Abstract
AIMS
In large randomized trials, thromboprophylaxis with fondaparinux in major orthopaedic surgery (MOS) has been shown to be superior to low molecular weight heparin (LMWH) prophylaxis with comparable safety. However, patients treated under trial conditions are different from unselected patients and efficacy and safety outcomes may be different in unselected patients in daily practice. We performed a retrospective cohort study to compare the efficacy and safety of venous thromboembolism (VTE) prophylaxis with fondaparinux or LMWH in 3896 consecutive patients undergoing major orthopaedic surgery at our centre.
METHODS
All patients undergoing MOS between January 2006 and December 2009 were retrospectively analyzed using patient charts, hospital admission and discharge database, quality management database, transfusion unit database and VTE event documentation. VTE standard prophylaxis at our institution was LMWH (3000–6000 aXa units once daily) from January 2006 to December 2007 or fondaparinux 2.5 mg from January 2008 to December 2009. In these two large cohorts of unselected consecutive patients, in-hospital incidences of VTE, surgical complications, severe bleeding and death were evaluated.
RESULTS
Symptomatic VTE was found in 4.1% of patients in the LMWH group (62/1495 patients; 95% CI 0.032, 0.052) compared with 5.6% of patients receiving fondaparinux (112/1994 patients, 95% CI 0.047, 0.067; P= 0.047). Distal deep vein thrombosis (DVT) was significantly more frequent in the fondaparinux group (3.9%, 95% CI 0.031, 0.048; vs. 2.5%; 95% CI 0.018, 0.034; P= 0.021). No significant differences in the rates of major VTE or death were found. Rates of severe bleeding, transfusion of RBC concentrates, plasma and platelet concentrates were comparable between both treatment groups. However, patients receiving fondaparinux had significantly lower rates of surgical revisions (1.6%, 95% CI 0.011, 0.022 vs. 3.7%, 95% CI 0.028, 0.047; P < 0.001). Multivariate analysis revealed previous VTE (HR 18.2, 95% CI 11.6, 28.5; P < 0.001) and female gender (HR 1.9, 95% CI 1.3, 2.7; P < 0.001), but not fondaparinux prophylaxis (HR1.3, 95% CI 0.9, 1.7; P= 0.184) to be associated with significantly increased VTE risk.
DISCUSSION
Thromboprophylaxis with fondaparinux is less effective to prevent distal VTE than LMWH in unselected patients undergoing MOS, but is equally effective with regard to rates of major VTE and death. However, differences in efficacy of LMWH or fondaparinux are of little relevance compared with a history of VTE or female gender, which were found to be the main VTE risk factors in MOS. The safety profile of fondaparinux was comparable with LMWH with regard to rates of severe bleeding complications, but patients receiving fondaparinux had significantly less surgical complications requiring surgical revisions. Both our efficacy and safety findings differ from data derived from large phase III trials testing fondaparinux against LMWH in MOS, where overall rates of symptomatic VTE were lower and the safety profile of fondaparinux was different.
CONCLUSION
We conclude that the strict patient selection and surveillance in phase-III trials results in lower VTE and bleeding event rates compared with unselected routine patients. Consequently, the efficacy and safety profile of thromboprophylaxis regimens needs to be confirmed in large registries or phase IV trials of unselected patients.
Keywords: bleeding, deep vein thrombosis, fondaparinux, venous thromboembolism, VTE prophylaxis
WHAT IS ALREADY KNOWN ABOUT THE TOPIC
Patients undergoing major orthopaedic surgery are at high risk of venous thromboembolism.
Medical thromboprophylaxis is effective to reduce this risk and in large trials, fondaparinux proved superiority over low molecular weight heparin (LMWH) in this indication with the downside of higher complication rates.
Patients in clinical trials are a selected subpopulation and efficacy and safety results need to be confirmed in large cohorts of unselected patients.
WHAT THIS STUDY ADDS
In unselected patients undergoing major orthopaedic surgery, thromboprophylaxis with fondaparinux was equally effective to prevent major venous thromboembolism (VTE), but inferior to prevent distal deep vein thrombosis (DVT) compared with low molecular weight heparin.
The rates of bleeding complications or blood transfusions were similar for patients receiving fondaparinux and LMWH, but use of fondaparinux was associated with significantly lower rates of surgical complications, which contributed to a shorter hospital stay.
Overall, we found much higher rates of symptomatic VTE and bleeding events in our large cohort of unselected patient in daily care compared with results of large phase III trials evaluating anticoagulants in this indication, indicating a selection bias in these prospective trials.
Introduction
Patients undergoing major orthopaedic surgery are at high risk for venous thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism (PE) [1, 2]. Pharmacological prophylaxis with low molecular weight heparins (LMWH) reduces VTE rates [3, 4]. Their use, however, is associated with an increased bleeding risk. Other side effects include allergic skin reactions and heparin-induced thrombocytopenia as LMWH are derived from animal sources. The indirect selective factor Xa inhibitor fondaparinux is a synthetic pentasaccharide. It has been developed to provide a selective anticoagulant effect and to overcome some side effects of LMWH such as allergic reactions [5–11].
A meta-analysis of large randomized controlled trials (RCTs) of VTE prophylaxis in major orthopaedic surgery [12, 13] found a 50% risk reduction for any VTE using fondaparinux 2.5 mg once daily compared with the LMWH enoxaparin (at doses of 40 mg once daily or 30 mg twice daily). Overall, the rates of bleeding, surgical complications and mortality were not different [12].
Based on these data, our institution switched from LMWH to fondaparinux for VTE prophylaxis in patients undergoing major orthopaedic surgery.
Translating results of RCTs into clinical practice is limited by the fact that selected study populations may not be representative for cohorts of unselected every day patients due to selection bias. Only selected institutions, often academic or academia-affiliated, participate in clinical trials and may not be representative for all institutions performing the intervention under investigation. At these sites, patients eligible for study participation are selected from all patients treated for this indication. The motivation of patients to or not to participate in a trial may also contribute to selection bias [14–17]. Finally, patients included in a trial have to fulfil strict inclusion and exclusion criteria and, therefore, are more homogenous than the whole group of patients [16, 18]. Consequently, results of RCTs have to be confirmed in large cohorts of unselected patients.
The objective of this retrospective cohort study was to compare the efficacy and safety of VTE prophylaxis with fondaparinux or LMWH in 3896 consecutive patients undergoing major orthopaedic surgery at our centre.
Methods
Patients
All patients undergoing major orthopaedic surgery at the University Clinic ‘Carl Gustav Carus’ in Dresden, Germany between January 2006 and December 2009 were retrospectively analyzed.
