Abstract
Background
Chronic recurrent sinusitis (CRS) is one of the most common chronic conditions in the United States. There is a significant subpopulation of CRS patients who remain resistant to cure despite rigorous treatment regimens including surgery, allergy therapy and prolonged antibiotic therapy. Antimicrobial photodynamic therapy (aPDT) is a non-invasive non-antibiotic broad spectrum antimicrobial treatment. Our previous in vitro studies demonstrated that aPDT reduced CRS polymicrobial biofilm and planktonic bacteria and fungi by >99.9% after a single treatment. Prior to human treatment however, aPDT treatment must be demonstrated to not result in histologic damage to the sinus ciliated respiratory epithelium. The objective of this study was to demonstrate the safety of aPDT treatment on a living human ciliated respiratory mucosal model (EpiAirway™).
Methods
A study of aPDT treatment of EpiAirway™ was performed. Treatment groups included a non-treatment control, laser light alone, photosensitizer alone and therapeutic photosensitizer and light combination (aPDT). At completion of treatment, the EpiAirway™ tissue was fixed in 10% formalin, paraffin-embedded, sectioned, H &E stained and mounted. All samples were blinded and microscopically examined by a human pathologist to assess any effect of aPDT on the tissue, cilia or mucosal glands. The results were correlated with the treatment parameters.
Results
The EpiAirway™ histologic study demonstrated no histologic alteration of the respiratory cilia or mucosal epithelium in any of the treatment groups.
Conclusions
aPDT is a safe treatment for CRS resulting in no histologic alteration of human ciliated respiratory mucosa as is found in the human sinuses.
Keywords: Antimicrobial photodynamic therapy, chronic recurrent sinusitis, ciliated respiratory epithelium
Introduction
Chronic recurrent sinusitis (CRS) is an inflammatory disease of the facial sinuses and nasal passages that is defined as lasting longer than 12 weeks or occurring more than 4 times per year with symptoms usually lasting more than 20 days.1 The National Institute for Health Statistics estimates that CRS is one of the most common chronic conditions in the United States affecting an estimated 37 million Americans.2 The potential etiologies of CRS include bacteria, viruses, allergies, fungi, superantigens, exotoxins and microbial biofilms. Importantly, CRS is also considered to be a significant factor that can exacerbate asthma, chronic lung diseases, eczema, otitis media and chronic fatigue.4–7
In clinical practice there is a significant subpopulation of patients with CRS who remain resistant to cure despite rigorous treatment regimens including surgery, allergy therapy and prolonged antibiotic therapy.1,8–21 One theory accounting for treatment failure is the destruction of the sinus mucociliary defense by the chronic sinus infection resulting in the development of secondary antibiotic resistant microbial colonization of the sinuses and biofilm formation.1,8–13,20 It is increasingly reported that methicillin resistant Staphylococcus aureus (MRSA) and multidrug resistant Pseudomonas aerugenosa are found in the clinical isolates of CRS patients and are a cause of antibiotic treatment failures.1,8–13
Numerous investigators have reported the presence of biofilms in the sinuses of patients with CRS and consider biofilm as a cause for the recalcitrant nature of persistent CRS.1,8–22 Antibiotic resistant strains of these bacteria also significantly contribute to poor clinical results with the presence of antibiotic resistant bacteria in clinical isolates as high as 30%.23 CRS with its chronic indolent course, resistance to antibiotics and acute exacerbations has a clinical course that parallels that of other persistent biofilm related inflammatory diseases.17,23 Due to the failure of standard therapies to control and cure CRS, other novel non-antibiotic therapies that are able to destroy biofilms and antibiotic resistant bacteria are needed.
Our previous studies have demonstrated the in vitro effectiveness of methylene blue based antimicrobial photodynamic therapy (aPDT) as a broad spectrum non-antibiotic polymicrobial treatment that is able to completely eradicate antibiotic resistant planktonic bacteria and fungi as well as significantly reduce CRS polymicrobial antibiotic resistant biofilm by >99.9% after a single treatment.24 Prior to human treatment however, MB aPDT treatment must be demonstrated to be safe and not result in histologic damage to the sinus ciliated respiratory epithelium. The current study sought to examine the histomorphologic effect of MB and MB aPDT on a validated living human ciliated respiratory mucosa model (EpiAirway™).
