Abstract
Birds are anosmic or at best microsmatic… This misbelief persisted until very recently and has strongly influenced the outcome of communication studies in birds, with olfaction remaining neglected as compared to acoustic and visual channels. However, there is now clear empirical evidence showing that olfaction is perfectly functional in birds and birds use olfactory information in a variety of ethological contexts. Although the existence of pheromones has never been formally demonstrated in this vertebrate class, different groups of birds, such as petrels, auklets and ducks have been shown to produce specific scents that could play a significant role in within-species social interactions. Behavioral experiments have indeed demonstrated that these odors influence the behavior of conspecifics. Additionally, in quail, deprivation of olfactory inputs decreases neuronal activation induced by sexual interactions with a female. It seems therefore well established that birds enjoy a functional sense of smell and a fast growing body of experimental evidence suggests that they use this channel of olfactory communication to control their social life. The unequivocal identification of an avian pheromone is, however, still ahead of us but there are now many exciting opportunities to unravel the behavioral and physiological particularities of chemical communication in birds.
Keywords: avian olfaction, ducks, Japanese quail, Procellariiforms, auklets, immediate early gene, kin recognition, sex recognition, homing, self-odor, MHC, chemosensory information
Introduction: pheromones in birds?
The existence of a functional olfactory system in birds has long been a matter of debate. This is partly due to the fact that birds do not display behaviors indicative of a reliance on olfactory cues (Roper 1999) contrary to mammals that often display specific behaviors when they actively sample the chemical composition of their environment. Mammals extend their neck, move their head, sniff and track the source of the odor (Sundberg et al. 1982). They also often adopt obvious scent-marking behaviors and sniff each other. Birds, in contrast, have developed a wide array of alternative communication signals and do not show behaviors typically associated with olfactory sampling of the environment. They often possess bright plumage colors, many species sing complex songs, some species perform elaborate sexual displays, their visual acuity is excellent and extends to the ultraviolet… With such a complex multi-channel communication system involving elaborate visual and acoustic features, one could legitimately wonder what kind of useful information would olfaction add to the system? The absence of sniffing-associated behaviors combined to the evolution of a complex visual-and acoustic-based communication have long promoted the belief that birds were poor smellers. However, many anatomical, physiological, and behavioral experiments have now demonstrated that the avian olfactory apparatus is similar in structure and function to that of other vertebrates (see below and reviews in: Hagelin 2007a; Balthazart and Taziaux 2009; Roper 1999).
The use of body odors in chemical signaling is not a new concept. The Ancient Greeks already noticed that the secretion of a female dog in heat attracts males, and Darwin included chemical signals alongside visual and acoustic signals in his conception of sexual selection (Wyatt 2003, 2009). The word “pheromone” was only proposed some fifty years ago by Karlson and Lüscher to define “substances that are secreted to the outside by an individual and received by a second individual of the same species, in which they release a specific reaction, for example, a definite behavior or a developmental process” (Karlson and Lüscher 1959). At first glance, this definition seems relatively broad and permissive. Many scientists considered that any kind of substances that is produced by another member of the same species and influences interactions between conspecifics, could be considered as a pheromone (Johnston 2003). However, this concept has faced a lot of controversy, especially among people studying chemical communication in vertebrates. Some researchers even suggested in the 1970s that the term “pheromone” should not be used for mammalian chemical signals mainly because they believed that a pheromone should not vary from one individual to another nor over time within one individual, that its recognition should not require any form of learning, and that a pheromone should be a single chemical compound rather than a mixture of multiple chemicals (Johnston 2003; Wyatt 2009). It was proposed to rather call complex olfactory signals in vertebrates a “signature odor”, a “pheromone blend” or a “mosaic signal” but not a pheromone per se. This nomenclature would severely restrict the array of chemical substances that could enter the category of pheromones. In addition to the difficulties associated with their isolation, identification and synthesis, scientists would indeed have to demonstrate that putative pheromones do not vary within a species and that their recognition is “innate”, which is practically very difficult if not impossible (but see Meredith 2001; Johnston 2003). Obviously, this restriction cannot be accepted by scientists studying “sex-pheromones” or “pheromone-based individual recognition” (Johansson and Jones 2007), since these chemical signals are by definition not invariable within a species (they distinguish males from females) or over time within an individual (they signal reproductive status).
Despite this semantic controversy, research on chemical communication in mammals has been extremely active for several decades and has characterized fairly stereotyped responses to chemical signals (see for example Schaal et al. 2003). In contrast, little attention was devoted to the potential existence of pheromones in birds. A recent review Hagelin (2007a) indicated: “Since we are only just beginning to understand the role of odors and chemosignals in birds, it is, as yet, premature to claim that any avian odor signal functions as a pheromone”. To date, the best evidence for the existence of pheromones in birds is limited to the demonstration that body odors can alter the behavior of a conspecific (e.g., Balthazart and Schoffeniels 1979; Hagelin et al. 2003; Bonadonna and Nevitt 2004). Although the chemical substances composing these odors have not been fully characterized yet, these scents currently represent our best link between pheromones and birds.
In the present paper, we first briefly review the experimental evidence accumulated over the past few decades demonstrating that birds are equipped with a functional olfactory system that allows them to detect odors present in the environment, in some cases at least, at biologically relevant concentrations. We then focus on the few groups of birds in which the presence of body odors has been documented and in which there is evidence that these odors modify specific aspects of the behavior. We finally conclude by summarizing the main gaps in our current knowledge and highlighting areas where additional research is clearly warranted.
Birds are not anosmic
It has long been believed that most birds were devoid of a functional olfactory system and this belief was textbook knowledge for at least the first three quarters of the twentieth century (Grassé 1950; Marshall 1961) and even more recently (del Hoyo et al. 1992). Experimental evidence started, however, accumulating in the 1960s indicating that this conception is probably wrong. The pioneering work of Bang and collaborators (Bang 1960; Bang and Cobb 1968) describing the anatomy of the olfactory cavity and comparing the size of the olfactory bulbs in multiple avian species first established the morphological bases of the sense of smell in birds. Subsequent neuroanatomical, physiological and behavioral studies demonstrated the complexity of avian olfactory structures and indicated the relative importance of olfaction in species as diverse as kiwis, vultures, petrels, but also in species with much smaller olfactory bulbs including various songbirds, pigeons, quail, and chickens.
Behavioral studies in particular have now demonstrated that many species of birds use olfaction in a multitude of biologically relevant contexts, including orientation in homing pigeons (reviewed by Wallraff 2004), food location in kiwis (Wenzel 1968; Cunningham et al. 2009), turkey vultures (Houston 1986; Graves 1992), and petrels (Hutchison and Wenzel 1980; Nevitt 2000; Cunningham et al. 2003; Nevitt 2008), recognition of various odors in domestic chicks (Burne and Rogers 1996; Marples and Roper 1996; Porter et al. 1999) and nest localization by petrels and other procellariiforms (Grubb 1974; Bonadonna et al. 2003a; Bonadonna and Nevitt 2004; see Roper 1999; Hagelin 2007a for reviews). This being said, research on the production or social use of bodily odors in birds has been largely neglected as compared to other vertebrate classes. Available data suggest nevertheless that species-specific odors are likely to play an important role in the control of social interactions in birds (e.g. Balthazart and Schoffeniels 1979; Hagelin et al. 2003; Bonadonna and Nevitt 2004).
Morphological and neuroanatomical evidence
The gross anatomy of the avian olfactory system is similar in birds and in other tetrapods including mammals. External nares situated near the base of the upper mandible of their beak communicate with two sequential nasal cavities that moisten the incoming air before it enters the third chamber that contains the olfactory epithelium (Jones and Roper 1997). This epithelium contains a diversity of seven trans-membrane G-coupled olfactory receptors (OR) that are encoded by a fairly large number of OR genes suggesting existence of a functional sense of smell developed to various degrees in different avian families. There are, for example, approximately 150 OR genes in the zebra fish (Danio rerio) genome and over 1000 in mammals. Birds are intermediate in this respect in that they possess between 100 and 650 OR genes depending on the species, with nocturnal species tending to possess larger number of genes than diurnal ones (Steiger et al. 2009a; Steiger et al. 2009b). The cells containing these receptors then project to the olfactory bulbs via paired olfactory nerves.
Avian olfactory bulbs exhibit a high degree of structural similarity with corresponding structures in other vertebrates (see Gomez and Celii 2008 for a recent review) and vary widely in size across species (Marshall 1961; Bang and Cobb 1968), in correlation with differences in lifestyles. This variation provides another indirect suggestion that the olfactory system is presumably functional at least in some species (Edinger 1908; Jones and Roper 1997). Organization of the olfactory bulbs in layers is, in birds, more similar to reptiles than to mammals and, in particular, the glomerular layer is relatively undifferentiated (See Hagelin 2007a for review). Mitral cells are, however, clearly differentiated and their number varies widely from species to species with some species possessing up to six times as many mitral cells as mice (Wenzel and Meisami 1987; Nevitt 2008). There is apparently no vomeronasal organ and no accessory olfactory bulbs in birds or it is vestigial, but this topic was never investigated in great detail (see Hagelin 2007a).
The olfactory bulbs in turn project to multiple brain areas including the piriform cortex, mesopallium (formerly hyperstriatum ventrale), the medial septal region and medial striatum (formerly lobus parolfactorius) (Rieke and Wenzel 1978; Reiner and Karten 1985; Bingman et al. 1994; see also Reiner et al. 2004 for the revision of the avian brain nomenclature). Olfactory information is thus potentially available to a variety of brain areas that are homologous to olfactory projection regions in mammals.
Electrophysiological evidence
Recordings of electrophysiological responses to odors collected on the olfactory nerves as well as in the olfactory bulbs and in the brain itself confirmed the presence of olfactory sensitivity. In rock doves (Columba livia) for example, electrodes implanted in the olfactory bulbs identified the classic olfactory spindles associated with detection of odor stimuli that had been previously recorded in mammalian olfactory bulbs and nerves (Sieck and Wenzel 1969). Similar results were obtained later in several other species (Wenzel and Sieck 1972). Already in the 1960s, action potentials could be recorded in fibers of the olfactory nerves in 14 avian species possessing a wide range of olfactory bulb sizes and therefore presumably variable olfactory abilities. This electrical activity is proportional to the concentration of the olfactory stimuli that the animal is exposed to (Tucker 1965).
Single unit responses to odors were similarly recorded from olfactory receptor cells in vultures as well as from the pigeon’s olfactory bulb and several studies demonstrated the effects of odor stimulation on the firing rate of olfactory bulb neurons (Sieck and Wenzel 1969; McKeegan et al. 2002; McKeegan and Lippens 2003). Central recordings also showed that the electrical activity observed in the olfactory nerves following the detection of olfactory stimuli is transmitted to the various parts of the brain. These include parts of the basal ganglia and multiple locations in associative zones implicated in the decoding and integration of information that the animal gathers from the outside world (Macadar et al. 1980).