Patients were identified using the hospital admission and discharge database and patient characteristics (age, gender, type of surgery, length of hospital stay) were extracted. For all patients, the performed surgical procedure was confirmed by cross-comparison with the quality management database of the Clinic of Orthopaedic Surgery. In this database, all complications such as postoperative VTE, amount of transfusions of red blood cells, fresh frozen plasma, platelet concentrates and fibrinogen concentrates, and surgical revisions are exactly documented. These data were extracted and cross-checked by comparison with the VTE database of the Department of Vascular Medicine and the transfusion database of the blood tranfusion unit of our hospital. Pre- and intra-operative protocols of the Department of Anaesthesiology were reviewed for demographic parameters (age, height and body weight, co-morbidities, co-medication) and for surgical complications. Again, these data were cross-checked with the quality management database of the Clinic of Orthopaedic Surgery. From the laboratory database, pre-operative laboratory coagulation values of platelet count, INR, activated partial thromboplastin time as well as creatinine were imported into the database. For a final cross-check, discharge letters were reviewed for consistency of VTE and bleeding complications, co-morbidity, co-medication and outcome for all patients. The flowchart of data analysis is given in Figure 1.
Figure 1.
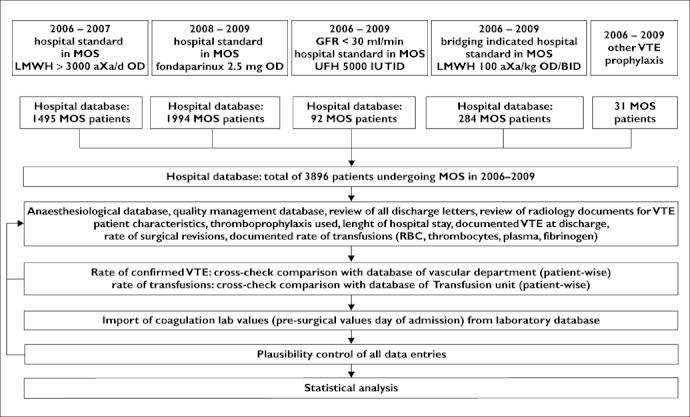
Flowchart of data analysis. Review of efficacy and safety of venous thromboembolism (VTE) prophylaxis in 3896 patient undergoing major orthopaedic surgery (MOS) between 2006 and 2009. LMWH = low molecular weight heparin; UFH = unfractionated heparin; aXa day−1= anti Factor Xa units day–1; OD = once daily; BID = twice daily; TID = three times daily; GFR = glomerular filtration rate; RBC = packed red blood cell concentrates
VTE prophylaxis
From January 2006 to December 2007, hospital guidelines recommended VTE prophylaxis with LMWH 3000 to 6000 aXa units once daily for patients undergoing major orthopaedic surgery, who had no contraindications for LMWH prophylaxis. Substance and dosage were chosen by the attending surgeon according to regulatory approval as well as national and hospital VTE prophylaxis guidelines. VTE prophylaxis with LMWH was started on the evening before surgery. In January 2008, hospital guidelines were changed to recommend VTE prophylaxis with fondaparinux 2.5 mg in major orthopaedic surgery starting on the evening after surgery. For all patients undergoing major orthopaedic surgery, continued thromboprophylaxis was recommended until day 35 post surgery. Patients with contraindications for LMWH or fondaparinux prophylaxis received other pharmacological interventions or mechanical thromboprophylaxis. These patients were also identified in our registry but excluded from further analysis. Therefore, two large cohorts of unselected, consecutive patients receiving thromboprophylaxis with either LMWH or fondaparinux were investigated in our study with regard to in-hospital incidences of VTE, surgical complications, severe bleeding and death.
Surgical procedures
Surgical procedures classified as major orthopaedic surgery included primary and secondary total hip and knee replacement. Surgical techniques and selection of prosthetic devices did not change substantially during the observational period. Furthermore, the standards and procedures of our Department of Anaesthesia for patients undergoing major orthopaedic surgery were not changed between 2006 and 2009.
Efficacy endpoints
Due to the retrospective design, only symptomatic VTE events were used as efficacy endpoints.
For all patients with suspected VTE events, reports and images of venous compression ultrasound, CT scans and ventilation perfusion scintigraphy were reviewed.
According to our hospital guidelines, all patients with clinical suspicion of DVT underwent immediate (within 24 h) bilateral complete compression ultrasound (CCUS) in our vascular unit by vascular specialists experienced in compression ultrasound using a standardized CCUS protocol [19, 20]. Proximal DVT was defined as any DVT occurring in a vein of the deep vein system of the lower extremity at the level of the popliteal vein or above. Distal DVT was defined as any DVT occurring in a vein of the deep vein system below the level of the popliteal vein, PE was defined as PE objectively confirmed in computed tomography (CT) or ventilation-perfusion (VQ) scan and major VTE was defined as the combination of symptomatic proximal DVT and symptomatic PE.
The adjudication criteria for VTE and VTE-related death were:
symptoms leading to CCUS, CT scan or VQ scan
presence of a thrombus in objective testing
autopsy confirmed VTE as cause of death
any sudden unexplained death.
For all patients dying during the postoperative period until hospital discharge, death certificates and autopsy reports were reviewed.
The efficacy endpoint was the rate of all VTE events. Furthermore, rates of VTE subtypes such as proximal or distal symptomatic DVT or symptomatic PE were evaluated at hospital discharge.
Safety endpoints
The primary endpoint for safety analysis was the rate of severe bleeding complications using a modification of the ISTH standard definition for ‘major bleeding’[21]. Since most patients develop a significant drop in haemoglobin levels after major orthopaedic surgery and time of postoperative blood sampling or blood transfusion are not standardized for a retrospective analysis, we excluded the standard criterion ‘asymptomatic drop of haemoglobin of at least 2 g dl–1’ from our analysis. Therefore, the endpoint of severe bleeding was defined as an overt or suspected bleeding with either
a documented transfusion of at least 2 units of packed red blood cells (RBC),
a surgical revision due to bleeding or
a bleeding into critical site such as intracranial, intra-ocular, intra-articular, retroperitoneal and overt gastrointestinal bleeding.
As strict hospital standards for transfusion tresholds have been established at our institution for years we analyzed transfusion requirements for all patients. The documentation of blood transfusions at our centre is complete, since all transfusion units have a track record and need to be documented patient-wise in the transfusion unit database of our hospital as well as in discharge documentations of every patient. Therefore, the transfusion rates of RBC units, plasma and platelet concentrates could be exactly determined for every patient and were used as a surrogate parameter to evaluate severe bleeding complications.
