Abstract
AIMS
To illustrate (i) the criteria and the development of the DRUID categorization system, (ii) the number of medicines that have currently been categorized, (iii) the added value of the DRUID categorization system and (iv) the next steps in the implementation of the DRUID system.
METHODS
The development of the DRUID categorization system was based on several criteria. The following steps were considered: (i) conditions of use of the medicine, (ii) pharmacodynamic and pharmacokinetic data, (iii) pharmacovigilance data, including prevalence of undesirable effects, (iv) experimental and epidemiological data, (v) additional data derived from the patient information leaflet, existing categorization systems and (vi) final categorization. DRUID proposed four tiered categories for medicines and driving.
RESULTS
In total, 3054 medicines were reviewed and over 1541 medicines were categorized (the rest were no longer on the EU market). Nearly half of the 1541 medicines were categorized 0 (no or negligible influence on fitness to drive), about 26% were placed in category I (minor influence on fitness to drive) and 17% were categorized as II or III (moderate or severe influence on fitness to drive).
CONCLUSIONS
The current DRUID categorization system established and defined standardized and harmonized criteria to categorize commonly used medications, based on their influence on fitness to drive. Further efforts are needed to implement the DRUID categorization system at a European level and further activities should be undertaken in order to reinforce the awareness of health care professionals and patients on the effects of medicines on fitness to drive.
Keywords: accidents, automobile driving, drug prescriptions, drug utilization, risk assessment, traffic
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Some commonly prescribed medications can be a hazardous to traffic safety.
Fifteen categorization systems are currently available in Europe. However, none of these systems clearly reports the methodology that was followed in order to categorize medications that impair driving.
None of the existing categorization systems are currently implemented at European level.
WHAT THIS STUDY ADDS
This study describes standardized and harmonized criteria to categorize medications according to their potential to impair fitness to drive.
This study proposes a European categorization system of medications that impair driving that covers all the most frequently prescribed medications.
The proposed categorization system can be seen as a tool to improve prescribing and dispensing procedures of medications that impair driving as well as an instrument to make patients aware of the role medications play in traffic safety.
Introduction
Driving a motor vehicle is a multifaceted task and it requires appropriate cognitive and psychomotor skills (e.g. alertness, concentration, reaction time, visual acuity) [1–3]. Medication can adversely affect these driving-related skills, and, consequently, be a hazard to traffic safety [4, 5].
The European Council Directive 83/570/EEC of October 1983 established that the summary of product characteristics (SmPC) has to contain information on medicines' ‘effects on the ability to drive and to use machines’[6]. In October 1991 the European Committee for Medicinal Products for Human Use (CHMP) provided a Note for Guidance for the SmPC in which it was stated that section 4.7 of medications registered from 1 January 1992 had to indicate, on the basis of the pharmacodynamic profile, reported adverse drug reactions (ADRs) and/or impairment of driving performance or performance related to driving based on three different levels of impairment with respect to the ability to drive and/or operate machines [7, 8]. However, this rule has never been implemented [9].
In September 2009, a new SmPC guideline was issued, which established that ‘on the basis of the pharmacodynamic and pharmacokinetic profile, reported adverse reactions and/or specific studies in a relevant target population addressing the performance related to driving and road safety or using machines, specify whether the medicinal product has: (i) no or negligible influence, (ii) minor influence, (iii) moderate influence or (iv) major influence on these abilities’[10]. These new guidelines were partly based on the proposal sent to the European Medicines Agency (EMA) by DRUID Work Package (WP) 4 partners during the consultation phase for the revision of the SmPC guidelines, in March 2008.
Despite the above-mentioned regulations, at this moment, a European categorization system has not yet been established, and warning systems for medications that potentially impair driving have mainly been developed and/or implemented at national levels [8, 11].
Existing categorization systems on medicines and driving
A review of the existing classification/categorization and labelling systems for medicines and driving was performed in 2008 and 15 different approaches were identified [12]. The categorization/labelling systems differed significantly and were not standardized, making them difficult to understand. In most cases [13–17], the categorization systems were developed by different and unrelated bodies, societies or researchers and were, in general, aimed at improving the prescription and dispensing of medicines to the patients and drivers. The identified categorization systems often included a limited number of medicines belonging to a few different therapeutic groups (e.g. antihistamines, anxiolytics, etc) and were not legally binding. However, the review also identified a couple categorization/labelling systems that were developed by regulatory bodies, included the use of pictograms and were legally binding [18, 19].
In 1973, the Netherlands became the first country to introduce a list of medications that can impair driving abilities. Besides the list, the use of a yellow warning sticker on medication boxes was established and implemented [20]. In 1981, Denmark, Finland, Iceland, Norway and Sweden adopted a warning label. The label consisted of a red triangle printed on packages of ‘especially dangerous’ medications, and it is currently still in use in Denmark, Finland, and Norway. Most recently, France [18] and Spain [19] developed a categorization/labelling of all available medicines using technical interdisciplinary groups formed from their respective national medicines regulatory agency [21, 22] The introduction of pictograms (three-tier labelling system in France and two-tier in Spain) to be added on the packages of certain medicines became legally binding in both countries.