Methods
EpiAirway AFT-100-AFB (MatTek Corp., Ashland, MA), a full thickness human ciliated mucus forming respiratory mucosa culture with 21 days growth without antibacterials or antifungals was used in the study. The EpiAirway grafts were handled per the directions provided by the manufacturer.25 MatTek's EpiAirway™ System is the only commercially available in vitro airway tissue model that originates from normal, human-derived tracheal/bronchial epithelial (NHBE) cells which have been cultured to form a 3-dimensional, pseudo-stratified ciliated highly differentiated model closely resembling the epithelial tissue of the respiratory tract. EpiAirway tissues are ideal for basic cell and molecular biology studies, pharmaceutical target validation and pre-clinical toxicity/efficacy determination, as well as inhalation toxicity studies of occupational or environmental air pollutants and toxins.25 EpiAirway provides a cost-effective and FDA recognized means of assessing various respiratory tract issues while avoiding species extrapolation and the use of laboratory animals. This study was performed to mimic the planned aPDT human sinus treatment with topical application of the MB photosensitizer to the sinus respiratory mucosa followed by the incubation and light conditions. Seven study groups, each with four specimens, were evaluated in order to assess the effect of various treatment conditions on the EpiAirway ciliated respiratory mucosal grafts. The optimal aPDT treatment conditions for CRS therapy have been previously determined to be MB 0.03% incubated for 3.5 minutes on the sinus mucosa followed by 670nm light at 150 mW/cm2 for 8 minutes. Therefore the seven study groups consisted of no treatment, phosphate buffered saline (PBS) administered for 11.5 minutes, PBS and 670nm light at 150 mW/cm2 for 8 minutes, 0.03% MB for 11.5 minutes with no light, 0.09% MB (3×) for 11.5 minutes with no light, 0.3% MB (10×) for 11.5 minutes with no light and 0.03% MB for 3.5 minutes incubation followed by 670nm light at 150 mW/cm2 for 8 minutes.
After completion of treatment of the EpiAirway™ ciliated respiratory mucosal tissue, each entire specimen was fixed in neutral buffered10% formalin, processed, paraffin-embedded, sectioned, H &E stained and mounted. All samples were microscopically sampled and read by a board-certified human pathologist to assess any effect of aPDT on the ciliated respiratory mucosal tissue and pathology reports issued. The pathologist was blinded to any of the treatment conditions on first examination. After completion of the official pathology reports, the treatment conditions were revealed to the pathologist and the histologic slides re-examined. The results were correlated with the treatment parameters using non-parametric tests.
Results
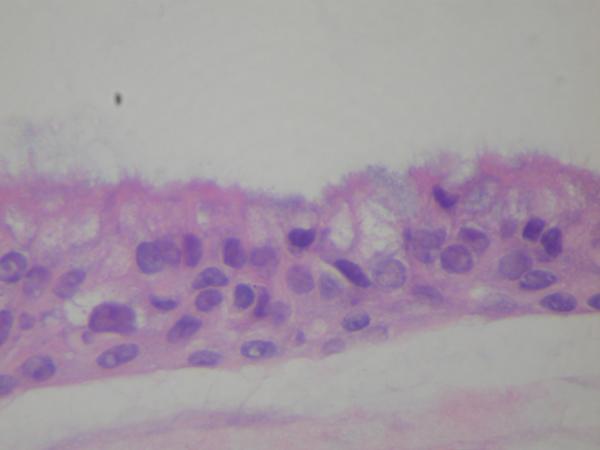
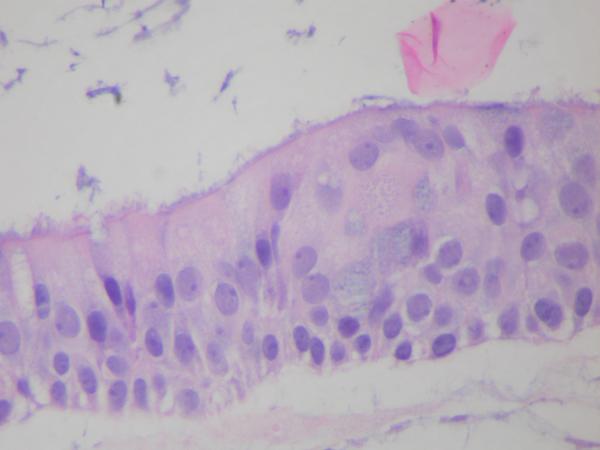
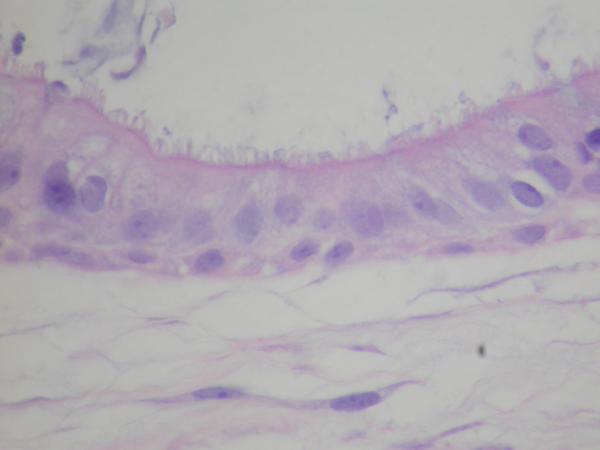
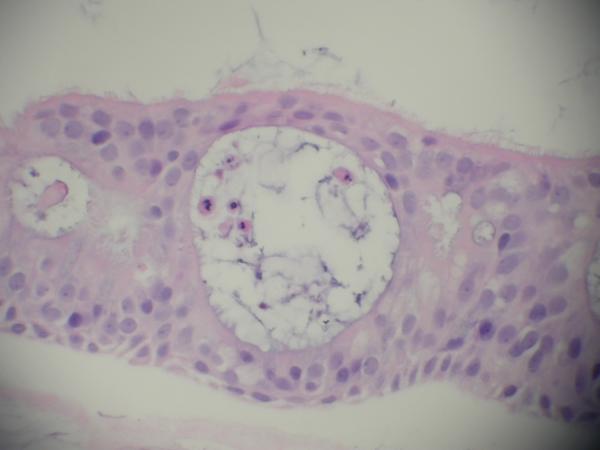
