Abstract
Objective
To investigate the effect of temperature, dilution, and pH on the viscosity of ocular lubricants.
Design
Laboratory based investigation of viscosity.
Participants
No human subjects.
Methods
Hypromellose 0.3%, sodium hyaluronate 0.4%, carboxymethylcellulose sodium 0.5%/glycerin 0.9%, and carmellose sodium 0.5% were investigated. Ostwald capillary viscometers were utilised for viscosity measurements. The kinematic viscosity of each lubricant was tested quantitatively from 22 to 40 °C, and over a pH range of 5–8 under isothermal conditions. The kinematic viscosity of each eye drop was also tested under dilution by varying the mass fraction of each eye drop under isothermal conditions.
Main outcome measure
Changes in kinematic viscosity.
Results
Hypromellose 0.3% had an initial pH of 8.34, while the other lubricants had a pH close to neutral. From 22 to 35 °C, the kinematic viscosity of sodium hyaluronate 0.4 fell by 36% from 37.8 to 24.4 mm2/s, carboxymethylcellulose sodium 0.5%/glycerin 0.9% fell by 35% from 16.98 to 11.1 mm2/s, hypromellose fell by 37% from 6.89 to 3.69 mm2/s, and carmellose sodium 0.5% fell by 25% from 2.77 to 1.87 mm2/s. At 32 °C only sodium hyaluronate 0.4%, and carboxymethylcellulose sodium 0.5%/glycerin 0.9% retained sufficient kinematic viscosity to maintain precorneal residence. Kinematic viscosities of all the topical lubricants were unaffected by pH but decreased significantly with dilution.
Conclusions
This study suggests that currently used ocular lubricants have limited bioavailability due to reductions in viscosity by temperature and dilutional changes under physiological conditions. Developing lubricants with stable viscosities may maximise therapeutic efficacy.
Keywords: lubricant, viscosity, rheology
Introduction
Normal sight is dependent on a moist ocular surface and the human tear film has an essential role on the maintenance of optical clarity, as well as nourishment, lubrication, and protection. Dry eye disease is one of the most frequently encountered problems in ophthalmology with estimates of prevalence ranging from 11 to 22%.1 Changes in ocular surface physiology and in the tear film occur in dry eye disease, but the mechanisms are not fully understood. The treatment of dry eye remains a significant clinical challenge, and topical lubricants form the mainstay of treatment.
The main limitation of current ocular lubricants is the short duration of symptom control. Various approaches have, therefore, been used to prolong the precorneal residence time such as occlusion of lacrimal puncta,2 and slow release artificial tears,3 but these have not had universal success.
An alternative approach is to use lubricants with high viscosity. Eye-drop instillation results in an increase in tear volume, and this slowly returns to its baseline level due to drainage through canaliculi, and because of fluid loss through other means such as transport across the ocular epithelia or evaporation.4 If the applied eye drops have a viscosity similar to that of tears, they are eliminated within a few minutes.5, 6, 7 It is, therefore, essential that ocular lubricants are of sufficient viscosity to maximise bioavailability.
Rheology is the study of deformation and the flow of complex fluids such as polymers, emulsions, suspensions, and biological systems.8 Viscosity is the most commonly sought after rheological parameter. Ocular lubricants can be treated as Newtonian viscous liquids, whereby the rate of viscous flow is proportional to the shear stress. For any Newtonian fluid, the dynamic viscosity η defined by the equation:
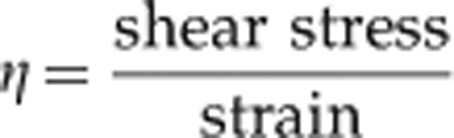 |
is a constant. The kinematic viscosity ν of a fluid is defined by:
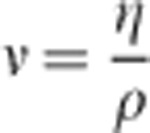 |
where ρ is the fluid density. The viscosity of real materials can be significantly affected by variables such as temperature, pressure, and composition.
When an ocular lubricant is applied to the eye, it will be affected by several factors. The temperature will increase from the ambient temperature to eye temperature, which ranges between 32.9 and 36 °C,9 it will undergo dilution, and it may be exposed to changes in pH. It must be remembered that it is a normal physical law that viscosity decreases when temperature increases and with dilution. In order to maximise bioavailability it is important that ocular lubricants, therefore, maintain their viscosity within the eye. The purpose of this study was to investigate the effect of temperature, dilution, and pH on the viscosity of ocular lubricants.
