Abstract
In birds as in other vertebrates, estrogens produced in the brain by aromatization of testosterone have widespread effects on behavior. Research conducted with male Japanese quail demonstrates that effects of brain estrogens on all aspects of sexual behavior, including appetitive and consummatory components as well as learned aspects, can be divided in two main classes based on their time-course. First, estrogens via binding to estrogen receptors regulate the transcription of a variety of genes involved primarily in neurotransmission. These neurochemical effects ultimately result in the activation of male copulatory behavior after a latency of a few days. Correlatively, testosterone and its aromatized metabolites increase the transcription of the aromatase mRNA resulting in an increased concentration and activity of the enzyme that actually precedes behavioral activation. Second, recent studies with quail demonstrate that brain aromatase activity (AA) can also be modulated within minutes by phosphorylation processes regulated by changes in intracellular calcium concentration such as those associated with glutamatergic neurotransmission. The rapid up or down-regulations of brain estrogen concentration presumably resulting from these changes in AA affect, by non-genomic mechanisms with relatively short latencies (frequency increases or decreases respectively within 10–15 min), the expression of male sexual behavior in quail and also in rodents. Brain estrogens thus affect behavior on different time-scales by genomic and non-genomic mechanisms similar to those of a hormone or a neurotransmitter.
Keywords: copulatory behavior, appetitive sexual behavior, sexual learning, rhythmic contractions of the cloacal gland sphincter, medial preoptic area, non genomic effects of steroids, aromatase, Japanese quail - estrogens
Introduction
One of the unexpected discoveries of behavioral (neuro)endocrinology around the middle on the 20th century was that differences in behaviors spontaneously exhibited by males and females do not originate, as previously believed, because adult males are exposed to “male-typical” hormones, primarily testosterone (T) secreted by their testes, while females are exposed to “female-typical” hormones, i.e., the ovarian steroids, 17β-estradiol (E2) and progesterone. Surprisingly E2 is able to activate the same behaviors as T in males (see examples in [1]). This finding progressively resulted in the idea that somehow T can be transformed in the brain into E2 before it produces its behavioral effects. The discovery that behaviors activated by androgens (e.g., T) can be blocked by a concomitant treatment with antiestrogens [2], and that estrogens very effectively mimic the central actions of T, especially on brain differentiation in rats (reviewed in [3, 4]) confirmed this notion. These studies thus promoted an active search for the brain enzyme that could produce estrogens from androgens and ultimately led to the discovery of brain aromatase activity in the early 1970s [5, 6].
During the following decades, important progress was made in the understanding of the role played by this enzyme in the control of male reproductive behavior as well as concerning the distribution of the enzyme in the brain and the control of its activity. It has in particular been demonstrated that aromatization of T within the preoptic area plays a critical role in the activation of many aspects of reproductive behaviors in a variety of vertebrate species [4]. Studies on birds, in particular the ring dove (Streptopelia risoria) and the Japanese quail (Coturnix japonica), have in this context played a critical role, namely because aromatase is expressed in the avian brain at higher concentrations than in the brains of mammals. The study of the neuroanatomical distribution of the enzyme and of the regulation of its activity is therefore markedly facilitated. The induction of brain aromatase activity by testosterone was for example discovered in ring doves [7] before being also identified in other birds and in mammals [8, 9].
Most of our own research has been carried out on male quail, a species that shows tremendous endocrine and neuroanatomical/neurochemical variation in response to changes in photoperiod (transfer from short days mimicking winter conditions to long days typical of the spring or summer) and in which activation of male-typical sexual behaviors is clearly linked to actions of sex steroids (Balthazart and Ball, 1998). Quail have also been selected for efficient reproduction and consequently males reliably show a variety of reproductive behaviors in standardized laboratory conditions, which greatly facilitates the analysis of the neuroendocrine controls of these behaviors. We have taken advantage of these useful features of quail to advance our understanding of the biological significance of brain aromatase in the control of the activation of male reproductive behavior. Much of the evidence presented below is thus derived from studies performed in this species but similar studies in other tetrapods, to the extent that they have been completed, largely confirm the conclusions drawn from quail studies.
Aromatase and activation of male copulatory behavior
Multiple studies performed first in the laboratory of Elizabeth Adkins-Regan at Cornell University and then subsequently in our own laboratory at the University of Liège have demonstrated beyond any doubt that, in quail, the activation of male copulatory behavior by T is limited by its aromatization into an estrogen. This conclusion is namely supported by the facts that 1) the behavior is activated by aromatizable (T, androstenedione) but not or much less by non-aromatizable androgens (5α-dihydrotestosterone, methyltrienolone), 2) the behavioral effects of T can be mimicked by treatments with estrogens such as E2 or diethylstilbestrol (DES), 3) the effects of T are blocked by simultaneous injections of anti-estrogens, i.e. compounds that block access of estrogens to their receptors but not or less so by anti-androgens, and finally 4) effects of T on copulatory behavior are blocked by injection of aromatase inhibitors, i.e. compounds that block the aromatization of the androgen (reviewed in: [4, 10]).
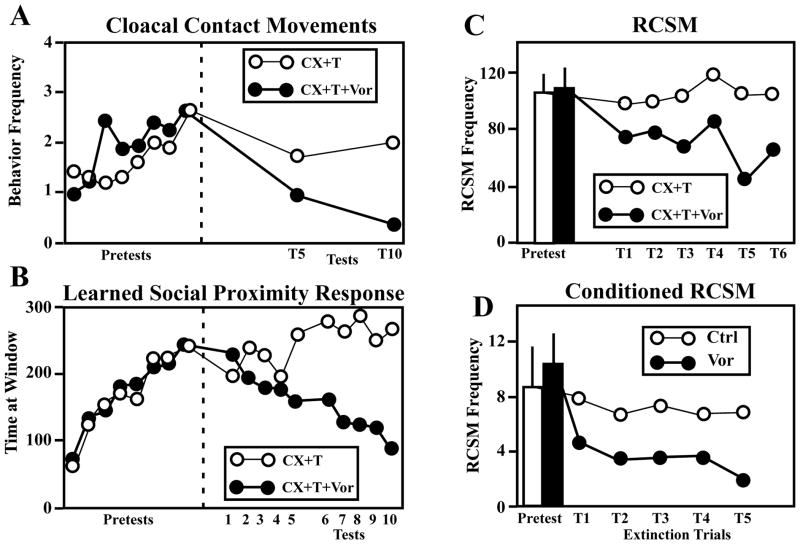
The results of one of these experiments is illustrated in Fig. 1A. One group of castrated male quail were in this experiment treated with a Silastic™ implant filled with T and then starting two weeks later, submitted to 8 behavioral tests during which they were presented to a sexually mature female for 5 min and their copulatory behavior (number of cloacal contact movements) was recorded. The birds were then assigned to one of two matched groups that had displayed equivalent amounts of behavior during the pretests. Subjects in one group were injected twice daily with the aromatase inhibitor, Vorozole™ while birds in the other group were injected with the vehicle. All birds were then submitted to two series of 5 tests measuring one aspect of their appetitive sexual behavior (see next section) and their copulatory behavior was again measured at the end of each series of tests. As expected, the frequency of cloacal contact movements (and other consummatory sexual behaviors including grabing the female’s neck feathers and mounting) was markedly inhibited in the Vorozole™-treated birds as compared to the other birds submitted to the same T treatment but without aromatase inhibitor. The frequency of these behaviors was very close to zero at the end of the experiment.
Figure 1.
Testosterone aromatization plays a key, limiting role in the activation by testosterone (T) of consummatory (A) and appetitive (B–C) aspects of male sexual behavior as well as on learned aspects of this behavior (D). A–B. Castrated male quail were treated with T and submitted to 8 pretests during with they could acquire the learned social proximity response (B) and express copulatory behavior (A). During the second part of the experiment (Tests 1–10, right of the dotted line), half of the subjects were injected twice daily with the aromatase inhibitor, Vorozole™ (CX+T+Vor) while other birds were injected with the vehicle (CX+T). Treatment with Vorozole™ markedly decreased the expression of copulatory behavior (A) and of the learned social proximity response (B). C. Effect of the treatment of castrated male quail with testosterone (CX+T) associated or not with the aromatase inhibitor Vorozole™ (CX+T+VOR) on the appetitive male sexual behavior measured by the frequency of rhythmic contractions of the cloacal sphincter muscles (RCSM). A progressive decline in RCSM frequency is observed during the 6 tests (T1–T6) performed during the 25 days when birds were exposed to the aromatase inhibitor. D. Effect of aromatase inhibition on the frequency of conditioned RCSM expressed in response to the visual presentation of a conditioned stimulus (see text for additional information). Adapted from data in [33, 44, 47].
