Exp Neurol. 1974;43(2):349–358. doi: 10.1016/0014-4886(74)90176-9. [DOI] [PubMed] [Google Scholar]
- 44.Foster AH, Carlson BM. Myotoxicity of local anesthetics and regeneration of the damaged muscle fibers. Anesth Analg. 1980;59(10):727–736. [PubMed] [Google Scholar]
- 45.Saito Y, Nonaka I. Initiation of satellite cell replication in bupivacaine-induced myonecrosis. Acta Neuropathol (Berl) 1994;88(3):252–257. doi: 10.1007/BF00293401. [DOI] [PubMed] [Google Scholar]
- 46.Louboutin JP, Fichter-Gagnepain V, Noireaud J. External calcium dependence of extensor digitorum longus muscle contractility during bupivacaine-induced regeneration. Muscle Nerve. 1996;19(8):994–1002. doi: 10.1002/(SICI)1097-4598(199608)19:8<994::AID-MUS7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 47.Nonaka I, Takagi A, Ishiura S, Nakase H, Sugita H. Pathophysiology of muscle fiber necrosis induced by bupivacaine hydrochloride (Marcaine) Acta Neuropathol (Berl) 1983;60(3–4):167–174. doi: 10.1007/BF00691863. [DOI] [PubMed] [Google Scholar]
- 48.Mozdziak PE, Pulvermacher PM, Schultz E. Unloading of juvenile muscle results in a reduced muscle size 9 wk after reloading. Journal of Applied Physiology. 2000;88(1):158–164. doi: 10.1152/jappl.2000.88.1.158. [DOI] [PubMed] [Google Scholar]
- 49.Hardonk MJ, Harms G. The use of 5'-bromodeoxyuridine in the study of cell proliferation. Acta Histochemica Supplementband. 1990;39:99–108. [PubMed] [Google Scholar]
- 50.Dolbeare F, Selden JR. Immunochemical quantitation of bromodeoxyuridine: application to cell-cycle kinetics. Methods Cell Biol. 1994;41:297–316. [PubMed] [Google Scholar]
- 51.Taaffe DR, Pruitt L, Pyka G, Guido D, Marcus R. Comparative effects of high- and low-intensity resistance training on thigh muscle strength, fiber area, and tissue composition in elderly women. Clinical Physiology. 1996;16:381–392. doi: 10.1111/j.1475-097x.1996.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 52.Kadi F, Thornell LE. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol. 2000;113(2):99–103. doi: 10.1007/s004180050012. [DOI] [PubMed] [Google Scholar]
- 53.Hikida RS, Waslsh S, Barylski N, Campos G, Hagerman FC, Staron RS. Is Hypertrophy Limited in elderly Muscle Fibers? A Comparison of Elderly and Young Strength-Trained Men. Basic and Applied Myology. 1998;8(6):419–427. [Google Scholar]
- 54.Roth SM, Ferrell RF, Hurley BF. Strength training for the prevention and treatment of sarcopenia. J Nutr Health Aging. 2000;4(3):143–155. [PubMed] [Google Scholar]
- 55.Putman CT, Sultan KR, Wassmer T, Bamford JA, Skorjanc D, Pette D. Fiber-type transitions and satellite cell activation in low-frequency-stimulated muscles of young and aging rats. J Gerontol A Biol Sci Med Sci. 2001;56(12):B510–B519. doi: 10.1093/gerona/56.12.b510. [DOI] [PubMed] [Google Scholar]
- 56.Renault V, Thornell LE, Eriksson PO, Butler-Browne G, Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell. 2002;1(2):132–139. doi: 10.1046/j.1474-9728.2002.00017.x. [DOI] [PubMed] [Google Scholar]
- 57.Dedkov EI, Borisov AB, Wernig A, Carlson BM. Aging of skeletal muscle does not affect the response of satellite cells to denervation. J Histochem Cytochem. 2003;51(7):853–863. doi: 10.1177/002215540305100701. [DOI] [PubMed] [Google Scholar]
- 58.Carlson BM, Faulkner JA. The regeneration of noninnervated muscle grafts and marcaine-treated muscles in young and old rats. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 1996;51(1):B43–B49. doi: 10.1093/gerona/51a.1.b43. [DOI] [PubMed] [Google Scholar]