Materials and methods
Materials
Viscometer
The U-tube viscometer employed in this project had two bulbs (of similar radius in order to minimise surface tension errors) linked by a capillary as shown in Figure 1. There were three calibration marks, A, B, and C. The viscometers had been pre-calibrated against reference viscometers held at the manufacturer's laboratory at a temperature of 40 °C. The viscometer was mounted vertically in a temperature-controlled water bath using a special viscometer holder.
Figure 1.
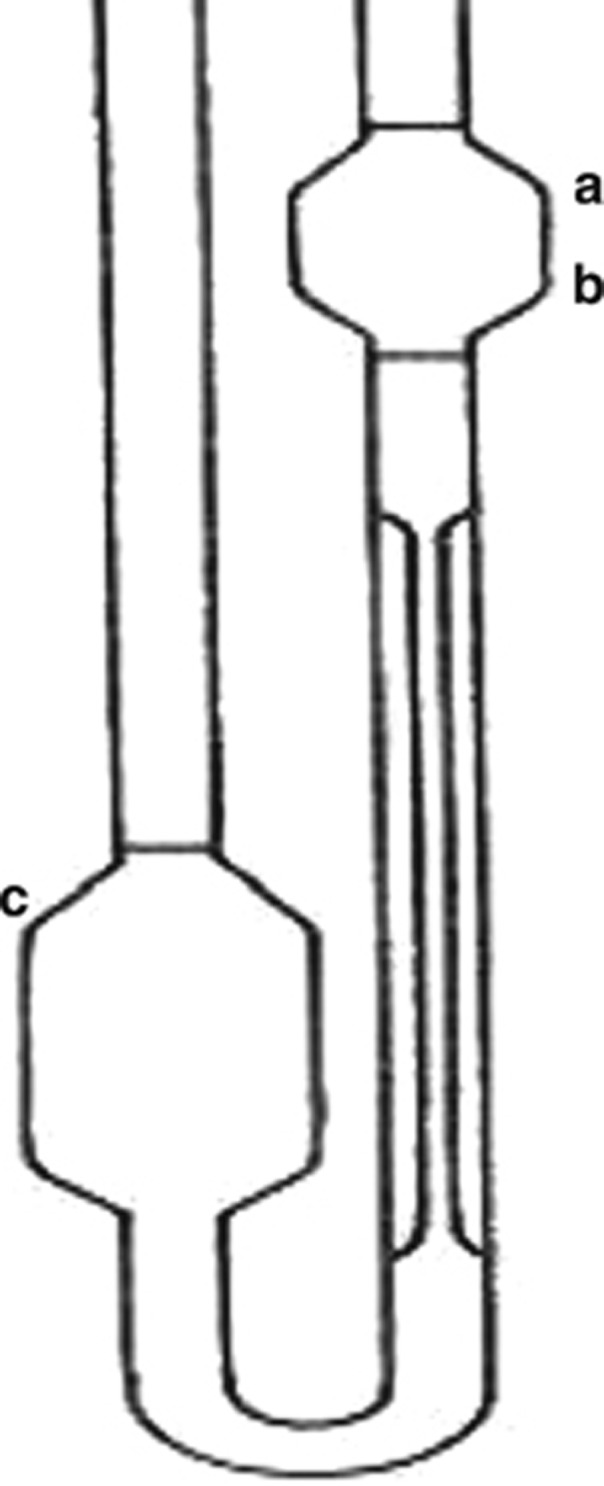
U-tube capillary viscometer.
The temperature of the bath was measured with a calibrated platinum resistance thermometer (PRT) of four-wire configuration, the resistance of which was measured an Agilent model 34970A data acquisition unit (Agilent Technologies, Winnersh, UK). The PRT was immersed in the constant-temperature bath, just beside the viscometer, and the overall uncertainty of the temperature was ±0.02 °C.
Eye-drop samples
The four eye-drop samples investigated were hypromellose 0.3% (this is the commercial name), carboxymethylcellulose sodium 0.5%/glycerin 0.9% (Optive, Allergan, Irvine, CA, USA), sodium hyaluronate 0.4% (Clinitas Soothe, Altacor, Cambridge, UK) and carmellose sodium 0.5% (Celluvisc 0.5%, Allergan).
