INTRODUCTION
The long-term mortality of myocardial infarction (MI) has been declining over the last decades. 1–3 Secondary prevention with beta blockers, angiotensin blocking agents (angiotensin-converter enzyme inhibitors, ACEI, or angiotensin2 receptor blockers, ARB) and statins (collectively post-MI drugs), as recommended by MI treatment guidelines, 4–7 is largely credited for this success. 2–3, 8 Although the use of these drugs has increased over time, 2, 9–11 far from all MI survivors receive them, suggesting that guidelines are applied to a variable degree.
Previous research has found that patient, 10–13 and provider 13 characteristics explain parts of the variation in prescribing secondary prevention for cardiovascular disease after initial hospital discharge. The extent of the variation by hospital and physician, and its change over time are not well characterized.
Therefore, in this study, we sought to describe temporal trends in initiating beta blockers, angiotensin-blocking agents and statins after discharge of an MI hospitalization from 1997 to 2004 in British Columbia, and analyze the variation in initiating these drugs adjusted for patient and provider characteristics, depending on the discharging hospital and physician, and the post-discharge physician responsible for secondary prevention decisions.
METHODS
Study population
We assembled a cohort of patients 18 years old and over hospitalized for a myocardial infarction in British Columbia between January 1997 and December 2004. We included only patients who were alive 30 days after discharge and whose medical care was paid for by the province-funded British Columbia Medical Services Plan, that provides medical care to 96% of British Columbia residents. 14 British Columbia health-care utilization data has been reported to be highly accurate and complete, 15 and has been extensively used for research on cardiovascular drugs and outcomes. 16,17
To define an MI hospitalization, we required a length of stay of 3–180 days with an ICD-9 code 410.xx in the first or second diagnosis position. This definition has been shown to have a positive predictive value of 94% in claims data. 18 When a patient had more than one MI during this period, we selected the first one. We further required enrollment in the Medical Services Plan for one year before the MI (baseline period) to assess the presence of medical conditions, health care utilization and medication use prior to the MI. We excluded patients with codes for prior MI or revascularization procedures in the baseline period, and those who were readmitted within 30 days of discharge from the index hospitalization
This study was approved by the Brigham and Women’s Hospital institutional review board and signed data use agreements were in place.
Study drug users
Pharmacy dispensing data was obtained through linkage of physician service claims, hospital discharge records and PharmaNet, a database that records every prescription dispensed in British Columbia. 19 We identified those patients who filled a prescription for a beta blocker, an angiotensin blocking agent (ACEI or ARB), or a statin, within 30 days of discharge after the index MI hospitalization. New users were those who had not filled prescriptions for any agent of the same class during the 12 months prior to the hospital admission. Patients treated with one class of drugs during the baseline period could initiate treatment with any other drug class. We classified statin claims as high-potency if the drug/strength combinations were expected to lower the LDL-cholesterol blood level by more than 40 % (details provided in the on-line appendix). 20–22
Hierarchical structure of the data and provider levels
Patients of one health care provider share measured and unobserved characteristics that impose a hierarchical structure to the individual-level data. These characteristics may influence the decision to initiate secondary prevention after an MI. Such clustering of patients by providers can be explicitly modeled. We identified from the hospital discharge records two provider levels: the hospital and the physician who was most responsible for the care of the patient during the MI hospitalization; we will refer to them as the discharging hospital and discharging physician. Hospitals that admitted 50 or more patients for MI from this cohort were considered high-volume hospitals; the cutoff for physicians was 20 or more.
Physician “responsible” for the secondary prevention prescribing decision
Because claims data do allow for the identification of physicians who should have prescribed secondary prevention but did not do so, we developed algorithms to identify the physician responsible for prescribing decisions without relying on the identification of the actual prescriber in patients who received secondary prevention. These definitions were based on those used previously in the literature, 23–27 and included the physician who before the MI and within 30 days after hospital discharge MI wrote most prescriptions for any drug (pre-MI and post-discharge prescriber) and for cardiac drugs (pre-MI and post-discharge cardiac prescriber), recorded most medical service claims (pre-MI and post-discharge provider) and claims only for cardiac diagnoses (pre-MI and post-discharge cardiac provider; a more detailed version of this analysis can be found in the on-line appendix). For patients who were prescribed secondary prevention, we calculated the agreement between the algorithm-identified physician and the prescribing physician for each definition and selected the algorithm that provided the highest agreement rates. The best performing definition was the post-discharge cardiac prescriber. The agreement rates were 88–91% for each of the three drug classes for continuing and new users combined and 90–92% for new-users. In order to identify the “responsible” physician we then applied this algorithm to the entire cohort.(i.e. patients who were and were not prescribed secondary prevention).
Model covariates
We identified the presence of the following patient characteristics: age at index MI, sex, and the presence of asthma, chronic obstructive pulmonary disease, atrioventricular block, heart failure, hypertension, chronic kidney disease or proteinuria, diabetes and myopathies, assessed during the baseline period and index hospitalization, as they are indications or contraindications for the drugs of interest. We also calculated the Romano comorbidity score and the number of distinct ‘cardiovascular’ medications used during the year prior to the MI hospitalization. 28
For each hospital, we calculated the annualized hospital admission volume as the number of patients in the cohort admitted to that hospital during the entire study period divided by the number of years the hospital admitted at least one cohort patient. For each physician, we calculated the annualized patient volume as defined above, and recorded the physician specialty as internal medicine, general practice, or other.
Statistical analysis
In a descriptive analysis, we calculated the yearly proportion of initiation among patients who had not been previously prescribed these drugs. To address the variation in drug initiation at the provider level, we used for the main analysis logistic regression models with random intercepts. 29 The random intercepts can be interpreted as the provider-specific deviation from the “average provider” probability of initiating a patient in each of the drug classes after controlling for patient and provider characteristics; they are assumed to have a normal distribution with a mean of zero and a variance determined by the data. This variance, the between-provider variance σb2, conveys the dispersion of provider-specific preference for initiating patients with the study drugs (model details in the on-line appendix).