Evidence derived from conditioning experiments in the laboratory
Numerous experiments were also performed in the laboratory to quantify avian olfactory sensitivity with behavioral techniques based largely but not exclusively on Pavlovian and Skinnerian conditioning. Common laboratory species such as pigeons or chicken were often the subjects of these experiments. Early studies showed, for example, that dilute concentrations of olfactory stimuli such as amyl acetate or pyridine significantly modify in a dose-response fashion the respiratory or cardiac rate of species as diverse as the greylag goose (Anser anser), shearwater (Puffinus puffinus) or pigeon (Columba livia) (Wenzel 1971a 1980; Walker et al. 1986). These responses were, however, difficult to quantify and subsequent studies utilized conditioning techniques developed mainly by psychologists to assess in a quantitative manner olfactory thresholds in birds.
Pavlovian (type 1) conditioning, in which an association is made between a previously neutral stimulus and a behavioral or physiological response, demonstrated for example that pigeons are sensitive to extremely low concentrations of chemical compounds such as butanol, butyl acetate and amyl acetate (Walker et al. 1986). Even passerines that have relatively small olfactory bulbs possess excellent olfactory capacities that are similar to those of other avian species and even of mascrosmic mammalian species such as rats or rabbits (Clark et al. 1993).
Skinnerian or operant (type 2) conditioning was similarly used to ask birds specific questions about their olfactory perception. In this procedure, the subject is trained to form an association between the presentation of a specific stimulus and a deliberate behavioral response (e.g., peck at a key) that must be produced when the stimulus is present. This approach confirmed that pigeons can detect subtle odors and are even very sensitive to small changes in the concentration of the stimuli. This represents a critical feature allowing a subject to locate the source of an olfactory signal and move in its direction based on the increase or decrease of the intensity of the stimulus. This differential sensitivity is in the same order of magnitude in pigeons as it is in rats (Davis 1973).
Together, these studies suggested that most, if not all, avian species detect olfactory stimuli, some of them at very low concentrations, and transfer corresponding signals to telencephalic sites that could then play a role in the control of a variety of physiological or behavioral responses.
Behavioral evidence for a role of avian olfaction in non-reproductive contexts
This prediction was followed by various experiments demonstrating that, indeed, olfactory information is used to control behavior and physiology in a variety of natural and experimental situations. A few key examples are reviewed below.
The use of olfaction in homing pigeons
In 1972, Floriano Papi working at the University of Pisa, Italy, discovered that pigeons who had their olfactory nerves sectioned where either unable to return to their loft or returned with much longer latencies (Papi et al. 1972). This result did not prove by itself that olfaction was involved in homing, but in a series of subsequent experiments Papi and his colleagues accumulated evidence strongly suggesting that this was indeed the case. According to the “olfactory navigation hypothesis” that was developed based on these studies, pigeons at their loft would build an olfactory map by associating the environmental odors carried by the winds with the directions from which they blow (Papi 1989, 1990).
This olfactory navigation hypothesis has been tested in multiple experiments using a variety of procedures. Most of these experiments have consistently produced results in agreement with the theory (for example Papi 1982; Papi and Casini 1990; Gagliardo et al. 1997; Wallraff 2000; Wallraff 2004; for review see Walcott 1996; Wallraff 1996; Wallraff 2004). Other scientists, however, disagree with this interpretation (e.g., Papi et al. 1978; Wiltschko 1996; Jorge et al. 2010) but it is beyond the scope of the present review to consider this controversy in detail.
Food detection by kiwis, new world vultures and procellariiforms
It has long been recognized that a few species, such as kiwis or vultures, use olfaction to guide food searching. Bernice Wenzel originally showed convincingly in controlled experiments that kiwis (Apteryx australis) locate their food during night based on its odor (Wenzel 1968, 1971b).
The vision of groups of vultures hovering over carrion is also familiar and clearly guided by the odors of decaying animal carcasses. Vultures are able to detect these preys many kilometers away (when vision is impossible) and will usually approach them flying upwind. Controlled field experiments showed that hidden carcasses or synthetic olfactory stimuli mimicking the odor of decaying meat such as ethyl mercaptans attract vultures from considerable distances (Stager 1964; Wenzel 1973; Graves 1992; Gomez et al. 1994).
Casual observations of spontaneous behavior had also suggested that several species of procellariiforms gather in areas of the sea that contain fish debris and approach this potential source of food in a zigzag movement navigating against the wind (presumably within the cone of odors originating in this food source). Careful field experiments formally confirmed that food location can be made exclusively based on olfactory signals by these birds (Grubb 1972; Hutchison and Wenzel 1980; Nevitt 2000; Nevitt et al. 2004).
Predator detection
Chemical cues from predators can represent an important source of information for preys. This is especially the case in species that use habitats in which visual detection of predators is constrained. Hole-nesting songbirds that use cavities for breeding are a typical example. Amo and collaborators (Amo et al. 2008) found that blue tits (Cyanistes caeruleus) delay their entry into the nest-box and refuse to enter the box when chemical cues of mustelids, a common predator, had been placed inside. It remains possible that the nest box avoidance when the mustelid odor was present related only to the potentially more prominent nature of this stimulus as compared to the control quail odor that was used in these experiments. These results are, however, consistent with a use of odors for predators’ detection, a conclusion also supported by recent work on kittiwakes (Leclaire et al. 2009) and additional studies would be warranted on this topic.
Olfactory communication during social interactions in birds
To this date, more or less direct evidence has been collected in only four groups of birds to suggest that olfactory signals originating in the subject’s body odor influence in some way the expression of socio-sexual behaviors. The relevant information is reviewed in the following sections.
Olfactory communication in ducks
During their annual cycle, mallard ducks alternate between periods of sexual quiescence during the summer molt and periods of intense sexual activity in the spring (March-May), when copulations and egg laying occur. Earlier in the breeding cycle (September-October to February), male mallards gather in groups of 4–10 individuals and exhibit a series of stereotyped behaviors called “social displays” directed either to other males or to females. These displays express competition between males for access to females and are additionally used to attract females.
We originally discovered that the secretion of the uropygial gland, a small gland located on the dorsal side of the tail, is sexually differentiated in ducks and varies seasonally in females. Ducks spend many hours every day taking care of their feathers. They spread on these feathers an oily secretion that is produced by the uropygial gland. This secretion plays a key role in maintaining the integrity of the plumage (Stettenheim 2000) and is thus critical for the bird’s survival. It may, however, also serve other functions.
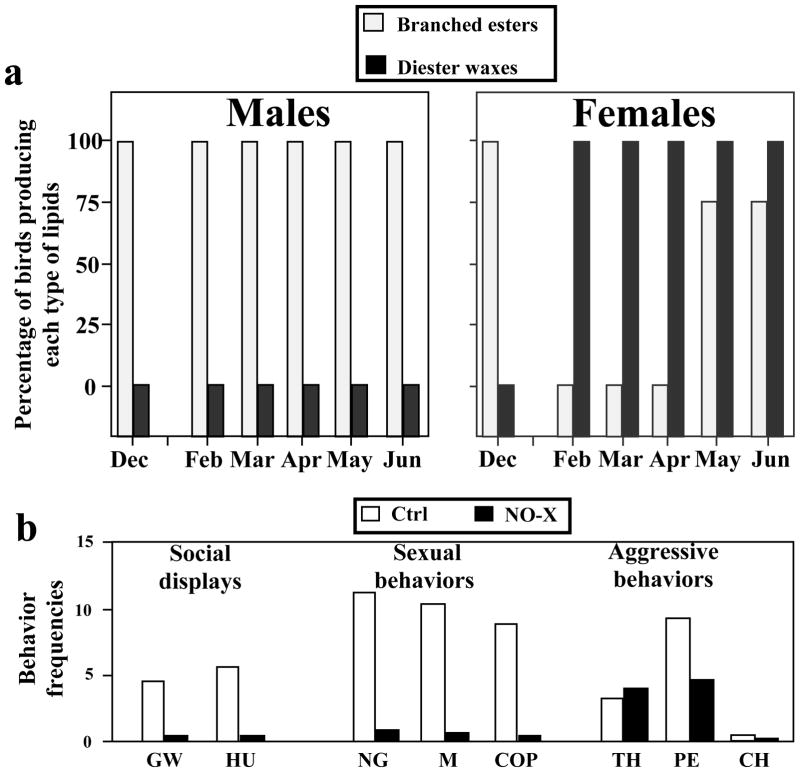
In collaboration with Dr. Jürgen Jacob, Ahrensburg, Germany, we analyzed by gas chromatography the composition of this secretion in birds of both sexes during an entire reproductive season from December to June (Jacob et al. 1979). This secretion contained large amounts of fatty acids belonging to three categories: ramified ester waxes, non-ramified ester waxes and diesters waxes (Jacob et al. 1979). In December, the secretion in both sexes was relatively similar: all birds had branched and unbranched ester waxes and none of them had diesters (Fig. 1A). This chemical composition remained stable in males during the next six months but, in contrast, all females stopped producing branched and unbranched esters waxes during the breeding season from January to April and only started secreting these compounds again after the period of active copulation (i.e. in May and June). In addition, the uropygial gland secretion of females contained diester waxes throughout the reproductive season that were never present in male secretions (Fig. 1A). The seasonal variation in the composition of the uropygial gland secretion in female birds was shown more recently to be under the control of estrogens (Bohnet et al. 1991).
Figure 1.
A. Seasonal variation (December to June) of the percentage of male (left) or female (right) domestic ducks synthesizing two types of lipids in their uropygial gland: branched ester waxes and diester axes. Prominent seasonal changes are seen in females but not in males. B. Behavior frequencies recorded during four hours of observations during January in male ducks that had their olfactory nerves sectioned bilaterally (NO-X; black bars) and in control subjects (Ctrl; open bars). A marked decrease in the expression of social displays and sexual behaviors was detected after the nerves section but aggressive behaviors were not affected. Abbreviations: GW: grunt-whistle; HU: head-up tail-up; NG: neck grab; M: mount; COP: copulation; TH: threat; PE: peck; CH: chase. Redrawn from data in Jacob et al. (1979) and Balthazart and Schoffeniels (1979).
The uropygial gland secretion could thus potentially represent a chemical signal used by males to recognize the sex of their partners and their endocrine condition. We tested this notion during two groups of behavioral experiments conducted in captivity.
In a first set of studies, one group of male domestic mallards were made anosmic by section of the olfactory nerves while another group of birds were submitted to the control manipulation (sham surgery without lesion of the nerves). The behavior of these birds was then tested regularly throughout the winter during standardized 30 min presentations of groups of 5 males to two females (Balthazart and Schoffeniels 1979). A profound inhibition of most social displays and most sexual behaviors sensu stricto (mounting and copulation) was observed in birds bearing olfactory nerve sections (Fig. 1B).
These data suggested that olfaction might play a role in the control of social interactions in ducks. However, it is known that, besides their role in the perception of olfactory stimuli, olfactory bulbs play a relatively complex role in behavioral arousal. The behavioral deficits observed following olfactory nerves sections (which results in a retrograde degeneration of the olfactory bulbs) could thus have been related to general effects on arousal independently of the perception of olfactory stimuli (Kenshalo and Isaac 1977) as observed repeatedly in behavioral studies of birds (Wenzel and Salzman 1968; Wenzel et al. 1969; Hutton et al. 1974; Wenzel and Rausch 1977). Available data do not permit to discriminate between these two interpretations but the second one appears, at present, less likely because other behaviors that are not directly linked to copulations such as aggressive behaviors were not affected (Fig. 1B).