The histomorphological evaluation of the EpiAirway specimens demonstrated persistence of ciliated respiratory epithelium in all the individual specimens for each study group. Intact epithelium with normal-appearing cilia were found in all preparations. (Figure 1, 2, 3) Each specimen (including the non-treatment control group) did demonstrate scattered intraepithelial vesicle formation with a few clusters of degenerated epithelial cells within the vesicles (Figure 4). This alteration was presumably not related to the aPDT treatments as it was found to a variable degree in all the preparations. In all preparations, there were areas of epithelial attenuation at the margins of individual specimens characterized by a single layer of flattened to low cuboidal epithelium without cilia. When the pathologist was subsequently not blinded to the specific treatment conditions, reevaluation of the slides demonstrated no specific histomorphological findings attributable to a specific treatment condition.
Figure 1.
EpiAirway Untreated Control (H&E, HPF 20×) demonstrating intact ciliated respiratory epithelium with intraepithelial vesicles.
Figure 2.
EpiAirway exposed to MB 0.3%, 10× therapeutic concentration, (H&E, HPF 20×) demonstrating intact ciliated respiratory epithelium.
Figure 3.
EpiAirway exposed to full PDT treatment, MB 0.03% and 670nm light at 150mW/cm2, (H&E, HPF 20×) demonstrating intact ciliated respiratory epithelium without destruction of cilia.
Figure 4.
EpiAirway specimen demonstrating scattered intraepithelial vesicle formation with a few clusters of degenerated epithelial cells within the vesicles
Discussion
CRS is one of the most common chronic conditions in the United States affecting an estimated 37 million Americans26 and a significant number of patients with CRS remain resistant to cure despite rigorous treatment regimens including surgery, allergy therapy and prolonged antibiotic therapy.3–17,27 One of the common theories for the cause of recalcitrant CRS is the polymicrobial biofilm colonization of the sinuses resulting in a local inflammatory response, edema and ultimate infection. Due to the failure of standard therapies to control and cure CRS, other novel non-antibiotic therapies that are able to destroy biofilms and antibiotic resistant bacteria and fungi which contribute to the chronic recurrent nature of CRS are needed. We have previously demonstrated that MB PDT is highly effective in the photoeradication of pathogenic Candida and various antibiotic resistant gram positive and gram negative bacteria that are commonly associated with chronic recurrent sinusitis. Importantly, the in vitro biofilm studies demonstrated a greater than 6.5 log reduction of antibiotic-resistant multi-specie bacterial biofilms after a single PDT treatment. Using a higher MB concentration and lower light parameters achieved greater than 7 logs of bacteria kill using two PDT light treatments.24 These results demonstrated that MB PDT results in a significant reduction in antibiotic-resistant multi-specie bacterial biofilms that are commonly found in CRS. These studies therefore indicated that MB PDT may be an effective treatment method for the control or eradication of antibiotic resistant bacteria, fungi and biofilms that are a major contributing cause of CRS. However, prior to human clinical use, MB aPDT treatment must be demonstrated to be safe and not result in histologic damage to the normal sinus ciliated respiratory epithelium.