For each drug class and biennium (1997–8, 1999–2000, 2001–2, 2003–4), we fitted one age-sex-adjusted and one fully-adjusted model where patients were clustered in hospitals, a second pair of models where patients were clustered in discharging physicians, and a third pair of models where patients were clustered in the physicians responsible for the prescribing decision. Age-sex adjusted models included patients’ age, age squared and sex only. Fully adjusted models incorporated asthma, chronic obstructive pulmonary disease, atrioventricular block, heart failure, hypertension, chronic kidney disease or proteinuria, Romano comorbidity score, number of cardiovascular medications, annualized volume and specialty (at physician-level analysis). We restricted the adjusted analyses to the patients of the high-volume providers, in the understanding that low-volume providers may not represent the providers overall. The low number of high-potency statin initiators in the earlier years of the study period limited the ability of the models to incorporate the covariates for asthma, atrioventricular block, heart failure, chronic kidney disease or proteinuria, and specialty. Similarly, the lack of variability in hypertension and heart failure for initiating ACEI/ARB at the post-discharge prescriber level at the end of the study period forced us to exclude these covariates from the fully-adjusted models at this provider level.
In order to facilitate the interpretation of the results, we centered the continuous covariates at their sample mean; and set the reference level for the binary covariates at their more frequent realization (male sex, absence of disease). The reference for physician’s specialty was general practice, which comprised over 3/4 of the physicians; the reference level for Romano score and number of ‘cardiovascular’ medications was 0, the most frequent values. Thus, the interpretation of the fixed intercept refers to an ‘average’ patient that has all the characteristics of the reference categories.
Analyses were conducted using SAS 9.1, SAS Institute Inc., Cary, NC, USA; multilevel, mixed-effects analyses were performed with proc glimmix.
RESULTS
We identified 35,994 adult patients who survived 30 days after hospital discharge for an MI in British Columbia from January 1997 to December 2004. Of those, 742 had a code for prior MI, 1,110 had revascularization procedures in the baseline year and 6,113 were readmitted within 30 days of discharge, leading to the exclusion of 7,381 patients. The final cohort comprised 28,613 patients.
The 28,613 subjects were admitted in 87 hospitals and treated by 2,810 discharging physicians; 25,840 (90%) study participants had an identifiable post-discharge cardiac prescriber. The cohort was predominantly male, with age ranging from 18 to 105 years. The median length of stay during the MI hospitalization was 7 days. Out of the 28,613 patients, 10,635 (37.17%) patients underwent revascularization procedures during the MI hospitalization. The patients who did not receive any study drug in the year before the index MI were younger, more likely to be male, and generally healthier than to those who received at least one study drug (Table 2). The number of study patients per hospital over the entire study period varied between 1 and 2,157. Among the 87 hospitals, 28 had revascularization facilities, all of which were high-volume hospitals. Each of the 2,810 discharging physicians had between 1 and 376 study patients during the study period. Among the 239 high-volume discharging physicians, 15 (6%) practiced general medicine and were responsible for the in-patient care of 3 % of patients of high-volume physicians; 201 (84%) practiced internal medicine and were responsible for 89% of patients; and 23 (10%) had other specialties and treated 8 % of patients. The figures were similar for the 205 high-volume post-discharge cardiac prescribers.
Table 2.
Estimated proportion of patients initiating study drugs by discharging hospital, discharging physician, and post-discharge physician by biennium; estimate, 95% interval and between-provider variance (σb2). Age-sex adjusted and fully-adjusted multilevel logistic regression models.
| Hospitals | Discharging physicians | Post-discharge physicians | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age-sex adjusted models* | Fully-adjusted models† | Age-sex adjusted models* | Fully-adjusted models† | Age-sex adjusted models* | Fully-adjusted models†‡ | ||||||||
