Abstract
AIMS
To evaluate the acute haemodynamic effects of a single oral dose of vardenafil and to study the drug concentration in relation to haemodynamic effects in patients with pulmonary hypertension (PH).
METHODS
Sixteen patients with PH (aged 29–85\ years), received one single oral dose of vardenafil (5, 10 or 20 mg). The haemodynamic effect was assessed over a 60 min period. Vardenafil plasma concentrations were measured after 15, 30, 45 and 60 min using liquid chromatography–tandem mass spectrometry.
RESULTS
At 60 min a reduction in mPAP with a median % decrease of −20.3% (range −48.3 to 3.0; P < 0.001) and an increase in cardiac output and the cardiac index with a median % change of 10.6% (range −25.0 to 88.1; P = 0.015) and 12.1% (range −24.0 to 94.4; P = 0.01) respectively was observed. The pulmonary vascular resistance (PVR) was reduced with a median % decrease of −28.9% (range −61.5 to −5.9; P < 0.001), and pulmonary selectivity was reflected by a median percent reduction of −16.9% (range −49.0 to 16.5; P = 0.002; n = 14) in the PVR/systemic vascular resistance ratio. There was a correlation between the plasma concentrations of vardenafil and change in mPAP (r = −0.579, P = 0.019) and between vardenafil concentrations and change in PVR (r = −0.662, P = 0.005).
CONCLUSIONS
Vardenafil causes rapid changes in cardiopulmonary haemodynamics and there is a correlation between plasma vardenafil drug concentration and the acute changes in mPAP as well as PVR in patients with PH.
Keywords: haemodynamics, pharmacokinetics, phosphodiesterase inhibitor, pulmonary hypertension, right heart catheterization, vardenafil
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Phosphodiesterase-5 (PDE5) inhibitor therapy is effective in the treatment of patients with pulmonary hypertension (PH). All available PDE5 inhibitors, sildenafil, tadalafil and vardenafil have been reported to cause pulmonary vasodilation acutely in patients with PH. There is a lack of information on the haemodynamic effects in relation to plasma concentrations for the available PDE5 inhibitors and the drug concentration to obtain optimal clinical effect is unknown.
WHAT THIS STUDY ADDS
This study provides information on the correlation between vardenafil plasma drug concentration and the acute changes in haemodynamics in patients with PH after a single oral dose of vardenafil.
Introduction
Pulmonary hypertension (PH) is a haemodynamic and pathophysiological state including a number of vascular diseases found in multiple clinical conditions, leading to right ventricular failure [1]. The presence of phosphodiesterase-5 (PDE5) in the pulmonary vasculature provides the basis for targeted treatment of pulmonary arterial hypertension (PAH) with PDE5 inhibitors [2–4]. Endogenous ligands such as nitric oxide (NO) and natriuretic peptides stimulate pulmonary vasodilatation by use of a common signalling pathway that involves generation of cyclic guanosine monophosphate (cGMP) as a second messenger to mediate the intracellular effects that finally result in vasodilatation [5]. The phosphodiesterases (PDEs) form a family of enzymes (PDE1-11) with different modes of regulation, localization, cellular expression, and inhibitor sensitivities that selectively catalyze the hydrolysis and thereby the degradation of cyclic adenosine monophosphate and cGMP [6]. In the pulmonary vasculature, the levels of cGMP are regulated mainly by PDE5 and PDE5 inhibition promotes the accumulation of cGMP allowing for prolonged and enhanced cGMP mediated pulmonary vasorelaxation [7].
Sildenafil, vardenafil and tadalafil are three selective PDE5 inhibitors currently widely used for the treatment of erectile dysfunction. Two large randomized, double-blind, placebo-controlled phase 3 clinical trials with sildenafil and tadalafil have demonstrated that these compounds are safe and beneficial in the treatment of patients with PAH, which has lead to their registration and approval for the treatment of PAH [8, 9]. Recently, it was demonstrated that vardenafil is effective and well tolerated in patients with PAH in the first randomized, double-blind, placebo-controlled study with this drug [10]. In this study, oral vardenafil improved exercise capacity, symptoms, haemodynamics and clinical outcome and it represents the first study in which monotherapy with a PDE5 inhibitor in treatment-naive PAH patients has been able to reduce significantly the occurrence of clinical worsening events [10]. Furthermore, beneficial therapeutic effects of vardenafil for treatment of patients with pulmonary hypertension (PH) including patients with PAH have been demonstrated in uncontrolled case reports and in a long term open label study [11–14]. In one of these studies, there are also data on the haemodynamic efficacy of vardenafil from a single dose evaluation up to 90 min [12]. Moreover, there are also data on the haemodynamic efficacy of vardenafil from a single dose evaluation study in which the acute haemodynamic effects of sildenafil and inhaled NO were studied and compared with the effects of different doses of vardenafil and tadalafil [15].
A dose-dependent effect has been demonstrated for tadalafil with regard to the distance walked in 6 min (6MWD) [9]. There is also some indication for a dose-dependent effect on lowering mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR) for sildenafil [8]. However there is only one peer reviewed publication where a study on haemodynamic effects in relation to actual plasma concentrations for one of the available PDE5 inhibitors (sildenafil) in patients with PH is described [16]. In the present study we have evaluated the acute haemodynamic response of a single dose of vardenafil in relation to plasma concentrations of vardenafil, in patients with PH during right heart catheterization (RHC).
