Abstract
AIMS
Erythromycin is a macrolide antibiotic indicated for respiratory tract infections, genital chlamydia and skin infections. It has recently been suggested that erythromycin use in the first trimester of pregnancy can increase the risk of congenital cardiovascular malformations. This study aimed to determine whether erythromycin exposure in the first trimester is associated with cardiovascular or other malformations.
METHODS
We studied 180 120 women in Norway who were pregnant during 2004–2007. Data on all live births stillbirths and induced abortions after 12 gestational weeks from The Medical Birth Registry of Norway (MBRN) were linked to information from the Norwegian prescription database (NorPD). We compared the pregnancy outcomes of women who had taken erythromycin (n= 1786, 1.0%), penicillin V (n= 4921, 2.7%) or amoxicillin (n= 1599, 0.9%) in their first trimester with outcomes of women who had not taken any systemic antibiotics (n= 163 653, 90.9%) during this period.
RESULTS
The risk of cardiovascular malformations was not significantly different with or without exposure to erythromycin in the first trimester (adjusted OR = 1.2 [95% CI 0.8, 1.8]) or in the most vulnerable period of heart formation (adjusted OR = 1.6 [95% CI 0.9–3.0]). Sub-analyses showed that the risk for any specific malformations was not increased with erythromycin, macrolides, penicillin V or amoxicillin compared with no antibiotic use in first trimester.
CONCLUSIONS
This large, population-based register study did not find that exposure to erythromycin or macrolides in the first trimester of pregnancy was associated with fetal cardiovascular or other malformations. These results suggest that the risk of erythromycin use during early pregnancy, if any, is low.
Keywords: antibiotics, cardiovascular malformations, drug safety, erythromycin, pregnancy outcome
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
It has generally been regarded as safe to take erythromycin during pregnancy. A recent study from Sweden found that erythromycin exposure in the first trimester was associated with congenital cardiovascular defects. The results led to warnings against using erythromycin in the first trimester from both the Swedish and Norwegian governments.
WHAT THIS STUDY ADDS
This large, population-based register study did not find that erythromycin or macrolide use in pregnancy was significantly associated with congenital malformations, cardiovascular malformations or any other specific malformation.
Introduction
Systemic antibacterials are among the drug groups most frequently used during pregnancy [1–5]. In a Norwegian register study among 106 000 pregnant women in 2004 to 2006, 10.0% received systemic anti-infectives in their first trimester, 12.2% in their second trimester and 13.0% in their third trimester [3]. Penicillins accounted for approximately 80% of these prescriptions and macrolides comprised the next most frequent class. The most commonly used macrolides were erythromycin, azithromycin, clarithromycin and spiramycin. All of these act by inhibiting bacterial protein biosynthesis. The antibacterial spectrum of macrolides is slightly broader than that of penicillins. Macrolides are a common substitute for patients with a penicillin allergy. The indications for prescribing antibiotic macrolides are primarily respiratory infections, bacterial skin infections and genital chlamydia.
Pneumonia and chlamydia have been associated with increased complications in pregnancy, and adequate pharmacological treatment is essential. Respiratory tract infections result in greater morbidity and mortality in the mother, due to the physiologic adaptations that occur during pregnancy [6, 7]. Chlamydia has been associated with adverse pregnancy outcomes, post partum endometritis and infections in the newborn child [8].
For a drug to be used during pregnancy, it must be documented as safe for both mother and child. The appropriateness of erythromycin for use during pregnancy has been internationally accepted [9, 10]. In a study of 229 101 pregnancies in the Michigan Medicaid programme, cited in the text book by Briggs et al., 6972 infants had been exposed to erythromycin during the first trimester. A total of 320 (4.6%) major birth defects were observed (297 expected), 77 of which were a cardiovascular defect (70 expected). Briggs et al. concluded that the data did not support an association between erythromycin and congenital formations, and that erythromycin was compatible with use during pregnancy [11]. However, a population based study from the Swedish Medical Birth Registry, with data from 1995–2002, showed an increased risk of congenital cardiovascular malformations among the children of mothers who had used erythromycin in early pregnancy [12]. Källén et al. found that among 1844 infants exposed to erythromycin in early pregnancy, 34 (1.8%) had cardiovascular malformations but, among 9110 infants exposed to penicillin V, 86 (0.9%) had these malformations (OR = 1.8, 95% CI 1.3, 2.6). The authors suggested that this association may represent an effect inherent to all macrolides, due to their ability to block a specific cardiac potassium channel. They also discussed alternative explanations to their results and acknowledged that the finding could be due to multiple testing. When the study was repeated with 496 073 infants from the Swedish Medical Birth Registry born in 2002–2006, no association was found between erythromycin and increased risk of congenital cardiovascular malformations (OR 1.31, 95% CI 0.78, 2.21) [13]. The authors have later estimated a common OR of 1.59, 95% CI 1.17, 2.17 [14].
In response to Källen et al.s' first study, the Swedish and Norwegian Regulatory Medicines Agencies have issued warnings against the use of macrolides during the first trimester [15, 16]. Other authorities have been less restrictive. Based on the categorization systems for prescribing drugs in pregnancy, erythromycin is currently assigned to category B in the US and category A in Australia [17, 18]. The WHO recommends erythromycin for chlamydia in pregnant women [19]. The UK Teratology Information Service summarizes as follows: ‘A recent study has suggested a possible increased risk of cardiovascular malformations and pyloric stenosis; however, causality has not been established, and the individual risk, if any, is thought to be low. If a macrolide antibiotic is required in pregnancy, then erythromycin would be considered the agent of choice’[20].
Cardiovascular malformations represent the most common category of congenital malformations. They affect up to 1% of live-born infants [21, 22]. Congenital cardiovascular malformations are a diverse group of conditions in terms of types and severity, and a proportion of cases is associated with chromosomal anomalies. The incidence has remained relatively constant over time and has varied little among different populations. This suggests that a greater aetiological contribution may arise from genetic factors than from environmental factors [23, 24]. Environmental risk factors include socio-economic status, maternal infections, drugs taken during pregnancy, nutritional factors and environmental pollution [25]. The timing of potential teratogenic exposure is critical. The primitive heart tube is formed 21 days after conception and the heart is developed at 7–8 weeks [26].