| Average provider (95% of the providers) |
σb2 | Average provider (95% of the providers) |
σb2 | Average provider (95% of the providers) |
σb2 | Average provider (95% of the providers) |
σb2 | Average provider (95% of the providers) |
σb2 | Average provider (95% of the providers) |
σb2 | ||
| Beta blockers | 1997 – 1998 | 0.59 (0.40-0.75) | 0.15 | 0.67 (0.47-0.82) | 0.18 | 0.62 (0.30-0.86) | 0.47 | 0.71 (0.39-0.91) | 0.49 | 0.69 (0.37-0.90) | 0.47 | 0.76 (0.42-0.93) | 0.59 |
| 1999 – 2000 | 0.67 (0.47-0.82) | 0.18 | 0.74 (0.56-0.87) | 0.18 | 0.70 (0.43-0.87) | 0.31 | 0.76 (0.51-0.91) | 0.32 | 0.76 (0.52-0.90) | 0.31 | 0.82 (0.59-0.93) | 0.34 | |
| 2001 – 2002 | 0.70 (0.49-0.85) | 0.20 | 0.76 (0.56-0.89) | 0.22 | 0.74 (0.49-0.89) | 0.31 | 0.78 (0.53-0.92) | 0.35 | 0.83 (0.56-0.95) | 0.45 | 0.86 (0.60-0.96) | 0.54 | |
| 2003 – 2004 | 0.75 (0.57-0.87) | 0.18 | 0.80 (0.64-0.91) | 0.19 | 0.77 (0.54-0.91) | 0.29 | 0.82 (0.60-0.93) | 0.32 | 0.83 (0.56-0.95) | 0.45 | 0.86 (0.60-0.96) | 0.51 | |
| ACEI/ARB | 1997 – 1998 | 0.36 (0.18-0.60) | 0.24 | 0.36 (0.17-0.60) | 0.25 | 0.39 (0.15-0.69) | 0.42 | 0.39 (0.16-0.68) | 0.39 | 0.46 (0.18-0.77) | 0.49 | 0.46 (0.18-0.77) | 0.49 |
| 1999 – 2000 | 0.51 (0.31-0.72) | 0.20 | 0.55 (0.36-0.72) | 0.15 | 0.57 (0.27-0.82) | 0.41 | 0.58 (0.30-0.81) | 0.35 | 0.64 (0.32-0.87) | 0.45 | 0.64 (0.36-0.85) | 0.36 | |
| 2001 – 2002 | 0.64 (0.40-0.83) | 0.25 | 0.69 (0.48-0.84) | 0.20 | 0.72 (0.35-0.92) | 0.63 | 0.74 (0.42-0.92) | 0.49 | 0.80 (0.39-0.96) | 0.89 | 0.81 (0.46-0.96) | 0.69 | |
| 2003 – 2004 | 0.70 (0.49-0.86) | 0.22 | 0.74 (0.55-0.87) | 0.19 | 0.75 (0.37-0.94) | 0.69 | 0.78 (0.46-0.94) | 0.51 | 0.81 (0.39-0.97) | 0.93 | 0.83 (0.49-0.96) | 0.68 | |
| All statins | 1997 – 1998 | 0.20 (0.06-0.48) | 0.45 | 0.26 (0.09-0.56) | 0.42 | 0.25 (0.06-0.64) | 0.74 | 0.29 (0.07-0.68) | 0.74 | 0.28 (0.06-0.70) | 0.86 | 0.30 (0.07-0.72) | 0.84 |
| 1999 – 2000 | 0.42 (0.18- 0.70) | 0.37 | 0.49 (0.22-0.75) | 0.36 | 0.47 (0.14-0.83) | 0.73 | 0.52 (0.16-0.85) | 0.75 | 0.53 (0.16-0.87) | 0.82 | 0.54 (0.17-0.87) | 0.81 | |
| 2001 – 2002 | 0.60 (0.24-0.87) | 0.62 | 0.68 (0.31-0.91) | 0.63 | 0.67 (0.32-0.90) | 0.58 | 0.72 (0.37-0.92) | 0.57 | 0.75 (0.35-0.95) | 0.78 | 0.77 (0.39-0.95) | 0.70 | |
| 2003 – 2004 | 0.72 (0.45-0.89) | 0.34 | 0.80 (0.58-0.92) | 0.29 | 0.80 (0.47-0.95) | 0.60 | 0.84 (0.54-0.96) | 0.58 | 0.86 (0.50-0.97) | 0.86 | 0.88 (0.60-0.98) | 0.70 | |
|
High-potency statins |
1997 – 1998 | 0.01 (0.00-0.02) | 0.39 | 0.01 (0.00-0.03) | 0.42 | 0.01 (0.00-0.04) | 0.85 | 0.01 (0.00-0.05) | 0.88 | 0.01 (0.00-0.05) | 0.84 | 0.01 (0.00-0.04) | 0.88 |
| 1999 – 2000 | 0.06 (0.01-0.24) | 0.74 | 0.06 (0.01-0.26) | 0.78 | 0.07 (0.01-0.38) | 1.20 | 0.07 (0.01-0.38) | 1.24 | 0.07 (0.01-0.40) | 1.19 | 0.07 (0.01-0.39) | 1.25 | |
| 2001 – 2002 | 0.17 (0.03-0.58) | 0.95 | 0.19 (0.03-0.62) | 1.00 | 0.21 (0.03-0.67) | 1.09 | 0.21 (0.03-0.67) | 1.11 | 0.23 (0.03-0.73) | 1.27 | 0.22 (0.03-0.71) | 1.25 | |
| 2003-2004 | 0.25 (0.07-0.60) | 0.57 | 0.28 (0.08-0.64) | 0.59 | 0.28 (0.04-0.77) | 1.17 | 0.31 (0.05-0.79) | 1.20 | 0.31 (0.04-0.83) | 1.47 | 0.34 (0.05-0.84) | 1.46 | |
Models are adjusted for age and age squared, and sex.
Models for beta blockers, ACEI/ARB and low-potency statins are adjusted for age and age squared, sex, asthma, COPD, atrioventricular block, heart failure, hypertension, renal diseases, diabetes, Romano comorbidity score, number of ‘cardiovascular’ medications, annualized volume, and specialty (for physician-level models only). Models for high-potency statins are adjusted for age and age squared, sex, Romano comorbidity score, hypertension, diabetes, number of ‘cardiovascular’ medications and annualized volume.
At the post-discharge physician level only, fully-adjusted models for ACEI/ARB are adjusted for age and age squared, sex, asthma, COPD, atrioventricular block, renal diseases, diabetes, Romano comorbidity score, number of ‘cardiovascular’ medications, annualized volume, and specialty (for physician-level models).
Medication use
Among the 28,613 cohort patients, 1,298 (5%) were on treatment with the three drug classes before MI hospitalizations. Of the 15,494 (54%) pre-MI non-users, 2,854 (18%) did not initiate treatment with any study drug; 3,819 (25%) initiated treatment with 1 drug, 4,662 (30%) with 2, and 4,159 (27%) with the 3 drug classes. In the time interval covering the baseline period and the thirty days after discharge, 9,890 (35%) out of 28,613 patients had filled prescriptions from all three study drug classes.
