In a recent paper that has appeared in Hormones and Behavior, Ben Sachs (Sachs, 2007) has written a stimulating and scholarly essay on the concept of sexual arousal. In the course of his very useful discussion about the complexities associated with this concept he expresses strong reservations about the use of the terms “appetitive” and “consummatory” as applied to considerations of male sexual behavior. In this critique he reviews well known concerns about the ability to assign clear category boundaries to these two classes of behavior and he reminds us that some authorities such as the ethologists Niko Tinbergen and Konrad Lorenz closely identified these terms with specific energy models of motivation that have been strongly criticized and are no longer considered useful in trying to understand the proximate control of behavior. We are among the authors noted by Sachs who continue to use these terms, especially in the context of the control of male sexual behavior. Although we find Professor Sachs’s overall discussion of how one might think about sexual arousal to be very valuable indeed we disagree with his critique of the use of the terms “appetitive” and “consummatory”. In this essay we would like to explain our perspective on the continuing use of these terms. Because many students trained today in contemporary behavioral neuroscience may not be familiar with the background behind these terms we have placed our discussion in a historical context.
The Origins of the Appetitive/Consummatory Distinction
First and foremost it is important to recognize that the appetitive/consummatory distinction was not proposed to support a particular model of metaphor for the functioning of the nervous system. Rather these terms were proposed as a way to capture variation in species- typical behavior that puzzled many comparative psychologists and ethologists. As noted by Sachs (2007), the specific terms “appetitive” and “consummatory” were first coined by Craig (1917). However, we agree with Marler and Hamilton (1966) that in the modern era the behavioral distinction that the appetitive/consummatory nomenclature captures was first clearly articulated by Sherrington (1906). Sherrington distinguished between “anticipatory” or “precurrent” reactions and “final” or “consummatory” ones. Craig (1917, 1918)) later made a similar distinction but substituted the word “appetitive” for “precurrent”. As noted by Marler and Hamilton (1966, p. 17 and p. 726) the main reason that this distinction was first proposed by Sherrington was based on behavioral observations, not on a theory or metaphor of nervous system functioning. The key issue that behavioral scientists were grappling with in the early 20th century was how to resolve conflicting observations about the species-typical stereotypy of many behaviors, dubbed Fixed Action Patterns by Lorenz (1937, 1950), with the highly variable and almost unpredictable attributes of other behaviors. Craig’s distinction of appetitive vs. consummatory helped resolve this issue. Appetitive behaviors are the more variable, searching phase of a behavioral sequence. Consummatory behaviors are the stereotypic phase and tend to result in the termination of a behavioral sequence (see Fig. 1).
Figure 1.
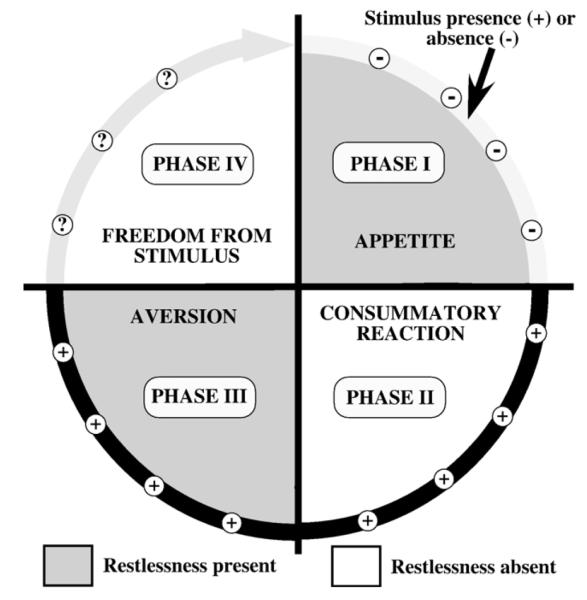
Model of « instinctive » behavior involving a 4-phase cycle according to Craig. In phase I, the relevant stimulus is absent and subjects show an appetite for that stimulus as indicated by restlessness (grayed quadrant), varied movements, effort and search. During phase II, the stimulus is present and releases the expression of the more stereotyped consummatory reaction to that stimulus. In phase III, the surfeit of that same stimulus becomes disturbing. The animal is in a state of aversion and expresses restlessness (grayed quadrant) and effort directed toward getting rid of the stimulus. Finally, during phase IV, the animal reaches a state of rest and freedom from the stimulus, which can be present or absent at that stage (question marks on figure). Figure drawn based on ideas in Craig (1917).