Secondary safety endpoints were
any transfusion of RBC, platelet or thrombocyte concentrates
any surgical revision
death from any cause
the length of hospital stay
The rate of surgical revisions (related and unrelated to bleeding complications) and other post surgical complications such as bleeding into a critical site are documented in the quality management database of the Clinic of Orthopaedic Surgery and could be determined exactly with detailed reports available. All safety endpoints were evaluated until hospital discharge.
Statistics
The two treatment groups were investigated for differences in baseline variables. Binary data were compared by Fisher's exact test, continuous data were examined for differences in means by Student's t-test and anova. The treatment groups differed with regard to the mean age and the proportions of men and women. Therefore, all comparisons were carried out using ancova including interaction effects. Binary data were analyzed in the same way using logistic regression models. 95% confidence intervals for proportions are given according to Blyth Still Casella.
For further analyses age, gender and BMI were considered to be potential confounders and were standardized by using the overall cohort's mean as the standard. The risk for VTE was modelled using Cox proportional hazard models. The proportional hazard assumption was not violated. In a first step, the crude hazard ratio was estimated. In a second step, this hazard ratio was adjusted for confounding factors. In a third step, both potential risk factors and their interaction terms were included into the model. In the last step, model terms contributing no significant effect to the model were removed.
Length of hospital stay was analyzed by Kaplan–Meier estimation and assessed by log-rank testing for the total cohort. Of note, subgroups with or without VTE or bleeding complications were also assessed by Kaplan–Meier estimation, but results are of descriptive value only, since interference of confounding factors, such as early discharge policy, did not allow for log-rank testing.
All statistical analyses were carried out using the IBM® SPSS® Statistics Version 19 and the StatXact 8.
The study protocol was approved by the local ethics committee. Due to the retrospective nature of our study and the strict pseudonymization of patient data, the local ethics committee and data protection authority did not request the patient's informed consent.
Results
Of the 3896 patients undergoing major orthopaedic surgery between January 2006 and December 2009, 2439 patients underwent hip arthroplasty (69.9%), 1278 patients underwent knee arthroplasty (36.6%) and 179 patients underwent surgery for explantation of artificial hip or knee joint (5.1%). During the observational period, all 3896 patients received VTE prophylaxis according to guidelines and hospital standard. Of these, 1495 patients (38.4%) received LMWH prophylaxis (hospital standard 2006–2007), 1994 patients (51.2%) received fondaparinux (hospital standard 2008–2009) and 407 patients (10.4%) received other prophylaxis (study drugs, unfractionated heparin due to renal insufficiency, therapeutic anticoagulation due to underlying disease) (Table 1). In the LMWH group, VTE prophylaxis was with certoparin 3000 IU once daily (62.3%), nadroparin 3000 IU once daily (14.7%), nadroparin 4000–6000 IU once daily (14.4%), enoxaparin 4000 IU once daily (8.5%) or tinzaparin 3500 IU once daily (0.1%). Of note, dose adjustments in the LMWH group were necessary, since certoparin is approved at a dosage of 3000 IU once daily, nadroparin at dosages of 3000, 4000 and 6000 IU day−1 (according to body weight and time after surgery), enoxaparin at 4000 IU once daily and tinzaparin at 3500 IU once daily.
Table 1A.
Patient characteristics and type of VTE prophylaxis in all patients undergoing major orthopaedic surgery between January 2005 and December 2009
| Prophylactic LMWH > 3000 aXa once daily mean ± SD | Fondaparinux 2.5 mg once daily mean ± SD | t-test P value | UFH 5000 IU three times daily mean ± SD | Therapeutic LMWH 100–200 aXa kg−1 day−1 mean ± SD | Other pharmacological or mechanical VTE prophylaxis mean ± SD | |
|---|---|---|---|---|---|---|
| n | 1495 | 1994 | 92 | 284 | 31 | |
| Male/Female | 667/828 | 721/1273 | <0.001 (Chi squared) | 47/45 | 149/135 | 12/19 |
| ratio | 0.8 | 0.6 | 1.0 | 1.1 | 0.6 | |
| History of VTE | 0.94% | 0.95% | >0.999 | 3.4% | 24.6% | 6.5% |
| yes/no % (n) | 14/1481 | 19/1975 | 3/89 | 70/214 | 2/31 | |
| Age (years) | 62.3 ± 13.3 | 66. 8 ± 11.9 | <0.001 | 72.1 ± 11.6 | 71.3 ± 9.9 | 67.7 ± 11.5 |
| Height (cm) | 168.5 ± 9.4 | 167.0 ± 9.7 | <0.001 | 167.7 ± 10.6 | 169.1 ± 10.0 | 168.8 ± 6.4 |
| Weight (kg) | 79.4 ± 16.5 | 80.9 ± 18.4 | 0.008 | 80.2 ± 18.3 | 84.4 ± 17.8 | 84.2 ± 14.1 |
| BMI (kg m–2) | 27.9 ± 5.2 | 29.0 ± 5.8 | 0.945 | 28.5 ± 5.9 | 29.4 ± 5.5 | 29.5 ± 5.3 |
| Platelet count (Gpt l–1) | 232.2 ± 83.0 | 207.1 ± 66.6 | <0.001 | 212.2 ± 120.2 | 205.7 ± 76.4 | 250.7 ± 58.0 |
| INR | 1.07 ± 0.12 | 1.11 ± 0.12 | <0.001 | 1.1 ± 0.2 | 1.2 ± 0.4 | 1.1 ± 0.2 |
| aPTT (s) | 28.9 ± 4.5 | 28.6 ± 7.1 | 0.181 | 31.6 ± 8.2 | 30.9 ± 5.1 | 28.4 ± 3.3 |
| Creatinine (µmol l–1) | 64.1 ± 23.1 | 63.1 ± 19.8 | 0.198 | 187.8 ± 146.9 | 72.0 ± 27.2 | 94.1 ± 108.6 |
LMWH = low molecular weight heparin; aXa = anti-Factor Xa units; UFH = unfractionated heparin.
Table 1B.