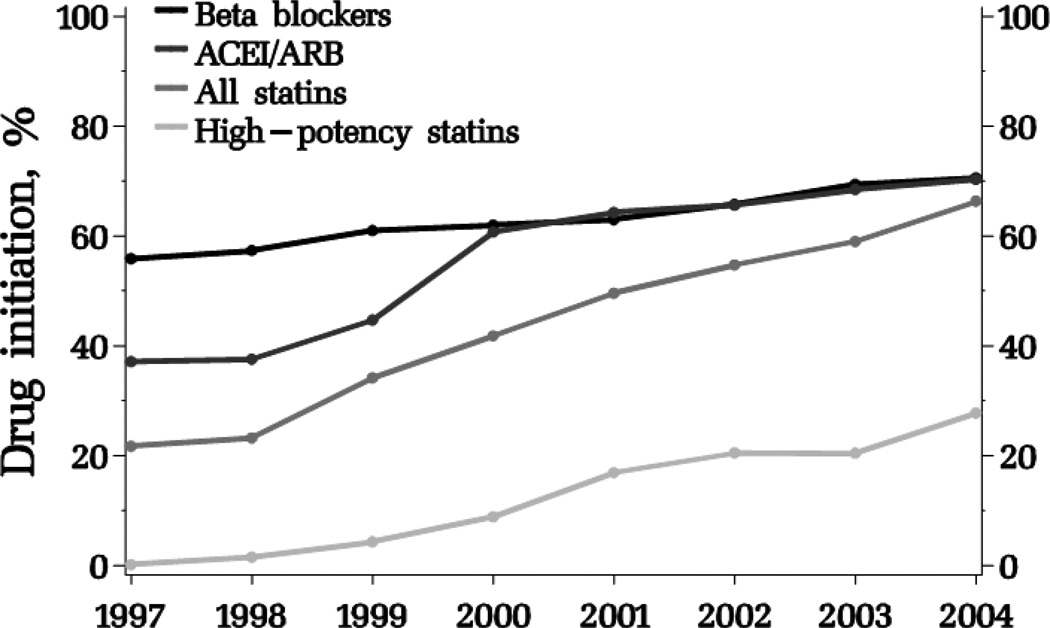
Post-MI drug initiation increased over the study period for all drug classes (Figure 1). Beta blockers had the highest percents of initiation at the beginning of the interval, but ACEI/ARB and statins initiation had risen to a similar level by 2004. Initiation on high-potency statins was rare in the first years of the study. In 2004; 14,287 (56%) patients had initiated beta blockers, out of 25,422 non-users; 10,973 (46%) had initiated ACEI/ARB out of 23,716 non-users; and 9,989 had initiated statins (7,171 low-potency and 2,818 high-potency statins) out of 25,848 non users.
Figure 1.
Proportion of patients newly initiating beta blockers, ACEI/ARB, all and high-potency statins after hospitalization for myocardial infarction by year.
ACEI/ARB: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers
Variation in treatment initiation
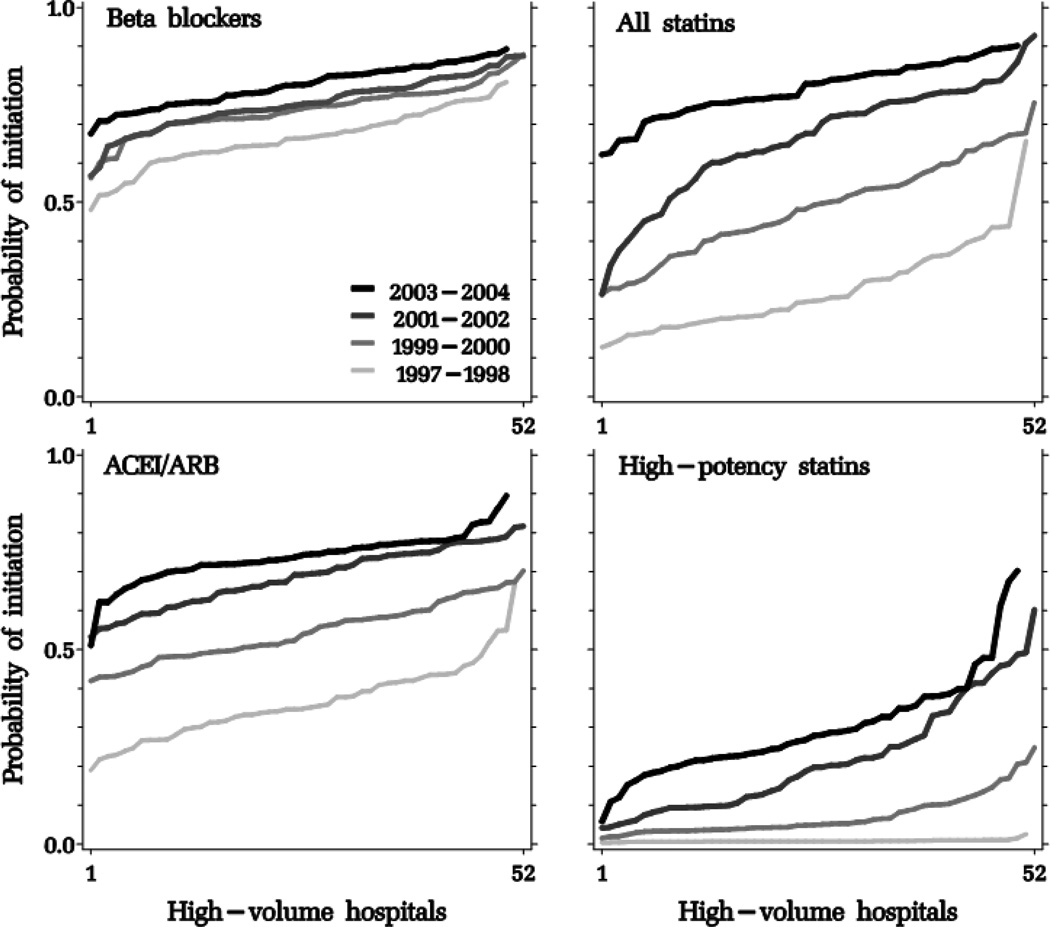
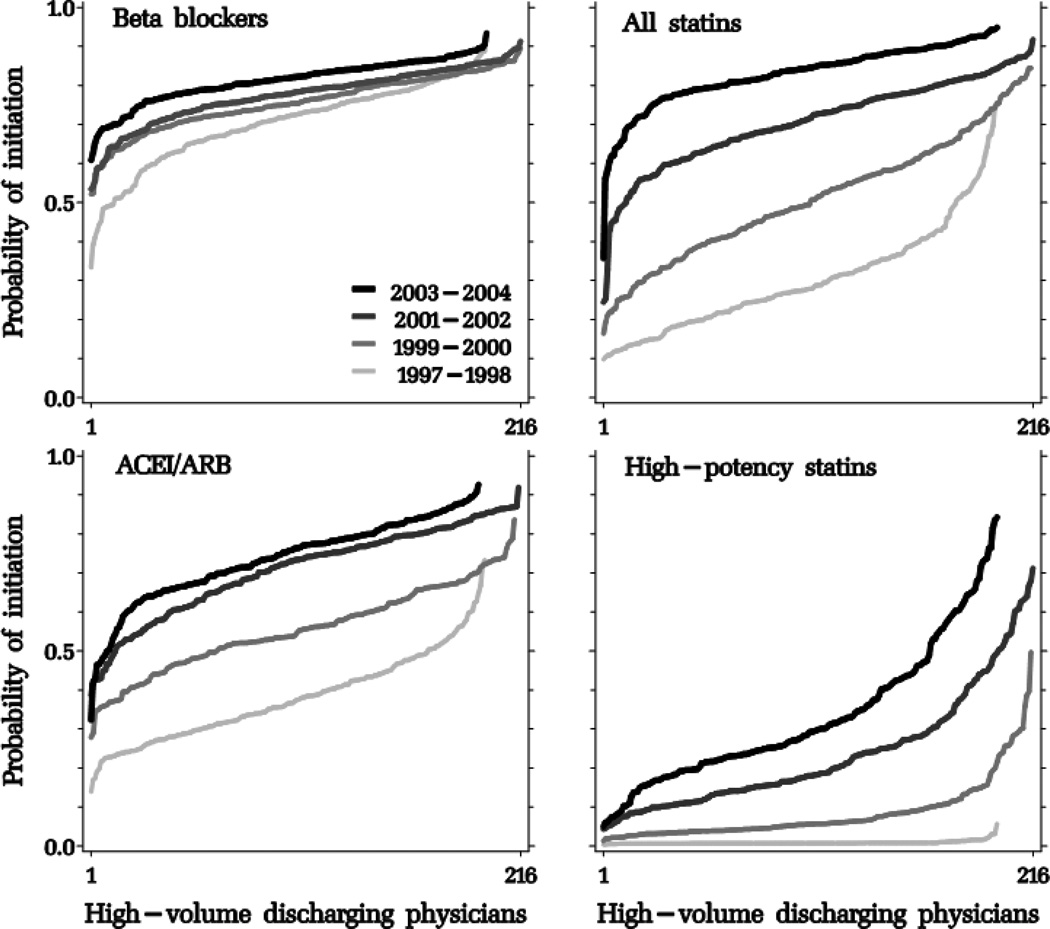
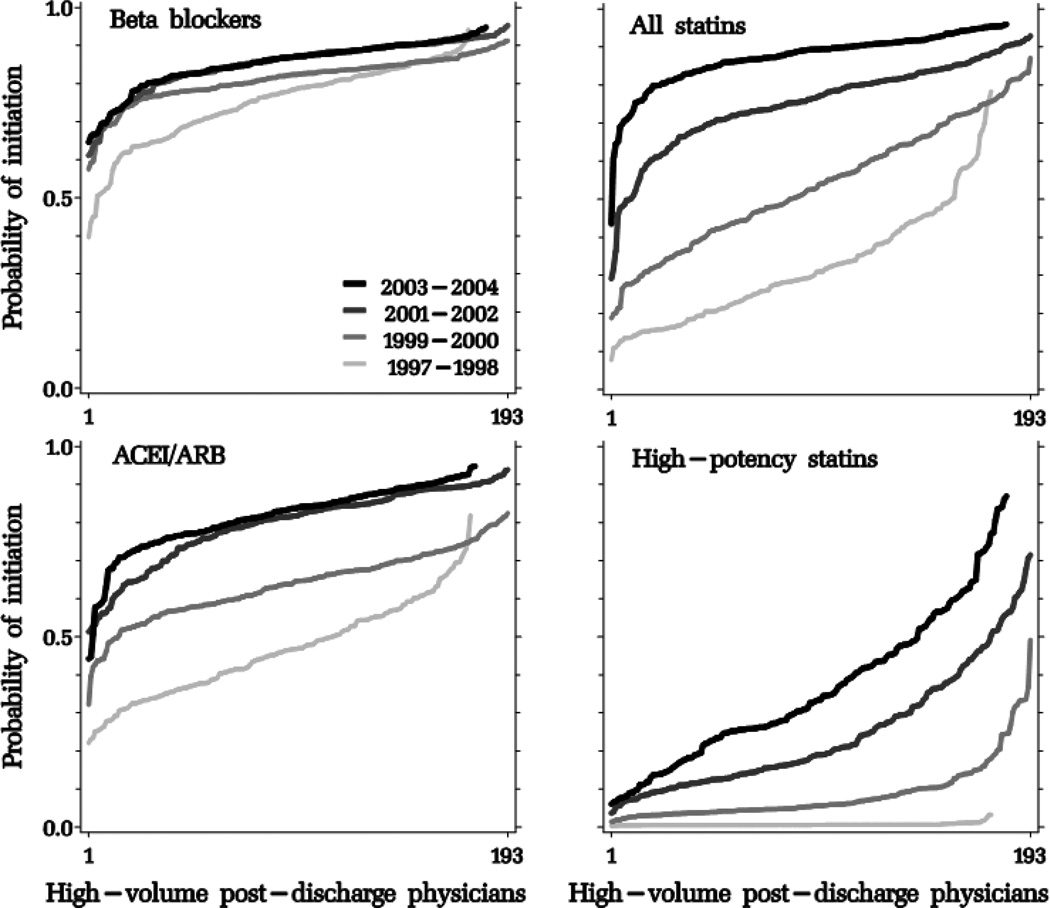
With each biennium, the hospital- and physician-specific probability of initiating an average patient in each drug class after an MI increased with a distinct pattern for each drug class (Figures 2, 3 and 4, and Table 3). The variation in provider-specific estimates is reflected in the y-axis range of each curve; its dispersion is summarized in the logit scale in the between-provider variance σb2. To facilitate the interpretation of σb2, we incorporated it in the equations described in the on-line appendix to estimate a 95% interval around the “average provider” point estimate, in the probability scale (Table 3). Compared to age-sex-adjusted models, the fully-adjusted models produced similar point estimates; provider-level variation was largest for high-potency statins, which increased until 2002 but began to decline afterwards at the discharging hospital and physician levels, but continued to increase until the end of the study period at the post-discharge cardiac prescriber level. Physician-level intervals were generally wider than hospital-level intervals. For example, while post-discharge cardiac prescribers in the 95% interval around the “average post-discharge cardiac prescriber” initiated an estimated 5 to 84% of their patients in high-potency statins in 2003–2004, the corresponding figures at the hospital level were 8% to 64%.