It is important to point out that although different categorization systems are currently available across Europe, the criteria for the establishment of a categorization system for potentially impairing medications has neither been clearly described nor published nor been officially adopted at European level [12].
The DRUID project and its categorization system on medicines and driving
The Driving under the Influence of Drugs, Alcohol and Medicines (DRUID) project is an integrated project funded by the European Commission. The main aim of DRUID is to give scientific support to European Union (EU) transport policy by establishing guidelines and measures that combat impaired driving [23].
The DRUID WP4 aims to provide the basis and the methodology for the development of a European classification/labelling system for medications with respect to their impact on fitness to drive. Furthermore, it also focuses on the development of a classification of relevant therapeutic groups that are currently on the market in Europe as well as new medications approved by the European Medicines Agency (EMA) in the years 2007–2009 [23].
Aims of the study
This paper illustrates: (i) the criteria and the development of the DRUID categorization system, (ii) the number of medicines that have currently been categorized and the distribution of the DRUID categories across the Anatomical Therapeutic Chemical (ATC) index, (iii) the importance of this system, its implications for health care professionals (HCPs) and patients, and its strengths and limitations and (iv) the next steps in the implementation of the DRUID system and some general recommendations.
Methods
The development of the DRUID categorization system was based on the criteria that were established by a group of experts in the field of medicines and driving, involved in the DRUID WP4, and based on their consensus [24].
The four DRUID categories on medicines and driving
In 2006, the DRUID group established and agreed that, according to its influence on fitness to drive, a medicine could be categorized as follows (Figure 1):
Figure 1.
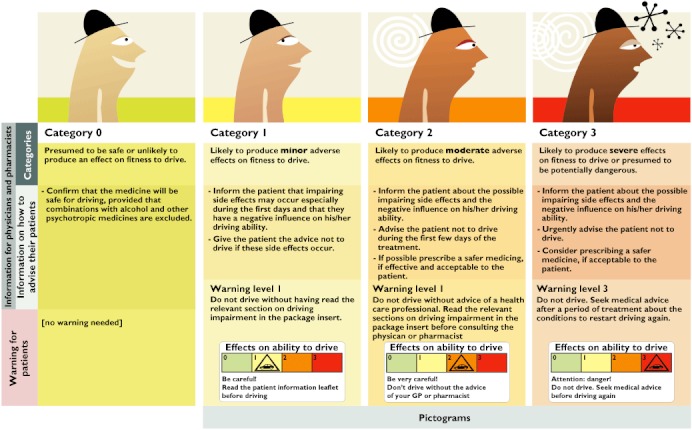
DRUID categorization system for medicines and driving
category 0 (no or negligible influence on fitness to drive),
category I (minor influence on fitness to drive),
category II (moderate influence on fitness to drive),
category III (severe influence on fitness to drive).
The proposed categorization is in line with the recently approved SmPC guidelines, which were adopted in September 2009 by the EMA [10].
Furthermore, the DRUID experts decided to develop, for each category, practical information to be used by HCPs for patient counselling purposes as well as simple warning labels that could be easily understood by patients (labelling) (Figure 1).
The DRUID categorization of medicines and driving
The ATC classification list [25] was used as a starting point for the selection of the relevant groups of medicines to be categorized. The aim was to categorize all available medicines on the European Union market for each selected ATC group.
Figure 2 shows the process that was followed in order to identify all those medications that are currently available on the EU market. In general, a medicine was considered available on the EU market if it was commercialized in at least two of the following European countries: Belgium, France, Germany, Greece, the Netherlands, Spain, United Kingdom and Ireland. If the above-mentioned criterion was not fulfilled, the medication was not included in the categorization process.
Figure 2.
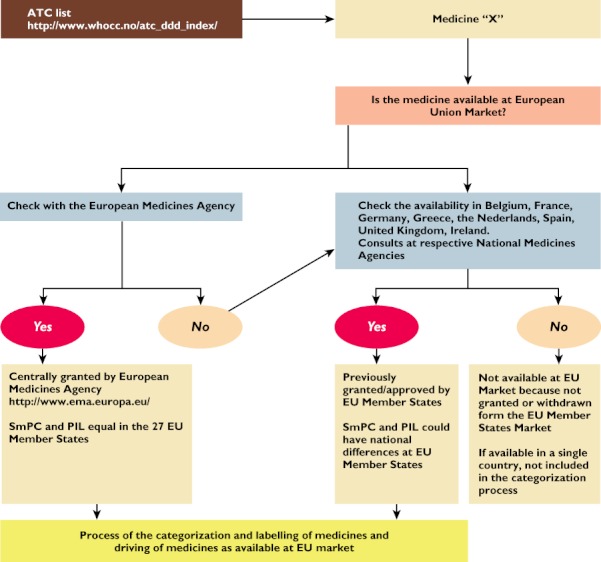
Identification process of medicines available on the EU market
After a meeting with the French Health Products Safety Agency (AFSSAPS) experts in categorizing medications affecting driving performance, the DRUID WP4 group decided to adopt a procedure similar to the one used in France and more specifically to evaluate the following information and data:
Conditions of use of the medicine in the EU market
Pharmacodynamic and pharmacokinetic data
Pharmacovigilance data (including prevalence of undesirable effects reported in the SmPC)
Experimental and epidemiological data
Additional data derived from the patient information leaflet (PIL) and existing categorization systems and information from other sources
Synthesis and final categorization.