Patient characteristics and type of VTE prophylaxis in patients receiving LMWH or fondaparinux prophylaxis between January 2005 and December 2009. anova group comparisons using covariate analysis for type of thromboprophylaxis, gender and demographic parameters as variables
| LMWH n= 1495 | Fondaparinux n= 1994 | |||||
|---|---|---|---|---|---|---|
| Parameter | Gender | Mean | 95% CI | Mean | 95% CI | P value |
| History of VTE [%] | Male | 0.31 | 0.05, 1.06 | 0.56 | 0.19, 1.39 | 0.751 |
| Female | 1.46 | 0.82, 2.48 | 1.11 | 0.65, 1.81 | ||
| BMI [kg m−2] | Male | 28.0 | 27.6, 28.3 | 29.1 | 28.7, 29.4 | <0.001 |
| Female | 27.8 | 27.5, 28.2 | 28.9 | 28.6, 29.2 | ||
| Platelet count (Gpt l−1) | Male | 220.1 | 215.3, 224.8 | 195.6 | 191.0, 200.1 | <0.001 |
| Female | 239.7 | 235.4, 244.1 | 215.2 | 211.4, 219.0 | ||
| INR | Male | 1.074 | 1.07, 1.08 | 1.117 | 1.11, 1.12 | <0.001 |
| Female | 1.068 | 1.06, 1.08 | 1.11 | 1.105, 1.12 | ||
| aPTT (s) | Male | 29.0 | 28.6, 29.4 | 28.6 | 28.2, 29.0 | 0.044 |
| Female | 28.9 | 28.6, 29.3 | 28.5 | 28.2, 28.8 | ||
| Creatinine (µmol l−1) | Male | 73.22 | 71.94, 74.51 | 72.36 | 71.13, 73.59 | 0.211 |
| Female | 57.99 | 56.81, 59.17 | 57.12 | 56.10, 58.14 | ||
LMWH = low molecular weight heparin; aXa = anti-Factor Xa units.
Patients in the fondaparinux group were more often female (63.8 vs. 55.4%), were older (66.8 vs. 62.3 years), had lower platelet counts (207 vs. 232 Gpt l−1) and a higher INR (1.2 vs. 1.1) compared with patients receiving prophylactic LMWH. These differences were statistically significant (Table 1). Of note, the number of patients with a history of VTE was low in both groups, since hospital standard at that time recommended therapeutic dosages of LMWH for these patients (Table 1).
Group differences in age, gender and BMI were regarded as clinically significant and in the following analyses, event rates were adjusted according to these parameters.
The rate of all symptomatic VTE events was 4.1% in the LMWH prophylaxis group (62/1495 patients, 95% CI 0.03, 0.05) and 5.6% in patients receiving fondaparinux (112/1994 patients, 95% CI 0.05, 0.07; P= 0.047). Between both treatment arms, no significant differences in rates for major VTE and death were found (Table 2A). Patients receiving thromboprophylaxis with fondaparinux had a significantly higher rate of distal DVT (3.9%, 95% CI 0.03, 0.05) compared with patients treated with LMWH (2.5%, 95% CI 0.02, 0.03; P= 0.021).
Table 2A.
Efficacy endpoints in all patients receiving prophylactic LMWH or fondaparinux for thromboprophylaxis after major orthopaedic surgery (95% Blyth-Still-Casella confidence intervals)
| LMWH n= 1495 | Fondaparinux n= 1994 | ||||
|---|---|---|---|---|---|
| Efficacy endpoints | % (n) | 95% CI | % (n) | 95% CI | P value |
| All VTE | 4.15 (62) | 3.19–5.23 | 5.62 (112) | 4.67–6.68 | 0.047 |
| Proximal DVT | 1.07 (16) | 0.64–1.69 | 1.30 (26) | 0.85–1.87 | 0.639 |
| PE | 0.54 (8) | 0.23–1.03 | 0.50 (10) | 0.27–0.89 | 0.999 |
| Distal VTE | 2.47 (37) | 1.78–3.37 | 3.91 (78) | 3.10–4.83 | 0.021 |
The safety of fondaparinux prophylaxis was comparable with LMWH prophylaxis with regard to the primary safety endpoint of severe bleeding as well as for the secondary safety endpoints, transfusions of units of RBCs, platelets or plasma concentrates (Table 2B). However, patients receiving LMWH prophylaxis had significantly more surgical complications leading to revision surgery (3.7%, 95% CI 0.03, 0.05 vs. 1.6%, 95% CI 0.01, 0.02; P < 0.001; Table 2B).
Table 2B.
Safety endpoints in all patients receiving prophylactic LMWH or fondaparinux for thromboprophylaxis after major orthopaedic surgery (95% Blyth-Still-Casella confidence intervals)
| LMWH n= 1495 | Fondaparinux n= 1994 | ||||
|---|---|---|---|---|---|
| Safety endpoints | % (n) | 95% CI | % (n) | 95% CI | P value |
| Severe bleeding | 12.58 (188) | 10.94, 14.35 | 11.13 (222) | 9.80, 12.59 | 0.202 |
| Transfusion > 2 RBC concentrates | 11.57 (173) | 9.99, 13.27 | 10.58 (211) | 9.27, 11.97 | 0.547 |
| Surgical revisions due to bleeding complications | 1.34 (20) | 0.85, 2.03 | 1.10 (22) | 0.69, 1.65 | 0.535 |
| Bleeding into critical site | 0.07 (1) | 0.001, 0.37 | 0.05 (1) | 0.001, 0.28 | 0.999 |
| Any surgical revision | 3.68 (55) | 2.81, 4.75 | 1.61 (32) | 1.10, 2.23 | <0.001 |
| Transfusion of plasma concentrates | 7.09 (106) | 5.84, 8.49 | 5.97 (119) | 4.97, 7.06 | 0.186 |
| Transfusion of platelet concentrates | 1.34 (20) | 0.85, 2.03 | 0.70 (14) | 0.41, 1.14 | 0.080 |
| Any death | 0.07 (1) | 0.001, 0.37 | 0.10 (2) | 0.02, 0.35 | 0.999 |
| Length of hospital stay (days) | 11.1 | 10.6, 11.3 | 9.3 | 9.1, 9.5 | <0.001 |
| Length of hospital stay (days) Median (25th and 75th percentile) | 9 (8, 11) | 9 (8, 9) | <0.001 | ||
Interestingly, efficacy and safety of LMWH and fondaparinux thromboprophylaxis differed in subgroups of patients according to the length of hospital stay (Table 3). Rates of VTE, bleeding and surgical complication events increased with prolonged hospitalization, indicating a causal relationship. However, significant differences between LMWH and fondaparinux prophylaxis were only seen in subgroups of patients. While VTE event rates were only numerically higher in fondaparinux patients discharged up until day 9, this difference became more pronounced and statistically significant in patients discharged later than day 9. On the other hand, bleeding complications were significantly more common in fondaparinux patients discharged before day 9 compared with LMWH prophylaxis. Finally, surgical revisions were more often seen in patients receiving LMWH prophylaxis and discharged after day 9.
Table 3.