Figure 2.
Adjusted probability of post-myocardial infarction discharge initiation of study drug classes, sorted by increasing probability by discharging hospitals, stratified by biennium.
ACEI/ARB: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers
Figure 3.
Adjusted probability of post-myocardial hospital discharge initiation of study drug classes, sorted by increasing probability by discharging physicians, stratified by biennium.
ACEI/ARB: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers
Figure 4.
Adjusted probability of post-myocardial hospital discharge initiation of study drug classes, sorted by increasing probability by post-discharge physicians, stratified by biennium.
ACEI/ARB: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers
DISCUSSION
In this population-based cohort of MI survivors in British Columbia, the initiation of beta blockers, ACEI/ARB and statins increased steadily in the period 1997–2004. We observed a wide variation between hospitals, and more so between physicians, in post-MI drug initiation, particularly in high-potency statins. Beyond adjustment for age and sex, additional patient- and provider-level characteristics did not meaningfully change this variation.
Our findings regarding drug utilization are similar to those previously reported in North America. In Ontario, in a population aged 65 and older, 90-day post-MI discharge utilization increased from 60 to 78%, 55 to 81% and 27 to 77%, respectively, in the same years. 30 In Quebec in 2004, 77% of post-MI patients filled prescriptions for beta blockers within 30 days of hospital discharge, 70% for ACEI/ARB and 72% for statins. 31 In the US, for New Jersey and Pennsylvania Medicare beneficiaries ≥ 65 years old, 30-day post-MI-discharge utilization increased from 51 to 72%, 42 to 50%, and 16 to 51%, respectively in the period from 1997 through 2004. 3 These results, though, are not directly comparable to ours: varying health-plan eligibility criteria determine dissimilar patient characteristics; moreover, these studies combined new users with continuing users, who are individuals for whom the treatment was perceived as effective and safe until the moment of their index MI. In contrast, the new-user design allowed us to assess post-MI drug initiation independently of patients’ prior beneficial or harmful experiences with the study drugs. In addition, it permitted us to address the very specific situation where the application of the MI treatment guidelines involves the prescription of new drugs, as opposed to the carry-over of an ongoing therapy.
An important question is whether variation in initiating post-MI drug use is associated with patient characteristics that may cluster patients in providers, or whether this is mostly due to provider preference. We found that, once age and sex are incorporated into multilevel regression models, the addition of 11 patient-specific characteristics and provider volume and specialty does not meaningfully change the variation in treatment initiation. This is compatible with preference being a main determinant of drug use in this population. A possible explanation is that the study participants were fairly homogeneous with regard to prescribing after age and sex were considered –they suffered a MI and survived at least 30 days without a readmission, they did not have a history of MI or revascularization procedures and they had not been on treatment with the drugs of interest in the year prior to the MI. Thus, the incorporation of more covariates in the models did not achieve further control of residual confounding.
The variation in use of high-potency statins was highest in 2001–2002 (at the hospital level), 1999–2000 (at the discharging physician level) and 2003–2004 (at the post-discharge cardiac prescriber level) as compared to prior years. A plausible explanation for the substantial time-dependent variation in initiating high-potency statins at the discharging hospital and physician levels is that, in 1997–1998, providers had no clear recommendations to follow for high-dose statin use after MI. Only a few of them would prescribe intensive statin therapy. As the information on the benefits of tight lipid control became available, early adopting providers would increasingly prescribe high-potency statins. The accumulation of evidence compiled in the 2004 guidelines 5 drove the late adopters to also prescribe high-potency statins, thus reducing variation at the discharging hospital and physician levels. The speed of adoption of guideline recommendations seemed to vary across provider levels and is a potential focus for quality of care-related educational efforts.
The steady increase in the initiation of secondary prevention throughout the study period may lead comparative effectiveness and comparative safety researchers to question the comparability of users and non-users over time; e.g., is it fair to contrast an individual initiating ACEI/ARB in 1997 to one who did not receive these drugs in 2004? Our results suggest that there may be substantial changes in prescribing patterns over time which should be accounted for in analyses of the comparative effectiveness and comparative safety of these drugs.
We assessed post-MI secondary prevention within a 30-day time-window after discharge for the index event. A longer identification period would have yielded slightly higher starting proportions but would not have changed meaningfully the observed provider variation. Guidelines establish that treatment should be started immediately after the MI; and more extended drug-initiation-defining windows may capture indications triggered by events that occurred later than the index MI. The provision of drug samples at hospital discharge or later in an ambulatory visit could delay the patient’s first outpatient filling of a prescription after the index MI. These individuals would be incorrectly identified as non-initiators, despite being treated. This should only raise concerns if the provision of samples covered more than 30 days in a substantial proportion of the patients, which is unlikely. Further, the last years of the study yield high proportions of initiation for all classes, suggesting that our method of capturing post-MI drug use is close to complete. Residual variation by patient or provider characteristics cannot be fully ruled out because information that is not recorded in administrative data, such as laboratory test results, may have an impact on the prescriber’s decision of treating a patient. We cannot assess the evolution of the trends we report after 2004 within our data. We speculate, though, that the trends carry on, as the evidence for these drugs’ benefits is well established and continues to accumulate.
In conclusion, the initiation of secondary prevention with beta blockers, ACEI/ARB and statins is increasing among MI survivors, but variation in initiation is wide among discharging hospitals and physicians, particularly for high-potency statin therapy.
Supplementary Material
Table 1.