To increase the current knowledge on the safety of erythromycin during pregnancy, we have taken a population-based approach to investigate the association between exposure to erythromycin during early pregnancy and congenital cardiovascular malformations. We also focused on exposure to macrolides as a group and effects on other congenital malformations. Secondary outcomes studied were neonatal survival, low birth weight, premature delivery, low Apgar scores and transfer to an intensive care unit (ICU).
Methods
This study was based on data retrieved after a linkage between two Norwegian population-based health registers, the Medical Birth Registry of Norway (MBRN) and the Norwegian prescription database (NorPD).
Study population
Data from the MBRN and NorPD were linked by using the unique Norwegian 11-digit personal identification number. The original data file included 187 632 pregnancy outcomes of all women who became pregnant after 31 December 2003 and who had a pregnancy outcome registered in the MBRN before 1 January 2008. Individual women may have had more than one entry due to several pregnancies within the study period. Infants born in a multiple birth (n= 6945) and infants with chromosomal anomalies (n= 573) were excluded. The final analyses included data on 180 120 women, corresponding to 96% of the original data file.
The medical birth registry of Norway
Since 1967, it has been compulsory in Norway to report all pregnancy outcomes (live births, stillbirths and induced abortions after week 12) to the MBRN. The standard form used by the hospitals and birth institution is usually filled in by the midwife. It is based on the antepartum obstetric record (completed at visits to a general practitioner, midwife or obstetrician during pregnancy) and the medical record for inpatient care (data recorded from before the time of birth until discharge). The MBRN contains demographic information about the mother and her health before and during the pregnancy, the mother's drug use, folic acid use, smoking and alcohol use before and during pregnancy, pregnancy and birth related complications and pregnancy outcome. The MBRN also contains data from neonatal and paediatric wards on congenital malformations, neonatal diagnoses and procedures performed on infants transferred to those units.
The Norwegian prescription database
In Norway, systemic antibiotics require a prescription. Since January 1 2004, the NorPD has maintained information about all prescription drugs that are dispensed from pharmacies to individual patients outside of medical institutions. Drugs were classified according to the Anatomical Therapeutic Chemical (ATC) codes [27]. Information about drugs includes the name, dosage, package size and the date the drug was dispensed.
Drug exposure
Each woman's unique personal identification number, which is allocated to every citizen in Norway, was used to link the NorPD to the MBRN data. With this linkage, we could identify women who had received medication during pregnancy. Date of conception was estimated by using the date of delivery and length of pregnancy. The gestational week that a woman started using a medication was estimated by the number of weeks from conception to the date the drug was dispensed. The timing and duration of exposure was estimated from the date the drug was dispensed, the daily defined dose (DDD) and the package size dispensed. The DDD is defined as the assumed average maintenance dose per day for a drug used for its primary indication in adults [27]. Any antibiotic that was dispensed was assumed to be consumed by the patient and this was counted as exposure to the antibiotic.
We defined three groups of antibiotics:
Erythromycin/macrolides
Penicillin V
Amoxicillin
The control group was defined as women who had not received any antibiotics. The comparison with women who had used penicillin V or amoxicillin was done to address the issue of confounding by indication, which in this study was the underlying maternal infection.
To investigate the risk of malformations, we defined the groups according to use of antibiotics in the first trimester of pregnancy (from conception to week 13). The control group included women who did not receive systemic antibiotics during that period. To investigate the risk of other adverse pregnancy outcomes, we defined different groups according to use of antibiotics during the entire pregnancy. That control group comprised women who did not receive any systemic antibiotics throughout pregnancy.
Pregnancy outcomes
We investigated all malformations, major malformations and 17 specific major malformations that are coded separately in the MBRN (defined/listed in: http://www.fhi.no/dokumenter/8105c63e8e.pdf). ‘All malformations’ is defined by the MBRN as all Q-diagnoses (Congenital malformations, deformations and chromosomal abnormalities) in ICD-10 plus P 83.5: Congenital hydrocele. Major birth defects include malformations considered to have adverse medical or social significance. The cardiovascular malformations studied included malformations of the heart and great vessels (ICD-10 code Q20 to Q26); other congenital malformations of the circulatory system were not included. A patent ductus arteriosus (ICD-10 code Q25.0) was not considered a cardiovascular malformation in premature infants; this included 254 infants (0.14%).
We also investigated these adverse pregnancy outcomes: stillbirths and neonatal deaths, low birth weight, premature delivery, Apgar score at 5 min and transfer to ICU. Stillbirth was defined as death of a foetus that was ≥22 weeks gestation or over 425 g. Neonatal death refers to a live-born baby that died within 28 days of birth. Low birth weight was defined as a live-born baby that weighed less than 2500 g. Premature birth refers to a birth that occurred before 37 weeks gestational age. Length of pregnancy in the MBRN is primarily based on ultrasound, and if missing, on the woman's reported first day in the last menstrual period (LMP). Pregnancy lengths over 44 weeks, and birth weights outside 3.5 standard deviations from the gender specific mean at each pregnancy week were considered miscoded and recoded as missing (0.1% and 0.4%, respectively).
Possible confounding variables
The following potential confounders, all from the MBRN, were included in the statistical analysis (categorized as shown in Table 2): Maternal age, parity, marital status, smoking during pregnancy, folic acid use, chronic diseases during pregnancy (asthma, cardiac disease, hypertensive illness, recurrent urinary tract infection and others), and previous late spontaneous abortion or stillbirth (pregnancy week 12–23).
Table 2.