Methods
Subjects
Sixteen patients with PH of different aetiologies, representing WHO group I-IV, according to the latest updated clinical classification of PH (Dana Point, 2008) were enrolled in the study between 2006 and 2007 [1]. The patient group consisted of females (n = 10) and males (n = 6), 29–85 years of age (median 63), World Health Organization (WHO) functional class II or III. All patients were receiving at least one of the conventional background therapies (diuretics, β-adrenoceptor blocker, ACE inhibitor, warfarin, angiotensin receptor blocker, digoxin, or Ca2+ antagonist). Four patients with a previous diagnosis of PAH were treated with a PAH-specific medication (three with bosentan and one with sildenafil). The patients on bosentan therapy are denoted in Table 1 and in Figure 1. These patients on bosentan were on stable doses (62.5 mg, 125 mg and 250 mg twice daily) with a treatment duration exceeding 3 months for all patients. The other 12 patients were diagnosed with PH or PAH at the time of enrolment after confirmation at RHC. The inclusion criteria were PH patients with a resting mPAP ≥ 25 mmHg measured by RHC and an age over 18 years. No patient met the exclusion criteria (severe liver dysfunction [Child-Pugh class C], or hypotension, [<90/50 mmHg]). Diagnostic procedures preceding patient recruitment included echocardiography, chest X-ray, lung function test, measurement of the 6MWD and in selected cases tomographic scan of the lung and pulmonary angiography. Patients were hospitalized 1 day before and 1 day after the RHC, and the indication for RHC was either haemodynamic follow-up of known PAH (n = 4) or for diagnostic confirmation of suspected PAH/PH (n = 12). All patients were fasting 24 h before the procedure, thus no medication was given prior to the RHC.
Table 1.
Baseline demographic and clinical characteristics
| Patient number | Age (years) | Gender (M/F) | Weight (kg) | BSA (m2) | Clinical classification of PH (WHO group I-IV) | WHO functional class I−IV | 6MWD (m) | Dose of vardenafil (mg) |
|---|---|---|---|---|---|---|---|---|
| 1 | 79 | M | 107 | 2.2 | IPAH (I) | III | 315 | 10 |
| 2 | 50 | F | 53 | 1.5 | PH due to left heart disease (II) | III | 350 | 5 |
| 3 | 60 | F | 73 | 1.8 | PH due to left heart disease (II) | II | 585 | 20 |
| 4* | 29 | F | 57 | 1.6 | IPAH (I) | II | 658 | 20 |
| 5 | 77 | M | 102 | 2.2 | PH due to left heart disease (II) | III | NA | 20 |
| 6 | 38 | F | 49 | 1.5 | IPAH (I) | III | 300 | 20 |
| 7 | 35 | M | 70 | 1.9 | PH due to left heart disease (II) | III | 422 | 20 |
| 8 | 45 | F | 69 | 1.8 | PAH associated with CTD (SSc) (I) | III | 347 | 20 |
| 9 | 85 | F | 78 | 1.8 | CTEPH (IV) | II | 251 | 10 |
| 10 | 76 | M | 69 | 1.9 | PH due to lungdisease (III) | III | 211 | 20 |
| 11 | 70 | M | 111 | 2.2 | IPAH (I) | III | 60 | 20 |
| 12 | 71 | F | 58 | 1.6 | PH due to left heart disease (II) | III | 25 | 20 |
| 13* | 44 | M | 90 | 2.2 | IPAH (I) | II | 570 | 20 |
| 14 | 66 | F | 60 | 1.6 | IPAH (I) | III | 390 | 20 |
| 15* | 72 | F | 65 | 1.7 | PAH associated with CTD (SSc) (I) | III | 180 | 20 |
| 16 | 59 | F | 63 | 1.7 | IPAH (I) | III | 300 | 20 |
M, male; F, female; BSA, body surface area; PH, pulmonary hypertension; WHO, World Health Organization; 6MWD, 6 min walk distance; IPAH, idiopathic pulmonary arterial hypertension; CTD, connective tissue disease; SSc, systemic sclerosis; CTEPH, chronic thromboembolic pulmonary hypertension; NA, not available. Roman numerals within brackets designate the clinical classification of PH (WHO groups I−IV). Patients on bosentan therapy are marked with an asterisk (*); patient 4 (bosentan 250 mg twice daily), patient 13 (bosentan 125 mg twice daily) and patient 15 (bosentan 62.5 mg twice daily).
Figure 1.
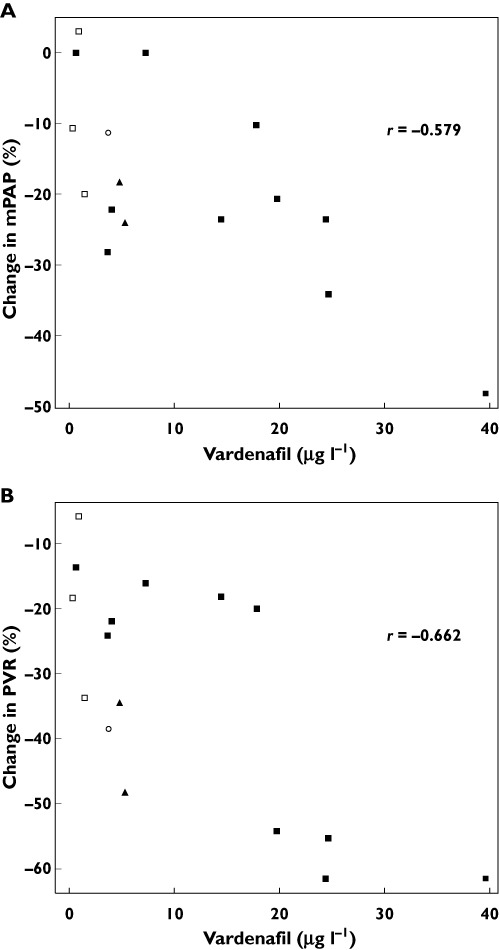
The association between vardenafil plasma concentration and change in mPAP (A) and PVR (B) at 60 min after a single oral dose of vardenafil in patients with pulmonary hypertension (n = 16). Thirteen patients received 20 mg of vardenafil ( and □), two patients received 10 mg (▴) and one patient received 5 mg (○). Open squares indicates patients exposed to bosentan (n = 3). Plasma vardenafil and change in mPAP and PVR correlated significantly (Spearman rank correlation r = −0.579, n = 16, P = 0.019 for mPAP and r = −0.662, n = 16, P = 0.005 for PVR)
and □), two patients received 10 mg (▴) and one patient received 5 mg (○). Open squares indicates patients exposed to bosentan (n = 3). Plasma vardenafil and change in mPAP and PVR correlated significantly (Spearman rank correlation r = −0.579, n = 16, P = 0.019 for mPAP and r = −0.662, n = 16, P = 0.005 for PVR)
Study design
The study was an open-label acute haemodynamic and pharmacokinetic trial performed at the regional PAH centre at Uppsala University Hospital. Each patient received one single oral dose of either 5 mg (n = 1), 10 mg (n = 2) or 20 mg (n = 13) vardenafil (Levitra®, Bayer Schering Pharma) depending on age and liver function (see Table 1). During RHC haemodynamic and mixed venous oxygen saturation measurements were performed at baseline and 15, 30, 45 and 60 min after vardenafil administration. Blood samples for determination of vardenafil concentrations were taken from the pulmonary artery at baseline and 15, 30, 45 and 60 min. Blood samples were collected into EDTA-tubes (BD Diagnostics) and were processed immediately in a thermostatic (20°C) centrifuge (Jouan BR 3.11) for 10 min at 3000 rev min–1. Plasma was separated and stored at −20°C until analysis. Approval for the study was obtained from a local ethics review committee and conducted in accordance with Good Clinical Practice guidelines and the Helsinki Declaration. Written informed consent was obtained from all patients.