In a second set of experiments, two groups of four intact male ducks that were visually isolated from each other were continuously exposed during the fall and winter (total period = six months) to females (two females for each group of males) that were impregnated with one of two artificial odors: amyl acetate (AA) for one group or ethyl acrylate (EA) for the other group (Balthazart and Schoffeniels 1979). During the next spring, the sexual preferences of these eight males were tested by presenting them in standard conditions to several pairs of unknown females, with one female in each pair carrying the AA odor and the other female carrying the EA odor. Males raised with AA females systematically directed their sexual activity to the AA female in the pair, but the reverse preference was not observed in the EA group. The EA birds directed their activity indiscriminately to both types of females. These data suggested that AA males had associated during the six months of training the AA odor with the notion of females and the performance of sexual behavior so that they oriented towards the females bearing this odor during the choice tests. This interpretation, however, raised the question of why EA males failed to develop a similar preference for EA females? The irritating odor of EA as compared to the more fruity odor of AA might be one explanation. Together experiments performed in ducks concur in suggesting that body odors from females, possibly originating from the uropygial gland, might influence the choice of the sexual partner by the male.
Recent work has identified the presence of seasonal variations in uropygial gland secretion in a variety of avian species. The function of these seasonal changes remains, however, elusive. They could represent an adaptation to the higher feather abrasion during incubation, help regulate the parasite load in the feathers or finally be a way of decreasing olfactory detection by predators or signaling mate quality (Reneerkens et al. 2002; Reneerkens et al. 2005; Reneerkens et al. 2006). Interestingly, the addition of uropygial gland secretions on a clutch of eggs modifies in a predictable manner the incubation behavior of female dark-eyed juncos (Junco hyemalis). Females reduce their incubation bout length on eggs bearing alien conspecific secretions and the effect is even more pronounced if secretions originated from another species. In contrast, females do not reduce their incubation bout length in response to the addition of their own uropygial gland secretion or of a vehicle-only control (Whittaker et al. 2009). Interestingly also, male chicken direct fewer mounts and copulations towards females that had their uropygial gland surgically removed than towards sham-operated females but this difference disappears if males are rendered anosmic by surgical destruction of the olfactory bulbs (Hirao et al. 2008). These experiments further suggest that the gland serves as a source of odors controlling sexual behaviors. The role of secretions from the uropygial gland as a source of olfactory signals in avian reproduction is therefore a topic that deserves additional research.
Brain activation by olfactory stimuli in quail
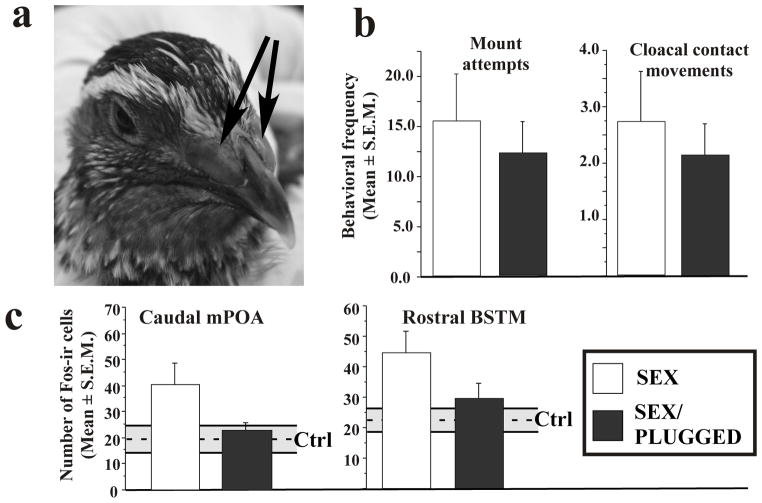
Over the past 30 years the Japanese quail (Coturnix japonica) has been the subject of a large number of laboratory studies on the endocrine mechanisms controlling male sexual behavior. This species has comparatively small olfactory bulbs and is usually considered to have a limited sense of smell, if any, even if quail are capable of discriminating between various chemical compounds and diets based on smell only (see Mills et al. 1997 for review). The potential role of olfaction in the control of sexual behavior in this species was recently tested in castrated male quail treated with exogenous testosterone. Their nares were mechanically occluded (blocked with a layer of rapid-drying dental cement; Fig. 2A) and it was confirmed that they could no longer detect prominent olfactory stimuli such as the odor of acetic acid.
Figure 2.
Occlusion of the nostrils in Japanese quail (A) does not lead to any significant change in sexual behavior (B) but alters the expression of immediate early gene fos in their brain (C). A. Photographs of the head of a male Japanese quail in which the nostrils were blocked by dental cement (arrow). B. Frequencies (± SEM) of mount attempts and cloacal contact movements recorded during a 10 min test in males that were either used as control (open columns; SEX) or had their nose blocked by dental cement (black columns; SEX/PLUGGED). C. Numbers of Fos-immunoreactive (Fos-ir) cells (±SEM) observed in the caudal medial preoptic area (mPOA) and rostral bed nucleus striae terminalis, medial part (BSTM) of male quail that had been allowed to copulate with a female either in the normal condition (SEX) or after their nose was blocked with dental cement (SEX/PLUGGED). The dotted lines and grey area represent the mean (±SEM) numbers of Fos-ir cells in control birds that stayed in their home cage and were not exposed to a female (Ctrl). Redrawn from data in Taziaux et al. (2008).
In standardized tests conditions with a female, most of these birds showed at least once the complete copulatory sequence and the frequency of their sexual behaviors was not obviously affected (Fig. 2B): the frequency of mount attempts and of cloacal contact movements were similar in control subjects and subjects with the nares occluded (Taziaux et al. 2008).
In brains collected 90 min after the end of this behavioral test an increased expression of the immediate early gene c-fos was found in brain regions associated with the control of sexual behavior such as the medial preoptic area (POA) and the medial part of the bed nucleus of the stria terminalis (BSTM) in birds that had sexual interactions with a female as compared to birds maintained in their home cage. Given the lack of behavioral inhibition, we discovered with some surprise that the number of Fos-immunoreactive cells in these two nuclei was significantly reduced in birds that had copulated with their nostrils plugged (Fig. 2C) (Taziaux et al. 2008). For unidentified reasons, we did not observe a reliable induction of the Fos protein in the olfactory bulbs of these subjects. However, another immediate early gene called zenk (also known as egr-1 or zif-268 in mammals; Mello et al. 1992) that is also induced by sexual stimulations in male quail (Ball et al. 1997; Charlier et al. 2005) was expressed in a larger number of cells in subjects allowed to copulate freely as compared to controls who stayed in their home cage or to birds who copulated with their nares occluded.
These experiments indicate that brain activity can be modulated by olfactory signals in quail, but raise a number of important questions. The absence of changes in behavior following nares occlusion could relate to the fact that male were not sexually naïve and relied potentially on visual cues only to control their behavior while olfactory signals might be required in naïve subjects (see Winans and Powers 1977; Pfeiffer and Johnston 1994 for similar data in hamsters). This leaves, however, open the question of the nature of the neural activation inhibited in birds copulating with plugged nostrils. Their copulatory behavior was expressed at normal rates so that the decrease in brain c-fos activation cannot be ascribed to a decrease in motor output. It is thus likely that the decrease in brain activation reflects the removal of olfactory inputs to the POA and BSTM, which would suggest that these inputs normally reach these brain nuclei during sexual behavior (Balthazart et al. 1998). This interpretation is also supported by the observed increase in Zenk in the olfactory bulbs of birds that copulated and its inhibition following blockade of the nostrils.
In mammals, sexual behavior-related olfactory information reaches the POA through a pathway that includes the cortico-medial amygdala and the bed nucleus of the stria terminalis (BSTM) (Sachs and Meisel 1988; Keverne 2004; Brennan and Zufall 2006). In quail, tract-tracing has identified an important projection from the arcopallium (homologous to parts of the mammalian amygdala) and in particular the nucleus taeniae of the amygdala to the medial preoptic nucleus (Balthazart and Absil 1997), suggesting that olfactory inputs could indeed reach the medial preoptic nucleus through a similar route in quail. Surprisingly, however, olfactory deprivation did not affect c-fos expression induced by male sexual behavior in the nucleus taeniae or in other nuclei such as the medial and lateral striatum, the medial septum or the piriform cortex that are also presumably part of the olfactory pathway (Rieke and Wenzel 1978; Reiner and Karten 1985; Brennan and Zufall 2006; Gagliardo et al. 2007). Additional work is thus needed to identify the reasons of this apparent discrepancy (olfactory information reaching the POA and BSTM of quail by another route; female olfactory not intense enough to induce c-fos expression in the first relays of olfactory pathway; olfactory signals activating other aspects of the functioning of these pathways that are not reflected in an increased c-fos expression).
The tangerine-scented auklets: a form of sexual attraction?
The crested auklet (Aethia cristatella) is a small seabird (family Alcidae; i.e. auks, guillemots, murres, …) that breeds in colonies on remote islands and coastlines around the Bering Sea and winters in flocks on nearby waters (Jones 1993). These relatively long-lived birds are monomorphic, socially monogamous, and breed in huge colonies mostly on cliffs and boulder fields facing the sea. Crested auklets are discernable thanks to a prominent facial crest ornament that is arched forward, and to a citrus- or tangerine-like body odor. Interestingly, both sexes share these characteristics. The crest reflects dominance status (Jones 1993) and plays an important role in sexual selection in both sexes (Jones and Hunter 1993). The citrus-like odor is detectable to human observers up to 1 km or more downwind of colonies or large flocks at sea (Jones 1993), but seems to be perceptible only during the Auklet’s breeding season (Hagelin et al. 2003; Hagelin 2007b; Douglas 2008a). The odor was suggested to be endogenously produced by wick feathers located in the interscapular region of the body (Douglas 2008a). The odorant composition is dominated by even-numbered, 6–12 carbon aldehydes similar to those found in heteropteran insects where they have defensive functions (Douglas et al. 2001).
Auklets in general, and crested auklets in particular, engage in complex and intense courtships both on large rocks where they congregate and at sea where copulations take place (Hunter and Jones 1999). Among the courtship patterns (see Jones 1993 for details), the “Ruff-Sniff” (mutual burying of bills in nape and neck feathers) and “Neck-twist” (mutual inter-twining of necks) displays are almost unique to crested auklets. It has been hypothesized that these displays could be triggered by the tangerine odor that is mainly noticed at the level of the nape and neck feathers, i.e. two regions of the body that are primarily involved in these courtships. The frictions involved may also spread the components over the bodies of the partners, particularly in those places that are hardly accessible through self-preening (Douglas 2008a).