Stereotaxic implantation of steroids in the brain, originally developed by Lisk [11], indicated that in mammals the preoptic area (POA) is the most important sex steroid-binding site for the activation of male sexual behavior. Subsequent experimental studies confirmed this conclusion in other vertebrate classes including birds (see [12, 13] for review) and quail are no exception to this rule. A suite of experiments indeed revealed that the medial POA is a necessary and sufficient site of T action for the activation of consummatory copulatory behavior in male quail [14]. Furthermore, a sexually dimorphic nucleus (volume larger in males than in females; [15]), identified as the medial preoptic nucleus (POM), that is located in the center of this area, is specifically involved in the control of this behavior. Indeed, electrolytic lesions of the POM, but not of the surrounding POA, suppress copulatory behavior activated in castrated males by subcutaneous Silastic™ implants containing T [16]. Conversely, stereotaxic implants filled with T activate all aspects of copulatory behavior in castrated males if their tip is located within the cytoarchitectonic boundaries of the POM but not if it is located in the adjacent POA [16, 17]. These data clearly indicate that T action in the POM is sufficient to activate copulatory behavior in adult male quail, although they do not rule out the possibility that the action of T at additional sites in the central nervous system may contribute to the behavioral activation under physiological conditions.
Subsequent stereotaxic work also demonstrated that T must be aromatized and estrogens act within the POM to activate sexual behavior. Indeed, implants of estrogens (E2 or DES) in the medial POA activate copulatory behavior in castrated quail like systemic treatments with the same compounds [14, 18], and conversely, the implantation in the medial POA of an aromatase inhibitor (Vorozole™) or of an antiestrogen (tamoxifen) inhibits the activation of sexual behavior produced by a systemic treatment with T [18–20].
Aromatase also mediates activation of appetitive sexual behavior
Theoretically, a motivated behavior can be divided in two successive phases, respectively called appetitive and consummatory [21]. Frank Beach originally introduced this distinction in the field of behavioral neuroendocrinology [22]. Although the usefulness of this classification has been questioned (see [23, 24] for recent discussions on this topic), it was used in multiple research projects investigating namely the potential separation between the neural structures controlling the appetitive and consummatory aspects of sexual behavior (in rats: [25, 26]; in quail: [13]).
Appetitive sexual behavior (ASB) can be defined as the suite of behaviors performed by an individual to localize and approach a sexual partner. These behaviors are usually quite variable and are potentially affected by learning processes (see next section). In contrast, consummatory sexual behavior (CSB) includes the behavior patterns directly associated with copulation and gamete transfer. They are more stereotyped and less susceptible to be influenced by learning processes.
In Japanese quail, two distinct behavioral responses have been used so far, in laboratory conditions, to quantify ASB: the learned social proximity response and the rhythmic contractions of the cloacal gland muscles.
The learned social proximity response
Michael Domjan and his colleagues at the University of Texas, Austin discovered that, when a male copulates with a female in a particular test arena, his behavior changes markedly and he will subsequently stand for hours in front of a window that provides him with visual access to the female. This is a type of associative learning that cannot easily be classified as either a form of classical conditioning or instrumental conditioning [27]; it has been called the learned social proximity response [28, 29]. This behavior appears to be a good indicator of appetitive sexual behavior, in that the male seems clearly to be engaging in this behavior in anticipation of copulatory behavior itself, and the behavior is expressed only in birds exposed to high T levels [30, 31].
Three types of experiments subsequently demonstrated that the learned social proximity response indicative of ASB depends, like copulatory behavior, on T aromatization. It was first demonstrated that the learned proximity response is acquired by castrated birds if they are systemically treated with either E2 or DES but not by untreated castrates [32]. A second experiment further demonstrated that once the proximity response had been acquired, its expression could be blocked by daily injections of the antiestrogen, tamoxifen [32] and finally, it was shown that daily injections of the aromatase inhibitor, Vorozole™, progressively inhibit the social proximity response previously acquired by castrated birds treated with T [33] (Fig. 1B) in parallel with the inhibition of CSB previously described (Fig. 1A).
The rhythmic contractions of the cloacal sphincter muscles (RCSM)
One problem with the social proximity response, however, is that the response is only learned if reinforced by the performance of CSB (although approach to an arbitrary stimulus has been successfully conditioned in male quail with paired visual access to a female [34]). This linkage prevents the assessment of ASB in subjects that fail to copulate. It is thus impossible to assess independently the appetitive and consummatory components of the behavior. A second behavioral procedure was more recently developed, based on studies conducted in the laboratory of Elizabeth Adkins-Regan [35, 36]. Male quail produce meringue-like foam that is transferred with sperm to the female cloaca during copulation and enhances the probability of egg fertilization [37]. Female quail have sperm storage tubules [38]. One function of the foam may be to increase the quantity of sperm stored after copulation. Female quail both lay eggs [39] and are most sexually receptive [40] in the afternoon. A hard-shell egg is thus likely to be in the oviduct during mating that occurs in the afternoon. If the cloacal foam of the male quail is manually expelled by the experimenter prior to afternoon matings, fewer eggs are fertilized than if the male has its normal foam quantity. The foam appears to increase the number of sperm reaching the storage tubules should oviposition occur prior to sperm storage [41].
This foam is produced by rhythmic movements of a sexually dimorphic striated cloacal sphincter muscle that is interdigitated with the proctodeal gland [36], an androgen-dependent structure [42] located at the caudal lip of the cloaca. These movements are greatly facilitated in males in the presence of a stimulus female [36]. This facilitation is also observed in sexually naive males [36] so that effects of endocrine manipulations on this behavior can be assessed independently of the activation of CSB. These RCSM are also facilitated nearly 20-fold in castrated males treated with T compared to castrated males receiving no T when provided with visual access to a female [43].
Interestingly, the activation by T of RCSM also seems to be limited by the aromatization of the androgen. During an experiment, two groups of castrated males that were treated with exogenous T and expressed RCSM with similar frequencies in response to the visual presentation of a female were injected twice daily with Vorozole™ or its vehicle during 25 days. They were then tested at regular intervals for the expression of RCSM during 2.5 min presentations to a female. As can be observed in Figure 1C, the aromatase inhibitor progressively decreased the RCSM frequency in treated subjects while the behavior was expressed at constant levels in control birds [44].
Together these data therefore support the notion that the mechanisms mediating the activation of appetitive and consummatory components of sexual behavior have a very similar if not identical steroid specificity. Both aspects of sexual behavior are activated by T but aromatization clearly limits the behavioral effects of the androgen. Interestingly, these two aspects of the behavior appear, in contrast, to be controlled by slightly different parts of the medial preoptic area. The data supporting this conclusion have, however, been reviewed recently [13] and their discussion is beyond the purpose of the present short review.
The learned components of sexual behavior also depend on aromatase
Successful reproduction requires the complex integration of a variety of internal and external stimuli. The male must indeed be able to react to the endocrine and neurochemical changes that mediate sexual motivation, identify the external stimuli related to the potential sexual partner, detect signs of sexual receptivity of this potential partner, approach and establish physical contact with this partner, and finally perform the relatively elaborate sequence of copulatory behaviors that will transfer gametes in the female’s reproductive tract. Many aspects of these tasks are spontaneously expressed by male quail that have been raised in isolation. They must therefore incorporate an innate component that develops in the absence of social imitation. The consummatory components of sexual behavior are largely innate in their form. Birds raised in isolation can produce the copulatory sequence including grabbing the female’s neck feathers, mounting and cloacal contact movements. There is however a form of learning that affects the coordination of these behaviors. During their first encounters with a female, males perform the correct sequence of movements but these are often poorly oriented (male will for example attempt to mount a female head to tail) and consequently male will often fail to achieve a successful copulation. However, with practice, the form and orientation of these behaviors will rapidly improve, allowing the efficient transfer of gametes to the female.