Miscellaneous
The following additional items were used: pipette sucker; count-up timer (reading to 0.01 s); 12 M hydrochloric acid to regulate pH; digital pH meter; pH calibration standards; digital-weighing machine; de-ionised water; sodium chloride to prepare saline solution; and appropriate glassware.
Methods
Viscometry
For normal-flow U-tube capillary viscometers, the kinematic viscosity of the standard viscosity liquids, ignoring the kinetic energy correction terms, is determined experimentally from the equation:
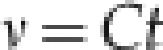 |
where ν is the kinematic viscosity at the stated temperature, t is the observed efflux time and C is the viscometer constant based upon an absolute value of 1.0034 mm2/s for the kinematic viscosity of pure water at 20 °C and normal atmospheric pressure. The efflux time is proportional to the kinematic viscosity of the fluid.
The viscometer was cleaned by rinsing carefully with running tap water and then with acetone. Oil-free compressed air was passed through the instrument, in order to remove any final traces of solvent. The viscometer was charged by introducing the sample lubricant through the left arm of the viscometer into the lower reservoir until the meniscus level was on calibration mark C. This was done by injecting the eye-drop sample via a syringe with 12 mm long hypodermic needle.
The viscometer was placed vertically in the holder and then inserted into the constant-temperature bath. Approximately 20 min were allowed for both the sample and viscometer to attain the bath temperature. The sample was then sucked into the right arm of the viscometer using a sucker, until the meniscus level was sufficiently above the top calibration mark A. The suction was then removed and the time taken for meniscus to fall under gravity from A to B (the efflux time) was measured.
The procedure was repeated isothermally until 3 or 4 readings were obtained and an average value was then calculated.
Effect of temperature
The general procedure described above of charging the viscometer with sample by applying and removing suction was followed throughout this project. Viscosity measurements of eye-drop samples were performed at five different temperatures: 22, 25, 30, 35 and 40 °C. The viscometer was carefully rinsed with water, followed by acetone and then dried before performing viscosity measurements at any new temperature.
Eye-drop dilution investigation
The mass of eye-drop sample just enough to fill the viscometer was weighed out precisely in a 100 ml plastic container. De-ionised water was then added drop by drop using a plastic pipette until an even mixture of 80% eye drop and 20% de-ionised water was obtained, that is, 2 g of water for every 8 g of eye drop. This procedure was performed in an enclosed weighing machine. The mass fraction and the initial mass of the diluted mixture were then recorded. The diluted sample was then injected into the lower reservoir of the viscometer and the mass of surplus mixture (not being used in the viscometer operation) was recorded. Viscosity measurements were then performed at a fixed temperature, by the methods described above.
Following measurements, the sample used in the viscometer was recovered using a syringe with a long hypodermic needle. The new total (used + surplus) mass of available 80% eye-drop sample was noted. Sufficient de-ionised water was then added to reduce the mass fraction of the original eye drop to 60%, utilising similar methods described above.
Before performing new viscosity measurements, the viscometer was carefully rinsed and dried. The procedure was then repeated to obtain results at mass fractions of 0.8, 0.6, 0.4, and 0.2. A bath temperature of 32 °C was maintained throughout the experiment. The viscosity value of de-ionised water at 32 °C was obtained via interpolation from the smooth curve through the data obtained at other temperatures. The entire procedure above was repeated using nine p.p.t. (parts-per-thousand) saline as the dilution medium instead of pure de-ionised water, for the same eye-drop sample and bath temperature.
Preparation of saline
Artificial tears were made of ∼9 p.p.t saline. First of all, 9 g of NaCl was weighed accurately in a weighing boat. The weighed sample was then transferred to a 1-l volumetric flask. De-ionised water was added to the flask until the meniscus was just below the graduation mark and the contents were thoroughly mixed. De-ionised water was then added drop by drop until the meniscus was exactly on the mark.
pH investigation
The pH meter was calibrated by using three standard buffer solutions having pH values of 4.01, 7.00, and 9.21 at 25 °C. The pH electrode was rinsed with distilled water and dried before performing pH measurements. The electrode was then inserted into a beaker containing the sample to be tested and the stabilised pH value from the meter was recorded.