Figures 3 and 4 summarize the methodology that was followed in order to assign a category to a selected medicine.
Figure 3.
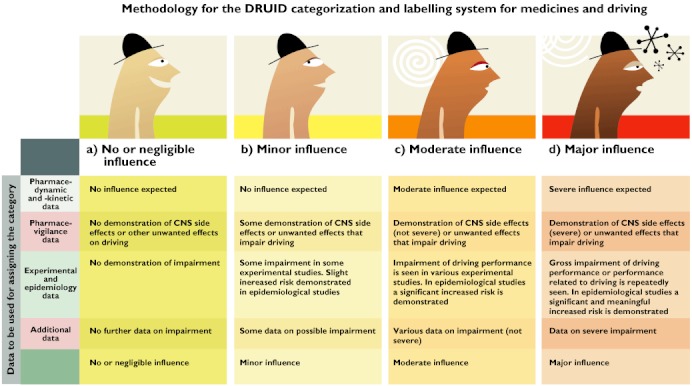
Methodology for the DRUID categorization system for medicines and driving
Figure 4.
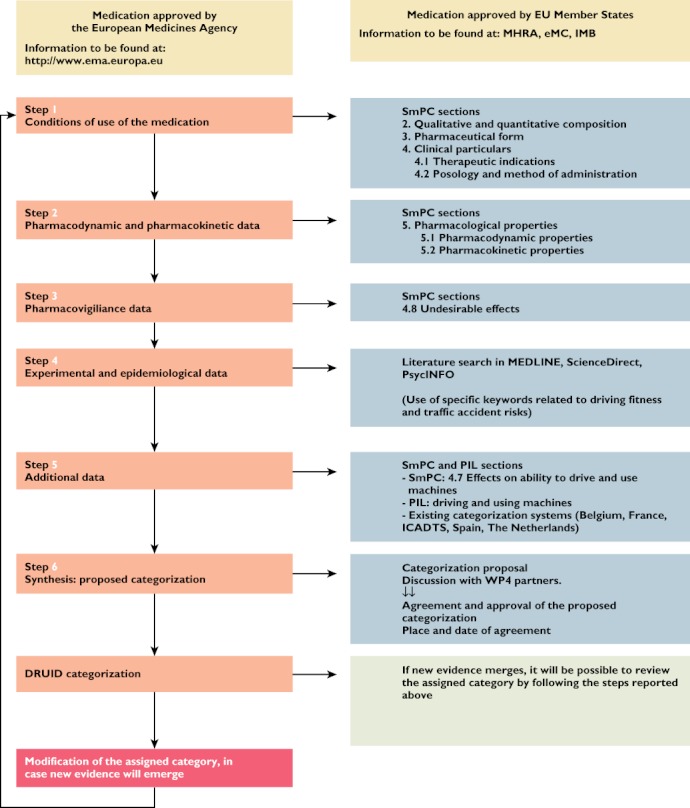
Flowchart representing the methodology that was followed during the DRUID categorization process. Legend: SmPC, Summary of Product Characteristics; PIL, Patient Information Leaflet; EMA, European Medicines Agency; MHRA, Medicines and Healthcare products Regulatory Agency; eMC, Electronic Medicines Compendium; IMB, Irish Medicines Board
The conditions of use of the medicine, pharmacodynamic, pharmacokinetic and pharmacovigilance data (including prevalence of undesirable effects) were derived from the SmPC [10], whereas point 4 (experimental and epidemiological data) was based on a scientific literature search.
The SmPC and PIL of the selected medications were found online, in one of the following websites: Medicines and Healthcare products Regulatory Agency (MHRA) [26], Electronic Medicines Compendium (eMC) [27], or Irish Medicines Board (IMB) [28], or retrieved from national medicines regulatory agencies as needed. In case of recently approved active substances, the SmPC was found on the EMA website [29]. The selection of the above mentioned medicines regulatory affairs agencies was simply based on the fact that the required information had to be available either in English or in a language that could be fully understood by DRUID WP4 partners.
Specific sections of the SmPC and PIL were used to retrieve details on the active substance presentations and strength, indications, posology, route of administration (step 1), pharmacodynamic and pharmacokinetic profile (step 2), effects on the ability to drive and use machines (step 5) and undesirable effects related to driving and operating machines (step 3).
With respect to the undesirable effects, their occurrence was considered as a key point, especially if experimental and epidemiological data were lacking or limited. This type of information was found in section 4.8 of the SmPC and, when not available, was retrieved from the available literature.
Generally speaking, only those adverse reactions that could affect the ability to drive and that were reported as common (>1/100, <1/10) or very common (>1/10) were considered to be relevant, as in accordance with the most recent EMA categorization on frequency of undesirable effects, side effects or adverse reactions. In cases of rare or very rare undesirable effects, or if certain severely impairing effects occur, for example sudden sleep attacks, the DRUID partners recommended that this should be mentioned in the PIL.