The effects and functions of the Auklet’s odor have been investigated using two different approaches. On the one hand, synthetic analogues of the auklet scent were shown to repel or impair ticks and mosquitos, and they even killed feather lice (Douglas et al. 2004; Douglas et al. 2005a; Douglas 2008a). As would be predicted, natural tick infection rates seem low in auklets despite the abundance of ticks in the breeding colonies (Douglas 2006). The auklet’s endogenously-produced odor may therefore have an ectoparasite repellent function (Douglas et al. 2001), that may be adaptive in birds breeding in dense colonies where parasites can spread easily. Interestingly, one study identified a trend for scent production (measured by octanal specifically) to be higher during the late rather than in the early incubation (no data are unfortunately available for the courtship period preceding egg laying; Douglas et al. 2008). This temporal pattern would be consistent with the idea that the main function of this scent is to repel parasites in the nest. It follows that the citrus- or tangerine-like odor could act as an honest signal of mate quality: birds secreting more aldehydes or high-quality aldehydes would signal a better protection against parasites that could be shared both with the partner and the chicks (through displays and allopreening). Accordingly, it was reported that the concentration of two components of the auklet scent, together with the length of the facial crest, predicted the social status of individuals (Hagelin 2007b).
Questions have, however, been raised concerning this ectoparasite repellency function of the auklet’s plumage scent. It has been pointed out that several of these experiments were performed with concentrations of odors higher than the ecologically relevant concentrations (Hagelin 2007a; Hagelin and Jones 2007). Furthermore, fresh auklet plumage did not always repel or increase morbidity of feather lice (Douglas et al. 2005b) and auklet ticks experimentally exposed to fresh piles of scented or unscented feathers were not repelled by scented plumage (Hagelin 2007a). However, it is known that fresh plumage looses a significant part of its scent within days after collection (Hagelin et al. 2003), meaning that the storage method and the time spent between feather collection and testing might also explain why plumage would not lead to convincing ectoparasitic repellency. Interestingly, some recent experiments conducted with biologically realistic concentrations have demonstrated a defensive effect of auklet-like scent against parasites (Douglas 2008a; Hagelin 2008). The potential role of the auklet scent on ectoparasitic repellency and in signaling immune capacities thus remains an open question (see Douglas 2008b; Hagelin 2008 for a discussion about this putative function of the auklet scent).
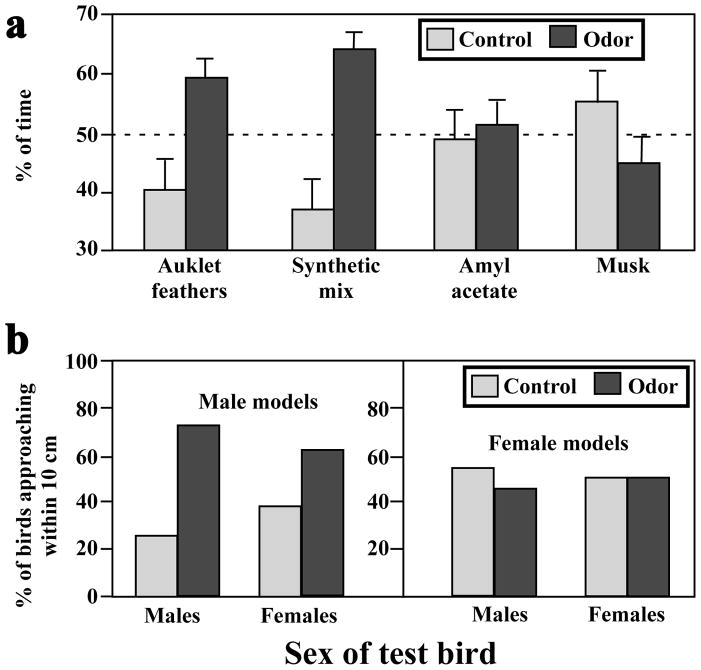
On the other hand, experiments have investigated the idea that the citrus-like scent could be used in chemical communication by auklets. T-maze experiments demonstrated that crested auklets preferentially orient towards the scent of fresh crested auklet feathers as compared to feathers from parakeet auklets that do not produce the citrus-like scent (Hagelin et al. 2003). Similar results were obtained using a mixture of two aldehydes that make up part of the tangerine feather scent, cis-4-decenal and octanal, whose concentration is elevated during the reproductive season (Fig. 3A). This demonstrated that a potentially very important social stimuli can be mimicked using synthetic chemicals (Hagelin et al. 2003).
Figure 3.
A. Percentage of time (mean ± SEM) spent by crested auklets in the arm of a T-maze next to a specific odor cue (black bars) as compared to the control arm containing no odor. A 50% choice (horizontal dashed line) would be expected in the absence of any attraction/repulsion by the odor. Significant preferences are observed for the auklet natural and synthetic odor, but not for biologically irrelevant odors such as amyl acetate (a chemical compound with a smell similar to banana) or mammalian musk. B. Percentage of male or female crested auklets that approached male or female decoys that had been scented or not with artificial scent (mixture of aldehydes) reproducing the tangerine-like odor produced by auklets during the breeding season. Redrawn from data in Hagelin et al. (2003), Jones et al. (2004) and Hagelin (2007b).
To test more specifically the social relevance of body scent in crested auklets, Jones and colleagues manipulated the odor of taxidermic models and observed in the colony whether free-living conspecifics differentially approached and displayed to decoys of different odor levels (Jones et al. 2004). Male decoys that were impregnated with the same synthetic mixture of aldehydes (cis-4-decenal and octanal) that was used by Hagelin and colleagues (Hagelin et al. 2003) were more likely to be approached by both sexes than were control decoys (Fig. 3B). However, when female decoys were used, reactions of the colony birds were not affected by the addition of synthetic aldehydes on the taxidermic models (Fig. 3B). Furthermore, wild birds did not perform sexual displays to any of the decoys, they simply approached them (Jones et al. 2004). Cues from different communication channels seem involved in prospective mate assessment in auklets (see above), like in many other bird species. The presence of an immobile bird, scented or not, may thus not be sufficient to elicit a full behavioral response. This might explain why birds were not displaying to the decoys and why approaching behavior did not lead to social and sexual interactions that would provide convincing evidence for a pheromonal control of behavior.
However, more recent experiments in the field confirmed that crested auklets will approach models scented with the synthetic components of the crested auklets or of a related species, the whiskered auklets (Douglas 2008a). But contrary to what had been observed in Jones et al. (2004) experiment, auklets here did rub their beaks and neck on these decoys, presumably to collect the scent, as normal birds do during the displays. Furthermore, tests performed with captive crested auklets in a zoo identified a significantly larger number of approaches to models scented with the aldehydes characterizing the crested auklet odor than with the control ethanol solution (the solvent of the adlehydes) and some of the birds even displayed to the aldehyde-scented model (Douglas 2008a).
The putative relationship between a conspicuous sexual display and a body scent that is only produced during the breeding season and might reflect individual quality in terms of parasitic resistance, suggests an interesting role of olfaction in the control of reproduction and in sexual selection mechanisms. Furthermore, the observation that body scent production strongly varies between individuals and populations (Douglas 2006; Hagelin 2007b), and is somewhat correlated with the seasonal patterns of progesterone concentrations (Douglas et al. 2008), a hormone closely involved in the control reproduction, lends further support to the notion that olfactory communication may be a significant part of these birds’ life-history traits.
Obviously, more work will still be necessary to complete this puzzle and raise the tangerine scent of these birds to a pheromonal status. For example, although individual variation in some components of the scent has been described, whether this variation has any relevance for the birds and constitutes an olfactory signature remains to be tested. Similarly, even if sexes do not seem to differ overall in their odor composition, a trend for higher production of octanal by males specifically during late incubation was reported (Douglas et al. 2008). The existence of a potential olfactory sex-discrimination has thus to be investigated.
Pheromonal communication in Procellariiforms?
Procellariiforms (petrels, albatrosses, shearwaters…) were among the first bird species known for their olfactory competence (Miller 1942; Bang 1960). They possess one of the most developed olfactory systems among birds (very complex olfactory epithelium and very large olfactory bulbs) (Bang 1966; Warham 1990, 1996), and their nostrils are often enclosed in an obvious tube on the dorsal surface of the bill. These long-lived birds spend nearly all of their lives in flight over the ocean, and are tied to land only for a few months each year (or every other year) to breed and rear a single offspring. Different species use olfactory cues to forage at different spatial scales. Several sub-Antarctic procellariform species have been shown to be highly sensitive to food odors and/or odors correlated with food such as dimethyl sulfide (DMS), fishy odors, and ammonia that may indicate the presence of prey species, island and colonies where they breed, or both (Grubb 1972; Verheyden and Jouventin 1994; Nevitt et al. 1995). Interestingly, blue petrels (Halobaena caerulea) can detect and follow food-related odors such as DMS even before they fledge (Cunningham et al. 2003), and sometimes at minute concentrations (approx. 10−12 mol l−1) (Bonadonna et al. 2006; see for review Nevitt 2008).
The olfactory abilities of Procellariiforms are not limited to foraging. They also use olfaction in the contexts of homing and individual recognition. Some species for example are able to locate their individual nest site in dense breeding colonies based on olfactory signals. Pioneering work on nest-site olfactory recognition was conducted in the Leach’s storm petrel (Oceanodroma leucorhoa) a species that breeds in burrows like most other petrel species (Warham 1990; Nevitt 2008). Petrels’ nests are characterized by a strong, musky odor that permeates the area around the burrow and is perceptible to the human nose at quite a distance (Grubb 1973). In the 1970s, Grubb conducted several observational studies and demonstrated that Leach’s storm petrels flying at night over the breeding colony were significantly more attracted by the scent of nest material collected in the colony than by forest leaf litter (Grubb 1973). He also compared the attractiveness of the playback of tape-recorded calls, and the attractiveness of the combination of both acoustic and olfactory cues. Although he found that the acoustic stimulus was more efficient than the olfactory stimulus, the combination of both caused petrels to approach and circle closer than they did to either stimulus separately. He noted that Leach’s storm petrels returning to their breeding grounds at night, land in the general vicinity of the burrow and then walk upwind to its entrance (Grubb 1974, 1979). Experiments in a Y-maze showed that petrels were significantly more attracted to the arm containing nesting material than to the arm containing forest litter (Grubb 1974).
More than two decades later, Bonadonna and colleagues tested the role of olfaction in the homing capacity of petrels in the sub-Antarctic Kerguelen archipelago with a technique of temporary smell deprivation by zinc sulfate, previously validated in Procellariiforms (Benvenuti et al. 1993). They caught blue petrels in their burrows and released them some 100–200 m further in the colony, during the night. All control birds returned to their burrow within two days, while none of the anosmic birds treated with zinc sulfate did (Bonadonna et al. 2001). Comparable, but less striking, results were obtained with thin-billed prions, Pachyptila belcheri (Bonadonna and Bretagnolle 2002). Recent work further indicated that blue petrels are able to discriminate based on olfactory cues the burrow of a blue petrel from the burrow of an Antarctic prion (Pachyptila desolata) (Bonadonna and Mardon 2010).