The pH of a particular eye-drop sample was decreased by adding small drops of 12 M hydrochloric acid and stirring until the pH meter read the desired pH value. Viscosity measurements were then carried out at several pH values, ranging from 3 to 7.
Results
The initial pH of the four eye-drops samples tested is shown in Table 1.
Table 1. The initial pH of the ocular lubricants tested.
| Sample | Initial pH |
|---|---|
| Hypromellose 0.3% | 8.34 |
| Carboxymethylcellulose sodium 0.5%/glycerin 0.9% | 6.75 |
| Sodium hyaluronate 0.4% | 6.95 |
| Carmellose sodium 0.5% | 7.24 |
All of the eye drops tested had a pH approaching neutral apart from hypromellose 0.3%, which had an alkaline pH.
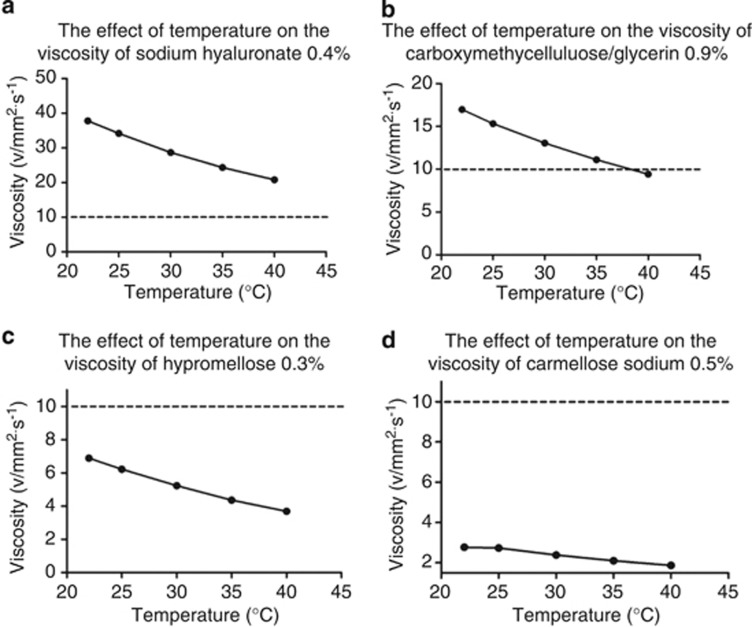
The effect of temperature on viscosity is shown in Figure 2. From 22 to 35 °C the kinematic viscosity of sodium hyaluronate 0.4% fell 36% from 37.8 to 24.35 8 mm2/s, carboxymethylcellulose sodium 0.5%/glycerin 0.9% fell 35% from 16.98 to 11.1 mm2/s, hypromellose 0.3 % fell 37% from 6.89 to 3.69 mm2/s, and carmellose sodium 0.5% fell 25% from 2.77 to 1.87 mm2/s.
Figure 2.
The effect of temperature on the viscosity of topical lubricants. (a) Sodium hyauronate 0.4%, (b) carboxymethylcellulose/glycerin 0.9%, (c) hypromellose 0.3%, (d) carmellose sodium 0.5%. Error bars of ±1.0% deviation have been applied and a second order best fit polynomial has been used to create best fit curves. The horizontal dotted line marks kinematic viscosity of 10 ν/mm2/s, which is the minimum viscosity required to maintain precorneal residence in man (see discussion).