Haemodynamic assessment
RHC was performed in all patients after an overnight fast. A fibreoptic thermodilution pulmonary artery catheter (Becton Dickinson Criticath SP5 107 HTD catheter) was inserted through the right internal jugular vein into the pulmonary artery. Correct position was verified by fluoroscopy. Central venous pressure (CVP), pulmonary artery wedge pressure (PAWP), cardiac output (CO) and cardiac index (CI) were measured at baseline and 60 min. Pulmonary artery pressure (PAP) was measured at baseline and 15, 30, 45 and 60 min. Pressures were registered with a Cathcor® system (Siemens) and the flow was calculated with thermodilution technique or with Fick's principle. Patients with tricuspid valve regurgitation >grade 1 were evaluated with Fick's principle due to greater variation with the thermodilution technique. The same physician performed all examinations, except patient 9, and the patients were in a resting supine position during the examination. Calculation was made of PVR, systemic vascular resistance (SVR) and PVR : SVR, a ratio that indicates pulmonary selectivity of the vasodilatory effect. During RHC some patients did not receive an arterial line because of either technical or procedural reasons or an arterial sampling was missed. Therefore we have missing data for the following set of haemodynamic variables (arterial saturation, aortic pressure, SVR and pulmonary arterial oxygen saturation).
Bioanalytical methods
Bioanalysis of plasma samples for the determination of vardenafil concentration was performed at the Swedish National Veterinary Institute (SVA) in Uppsala using liquid chromatography–tandem mass spectrometry (LC-MS/MS).
Vardenafil dihydrochloride and the internal standard pentadeuterated vardenafil (vardenafil-d5) were purchased from Toronto Research Chemicals (North York, ON, Canada). The water was purified using a Milli-Q water purification system (Millipore, Bedford, MA, USA). All other chemicals were of analytical grade or better and used without further purification.
The sample pretreatment was carried out as follows. To 1 ml of plasma, 50 µl of internal standard solution (vardenafil-d5 at 250 ng ml−1 in methanol) and 50 µl of methanol were added followed by 50 µl of 2.0 m NaOH (aq) and 5.0 ml of ethyl acetate. The samples were then shaken for 20 min and centrifuged at 1000 g for 10 min. The organic phases were transferred to new tubes and were evaporated to dryness under a gentle stream of nitrogen at 60°C. The samples were reconstituted in 50 µl of water/methanol 80:20 (v/v) whereafter they were transferred to vials for LC-MS/MS analysis.
A Surveyor MS pump was hyphenated to a TSQ Quantum Ultra tandem quadrupole mass spectrometer, with an electrospray interface operating in the positive mode (Thermo Fischer Scientific, San José, CA, USA). The column was a Luna C8 [2] with the dimensions: length 50 mm, i.d. 2.0 mm (Phenomenex, Torrence, CA, USA). The mobile phase consisted of (A) 0.1 % acetic acid in water and (B) acetonitrile. A gradient was run as follows: 10 % B for 1 min, increase from 10 % to 90 % B in 4 min, reduction to 10 % B in 0.10 min and constantly at 10 % B, for 2.90 min. The total run time was 8 min, the flow rate was 200 µl min−1 and the injection volume was 10 µl.
The data acquisition mode was Selected Reaction Monitoring (SRM) and the transitions were m/z 489 [M + H]+→ 151 for vardenafil (collision energy 40 V), and m/z 495 [M + H]+→ 151 for the internal standard vardenafil-d5 (collision energy 40 V). The dwell time was 0.10 s.
Stock solutions of vardenafil dihydrochloride and the internal standard were prepared in methanol at approximately 0.1 mg ml−1. These solutions were diluted and used to spike (50 µl) blank plasma to obtain calibration samples. Calibration was performed by linear curve fit (no weighting) of the peak area ratio (analyte : internal standard) as a function of the concentration. The calibration curve interval was 0.35–35 ng ml−1. Quality control samples were prepared by adding 50 µl of separately prepared working solutions. The lowest limit of quantification (LLOQ) was 0.35 ng ml−1 and the precision expressed as relative standard deviation was <2.2%.
Statistical analysis
Baseline data, when compiled, are given as the median and range (minimum to maximum). Differences of study parameters between baseline and post treatment values were evaluated by Wilcoxon's signed-rank test and differences between groups were evaluated by the Mann–Whitney U-test. The Spearman rank correlation test was performed to evaluate the association between plasma vardenafil exposure (plasma concentration or AUC) and change in hemodynamic parameters. Significance was defined as P < 0.05.