Maternal characteristics according to the systemic antibiotics used during the first trimester
| Erythromycin n= 1786 | Penicillin V n= 4921 | Amoxicillin n= 159 9 | Controls n= 163 653 | |
|---|---|---|---|---|
| Maternal age at delivery (years) | ||||
| <20 | 107 (6.0)* | 116 (2.4)*** | 69 (4.3)* | 3393 (2.1) |
| 20–29 | 840 (47.0) | 2232 (45.4) | 752 (47.0) | 73 952 (45.2) |
| 30–39 | 808 (45.2) | 2472 (50.2) | 743 (46.5) | 81 862 (50.0) |
| >39 | 31 (1.7) | 101 (2.1) | 35 (2.2) | 4446 (2.7) |
| Primiparity (%) | 755 (42.3) | 1467 (29.8)* | 629 (39.3) | 67 900 (41.5) |
| Married/cohabiting (%) | 1544 (86.5)* | 4549 (92.4) | 1427 (89.2)* | 152 307 (93.1) |
| Previous stillbirth or late miscarriage after gestation week 23 (%) | 46 (2.6) | 180 (3.7) | 81 (5.1)* | 5510 (3.4) |
| Cigarette smoking during pregnancy (%) | ||||
| Daily | 187 (10.5)* | 418 (8.5)* | 146 (9.1)* | 10 493 (6.4) |
| Sometimes | 26 (1.5) | 51 (1.0) | 11 (0.7) | 1190 (0.7) |
| No | 1189 (66.6) | 3395 (69.0) | 1091 (68.2) | 116 406 (71.1) |
| Missing | 384 (21.5) | 1057 (21.5) | 351 (22.0) | 35 564 (21.7) |
| Folic acid intake (%) | ||||
| Prior to and during pregnancy | 303 (17.0)* | 964 (19.6)* | 268 (16.8)* | 33 858 (20.7) |
| Prior to or during pregnancy | 638 (35.7) | 1746 (35.5) | 581 (36.3) | 53 797 (32.9) |
| No intake | 845 (47.3) | 2211 (44.9) | 750 (46.9) | 75 998 (46.4) |
| Maternal illness (%) | ||||
| Recurrent urinary tract infection | 62 (3.5) | 197 (4.0)* | 152 (9.5)* | 4624 (2.8) |
| Asthma | 142 (8.0)* | 290 (5.9)* | 101 (6.3)* | 6690 (4.1) |
| Heart disease | 21 (1.2)* | 43 (0.9)* | 17 (1.1)* | 1008 (0.6) |
| Other chronic illness† | 77 (4.3) | 215 (4.4)** | 85 (5.3)* | 6068 (3.6) |
| Hypertensive illness during pregnancy | 121 (6.8)*** | 291 (5.9) | 83 (5.2) | 9176 (5.6) |
P < 0.001,
P < 0.005,
P < 0.05 Pearson's chi square test was used to compare each of the antibiotic groups with controls as the reference group.
Other chronic maternal illnesses included thyroid disorders, rheumatic disease, chronic kidney disease, diabetes and epilepsy.
Statistical analysis
Crude and adjusted odds ratios (cORs and aORs, respectively) with 95% confidence intervals (CI) were estimated with Pearson's chi-square tests and logistic regression analyses. Associations between the use of erythromycin, macrolides, penicillin V or amoxicillin during the first trimester and congenital malformations were investigated for all malformations together, for major malformations, for cardiovascular malformations, for atrial and ventricular septal defects (ASDs and VSDs) jointly and for the 17 malformations predefined by the MBRN. Sub-analyses were performed to determine associations between exposure to antibiotics during the most vulnerable period of heart formation (defined as day 28–56) [26] and congenital heart malformations.
In the multivariate analyses, different potential confounding factors were chosen for each pregnancy outcome, depending on their clinical plausibility and the results from exploratory data analysis. The analytical strategy was that only significant confounding factors were retained in the final models. Adjusted analyses were performed if there were at least three exposed cases. The Hosmer–Lemeshow test was used to assess the robustness of the models. One-tail, two sample tests with percentage values were used in the power calculations. We estimated that, to demonstrate a 50% increase in cardiovascular malformations after exposure to erythromycin in the first trimester with 80% power and P < 0.05, it was necessary to obtain a total sample size of 192 500, given a baseline prevalence of 1% for cardiovascular malformations, given 1.5% exposure to erythromycin and given 10% exposure to any antibiotics in first trimester. This implied 2888 erythromycin exposures. In the final data set, erythromycin use in the population was lower and the final sample size was slightly lower than expected. Post hoc power analyses revealed that with 80% power and P < 0.05, our data could have demonstrated a 65% or higher increase in cardiovascular malformations among women exposed to erythromycin in first trimester. Statistical analysis was performed with the Predictive Analytics Software (PASW) Statistics version 18.0 (SPSS Inc., Chicago, IL, USA).
Ethical aspects
This study was approved by the Regional Committees for Medical and Health Research Ethics (REK). No written informed consent was required.
Results
The final data file included 180 120 pregnancies, of which 178 142 (98.8%) resulted in a live birth, 1176 (0.7%) in a stillbirth, 359 (0.2%) in a neonatal death and 433 (0.2%) in an induced abortion after week 12. The overall rate of non-chromosomal congenital malformations was 4.9% and the rate of cardiovascular malformations was 1.0%. The mean birth weight was 3545 g (SD 565 g) and the mean gestational age was 39.9 weeks (SD 1.9 weeks) among the live-born infants.
A total of 49 231 (27.3%) women received one or more antibacterials for systemic use during pregnancy. The most common antibacterial was penicillin V (Table 1). In the first trimester, 16 467 (9.1%) women had received systemic antibiotics; 15 173 (8.4%) received one course of antibiotics, 1236 (0.7%) received two courses and 59 received three or four courses. Erythromycin had been dispensed to 1786 (1.0%) women in the first trimester. Of these women, 20.1% had also received other antibiotics during that period. In all, 163 653 (90.9%) women did not receive antibiotics during the first trimester (control group in Tables 2 and 3) and 130 889 (72.7%) women did not receive any antibiotics during pregnancy (control group in Table 5).
Table 1.