Table 1 reports the criteria used for assigning a medicine to a specific category whenever experimental or epidemiological data were lacking or limited.
Table 1.
Relationship of the undesirable effects category in the SmPC to the DRUID categorization system
| Declaration of undesirable effects that can potentially impair the fitness to drive safely | DRUID category |
|---|---|
| Very common (>1/10) | Category II or III |
| Common (>1/100, <1/10) | Category I |
| Rare (>1/10 000, <1/1000) or very rare (<1/10 000) | Category 0 |
Table 2 lists the undesirable effects that could impair the ability to drive, and, therefore, were taken into account in the categorization process.
Table 2.
List of undesirable effects that can impair driving ability that were considered for the categorization of active substances based on their level of driving impairment
| System organ class | Selection of side effects that can impair the ability to drive safely |
|---|---|
| Nervous system disorders | • Somnolence, dizziness, drowsiness |
| • Confusion – cognitive disorder- disorientation | |
| • Involuntary movement disorders: ataxia, tremor, parkinsonism, acute dystonic (dyskinesia) and dyskinetic reactions (dystonia) | |
| • Convulsions – seizures | |
| Psychiatric disorders | • Perception disturbances (hallucination, visual hallucination, auditory hallucination, illusion) |
| • Psychotic reactions and psychotic disorder (including paranoia psychosis) | |
| • [Other: emotional lability, mood swings, aggression, nervousness, irritability, personality disorders, thinking abnormal, abnormal behaviour, euphoric mood, restlessness (emotional state of excitement), depersonalization] | |
| Eye disorders | • Diplopia or double vision, |
| • Blurred vision | |
| • Accommodation disorders | |
| • Visual acuity reduced | |
| • Photophobia | |
| • [Other: visual field defect, peripheral vision loss, altered visual depth perception, oculogyric crisis]. | |
| Ear and labyrinth disorders | • Vertigo |
| • Hearing loss | |
| • [Other: buzzing, tinnitus] | |
| Metabolism and nutrition disorders | • Hypoglycaemia |
| Vascular disorders | • Hypotension |
Data sources for the scientific literature evaluation included the electronic databases Medline, Science Direct and PsycINFO. The search was performed by using these combinations of keywords: ‘active substance name and psychomotor performance’, ‘active substance name and automobile driving’ and ‘active substance name and traffic accidents’. The final data selection was limited to full text articles published in English and other languages that included references to side effects, experimental and pharmacoepidemiological studies and case reports on each active substance to be categorized and its possible driving impairment. No restrictions concerning the publication year were applied.
Additional steps consisted of reviewing section 4.7 of the SmPC ‘Effects on ability to drive and use machines’ and the PIL section on ‘Driving and using machines’ as well as reviewing the previous categorization (if available) of the medicine in Belgium, France, the Netherlands, Spain and the International Council on Alcohol, Drugs and Traffic Safety (ICADTS) list.
In the cases of severely impairing medicines, recently approved medications, or medicines belonging to the ATC groups N and R06, all the collected data were compiled in fact sheets with a standardized lay-out, which were used during the active substance evaluation procedure and the approval of its final category.
After evaluating all the available data, a provisional category was assigned to each active substance. The provisional category was proposed and discussed during WP4 meetings, where a final and definitive category was assigned and approved by all WP4 partners.
It is important to note that the DRUID methodology on the categorization of medicines affecting driving fitness allows not only to categorize an active substance but also to revise a previously assigned category, in cases where new evidence emerges, by following the same 5 step approach (Figure 4).
Medicines to be categorized
The following ATC groups were considered in the categorization process:
A – Alimentary tract and metabolism
B – Blood and blood forming organs
C – Cardiovascular system
D – Dermatologicals
M – Musculoskeletal system
N – Nervous system
R – Respiratory system
S – Sensory organs
Results
Three thousand fifty-four medicines were considered for inclusion into the categorization process. Of these 3054 medicines, 1513 were not categorized because they were not available on the EU market.
The distribution of the 1541 categorized medicines (see supplementary data) was as follows (Figure 5): Category 0 50.3%, Category I 26.0%, Category II 11.2%, Category III 5.8%, Multiple categories 4.4% and Depending on the medicine in combination 2.3%. This figure shows that the majority of medications belong to either category 0 or category I (Figure 5).
Figure 5.
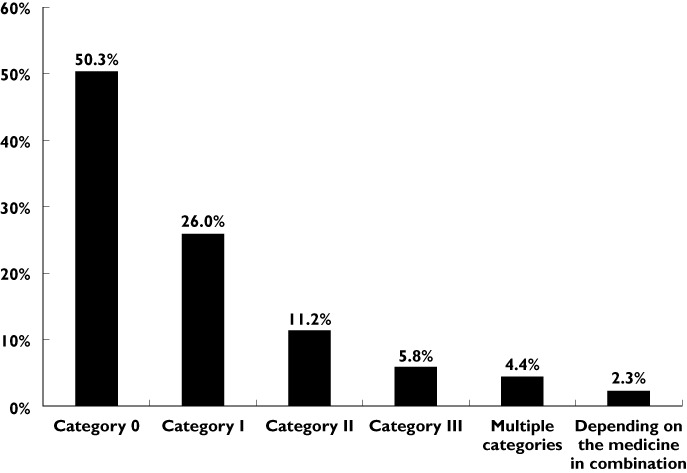
Distribution of the 1541 categorized medicines within the different DRUID categories
It is important to note that the term ‘multiple categories’ refers to the fact that a certain medication could be included in more than one category. There could be several reasons for this, such as different routes of administration of the same active substances (e.g. topical, oral, parenteral, etc), different pharmaceutical formulations (e.g. aqueous vehicle, cream, drops or ointment, etc.), different dosages administered, etc.