The photodynamic mechanism of bacterial and fungal cell destruction is by perforation of the cell membrane or wall by PDT induced singlet oxygen and oxygen radicals thereby allowing the dye to be further translocated into the cell. Once there, the photodynamic photosensitizer, in its new sites, photodamages inner organelles and induces cell death.28 This mechanism of action is very acute29–34 and therefore and PDT related cellular damage can be observed histomorphologically immediately after PDT treatment. Therefore, the present study evaluated the histomorphology of MB aPDT treatment on human ciliated respiratory epithelium as is found in the human paranasal sinuses in order to determine whether there is any effect of MB aPDT treatment on the normal sinus epithelium.
The use of light microscopy to determine the effects of various chemicals and treatments on the histomorphologic structure of nasal and sinus ciliated respiratory epithelium is well established in the literature.35–40 As well, any changes in the histomorphologic structure of the paranasal sinus epithelium has correlated with functional sinus mucosal changes, such as a change in ciliary beat frequency.35–40 Furthermore, the human highly differentiated ciliated respiratory EpiAirway tissues used in this study are ideal for basic cell and molecular biology studies, pharmaceutical target validation and pre-clinical toxicity/efficacy determination, as well as inhalation toxicity studies of occupational or environmental air pollutants and toxins.25 This EpiAirway tissue model provides a cost-effective and FDA recognized means of assessing various respiratory tract issues while avoiding species extrapolation and the use of laboratory animals.
Conclusion
The present study of the effect of MB alone and MB aPDT, at concentrations and light doses necessary to treat CRS, on the ciliated respiratory epithelial in vivo EpiAirway tissue demonstrated no histologic evidence of respiratory epithelial damage that could be attributed to aPDT. Intact ciliated respiratory epithelium remained in all instances regardless of study group. Accordingly, we believe that MB aPDT is not associated with significant histological ciliary or epithelial injury and is safe for human treatment. Further studies to evaluate the effect of MB aPDT treatment on ciliary beat frequency are presently underway.
Acknowledgment
Supported by NIH Grant R43 AI094706
Footnotes
Financial disclosure: MAB is Chief Scientific Officer of PhotoBiologix, Inc and a shareholder and Chief Medical Officer of Sinuwave, Inc.; JWJ: none; LP: none; NL: shareholder Sinuwave, Inc.
References
- 1.Marple BF, Stankiewicz JA, Baroody FM, et al. Diagnosis and Management of Chronic Rhinosinusitis in Adults. Postgraduate Medicine. 2009;121(6):121–39. doi: 10.3810/pgm.2009.11.2081. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics, NCHS . Summary Health Statistics for US Adults, 2002. Centers for Disease Control, US Department of Health and Human Services; Hyattsville, MD: 2002. Chronic sinusitis. [Google Scholar]
- 3.Murphy MP, Fishman P, Short SO, et al. Health care utilization and cost amount adults with chronic rhinosinusitis enrolled in a health maintenance organization. Otolaryngol Head Neck Surg. 2002;127(5):367–76. doi: 10.1067/mhn.2002.129815. [DOI] [PubMed] [Google Scholar]
- 4.Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg. 1995;113:104–9. doi: 10.1016/S0194-59989570152-4. [DOI] [PubMed] [Google Scholar]
- 5.Hamilos DL. Chronic sinusitis. J Allergy Clin Immunol. 2000;106:213–27. doi: 10.1067/mai.2000.109269. [DOI] [PubMed] [Google Scholar]