Systemic antibiotics used during pregnancy (n= 180 120 women)*
| First trimester | Second and third trimester | First–third trimester | |
|---|---|---|---|
| Oral antibiotic | n (%) | n (%) | n (%) |
| Macrolides | 2549 (1.4) | 4437 (2.5) | 6744 (3.7) |
| Erythromycin | 1786 (1.0) | 4122 (2.3) | 5729 (3.2) |
| Azitromycin | 643 (0.4) | 280 (0.2) | 913 (0.5) |
| Clarithromycin | 229 (0.1) | 77 (0.04) | 304 (0.2) |
| Spiramycin | 33 (0.02) | 86 (0.05) | 116 (0.1) |
| Penicillins | 12 049 (6.7) | 31 360 (17.4) | 39 658 (22.0) |
| Penicillin V | 4921 (2.7) | 12 477 (6.9) | 16 695 (9.3) |
| Amoxicillin | 1599 (0.9) | 5445 (3.0) | 6824 (3.8) |
| Nitrofurantoin | 1334 (0.7) | 4714 (2.6) | 5794 (3.2) |
| Sulfonamide/trimetoprim | 924 (0.5) | 2512 (1.4) | 3398 (1.9) |
| Tetracyclines | 651 (0.4) | 65 (0.04) | 712 (0.4) |
| Cefalosporines | 201 (0.1) | 521 (0.3) | 710 (0.4) |
| Fluoroquinolones | 114 (0.1) | 33 (0.02) | 147 (0.1) |
The use of one or more courses of the same antibiotics was counted once; the use of different antibiotics was counted as one per course.
Table 3.
Congenital malformations after exposure in the first trimester to macrolides, erythromycin, penicillin V or amoxicillin
| Total n= 180 120 | Macrolides first trimester n= 2549 | Erythromycin first trimester n= 1786 | Penicillin V first trimester n= 4921 | Amoxicillin first trimester n= 1599 | Controls n= 163 653 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | aOR (95% CI) | n (%) | aOR (95% CI) | n (%) | aOR (95% CI) | n (%) | aOR (95% CI) | n (%) | |
| All malformations* | 8865 (4.9) | 127 (5.0) | 1.02 (0.86, 1.23) | 90 (5.0) | 1.04 (0.84, 1.29) | 218 (4.4) | 0.93 (0.81, 1.06) | 68 (4.3) | 0.87 (0871, 1.11) | 8073 (4.9) |
| Major malformations† | 5065 (2.8) | 69 (2.7) | 0.96 (0.76, 1.22) | 51 (2.9) | 1.02 (0.77, 1.35) | 139 (2.8) | 1.03 (0.87, 1.22) | 36 (2.3) | 0.78 (0.57, 1.11) | 4616 (2.8) |
| Cardiovascular defects‡ | 1809 (1.0) | 25 (1.0) | 0.96 (0.65, 1.43) | 21 (1.2) | 1.16 (0.75, 1.78) | 46 (0.9) | 0.92 (0.69, 1.24) | 12 (0.8) | 0.74 (0.42, 1.31) | 1653 (1.0) |
| ASD / VSD | 1228 (0.7) | 19 (0.7) | 1.08 (0.67, 1.71) | 15 (0.8) | 1.22 (0.73, 2.04) | 29 (0.6) | 0.87 (0.60, 1.25) | 10 (0.6) | 0.93 (0.50, 1.73) | 1124 (0.7) |
All cardiac defects included those classified as International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) code Q20 to Q26. ASD, atrial septum defect; VSD, ventricular septum defect; aOR, adjusted odds ratio; CI, confidence interval. Adjusted odds ratios were calculated for each antibiotic group with controls (women who did not receive any antibiotics during the first trimester) as the reference.
Adjusted for: age, parity, marital status, previous stillbirth or late abortion, cardiac disease, other maternal chronic illness, smoking during pregnancy and folic acid use.
Adjusted for: age, parity, cardiac disease, other maternal chronic illness, smoking during pregnancy and folic acid use.
Adjusted for: age, parity, previous stillbirth or late abortion, recurrent urinary tract infections, cardiac disease, other maternal chronic illness, smoking during pregnancy and folic acid use.
Table 5.
Pregnancy outcome after exposure to erythromycin, penicillin V and amoxicillin during pregnancy
| Total n= 180 120 | Erythromycin n= 5729 | Penicillin V n= 16 695 | Amoxicillin n= 6824 | Controls n= 130 889 | ||||
|---|---|---|---|---|---|---|---|---|
| Pregnancy outcome | n (%) | n (%) | aOR (95% CI) | n (%) | aOR (95% CI) | n (%) | aOR (95% CI) | n (%) |
| Stillbirth and neonatal mortality* | 1519/179 677 (0.9) | 41 (0.7) | 0.77 (0.56, 1.06) | 118 (0.7) | 0.80 (0.66, 0.97) | 62 (0.9) | 0.98 (0.76, 1.27) | 1135 (0.9) |
| Low birth weight† | 7074/179 401 (3.9) | 204 (3.6) | 0.85 (0.68, 1.05) | 587 (3.5) | 1.00 (0.88, 1.14) | 258 (3.8) | 0.88 (0.73, 1.06) | 5177 (4.0) |
| Preterm delivery† | 11 168/179 979 (6.2) | 346 (6.0) | 0.96 (0.86, 1.07) | 905 (5.4) | 0.90 (0.84, 0.97) | 450 (5.2) | 1.04 (0.94, 1.15) | 8143 (6.2) |
| Transfer to ICU‡ | 14 883/174 710 (8.5) | 507 (9.2) | 1.12 (1.02, 1.23) | 1326 (8.2) | 1.04 (0.98, 1.10) | 621 (9.4) | 1.12 (1.03, 1.22) | 10 616 (8.4) |
| Apgar score < 7 at 5 min§ | 3060/179 026 (1.7) | 96 (1.7) | 1.01 (0.82, 1.25) | 264 (1.6) | 1.02 (0.89, 1.16) | 122 (1.8) | 1.07 (0.89, 1.28) | 2175 (1.7) |
aOR: adjusted odds ratio; CI: confidence interval. The control group was women who did not receive any antibiotics during pregnancy.
For each outcome, a proportion of the total was excluded or missing, as follows: Stillbirth and neonatal mortality: 433 elective abortions excluded (0.2%); Low birth weight: n= 719 (0.4%) missing; Preterm delivery: n= 141 (0.1%) missing; Transfer to ICU: n= 5410 (3.0%) missing; Apgar score at 5 min: n= 1094 (0.6%) missing.