With respect to the terminology ‘depending on medicines in combination’, it is relevant to observe that this approach was used when the categorization depended on the combination of the medication under evaluation with another active substance. In these cases, since the ATC classification [18] often did not report the medicine used in combination, it was decided not to use a final category but to follow the above-mentioned approach.
Table 3 gives an overview of the distribution of the medicines in each category, stratified by ATC group. It is apparent from this table that the N group contains the highest number of category III medications. A detailed description of the category distribution within the N group is depicted in Table 4. The N05 sub-group shows the highest number of category III medicines, followed by the N01 sub-group. The N05 sub-group also contains the highest number of medications assigned to more than one category.
Table 3.
Number of medicines categorized by ATC group
| DRUID categorization | ||||||||
|---|---|---|---|---|---|---|---|---|
| ATC group | Not evaluated or not available at EU market | 0 | I | II | III | Multiple categories | Depending on the medicine in combination | Total |
| A – alimentary tract and metabolism | 243 | 234 | 69 | 8 | 1 | 4 | 4 | 563 |
| B – blood and blood forming organs | 86 | 135 | 1 | 1 | 2 | 225 | ||
| C – cardiovascular system | 246 | 90 | 200 | 11 | 1 | 548 | ||
| D – dermatologicals | 156 | 192 | 1 | 4 | 353 | |||
| M – musculo-skeletal system | 88 | 22 | 44 | 28 | 15 | 197 | ||
| N – nervous system | 346 | 9 | 30 | 86 | 53 | 36 | 560 | |
| R – respiratory system | 195 | 62 | 24 | 32 | 10 | 5 | 14 | 342 |
| S – sensory organs | 153 | 31 | 31 | 6 | 11 | 18 | 16 | 266 |
| Total | 1513 | 775 | 400 | 172 | 90 | 68 | 36 | 3054 |
Table 4.
Number of medicines from the ATC group, N-NERVOUS SYSTEM MEDICINES, categorized in each DRUID category
| Druid categorization | ||||||||
|---|---|---|---|---|---|---|---|---|
| ATC group N – nervous system | Not evaluated or not available in EU market | 0 | I | II | III | Multiple categories | Depending on the medicine in combination | Total |
| N01 ANAESTHETICS | 31 | 3 | 3 | 1 | 12 | 10 | 60 | |
| N01A Anaesthetics, general | 20 | 11 | 1 | 32 | ||||
| N01B Anaesthetics, local | 11 | 3 | 3 | 1 | 1 | 9 | 28 | |
| N02 ANALGESICS | 93 | 2 | 7 | 10 | 3 | 7 | 122 | |
| N02A Opioids | 31 | 2 | 7 | 40 | ||||
| N02B Other analgesics and antipyretics | 52 | 2 | 6 | 1 | 1 | 62 | ||
| N02C Antimigraine preparations | 10 | 1 | 9 | 20 | ||||
| N03 ANTIEPILEPTICS | 23 | 14 | 4 | 2 | 43 | |||
| N03A Antiepileptics | 23 | 14 | 4 | 2 | 43 | |||
| N04 ANTIPARKINSON | 16 | 3 | 16 | 1 | 36 | |||
| N04A Anticholinergic agents | 10 | 4 | 1 | 15 | ||||
| N04B Dopaminergic agents | 6 | 3 | 12 | 21 | ||||
| N05 PSYCHOLEPTICS | 107 | 4 | 16 | 26 | 12 | 165 | ||
| N05A Antipsychotics | 31 | 13 | 8 | 9 | 65 | |||
| N05B Anxiolytics | 23 | 1 | 3 | 7 | 1 | 35 | ||
| N05C Hypnotics and sedatives | 53 | 3 | 11 | 2 | 69 | |||
| N06 PSYCHOANALEPTICS | 62 | 2 | 10 | 20 | 7 | 1 | 102 | |
| N06A Antidepressants | 37 | 1 | 7 | 12 | 7 | 1 | 65 | |
| N06B Psychostimulants, agents used for ADHD and nootropics | 22 | 3 | 4 | 29 | ||||
| N06C Psycholeptics and psychonaleptics in combination | 2 | 2 | ||||||
| N06D Anti-dementia drugs | 1 | 1 | 4 | 6 | ||||
| N07 OTHER NERVIOUS SYSTEM DRUGS | 14 | 2 | 3 | 9 | 1 | 3 | 32 | |
| N07A Parasympathomimetics | 6 | 2 | 1 | 9 | ||||
| N07B Drugs used in addictive disorders | 2 | 2 | 1 | 4 | 1 | 2 | 12 | |
| N07C Antivertigo preparations | 2 | 1 | 2 | 5 | ||||
| N07X Other nervous system drugs | 4 | 1 | 1 | 6 | ||||
| Total | 346 | 9 | 30 | 86 | 53 | 36 | 560 | |
Discussion
The current DRUID categorization system establishes and defines standardized and harmonized criteria to categorize commonly prescribed medicines based on their influence on fitness to drive. To date, this system nearly embraces the full ATC index and it intends to provide a complete coverage of the most commonly prescribed medications in Europe. This categorization procedure is developed by a European group of experts and is meant to go beyond the national context to address a broader European scenario and involve different facets of health care practice.