Patient characteristics assessed during the year before the MI hospital admission
| All patients (N = 28,613) |
No prior use of study drugs (N =15,494 ) |
Prior use of at least one study drug (N = 13,119) |
||||
|---|---|---|---|---|---|---|
| Mean ± SD or n (%) | ||||||
| Demographics | ||||||
| Age (years) | 68±13 | 66±14 | 71±12 | |||
| Sex (male) | 19,026 | (66%) | 11,066 | (71%) | 7,960 | (61%) |
| Medical conditions | ||||||
| Arrhythmias | 559 | (2%) | 162 | (1%) | 397 | (3%) |
| Hypertension | 10,679 | (37%) | 3,214 | (21%) | 7,465 | (57%) |
| Heart-valve diseases | 800 | (3%) | 304 | (2%) | 496 | (4%) |
| Heart failure admissions | 410 | (1%) | 68 | (0%) | 342 | (3%) |
| Stroke | 1,169 | (4%) | 410 | (3%) | 759 | (6%) |
| Chronic obstructive pulmonary disease | 1,443 | (5%) | 708 | (5%) | 735 | (6%) |
| Asthma | 1,230 | (4%) | 651 | (4%) | 579 | (4%) |
| Diabetes mellitus | 3,496 | (12%) | 917 | (6%) | 2,579 | (20%) |
| Renal diseases | 560 | (2%) | 103 | (1%) | 457 | (3%) |
| Osteoarthrosis | 3,328 | (12%) | 1,625 | (10%) | 1,703 | (13%) |
| Depression | 1,718 | (6%) | 907 | (6%) | 811 | (6%) |
| Malignancies | 2,285 | (8%) | 1,098 | (7%) | 1,187 | (9%) |
| Health care utilization | ||||||
| Number of physicians visited in previous year | 7±5 | 6±4 | 9±5 | |||
| Nursing home days in previous year | 1±15 | 1±13 | 1±17 | |||
| Drug utilization | ||||||
| Number of 'cardiovascular' drugs | 2±2 | 0±1 | 3±2 | |||
| Calcium-channel blockers | 5,003 | (17%) | 1,346 | (9%) | 3,657 | (28%) |
| Nitrates | 3,346 | (12%) | 663 | (4%) | 2,683 | (20%) |
| Loop diuretics | 2,239 | (8%) | 433 | (3%) | 1,806 | (14%) |
| Thiazides | 3,117 | (11%) | 719 | (5%) | 2,398 | (18%) |
| Digoxin | 1,411 | (5%) | 359 | (2%) | 1,052 | (8%) |
| Beta blockers | 5,626 | (20%) | 0 | (0%) | 5,626 | (43%) |
| ACEI/ARB | 8,393 | (29%) | 0 | (0%) | 8,393 | (64%) |
| Statins | 5,481 | (19%) | 0 | (0%) | 5,481 | (42%) |
| Fibrates | 494 | (2%) | 96 | (1%) | 398 | (3%) |
MI: myocardial infarction
ACEI/ARB: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers
Acknowledgments
Sources of funding: Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Boston, MA, USA, National Heart Lung and Blood Institute RC4-HL102023 (Dr. Schneeweiss)
SS is a paid member of the Scientific Advisory Board of HealthCore and consultant to HealthCore, WHISCON and RTI, and recipient of investigator-initiated grants from Pfizer and Novartis. He is Principal Investigator of the Brigham and Women’s Hospital DEcIDE Research Center for comparative effectiveness research funded by the Agency for Healthcare Research and Quality.
Footnotes
Conflicts of interest:
AVM, NKC and CRD do not have potential conflicts of interest to disclose.
REFERENCES
- 1.Tu JV, Naylor CD, Austin P. Temporal changes in the outcomes of acute myocardial infarction in Ontario, 1992–1996. CMAJ. 1999;161:1257–1261. [PMC free article] [PubMed] [Google Scholar]
- 2.Pilote L, Lavoie F, Ho V, et al. Changes in the treatment and outcomes of acute myocardial infarction in Quebec, 1988–1995. CMAJ. 2000;163:31–36. [PMC free article] [PubMed] [Google Scholar]
- 3.Setoguchi S, Glynn RJ, Avorn J, et al. Improvements in long-term mortality after myocardial infarction and increased use of cardiovascular drugs after discharge: a 10-year trend analysis. J Am Coll Cardiol. 2008;51:1247–1254. doi: 10.1016/j.jacc.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 4.Ryan TJ, Anderson JL, Antman EM, et al. ACC/AHA guidelines for the management of patients with acute myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction) J Am Coll Cardiol. 1996;28:1328–1428. doi: 10.1016/s0735-1097(96)00392-0. S0735109796003920 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction) Circulation. 2004;110:588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong PW, Bogaty P, Buller C, et al. The 2004 ACC/AHA guidelines: a perspective and adaptation for Canada by the Canadian Cardiovascular Society Working Group. Can J Cardiol. 2004;20:1075–1079. [PubMed] [Google Scholar]
- 7.Ryan TJ, Antman EM, Brooks NH, et al. 1999 update: ACC/AHA Guidelines for the Management of Patients With Acute Myocardial Infarction: Executive Summary and Recommendations: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction) Circulation. 1999;100:1016–1030. doi: 10.1161/01.cir.100.9.1016. [DOI] [PubMed] [Google Scholar]
- 8.Quan H, Cujec B, Jin Y, et al. Acute myocardial infarction in Alberta: temporal changes in outcomes, 1994 to 1999. Can J Cardiol. 2004;20:213–219. [PubMed] [Google Scholar]
- 9.Setoguchi S, Glynn RJ, Avorn J, et al. Ten-year trends of cardiovascular drug use after myocardial infarction among community-dwelling persons >or =65 years of age. Am J Cardiol. 2007;100:1061–1067. doi: 10.1016/j.amjcard.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 10.Yarzebski J, Granillo E, Spencer FA, et al. Changing trends (1986–2003) in the use of lipid lowering medication in patients hospitalized with acute myocardial infarction: a community-based perspective. Int J Cardiol. 2009;132:66–74. doi: 10.1016/j.ijcard.2007.10.055. S0167-5273(07)01971-7 [pii] 10.1016/j.ijcard.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan AT, Yan RT, Tan M, et al. Optimal medical therapy at discharge in patients with acute coronary syndromes: temporal changes, characteristics, and 1-year outcome. Am Heart J. 2007;154:1108–1115. doi: 10.1016/j.ahj.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 12.Gnavi R, Migliardi A, Demaria M, et al. Statins prescribing for the secondary prevention of ischaemic heart disease in Torino, Italy. A case of ageism and social inequalities. Eur J Public Health. 2007;17:492–496. doi: 10.1093/eurpub/ckm005. ckm005 [pii] 10.1093/eurpub/ckm005 [doi] [DOI] [PubMed] [Google Scholar]