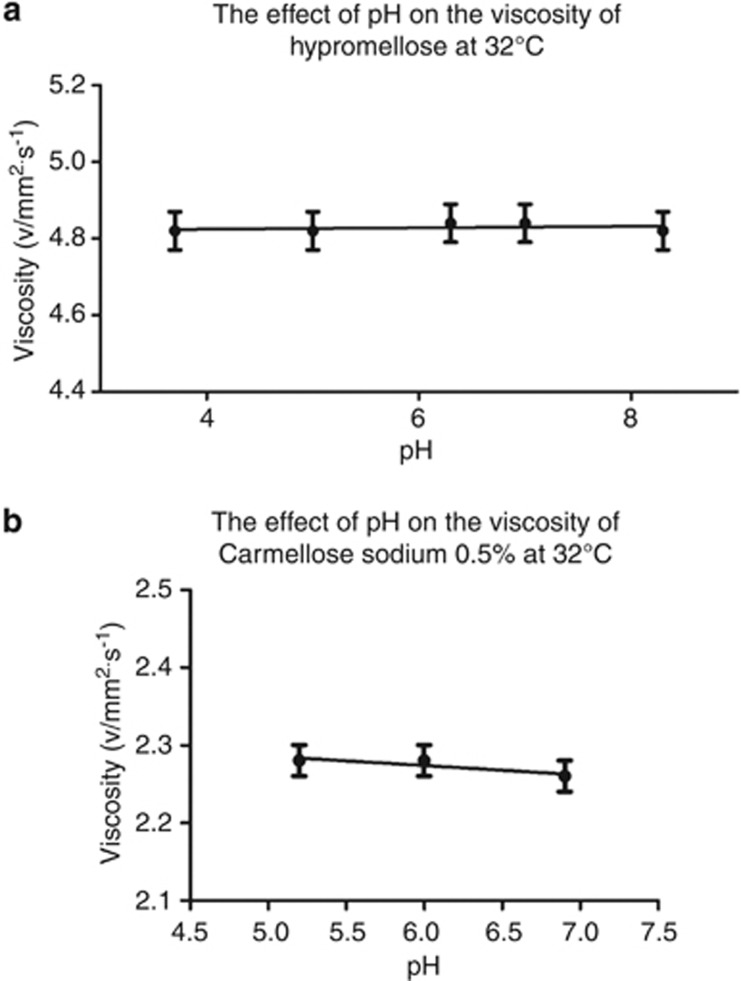
The effect of pH on the kinematic viscosity of hypromellose 0.3%, and carmellose sodium 0.5%, are shown in Figure 3. The kinematic viscosities of hypromellose 0.3% and carmellose sodium 0.5% were unaffected by pH and remained constant around 4.82 mm2/s for hypromellose 0.3% and 2.27 mm2/s for carmellose sodium 0.5%.
Figure 3.
The effect of pH on the kinematic viscosity of topical lubricants. (a) Hypromellose 0.3% at 32 °C. (b) Carmellose sodium 0.5% at 32 °C. Error bars of ±1.0% deviation have been applied and linear regression has been used to create a best fit line.
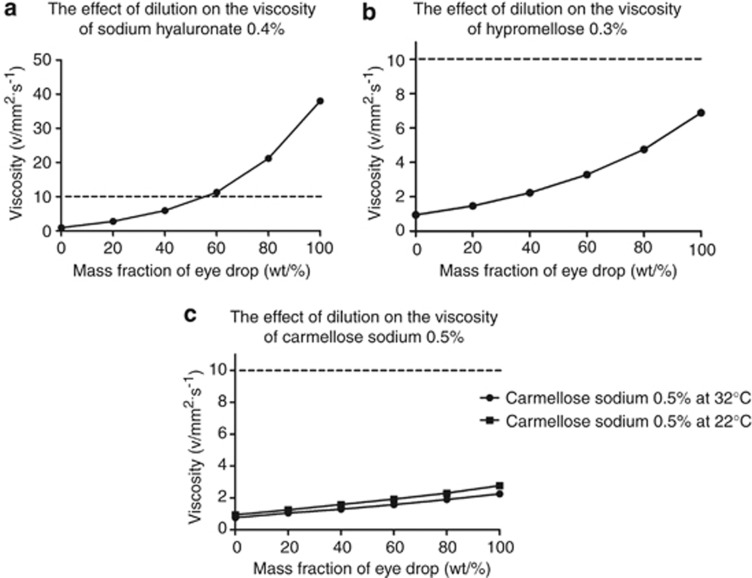
The effect of dilution on the kinematic viscosities of hypromellose 0.3%, carboxymethylcellulose sodium 0.5%/glycerin 0.9% and sodium hyaluronate 0.4% are shown in Figure 4. The viscosities of all the lubricants tested decreased rapidly with dilution. Sodium hyaluronate showed the highest fall in kinematic viscosity with dilution; its viscosity fell by 84% from 38 to 5.94 mm2/s when the mass fraction was reduced from 100 to 40%.