Event rates for VTE, bleeding complications and surgical revisions according to type of thromboprophylaxis and length of hospital stay. Rates of events increased in all groups according to duration of hospitalization, indicating causal relationship. Of note, significant differences between LMWH and fondaparinux prophylaxis were only seen in subgroups of patients
| Type of thromboprophylaxis | ||||||||
|---|---|---|---|---|---|---|---|---|
| LMWH (n= 1474) | Fondaparinux (n= 1973) | |||||||
| Event | Length of hospital stay (days) | n | % | 95% CI | n | % | 95% CI | P value |
| Any VTE | 0–8 | 6/502 | 1.20 | 0.4, 2.6 | 13/903 | 1.44 | 0.8, 2.4 | 0.813 |
| 9 | 19/487 | 3.90 | 2.4, 6.0 | 30/583 | 5.15 | 3.5, 7.3 | 0.380 | |
| >9 | 35/485 | 7.22 | 5.1, 9.9 | 65/487 | 13.35 | 10.5, 16.7 | 0.002 | |
| Severe bleeding | 0–8 | 11/502 | 2.19 | 1.1, 3.9 | 41/903 | 4.54 | 3.3, 6.1 | 0.027 |
| 9 | 20/487 | 4.11 | 2.5, 6.3 | 38/583 | 6.52 | 4.7, 8.8 | 0.103 | |
| >9 | 150/485 | 30.93 | 26.8, 35.3 | 140/487 | 28.75 | 24.8, 33.0 | 0.483 | |
| Surgical revision | 0–8 | 2/502 | 0.40 | 0.0, 1.4 | 3/903 | 0.33 | 0.1, 1.0 | 0.999 |
| 9 | 1/487 | 0.21 | 0.0, 1.1 | 3/583 | 0.51 | 0.1, 1.5 | 0.630 | |
| >9 | 50/485 | 10.31 | 7.7, 13.4 | 26/487 | 5.34 | 3.5, 7.7 | 0.004 | |
Three patients died during hospital stay (one treated with LMWH and two treated with fondaparinux). All deaths were caused by septic complications leading to multi-organ failure. One of the two patients treated with fondaparinux who died, was a 74-year-old lady with Alzheimer's disease and septic hip infection who developed relevant wound haematoma as well as proximal DVT after surgery, both of which which did not cause death.
The mean length of hospital stay was significantly shorter in the fondaparinux group (9.3 days, 95% CI 9.1, 9.5 vs. 10.9 days, 95% CI 10.6, 11.3; P < 0.001). This finding was caused by a statistically significant difference in the 75th percentile (Table 2B) between both treatment groups, who had the same 25th and 50th percentile for length of hospitalization. Furthermore, subgroup analyses were carried out describing the influence of complications or type of thromboprophylaxis on length of hospitalization (Figure 2). Of note, due to the interference of confounding factors such as early discharge policy, the Kaplan–Meier curves in Figure 2 are of descriptive nature only and were not statistically tested for significance. A difference in length of hospitalization was found in patients without any VTE, bleeding or surgical complications (Figure 2A), indicating that the existing trend to early discharge due to economic considerations contributed to this finding. Interestingly, the occurrence of VTE complications contributed to a comparable prolongation of hospitalization in both treatment groups (Figure 2B).
Figure 2.
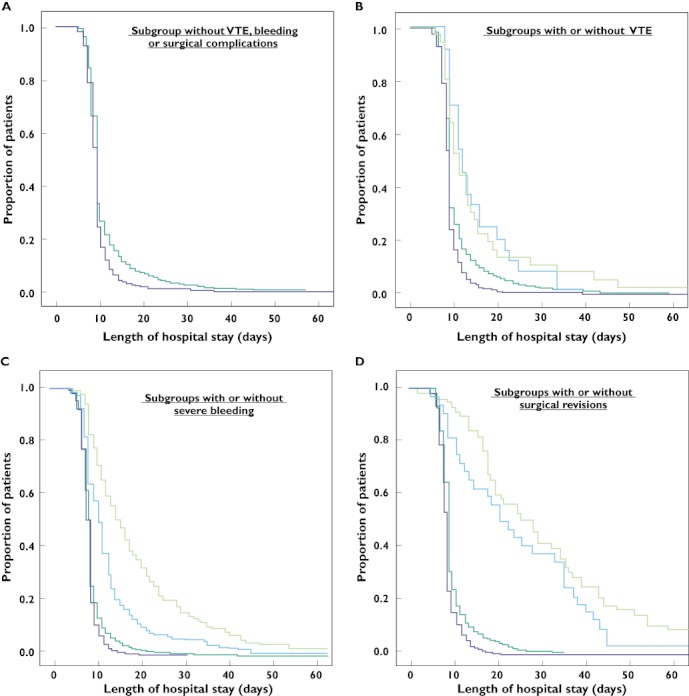
Kaplan–Meier analysis of hospital discharge (length of hospital stay in days) according to treatment group (A), subgroups with and without VTE (B), subgroups with and without severe bleeding (C) and subgroups with and without surgical revisions (D).  , LMWH;
, LMWH;  , fondaparinux;
, fondaparinux;  , LMWH without symptomatic VTE;
, LMWH without symptomatic VTE;  , LMWH with symptomatic VTE;
, LMWH with symptomatic VTE;  , fondaparinux without symptomatic VTE;
, fondaparinux without symptomatic VTE;  , fondaparinux with symptomatic VTE;
, fondaparinux with symptomatic VTE;  , LMWH without severe bleeding;
, LMWH without severe bleeding;  , LMWH with severe bleeding;
, LMWH with severe bleeding;  , fondaparinux without severe bleeding;
, fondaparinux without severe bleeding;  , fondaparinux with severe bleeding;
, fondaparinux with severe bleeding;  , LMWH without revision;
, LMWH without revision;  , LMWH with revision;
, LMWH with revision;  , fondaparinux without revision;
, fondaparinux without revision;  , fondaparinux with revision
, fondaparinux with revision
In contrast, in cases of bleeding or surgical complications, our analysis indicated that these patients had longer hospitalizations with LMWH than with fondaparinux prophylaxis (Figure 2C,D), which also contributed to the finding of a shorter hospital stay in the fondaparinux group.
Risk factors for VTE
Using the type of thromboprophylaxis as co-variables, a multivariate analysis using a Cox logistic regression model was used to evaluate potential risk factors for the occurrence of VTE complications. A total number of 3447 patients had complete data for this analysis (1474 LMWH and 1973 fondaparinux), who experienced a total of 168 VTE events (60 and 108, respectively).
The first step of the risk analysis included type of thromboprophylaxis only and found the use fondaparinux to be related to an increased risk for VTE (HR 2.29, 95% CI 1.66, 3.16). After adjustment for age- and gender-related differences, the HR decreased to 1.80 (95% CI 0.52, 6.24), which failed to reach statistical significance.