Adjusted for: age, marital status, previous stillbirth or late abortion, smoking during pregnancy, folic acid use and asthma.
Adjusted for: age, parity, marital status, previous stillbirth or late abortion, smoking during pregnancy, folic acid use, recurrent urinary tract infections, cardiac disease and other maternal chronic illness.
Adjusted for: age, parity, marital status, previous stillbirth or late abortion, smoking during pregnancy, folic acid use, cardiac disease, asthma and other maternal chronic illness.
Adjusted for: age, parity, marital status, previous stillbirth or late abortion, smoking during pregnancy, folic acid use and other maternal chronic illness.
Maternal characteristics
Table 2 shows the women who received erythromycin, penicillin V or amoxicillin in the first trimester compared with those who did not receive any antibiotics in this first period of pregnancy. Women who had used erythromycin were younger, were more often unmarried, were smokers, did not take folic acid and they had a higher prevalence of asthma and other maternal illnesses compared with the controls. A similar pattern was observed for women who had used penicillin V or amoxicillin.
Congenital malformations
Logistic regression analyses showed that the risk of infant cardiovascular malformations was not significantly higher among women who used erythromycin, macrolides, penicillin V or amoxicillin in the first trimester or in the most vulnerable period of foetal heart formation compared with the control group. Of the 1785 pregnant women exposed to erythromycin in the first trimester, 90 had an infant with one or more non-chromosomal congenital malformations (5.0%) (aOR = 1.04, 95% CI 0.84, 1.29) and 21 had an infant with a cardiovascular malformation (1.2%; aOR = 1.16, 95% CI 0.75, 1.78) (Table 3 and Table 4).
Table 4.
Description of the 21 pregnancies that resulted in heart malformations after exposure to erythromycin in the first trimester
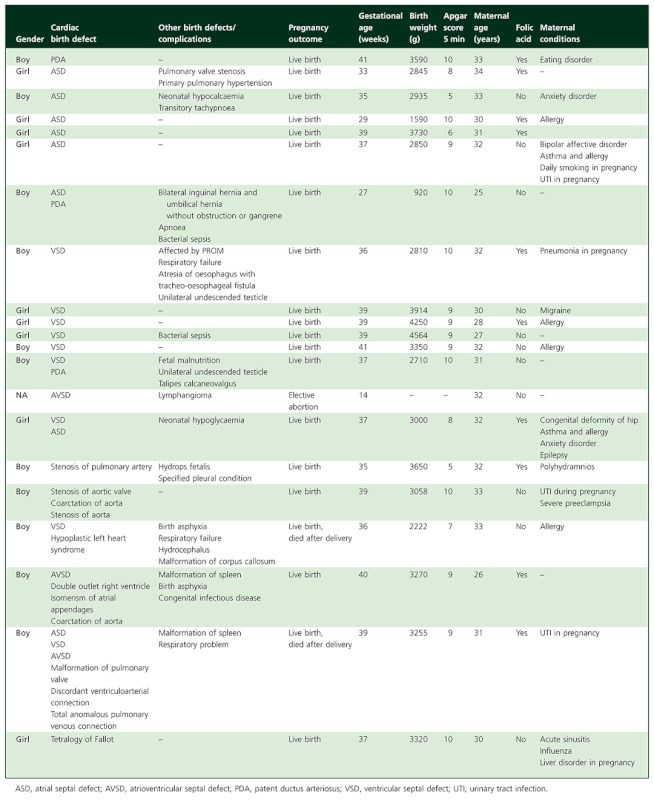 |
In all, 611 (34%) women had received erythromycin during the most sensitive period of cardiac organogenesis. Ten (1.6%) of their infants had cardiovascular malformations and six (1.0%) had ASDs or VSDs. We found no significant associations between erythromycin use during days 28–56 of gestation and cardiovascular malformations (aOR = 1.62 95% CI 0.86, 3.02) or ASD/VSD (aOR = 1.41, 95% CI 0.63, 3.17).
Among 2549 women who had received any macrolide during the first trimester, we found no associations with any congenital malformation (aOR = 1.02, 95% CI 0.86, 1.23), with major malformations (aOR = 0.96, 95% CI 0.76, 1.22) or with a cardiovascular malformation (aOR = 0.96, 95% CI 0.65, 1.43). Similarly, among 798 women exposed to macrolides during days 28–56 of gestation, exposure was not associated with any cardiovascular malformation (aOR 1.36, 95% CI 0.75, 2.47) or with an ASD/VSD (aOR = 1.26, 95% CI 0.60, 2.65).
In adjusted sub-analyses, we found no association between exposure to erythromycin, macrolide, penicillin V or amoxicillin use in the first trimester and the risk for any of the 17 pre-defined specific malformations.
Other pregnancy outcomes
In logistic regression analysis, other pregnancy outcomes were compared in women who used erythromycin, penicillin V or amoxicillin at any time in pregnancy and women who had not used any antibiotics in pregnancy (Table 5). The rate of stillbirth, neonatal deaths and preterm deliveries was slightly lower among those who had used penicillin V compared with the control group. Slightly more infants were transferred to an ICU for women who had used erythromycin or amoxicillin during pregnancy.
Discussion
In this large, population-based, register study, we found no significant associations between erythromycin use in pregnancy and congenital malformations, cardiovascular malformations or any other specific malformations in infants. There were no associations between exposure to macrolides as a group and congenital malformations. These findings were in accordance with the majority of existing studies [11, 28–33].
Risk of cardiovascular malformations
In contrast to the data from the Swedish Medical Birth Registry, we did not find an association between erythromycin use in the first trimester and congenital cardiovascular malformations. This finding is reassuring, but should be interpreted with caution. In a review of noninherited risk factors for cardiovascular malformations, Jenkins et al. highlight the possibility of an effect dilution in epidemiological studies resulting from grouping phenotypes with different inherent susceptibilities [25], and ideally, each of the congenital cardiovascular defects should be assessed separately. Källén et al. have suggested that erythromycin may actually be weakly teratogenic due to the ability of erythromycin to inhibit a specific cardiac potassium channel [12, 34–37]. In animal embryo and in vitro studies, drugs that inhibit this channel have induced dose-dependent bradycardia and, at high concentrations, arrhythmia [38]. Experimentally, arrhythmia in the embryonic heart can lead to pressure changes and misdirection of blood flow; in turn, that can lead to hypoxia and reoxygenation damage and to cardiac and vessel defects. In preclinical animal studies, antiepileptic drugs and class III anti-arrhythmics, which also block this channel, have been shown to cause palate-cleft malformations, digit malformations and cardiovascular malformations [38].