Results
Haemodynamic parameters
Baseline demographic and clinical characteristics of the study population are shown in Table 1. The baseline haemodynamic variables and the observed changes in haemodynamics for the patients after a single 5, 10 or 20 mg oral dose of vardenafil from baseline to 60 min are shown in Table 2. Due to high age and/or liver failure one patient received 5 mg and two patients received 10 mg and all of the other patients received 20 mg.
Table 2.
Median % changes (range minimum-maximum) in haemodynamic parameters during right heart catheterization from baseline to 60 min and the pharmacokinetic parameters of vardenafil after a single oral dose
| n | Baseline median (min, max) | 60 min median (min, max) | *Change (%) median (min, max) | P value1 | |
|---|---|---|---|---|---|
| Heart rate (beats min−1) | 16 | 71 (56, 94) | 72 (50, 102) | −0.9 (−19.2, 19.4) | 0.978 |
| Aortic pressure (mmHg) | 14 | 135 (118, 224) | 127.50 (104, 321) | −5.8 (−26.2, 122.9) | 0.153 |
| Mean aortic pressure (mmHg) | 14 | 94 (81, 139) | 88 (68, 119) | −6.0 (−27.0, 2.1) | <0.001 |
| Systolic PAP (mmHg) | 16 | 65.50 (41, 98) | 52.50 (26, 89) | −17.2 (−36.6, 3.8) | <0.001 |
| mPAP (mmHg) | 16 | 40 (29, 65) | 34.50 (15, 58) | −20.3 (−48.3, 3.0) | <0.001 |
| mPAWP (mmHg) | 16 | 11 (4, 32) | 10.50 (3, 32) | 0.0 (−40.0, 50.0) | 0.977 |
| mRAP (mmHg) | 16 | 8.50 (2, 28) | 6.50 (1, 23) | −13.1 (−83.3, 100.0) | 0.720 |
| CO (l min−1) | 16 | 3.95 (2.10, 5.83) | 4.95 (2.60, 7.90) | 10.6 (−25.0, 88.1) | 0.015 |
| CI (l min−1 m−2) | 16 | 2.30 (1.40, 3.40) | 2.75 (1.70, 4.50) | 12.1 (−24.0, 94.4) | 0.010 |
| PVR (dyn .s cm−5) | 16 | 612 (192, 1144) | 404 (80, 816) | −28.9 (−61.5, −5.9) | <0.001 |
| SVR (dyn .s cm−5) | 14 | 1852 (848, 2968) | 1444 (640, 2248) | −20.6 (−61.2, 1.7) | <0.001 |
| PVR : SVR | 14 | 0.28 (0.09, 0.53) | 0.25 (0.09, 0.50) | −16.9 (−49.0, 16.5) | 0.002 |
| PA sat (%) | 13 | 60.50 (45.90, 69.40) | 62.45 (51, 76.50) | 2.6 (−7.7, 36.1) | 0.168 |
| Art sat (%) | 11 | 91.20 (85, 93.70) | 90.40 (79.70, 96.55) | −1.3 (−6.6, 5.2) | 0.375 |
| Vardenafil concentration (µg l−1) | 16 | 5.07 (0.33, 39.6) | |||
| Vardenafil AUC(0,45 min) (µg l−1 h) | 15 | 2.53 (0.00, 17.9) | |||
| Vardenafil AUC(0,60 min) (µg l−1 h) | 15 | 3.88(0.04, 23.08) |
Wilcoxon's signed-rank test.
Values shown represent the median % change (with range minimum to maximum). m, mean; PAP, pulmonary artery pressure; PAWP, pulmonary arterial wedge pressure; RAP, right atrial pressure; CO, cardiac output; CI, cardiac index; PVR, pulmonary vascular resistance; SVR, systemic vascular resistance; PA, pulmonary artery; Art, arterial; AUC(0,45 min) and (0,60 min), area under the plasma concentration−time curve from time zero to 45 and 60 min, respectively.
A significant reduction in mPAP with a median % decrease of −20.3% (range −48.3 to 3.0; P < 0.001; n = 16) was observed at 60 min. Furthermore at 60 min there was a significant increase in CO and the CI with a median % change of 10.6% (range −25.0 to 88.1; P = 0.015; n = 16) and 12.1% (range −24.0 to 94.4; P = 0.01; n = 16) respectively. PVR was reduced significantly with a median % decrease of −28.9% (range −61.5 to −5.9; P < 0.001; n = 16) and pulmonary selectivity of the vasodilatory effect was reflected by a median % reduction of −16.9% (range −49.0 to 16.5; P = 0.002; n = 14) in the PVR : SVR ratio.
The PAH patients (representing WHO group I) on bosentan (n = 3) demonstrated a median decrease of mPAP with −10.8% at 60 min (range −20.0 to 3.0) as compared with −23.8% (range −34.1 to 0) for the other PAH patients not exposed to bosentan (n = 6) (P = 0.095 for difference in response). There were no statistically significant differences in the haemodynamic response at 60 min between the three PAH patients exposed to bosentan in comparison with the rest of the group of PH/PAH patients (n = 13) (data not shown). In addition, there were no statistically significant differences between patients with PAH (n = 9) and PH (representing WHO group II-IV) (n = 7) with regard to haemodynamic response at 60 min. The PAH patients demonstrated a median decrease of mPAP of −22.2% (range −34.1 to 3.0) as compared with −18.4% (range −48.3 to 0) for the PH group (P = 0.738 for difference in response).