- 13.Kulik A, Brookhart MA, Levin R, et al. Impact of statin use on outcomes after coronary artery bypass graft surgery. Circulation. 2008;118:1785–1792. doi: 10.1161/CIRCULATIONAHA.108.799445. CIRCULATIONAHA.108.799445 [pii] 10.1161/CIRCULATIONAHA.108.799445 [doi] [DOI] [PubMed] [Google Scholar]
- 14.Canadian Institute for Health Information, Physicians in Canada: Fee-for-Service Utilization, 2005–2006. Ottawa: CIHI; 2008. [Google Scholar]
- 15.Schneeweiss S, Walker AM, Glynn RJ, et al. Outcomes of reference pricing for angiotensin-converting-enzyme inhibitors. N Engl J Med. 2002;346:822–829. doi: 10.1056/NEJMsa003087. 10.1056/NEJMsa003087 346/11/822 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Brookhart MA, Patrick AR, Schneeweiss S, et al. Physician follow-up and provider continuity are associated with long-term medication adherence: a study of the dynamics of statin use. Arch Intern Med. 2007;167:847–852. doi: 10.1001/archinte.167.8.847. [DOI] [PubMed] [Google Scholar]
- 17.Rassen JA, Choudhry NK, Avorn J, et al. Cardiovascular outcomes and mortality in patients using clopidogrel with proton pump inhibitors after percutaneous coronary intervention or acute coronary syndrome. Circulation. 2009;120:2322–2329. doi: 10.1161/CIRCULATIONAHA.109.873497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiyota Y, Schneeweiss S, Glynn RJ, et al. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Hanley GE, Morgan S, Hurley J, et al. Distributional consequences of the transition from age-based to income-based prescription drug coverage in British Columbia, Canada. Health Econ. 2008;17:1379–1392. doi: 10.1002/hec.1337. 10.1002/hec.1337. [DOI] [PubMed] [Google Scholar]
- 20.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choudhry NK, Levin R, Winkelmayer WC. Statins in elderly patients with acute coronary in typical practice syndrome: an analysis of dose and class effects. Heart. 2007;93:945–951. doi: 10.1136/hrt.2006.110197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plosker GL, Dunn CI, Figgitt DP. Cerivastatin: a review of its pharmacological properties and therapeutic efficacy in the management of hypercholesterolaemia. Drugs. 2000;60:1179–1206. doi: 10.2165/00003495-200060050-00011. [DOI] [PubMed] [Google Scholar]
- 23.Tu JV, Austin PC, Chan BT. Relationship between annual volume of patients treated by admitting physician and mortality after acute myocardial infarction. Jama. 2001;285:3116–3122. doi: 10.1001/jama.285.24.3116. [DOI] [PubMed] [Google Scholar]
- 24.Choudhry NK, Anderson GM, Laupacis A, et al. Impact of adverse events on prescribing warfarin in patients with atrial fibrillation: matched pair analysis. BMJ. 2006;332:141–145. doi: 10.1136/bmj.38698.709572.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choudhry NK, Soumerai SB, Normand SL, et al. Warfarin prescribing in atrial fibrillation: the impact of physician, patient, and hospital characteristics. Am J Med. 2006;119:607–615. doi: 10.1016/j.amjmed.2005.09.052. S0002-9343(05)00911-3 [pii] 10.1016/j.amjmed.2005.09.052 [doi] [DOI] [PubMed] [Google Scholar]
- 26.Kulik A, Levin R, Ruel M, et al. Patterns and predictors of statin use after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2007;134:932–938. doi: 10.1016/j.jtcvs.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 27.Block AE, Solomon DH, Cadarette SM, et al. Patient and physician predictors of post-fracture osteoporosis management. J Gen Intern Med. 2008;23:1447–1451. doi: 10.1007/s11606-008-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneeweiss S, Seeger JD, Maclure M, et al. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154:854–864. doi: 10.1093/aje/154.9.854. [DOI] [PubMed] [Google Scholar]
- 29.Krumholz HM, Brindis RG, Brush JE, et al. Standards for statistical models used for public reporting of health outcomes: an American Heart Association Scientific Statement from the Quality of Care and Outcomes Research Interdisciplinary Writing Group: cosponsored by the Council on Epidemiology and Prevention and the Stroke Council. Endorsed by the American College of Cardiology Foundation. Circulation. 2006;113:456–462. doi: 10.1161/CIRCULATIONAHA.105.170769. CIRCULATIONAHA.105.170769 [pii] 10.1161/CIRCULATIONAHA.105.170769. [DOI] [PubMed] [Google Scholar]
- 30.Austin PC, Tu JV, Ko DT, et al. Use of evidence-based therapies after discharge among elderly patients with acute myocardial infarction. CMAJ. 2008;179:895–900. doi: 10.1503/cmaj.071481. 179/9/895 [pii] 10.1503/cmaj.071481 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudson M, Richard H, Pilote L. Parabolas of medication use and discontinuation after myocardial infarction--are we closing the treatment gap? Pharmacoepidemiol Drug Saf. 2007;16:773–785. doi: 10.1002/pds.1414. 10.1002/pds.1414 [doi] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.