If a weak adverse effect of erythromycin or macrolides exists, it will be underestimated in analyses of exposure in the first trimester because erythromycin use in the first weeks after conception is unlikely to affect the foetus. When restricting erythromycin exposure to the most vulnerable period of heart formation, we found an insignificantly higher odds for congenital cardiovascular malformations. The lack of significance may have been due to the lack of statistical power, and we cannot rule out a true association either with the drug or the underlying infection. On the other hand, other potential causes for this observation are pure coincidence, bias, confounding factors, like an underlying disease that predisposed for infections or a teratogenic effect of the infection that led to the erythromycin prescription. Although the studies are not directly comparable, the Swedish study of women exposed to erythromycin in the first trimester had a similar OR for cardiovascular malformations as we found in the subgroup of women exposed in week 5–8 of pregnancy, and in their study the association was significant. In Källen et al.'s as well as our study, smoking was more common and folic acid use less common among the antibiotic users. Use of multivitamins containing folic acid may reduce the risk of cardiovascular defects similar to the risk reduction for neural tube defects [25]. Källén et al. also report a higher concomitant use of a number of drugs, including drugs given at upper respiratory infections and asthma, oral contraceptives, minor analgetics, NSAIDs, opioids, systemic corticosteroids, sedatives and hypnotics [14]. Co-medication indicates a higher degree of maternal co-morbidity. If the women have used drugs during the infection with teratogenic properties, as might be the case with NSAIDs, this could affect the risk estimates [25]. As teratogenic drugs often causes malformations in more than one organ, the presence of combinations of apparently unrelated malformations increases the likelihood of teratogenicity [39]. We found no such tendencies in the presented details of the clinical cases in this study. Maternal co-morbidity was present in several of the exposed cases. The lack of consistent results across epidemiological studies also reduces the likelihood of erythromycin being an important teratogenic agent [39].
Other pregnancy outcomes
In adjusted analyses, we found no differences in preterm delivery, low birth weight or low Apgar scores between infants exposed to erythromycin, penicillin V or amoxicillin and those not exposed to any antibiotics during pregnancy. The risk of perinatal infant mortality was lower among women who used penicillin V during pregnancy. The risk of transferring the infant to the ICU was marginally higher among women who used erythromycin and amoxicillin. We found no large differences or obvious patterns. Overall, these results suggest that these antibiotics are safe to take in the second and third trimester.
Advantages and limitations
There are several advantages to this cohort study. The most obvious strength is that it was large and population-based. It consisted of over 180 000 pregnant women and their infants, the entire birthing population of Norway over 4 years. This represents one of the largest populations studied for the effects of erythromycin during pregnancy. Antibiotics are not sold over the counter in Norway and, thus, we had complete information on all antibiotics dispensed to pregnant women in the study period. As data on medication use and maternal health was collected prospectively, recall bias was avoided. Validation studies of congenital malformations in the MBRN have shown that the ascertainment of cases is related to type and severity. Cardiovascular anomalies have not been studied specifically, but the registration of serious malformations has been found satisfactory [40, 41]. It is unlikely that the ascertainment of malformations is affected by antibiotic use, and as long as exposure or outcome data are obtained without a bias, incomplete data will not affect the odds ratio estimates [42].
Several limitations should be mentioned. Firstly, although we included nearly 1800 women with erythromycin exposure during the first trimester, we did not reach the target statistical power. With an adjusted odds ratio ranging from 0.75 to 1.78, we cannot exclude a weak increase in the risk for foetal cardiovascular malformations in this group of women compared with the control group. For women exposed to erythromycin in weeks 5–8, the confidence interval for the adjusted odds ratio is wider and does not exclude an increased risk of three or less. Secondly, a major challenge in pharmacoepidemiologocal studies is the accuracy of the exposure assessment. We used dispensed antibiotics as a measure of antibiotic exposure: The women have received a prescription for an acute infection from the physician, brought it to the pharmacy and paid for the medication. Nevertheless, not all women will have used the full course of antibiotics, and this will bias the analysis towards 1.0. Thirdly, the NorPD did not contain information on the indication for drug use or the prescribed doses. This made it necessary to estimate the duration of antibiotic exposure based on the DDD. Furthermore, the NorPD have no information on antibiotics or other drugs provided in hospitals or other institutions.
Finally, although we adjusted for a variety of confounders available, the adjustment may not have been complete due to inaccurate registration in the MBRN, and some confounding factors are not adjusted for, such as concomittant drugs. Confounding by indication is a strong and difficult to control confounder which to a certain extent was reduced by the fact that exposure to penicillin V and amoxicillin was not associated with congenital cardiovascular disease in this study. These two antibiotics are often, but not fully, prescribed for similar conditions as the macrolides. In a Norwegian study of prescription patterns for acute respiratory tract infections, penicillin V was more often prescribed for tonsillitis and macrolides were more often prescribed for acute bronchitis and cough [43]. Further, only amoxicillin is prescribed for urinary tract infections and only erythromycin or azithromycin for genital chlamydia. Confounding by underlying disease may also affect the results.The validity of asthma, diabetes and epilepsy registrations in the MBRN have been estimated to be 51%, 72%, and 74%, respectively [44]. We find that underlying maternal disease is more common among women who have been using antibiotics than among women who have not been using antibiotics. If the percentage of under-reporting is identical in the two groups, the relative under-reporting will be higher among women using antibiotics. Maternal diabetes and epilepsy have been previously associated with congenital cardiovascular malformations [25]. Suboptimal control for these underlying maternal diseases in this cohort may have led to an overestimation of adverse pregnancy outcomes among pregnant women exposed to antibiotics. The control for cigarette smoking may also have led to a similar overestimation. It is likely to be under reported, it is more common among the antibiotic users and it is previously associated with cardiovascular defects in some studies [25].