- 6.Somerville LL. Hidden factors in asthma. Allergy Asthma Proc. 2001;22:341–5. [PubMed] [Google Scholar]
- 7.Chester AC. Health impact of chronic sinusitis. Otolaryngol Head Neck Surg. 1999;114:842–9. doi: 10.1016/S0194-59989670122-5. [DOI] [PubMed] [Google Scholar]
- 8.Desrosiers MY, Kilty SJ. Treatment alternatives for chronic rhinosinusitis persisting after ESS: What to do when antibiotics, steroids and surgery fail. Rhinology. 2008;46:3–14. [PubMed] [Google Scholar]
- 9.Cohen M, Kofonow J, Nayak JV, et al. Biofilms in chronic rhinosinusitis: A review. Am J Rhinol Allergy. 2009 May-Jun;23(3):255–60. doi: 10.2500/ajra.2009.23.3319. [DOI] [PubMed] [Google Scholar]
- 10.Kilty SJ, Desrosiers MY. Are Biofilms the Answer in the Pathophysiology and Treatment of Chronic Rhinosinusitis? Immunol Allergy Clin N Am. 2009;29:645–56. doi: 10.1016/j.iac.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Foreman A, Psaltis AJ, Tan LW, Wormald P. Characterization of bacterial and fungal biofilms in chronic rhinosinusitis. Am J Rhinol Allergy. 2009;23(6):556–61. doi: 10.2500/ajra.2009.23.3413. [DOI] [PubMed] [Google Scholar]
- 12.Hunsaker DH, Leid JG. The relationship of biofilms to chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 16:237–41. doi: 10.1097/MOO.0b013e3282fdc6d5. [DOI] [PubMed] [Google Scholar]
- 13.Kilty SJ, Desrosiers MY. The Role of Bacterial Biofilms and the Pathophysiology of Chronic Rhinosinusitis. Current Allergy and Asthma Reports. 2008;8:227–33. doi: 10.1007/s11882-008-0038-2. [DOI] [PubMed] [Google Scholar]
- 14.Daele JJ. Chronic sinusitis in children. Acta Otorhinolaryngol Belg. 1997;51:285–304. [PubMed] [Google Scholar]
- 15.Sanderson AR, Leid JG, Hunsaker D. Bacterial Biofilms on the Sinus Mucosa of Human Subjects with Chronic Rhinosinusitis. Laryngoscope. 2006 Jul;116:1121–6. doi: 10.1097/01.mlg.0000221954.05467.54. [DOI] [PubMed] [Google Scholar]
- 16.Cryer J, Schipor L, Perloff JR, Palmer JN. Evidence of Bacterial Biofilms in Human Chronic Sinusitis. ORL. 2004 Jun;66:155–8. doi: 10.1159/000079994. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson BJ, Stolz DB. Demonstration of Biofilm in Human Bacterial Chronic Rhinosinusitis. Am J of Rhinology. 2005 Sep-Oct;19(5):452–7. [PubMed] [Google Scholar]
- 18.Palmer JN. Bacterial Biofilms: Do They Play a Role in Chronic Sinusitis. Otolaryngol Clin N Am. 2005;38:1193–1201. doi: 10.1016/j.otc.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Harvey RJ, Lund VJ. Biofilms and Chronic Rhinosinusitis: Systematic Review of Evidence, Current Concepts and Directions for Research. Rhinology. 2007;45:3–13. [PubMed] [Google Scholar]
- 20.Palmer JN. Bacterial Biofilms in Chronic Rhinosinusitis. Ann Otol Rhinol Laryngol. 115(9 Suppl 196):35–9. doi: 10.1177/00034894061150s906. [DOI] [PubMed] [Google Scholar]
- 21.Ramadan HH, Sanclement JA, Thomas JG. Chronic Rhinosinusitis and Biofilms. Otolaryngol Head Neck Surg. 2005;132:414–7. doi: 10.1016/j.otohns.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Sanclement JA, Webster P, Thomas J, Ramadan HH. Bacterial Biofilms in Surgical Specimens of Patients with Chronic Rhinosinusitis. Laryngoscope. 2005 Apr;115:578–82. doi: 10.1097/01.mlg.0000161346.30752.18. [DOI] [PubMed] [Google Scholar]
- 23.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001 Jul 14;358(9276):135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 24.Biel MA, Sievert C, Usacheva M, Teichert M, Balcom J. Antimicrobial photodynamic therapy treatment of chronic recurrent sinusitis biofilms. International Forum of Allergy & Rhinology. 2011;1(5):329–34. doi: 10.1002/alr.20089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. http://www.mattek.com/pages/products/epiairway.