Plasma concentrations of vardenafil and haemodynamic response
A full set of routine haemodynamic variables was available for the patients at baseline and 60 min after administration of a single dose of vardenafil. mPAP was registered at 15, 30 and 45 min as well. The median plasma concentration of vardenafil, 60 min after a single oral dose of vardenafil (5, 10 or 20 mg) was 5.07 ug l−1 (n = 16, Table 2), with a median area under the curve (AUC)(0,45 min) and AUC(0,60 min) value (n = 15, Table 2) of 2.53 µg h l−1 and 3.88 µg h l−1 respectively. The individual plasma concentrations of vardenafil at 60 min are illustrated in Figure 1. The three PAH patients exposed to bosentan (Table 1) displayed a significantly lower median plasma vardenafil concentration at 60 min [0.33 µg l−1 (patient 4); 0.88 µg l−1 (patient 13); 1.52 µg l−1 (patient 15)] as compared with the other PH/PAH patients (7.3 µg l−1, range 0.68 to 39.6, n = 13) (P = 0.014 for difference in concentration).
The relationship between plasma vardenafil exposure and haemodynamic response 60 min after vardenafil administration is presented in Table 3. In the non-parametric Spearman rank correlation test, there was a statistically significant correlation between the plasma concentrations of vardenafil and change in mPAP (n = 16, r = −0.579, P = 0.019), and between vardenafil concentrations and change in PVR (n = 16, r = −0.662, P = 0.005), illustrated in Figure 1. Similarly as shown in Table 3, there was a significant correlation between vardenafil AUC and change in mPAP (n = 15, r = −0.668, P = 0.007 for AUC(0,45 min), and n = 15, r = −0.744, P = 0.001 for AUC(0,60 min)) and between vardenafil AUC and change in PVR (n = 15, r = −0.540, P = 0.038 for AUC(0,45 min) and n = 15, r = −0.588, P = 0.021 for AUC(0,60 min)). After a single dose of vardenafil, plasma drug concentrations and mPAP measurements from baseline, 15, 30, 45 and 60 min were available from 14 patients, presented in Table 4. A significant change in mPAP with a median % decrease of −10.54% (P = 0.013, Table 4) was already observed at 15 min. The significant decrease in mPAP in relation to baseline was seen also at 30, 45 and 60 min (Table 4). A clear trend with increasing median % reduction in mPAP was registered in parallel with increasing plasma concentrations of vardenafil from baseline to 60 min after drug administration.
Table 3.
Spearman's rank correlation between vardenafil exposure and % change in haemodynamic parameters at 60 min after a single oral dose of vardenafil
| Vardenafil concentration | Vardenafil AUC(0,45 min) | Vardenafil AUC(0,60 min) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Spearman's rho | P value | n | Spearman's rho | P value | n | Spearman's rho | P value | |
| Heart rate (beats min−1) | 16 | 0.291 | 0.274 | 15 | 0.018 | 0.950 | 15 | 0.054 | 0.850 |
| Aortic pressure (mmHg) | 14 | −0.552 | 0.041 | 14 | −0.174 | 0.553 | 14 | −0.270 | 0.350 |
| Mean aortic pressure (mmHg) | 14 | −0.582 | 0.029 | 14 | −0.424 | 0.131 | 14 | −0.481 | 0.081 |
| Systolic PAP (mmHg) | 16 | −0.397 | 0.128 | 15 | −0.422 | 0.117 | 15 | −0.504 | 0.056 |
| mPAP (mmHg) | 16 | −0.579 | 0.019 | 15 | −0.668 | 0.007 | 15 | −0.744 | 0.001 |
| mPAWP (mmHg) | 16 | −0.148 | 0.585 | 15 | −0.065 | 0.818 | 15 | −0.087 | 0.757 |
| mRAP (mmHg) | 16 | −0.397 | 0.128 | 15 | −0.360 | 0.188 | 15 | −0.362 | 0.184 |
| CO (l min−1) | 16 | 0.282 | 0.289 | 15 | −0.120 | 0.671 | 15 | 0.021 | 0.940 |
| CI (l min−1 m−2) | 16 | 0.308 | 0.247 | 15 | −0.272 | 0.327 | 15 | −0.129 | 0.648 |
| PVR (dyn .s cm−5) | 16 | −0.662 | 0.005 | 15 | −0.540 | 0.038 | 15 | −0.588 | 0.021 |
| SVR (dyn .s cm−5) | 14 | −0.486 | 0.078 | 14 | −0.095 | 0.748 | 14 | −0.222 | 0.446 |
| PVR : SVR | 14 | −0.191 | 0.513 | 14 | −0.521 | 0.056 | 14 | −0.433 | 0.122 |
| PA sat (%) | 13 | −0.137 | 0.655 | 12 | −0.084 | 0.795 | 12 | −0.077 | 0.812 |
| Art sat (%) | 11 | −0.264 | 0.433 | 11 | −0.218 | 0.519 | 11 | −0.191 | 0.574 |
m, mean; PAP, pulmonary artery pressure; PAWP, pulmonary arterial wedge pressure; RAP, right atrial pressure; CO, cardiac output; CI, cardiac index; PVR, pulmonary vascular resistance; SVR, systemic vascular resistance; PA, pulmonary artery; Art, arterial; AUC(0,45 min) and (0,60 min), area under the plasma concentration−time curve from time zero to 45 and 60 min, respectively.
Table 4.
Spearman's rank correlation between vardenafil concentration and % change in mPAP after a single oral dose of vardenafil at different time points up to 60 min after vardenafil administration
| n = 14 Time(min) | Vardenafil concentration (µg l−1) median | mPAP (mmHg) median | % change median | P value1 | Spearman's rho* | P value |
|---|---|---|---|---|---|---|
| 0 | 0.00 | 40.00 | 0.00 | . | . | |
| 15 | 1.01 | 38.50 | −10.54 | 0.013 | −0.263 | 0.364 |
| 30 | 5.99 | 36.50 | −16.15 | 0.002 | −0.528 | 0.053 |
| 45 | 5.43 | 35.50 | −17.51 | <0.001 | −0.488 | 0.076 |
| 60 | 6.30 | 34.50 | −20.34 | <0.001 | −0.634 | 0.015 |
Wilcoxon's signed-rank test.