Learning processes are more important and complex at the level of appetitive aspects of sexual behavior. Males indeed need to develop knowledge of the specific stimuli that indicate the female’s sexual receptivity and of the situations in which they can successfully copulate with her. Learning thus allows males to predict the success of copulation. We already mentioned the learned social proximity response that male quail will express only after they have experienced sexual interactions in a specific test arena. This response is obviously adaptive since it will increase the attention paid by the male to the female and will consequently improve his chances of achieving successful copulations.
Work on RCSM has also demonstrated the potential importance of learning in the expression of this measure of ASB and the functional significance of this learning process. Adkins-Regan and MacKillop demonstrated that quail inseminations are more likely to fertilize eggs if they occur in a context that predicts the arrival of a conspecific, i.e. a context where males have previously copulated with a quail hen [45]. The mechanisms underlying this increased efficiency are not fully understood but if exposure to a sexual conditioned stimulus (CS) also promotes the expression of RCSM, then the benefits of increased RCSM and early foam production could account for the increased fertilization rates reported in male quail exposed to a sexual CS prior to copulation.
We therefore initiated investigations to explore whether RCSM could be elicited by a CS unrelated to copulation and indeed demonstrated that repeated exposure to an arbitrary stimulus paired with the presentation of a female progressively elicits after repeated testing the expression of RCSM in response to the CS only. In these experiments, male quail were placed in a two chamber test arena in which one large chamber contained the experimental subject and one smaller chamber located on the side could slide and provide a view of either an arbitrary stimulus (two terry-cloth covered cylinders placed at right angle, the CS) or a live quail hen (the unconditioned stimulus [US]). In this arena, males were repeatedly exposed in sequence to the CS for 30-s followed by visual access to the female (US) for 2 min. A rapid and orderly acquisition of the conditioned RCSM response was observed in these conditions and a plateau in responding was usually obtained after 5 to 10 conditioning trials [46].
These conditioned RCSM are testosterone-dependent. Like RCSM made in response to a female [43], they almost completely disappear during non-reinforced test trials in castrated birds from which Silastic™ implants providing exogenous T are removed, and they are rapidly restored when the treatment with exogenous testosterone is reinstated. This observation thus suggests an additional functional significance: the performance of learned-RCSM conditioned during one breeding season may transfer across a non-breeding period to a subsequent breeding season. Their expression in response to the sexual CS would simply disappear during the winter when plasma T concentration are low but would reappear without any need for relearning in the following spring as soon as testicular activity is stimulated by the increase in day length and plasma T concentrations return to breeding level.
Interestingly, like most other aspects of reproductive behaviors, the conditioned RCSM also depend on T aromatization for their expression. In one experiment, castrated, T-treated, male quail were again presented with paired presentations of the same arbitrary focal CS and visual access to a female. Once conditioned RCSM had developed, subjects were injected twice daily with the aromatase inhibitor Vorozole™ during a series of non-reinforced test presentations of the CS. As shown in Figure 1D, injections of Vorozole™ significantly decreased the number of RCSM elicited by the sexual CS. This decrease was specific to sexual RCSM [47]. Conditioned sexual RCSM are therefore mediated by the aromatization of T, most likely due to effects on central aromatase activity related to sexual motivation.
The controls of aromatase activity: genomic and non-genomic mechanisms
As already mentioned, the high level of expression of aromatase in the avian brain has facilitated the analysis of the distribution of the enzyme in the brain. Converging evidence coming from assays of enzyme activity in nuclei microdissected by the Palkovits punch technique, from immunhistochemistry of the enzymatic protein and from in situ hybridization of the corresponding mRNA indicates that in birds aromatase is mainly expressed in the medial preoptic area, the medial portion of bed nucleus of the stria terminalis, and the mediobasal hypothalamus from the level of the ventromedial nucleus to the caudal end of this structure at the level of the infundibulum. This information has been reviewed several times [10, 48–50] and will not be considered here in more detail. The distribution of the enzyme is interestingly very similar in mammalian species [51] but analysis of the protein by immunohistochemistry remains difficult at this time, at least in the adult brain due apparently to the low concentration of this protein.
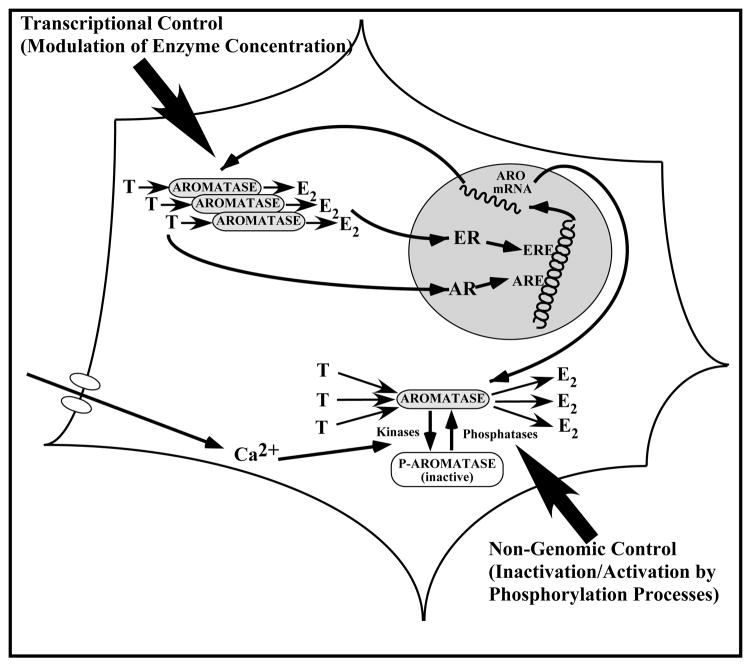
The mechanisms that regulate brain aromatase activity have been largely revealed based on studies in birds (ring doves and quail) but appear to be very similar to the mechanisms operating in mammals. In all species of tetrapods investigated so far, T increases aromatase activity in the POA. A parallel increase in the mRNA of the enzyme has also been demonstrated in several species including rodents (e.g., [52] ), suggesting that the control of the enzymatic activity by steroids results from a change in the transcription of the aromatase gene. In quail, this control of aromatase by T has been investigated independently at the level of the enzymatic activity, the protein (assessed semi-quantitatively by immunocytochemistry) and the corresponding mRNA (quantified by RT-PCR or in situ hybridization). These studies have demonstrated that the induction of aromatase activity by a chronic treatment with exogenous T of castrated male quail has approximately the same magnitude (6 fold increase) as the increase in the number of aromatase-immunoreactive neurons in the POM (5 fold increase) or the increase in aromatase mRNA concentration measured by RT-PCR (4 fold increase) [53, 54]. This suggests that the control by T of aromatase activity takes place mainly if not exclusively at the transcriptional (or at least pre-translational) level (Fig. 2, left part).
Figure 2.
Schematic representation of the genomic (left part of the figure) and non-genomic (right part of the figure) mechanisms controlling the activity of aromatase in the quail preoptic area. Genomic. Testosterone (T) and its aromatized metabolite, estradiol (E2) bind to their cognate nuclear receptors (the androgen and estrogen receptors, AR and ER respectively). When activated, these receptors interact with their specific responsive elements (androgen and estrogen responsive elements, ARE and ERE shown here but other possibilities also exist) and regulate the transcription of specific steroid-sensitive genes. Transcription of the gene Cyp19 encoding aromatase in increased in the presence of T or E2 and the resulting increase in the amount of enzymatic protein ultimately results in increased enzymatic activity. Non-genomic. The aromatase protein can be phosphorylated, namely under the influence of changes in intracellular calcium concentrations. The phosphorylated aromatase is less active than its non-phosphorylated form. These changes result within minutes in very large variations in aromatase activity that are not associated with changes in enzyme concentration. See text for additional explanation.
These effects of T on aromatase transcription appear to be largely mediated by the interaction of the steroid with androgen receptors in rats [9, 55], but mostly by an action of locally produced estrogens in birds [56, 57]. There is, however, in both species a clear synergism between non-aromatizable androgens and estrogens in the regulation of aromatase, but androgens play the major role in mammals, while estrogens play the major role in birds. This synergism has been observed in quail at the three different levels at which aromatase has been studied: the mRNA concentration, the protein as assessed semi-quantitatively by immunocytochemistry and the enzyme activity (see [54, 58] for reviews).