Figure 4.
The effect of dilution on the kinematic viscosity of topical lubricants. (a) Hypromellose 0.3%, (b) carboxymethylcellulose sodium 0.5%/glycerin 0.9%, (c) sodium hyaluronate 0.4%. Error bars of ±1.0% deviation have been applied and a second order best fit polynomial has been used to create best fit curves. The horizontal dotted line marks kinematic viscosity of 10 ν/mm2/s, which is the minimum viscosity required to maintain precorneal residence in man (see discussion).
Discussion
The authors believe that this is the first study in the current literature to investigate the effects of physiological factors such as pH, temperature and dilutional effects on eye-drop viscosity. It has previously been demonstrated experimentally that solutions with high viscosity have longer precorneal residence times.10, 11, 12 It is, therefore, desirable when formulating topical lubricants, to ensure that there is sufficient viscosity to maintain bioavailability.
The minimum kinematic viscosity required to maintain bioavailability has not been investigated in the current literature. However, the minimum shear viscosity of eye drops required to maintain precorneal residence in man has been reported to be 10 mPa s.12 The kinematic viscosity measured by capillary viscometers like those used in our study, is simply the shear viscosity divided by the density. In practical terms a shear viscosity of 10mPa s is equivalent to a kinematic viscosity of 10 mm2/s in dilute aqueous solutions.
Our study, therefore, suggests that carmellose sodium 0.5% with a kinematic viscosity of 4.82 mm2/s at 32 °C and hypromellose 0.3% with a kinematic viscosity of 2.27 mm2/s at 32 °C do not have sufficient absolute kinematic viscosity to maintain precorneal residence regardless of temperature, dilution, or pH. Although there have been many studies investigating ocular lubricants, no unambiguous statistical differences have been reported between the product types.13, 14 However, a recent systematic review of different ocular lubricants found that eye drops such as hypromellose had the least net changes in improvement compared with carbomer gels and hyaluronic acid products, and low viscosities may explain this.14
Sodium hyaluronate 0.4% and carboxymethylcellulose sodium 0.5%/glycerin 0.9%, had the highest absolute kinematic viscosities, but also showed steep declines in viscosity with increasing temperature. Our study suggests that under physiological conditions, when increasing from room temperature (22 °C) to approximate eye temperature (35 °C), kinematic viscosities for these two lubricants falls by about one third, but remains above the critical level of 10 mm2/s.
Moreover, the kinematic viscosities of all the ocular lubricants tested were strongly affected by dilutional changes, with the viscosity of sodium hyaluronate 0.4% being particularly sensitive to changes in concentration. This is of particular clinical relevance, as ocular lubricants will be constantly subjected to dilutional effects due to tear production and lacrimal drainage replacing the contents of the conjunctival sac. Human tears turn over at about 16%/minute with a normal blink rate of 15–20 blinks/min.15 The concentration of eye drops, therefore, has the potential to decrease by 50% within 3–4 minutes of instillation.
Changes in pH did not have a significant effect on the kinematic viscosity of the ocular lubricants tested. Our initial measurement of pH found that hypromellose 0.3% had an alkaline pH of 8.34. As well as being potentially harmful to a compromised eye surface, this may further decrease bioavailablity as an alkaline pH is likely to stimulate tear production and blinking.
Our study of four commonly available ocular lubricants, therefore, suggests that sodium hyaluronate 0.4%, and carboxymethylcellulose sodium 0.5%/glycerin 0.9% are the best performing medications.
A possible limitation of this study is that it has been assumed that all the eye drops studied are Newtonian viscous fluids, which offer a constant amount of resistance to shear to all rates of shear. However, studies suggest that sodium hyaluronate may exhibit non-Newtonian or pseudoplastic behavior.5 It is assumed that sodium hyaluronate may undergo reductions in viscosity when exposed to high shear rates during blinking. However, any reduction in viscosity when blinking is likely to exacerbate its sensitivity to temperature and dilutional changes, and so will not affect the findings of this study.