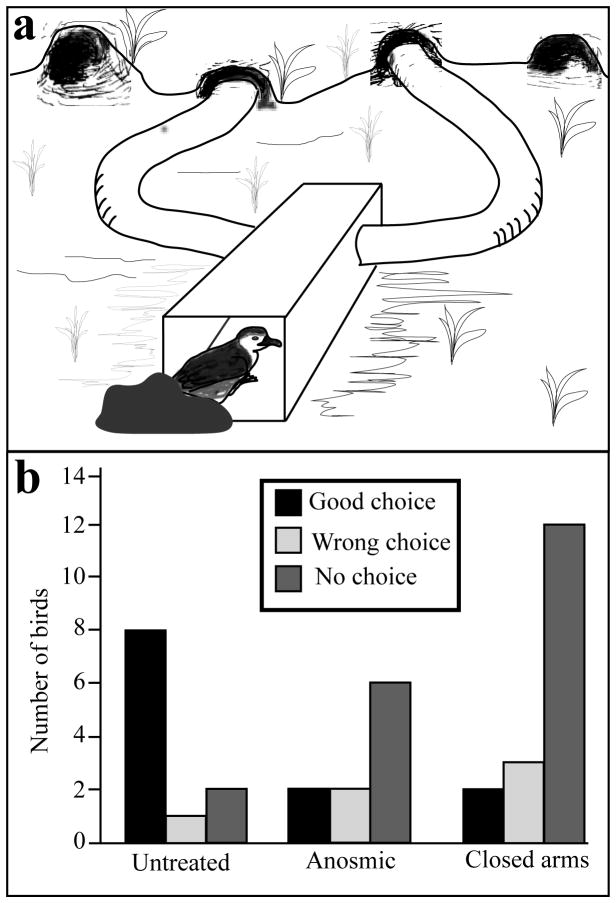
The most convincing set of experiments on burrow recognition by its olfactory signature has probably been conducted in the Antarctic prion tested in a Y maze. Instead of testing whether prions would recognize their own nesting material from forest litter, Bonadonna and colleagues (Bonadonna et al. 2003b) tested whether prions were able to discriminate between their own burrow scent and the burrow scent of a conspecific by placing in the field site a portable maze in which each arm was directly connected to the entrance of one burrow (Fig. 4A). Using intact, anosmic (zinc sulfate-injected) and sham-operated birds, they demonstrated that intact and sham-operated Antarctic prions are able to recognize their own burrow scent signature, whereas most anosmic birds did not make any choice or choose randomly between the two burrows (Fig. 4B). In addition, to rule out any positioning effect of the maze that could be used as a navigational aid and to prove that the bird’s movements and orientation in the maze were led by odors, they placed the maze in front of two burrows as in the preceding experiment, one of them being the prion’s own burrow, but they closed the ends of the arms instead of placing them in the burrow entrances. In this particular situation, most birds did not make any choice (Fig. 4B) (Bonadonna et al. 2003b).
Figure 4.
Experimental maze used to investigate olfactory orientation towards the burrow in Antarctic prions (A) and results of the choice tests performed in a variety of conditions (B). A. A flexible T-maze was located in front of two burrows and the end of one arm was connected to the entrance of the burrow of the bird being tested while the other arm was connected to the entrance of a neighboring burrow. In control experiments, the same maze was used and placed in front of burrows but the end of each arm was closed so that no olfactory stimulus could be detected in the maze. B. Results of these maze orientation experiments performed in a variety of conditions: birds were either untreated (left) or were made anosmic by injection of zinc sulphate in the olfactory chambers (middle) or were tested in a control situation in which the end of the arms were closed so that olfactory stimuli from the burrows could not enter the maze (right). Black= choice of the correct arm leading to the nest, Light grey= wrong choice (neighbor’s burrow), Dark grey = no choice. Redrawn from data in Bonadonna et al. (2003b).
As previously mentioned in the case for food odors (see above and Bonadonna et al. 2006), it could also be established that olfactory recognition of the nest already exists in the young petrel chicks. Minguez demonstrated using both homing and maze techniques that chicks of European storm petrel (Hydrobates pelagicus) on Benidorm island are already able to track the odor of their nest. Intact birds were able to walk various distances to their nest, while chicks with their nares plugged were less susceptible to locate it (Minguez 1997). Furthermore, 8 out of 9 intact birds did discriminate in a maze between their own nest material compared to the nest material of a neighbor.
These nest-recognition experiments did not tell us anything about the origin of the odor used to locate the nest, but they opened the door to a possible recognition mechanism allowing birds to discriminate their own odor from that of a conspecific. If the nest was truly located based on the scent of the owner imprinted in nest material, these experiments had then identified an olfactory based self–no-self discrimination as described in mammals (Mateo and Johnston 2000) or fishes (Thunken et al. 2009). This would be a critical step towards the demonstration of the existence of pheromones in birds.
The burrow recognition studies described so far did not, however, exclude the possibility that nest-related odors other than those produced by the birds themselves (e.g. food remains) could be used in nest location processes. Subsequent work provided a clear demonstration that European storm-petrels and Antarctic prions can recognize their own body scent and, in the case of the Antarctic prions and Wilson’s storm petrels (Oceanites oceanicus), the odor of their mating-partner (De Leon et al. 2003; Bonadonna and Nevitt 2004; Jouventin et al. 2007). The occurrence of individual olfactory recognition was first suggested by experiments in European storm petrel showing that chicks preferentially oriented to a maze arm in which they had walked earlier, as compared to a similar arm in which another conspecific chick had walked before. The authors argued that the maze arms become impregnated with the body scent of the chicks, which then formed the basis of the discrimination (De Leon et al. 2003).
Subsequent studies investigating social odor recognition in Procellariiforms used a more direct methodology for collecting odor samples used in discrimination tests. Whereas Jouventin et al. (Jouventin et al. 2007) used living birds as odor sources placed at the end of each maze arm, Bonadonna and Nevitt (Bonadonna and Nevitt 2004) caught their stimulus subjects prior to the behavioral tests and kept them in cotton bags for an hour. In contact with the bird’s feathers, the bag was permeated with the body scent of the bird and was subsequently used as an odor source in the maze. With this method, Antarctic prions (Bonadonna and Nevitt 2004), Wilson’s storm petrels (Jouventin et al. 2007) and blue petrels (Mardon and Bonadonna 2009) were all shown to be preferentially attracted to their mate’s odor when tested against the odor of another conspecific. Surprisingly however, experimental birds avoided their own-odor when presented with a choice between their own body scent and the body scent of their partner or of another conspecific (Bonadonna and Nevitt 2004; Mardon and Bonadonna 2009). This finding suggests the existence of a mechanism of self-avoidance based on odors that could be linked to mechanisms of genetic relatedness (kin or non-kin) recognition and inbreeding prevention (Potts and Wakeland 1990; Heth et al. 1998; Zelano and Edwards 2002).
Besides this behavioral evidence, recent studies by gas chromatography and mass spectrometry of feather extracts collected in Antarctic prions have additionally suggested the existence of an olfactory signature that could potentially differentiate individuals. These analyses revealed that lipids, presumably produced by the uropygial gland, were more similar within an individual when repeatedly measured over three years, than they were between individuals (Bonadonna et al. 2007). There was also a strong statistical trend suggesting the presence of a sex difference in some of these fatty acids compounds, lending support to a potential olfactory mechanism of sex discrimination in petrels. Chemical analyses of the uropygial gland secretions of Antarctic prions and blue petrels associated with sophisticated analyzes of the results by multivariate statistical tools have recently confirmed the presence in these secretions of specific sets of molecules potentially conveying information about the species, sex and individual characteristics of the donor (Mardon et al. 2010). The use of this information has, however, not been confirmed so far by behavioral experiments. When Antarctic prions were asked in Y-maze to choose between male and female odors, they chose randomly (Bonadonna et al. 2009). These experiments thus suggest that these prions recognize the specific odor of their mate as an individual but do not generalize this identification to the sex of the birds.
Conclusions and future prospects
The amount of knowledge accumulated by professional scientists and amateur ornithologists on reproductive behaviors in birds in the lab and in the field is more extensive than for any other vertebrate class (Ball and Balthazart 2002). This is particularly true when it comes to communication studies since birds have evolved a wide array of obvious social signaling pathways that are easily quantified and manipulated. This conspicuousness makes birds easy to study. Chemical communication has, however, remained poorly represented in this wealth of studies, mainly due to persistent misbelieves. Time has come for changes: olfaction in birds is well developed and the future of chemical communication in birds will probably be as exciting as it has been unexplored. However, in order to establish that birds actually use odors to regulate their social interactions and determine whether these odors can be qualified as pheromones, a lot of research remains to be carried out. The first step toward these goals is to isolate and characterize the compounds present in the odor bouquets produced by the birds that induce reproducible behavioral effects. If odors modulate/control avian behaviors, they obviously do so by affecting neural activity and, as a consequence, a number of physiological mechanisms (Johnston 2003). The detail of the electrophysiological events induced by the active compounds (pheromones) should thus be investigated. In addition, the relative power of odors compared to other social cues (song, ornaments, receptive behaviors of females,…), in eliciting complex behavioral reactions (e.g. courtship behaviors in ducks; Balthazart and Schoffeniels 1979) or in inducing physiological responses, needs further investigation.
If birds use olfaction as a proximate mechanism for kin recognition, one could argue that the major histocompatibility complex (MHC) should become a primary focus of research in birds, as it is already in mammals. The MHC complex encodes molecules that determine immunological identity at the tissue level (Brennan and Zufall 2006). It is the most variable region of the genome (Johnston 2003), making each individual unique. One particularly interesting characteristics of the MHC for chemical communication studies is that it influences the volatile constituents of urine in mammals (Brennan and Zufall 2006). Pioneering studies linking MHC to olfaction have demonstrated that highly trained mice could discriminate between urines coming from two strains that only differ in some of their MHC loci (Yamaguchi et al. 1981). In a mate-choice context, mice also avoid mates that have a MHC type similar to their own and this has been suggested to be an adaptive mechanism for avoiding inbreeding and increasing offspring fitness by maintaining a high level of MHC heterozygosity, which may favor resistance to pathogens (Yamazaki et al. 1976; Potts and Wakeland 1990). In songbirds, MHC genes are subject to the same types of diversifying forces as those observed in mammals (Edwards et al. 1995; O’Dwyer and Nevitt 2009) and non-random mate-choice with respect to MHC characteristics has been observed is some species (e.g. house sparrow, Passer domesticus; Bonneaud et al. 2006). Whether birds identify MHC polymorphism through olfaction and use it in a mate-choice context is not known yet, but ongoing research should rapidly clarify that possibility (Bonadonna 2009; O’Dwyer and Nevitt 2009).
Despite an important research effort, there are only few pheromones that have been chemically identified among mammals, mainly because mammalian pheromones are complex signals containing several compounds (Wyatt 2003). If this chemical intricacy is related to the complexity of behavioral and physiological regulations in higher vertebrates, we might expect the same difficulty to occur in birds, with complex mixtures of chemical components encoding individual identities, sex or physiological status (Smith et al. 2001).