The third step included ‘platelet count’, ‘positive history of VTE’ and ‘BMI’, which improved analysis. The last step excluded group-specific age- and gender-corrections from analysis. The results of multivariate analysis are given in Table 4.
Table 4.
Multivariate analysis using Cox logistic regression model to evaluate potential risk factors for the occurrence of VTE in patients receiving prophylactic LMWH or fondaparinux for thromboprophylaxis after major orthopaedic surgery (n= 3447)
| Patients with/without VTE | |||
|---|---|---|---|
| HR | 95% CI | P value | |
| Use of fondaparinux | 1.25 | 0.90, 1.75 | 0.184 |
| Female gender | 1.89 | 1.30, 2.75 | 0.001 |
| Age (per year) | 1.03 | 1.01, 1.04 | <0.001 |
| Platelet count | 0.99 | 0.99, 1.00 | 0.024 |
| Previous VTE | 18.21 | 11.64, 28.48 | <0.001 |
| BMI (per unit) | 1.05 | 1.02, 1.08 | <0.001 |
HR = hazard ratio.
A positive history of VTE (HR 18.3) followed by female gender (HR 1.9) were found to be the most relevant independent risk factors for VTE in our cohort. In contrast, fondaparinux use (HR 1.3) was not an independent VTE risk factor.
Discussion
High rate of symptomatic VTE in unselected routine patients
The rate of symptomatic VTE in these large unselected cohorts of routine patients presented was 4.1–5.6% (1.6–1.8% major VTE, 0.5% PE) and thereby nearly 10-fold higher compared with phase III trials evaluating fondaparinux or LMWH (symptomatic VTE rate about 0.5%, PE rate 0.2%) [12, 13].
A possible explanation for the low incidence of symptomatic VTE in phase III trials might be that screening of asymptomatic patients around day 11 established asymptomatic DVT and led to therapeutic interventions, which may prevent thrombus extension and development of symptomatic VTE [22]. On the other hand, the high rate of distal symptomatic DVT in our study cohorts suggests that a large proportion of VTE events in major orthopaedic surgery patients become symptomatic in the early phase of thrombus development. This would have been expected to occur to a similar extent before screening on day 11 in prospective phase III trials. The high rate of symptomatic VTE in our cohorts was not due to insufficient prophylaxis, since strict hospital guidelines assured that dosage of prophylaxis was chosen according to regulatory approval and national and hospital guidelines. LMWH prophylaxis was started the evening before surgery and fondaparinux started within 12 h after surgery. All patients received graduated compression stockings (if not contraindicated) and early mobilization. Differences in VTE rates were also not explained by different observation periods. Our retrospective evaluation documented symptomatic VTE events until hospital discharge (mean 10.5 days), while phase III trials in this indication evaluated symptomatic VTE events until day 11 [8, 10, 11].
Another explanation for the different results obtained in a retrospective comparison of two consecutive cohorts, the latter one using a new compound may also be that doctors are more cautious if a patient claims symptoms with the new drug, thereby indicating more ultrasound examinations, which could account for the higher rate of DVT detected in the second cohort. However, the annual rate of CCUS examinations was fairly constant around 13 to 15% of all patients undergoing MOS in our hospital throughout the observation period.
We conclude that the significantly higher rates of symptomatic DVT and PE in unselected routine patients compared with patients in prospective phase III trials are most likely due to different population characteristics. This is supported by the fact that phase III trials of thromboprophylaxis mainly included selected patients undergoing elective hip and knee arthroplasty, whereas in clinical practice at major reference centers a significant proportion of major orthopaedic surgery consist of revision arthroplasty in unselected patients with relevant comorbidities, who are at higher risk of VTE.
Prevention of VTE with fondaparinux or LMWH
In our cohorts of patients undergoing major orthopaedic surgery VTE prophylaxis with fondaparinux resulted in comparable rates of symptomatic proximal DVT and major VTE events. However, patients receiving fondaparinux had a higher rate of distal symptomatic DVT compared with patients receiving LMWH. This contrasts to the results of a meta-analysis of phase III trials evaluating fondaparinux compared with enoxaparin for this indication [12, 13], in which fondaparinux resulted in a 50% risk reduction for any VTE event. This was mainly due to a reduction in asymptomatic DVT whereas the rate of symptomatic DVT did not differ. Interestingly, we found that efficacy of fondaparinux was comparable with LMWH until day 9, but was significantly inferior to LMWH prophylaxis in patients with prolonged hospitalization. The reasons for the lower efficacy of fondaparinux compared with LMWH in the prevention of symptomatic distal VTE and in patients with prolonged hospitalization in our cohorts remain unclear. On the other hand, most VTE events presented as distal VTE and the clinical and prognostic relevance of isolated distal DVT is still a matter of debate [23–26], especially since a benefit of anticoagulant treatment has not yet been proven.
Safety of thromboprophylaxis with fondaparinux or LMWH
The rates of severe bleeding complications were similar between both treatment groups. However, we found a trend towards higher transfusion rates of plasma concentrates and platelet concentrates in patients treated with LMWH. In addition, we found a significantly lower rate of surgical complications leading to secondary surgery in patients receiving fondaparinux compared with LMWH. This is in contrast to published data from prospective RCTs: bleeding complications were more frequent in patients receiving fondaparinux compared with enoxaparin, but the rates of postoperative complications were comparable [12]. These differing findings cannot be explained by differences in trial design, observational period or study-related interventions. While in a retrospective analysis, data on complications might be documented incompletely, the information used for our analysis was directly extracted from the quality management database of the Clinic of Orthopaedic Surgery, in which all treatment-associated complications are prospectively entered during hospitalization and reviewed at discharge. This led to a nearly complete set of data. Review of surgical procedures and standards at our hospital did not identify a change of indications for surgical revisions, surgical technique or expertise during the study period. Therefore, the influence of confounding factors on safety outcomes was limited.
On the other hand, some of the observed findings may have been influenced by the LMWH regimen used in our study. Patients in the LMWH cohort were treated with the approved dosages of either certoparin, nadroparin, enoxaparin or tinzaparin and recommendations of LMWH substance and dosage had been implemented for patients undergoing major orthopaedic surgery during the observational period. However, we cannot completely rule out that subjective risk assessment lead treating physicians to use another LMWH or a different dosage in individual patients. Furthermore, also secondary joint replacements and explantation surgery were included in our study. In contrast to this, large RCTs routinely use highly standardized thromboprophylaxis with enoxaparin 40 mg once daily or 30 mg twice daily only and exclude explantation surgery.