Spearman's rank correlation between vardenafil concentration and median change (%) in mPAP. mPAP, mean pulmonary artery pressure.
Safety
Vardenafil was well tolerated and there were no adverse events observed in this study after the administration of a single oral dose of vardenafil. No hypotension or clinically significant changes in haematological or biochemical parameters were seen.
Discussion
We have shown that a single dose of oral vardenafil, a PDE5 inhibitor, is a potent and selective pulmonary vasodilator displaying a rapid onset of effect on pulmonary haemodynamics.
A major and novel finding in this study is the correlation between vardenafil plasma drug concentration and the acute changes in mPAP as well as PVR. Among the available PDE5 inhibitors, vardenafil was chosen because of its rapid onset of effect [15]. Moreover, vardenafil is more potent and selective than sildenafil and tadalafil on its inhibitory activity on PDE5 [17–19].
In our study vardenafil displayed pulmonary selectivity acutely, reflected by a median % reduction of −16.9% in the PVR/SVR ratio.
In conflict with these results, Ghofrani et al. found that acutely only sildenafil and tadalafil caused a significant reduction the PVR : SVR ratio, thus showing selectivity for the pulmonary circulation, corroborated by a small case report from Aizawa et al. where the acute effects of vardenafil were studied and no pulmonary selectivity was seen [12, 15]. However pulmonary selectivity for vardenafil was noted in the latter study by Aizawa et al. who observed a significant decrease in PVR : SVR ratio by −20.7% after 3 months of treatment [12]. Pulmonary selectivity for vardenafil has also been demonstrated in a long term study by Jing et al., as reflected by a reduction in the PVR : SVR ratio of −13.2% [14]. This disceprancy between our results and others regarding pulmonary selectivity for vardenafil after acute administration could be explained by differences in study population size, where Ghofrani et al. included seven and nine patients in the vardenafil groups and Aizawa et al. studied five patients whereas we had 14 patients for which this variable was calculated. As in the study by Ghofrani et al., vardenafil caused significant acute changes in the clinically relevant haemodynamic variables mPAP, CI and PVR.
In our study, a significant change in mPAP with a median % decrease of −10.54% was observed already at 15 min after drug administration. Interestingly it has been shown that vardenafil, contrary to the other two PDE5 inhibitors, has other pharmacological effects apart from PDE5 inhibition, also blocking Ca2+ fluxes, thus enhancing its vasorelaxing properties [19]. This unique dual effect of vardenafil might explain the rapid decrease of mPAP in our study.
We observed a linear correlation between increasing vardenafil concentrations and fall in mPAP and PVR. Regarding the other PDE5 inhibitors it has been observed that both tadalafil and sildenafil increased the 6MWD in a dose-dependent manner [9, 20].
The patients in our study that were on bosentan treatment displayed very low concentrations of vardenafil at 60 min, indicating a possible drug interaction between vardenafil and bosentan. It was noted that the patient on the highest dose of bosentan (250 mg twice daily) displayed the lowest plasma concentration of vardenafil whereas the patient on the lowest bosentan dose (62.5 mg twice daily) displayed almost five times higher plasma vardenafil concentration. The significant difference in plasma vardenafil concentrations at 60 min between the patients exposed to bosentan and the others was not reflected by a significant difference in the haemodynamic response. Even though the difference in mPAP decrease at 60 min between these groups was relatively large, it did not reach statistical significance most likely due to the small sample size. For tadalafil and sildenafil there is a known drug interaction with bosentan mediated by the cytochrome P450 3A4 leading to a reduction of plasma concentrations of tadalafil and sildenafil by approximately 40% and 60% respectively [21–23]. In fact, in the pivotal study for tadalafil a significant effect on the placebo-corrected 6MWD could not be seen in the patients who were on background bosentan [9]. Moreover, the effect on all secondary endpoints tended to be better in patients on tadalafil monotherapy [9]. In a small study by Hatano et al., the acute haemodynamic effects of a single dose of sildenafil (50 mg) with simultaneous measurements of plasma concentrations of sildenafil and its major metabolite were examined [16]. In this study only eight patients with PAH were included and they were divided in two groups (four patients in each group) with and without chronic bosentan treatment. In both groups sildenafil caused pulmonary artery dilatation with reduction of mPAP and PVR. The small sample size did not allow for distinguishing statistical differences between the groups with regard to haemodynamic effects. However, a numerically smaller maximal reduction of mPAP and PVR in the patients who were chronically treated with bosentan was seen [16]. In support of our results, Pfizer has submitted the results obtained from the study A 1481024 to the European Medicines Agency in which pharmacokinetic and pharmacodynamic data show that therapeutic plasma concentrations of sildenafil should be in the range of 10 and 100 ug l−1 to obtain significant effect on mPAP and PVR [24]. The maximal reductions in mPAP and PVR were seen at 100 ug l−1.
We have not found any available data for tadalafil or vardenafil regarding the optimal plasma concentration for maximal reduction in mPAP or PVR. It is however reasonable to assume that there is a drug class effect for the PDE5 inhibitors regarding concentration dependent effects on the relevant pulmonary parameters in patients with PH. In our study, considerable variation in plasma vardenafil concentrations at 60 min was observed, which were significantly correlated to changes in mPAP and PVR. Even in the more uniform subgroup of patients not co-medicated with bosentan and receiving 20 mg vardenafil (n = 10) the variability was surprisingly high reflected by a range in plasma vardenafil concentrations of 0.68 µg l−1 to 39.6 µg l−1 and change in mPAP and PVR ranging from 0% to −48.3% and −13.6% to −61.5% respectively (as illustrated in Figure 1). This variation in plasma vardenafil concentrations could serve as an explanation for the absence of a clear dose-dependent response in haemodynamics for vardenafil in the study by Ghofrani et al. [15]. In that study, at single oral doses of 10 mg and 20 mg, vardenafil caused a significant decrease of mPAP of −14.3% and −12.1% respectively compared with baseline. In the same study the acute haemodynamic response to tadalafil at three different single oral doses was explored (20, 40 and 60 mg). Despite the large difference in administered tadalafil dose, a significant difference in the acute haemodynamic response could not be detected [15].