Available evidence, therefore, suggests that the control of brain aromatase activity mainly takes place via changes in the transcription of the corresponding gene and consequently of the enzyme concentration. There are, however, other ways through which the activity of an enzyme can be modified without changing its concentration. The presence of various types of endogenous inhibitors can, for example, modify either the maximum velocity or the apparent affinity of an enzyme and thus its activity in vivo. In addition, changes in the structure of the enzyme can also affect its activity and in this context, a substantial amount of data indicates that the specific phosphorylation of serine, threonine, or tyrosine residues of an enzymatic protein can dramatically change its activity [59, 60]. There was anecdotal evidence in the literature that divalent cations such as Ca2+ and Mg2+ acutely modify aromatase activity [61–63] and because these cations are also known to modulate phosphorylation processes [64], we hypothesized that brain aromatase activity could be modified by phosphorylations.
As expected under this hypothesis, we observed a profound (80–90%) inhibition of aromatase activity within 10–15 min after the addition of suitable physiological concentrations of ATP, Mg2+ and Ca2+ to quail brain homogenates (Fig. 2, Right side). This inhibition was blocked by agents that chelate divalent ions such as EGTA or EDTA as well as after the addition of kinase inhibitors such as staurosporine (a serine/threonine kinase inhibitor), or inhibitors of protein kinase C (PKC), or A (PKA) clearly indicating that inhibitory effects of ATP/Mg/Ca are mediated by protein phosphorylations [65, 66]. This interpretation is also consistent with the finding that the quail aromatase sequence contains several consensus sites of phosphorylation that are present in the avian as well as mammalian aromatase sequences (e.g. [66–72]). Interestingly, these effects are also by far much faster (a few minutes) than the changes in activity related to increases in the transcription of the enzyme (hours to days).
We also wondered whether similar rapid regulations of aromatase activity occur in a more natural system containing intact neurons. Explants of the preoptic-hypothalamic area were therefore incubated in vitro in the presence of [1β-3H]-androstenedione. Cumulative aromatase activity in these explants was then measured every 5 min by quantifying the amount of tritiated water that had been released by aromatization of radioactive androstenedione. Experimental manipulations designed to manipulate the intracellular calcium concentration in the explants, such as a depolarization by addition of potassium in the incubation medium or the exposure to thapsigargin, a lactone known for its capacity to mobilize intracellular pools of Ca2+, inhibited within 5 min and in a reversible manner the enzymatic activity [65]. Furthermore, the activation of glutamatergic receptors of the AMPA (a-amino-methyl-4-isoxazole propionic acid) or kainate subtypes similarly reduced aromatase activity within 5 min and in a transient manner. This effect could be blocked by preincubation with specific antagonists such as NBQX or CNQX indicating that it results from a receptor-mediated mechanisms. Rapid modulations of aromatase activity in these explants possibly involves also a modification of intracellular Ca2+ concentrations (entrance of extracellular Ca2+ or mobilization of intracellular Ca2+)[73].
All these experiments thus indicated that brain aromatase activity can be rapidly regulated in vitro, both in brain homogenates and in brain explants, by calcium-dependent phosphorylations potentially mediated in physiological conditions by glutamate release. Interestingly rapid changes (after 5–15 min) in preoptic aromatase activity have also been detected in vivo in male quail that had just been stressed [54] or had copulated with a female [74]. There is to this date no direct experimental evidence that these changes of aromatase activity observed in vivo are mediated by the same mechanisms as in vitro (i.e., by calcium-dependent enzyme phosphorylations) but their latency precludes a transcriptional control modifying the concentration of the enzyme. A change in enzyme conformation (e.g., phosphorylation) is therefore a parsimonious interpretation, but additional studies should be carried out to test this interpretation.
In summary, two apparently independent types of mechanisms have been identified that modulate the activity of aromatase in the quail preoptic area. The enzyme activity can be increased via changes in the transcription of the enzymatic protein, mainly controlled in the brain by sex steroids. This control mechanism is relatively slow and takes hours to days to affect in a significant manner the activity of the enzyme [75]. In parallel, changes in the phosphorylation of the existing molecules of enzymatic protein (and possibly other less characterized mechanisms such as the fixation of calmodulin, see [76]) are also able to change within minutes the enzymatic activity without changing the enzyme concentration. These mechanisms are schematically presented in Figure 2.
Rapid behavioral effects of rapid changes in brain aromatase activity
The concentration of estrogens in the brain can thus be affected on different time-scales by genomic and non-genomic mechanisms. We previously described effects of brain estrogens on different aspects of sexual behavior (consummatory, appetitive, and learned) that are produced after days of exposure to the steroid. These behavioral effects are mediated, by and large, by genomic mechanisms of action of estrogens as indicated by their latency and by the demonstration that they are blocked by administration of estrogen receptor antagonists such as tamoxifen or CI-628 [18, 77]. Because brain aromatase and thus presumably brain estrogens concentration can also be rapidly modified by non genomic mechanisms, we wondered whether these rapid changes in estrogen bioavailabilty could have functional consequences at the behavioral level. This question was additionally prompted by the observation that aromatase immunoreactivity and aromatase activity can be identified at the level of presynaptic boutons [51, 78, 79] where the enzyme presumably produces estrogens that could act directly by non genomic mechanisms at the synapse level. Evidence had accumulated indicating the existence of rapid cellular effects of steroids in the brain, in particular of estrogens, but little information was available concerning the existence of such effects at the level of the entire organism. One study had, however, shown that in rats, systemic injections of estradiol in castrated males increase within 35 min the expression of some aspects of reproductive behaviors including anogenital olfactory investigations and mounts of the female by the male [80]. This study indicated that estrogens could possibly modify sexual behavior with short latencies presumably incompatible with a genomic mode of action. We decided therefore to investigate this possibility in quail, a species in which estrogen production in the brain had been shown to vary within minutes.
Experiments were carried out in which effects of rapid up- or down-regulations of brain aromatase activity were mimicked by either injecting a large dose of exogenous estrogen or a single dose of an aromatase inhibitor, respectively. Sexual behavior was then quantified at various latencies between 5 min and one hour after these manipulations. The most salient features of the results obtained in these studies will be briefly summarized. Additional discussion can be found in previous reviews [50, 54, 81, 82].
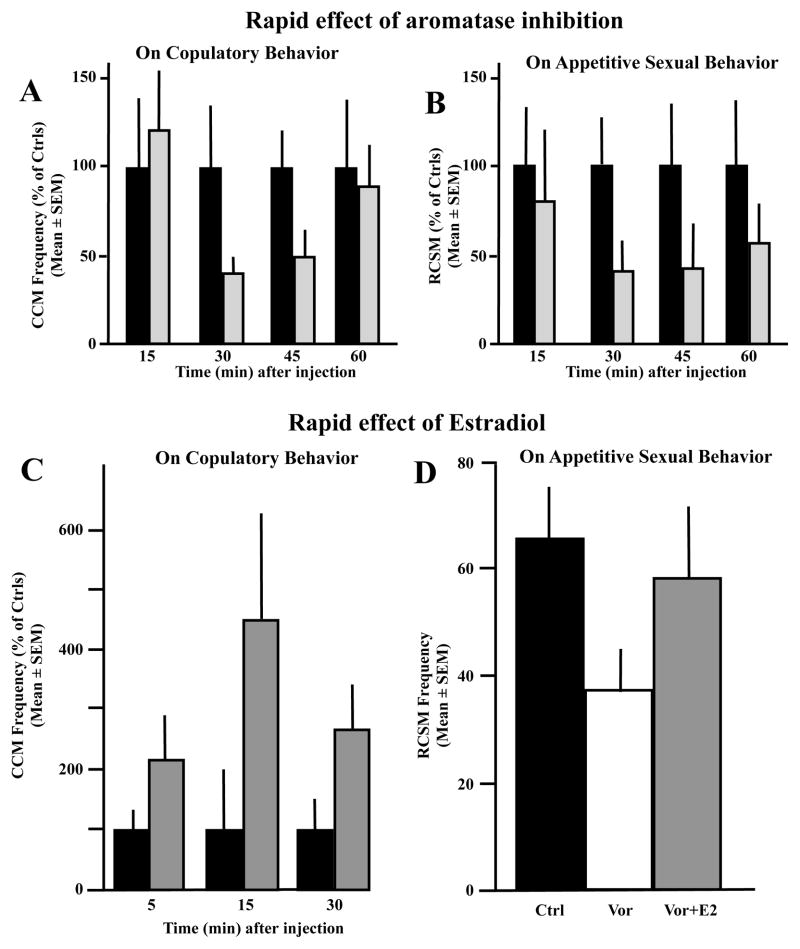
A single injection of the aromatase inhibitor Vorozole™ (VOR), a non-steroidal aromatase inhibitor [83] induced in gonadally intact sexually mature males or in castrates treated with Silastic™ testosterone implants (CX+T40) a major decline in the frequency of copulatory behaviors that reached its maximal magnitude 30 to 45 min after the injection [84]. This inhibition nearly disappeared after one hour (see Fig. 3A for one example of such an experiment; [84]). Quite interestingly, appetitive sexual behavior as measured by the frequency of rhythmic contraction of the cloacal sphincter muscles (RCSM) was similarly affected (Fig. 3B). A statistically significant decline in the frequency of these contractions in response to the view of a female was observed 30 to 45 min after the injection of Vorozole™, and the behavior was beginning to recover at 60 min post injection [84].