In conclusion, if we stay with the original definition of pheromones proposed by Karlson and Lüscher (see introduction), it now seems highly probable, if not established, that such pheromones do exist in birds. It was suggested thirty years ago that secretions of the uropygial gland affect socio-sexual behaviors in ducks. More recently, deprivation of olfactory stimuli was shown to affect activation of brain areas implicated in the control of sexual behavior in quail. Tangerine-scented body odors have been identified in auklets and shown to reliably attract birds of both sexes. In petrels, individual recognition is possible based on olfactory signals produced by the birds. Together these different observations meet all original criteria included in the definition of pheromone, namely production by the animal (ducks, auklets, petrels), detection by a conspecific (all four examples), and release of a specific reaction (ducks, auklets and petrels). There are of course many missing pieces of information and different types of information often refer to different avian species. The active compounds have only been fully identified in auklets (with some partial information being available on the nature of uropygial gland secretions in ducks, petrels and other birds), the specific behavioral reactions that are controlled are not always fully specified (auklets approach but will they court reliably, petrels recognize their mate but could they also identify other conspecifics, their sex?) and the underlying physiological processes are poorly described (there is a differential brain activation in quail but what is its function?). The learned versus “innate” nature of the behavioral reaction to body odors has also not been investigated in birds. However, in our view, all these limitations relate only to the relative paucity of studies on the topic that has not been considered worth investigating until quite recently. Avian pheromones are probably not a myth; they just need to be investigated.
Acknowledgments
We thank Caroline Nieberding, Francesco Bonadonna, Julie Hagelin and one anonymous reviewer for helpful comments on a previous version of this manuscript. Preparation of this review and the experimental work from the J.B. laboratory that is described were supported by grants from the National Institutes of Health (R01 NIH MH50388) and the Belgian FRFC2.4537.09 to J.B. S.P.C. received a Léon Speeckaert Fund postdoctoral fellowship from the King Baudouin Foundation and the Belgian American Educational Foundation (BAEF)
References
- Amo L, Galvan I, Tomas G, Sanz JJ. Predator odour recognition and avoidance in a songbird. Functional Ecology. 2008;22:289–293. [Google Scholar]
- Ball GF, Balthazart J. Neuroendocrine mechanisms regulating reproductive cycles and reproductive behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Academic Press; San Diego: 2002. pp. 649–798. [Google Scholar]
- Ball GF, Tlemçani O, Balthazart J. Induction of the Zenk protein after sexual interactions in male Japanese quail. Neuroreport. 1997;8:2965–2970. doi: 10.1097/00001756-199709080-00032. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P. Identification of catecholaminergic inputs to and outputs from aromatase-containing brain areas of the Japanese quail by tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Comp Neurol. 1997;382:401–428. [PubMed] [Google Scholar]
- Balthazart J, Absil P, Gérard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J Neurosci. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Schoffeniels E. Pheromones are involved in the control of sexual-behavior in birds. Naturwissenschaften. 1979;66:55–56. doi: 10.1007/BF00369365. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Taziaux M. The underestimated role of olfaction in avian reproduction? Behavioural Brain Research. 2009;200:248–259. doi: 10.1016/j.bbr.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang BG. Anatomical evidence for olfactory function in some species of bird. Nature. 1960;4750:547–549. doi: 10.1038/188547a0. [DOI] [PubMed] [Google Scholar]
- Bang BG. Olfactory apparatus of tubenosed birds. Acta Anat. 1966;65:391. doi: 10.1159/000142884. [DOI] [PubMed] [Google Scholar]
- Bang BG, Cobb S. The size of the olfactory bulb in 108 species of birds. Auk. 1968;85:56–61. [Google Scholar]
- Benvenuti S, Ioale P, Massa B. Olfactory experiments on cory shearwater (Calonectris diomedea): The effect of intranasal zinc sulphate treatment on short-range homing behaviour. Bollettino Di Zoologia. 1993;60:207–210. [Google Scholar]
- Bingman VP, Casini G, Nocjar C, Jones T-J. Connections of the piriform cortex in homing pigeons (Columba livia) studied with fast blue and WGA-HRP. Brain Behav Evol. 1994;43:206–218. doi: 10.1159/000113635. [DOI] [PubMed] [Google Scholar]
- Bohnet S, Rogers L, Sasaki G, Kolattukudy PE. Estradiol induces proliferation of peroxisome-like microbodies and the production of 3-hydroxy fatty acid diesters, the female pheromones, in the uropygial glands of male and female mallards. J Biol Chem. 1991;266:9795–9804. [PubMed] [Google Scholar]
- Bonadonna F. In: Olfaction in petrels: from homing to self-odor avoidance. Finger TE, editor. Blackwell Publishing; 2009. pp. 428–433. [DOI] [PubMed] [Google Scholar]
- Bonadonna F, Bretagnolle V. Smelling home: a good solution for burrow-finding in nocturnal petrels? Journal of Experimental Biology. 2002;205:2519–2523. doi: 10.1242/jeb.205.16.2519. [DOI] [PubMed] [Google Scholar]
- Bonadonna F, Mardon J. One house two families: petrel squatters get a sniff of low-cost breeding opportunities. Ethology. 2010;116:176–182. [Google Scholar]
- Bonadonna F, Nevitt GA. Partner-specific odor recognition in an Antarctic seabird. Science. 2004;306:835. doi: 10.1126/science.1103001. [DOI] [PubMed] [Google Scholar]
- Bonadonna F, Caro SP, Brooke ML. Olfactory sex recognition investigated in Antarctic prions. PLoS ONE. 2009;4:e4148. doi: 10.1371/journal.pone.0004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadonna F, Caro SP, Jouventin P, Nevitt GA. Evidence that blue petrel, Halobaena caerulea, fledglings can detect and orient to dimethyl sulfide. Journal of Experimental Biology. 2006;209:2165–2169. doi: 10.1242/jeb.02252. [DOI] [PubMed] [Google Scholar]
- Bonadonna F, Cunningham GB, Jouventin P, Hesters F, Nevitt GA. Evidence for nest-odour recognition in two species of diving petrel. Journal of Experimental Biology. 2003a;206:3719–3722. doi: 10.1242/jeb.00610. [DOI] [PubMed] [Google Scholar]
- Bonadonna F, Hesters F, Jouventin P. Scent of a nest: discrimination of own-nest odours in Antarctic prions, Pachyptila desolata. Behavioral Ecology and Sociobiology. 2003b;54:174–178. [Google Scholar]
- Bonadonna F, Miguel E, Grosbois V, Jouventin P, Bessiere JM. Individual odor recognition in birds: An endogenous olfactory signature on petrels’ feathers? Journal of Chemical Ecology. 2007;33:1819–1829. doi: 10.1007/s10886-007-9345-7. [DOI] [PubMed] [Google Scholar]
- Bonadonna F, Spaggiari J, Weimerskirch H. Could osmotaxis explain the ability of blue petrels to return to their burrows at night? Journal of Experimental Biology. 2001;204:1485–1489. doi: 10.1242/jeb.204.8.1485. [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Chastel O, Federici P, Westerdahl H, Sorci G. Complex Mhc-based mate choice in a wild passerine. Proc R Soc B-Biol Sci. 2006;273:1111–1116. doi: 10.1098/rspb.2005.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444:308–315. doi: 10.1038/nature05404. [DOI] [PubMed] [Google Scholar]
- Burne THJ, Rogers LJ. Responses to odorants by the domestic chick. Physiol Behav. 1996;60:1441–1447. doi: 10.1016/s0031-9384(96)00300-9. [DOI] [PubMed] [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Sexual behavior activates the expression of the immediate early genes c-fos and Zenk (egr-1) in catecholaminergic neurons of male Japanese quail. Neuroscience. 2005;131:13–30. doi: 10.1016/j.neuroscience.2004.09.068. [DOI] [PubMed] [Google Scholar]
- Clark L, Avilova KV, Bean NJ. Odor thresholds in passerines. Comp Biochem Physiol. 1993;104A:305–312. [Google Scholar]
- Cunningham GB, Van Buskirk RW, Bonadonna F, Weimerskirch H, Nevitt GA. A comparison of the olfactory abilities of three species of procellariiform chicks. Journal of Experimental Biology. 2003;206:1615–1620. doi: 10.1242/jeb.00286. [DOI] [PubMed] [Google Scholar]
- Cunningham SJ, Castro I, Potter MA. The relative importance of olfaction and remote touch in prey detection by North Island brow kiwis. Anim Behav. 2009;78:899–905. [Google Scholar]
- Davis RG. Olfactory pyschophysical parameters in man, rat, dog, and pigeon. J Comp Physiol Psychol. 1973;85:221–232. doi: 10.1037/h0035053. [DOI] [PubMed] [Google Scholar]
- De Leon A, Minguez E, Belliure B. Self-odour recognition in European storm-petrel chicks. Behaviour. 2003;140:925–933. [Google Scholar]
- del Hoyo J, Elliott A, Sargatal J. Handbook of the birds of the world. Vol. 1. Lynx Edition; Barcelona: 1992. [Google Scholar]
- Douglas HD. Measurement of chemical emissions in crested auklets (Aethia cristatella) Journal of Chemical Ecology. 2006;32:2559–2567. doi: 10.1007/s10886-006-9164-2. [DOI] [PubMed] [Google Scholar]
- Douglas HD. Prenuptial perfume: Alloanointing in the social rituals of the crested auklet (Aethia cristatella) and the transfer of arthropod deterrents. Naturwissenschaften. 2008a;95:45–53. doi: 10.1007/s00114-007-0294-3. [DOI] [PubMed] [Google Scholar]
- Douglas HD. In defense of chemical defense: Quantification of volatile chemicals in feathers is challenging. The Auk. 2008b;125:496–497. [Google Scholar]
- Douglas HD, Co JE, Jones TH, Conner WE. Heteropteran chemical repellents identified in the citrus odor of a seabird (crested auklet : Aethia cristatella): evolutionary convergence in chemical ecology. Naturwissenschaften. 2001;88:330–332. doi: 10.1007/s001140100236. [DOI] [PubMed] [Google Scholar]
- Douglas HD, Co JE, Jones TH, Conner WE. Interspecific differences in Aethia spp. auklet odorants and evidence for chemical defense against ectoparasites. Journal of Chemical Ecology. 2004;30:1921–1935. doi: 10.1023/b:joec.0000045586.59468.de. [DOI] [PubMed] [Google Scholar]