Future directions
In this large, pharmacoepidemiological study, we found no increased risk of congenital cardiovascular malformation among women who had used erythromycin in the first trimester of pregnancy compared with controls without antibiotic exposure. These results were consistent with the majority of studies to date, which suggest that the risk of erythromycin use during early pregnancy is low and in most cases will outweigh the risk of the maternal infection.
Acknowledgments
We thank the Norwegian Antibiotic Centre for Primary Care for financial support in obtaining the data and the Medical Birth Registry of Norway for advice during the process.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Andrade SE, Gurwitz JH, Davis RL, Chan KA, Finkelstein JA, Fortman K, McPhillips H, Raebel MA, Roblin D, Smith DH, Yood MU, Morse AN, Platt R. Prescription drug use in pregnancy. Am J Obstet Gynecol. 2004;191:398–407. doi: 10.1016/j.ajog.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Bakker MK, Jentink J, Vroom F, Van Den Berg PB, De Walle HE, De Jong-Van Den Berg LT. Drug prescription patterns before, during and after pregnancy for chronic, occasional and pregnancy-related drugs in the Netherlands. BJOG. 2006;113:559–68. doi: 10.1111/j.1471-0528.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- 3.Engeland A, Bramness JG, Daltveit AK, Ronning M, Skurtveit S, Furu K. Prescription drug use among fathers and mothers before and during pregnancy. A population-based cohort study of 106,000 pregnancies in Norway 2004-2006. Br J Clin Pharmacol. 2008;65:653–60. doi: 10.1111/j.1365-2125.2008.03102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gagne JJ, Maio V, Berghella V, Louis DZ, Gonnella JS. Prescription drug use during pregnancy: a population-based study in Regione Emilia-Romagna, Italy. Eur J Clin Pharmacol. 2008;64:1125–32. doi: 10.1007/s00228-008-0546-y. [DOI] [PubMed] [Google Scholar]
- 5.Olesen C, Steffensen FH, Nielsen GL, de Jong-van den Berg, Olsen J, Sorensen HT. Drug use in first pregnancy and lactation: a population-based survey among Danish women. The EUROMAP group. Eur J Clin Pharmacol. 1999;55:139–44. doi: 10.1007/s002280050608. [DOI] [PubMed] [Google Scholar]
- 6.Graves CR. Pneumonia in pregnancy. Clin Obstet Gynecol. 2010;53:329–36. doi: 10.1097/GRF.0b013e3181de8a6f. [DOI] [PubMed] [Google Scholar]
- 7.Laibl V, Sheffield J. The management of respiratory infections during pregnancy. Immunol Allergy Clin North Am. 2006;26:155–72. doi: 10.1016/j.iac.2005.11.003. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rours GI, Duijts L, Moll HA, Arends LR, de GR, Jaddoe VW, Hofman A, Steegers EA, Mackenbach JP, Ott A, Willemse HF, van der Zwaan EA, Verkooijen RP, Verbrugh HA. Chlamydia trachomatis infection during pregnancy associated with preterm delivery: a population-based prospective cohort study. Eur J Epidemiol. 2011;26:493–502. doi: 10.1007/s10654-011-9586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2010. Atlanta, GA: Centers for Disease Control and Prevention; 2011. Available at http://www.cdc.gov/std/treatment/2010/toc.htm (last accessed 30 April 2012) [Google Scholar]
- 10.World Health Organization. Sexually Transmitted and Other Reproductive Tract Infections. A Guide to Essential Practice. Geneva: World Health Organization; 2005. [Google Scholar]
- 11.Briggs GG, Freeman RK, Yaffe SJ, editors. Drugs in Pregnancy and Lactation. A Reference Guide to Fetal and Neonatal Risk, 7th Edition. Baltimore, MD: Lippincott Williams & Wilkins; 2005. pp. 588–90. [Google Scholar]
- 12.Kallen BA, Otterblad OP, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol. 2005;20:209–14. doi: 10.1016/j.reprotox.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Källen KBM. Maternal drug use in early pregnancy and risk of cardiovascular defect in infant – A follow-up study. Reprod Toxicol. 2008;26:64–5. doi: 10.1016/s0890-6238(03)00012-1. (Abstract presented at the 36th Annual Conference of the European Teratology Society, Sept 21-24 2008; Edinburgh, Scotland) [DOI] [PubMed] [Google Scholar]
- 14.Källén BA. Drugs during Pregnancy. New York: Nova Science Publishers Inc; 2009. [Google Scholar]
- 15.Norwegian Medicines Agency. [Drugs during pregnancy][Internet] 2007. Norwegian. Available at http://www.slv.no/templates/InterPage____28568.aspx (last accessed 30 April 2012)
- 16.The Swedish Medical Products Agency. [Erythromycin should be avoided in early pregnancy] [Internet] 2006. Swedish. Available at http://www.lakemedelsverket.se/Alla-nyheter/NYHETER–2005/Erytromycin-bor-undvikas-under-tidig-graviditet/ (last accessed 30 April 2012)
- 17.Therapeutic Goods Administration. Prescribing medicines in pregnancy database [Internet] 2012. Department of Health and Ageing, Australian Government. Available at http://www.tga.gov.au/hp/medicines-pregnancy.htm (last accessed 30 April 2012)
- 18.Drugs Information Online. Eythromycin Tablets [Internet] 2012. Available at http://www.drugs.com (last accessed 30 April 2012)
- 19.World Health Organization. Guidelines for the Management of Sexually Transmitted Infections. Geneva: World Health Organization; 2003. [Google Scholar]
- 20.Jones D. Use of erythromycin in pregnancy. [Internet]. UK Teratology Information Service. 2009. Available at http://www.nelm.nhs.uk/en/NeLM-Area/Evidence/Drugs-in-Pregnancy/Use-of-erythromycin-in-pregnancy/ (last accessed 30 April 2012)