With the articulation of this distinction enduring conflicts related to the mechanistic- vitalist controversy could be resolved. Appetitive behaviors do indeed appear to be more “spontaneous” and less dependent on a clear external triggering stimulus but this does not make their control mechanisms less subject to a mechanistic analysis of their regulation (Marler and Hamilton, 1966, p 18).
Relationship between the appetitive/consummatory distinction and energy models of motivation
The pioneers of modern ethology Konrad Lorenz and Niko Tinbergen both embraced the appetitive/consummatory distinction as a useful first step in behavioral description (Lorenz, 1950; Tinbergen, 1951). A careful reading of their early discussion of this concept reveals that they found it useful precisely because it provided a terminology to organize many behavioral phenomena for a causal analysis. However, both Lorenz and Tinbergen adopted models or metaphors of nervous system function that were designed to explain the control of species-typical behavior (see Fig. 2).
Figure 2.
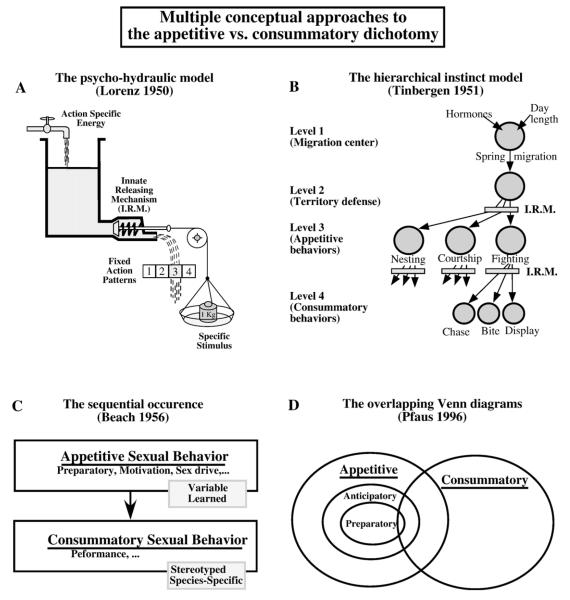
Diagrammatic presentation of diverse approaches to the notions of appetitive and consummatory behavior in the ethological literature (A-B) and in behavioral neuroendocrinology (C-D). A. According to the “psycho-hydraulic” model of Lorenz (1950) a behavior is associated with an action specific energy (“motivation”, “drive”), which accumulates until the animal encounters a specific stimulus (Sign stimulus) that will active a neural innate releasing mechanism (I.R.M.). If enough energy (water in the model) has accumulated and the stimulus is present, the animal will then display the fixed actions patterns that will release the energy and are thus considered as consummatory behaviors. In the absence of stimulus, the animal engages in various forms of appetitive behavior to search for the specific stimulus. B. In the hierarchical model of Tinbergen (1951), a behavioral system, illustrated here by the reproductive behavior of the stickleback, is organized in multiple levels from the motivation centers at higher levels down to fine-grained motor and neural controls at lower levels. In the spring, under the influence of increasing day lengths and of hormones, fishes migrate to their breeding river (Level 1), establish a territory (Level 2), and then motivation, gated by the innate releasing mechanisms (I.R.M) will lead to the display of various forms of appetitive behavior (Level 3). Depending on the stimuli that will be encountered and that will activate other I.R.M., consummatory behaviors will subsequently be expressed, resulting in a decrease in motivation (Level 4). C. Frank Beach (1956) introduced the notion of appetitive and consummatory behaviors in the field of behavioral endocrinology and applied them to male sexual behavior. In his view, appetitive behaviors are variable behaviors produced by an animal that will enhance the probability of subsequently encountering specific stimuli that will allow expression of more stereotyped behaviors resulting in a functional outcome associated with a reduction of motivation, the consummatory behavior. In his conceptualization, appetitive and consummatory behaviors occur in sequence. D. In contrast, in his incentive sequence model, Pfaus (1996) recognizes that the border between appetitive and consummatory behavior is not sharply defined and that the animal can move back and forth between these two aspects of the behavioral sequence. In his model, the behavioral stream moves initially from left to right in the actions symbolized by overlapping Venn diagrams but then can go back towards the left again during consummatory interactions in which the behaviors occur in bouts.