Figure 3.
A single injection of the aromatase inhibitor Vorozole™ or of estradiol (E2) respectively inhibits or stimulates within a few minutes the expression of both the consummatory and the appetitive components of male sexual behavior in quail. A) Effects of a single injection of Vorozole™ (30 mg/kg) on the frequency of cloacal contact movements (CCM) in sexually mature, gonadally intact male. Data are expressed as percentage of control levels to allow direct comparison. B) Effects of a single injection of Vorozole™ (30 mg/kg) on the frequency of rhythmic contraction of the cloacal sphincter muscles (RCSM) in sexually mature, gonadally intact male. Data are expressed as percentage of control levels. C) Effect of an acute injection of 500 μg E2/kg on the frequency of cloacal contact movements (CCM) expressed during tests performed 5, 15 or 30 min later. A transient stimulation of behavior was observed at 15 min post-injection. D) Reversibility of the inhibitory effects of Vorozole™ on the expression of rhythmic contraction of the cloacal sphincter muscles (RCSM). Gonadally intact males were injected 30 min before testing with either 30 mg/kg Vorozole™ alone or the same amount or aromatase inhibitor in association with 500 μg/kg E2. The Vorozole™-induced inhibition was largely reversed by the concurrent treatment with E2. Redrawn from data in [84, 85].
Conversely, the injection of a single large dose of estradiol (500 μg/kg) elicited within 15 min a significant increase in the frequency of male copulatory behaviors (see Fig. 3C for a representative experiment). This effect was, however, not observed in castrated birds but was obtained only if castrated males were chronically pretreated for at least one week with a very small dose of exogenous testosterone that activated a weak and infrequent sexual behavior. This small dose of testosterone is by itself insufficient to fully activate sexual behavior, but must be administered to make the acute injection of the bolus of estradiol behaviorally effective [85]. Rapid effects of estrogens thus cannot be obtained in subjects that have not been previously primed with a suboptimal dose of testosterone, presumably reflecting the fact that there is an interaction between genomic and non-genomic effects of steroids in the activation of male copulatory behavior. A similar synergism has been demonstrated in the mechanisms controlling female receptivity in rats as well as for the activation of a number of neurochemical effects of estrogens in the rat preoptic area-hypothalamus[86, 87].
Rapid effects of estradiol on the expression of appetitive sexual behavior could also be observed in male quail. As illustrated in Fig. 3D, male quail that had been injected 30 min before the behavioral test with a single dose of the aromatase inhibitor Vorozole™ showed a significant decrease in the frequency of rhythmic contractions of the sphincter muscles when presented with a female, but this inhibition was almost completely reversed by a single injection of estradiol given concurrently with the aromatase inhibitor, that is 30 min before the test [84].
Similar rapid effects of estradiol additionally seem to be affecting responses to nociceptive stimuli in male quail [88], and a recent set of studies also indicated that the acute injection of an aromatase inhibitor or of estradiol respectively inhibits or stimulate with latencies of 15 to 30 min the expression of copulatory behavior in male mice [89]. The existence of such rapid behavioral effects of estradiol seems to be an ancient conserved feature in vertebrates since they are already observed in fishes. Injection of estradiol indeed modulates within minutes sound production (vocal communication) in the plainfin midshipman fish [90].
Conclusions
During the reproductive season, all animals must express a suite of behavioral patterns that allow them to locate and encounter a conspecific of the opposite sex and then to copulate with him/her. In order to lead to a successful reproduction, these behaviors must be expressed in a coordinated manner and also must be adjusted to the behavior of the partner in response to adequate social stimuli. The expression of this behavioral sequence is largely controlled by “innate” mechanisms that do not require any special social learning. The copulatory sequence, for example, is expressed in its species-typical form by male quail that have been raised independently of adult birds from the previous generation and had therefore no model to copy their behavioral patterns. The orientation of the copulatory patterns and the nature of the objects that trigger the expression of appetitive sexual behavior are, however, learned at least in part during the first interactions with the partner of the other sex.
Because these different aspects of sexual behavior must obviously be coordinated to ensure the success of reproduction, it could on a priori bases be expected that they are controlled by endocrine and neural mechanisms that are themselves closely related. Our research in Japanese quail has indeed demonstrated that the neural circuits controlling appetitive and consummatory sexual behavior are very similar even if some subtle differences can be observed namely in the loci within the preoptic area that seem to be implicated in the control of these two aspects of behavior (see [13, 91] for review). Furthermore, all aspects of sexual behavior in quail are also the object of a strong synchronization by endocrine stimuli and actually depend on similar if not completely identical neuroendocrine mechanisms. Both the appetitive and consummatory aspect of male sexual behavior as well as the learned components of this behavior all depend for their activation of the action of testosterone that must be aromatized into estradiol in order to exert its activating effects on behavior (Fig. 4).
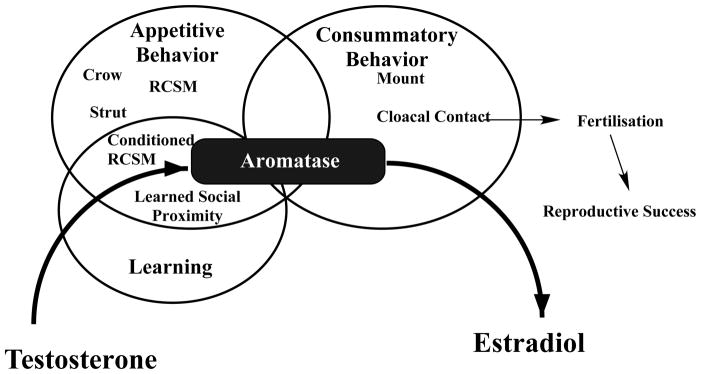
Figure 4.
Appetitive sexual behavior in male quail consists of a number of behaviors used to locate and then approach a female. These behaviors include a vocalization (crowing), a pre- (but also often post-) copulatory display (strutting), the rhythmic contractions of the cloacal sphincter muscles (RCSM) and an approach behavior observed in specific laboratory conditions called, learned social proximity response. This behavioral sequence eventually leads to the copulatory sequence sensu stricto, including mounts and cloacal contacts, also called consummatory sexual behavior. Aspects of the appetitive sexual behavior such as RCSM can be conditioned and are, after training, expressed following presentation of an arbitrary conditioned stimulus. These three aspects of sexual behavior (appetitive, consummatory and learned) are all activated by testosterone and the androgen must be aromatized into an estrogen such as estradiol to produce these behavioral effects. Aromatase thus plays a critical role in all steps of the reproductive behavior that lead to a successful reproduction. See also text for additional comments.