The categorization system could be seen as a tool to improve prescribing and dispensing procedures both at a national and European level and, therefore, as an instrument to inform and involve HCPs better [11, 30]. In this respect, it is important that HCPs know the fundamentals of the categorization system and use it properly in order to inform fully their patients about the risks of driving under the influence of impairing medicines. Furthermore, HCPs should be able to distinguish between the four levels of impairment and, if possible, choose the least impairing medication within the same therapeutic group. Moreover, this system should encourage HCPs to update their knowledge on medicines and driving in order to be prepared to answer questions that patients might have on this topic [8, 11].
The DRUID categorization system should also be used as a tool to motivate HCPs to provide patients with clear information, communicate to patients the risk associated with driving under the influence of medicines and catalyze health care professional-patient discussions, leading to both safer prescriptions and patients who are more conscientious about their decision on whether or not to drive [8, 11, 30].
This classification could be a useful tool in helping patients be more involved in the decision-making process, understand the hazards of some medications to traffic safety and remind them to use caution while driving until their individual responses to their therapy have been well established.
To our knowledge, this is the first time that the European Commission assigned an expert group in the field of medicines and driving the task of establishing the criteria for a European classification system and developing a categorization system for relevant therapeutic groups of medications with respect to their impact on driving skills. The categorization efforts were carried out by an international group of DRUID partners, coming from six different institutions in Europe, and gathered all their scientific competence, knowledge, expertise, and experience in the field of road safety research and practice. All the available data from multiple sources were collected according to a standardized step-by-step procedure, which allows for the future maintenance and/or revision of the current DRUID categorization system as new evidence emerges in the future, and it also allows for the constitution of a consistent evidence-based classification methodology to categorize new medications prior to their market authorization. Last but not least, as reported above, the DRUID categorization system encompasses the entire ATC list. Therefore, it is the first categorization system to provide a nearly complete overview of the influence of frequently prescribed medications on the ability to drive. Additionally, in the cases of severely impairing medications (e.g. medicines from the N group), the system is integrated with fact sheets which concisely emphasize the key points of the categorization and can be easily used as a support mechanism in HCPs' daily practice [24].
Lastly, some limitations of the DRUID categorization system should be considered. In particular, special attention should be paid to the fact that a category is attributed to the single medicine, given to an adult, for its main indication, in a normal dosage, and at the start of the treatment [7, 8, 17]. Therefore, if a medication is not prescribed according to these conditions, it is crucial to bear in mind that the categorization system can only be used as background information, and it is necessary to carefully assess all the individual risk factors and avoid strict adherence to the medication classification. Furthermore, the system is focused on the effects of medications on fitness to drive and, consequently, the role of the disease, which could also influence fitness to drive, is not considered and certainly needs further attention while counselling the patient [7, 8].
Finally, the categorization system should always be associated with proper patient counselling in order to avoid any misunderstandings from the patient's side and to ensure that the patient receives adequate information allowing him/her to make a consistent decision with the message given by the medication category.
Next steps and recommendations
The categorization system presented in this manuscript was developed within the DRUID project and, therefore, in a European context. As a consequence, the DRUID partners agreed that the European regulatory authorities should to be informed about this categorization process. This should lead to discussion and consensus on the criteria hereby proposed and special efforts should be carried out to implement the current system at both international and national level, with consideration country specific circumstances.
In this respect, it is important to underline that the DRUID consortium [31] previously approached the EMA Pharmacovigilance Working Party (PhVWP) in order to obtain its contribution in relation to the development of the categorization/labelling system for medications that impair driving [32]. In June 2011, the PhVWP agreed that any information on the influence of medicines on driving ability should be simple and helpful to the patient and, therefore, be reflected in the package leaflet. Furthermore, the PhVWP recommended including in the package leaflet a two-tier risk classification system differentiating between medicinal products with a potential for relevant influence on driving (moderate or major influence) and medicinal products without a potential for relevant influence (no or minor influence). Finally, the PhVWP recognized that this two-tier risk classification system could be further divided to include a maximum of four categories at the discretion of Member States [32]. This consensus is an important step in the harmonization of information on the potential for a medicine's impairing effects on fitness to drive. However, it would be desirable for Member States to be provided with further discretionary activities, which could be used to reinforce the awareness of HCPs and patients on the effects of medicines on fitness to drive.
Since the categorization requires constant revision, it is also advised that an expert working group on medicines and driving be established to keep the system functional, up-to-date, and reliable.