These models tied the occurrence of consummatory behavior specifically with the release of an action specific energy. Lorenz (1950) proposed a hydraulic model to explain how the management of action specific energy can be linked with the occurrence of fixed action patterns (Fig. 2A). According to his view a consummatory behavior would release the reservoir of energy and thus lead to the termination of the behavior in question. Tinbergen’s model stressed a hierarchy of neural “centers” that organized behaviors with the control of appetitive behaviors being just prior in the hierarchy to the occurrence of consummatory behaviors (Fig. 2B) (Tinbergen, 1951). These ethological models of motivation were criticized (e.g., Hinde, 1956, 1970) based on several criteria. It was pointed out, for example, that several aspects of the Innate Releasing Mechanisms conceived as a “block” or a “key-lock” structure in the Models of Lorenz and Tinbergen involved properties that were presumably not present in the central nervous system (Hinde, 1956). As stated by Hinde (1956, p.330) problems arose mostly “when the properties of the model were not clearly differentiated from those of the original”. What he means is that one must remember that a model tries to capture properties of a genuine physiological process to facilitate research but when one starts to think of the model as synonymous with the physiological process under investigation one actually can have “….retarding influences on research” (Hinde, 1956, p. 330). When appropriately understood, these models were helpful in guiding research but the length of time that they would serve a useful function was limited. These models certainly no longer guide research on the mechanisms of behavior and one can argue that they are not even widely known today. But because these terms were associated by some authors with models of nervous system that are no longer relevant, as noted by Sachs (2007), many authors such as Manning and Dawkins have argued against the use of the terms appetitive and consummatory because they were in the past so closely linked to these models (Manning and Dawkins, 1998).
The Current Utility of the Appetitive/Consummatory Distinction as a Description of Behavior Related to an Analysis of the Mechanistic Control of Behavior
Modern training in neuroethology and behavioral neuroscience no longer devotes much attention to the models of drive proposed by Tinbergen and Lorenz. A survey of relevant textbooks in behavioral neuroscience and neuroethology reveals little consideration of such models outside of a historical context (e.g., Carew, 2000; Rosenzweig, Breedlove, and Watson, 2004; Zupanc, 2004). We would argue that the linkage of the terms appetitive/consummatory with these now defunct motivational models is not an impediment to the appropriate use of these terms by modern students of behavior. Other terms in behavioral science have endured despite the fact that they have been linked to a particular mechanistic theory. For example, the term “imprinting” was coined by Lorenz to describe the selective learning by a gallinaceous chick to follow a moving stimulus encountered shortly after hatching and for this following response that endures until the chick reached sexual maturity. Many investigators have tied this description of a very particular behavioral phenomenon to different theories about neural mechanisms. The theories of control have changed over the years but the term has remained useful. This is also the case for the appetitive/consummatory distinction.
However, there have been criticisms of the appetitive/consummatory distinction related to its utility as a way to describe behavior. The most obvious and commonly stated problem is that the distinction between the two categories is not always clear. In regards to this problem, we would like to make the general observation that many biological categories have poorly defined boundaries. One need only consider the challenges associated with the definition of a species or a gene to realize how difficult it can be to definitively define the boundary of a biological category. In the case of the appetitive/consummatory distinction it is not always apparent when the transition occurs.