Interestingly both appetitive and consummatory sexual behaviors are affected by estrogens derived from brain aromatization of testosterone in a manner that seems to include an interaction between genomic and non-genomic effects of the steroid. Rapid effects of changes in brain estrogen concentrations (up- or down-regulations) were shown to modulate the expression of both appetitive and consummatory sexual behavior but only in birds that were exposed to sub-threshold doses of testosterone activating a minimal amount of sexual behavior. The rapid stimulatory effects of exogenous estrogens could not be seen in castrated males if they were not pretreated with a small dose of testosterone. At this time, rapid effects of estrogens on components of sexual behavior that are learned, such as the rhythmic contractions of the cloacal sphincter muscles expressed in reaction to a conditioned stimulus, have not been specifically tested. However, inhibition of aromatase acutely blocked the expression of the learned social proximity response indicating that at least some aspects of a partly learned sexual behavior are affected by rapid changes in estrogens bioavailability.
Rapid changes in brain estrogens thus seem to affect all aspects of sexual behavior. They also seem to be widespread among vertebrates since such effects have been detected in representative species from three different vertebrate classes, fishes, birds and mammals [80, 84, 85, 89, 90]. It appears likely that future research will be able to evidence them broadly across a variety of species. These findings do obviously raise a large number of questions that will only be considered briefly here due to space limitations. A more extensive discussion of these issues can be found in previous review papers [50, 54, 81, 82].
Cellular mechanisms
The work summarized here clearly indicates that estrogens can exert rapid physiological and behavioral effects that concern the entire organism. This raises the question of the cellular mechanisms mediating these effects. There is to this date a very substantial literature describing cellular effects of estrogens (and steroids in general) that are not mediated by their interaction with nuclear receptors and it is beyond the scope of the present paper to review these data. An entire volume of Frontiers in Neuroendocrinology was recently devoted to this issue (vol 29/2, 2008), including two chapters on estrogens [87, 92].
Because aromatase is expressed in synaptic boutons in quail and in various species of mammals [79], the release of estrogens at the synapse level might conceivably act on the postsynaptic membrane, through an interaction with GABA or glutamate receptors, and/or with one of the putative membrane estrogen receptors (or estrogen-sensitive systems) that have been tentatively identified. These include (a) intracellular nuclear estrogen receptors (i.e. α and β subtypes) that become associated with the cell membrane, or receptors of a different nature that at least show specific immune cross-reactivity with the nuclear receptors, (b) forms of receptors associated with membrane caveolae, (c) the newly identified ER-X receptor, or (d) other novel membrane estrogen receptors, including G-protein-coupled seven transmembrane domain receptors such as GPR30. There are therefore multiple transduction systems for fast signaling by estrogens at the membrane level. Furthermore fast estrogen signaling can also take place within the neuronal perikarya and, for example, estrogens have been shown to modulate the activity of various kinases (e.g. PKA, PKC, MAPK or phosphatidylinositol-3 kinase), thus the affecting phosphorylation of various molecules implicated in second messenger signaling cascades (e.g. CREB) or in neurotransmission (e.g. tyrosine hydroxylase phosphorylation) (see [81, 82, 93, 94] for references). Additional research will be needed to identify aspects of these cellular actions that mediate the rapid effects of estrogen on sexual behavior
Termination of estrogen action
The observation that various aspects of sexual behavior are rapidly inhibited following a single injection of an aromatase inhibitor reveals that some mechanism(s) must be present in the brain to terminate estrogen action when the synthesis of the steroid is interrupted. The half-life of estrogens in the periphery is relatively short and the steroid can be metabolized by a variety of enzymes. Some of these enzymes have been shown to be expressed in the brain, and they are likely to play a significant role in the catabolism of brain-derived estrogens [82]. Alternatively, it is also conceivable that estrogens are produced in high concentration at the level of specific synapses implicated in the control of sexual behavior but that diffusion rapidly equilibrates these high local concentrations with the much lower average brain concentration that reflects peripheral concentrations. This lower concentration would then be unable to trigger the fast effects of estrogens that have been discussed here that are known to depend on steroid concentrations much higher than those found in the periphery (see [50, 82] for additional discussion).
Functional significance
Finally, the functional significance of such a dual control of behavior through a synergistic action of genomic and non-genomic mechanisms remains partly unclear at present. Why would evolution have allowed the co-existence of a double regulation of behavior by estrogens with latencies of effects that differ by several orders of magnitude? It must be noted that behavior itself is and must be regulated on several different time scales. In the long term, species breeding in the temperate zone have adjusted to the variable environmental conditions so that reproduction takes place only when food will be abundant to raise a brood successfully. In quail and many other avian and mammalian species, this seasonal control is exerted by the changes in photoperiod that control testicular (and ovarian) activity. In quail, it is only during spring, under the influence of increasing day lengths, that the testes will develop and secrete large amounts of testosterone that will then act on the brain to activate the transcription of aromatase and the activation of sexual behaviors.
However, even during the reproductive season, birds have to attend other activities than reproduction (search for food, hide from predators, etc…), and it is appropriate that sexual behavior is only expressed during specific time windows when a partner of the opposite sex is present and sexually receptive. It has usually been considered that this short-term regulation of behavior expression was controlled by neurotransmitter activity (e.g. dopaminergic, noradrenergic, or glutamatergic inputs to steroid-sensitive areas). With the discovery of rapid changes in estrogen production in the brain and of rapid effects of estrogens on reproductive behaviors, this notion may have to be reconsidered and it appears likely that estrogens actually play a substantial role in the control of both the long-term and short-term variations in sexual behavior. We have actually argued that in the short-term context, estradiol might display most if not all functional characteristics of a neurotransmitter or at least a neuromodulator [82]. The functional significance and the mechanisms underlying such effects represent an emerging field of investigations that is likely to substantially affect our current understanding of steroid action in the brain.
Acknowledgments
The preparation of this review and the experimental work described was supported by grants from the NIMH (Grant number RO1 MH50388) to Gregory F. Ball and from the Belgian Fonds de la Recherche Fondamentale Collective (Grant number 2.4562.05) to JB. CAC is FNRS post-doctoral Researcher.