- 59.Carlson BM, Dedkov EI, Borisov AB, Faulkner JA. Skeletal muscle regeneration in very old rats. J Gerontol A Biol Sci Med Sci. 2001;56(5):B224–B233. doi: 10.1093/gerona/56.5.b224. [DOI] [PubMed] [Google Scholar]
- 60.Connor NP, Ota F, Nagai H, Russell JA, Leverson GE. Differences in age-related alterations in muscle contraction properties in rat tongue and hindlimb. J Speech Lang Hear Res. 2008;51:818–827. doi: 10.1044/1092-4388(2008/059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walz PC, Hiatt KK, Naidu M, Halum SL. Characterization of laryngeal muscle stem cell survival and proliferation. Laryngoscope. 2008;118(8):1422–1426. doi: 10.1097/MLG.0b013e318173e188. [DOI] [PubMed] [Google Scholar]
- 62.Harel I, Nathan E, Tirosh-Finkel L, et al. Distinct origins and genetic programs of head muscle satellite cells. Dev Cell. 2009;16(6):822–832. doi: 10.1016/j.devcel.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas LB, Harrison AL, Stemple JC. Aging thyroarytenoid and limb skeletal muscle: Lessons in contrast. J Voice. 2008;22:430–450. doi: 10.1016/j.jvoice.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 64.Malmgren LT, Jones CE, Bookman LM. Muscle fiber and satellite cell apoptosis in the aging human thyroarytenoid muscle: a stereological study with confocal laser scanning microscopy. Otolaryngology. 2001;125(1):34–39. doi: 10.1067/mhn.2001.116449. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki T, Connor NP, Lee K, Bless DM, Ford CN, Inagi K. Age-related alterations in myosin heavy chain isoforms in rat laryngeal muscles. Ann Otol Rhinol Laryngol. 2002;111:962–967. doi: 10.1177/000348940211101102. [DOI] [PubMed] [Google Scholar]
- 66.Nagai H, Ota F, Konopacki R, Connor NP. Discoordination of laryngeal and respiratory movements in aged rats. Am J Otolaryngol. 2005;26(6):377–382. doi: 10.1016/j.amjoto.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 67.McMullen CA, Andrade FH. Functional and morphological evidence of age-related denervation in rat laryngeal muscles. J Gerontol A Biol Sci Med Sci. 2009;64(4):435–442. doi: 10.1093/gerona/gln074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McMullen CA, Andrade FH. Contractile dysfunction and altered metabolic profile of the aging rat thyroarytenoid muscle. Journal of Applied Physiology. 2006;100:602–608. doi: 10.1152/japplphysiol.01066.2005. [DOI] [PubMed] [Google Scholar]
- 69.Russell JA, Nagai H, Connor NP. Effect of aging on blood flow in rat larynx. Laryngoscope. 2008;118:559–563. doi: 10.1097/MLG.0b013e31815bac17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doherty TJ. Aging and Sarcopenia. Journal of Applied Physiology. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 71.Li ZB, Lehar M, Samlan R, Flint PW. Proteomic analysis of rat laryngeal muscle following denervation. Proteomics. 2005;5(18):4764–4776. doi: 10.1002/pmic.200401329. [DOI] [PubMed] [Google Scholar]
- 72.Miyamaru S, Kumai Y, Ito T, Yumoto E. Effects of long-term denervation on the rat thyroarytenoid muscle. Laryngoscope. 2008;118(7):1318–1323. doi: 10.1097/MLG.0b013e31816f693f. [DOI] [PubMed] [Google Scholar]
- 73.Donghui C, Shicai C, Wei W, et al. Functional modulation of satellite cells in long-term denervated human laryngeal muscle. Laryngoscope. 2010;120(2):353–358. doi: 10.1002/lary.20796. [DOI] [PubMed] [Google Scholar]