Although vardenafil has been found to be effective and well tolerated in patients with PAH at a dose of 5 mg twice daily in a well designed study [10], clinical trial data from dosing regimens exceeding 10 mg day−1 are lacking. The results from the present study warrant further characterization of the pharmacokinetic profile of vardenafil in patients with PH/PAH.
Furthermore there is a need for a long term study to evaluate the dose–concentration−effect relationships of vardenafil in patients with PH/PAH.
Although the current licensed indication for sildenafil and tadalafil is PAH, studies on patients with PH due to chronic thromboembolic disease [25, 26], left heart disease [27, 28] and lung diseases [29–31] have demonstrated positive treatment effects with PDE5 inhibition. The majority of the patients in our study had PAH, but we also included patients with PH secondary to other conditions such as chronic thromboembolic disease, lung disease and chronic left ventricular failure, thus reflecting the patient spectra that currently in clinical practice are treated with PDE5 inhibition. In our study there was no difference in haemodynamic response at 60 min between PAH patients and patients with PH (WHO group II-IV).
In summary, we show that there is a correlation between plasma vardenafil drug concentration and the acute changes in mPAP as well as PVR. Since mPAP and PVR are haemodynamic variables that are strongly associated with mortality in patients with PAH we suggest that the optimal plasma concentration of PDE5 inhibition in relation to relevant endpoints in patients with PH should be further studied [32].
Acknowledgments
The authors would like to thank Mrs. Lisa Wernroth (Uppsala Clinical Research Center, Uppsala, Sweden) for help with the statistical analysis.
Competing Interests
G. Wikström has received lecture fees from Actelion Pharmaceuticals, Pfizer, Bayer Schering Pharma and GlaxoSmithKline. The other authors have no competing interest to declare.
REFERENCES
- 1.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S43–54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Rabe KF, Tenor H, Dent G, Schudt C, Liebig S, Magnussen H. Phosphodiesterase isozymes modulating inherent tone in human airways: identification and characterization. Am J Physiol. 1993;264:L458–64. doi: 10.1152/ajplung.1993.264.5.L458. [DOI] [PubMed] [Google Scholar]
- 3.Rabe KF, Tenor H, Dent G, Schudt C, Nakashima M, Magnussen H. Identification of PDE isozymes in human pulmonary artery and effect of selective PDE inhibitors. Am J Physiol. 1994;266:L536–43. doi: 10.1152/ajplung.1994.266.5.L536. [DOI] [PubMed] [Google Scholar]
- 4.Corbin JD, Beasley A, Blount MA, Francis SH. High lung PDE5: a strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem Biophys Res Commun. 2005;334:930–8. doi: 10.1016/j.bbrc.2005.06.183. [DOI] [PubMed] [Google Scholar]
- 5.Klinger JR. The nitric oxide/cGMP signaling pathway in pulmonary hypertension. Clin Chest Med. 2007;28:143–67. doi: 10.1016/j.ccm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 7.Cohen AH, Hanson K, Morris K, Fouty B, McMurty IF, Clarke W, Rodman DM. Inhibition of cyclic 3′-5′-guanosine monophosphate-specific phosphodiesterase selectively vasodilates the pulmonary circulation in chronically hypoxic rats. Clin Invest. 1996;97:172–9. doi: 10.1172/JCI118386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G Sildenafil use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–57. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 9.Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A, Frumkin L, Barst RJ. Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) Study Group. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 10.Jing ZC, Yu ZX, Shen JY, Wu BX, Xu KF, Zhu XY, Pan L, Zhang ZL, Liu XQ, Zhang YS, Jiang X, Galiè N, for the Efficacy and safety of VArdenafiL in the treatment of pUlmonary Arterial hyperTensION (EVALUATION) Study Group Vardenafil in pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2011;183:1723–9. doi: 10.1164/rccm.201101-0093OC. [DOI] [PubMed] [Google Scholar]
- 11.Jochmann N, Kiecker F, Borges AC, Hofmann MA, Eddicks S, Sterry W, Baumann G, Trefzer U. Long-term therapy of interferon-alpha induced pulmonary arterial hypertension with different PDE-5 inhibitors: a case report. Cardiovasc ultrasound. 2005;3:26. doi: 10.1186/1476-7120-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aizawa K, Hanaoka T, Kasai H, Kogashi K, Kumazaki S, Koyama J, Tsutsui H, Yazaki Y, Watanabe N, Kinoshita O, Ikeda U. Long-term vardenafil therapy improves hemodynamics in patients with pulmonary hypertension. Hypertens Res. 2006;29:123–8. doi: 10.1291/hypres.29.123. [DOI] [PubMed] [Google Scholar]
- 13.Giacomini M, Borotto E, Bosotti L, Denkewitz T, Reali-Forster C, Carlucci P, Centanni S, Mantero A, Iapichino G. Vardenafil and weaning from inhaled nitric oxide: effect on pulmonary hypertension in ARDS. Anaesth Intensive Care. 2007;35:91–3. doi: 10.1177/0310057X0703500113. [DOI] [PubMed] [Google Scholar]
- 14.Jing ZC, Jiang X, Wu BX, Xu XQ, Wu Y, Ma CR, Wang Y, Yang YJ, Pu JL, Gao W. Vardenafil treatment for patients with pulmonary arterial hypertension: a multicentre, open-label study. Heart. 2009;95:1531–6. doi: 10.1136/hrt.2009.169417. [DOI] [PubMed] [Google Scholar]