References
- 1.Beach FA. Hormones and behavior. Paul B. Hoeber, Inc; New York: 1948. [Google Scholar]
- 2.Beyer C, et al. Effect of some antiestrogens and aromatase inhibitors on androgen-induced sexual behavior in castrated male rats. Horm Behav. 1976;7:353–363. doi: 10.1016/0018-506x(76)90040-4. [DOI] [PubMed] [Google Scholar]
- 3.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1303. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 4.Balthazart J. Steroid metabolism and the activation of social behavior. In: Balthazart J, editor. Advances in Comparative and Environmental Physiology. Vol. 3. Springer Verlag; Berlin: 1989. pp. 105–159. [Google Scholar]
- 5.Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by the anterior hypothalamus of adult male and female rats. Endocrinology. 1972;90:295–298. doi: 10.1210/endo-90-1-295. [DOI] [PubMed] [Google Scholar]
- 6.Naftolin F, et al. The formation of estrogens by central neuroendocrine tissues. Rec Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- 7.Steimer T, Hutchison JB. Androgen increases formation of behaviourally effective oestrogen in dove brain. Nature. 1981;292:345–347. doi: 10.1038/292345a0. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher M, Balthazart J. Testosterone-induced brain aromatase is sexually dimorphic. Brain Res. 1986;370:285–293. doi: 10.1016/0006-8993(86)90483-x. [DOI] [PubMed] [Google Scholar]
- 9.Roselli CE, Resko JA. Androgens regulate brain aromatase activity in adult male rats through a receptor mechanism. Endocrinology. 1984;114:2183–2189. doi: 10.1210/endo-114-6-2183. [DOI] [PubMed] [Google Scholar]
- 10.Ball GF, Balthazart J. Neuroendocrine mechanisms regulating reproductive cycles and reproductive behavior in birds. In: Pfaff DW, et al., editors. Hormones, Brain and Behavior. Vol. 2. Academic Press; San Diego, CA: 2002. pp. 649–798. [Google Scholar]
- 11.Lisk RD. Diencephalic placement of estradiol and sexual receptivity in the female rat. Am J Physiol. 1962;203:493–496. doi: 10.1152/ajplegacy.1962.203.3.493. [DOI] [PubMed] [Google Scholar]
- 12.Hutchison JB. Hypothalamic regulation of male sexual responsiveness to androgen. In: Hutchison JB, editor. Biological determinants of sexual behaviour. John Wiley; Chichester: 1978. pp. 277–319. [Google Scholar]
- 13.Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson JT, Adkins-Regan E. Activation of sexual behavior by implantation of testosterone propionate and estradiol benzoate into the preoptic area of the male Japanese quail (Coturnix japonica) Horm Behav. 1989;23:251–268. doi: 10.1016/0018-506x(89)90065-2. [DOI] [PubMed] [Google Scholar]
- 15.Panzica GC, et al. Sexual differentiation and hormonal control of the sexually dimorphic preoptic medial nucleus in quail. Brain Res. 1987;416:59–68. doi: 10.1016/0006-8993(87)91496-x. [DOI] [PubMed] [Google Scholar]
- 16.Balthazart J, Surlemont C. Copulatory behavior is controlled by the sexually dimorphic nucleus of the quail POA. Brain Res Bull. 1990;25:7–14. doi: 10.1016/0361-9230(90)90246-v. [DOI] [PubMed] [Google Scholar]
- 17.Balthazart J, Surlemont C, Harada N. Aromatase as a cellular marker of testosterone action in the preoptic area. Physiol Behav. 1992;51:395–409. doi: 10.1016/0031-9384(92)90158-x. [DOI] [PubMed] [Google Scholar]
- 18.Balthazart J, Surlemont C. Androgen and estrogen action in the preoptic area and activation of copulatory behavior in quail. Physiol Behav. 1990;48:599–609. doi: 10.1016/0031-9384(90)90198-d. [DOI] [PubMed] [Google Scholar]
- 19.Watson JT, Adkins-Regan E. Testosterone implanted in the preoptic area of male Japanese quail must be aromatized to activate copulation. Horm Behav. 1989;23:432–447. doi: 10.1016/0018-506x(89)90055-x. [DOI] [PubMed] [Google Scholar]
- 20.Balthazart J, Evrard L, Surlemont C. Effects of the non-steroidal aromatase inhibitor, R76713 on testosterone-induced sexual behavior in the Japanese quail (Coturnix coturnix japonica) Horm Behav. 1990;24:510–531. doi: 10.1016/0018-506x(90)90039-z. [DOI] [PubMed] [Google Scholar]
- 21.Timberlake W, Silva KM. Appetitive behavior in ethology, psychology, and behavior systems. In: Thompson NS, editor. Perspectives in Ethology, Volume 11: Behavioral Design. Plenum Press; New York: 1995. pp. 211–253. [Google Scholar]
- 22.Beach FA. Characteristics of masculine “sex drive”. Nebraska Symposium on Motivation. 1956;4:1–32. [Google Scholar]
- 23.Ball GF, Balthazart J. How useful is the appetitive and consummatory distinction for our understanding of the neuroendocrine control of sexual behavior? Horm Behav. 2008 doi: 10.1016/j.yhbeh.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachs BD. The appetitive-consummatory distinction: Is this 100-year-old baby worth saving? Reply to Ball and Balthazart Horm Behav. 2008;53:315–318. doi: 10.1016/j.yhbeh.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses in male rats. Neurosci Biobehav Rev. 1990;14:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- 26.Everitt BJ. Neuroendocrine mechanisms underlying appetitive and consummatory elements of masculine sexual behavior. In: Bancroft J, editor. The pharmacology of sexual function and dysfunction. Elsevier; Amsterdam: 1995. pp. 15–31. [Google Scholar]
- 27.Domjan M, Hollis KL. Reproductive behavior: a potential model system for adaptative specializations in learning. In: Bolles RC, Beecher MD, editors. Evolution and learning. Hillsdale. NJ: 1988. pp. 213–237. [Google Scholar]
- 28.Domjan M, Hall S. Determinants of social proximity in Japanese quail (Coturnix coturnix japonica): male behavior. J Comp Psychol. 1986;100:59–67. [PubMed] [Google Scholar]
- 29.Domjan M, Hall S. Sexual dimorphism in the social proximity behavior of Japanese quail (Coturnix coturnix japonica) J Comp Psychol. 1986;100:68–71. [PubMed] [Google Scholar]
- 30.Domjan M. Photoperiodic and endocrine control of social proximity behavior in male Japanese quail (Coturnix coturnix japonica) Behav Neurosci. 1987;101:385–392. doi: 10.1037//0735-7044.101.3.385. [DOI] [PubMed] [Google Scholar]
- 31.Balthazart J, Ball GF. The Japanese quail as a model system for the investigation of steroid-catecholamine interactions mediating appetitive and consummatory aspects of male sexual behavior. Ann Rev Sex Res. 1998;9:96–176. [PubMed] [Google Scholar]
- 32.Balthazart J, et al. Appetitive as well as consummatory aspects of male sexual behavior in quail are activated by androgens and estrogens. Behav Neurosci. 1995;109:485–501. [PubMed] [Google Scholar]
- 33.Balthazart J, Castagna C, Ball GF. Aromatase inhibition blocks the activation and sexual differentiation of appetitive male sexual behavior in Japanese quail. Behav Neurosci. 1997;111:381–397. [PubMed] [Google Scholar]
- 34.Holloway KS, Domjan M. Sexual approach conditioning: tests of unconditional stimulus devaluation using hormone manipulations. J Exp Psychol. 1993;19:47–55. doi: 10.1037//0097-7403.19.1.47. [DOI] [PubMed] [Google Scholar]
- 35.Thompson RR, et al. Role of the archistriatal nucleus taeniae in the sexual behavior of male Japanese quail (Coturnix japonica): A comparison of function with the medial nucleus of the amygdala in mammals. Brain Behav Evol. 1998;51:215–229. doi: 10.1159/000006539. [DOI] [PubMed] [Google Scholar]
- 36.Seiwert CM, Adkins-Regan E. The foam production system of the male Japanese quail: Characterization of structure and function. Brain Behav Evol. 1998;52:61–80. doi: 10.1159/000006553. [DOI] [PubMed] [Google Scholar]
- 37.Cheng KM, Hickman AR, Nichols CR. Role of the proctodeal gland foam of male Japanese quail in natural copulations. The Auk. 1989;106:279–285. [Google Scholar]
- 38.Birkhead TR, Fletcher F. Sperm storage and the release of sperm from the sperm storage tubules in Japanese quail. 1994:101–105. Ibis. [Google Scholar]
- 39.Doi O, et al. Changes in the pituitary and plasma LH, plasma and follicular progesterone, and estradiol, and plasma testosterone and estrone concentrations during the ovulatory cycle of the quail (Coturnix coturnix japonica) Gen Comp Endocrinol. 1980;41:156–163. doi: 10.1016/0016-6480(80)90139-2. [DOI] [PubMed] [Google Scholar]
- 40.Delville Y, Sulon J, Balthazart J. Diurnal variations of sexual receptivity in the the female Japanese quail. Horm Behav. 1986;20:13–33. doi: 10.1016/0018-506x(86)90026-7. [DOI] [PubMed] [Google Scholar]
- 41.Adkins-Regan E. Foam produced by male Coturnix quail: what is its function ? The Auk. 1999;116:184–193. [Google Scholar]
- 42.Sachs BD. Photoperiodic control of the cloacal gland of the Japanese quail. Science. 1967;157:201–203. doi: 10.1126/science.157.3785.201. [DOI] [PubMed] [Google Scholar]