- 26.Wainwright M, Phoenix DA, Nickson PB, Morton G. The use of new methylene blue in Pseudomonas aeruginosa biofilm destruction. Biofouling. 2002;18(4):247–9. [Google Scholar]
- 27.Soukos NS, Wilson M, Burns T, et al. Photodynamic effects of toluidine blue on human oral keratinocytes and fibroblasts and Streptococcus sanguis evaluated in vitro. Lasers Surg Med. 1996;18(3):253–9. doi: 10.1002/(SICI)1096-9101(1996)18:3<253::AID-LSM6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 28.Harris F, Chatfield LK, Phoenix DA. Phenothiazinium based photosensitisers - photodynamic agents with a multiplicity of cellular targets and clinical applications. Curr Drug Targets. 2005;6(5):615–27. doi: 10.2174/1389450054545962. [DOI] [PubMed] [Google Scholar]
- 29.Gorman AA, Rodgers MA. Lifetime and reactivity of singlet oxygen in an aqueous micellar system: A pulsed nitrogen laser study. Chem Phys Lett. 1978;55:52–4. [Google Scholar]
- 30.Kato S, Morita M, Koisumi M. Studies of the transient intermediates in the photoreduction of methylene blue. Bull Japan Chem Soc. 1964;37:117–24. [Google Scholar]
- 31.Martin JP, Logsdon N. Oxygen radicals are generated by dye-mediated intracellular photooxidations: a role for superoxide in photodynamic effects. Arch Biochem Biophys. 1987;256:39–49. doi: 10.1016/0003-9861(87)90423-1. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson R, Merkel PB, Kearns DR. Kinetic properties of the triplet states of methylene blue and other photosensitizing dyes. Photochem Photobiol. 1972;16:109–16. doi: 10.1111/j.1751-1097.1972.tb07342.x. [DOI] [PubMed] [Google Scholar]
- 33.Obata H, et al. Photochemical reactions between methylene blue and tri-, di- and monomethylamuine, III. The behavior of methylene blue in the presence of oxygen. Bull Japan Chem Soc. 1959;32:125–32. [Google Scholar]
- 34.Somer G, Green M. Photoreduction of methylene blue in water. Photochem Photobiol. 1963;17:179–90. [Google Scholar]
- 35.Kilty SJ, Dakheelallah A, Duval M, Groleau MA, De Nanassy J, Gomes MM. Manuka honey: Histological effect on respiratory mucosa. Am J of Rhinology & Allergy. 2010;24(2):e63–e66. doi: 10.2500/ajra.2010.24.3453. [DOI] [PubMed] [Google Scholar]
- 36.Bolukbasi S, Habesoglu TE, Habesoglu M, Samanci B, Erol Y, Gumrukcu G, Egeli E. Histopathological changes of rat larynx mucosa with exposure to chronic thinner inhalation. Otolaryngol Head Neck Surg. 2009;141:75–80. doi: 10.1016/j.otohns.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 37.Berg OH, Lie K, Steinsvag S. The Effect of Decongestive Nosedrops on Human Respiratory Mucossa In Vitro. Laryngoscope. 1994;104:1153–8. doi: 10.1288/00005537-199409000-00018. [DOI] [PubMed] [Google Scholar]
- 38.Gaafar H, Girgis R, Hussein M, El-Nemr F. The effect of ammonia on the respiratory nasal mucosa of mice. Acta Otolaryngol. 1992;112(2):339–42. doi: 10.1080/00016489.1992.11665429. [DOI] [PubMed] [Google Scholar]
- 39.Dowling RB, Johnson M, Cole PH, Wilson R. Effect of fluticasone propionate and salmeterol on Pseudomonas aeruginosa infection of the respiratory mucosa in vitro. Eur Respir J. 1999;14(2):363–9. doi: 10.1034/j.1399-3003.1999.14b21.x. [DOI] [PubMed] [Google Scholar]
- 40.Matulionis DH. Light and Electron Microscopic Study of the Effects of ZnSO4 on Mouse Nasal Respiratory Epithelium and Subsequent Responses. Anat Rec. 1975;183(1):63–82. doi: 10.1002/ar.1091830107. [DOI] [PubMed] [Google Scholar]