- 21.Eurocat. Eurocat Special Report. Congenital heart defects in Europe 2000-2005. University of Ulster: eurocat central registry. 2009. Available at http://www.eurocat-network.eu/content/Special-Report-CHD.pdf (last accessed 30 April 2012)
- 22.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–72. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 23.Brennan P, Young ID. Congenital heart malformations: aetiology and associations. Semin Neonatol. 2001;6:17–25. doi: 10.1053/siny.2000.0032. [DOI] [PubMed] [Google Scholar]
- 24.Pierpont ME, Basson CT, Benson DW, Jr, Gelb BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D, Webb CL. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:3015–38. doi: 10.1161/CIRCULATIONAHA.106.183056. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, Elixson M, Warnes CA, Webb CL. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:2995–3014. doi: 10.1161/CIRCULATIONAHA.106.183216. [DOI] [PubMed] [Google Scholar]
- 26.Sadler TW. Langman's Medical Embryology, 6th Edition. Baltimore, MD: Williams & Wilkins; 1990. [Google Scholar]
- 27.WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment 2011. Oslo: World Health Organization; 2010. [Google Scholar]
- 28.Bar-Oz B, Diav-Citrin O, Shechtman S, Tellem R, Arnon J, Francetic I, Berkovitch M, Ornoy A. Pregnancy outcome after gestational exposure to the new macrolides: a prospective multi-center observational study. Eur J Obstet Gynecol Reprod Biol. 2008;141:31–4. doi: 10.1016/j.ejogrb.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, Hu DJ. Antibacterial medication use during pregnancy and risk of birth defects: national Birth Defects Prevention Study. Arch Pediatr Adolesc Med. 2009;163:978–85. doi: 10.1001/archpediatrics.2009.188. [DOI] [PubMed] [Google Scholar]
- 30.Czeizel AE, Rockenbauer M, Sorensen HT, Olsen J. A population-based case-control teratologic study of oral erythromycin treatment during pregnancy. Reprod Toxicol. 1999;13:531–6. doi: 10.1016/s0890-6238(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 31.Drinkard CR, Shatin D, Clouse J. Postmarketing surveillance of medications and pregnancy outcomes: clarithromycin and birth malformations. Pharmacoepidemiol Drug Saf. 2000;9:549–56. doi: 10.1002/pds.538. [DOI] [PubMed] [Google Scholar]
- 32.Einarson A, Phillips E, Mawji F, D’Alimonte D, Schick B, Addis A, Mastroiacova P, Mazzone T, Matsui D, Koren G. A prospective controlled multicentre study of clarithromycin in pregnancy. Am J Perinatol. 1998;15:523–5. doi: 10.1055/s-2007-994053. [DOI] [PubMed] [Google Scholar]
- 33.Louik C, Werler MM, Mitchell AA. Erythromycin use during pregnancy in relation to pyloric stenosis. Am J Obstet Gynecol. 2002;186:288–90. doi: 10.1067/mob.2002.119718. [DOI] [PubMed] [Google Scholar]
- 34.Cubeddu LX. Iatrogenic QT abnormalities and fatal arrhythmias: mechanisms and clinical significance. Curr Cardiol Rev. 2009;5:166–76. doi: 10.2174/157340309788970397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simko J, Csilek A, Karaszi J, Lorincz I. Proarrhythmic potential of antimicrobial agents. Infection. 2008;36:194–206. doi: 10.1007/s15010-007-7211-8. [DOI] [PubMed] [Google Scholar]
- 36.Stanat SJ, Carlton CG, Crumb WJ, Jr, Agrawal KC, Clarkson CW. Characterization of the inhibitory effects of erythromycin and clarithromycin on the HERG potassium channel. Mol Cell Biochem. 2003;254:1–7. doi: 10.1023/a:1027309703313. [DOI] [PubMed] [Google Scholar]
- 37.Owens RC, Jr, Nolin TD. Antimicrobial-associated QT interval prolongation: points of interest. Clin Infect Dis. 2006;43:1603–11. doi: 10.1086/508873. [DOI] [PubMed] [Google Scholar]
- 38.Danielsson BR, Skold AC, Azarbayjani F. Class III antiarrhythmics and phenytoin: teratogenicity due to embryonic cardiac dysrhythmia and reoxygenation damage. Curr Pharm Des. 2001;7:787–802. doi: 10.2174/1381612013397744. [DOI] [PubMed] [Google Scholar]
- 39.Shepard TH. ‘Proof’ of human teratogenicity. Teratology. 1994;50:97–8. doi: 10.1002/tera.1420500202. [DOI] [PubMed] [Google Scholar]
- 40.Kubon C, Sivertsen A, Vindenes HA, Abyholm F, Wilcox A, Lie RT. Completeness of registration of oral clefts in a medical birth registry: a population-based study. Acta Obstet Gynecol Scand. 2007;86:1453–7. doi: 10.1080/08037050701645090. [DOI] [PubMed] [Google Scholar]
- 41.Melve KK, Lie RT, Skjaerven R, Van Der Hagen CB, Gradek GA, Jonsrud C, Braathen GJ, Irgens LM. Registration of Down syndrome in the medical birth registry of Norway: validity and time trends. Acta Obstet Gynecol Scand. 2008;87:824–30. doi: 10.1080/00016340802217184. [DOI] [PubMed] [Google Scholar]
- 42.Källén BA. Methodological issues in the epidemiological study of the teratogenicity of drugs. Congenit Anom. 2005;45:44–51. doi: 10.1111/j.1741-4520.2005.00062.x. [DOI] [PubMed] [Google Scholar]
- 43.Gjelstad S, Straand J, Dalen I, Fetveit A, Strøm H, Lindbæk M. Do general practicioners' consultation rates influence their prescribing patterns for acute respiratory tract infections? J Antimicrob Chemother. 2011;66:2425–33. doi: 10.1093/jac/dkr295. [DOI] [PubMed] [Google Scholar]
- 44.Engeland A, Bjorge T, Daltveit AK, Vollset SE, Furu K. Validation of disease registration in pregnant women in the Medical Birth Registry of Norway. Acta Obstet Gynecol Scand. 2009;88:1083–9. doi: 10.1080/00016340903128454. [DOI] [PubMed] [Google Scholar]