- 43.Balthazart J, et al. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J Neurosci. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taziaux M, Cornil CA, Balthazart J. Aromatase inhibition blocks the expression of sexually-motivated cloacal gland movements in male quail. Behav Processes. 2004;67:461–469. doi: 10.1016/j.beproc.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Adkins-Regan E, MacKillop EA. Japanese quail (Coturnix japonica) inseminations are more likely to fertilize eggs in a context predicting mating opportunities. Proc Biol Sci. 2003;270:1685–1689. doi: 10.1098/rspb.2003.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holloway KS, Balthazart J, Cornil CA. Androgen mediation of conditioned rhythmic cloacal sphincter movements in Japanse quail (Coturnix japonica) J Comp Psychol. 2005;119:49–57. doi: 10.1037/0735-7036.119.1.49. [DOI] [PubMed] [Google Scholar]
- 47.Cornil CA, et al. The effects of aromatase inhibition on testosterone-dependent conditioned rhythmic cloacal sphincter movements in male Japanese quail. Physiol Behav. 2004;83:99–105. doi: 10.1016/j.physbeh.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Foidart A, et al. Critical re-examination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J Chem Neuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- 49.Balthazart J, Ball GF. New insights into the regulation and function of brain estrogen synthase (aromatase) TINS. 1998;21:243–249. doi: 10.1016/s0166-2236(97)01221-6. [DOI] [PubMed] [Google Scholar]
- 50.Balthazart J, et al. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol Behav. 2004;83:247–270. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 51.Naftolin F, Horvath TL, Balthazart J. Estrogen synthetase (aromatase) immunohistochemistry reveals concordance between avian and rodent limbic systems and hypothalami. Proc Soc Exp Biol Med. 2001;226:717–725. doi: 10.1177/153537020222600802. [DOI] [PubMed] [Google Scholar]
- 52.Abdelgadir SE, et al. Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology. 1994;135:395–401. doi: 10.1210/endo.135.1.8013375. [DOI] [PubMed] [Google Scholar]
- 53.Balthazart J, Foidart A. Brain aromatase and the control of male sexual behavior. J Steroid Biochem Mol Biol. 1993;44:521–540. doi: 10.1016/0960-0760(93)90256-v. [DOI] [PubMed] [Google Scholar]
- 54.Balthazart J, et al. Estradiol, a key endocrine signal in the sexual diferentiation and activation of reproductive behavior in quail. J Exp Zool Pt A, Ecol Genetics and Physiol. 2008;309A doi: 10.1002/jez.464. [DOI] [PubMed] [Google Scholar]
- 55.Roselli CE, Horton LE, Resko JA. Time-course and steroid specificity of aromatase induction in rat hypothalamus-preoptic area. Biol Reprod. 1987;37:628–633. doi: 10.1095/biolreprod37.3.628. [DOI] [PubMed] [Google Scholar]
- 56.Hutchison JB, Steimer TH. Formation of behaviorally effective 17beta-estradiol in the dove brain: steroid control of preoptic aromatase. Endocrinology. 1986;118:2180–2187. doi: 10.1210/endo-118-6-2180. [DOI] [PubMed] [Google Scholar]
- 57.Harada N, et al. Synergism between androgens and estrogens in the induction of aromatase and its messenger RNA in the brain. Brain Res. 1993;622:243–256. doi: 10.1016/0006-8993(93)90825-8. [DOI] [PubMed] [Google Scholar]
- 58.Absil P, et al. The control of preoptic aromatase activity by afferent inputs in Japanese quail. Brain Res Rev. 2001;37:38–58. doi: 10.1016/s0165-0173(01)00122-9. [DOI] [PubMed] [Google Scholar]
- 59.Albert KA, et al. Calcium/phospholipid-dependent protein kinase (protein kinase C) phosphorylates and activates tyrosine hydroxylase. Proc Natl Acad Sci USA. 1984;81:7713–7717. doi: 10.1073/pnas.81.24.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daubner SC, et al. Site-directed mutagenesis of serine 40 of rat tyrosine hydroxylase. J Biol Chem. 1992;267:12639–12646. [PubMed] [Google Scholar]
- 61.Onagbesan OM, Podie MJ. Calcium-dependent stimulation of estrogen secretion by FSH from theca cells of the domestic hen (Gallus domesticus) Gen Comp Endocrinol. 1989;75:177–186. doi: 10.1016/0016-6480(89)90069-5. [DOI] [PubMed] [Google Scholar]
- 62.Hochberg Z, et al. The dual effect of calcium on aromatization by cultured human trophoblast. J Steroid Biochem. 1986;24:1217–1219. doi: 10.1016/0022-4731(86)90386-9. [DOI] [PubMed] [Google Scholar]
- 63.Steimer T, Hutchison JB. Micromethods for the in vitro study of steroid metabolism in the brain using radiolabelled tracers. In: Greenstein B, editor. Neuroendocrine research methods. Vol. 2. Harwood Academic Publishers; Chur, Switzerland: 1991. pp. 875–919. [Google Scholar]
- 64.Ames MM, Lerner P, Lovenberg W. Tyrosine hydroxylase: activation by protein phosphorylation and end product inhibition. J Biol Chem. 1978;253:27–31. [PubMed] [Google Scholar]
- 65.Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol. 2001;13:61–71. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- 66.Balthazart J, et al. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur J Neurosci. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- 67.Corbin CJ, et al. Isolation of a full-length cDNA insert encoding human aromatase system cytochrome P-450 and its expression in nonsteroidogenic cells. Proc Natl Acad Sci USA. 1988;85:8948–8952. doi: 10.1073/pnas.85.23.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harada N. Cloning of a complete cDNA encoding human aromatase: Immunochemical identification and sequence analysis. Biochem Biophys Res Commun. 1988;156:725–732. doi: 10.1016/s0006-291x(88)80903-3. [DOI] [PubMed] [Google Scholar]
- 69.Shen P, et al. Isolation and characterization of a zebra finch aromatase cDNA: In situ hybridization reveals high aromatase expression in brain. Mol Brain Res. 1994;24:227–237. doi: 10.1016/0169-328x(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 70.Harada N. Novel properties of human placental aromatase as cytochrome P-450: purification and characterization of a unique form of aromatase. J Biochem. 1988;103:106–113. doi: 10.1093/oxfordjournals.jbchem.a122213. [DOI] [PubMed] [Google Scholar]
- 71.McPhaul MJ, et al. The expression of a functional cDNA encoding the chicken cytochrome P-450arom (aromatase) that catalyzes the formation of estrogen from androgen. J Biol Chem. 1988;263:16358–16363. [PubMed] [Google Scholar]
- 72.Means GD, et al. Structural analysis of the gene encoding human aromatase cytochrome P-450, the enzyme responsible for estrogen biosynthesis. J Biol Chem. 1989;264:19385–19391. [PubMed] [Google Scholar]
- 73.Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- 74.Cornil CA, et al. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology. 2005;146:3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balthazart J, Foidart A, Hendrick JC. The induction by testosterone of aromatase activity in the preoptic area and activation of copulatory behavior. Physiol Behav. 1990;47:83–94. doi: 10.1016/0031-9384(90)90045-6. [DOI] [PubMed] [Google Scholar]
- 76.Balthazart J, et al. Effects of calmodulin on aromatase activity in the preoptic area. J Neuroendocrinol. 2005;17:664–671. doi: 10.1111/j.1365-2826.2005.01355.x. [DOI] [PubMed] [Google Scholar]
- 77.Adkins EK, Nock BL. The effects of the antiestrogen CI-628 on sexual behavior activated by androgen and estrogen in quail. Horm Behav. 1976;7:417–429. doi: 10.1016/0018-506x(76)90013-1. [DOI] [PubMed] [Google Scholar]
- 78.Schlinger BA, Callard GV. Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinol. 1989;49:434–441. doi: 10.1159/000125149. [DOI] [PubMed] [Google Scholar]
- 79.Naftolin F, et al. Aromatase immunoreactivity in axon terminals of the vertebrate brain - An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinol. 1996;63:149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- 80.Cross E, Roselli CE. 17beta-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1346–R1350. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- 81.Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: Where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 83.De Coster R, et al. New non-steroidal aromatase inhibitors: Focus on R76713. J Steroid Biochem. 1990;37:335–341. doi: 10.1016/0960-0760(90)90482-z. [DOI] [PubMed] [Google Scholar]
- 84.Cornil CA, et al. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm Behav. 2006;49:45–67. doi: 10.1016/j.yhbeh.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cornil CA, et al. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res. 2006;166:110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 86.Kow L-M, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. PNAS. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 88.Evrard HC, Balthazart J. Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. J Neurosci. 2004;24:7225–7229. doi: 10.1523/JNEUROSCI.1638-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taziaux M, et al. Sexual behavior activity tracks rapid changes in brain estrogen concentrations. J Neurosci. 2007;27:6563–6572. doi: 10.1523/JNEUROSCI.1797-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J Neurosci. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol Behav. 2004;83:329–346. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 92.Spencer JL, et al. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 94.Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73:985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]