- Douglas HD, Co JE, Jones TH, Conner WE, Day JF. Chemical odorant of colonial seabird repels mosquitoes. Journal of Medical Entomology. 2005a;42:647–651. doi: 10.1093/jmedent/42.4.647. [DOI] [PubMed] [Google Scholar]
- Douglas HD, Kitaysky AS, Kitaiskaia EV. Seasonal covariation in progesterone and odorant emissions among breeding crested auklets (Aethia cristatella) Horm Behav. 2008;54:325–329. doi: 10.1016/j.yhbeh.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Douglas HD, Malenke JR, Clayton DH. Is the citrus-like plumage odorant of Crested Auklets (Aethia cristatella) a defense against lice? J Ornithol. 2005b;146:111–115. [Google Scholar]
- Edinger L. The relations of comparative anatomy to comparative psychology. J Comp Neurol Psychol. 1908;18:434–457. [Google Scholar]
- Edwards SV, Wakeland EK, Potts WK. Contrasting histories of avian and mammalian Mhc genes revealed by class II B sequences from songbirds. Proc Natl Acad Sci U S A. 1995;92:12200–12204. doi: 10.1073/pnas.92.26.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardo A, Mazzotto M, Bingman VP. Piriform cortex ablations block navigational map learning in homing pigeons. Behav Brain Res. 1997;86:143–148. doi: 10.1016/s0166-4328(96)02253-x. [DOI] [PubMed] [Google Scholar]
- Gagliardo A, Pecchia T, Savini M, Odetti F, Ioale P, Vallortigara G. Olfactory lateralization in homing pigeons: initial orientation of birds receiving a unilateral olfactory input. Eur J Neurosci. 2007;25:1511–1516. doi: 10.1111/j.1460-9568.2007.05378.x. [DOI] [PubMed] [Google Scholar]
- Gomez G, Celii A. The peripheral olfactory system of the domestic chicken: physiology and development. Brain Res Bull. 2008;76:208–216. doi: 10.1016/j.brainresbull.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Gomez LG, Houston DC, Cotton P, Tye A. The role of greater yellow-headed vultures Cathartes melambrotus as scavengers in neotropical forest. Ibis. 1994;136:193–196. [Google Scholar]
- Grassé P. Traité de Zoologie. Vol. 15. Masson; Paris: 1950. [Google Scholar]
- Graves GR. Greater yellow-headed vulture (Cathartes melambrotus) locates food by olfaction. Journal of Raptor Research. 1992;26:38–39. [Google Scholar]
- Grubb TC. Smell and foraging in shearwaters and petrels. Nature 1972 [Google Scholar]
- Grubb TC. Colony location by Leach’s petrel. Auk. 1973;90:78–82. [Google Scholar]
- Grubb TC. Olfactory navigation to the nesting burrow in Leaches petrel Oceanodroma leucorrhoa. Anim Behav. 1974;22:192–202. doi: 10.1016/s0003-3472(74)80069-2. [DOI] [PubMed] [Google Scholar]
- Grubb TC. Olfactory guidance of Leach’s storm petrel to the breeding island. Wilson Bulletin. 1979;91:141–143. [Google Scholar]
- Hagelin JC. Odors and chemical signaling. In: Jamieson BGM, editor. Reproductive biology and phylogeny of birds. Science Publishers; Enfield (NH) Jersey Plymouth: 2007a. pp. 75–119. [Google Scholar]
- Hagelin JC. The citrus-like scent of crested auklets: reviewing the evidence for an avian olfactory ornament. Journal of Ornithology. 2007b;148:S195–S201. [Google Scholar]
- Hagelin JC, Jones IL. Bird odors and other chemical substances: A defense mechanism or overlooked mode of intraspecific communication? Auk. 2007;124:741–761. [Google Scholar]
- Hagelin JC. New data and new questions for Crested Auklet research. The Auk. 2008;125:497–498. [Google Scholar]
- Hagelin JC, Jones IL, Rasmussen LEL. A tangerine-scented social odour in a monogamous seabird. Proceedings of the Royal Society of London Series B-Biological Sciences. 2003;270:1323–1329. doi: 10.1098/rspb.2003.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heth G, Todrank J, Johnston RE. Kin recognition in golden hamsters: evidence for phenotype matching. Anim Behav. 1998;56:409–417. doi: 10.1006/anbe.1998.0747. [DOI] [PubMed] [Google Scholar]
- Hirao A, Aoyama M, Sugita S. The role of uropygial gland on sexual behavior in domestic chicken Gallus gallus domesticus. Behav Processes. 2008;80:115–120. doi: 10.1016/j.beproc.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Houston DC. Scavenging efficiency of turkey vultures in tropical forest. Condor. 1986;88:318–323. [Google Scholar]
- Hunter FM, Jones IL. The frequency and function of aquatic courtship and copulation in Least, Crested, Whiskered, and Parakeet Auklets. Condor. 1999;101:518–528. [Google Scholar]
- Hutchison LV, Wenzel BM. Olfactory guidance in foraging by procellariiforms. Condor. 1980;82:314–319. [Google Scholar]
- Hutton RS, Wenzel BM, Baker T, Homuth M. Two-way avoidance learning in pigeons after olfactory nerve section. Physiol Behav. 1974;13:57–62. doi: 10.1016/0031-9384(74)90306-0. [DOI] [PubMed] [Google Scholar]
- Jacob J, Balthazart J, Schoffeniels E. Sex differences in the chemical composition of uropygial gland waxes in domestic ducks. Biochem Syst Ecol. 1979;7:149–153. [Google Scholar]
- Johansson BG, Jones TM. The role of chemical communication in mate choice. Biological Reviews. 2007;82:265–289. doi: 10.1111/j.1469-185X.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Chemical communication in rodents: From pheromones to individual recognition. Journal of Mammalogy. 2003;84:1141–1162. [Google Scholar]
- Jones IL. Crested auklet (Aethia cristatella) In: Poole A, Gill F, editors. The birds of North America. Philadelphia: The Academy of Natural Sciences; Washington, D.C: The American Ornithologist’s Union; 1993. [Google Scholar]
- Jones IL, Hagelin JC, Major HL, Rasmussen LEL. An experimental field study of the function of Crested Auklet feather odor. Condor. 2004;106:71–78. [Google Scholar]
- Jones IL, Hunter FM. Mutual sexual selection in a monogamous seabird. Nature. 1993;362:238–239. [Google Scholar]
- Jones RB, Roper TJ. Olfaction in the domestic fowl: A critical review. Physiol Behav. 1997;62:1009–1018. doi: 10.1016/s0031-9384(97)00207-2. [DOI] [PubMed] [Google Scholar]
- Jorge PE, Marques PAM, Phillips JB. Activational effects of odours on avian navigation. Proc R Soc B-Biol Sci. 2010;277:45–49. doi: 10.1098/rspb.2009.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouventin P, Mouret V, Bonadonna F. Wilson’s storm petrels Oceonites oceonicus recognise the olfactory signature of their mate. Ethology. 2007;113:1228–1232. [Google Scholar]
- Karlson P, Lüscher M. Pheromones - New term for a class of biologically active substances. Nature. 1959;183:55–56. doi: 10.1038/183055a0. [DOI] [PubMed] [Google Scholar]
- Kenshalo DRJ, Isaac W. Informational and arousal properties of olfaction. Physiol Behav. 1977;6:1085–1087. doi: 10.1016/0031-9384(77)90015-4. [DOI] [PubMed] [Google Scholar]
- Keverne EB. Importance of olfactory and vomeronasal systems for male sexual function. Physiol Behav. 2004;83:177–187. doi: 10.1016/j.physbeh.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Leclaire S, Mulard H, Wagner RH, Hatch SA, Danchin E. Can kittiwakes smell? Experimental evidence in a larid species. Ibis. 2009;151:584–587. [Google Scholar]
- Macadar AW, Rausch LJ, Wenzel BM, Hutchison LV. Electrophysiology of the olfactory pathway in the pigeon. J Comp Physiol A. 1980;137:39–46. [Google Scholar]
- Mardon J, Bonadonna F. Atypical homing or self-odour avoidance? Blue petrels (Halobaena caerulea) are attracted to their mate’s odour but avoid their own. Behavioral Ecology and Sociobiology. 2009;63:537–542. [Google Scholar]
- Mardon J, Saunders SM, Anderson MJ, Couchoux C, Bonadonna F. Species, Gender, and Identity: Cracking Petrels’ Sociochemical Code. Chem Senses. 2010 doi: 10.1093/chemse/bjq021. in press. [DOI] [PubMed] [Google Scholar]
- Marples NM, Roper TJ. Effects of novel colour and smell on the response of naive chicks towards food and water. Anim Behav. 1996;51:1417–1424. [Google Scholar]
- Marshall JC. Biology and Comprarative Physiology of Birds. Academic Press; New York: 1961. [Google Scholar]
- Mateo JM, Johnston RE. Kin recognition and the ‘armpit effect’: evidence of self-referent phenotype matching. Proceedings of the Royal Society of London Series B-Biological Sciences. 2000;267:695–700. doi: 10.1098/rspb.2000.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeegan DE, Demmers TG, Wathes CM, Jones RB, Gentle MJ. Stimulus-response functions of single avian olfactory bulb neurones. Brain Res. 2002;953:101–111. doi: 10.1016/s0006-8993(02)03275-4. [DOI] [PubMed] [Google Scholar]
- McKeegan DE, Lippens N. Adaptation responses of single avian olfactory bulb neurones. Neurosci Lett. 2003;344:83–86. doi: 10.1016/s0304-3940(03)00449-x. [DOI] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci U S A. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith M. Human vomeronasal organ function: A critical review of best and worst cases. Chem Senses. 2001;26:433–445. doi: 10.1093/chemse/26.4.433. [DOI] [PubMed] [Google Scholar]
- Miller L. Some tagging experiments with Black-footed albatrosses. Condor. 1942;44:3–9. [Google Scholar]
- Mills AD, Crawford LL, Domjan M, Faure JM. The behavior of the Japanese or domestic quail Coturnix japonica. Neurosci Biobehav Rev. 1997;21:261–281. doi: 10.1016/s0149-7634(96)00028-0. [DOI] [PubMed] [Google Scholar]
- Minguez E. Olfactory nest recognition by British storm-petrel chicks. Anim Behav. 1997;53:701–707. [Google Scholar]
- Nevitt GA. Olfactory foraging by Antarctic procellariiform seabirds: life at high Reynolds numbers. Biol Bull. 2000;198:245–253. doi: 10.2307/1542527. [DOI] [PubMed] [Google Scholar]
- Nevitt GA. Sensory ecology on the high seas: the odor world of the procellariiform seabirds. J Exp Biol. 2008;211:1706–1713. doi: 10.1242/jeb.015412. [DOI] [PubMed] [Google Scholar]
- Nevitt GA, Reid K, Trathan P. Testing olfactory foraging strategies in an Antarctic seabird assemblage. J Exp Biol. 2004;207:3537–3544. doi: 10.1242/jeb.01198. [DOI] [PubMed] [Google Scholar]
- Nevitt GA, Veit RR, Kareiva P. Dimethyl sulfide as a foraging cue for Antarctic procellariiform seabirds. Nature. 1995;376:680–682. [Google Scholar]
- O’Dwyer TW, Nevitt GA. Individual Odor Recognition in Procellariiform Chicks Potential Role for the Major Histocompatibility Complex. International Symposium on Olfaction and Taste. 2009;1170:442–446. doi: 10.1111/j.1749-6632.2009.03887.x. [DOI] [PubMed] [Google Scholar]