Furthermore, it is recommended that special attention be paid to educating those who might play an active role in traffic safety. In this respect, medical and pharmacy schools should develop targeted educational programmes covering the issue of medication use and driving. Police officers and driving instructors should be adequately trained on this topic so that they are able to transfer knowledge about the effects of certain medications on a person's ability to drive to potential patients who may drive in traffic.
Finally, a guideline should be developed to explain the use of the categorization system to HCPs and to serve as a support mechanism in the decision making process. On the other hand, since the PIL is the most accessible source of information for patients, it would also be advisable to develop an effective strategy to communicate the risk related to the use of medicines and driving. For instance, a straightforward grading system could be included in the patient package leaflet and warning labels in the form of pictograms could be printed on the medication box to provide clear instructions about the use of the medication and driving to patients.
Disclaimers
This document has been produced under the project ‘Driving under Influence of Drugs, Alcohol and Medicines’ (DRUID) financed by the European Community within the framework of the EU 6th Framework Programme (Contract No TREN-05-FP6TR-S07.61320–518404-DRUID).
This document reflects only the authors' view. The European Community is not liable for any use that may be made of the information contained therein.
Acknowledgments
The authors would like to acknowledge all the DRUID Project WP4 Partners: Kristof Pil, Alain Verstraete (Ghent University – Belgium); Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (University of Grenoble, Centre Régional de Pharmacovigilance – France); Michael Heißing (BASt, Bundesanstalt für Straßenwesen – Germany); Katerina Touliou (CERTH-HIT, Centre for Research and Technology Hellas – Greece); Inmaculada Fierro (University of Valladolid – Spain).
Figures 1–4 were produced by Soldegato (http://www.soldegato.com) for the University of Valladolid (Valladolid – Spain).
Competing Interests
The authors declare that they do not have any competing interests.
REFERENCES
- 1.Kelly E, Darke S, Ross J. A review of drug use and driving: epidemiology, impairment, risk factors and risk perceptions. Drug Alcohol Rev. 2004;23:319–44. doi: 10.1080/09595230412331289482. [DOI] [PubMed] [Google Scholar]
- 2.Fishbain DA, Cutler RB, Rosomoff HL, Rosomoff RS. Can patients taking opioids drive safely? A structured evidence-based review. J Pain Palliat Care Pharmacother. 2002;16:9–28. [PubMed] [Google Scholar]
- 3.Hogan DB. Which older patients are competent to drive? Approaches to office-based assessment. Can Fam Physician. 2005;51:362–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Drummer OH. The role of drugs in road safety. Aust Prescr. 2008;31:33–5. [Google Scholar]
- 5.Augsburger M, Rivier L. Drugs and alcohol among suspected impaired drivers in Canton de Vaud (Switzerland) Forensic Sci Int. 1997;85:95–104. doi: 10.1016/s0379-0738(96)02084-1. [DOI] [PubMed] [Google Scholar]
- 6.Council Directive 83/570/EEC of 26 October 1983 amending Directives 65/65/EEC, 75/318/EEC and 75/319/EEC on the approximation of provisions laid down by law, regulation or administrative action relating to proprietary medicinal products. OJL. :1–10. 332, 28.11.1983, Available at http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:31983L0570:EN:HTML (Accessed July 2011) [Google Scholar]
- 7.Alvarez FJ, del Rio MC. Drugs and driving. Lancet. 1994;344:282. doi: 10.1016/s0140-6736(94)91335-8. [DOI] [PubMed] [Google Scholar]
- 8.de Gier JJ, Alvarez FJ, Mercier-Guyon C, Verstraete AG. Prescribing and dispensing guidelines for medicinal drugs affecting driving performance. In: Verster JC, Pandi-Perumal SR, Ramaekers JG, de Gier JJ, editors. Drugs, Driving and Traffic Safety. Basel: Birkaeuser Verlag AG; 2009. pp. 121–34. [Google Scholar]
- 9.Álvarez FJ, Del Río MC. Medicinal drugs and driving: from research to clinical practice. Trends Pharmacol Sci. 2002;23:441–5. doi: 10.1016/s0165-6147(02)02083-7. [DOI] [PubMed] [Google Scholar]
- 10.European Commission. Enterprise and Industry Directorate-GeneralA guideline on summary of product characteristics (SmPC) 2009. [online]. Available at http://ec.europa.eu/health/files/eudralex/vol-2/c/smpc_guideline_rev2_en.pdf and http://ec.europa.eu/health/documents/eudralex/vol-2/index_en.htm (Accessed July 2011)
- 11.de Gier JJ. Drugs and driving research: application of results by drug regulatory authorities. Hum Psychopharmacol Clin Exp. 1998;13:S133–S136. [Google Scholar]
- 12.Pil K, Raes E, van den Neste T, Goessaert AS, Veramme J, Verstraete AG. Review of existing classification efforts (DRUID Deliverable 4.1.1.) 2008. Available at http://www.druid-project.eu/cln_007/nn_107534/Druid/EN/deliverales-list/deliverables-list-node.html?__nnn=true (Accessed July 2011)
- 13.Wolschrijn H, de Gier JJ, de Smet PAGM. Drugs and driving: a new categorization system for drugs affecting psychomotor performance. Institute for Drugs, Safety and Behavior, University of Limburg, the Netherlands. 1991. Tech Report.