This problem can be discerned when one considers the organization of male sexual behavior. In Japanese quail, the species we have studied for many years, males when alone will produce a vocalization called a “crow” that functions to attract females (Goodson and Adkins-Regan, 1999). Once the male is in the presence of a female the crow vocalizations declines (Potash, 1974). Subsequently, in the presence of a female a male will often exhibit a display called strutting. This will be followed by a copulatory sequence of behaviors during which the male will grab the female’s neck feathers with his beak, attempt to mount on her back and then eventually succeed in apposing his cloaca to the female’s cloaca so that sperm transfer can occur. This last behavior is known as cloacal contact movements and is associated with the male opening his wings, lowering his tail and making contact with the female’s cloaca while falling backwards. We generally think of the neck grab — mount — cloacal contact movement sequence as a consummatory response (Balthazart, Reid, Absil, Foidart, and Ball, 1995). In contrast, crowing and searching for a female in her absence are examples of appetitive behaviors. Strutting also appears to be appetitive in that it is highly variable and often precedes copulation. However, it is not exclusively pre-copulatory but can be post-copulatory as well and it is not linked in any close way with the copulatory sequence. Does this distinction work well when describing behavior in a quantitative fashion? Quantifying consummatory behavior is relatively easy, domesticated Japanese quail when paired in a small arena will start copulating very rapidly and the consummatory sequence is easy to quantify. Identifying ways to quantify appetitive behavior has been more of a challenge and this is not surprising given how variable these responses can be. We have utilized measures of appetitive behavior that have been developed by others. For example, Domjan and colleagues discovered that male quail after a single copulation with a female will, if left in the same chamber, stand stare through a window at any female literally for hours (Domjan, 1998; Domjan and Hall, 1986a, 1986b). This learned social proximity response provides a good measure of a male who is focused on pursuing and attaining a female for purposes of copulation, i.e., a male in an appetitive behavioral phase. Overall, this behavioral distinction has worked well for us in our mechanistic investigations in that we have characterized the hormonal control of both types of behavior (e.g., Balthazart, Castagna, and Ball, 1997; Balthazart et al., 1995) and have identified neural sites that are differentially involved in these different aspects of the behavior (Balthazart, Absil, Gèrard, Appeltants, and Ball, 1998; Balthazart and Ball, 2007; Taziaux, Cornil, Dejace, Arckens, Ball, and Balthazart, 2006). This does not mean that there are not complexities in applying the distinction. For example, some authors who study mammalian species have advocated that mount attempts are appetitive in nature (e.g., Zumpe, Bonsall, and Michael, 1993). With this view the true consummatory response in quail would only be the cloacal contact movements. We agree that a case can be made for such as view. But as argued by Pfaus (1996) appetitive and consummatory behaviors consist of a continuous stream of behaviors that focuses on a particular incentive or goal. The final goal involves behaviors that require direct contact with the primary incentive (i.e. the female quail in this case) and thus we argue that the entire copulatory sequence is part of the consummatory act. However, in cases such as the copulatory sequence in quail it is impossible to draw an exact line between where the appetitive phase ends and the consummatory phase begins but one simple solution as advocated by Pfaus (1996, Fig. 2D) is to recognize this overlap and take it into account when implementing a behavioral analysis. Where one wants to draw the line may even change with experience, a preparatory act in an inexperienced male may be highly variable and only partially linked in a temporal manner but then become highly stereotypic and almost automatic with experience (Pfaus, 1996). Despite these behavioral complexities the distinction has been useful to us in guiding our mechanistic analyses.