- 15.Ghofrani HA, Voswinckel R, Reichenberger F, Olschewski H, Haredza P, Karadaş B, Schermuly RT, Weissmann N, Seeger W, Grimminger F. Differences in hemodynamic and oxygenation responses to three different phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension: a randomized prospective study. J Am Coll Cardiol. 2004;44:1488–96. doi: 10.1016/j.jacc.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 16.Hatano M, Yao A, Kinugawa K, Hirata Y, Nagai R. Acute effect of sildenafil is maintained in pulmonary arterial hypertension patients chronically treated with bosentan. Int Heart J. 2011;52:233–9. doi: 10.1536/ihj.52.233. [DOI] [PubMed] [Google Scholar]
- 17.Blount MA, Beasley A, Zoraghi R, Sekhar KR, Bessay EP, Francis SH, Corbin JD. Binding of tritiated sildenafil, tadalafil, or vardenafil to the phosphodiesterase-5 catalytic site displays potency, specificity, heterogeneity, and cGMP stimulation. Mol Pharmacol. 2004;66:144–52. doi: 10.1124/mol.66.1.144. [DOI] [PubMed] [Google Scholar]
- 18.Corbin JD, Beasley A, Blount MA, Francis SH. Vardenafil: structural basis for higher potency over sildenafil in inhibiting cGMP-specific phosphodiesterase-5 (PDE5) Neurochem Int. 2004;45:859–63. doi: 10.1016/j.neuint.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Toque HA, Teixeira CE, Priviero FB, Morganti RP, Antunes E, De Nucci G. Vardenafil, but not sildenafil or tadalafil, has calcium-channel blocking activity in rabbit isolated pulmonary artery and human washed platelets. Br J Pharmacol. 2008;154:787–96. doi: 10.1038/bjp.2008.141. Epub 2008 Apr 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spring RM, lrich S, Huber LC, Speich R, Maggiorini M, Treder U, Fischler M. Sildenafil for pulmonary hypertension: dose-dependent improvement in exercise performance. Pulm Pharmacol Ther. 2008;21:516–21. doi: 10.1016/j.pupt.2007.11.006. Epub 2007 Dec 23. [DOI] [PubMed] [Google Scholar]
- 21.Burgess G, Hoogkamer H, Collings L, Dingemanse J. Mutual pharmacokinetic interactions between steady-state bosentan and sildenafil. Eur J Clin Pharmacol. 2008;64:43–50. doi: 10.1007/s00228-007-0408-z. Epub 2007 Nov 27. [DOI] [PubMed] [Google Scholar]
- 22.Paul GA, Gibbs JS, Boobis AR, Abbas A, Wilkins MR. Bosentan decreases the plasma concentration of sildenafil when coprescribed in pulmonary hypertension. Br J Clin Pharmacol. 2005;60:107–12. doi: 10.1111/j.1365-2125.2005.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wrishko RE, Dingemanse J, Yu A, Darstein C, Phillips DL, Mitchell MI. Pharmacokinetic interaction between tadalafil and bosentan in healthy male subjects. J Clin Pharmacol. 2008;48:610–8. doi: 10.1177/0091270008315315. Epub 2008 Feb 27. [DOI] [PubMed] [Google Scholar]
- 24.European Medicines Agency. Revatio® scientific discussion (report EMEA/H/C/638). European Public Assessment Report. 2005.
- 25.Ghofrani HA, Schermuly RT, Rose F, Wiedemann R, Kohstall MG, Kreckel A, Olschewski H, Weissmann N, Enke B, Ghofrani S, Seeger W, Grimminger F. Sildenafil for long-term treatment of nonoperable chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2003;167:1139–41. doi: 10.1164/rccm.200210-1157BC. Epub 2003 Jan 24. [DOI] [PubMed] [Google Scholar]
- 26.Suntharalingam J, Treacy CM, Doughty NJ, Goldsmith K, Soon E, Toshner MR, Sheares KK, Hughes R, Morrell NW, Pepke-Zaba J. Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest. 2008;134:229–36. doi: 10.1378/chest.07-2681. Epub 2008 Feb 8. [DOI] [PubMed] [Google Scholar]
- 27.Jabbour A, Keogh A, Hayward C, Macdonald P. Chronic sildenafil lowers transpulmonary gradient and improves cardiac output allowing successful heart transplantation. Eur J Heart Fail. 2007;9:674–7. doi: 10.1016/j.ejheart.2007.01.008. Epub 2007 Mar 7. [DOI] [PubMed] [Google Scholar]
- 28.Tedford RJ, Hemnes AR, Russell SD, Wittstein IS, Mahmud M, Zaiman AL, Mathai SC, Thiemann DR, Hassoun PM, Girgis RE, Orens JB, Shah AS, Yuh D, Conte JV, Champion HC. PDE5A inhibitor treatment of persistent pulmonary hypertension after mechanical circulatory support. Circ Heart Fail. 2008;1:213–9. doi: 10.1161/CIRCHEARTFAILURE.108.796789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghofrani HA, Wiedemann R, Rose F, Schermuly RT, Olschewski H, Weissmann N, Gunther A, Walmrath D, Seeger W, Grimminger F. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet. 2002;360:895–900. doi: 10.1016/S0140-6736(02)11024-5. [DOI] [PubMed] [Google Scholar]
- 30.Madden BP, Allenby M, Loke TK, Sheth A. A potential role for sildenafil in the management of pulmonary hypertension in patients with parenchymal lung disease. Vascul Pharmacol. 2006;44:372–6. doi: 10.1016/j.vph.2006.01.013. Epub 2006 Mar 29. [DOI] [PubMed] [Google Scholar]
- 31.Collard HR, Anstrom KJ, Schwarz MI, Zisman DA. Sildenafil improves walk distance in idiopathic pulmonary fibrosis. Chest. 2007;131:897–9. doi: 10.1378/chest.06-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swiston JR, Johnson SR, Granton JT. Factors that prognosticate mortality in idiopathic pulmonary arterial hypertension: a systematic review of the literature. Respir Med. 2010;104:1588–607. doi: 10.1016/j.rmed.2010.08.003. [DOI] [PubMed] [Google Scholar]