- Papi F. Olfaction and homing in pigeons: ten years of experiments. In: Papi F, Wallraff HG, editors. Avian Navigation. Springer Verlag; Berlin, Heidelberg: 1982. pp. 149–159. [Google Scholar]
- Papi F. Pigeons use olfactory cues to navigate. Ethology,Ecology and Evolution. 1989;1:219–231. [Google Scholar]
- Papi F. Olfactory navigation in birds. Experientia. 1990;46:352–363. [Google Scholar]
- Papi F, Casini G. Pigeons with ablated pyriform cortex home from familiar but not from unfamiliar sites. Proc Natl Acad Sci U S A. 1990;87:3783–3787. doi: 10.1073/pnas.87.10.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papi F, Fiore L, Fiaschi V, Benvenuti S. Olfaction and homing in pigeons. Monit Zool Ital. 1972;6:85–95. [Google Scholar]
- Papi F, Keeton WT, Brown AI, Benvenuti S. Do American and Italian pigeons rely on different homing mechanisms. J Comp Physiol [A] 1978;128:303–317. [Google Scholar]
- Pfeiffer CA, Johnston RE. Hormonal and behavioral responses of male hamsters to females and female odors: roles of olfaction, the vomeronasal system, and sexual experience. Physiol Behav. 1994;55:129–138. doi: 10.1016/0031-9384(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Porter RH, Hepper PG, Bouchot C, Picard M. A simple method for testing odor detection and discrimination in chicks. Physiol Behav. 1999;67:459–462. doi: 10.1016/s0031-9384(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Potts WK, Wakeland EK. Evolution of diversity at the Major Histocompatibility Complex. Trends in Ecology & Evolution. 1990;5:181–187. doi: 10.1016/0169-5347(90)90207-T. [DOI] [PubMed] [Google Scholar]
- Reiner A, Karten HJ. Comparison of olfactory bulb projections in pigeons and turtles. Brain Behav Evol. 1985;27:11–27. doi: 10.1159/000118717. [DOI] [PubMed] [Google Scholar]
- Reiner AD, Perkel J, Bruce L, Butler A, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneerkens J, Piersma T, Damste JS. Switch to diester preen waxes may reduce avian nest predation by mammalian predators using olfactory cues. J Exp Biol. 2005;208:4199–4202. doi: 10.1242/jeb.01872. [DOI] [PubMed] [Google Scholar]
- Reneerkens J, Piersma T, Sinninghe Damste JS. Sandpipers (Scolopacidae) switch from monoester to diester preen waxes during courtship and incubation, but why? Proc Biol Sci. 2002;269:2135–2139. doi: 10.1098/rspb.2002.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneerkens J, Piersma T, Sinninghe Damste JS. Discerning adaptive value of seasonal variation in preen waxes: comparative and experimental approaches. Acta Zoologica Sinica. 2006;52 (Suppl):272–275. [Google Scholar]
- Rieke GK, Wenzel BM. The forebrain projections of the pigeon olfactory bulb. J Morphol. 1978;158:41–56. doi: 10.1002/jmor.1051580105. [DOI] [PubMed] [Google Scholar]
- Roper TJ. Olfaction in birds. Advances in the Study of Behavior. 1999:247–332. [Google Scholar]
- Sachs BD, Meisel RL. The physiology of male sexual behavior. In: Knobil E, Neill J, et al., editors. The physiology of reproduction. Raven Press; New York: 1988. pp. 1393–1485. [Google Scholar]
- Schaal B, Coureaud G, Langlois D, Giniès C, Sémon E, Perrier G. Chemical and behavioural characterization of the rabbit mammary pheromone. Nature. 2003;424:68–72. doi: 10.1038/nature01739. [DOI] [PubMed] [Google Scholar]
- Sieck MH, Wenzel BM. Electrical activity of the olfactory bulb of the pigeon. Electroenceph Clin Neurophysiol. 1969;26:62–69. doi: 10.1016/0013-4694(69)90034-0. [DOI] [PubMed] [Google Scholar]
- Smith TE, Tomlinson AJ, Mlotkiewicz JA, Abbott DH. Female marmoset monkeys (Callithrix jacchus) can be identified from the chemical composition of their scent marks. Chem Senses. 2001;26:449–458. doi: 10.1093/chemse/26.5.449. [DOI] [PubMed] [Google Scholar]
- Stager KE. The role of olfaction in food location by the Turkey vulture (Cathartes aura) Los Angeles County Mus Contrib Sci. 1964;81:3–63. [Google Scholar]
- Steiger SS, Fidler AE, Kempenaers B. Evidence for increased olfactory receptor gene repertoire size in two nocturnal bird species with well-developed olfactory ability. BMC Evol Biol. 2009a;9:117. doi: 10.1186/1471-2148-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger SS, Kuryshev VY, Stensmyr MC, Kempenaers B, Mueller JC. A comparison of reptilian and avian olfactory receptor gene repertoires: species-specific expansion of group gamma genes in birds. BMC Genomics. 2009b;10:446. doi: 10.1186/1471-2164-10-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettenheim PR. The Integumentary Morphology of Modern Birds—An Overview. American Zoologist. 2000;40:461–477. [Google Scholar]
- Sundberg H, Doving K, Novikov S, Ursin H. A method for studying responses and habituation to odors in rats. Behavioral and Neural Biology. 1982;34:113–119. doi: 10.1016/s0163-1047(82)91501-1. [DOI] [PubMed] [Google Scholar]
- Taziaux M, Keller M, Ball GF, Balthazart J. Site specific effects of anosmia and cloacal gand anesthesia on Fos expression induced in male quail by sexual behavior. Behav Brain Res. 2008;194:52–65. doi: 10.1016/j.bbr.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thunken T, Waltschyk N, Bakker TCM, Kullmann H. Olfactory self-recognition in a cichlid fish. Anim Cogn. 2009;12:717–724. doi: 10.1007/s10071-009-0231-2. [DOI] [PubMed] [Google Scholar]
- Tucker D. Electrophysiological evidence of olfactory function in birds. Nature. 1965;207:34–36. doi: 10.1038/207034a0. [DOI] [PubMed] [Google Scholar]
- Verheyden C, Jouventin P. Olfactory behavior of foraging Procellariiforms. Auk. 1994;111:285–291. [Google Scholar]
- Walcott C. Pigeon homing: Observations, experiments and confusions. J Exp Biol. 1996;199:21–27. doi: 10.1242/jeb.199.1.21. [DOI] [PubMed] [Google Scholar]
- Walker JC, Walker DB, Tambiah CR, Gilmore KS. Olfactory and nonolfactory odor detection in pigeons: elucidation by a cardiac acceleration paradigm. Physiol Behav. 1986;38:575–580. doi: 10.1016/0031-9384(86)90428-2. [DOI] [PubMed] [Google Scholar]
- Wallraff HG. Seven theses on pigeon homing deduced from empirical findings. J Exp Biol. 1996;199:105–111. doi: 10.1242/jeb.199.1.105. [DOI] [PubMed] [Google Scholar]
- Wallraff HG. Simulated navigation based on observed gradients of atmospheric trace gases (models on pigeon homing, part 3) J Theor Biol. 2000;205:133–145. doi: 10.1006/jtbi.2000.2052. [DOI] [PubMed] [Google Scholar]
- Wallraff HG. Avian olfactory navigation: its empirical foundation and conceptual state. Animal Behaviour. 2004;67:189–204. [Google Scholar]
- Warham J. The Petrels: Their Ecology and Breeding Systems. Academic Press; London: 1990. [Google Scholar]
- Warham J. The behaviour, population biology and physiology of the petrels. Academic Press; London: 1996. [Google Scholar]
- Wenzel BM. Olfactory prowess of the kiwi. Nature. 1968;220:1133–1134. doi: 10.1038/2201133a0. [DOI] [PubMed] [Google Scholar]
- Wenzel BM. Olfaction in birds. In: Beidler LM, editor. Handbook of Sensory Physiology IV Chemical senses 1 Olfaction. Springer Verlag; Berlin: 1971a. pp. 432–448. [Google Scholar]
- Wenzel BM. Olfactory sensation in the kiwi and other birds. Ann NY Acad Sci. 1971b;188:183–193. doi: 10.1111/j.1749-6632.1971.tb13097.x. [DOI] [PubMed] [Google Scholar]
- Wenzel BM. Chemoreception. In: Farner DS, King JR, Parkes KC, editors. Avian Biology. Vol. 3. Academic Press; New York: 1973. pp. 389–415. [Google Scholar]
- Wenzel BM. Chemoreception in seabirds. In: Burger J, Olla BL, Winn HE, editors. Behavior of marine animals. Vol. 4. Plenum Publishing Co; New York: 1980. pp. 41–67. [Google Scholar]
- Wenzel BM, Albritton PF, Salzman A, Oberjat TE. Behavioral changes in pigeons following olfactory nerve section or bulb ablation. In: Pfaffman C, editor. Olfaction and taste. Vol. 3. Rockefeller University; New York: 1969. pp. 278–287. [Google Scholar]
- Wenzel BM, Meisami E. Number, size and density of mitral cells in the olfactory bulbs of the northern fulmar and rock dove. Ann NY Acad Sci. 1987;510:700–702. [Google Scholar]
- Wenzel BM, Rausch LJ. Does the olfactory system modulate affective behavior in the pigeon? In: Wenzel BM, Ziegler HP (eds) Tonic functions of sensory systems. Ann NY Acad Sci. 1977:314–330. doi: 10.1111/j.1749-6632.1977.tb39735.x. [DOI] [PubMed] [Google Scholar]
- Wenzel BM, Salzman A. Olfactory bulb ablation or nerve section and pigeon’s behavior in non-olfactory learning. Exp Neurol. 1968;22:472–479. doi: 10.1016/0014-4886(68)90011-3. [DOI] [PubMed] [Google Scholar]
- Wenzel BM, Sieck MH. Olfactory perception and bulbar electrical activity in several avian species. Physiol Behav. 1972;9:287–293. doi: 10.1016/0031-9384(72)90147-3. [DOI] [PubMed] [Google Scholar]
- Whittaker DJ, Reichard DG, Dapper AL, Keterson ED. Behavioral responses of nesting female dark-eyed juncos Junco hyemalis to hetero- and conspecific passerine preen oils. J Avian Biol. 2009;40:579–583. [Google Scholar]
- Wiltschko R. The function of olfactory input in pigeon orientation: Does it provide navigational information or play another role? J Exp Biol. 1996;199:113–119. doi: 10.1242/jeb.199.1.113. [DOI] [PubMed] [Google Scholar]
- Winans SS, Powers JB. Olfactory and vomeronasal deafferentation of male hamsters: histological and behavioral analyses. Brain Res. 1977;126:325–344. doi: 10.1016/0006-8993(77)90729-6. [DOI] [PubMed] [Google Scholar]
- Wyatt TD. Pheromones and animal behaviour: Communication by smell and taste. Cambridge University Press; Cambridge: 2003. [Google Scholar]
- Wyatt TD. Fifty years of pheromones. Nature. 2009;457:262–263. doi: 10.1038/457262a. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Yamazaki K, Beauchamp GK, Bard J, Thomas L, Boyse EA. Distinctive urinary odors governed by the major histocompatibility locus of the mouse. Proc Nat Acad Sci USA. 1981;78:5817–5820. doi: 10.1073/pnas.78.9.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Boyse EA, Mike V, Thaler HT, Mathieson BJ, Abbott J, Boyse J, Zayas ZA, Thomas L. Control of mating preferences in mice by genes in major histocompatibility complex. J Exp Med. 1976;144:1324–1335. doi: 10.1084/jem.144.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelano B, Edwards SV. An Mhc component to kin recognition and mate choice in birds: Predictions, progress, and prospects. Am Nat. 2002;160:S225–S237. doi: 10.1086/342897. [DOI] [PubMed] [Google Scholar]