Conclusion
Our recommendation is that the appetitive/consummatory distinction be maintained for studies of sexual behavior. We recognize the criticisms made by Sachs (2007) but do not think they are serious enough to warrant a complete ban on the use of these terms. Like many terms in the biological and behavioral sciences the terms appetitive and consummatory should be used in a precise manner and with caution. Again a consideration of the fate of related terms whose usefulness has been criticized can be helpful. For example, for some years it looked as if the term “motivation” would be banned from the lexicon of scientific terms appropriate for use by behavioral scientists but it has been rehabilitated for the fundamental reason that it reflects an underlying reality about how endogenous physiological events can change a behavioral response to a constant stimulus (e.g., Pfaff, 1999). Similarly the appetitive/consummatory distinction continues to help guide the causal analysis of sexual behavior in a productive way. The work of Everitt illustrates this well (Everitt, 1990; Everitt, 1995). He marshaled a research program analyzing the neural circuit for male sexual behavior in rats that was guided by behavioral tasks designed to separate out appetitive and consummatory components. One may well want to quibble with some of the conclusions he drew about the functional neuroanatomy of the male sexual behavior circuit, but there is no question that the endeavor was a useful one overall that has inspired subsequent research including our own. The usefulness of the appetitive/consummatory distinction is not limited to studies of male sexual behavior (Timberlake and Silva, 1995). For example, investigators working in the ingestive behavior field (e.g., Baird, Gray, and Fischer, 2006) as well as in the drug addiction field (e.g., Simon and Setlow, 2006) have employed these terms as a way to characterize the behavioral organization of the behaviors that they are interested in studying in productive ways in relation to their respective mechanistic analyses. The appetitive/consummatory distinction should therefore be maintained for the same reasons that it was first proposed by Craig nearly 100 years ago. It captures an important element of behavioral organization for many goal-directed behavioral systems. Recognizing this organizational principle helps guide our mechanistic analyses in productive ways. Of course the distinction has problems when subjected to a critical analysis, but let’s not throw the baby out with the proverbial bathwater! We often have trouble categorizing complex behavioral and biological phenomenon and this requires that we utilize many terms in a productive manner despite the fact that they are at times hard to define at their definitional boundaries.
Acknowledgements
The preparation of this review and the experimental work described was supported by grants from the NIMH (Grant number RO1 MH50388) to GFB and from the Belgian Fonds de la Recherche Fondamentale Collective (Grant number 2.4562.05) to JB. We thank Peter Holland for discussions about the appetitive/consummatory distinction.
References
- Baird JP, Gray NE, Fischer SG. Effects of neuropeptide Y on feeding microstructure: Dissociation of appetitive and consummatory actions. Behav. Neurosci. 2006;120:937–951. doi: 10.1037/0735-7044.120.4.937. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Gèrard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J. Neurosci. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front. Neuroendocrinol. 2007 doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Castagna C, Ball GF. Aromatase inhibition blocks the activation and sexual differentiation of appetitive male sexual behavior in Japanese quail. Behav. Neurosci. 1997;111:381–397. [PubMed] [Google Scholar]
- Balthazart J, Reid J, Absil P, Foidart A, Ball GF. Appetitive as well as consummatory aspects of male sexual behavior in quail are activated by androgens and estrogens. Behav. Neurosci. 1995;109:485–501. [PubMed] [Google Scholar]
- Beach FA. Characteristics of masculine “sex drive”. Nebraska Symposium on Motivation.1956. pp. 1–32. [Google Scholar]
- Carew TJ. Behavioral neurobiology: The cellular organization of natural behavior. Sinauer Associates; Sunderland MA: 2000. [Google Scholar]