Patients receiving fondaparinux were found to have a significantly shorter hospital stay compared with the LMWH group. As the length of hospital stay in general has decreased over the years mainly due to health economic reasons the analysis of two cohorts of consecutive patients covering a time period of 4 years may have been influenced by this development. The analysis of the Kaplan–Meier curves of hospital stay overlapped for about 70% of all patients treated with LMWH or fondaparinux. The 25th and 50th percentiles of the length of hospital stay were not different between both cohorts, but the 75th percentile of hospital stay was significantly longer in the LMWH cohort. Interestingly, patients receiving fondaparinux had a shorter hospitalization also in the subgroup of patients without any VTE, bleeding or surgical complications (Figure 2A), indicating that the trend to early discharge due to economic considerations contributed to this finding. On the other hand, the observed difference in the length of hospital stay was also found in a subgroup analysis of the last 500 patients treated with LMWH (end of 2007) and the first 500 patients treated with fondaparinux (beginning of 2008), which indicated that differences in hospitalization were seen in much shorter time intervals, which can hardly be explained by a trend to earlier discharge due to economic considerations alone and further Kaplan–Meier analysis indicated, that also lower rates of revisions and earlier discharge of patients with bleeding or surgical complications in the fondaparinux cohort rather than a change in discharge policy alone led to the shorter duration of hospitalization in this group.
A possible explanation for the higher rates of surgical complications in the LMWH group could be the timing of thromboprophylaxis. Whereas fondaparinux is started 12 h after surgery, LMWH prophylaxis in major orthopaedic surgery requires initiation of anticoagulant therapy the evening before surgery according to European recommendations and drug approval. Therefore, patients receiving LMWH are operated while on treatment, which may increase surgical complications. Our data do not allow an estimate of the impact of pre or post surgical initiation of LMWH thromboprophylaxis on surgical complication rates as all patients in the LMWH group received the first dose of anticoagulation the evening before surgery. However, our data provide reliable insight into the complication rates of large cohorts of unselected receiving LMWH started pre-surgery and fondaparinux started post-surgery.
Limitations
There are several limitations of our study. First, the retrospective design does not allow for randomization. Therefore significant differences of certain demographic characteristics exist between both groups. Patients in the fondaparinux group were older, had a higher INR and lower platelet counts, which may result in an increased risk of surgical complications. However, patients receiving fondaparinux had lower rates of surgical complications. Therefore, our analysis may even have underestimated the safety benefit of fondaparinux. Furthermore, in uni- and bivariate analysis, no significant influence of gender, INR or platelet count on the efficacy and safety results of both LMWH and fondaparinux could be found. Therefore, the lower rate of complications in this group has not been caused by the differences in baseline characteristics. Second, due to the retrospective, non-concurrent cohort study design, changes in patient management over time besides changes in thromboprophylaxis need to be considered. During the 4 years study period no significant changes in surgical technique or anaesthesia were introduced. Therefore, differences between treatment arms cannot be explained by such bias. Third, the standard definition of bleeding complications including drop in haemoglobin values as used in large prospective phase III trials could not be used. Most patients developed a drop of haemoglobin and times of blood sampling (during surgery, post surgery or post transfusion) were not predefined. Therefore, haemoglobin values over time could not be analyzed. However, in our cohort of nearly 3500 patients as many as 40% received RBC concentrates and 11% required transfusions of more than 2 units of RBC. Hospital standards regarding indications for transfusions did not change over time. Therefore, the rate of transfusions could be used as a surrogate parameter for bleeding complications. Furthermore, the standard criteria of ‘bleeding requiring intervention or surgical revision’ and ‘bleeding into a critical organ site’ were also included in our endpoint definition.
Finally, no information on the rates of VTE, bleeding complication and death were available for the post discharge period. Therefore, the total event rate might be even higher than found in our in-hospital analysis.
The strengths of our study are first the size of our cohort, comparing nearly 1500 patients treated with LMWH with about 2000 patients receiving fondaparinux, which compares with the sample size of RCTs. Second we used clinically relevant endpoints (objectively confirmed symptomatic VTE, severe bleeding complications, surgical revisions, length of hospital stay).
In conclusion, thromboprophylaxis with fondaparinux was numerically less effective in preventing symptomatic distal VTE than LMWH in unselected patients undergoing major orthopaedic surgery, but was equally safe in preventing major VTE and death in these patients. However, the lower efficacy of fondaparinux to prevent distal VTE was statistically significant only in a subgroup of patients with prolonged hospitalization and in multivariate analysis of all patients, the use of fondaparinux was not an independent risk factor for VTE. Furthermore, we found fondaparinux prophylaxis to be equally safe with regard to severe bleeding and surgical complications. These findings are in contrast with the results of large phase III trials, in which fondaparinux prophylaxis was associated with a reduction of VTE events and similar rates of surgical complications compared with LMWH. Differences in trial and real world populations and differences in the management of trial and real world patients are most likely the main confounding factors for these findings. Therefore, results of clinical trials evaluating efficacy of VTE prophylaxis need to be confirmed in large registries or phase IV trials of unselected patients, since both prospective RCTs and data from unselected patients in daily care provide valuable information for a balanced appreciation of drug effects.
Acknowledgments
We want to thank Stephan Kirschner, MD, Marlene Döring and Josephine Schmidt, who are responsible for the quality management database in the Clinic of Orthopaedic Surgery, University Hospital ‘Carl Gustav Carus’ Dresden. Furthermore, we are grateful to Gerhard Prosinger from the Department of Medical Controlling, to Ben Heyne from the Department of Clinical Chemistry and Günther Böhme from the Department of Anaesthesia. All of them repeatedly helped with the data extraction from their respective databases and assured high data quality by numerous quality checks.
Competing Interests
The Ortho-TEP registry was supported by a grant of Bayer Healthcare, providing funding for a documentation assistant. All authors declare that the sponsor had no influence on the conduct of the study, data collection, statistical analysis or written presentation of study findings.