- 14.Toxicological Society of Belgium and Luxembourg asbl. Influence des médicaments sur les capacités de conduire. Bruxelles: Toxicological Society of Belgium and Luxembourg asbl; 1999. [Google Scholar]
- 15.Del Río MC, Alvarez FJ, González-Luque JC. Guía De Prescripción Farmacológica Y Seguridad Vial [The Pharmaceutical Prescription Guidelines and Road Safety] 2nd edn. Madrid: Dirección General de Tráfico; 2002. [Google Scholar]
- 16.Agency for Medicinal Products and Medical Devices of the Republic of Slovenia. 2006. Rules on labelling of medicinal products and on the packaging leaflet. Official Journal of the Republic of Slovenia, No. 54/06 and the Medicinal Products Act (Official Journal of the Republic of Slovenia, No. 31/06)
- 17.Prescribing and Dispensing Guidelines for Medicinal Drugs Affecting Driving Performance. Utrecht: International Council on Alcohol, Drugs and Traffic Safety; 2001. ICADTS Working Group on Prescribing and Dispensing Guidelines for Medicinal Drugs Affecting Driving Performance. Available at http://www.icadts.nl/medicinal.html (Accessed July 2011) [Google Scholar]
- 18. Ministère de la Santé et des Solidarités. Direction Generale de la Santé. Arrêté du 18 Juillet 2005 pris pour l'application de l'article R.5121-139 du code de la santé publique et relative à l'opposition d'un pictogramme sur le conditionnement extérieur de certain médicaments et produitsJournal Officiel de la République Française. Août 2005 (SAN/P0522726A)
- 19.Real Decreto 1345/2007, de 11 de octubre, por el que se regula el procedimiento de autorización, registro y condiciones de dispensación de los medicamentos de uso humano fabricados industrialmente. pp. 45652–45698. BOE de 7 de Noviembre de 2007.
- 20.Koninklijke Nederlandse Maatschappij ter bevordering der Pharmacie (KNMP) Contra-indicatie Verkeersdeelname. Contra-indication ‘Participation in traffic’ (in Dutch)Instituut voor Verantwoord Medicijngebruik. 2008. [online]. Available at http://www.geneesmiddeleninhetverkeer.nl (Accessed July 2011)
- 21.Agence Française de Sécurité Sanitaire des Produits de Santé (AFSSAPS) – Médicaments et conduite automobile – Mise au point. 6/04/2009. Available at http://www.afssaps.fr/Infos-de-securite/Recommandations/Medicaments-et-conduite-automobile-Mise-au-point/(language)/fre-FR (Accessed July 2011) [DOI] [PubMed]
- 22. Agencia Española de Medicamentos y Productos Sanitarios. Medicamentos y Conduccion. Available at http://www.aemps.gob.es/industria/etiquetado/conduccion/home.htm (Accessed July 2011)
- 23. Driving Under the Influence of Drugs, Alcohol and Medicines – DRUID. Available at http://www.druid-project.eu/ (Accessed July 2011)
- 24.Gómez-Talegón T, Fierro I, Del Río MC, Álvarez FJ. Establishment of framework for classification/categorisation and labelling of medicinal drugs and driving. Deliverable 4.3.1, 2011. DRUID (Driving under the Influence of Drugs, Alcohol and Medicines). 6th Framework programme. Available at http://www.druid-project.eu/ (Accessed July 2011)
- 25. Collaborating WHO Centre for Drug Statistics Methodology. ATC/DDD index. Available at http://www.whocc.no/atc_ddd_index/ (Accessed July 2011)
- 26. Medicines and Healthcare Products Regulatory Agency- MHRA. Available at http://www.mhra.gov.uk/index.htm (Accessed July 2011)
- 27. Electronic Medicines Compendium – eMC. Available at http://www.medicines.org.uk/EMC/default.aspx (Accessed July 2011)
- 28. Irish Medicines Board – IMB. Available at http://www.imb.ie/ (Accessed July 2011)
- 29. European Medicines Agency – EMAHuman Medicines. European public assessment report. Available at http://www.ema.europa.eu/ema/index.jsp (Accessed July 2011)
- 30.Talbot J, Stephens MDB. Clinical trials: – collection of safety data and establishing the adverse drug reaction profile. In: Talbot J, Stephens MDB, editors. Stephens' Detection of New Adverse Drug Reactions. 5thedn. Chichester: John Wiley and Sons, Ltd; 2004. pp. 167–242. [Google Scholar]
- 31.de Gier JJ, Ravera S, Monteiro SP, Álvarez FJ. 2011. Establishment of criteria for a European categorisation system for medicinal drugs and driving. Deliverable 4.2.1, 2011. DRUID (Driving under the Influence of Drugs, Alcohol and Medicines). 6th Framework programme. Available at http://www.druid-project.eu/ (Accessed July 2011)
- 32.EMA. Pharmacovigilance Working Party (PhVWP) Monthly report Issue number: 1106. June 2011 plenary meeting. EMA/CHMP/PhVWP/486894/2011. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Report/2011/06/WC500108098.pdf (Accessed October 2011)