- Craig W. Appetites and aversions as constituents of instincts. Proc. Natl. Acad. Sci. USA. 1917;3:685–688. doi: 10.1073/pnas.3.12.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. Appetites and aversions as constituents of instincts. Biol. Bull. Marine Biology Woods Hole. 1918;34:91–107. [Google Scholar]
- Domjan M. Going wild in the laboratory: Learning about species-typical cues. In: Medin D, editor. The Psychology of Learning and Motivation. Vol. 38. Academic Press; San Diego: 1998. pp. 155–186. [Google Scholar]
- Domjan M, Hall S. Determinants of social proximity in Japanese quail (Coturnix coturnix japonica): male behavior. J. Comp. Psychol. 1986a;100:59–67. [PubMed] [Google Scholar]
- Domjan M, Hall S. Sexual dimorphism in the social proximity behavior of Japanese quail (Coturnix coturnix japonica) J. Comp. Psychol. 1986b;100:68–71. [PubMed] [Google Scholar]
- Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses in male rats. Neurosci. Biobehav. Rev. 1990;14:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- Everitt BJ. Neuroendocrine mechanisms underlying appetitive and consummatory elements of masculine sexual behavior. In: Bancroft J, editor. The pharmacology of sexual function and dysfunction. Elsevier; Amsterdam: 1995. pp. 15–31. [Google Scholar]
- Goodson JL, Adkins-Regan E. Playback of crow of male Japanese quail elicits female phonotaxis. Condor. 1999;99:990–993. [Google Scholar]
- Hinde RA. Ethological models and the concept of ‘drive’. British Journal for the Philosophy of Science. 1956;6:321–331. [Google Scholar]
- Hinde RA. Animal behaviour. 2nd Edition McGraw-Hill; New York: 1970. [Google Scholar]
- Lorenz K. Uber die Bildung des Instinktbergiffes (The establishment of the instinct concept) Die Naturwissenschaften. 1937;25:280–300. 307-318, 325-331. [Google Scholar]
- Lorenz K. The comparative method in studying innate behavior patterns. Symp. Soc. Exp. Biol. 1950;4:221–268. [Google Scholar]
- Manning A, Dawkins MS. 5th ed Cambridge Unniversity Press; Cambridge UK: 1998. An introduction to animal behavior. [Google Scholar]
- Marler P, Hamilton WJI. Mechanisms of animal behavior. Wiley and Sons; New York: 1966. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Neurobiological and molecular mechanisms of sexual motivation. The MIT Press; Cambridge, Mass.: 1999. Drive. [Google Scholar]
- Pfaus JG, Frank A. Beach Award - Homologies of animal and human sexual behaviors. Horm. Behav. 1996;30:187–200. doi: 10.1006/hbeh.1996.0024. [DOI] [PubMed] [Google Scholar]
- Potash LM. An experimental analysis of the use of location calls by Japanese quail, Coturnix coturnix japonica. Behaviour. 1974;54:153–180. [Google Scholar]
- Rosenzweig MR, Breedlove SM, Watson NV. 4th ed Sinauer Associates; Sunderland MA: 2004. Biological psychology: An introduction to behavioral and cognitive neuroscience. [Google Scholar]
- Sachs BD. A contextual definition of male sexual arousal. Horm. Behav. 2007;51:569–78. doi: 10.1016/j.yhbeh.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The integrative action of the nervous system. Scribner; New York: 1906. [Google Scholar]
- Simon NW, Setlow B. Post-training amphetamine administration enhances memory consolidation in appetitive Pavlovian conditioning: Implications for drug addiction. Neurobiology of Learning. 2006;86:305–310. doi: 10.1016/j.nlm.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Taziaux M, Cornil CA, Dejace C, Arckens L, Ball GF, Balthazart J. Neuroanatomical specificity in the expression of the immediate early gene c-fos following expression of appetitive and consummatory male sexual behaviour in Japanese quail. Eur. J. Neurosci. 2006;23:1869–87. doi: 10.1111/j.1460-9568.2006.04719.x. [DOI] [PubMed] [Google Scholar]
- Timberlake W, Silva KM. Appetitive behavior in ethology, psychology, and behavior systems. In: Thompson NS, editor. Perspectives in Ethology, Volume 11: Behavioral Design. Plenum Press; New York: 1995. pp. 211–253. [Google Scholar]
- Tinbergen N. The study of instinct. Clarendon Press; Oxford: 1951. [Google Scholar]
- Zumpe D, Bonsall RW, Michael RP. Effects of the nonsteroidal aromatase inhibitor, fadrozole, on the sexual behavior of male cynomolgus monkeys (Macaca fascicularis) Horm. Behav. 1993;27:200–215. doi: 10.1006/hbeh.1993.1015. [DOI] [PubMed] [Google Scholar]
- Zupanc GKH. Behavioral neurobiology: An integrative approach. Oxford University Press; New York: 2004. [Google Scholar]