REFERENCES
- 1.Nicolaides AN, Breddin HK, Fareed J, Goldhaber S, Haas S, Hull R, Kalodiki E, Myers K, Samama M, Sasahara A Cardiovascular Disease Educational and Research Trust and the International Union of Angiology. Prevention of venous thromboembolism. International consensus statement. Guidelines compiled in accordance with the scientific evidence. Int Angiol. 2001;20:1–37. [PubMed] [Google Scholar]
- 2.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-based Clinical Practice Guidelines (8th edition) Chest. 2008;133(Suppl.):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 3.Lassen MR, Borris LC, Christiansen HM, Schøtt P, Olsen AD, Sørensen JV, Rahr H, Jensen HP. Clinical trials with low molecular weight heparins in the prevention of postoperative thromboembolic complications: a meta-analysis. Semin Thromb Hemost. 1991;17(Suppl. 3):284–90. [PubMed] [Google Scholar]
- 4.Leizorovicz A, Haugh MC, Chapuis FR, Samama MM, Boissel JP. Low molecular weight heparin in prevention of perioperative thrombosis. BMJ. 1992;305:913–20. doi: 10.1136/bmj.305.6859.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen AT, Davidson BL, Gallus AS, Lassen MR, Prins MH, Tomkowski W, Turpie AG, Egberts JF, Lensing AW ARTEMIS Investigators. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332:325–9. doi: 10.1136/bmj.38733.466748.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Büller HR, Davidson BL, Decousus H, Gallus A, Gent M, Piovella F, Prins MH, Raskob G, Segers AE, Cariou R, Leeuwenkamp O, Lensing AW Matisse Investigators. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med. 2004;140:867–73. doi: 10.7326/0003-4819-140-11-200406010-00007. [DOI] [PubMed] [Google Scholar]
- 7.Büller HR, Davidson BL, Decousus H, Gallus A, Gent M, Piovella F, Prins MH, Raskob G, van den Berg-Segers AE, Cariou R, Leeuwenkamp O, Lensing AW Matisse Investigators. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med. 2003;349:1695–702. doi: 10.1056/NEJMoa035451. [DOI] [PubMed] [Google Scholar]
- 8.Turpie AG, Bauer KA, Eriksson BI, Lassen MR PENTATHALON 2000 Study Steering Committee. Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip-replacement surgery: a randomised double-blind trial. Lancet. 2002;359:1721–6. doi: 10.1016/S0140-6736(02)08648-8. [DOI] [PubMed] [Google Scholar]
- 9.Lassen MR, Bauer KA, Eriksson BI, Turpie AG European Pentasaccharide Elective Surgery Study (EPHESUS) Steering Committee. Postoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip-replacement surgery: a randomised double-blind comparison. Lancet. 2002;359:1715–20. doi: 10.1016/S0140-6736(02)08652-X. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson BI, Bauer KA, Lassen MR, Turpie AG Steering Committee of the Pentasaccharide in Hip-Fracture Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med. 2001;345:1298–304. doi: 10.1056/NEJMoa011100. [DOI] [PubMed] [Google Scholar]
- 11.Bauer KA, Eriksson BI, Lassen MR, Turpie AG Steering Committee of the Pentasaccharide in Major Knee Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med. 2001;345:1305–10. doi: 10.1056/NEJMoa011099. [DOI] [PubMed] [Google Scholar]
- 12.Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomized double-blind studies. Arch Intern Med. 2002;162:1833–40. doi: 10.1001/archinte.162.16.1833. [DOI] [PubMed] [Google Scholar]
- 13.Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Superiority of fondaparinux over enoxaparin in preventing venous thromboembolism in major orthopedic surgery using different efficacy end points. Chest. 2004;126:501–8. doi: 10.1378/chest.126.2.501. [DOI] [PubMed] [Google Scholar]
- 14.Casteels M, Flamion B. Open-label trials and drug registration: a European regulator's view. J Thromb Haemost. 2008;6:232–4. doi: 10.1111/j.1538-7836.2008.02835.x. [DOI] [PubMed] [Google Scholar]
- 15.Halpern SD. Evaluating preference effects in partially unblinded, randomized clinical trials. J Clin Epidemiol. 2003;56:109–15. doi: 10.1016/s0895-4356(02)00598-x. [DOI] [PubMed] [Google Scholar]
- 16.Crowther MA, Ginsberg JS, Julian J, Denburg J, Hirsh J, Douketis J, Laskin C, Fortin P, Anderson D, Kearon C, Clarke A, Geerts W, Forgie M, Green D, Costantini L, Yacura W, Wilson S, Gent M, Kovacs MJ. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med. 2003;349:1133–8. doi: 10.1056/NEJMoa035241. [DOI] [PubMed] [Google Scholar]
- 17.Berger VW, Exner DV. Detecting selection bias in randomized clinical trials. Control Clin Trials. 1999;20:319–27. doi: 10.1016/s0197-2456(99)00014-8. [DOI] [PubMed] [Google Scholar]
- 18.Tinetti ME, Bogardus ST, Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351:2870–4. doi: 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz T, Schmidt B, Schmidt B, Schellong SM. Interobserver agreement of complete compression ultrasound for clinically suspected deep vein thrombosis. Clin Appl Thromb Hemost. 2002;8:45–9. doi: 10.1177/107602960200800106. [DOI] [PubMed] [Google Scholar]
- 20.Schellong SM, Schwarz T, Halbritter K, Beyer J, Siegert G, Oettler W, Schmidt B, Schroeder HE. Complete compression ultrasonography of the leg veins as a single test for the diagnosis of deep vein thrombosis. Thromb Haemost. 2003;89:228–34. [PubMed] [Google Scholar]
- 21.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 22.Chagnon I, Bounameaux H, Aujesky D, Roy PM, Gourdier AL, Cornuz J, Perneger T, Perrier A. Comparison of two clinical prediction rules and implicit assessment among patients with suspected pulmonary embolism. Am J Med. 2002;113:269–75. doi: 10.1016/s0002-9343(02)01212-3. [DOI] [PubMed] [Google Scholar]
- 23.Schellong SM. Distal DVT: worth diagnosing? Yes. J Thromb Haemost. 2007;5:51–4. doi: 10.1111/j.1538-7836.2007.02490.x. [DOI] [PubMed] [Google Scholar]
- 24.Righini M, Paris S, Le Gal G, Laroche JP, Perrier A, Bounameaux H. Clinical relevance of distal deep vein thrombosis. Review of literature data. Thromb Haemost. 2006;95:56–64. [PubMed] [Google Scholar]
- 25.Seinturier C, Bosson JL, Colonna M, Imbert B, Carpentier PH. Site and clinical outcome of deep vein thrombosis of the lower limbs: an epidemiological study. J Thromb Haemost. 2005;3:1362–7. doi: 10.1111/j.1538-7836.2005.01393.x. [DOI] [PubMed] [Google Scholar]
- 26.Iskander GA, Nelson RS, Morehouse DL, Tenquist JE, Szlabick RE. A: incidence and propagation of infrageniculate deep venous thrombosis in trauma patients. J Trauma. 2006;61:695–700. doi: 10.1097/01.ta.0000210453.70742.7f. [DOI] [PubMed] [Google Scholar]