In vitro studies such as this do not always correspond with in vivo findings. However, previous in vivo work has found that sodium hyaluronate does indeed have the highest precorneal residence times compared with other ocular lubricants such as polyvinyl alcohol or hydroxypropylmethylcellulose.16
In summary, the kinematic viscosities of ocular lubricants are independent of pH, but are strongly dependant on changes in temperature and dilutional changes. These are likely to reduce bioavailability under physiological conditions during instillation in the eye. Developing future lubricants with viscosities that are more resilient to temperature and dilution will maximise therapeutic efficacy.
The authors declare no conflict of interest.
References
- Brewitt H, Sistani F. Dry eye disease: the scale of the problem. Surv Ophthalmol. 2001;45 (Suppl 2:S199–S202. doi: 10.1016/s0039-6257(00)00202-2. [DOI] [PubMed] [Google Scholar]
- Dohlman CH. Punctal occlusion in keratoconjunctivitis sicca. Ophthalmology. 1978;85 (12:1277–1281. doi: 10.1016/s0161-6420(78)35555-x. [DOI] [PubMed] [Google Scholar]
- Werblin TP, Rheinstrom SD, Kaufman HE. The use of slow-release artificial tears in the long-term management of keratitis sicca. Ophthalmology. 1981;88 (1:78–81. doi: 10.1016/s0161-6420(81)35074-x. [DOI] [PubMed] [Google Scholar]
- Maurice DM. The dynamics and drainage of tears. Int Ophthalmol Clin. 1973;13 (1:103–116. doi: 10.1097/00004397-197301310-00009. [DOI] [PubMed] [Google Scholar]
- Snibson GR, Greaves JL, Soper ND, Prydal JI, Wilson CG, Bron AJ, et al. Precorneal residence times of sodium hyaluronate solutions studied by quantitative gamma scintigraphy. Eye. 1990;4 (Pt 4:594–602. doi: 10.1038/eye.1990.83. [DOI] [PubMed] [Google Scholar]
- Yokoi N, Komuro A. Non-invasive methods of assessing the tear film. Exp Eye Res. 2004;78 (3:399–407. doi: 10.1016/j.exer.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Meadows DL, Paugh JR, Joshi A, Mordaunt J. A novel method to evaluate residence time in humans using a nonpenetrating fluorescent tracer. Invest Ophthalmol Vis Sci. 2002;43 (4:1032–1039. [PubMed] [Google Scholar]
- Barnes HA. An Introduction to Rheology. Elsevier International: Oxford, UK; 1993. [Google Scholar]
- Purslow C, Wolffsohn JS. Ocular surface temperature: a review. Eye Contact Lens. 2005;31 (3:117–123. doi: 10.1097/01.icl.0000141921.80061.17. [DOI] [PubMed] [Google Scholar]
- Chrai SS, Robinson JR. Ocular evaluation of methylcellulose vehicle in albino rabbits. J Pharm Sci. 1974;63 (8:1218–1223. doi: 10.1002/jps.2600630810. [DOI] [PubMed] [Google Scholar]
- Bach FC, Adam JB, McWhirter HC, Johnson JE. Ocular retention of artificial tear solutions. Comparison of hydroxypropyl methylcellulose and polyvinyl alcohol vehicles using an argyrol marker. Ann Ophthalmol. 1972;4 (2:116–119. [PubMed] [Google Scholar]
- Zaki I, Fitzgerald P, Hardy JG, Wilson CG. A comparison of the effect of viscosity on the precorneal residence of solutions in rabbit and man. J Pharm Pharmacol. 1986;38 (6:463–466. doi: 10.1111/j.2042-7158.1986.tb04611.x. [DOI] [PubMed] [Google Scholar]
- Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405–412. doi: 10.2147/opth.s5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty MJ, Glavin S. Efficacy of different dry eye treatments with artificial tears or ocular lubricants: a systematic review. Ophthalmic Physiol Opt. 2009;29 (6:573–583. doi: 10.1111/j.1475-1313.2009.00683.x. [DOI] [PubMed] [Google Scholar]
- Forrester J. The Eye: Basic Sciences in Practise. WB Saunders: London; 2002. [Google Scholar]
- Snibson GR, Fitzgerald P, Hardy JG, Wilson CG. Ocular surface residence times of artificial tear solutions. Cornea. 1992;11 (4:288–293. doi: 10.1097/00003226-199207000-00003. [DOI] [PubMed] [Google Scholar]