Abstract
Japanese quail (Coturnix japonica; referred to simply as quail in this article) readily exhibit sexual behavior and related social behaviors in captive conditions and have therefore proven valuable for studies of how early social experience can shape adult mate preference and sexual behavior. Quail have also been used in sexual conditioning studies illustrating how natural stimuli predict successful reproduction via Pavlovian processes. In addition, they have proven to be a good model to study how variation in photoperiod regulates reproduction and how variation in gonadal steroid hormones controls sexual behavior. For example, studies have shown that testosterone activates male-typical behaviors after being metabolized into estrogenic and androgenic metabolites. A critical site of action for these metabolites is the preoptic medial nucleus (POM), which is larger in males than in females. The enzyme aromatase converts testosterone to estradiol and is enriched in the POM in a male-biased fashion. Quail studies were the first to show that this enzyme is regulated both relatively slowly via genomic actions of steroids and more quickly via phosphorylation. With this base of knowledge and the recent cloning of the entire genome of the closely related chicken, quail will be valuable for future studies connecting gene expression to sexual and social behaviors.
Keywords: appetitive sexual behavior, aromatase, consummatory sexual behavior, Japanese quail (Coturnix japonica), preoptic area (POA), sexual learning, sex differences, sexual differentiation
Introduction
This review concerns the usefulness of investigations using Japanese quail (Coturnix japonica) to illustrate general principles about the hormonal control of reproductive and related social behaviors. Japanese quail and other avian species have played an important role in studies of these questions throughout the history of behavioral endocrinology (Konishi et al. 1989). We begin with a few general observations on the use of birds in neuroendocrine research and then follow with a specific review of the value of Japanese quail (which we simply call quail for the sake of brevity).
The study of hormone, brain, and behavior interrelationships emerged as an identifiable field in the mid-20th century (Beach 1948), 1975, 1981). The origins of behavioral endocrinology are truly interdisciplinary in that the field attracted investigators trained in the behavioral sciences such as experimental psychology or ethology as well as those trained in endocrinology or neurobiology (Beach 1981); Marler 2005). These investigators have used a variety of animal species in their investigations (Adkins-Regan 1990; Marler 2005); in particular, ethologists and comparative psychologists interested in the study of the mechanistic control of behavior came from a tradition in which birds were widely used (Marler 2005).
There are many advantages to studying birds; for example, they exhibit a wide range of social behaviors, can be easily observed in both the laboratory and field settings, and have neuroendocrine systems that share fundamental properties with other vertebrate species including mammals (Konishi et al. 1989; Wingfield 2005). In the 1960s Lehrman (1961, 1965) took the lead in illustrating the value of birds to studies of the hormonal control of reproductive behavior, based on his now classic studies on ring doves, and Hinde (1965) conducted parallel studies on canaries. An important insight that emerged from their work is that the concentration of a steroid hormone in the blood can profoundly affect the probability that an animal will respond to a reproductive stimulus and that this response will itself markedly change the animal’s hormonal milieu. These investigations formed one of the threads that has led to the field endocrinology movement spearheaded by Wingfield and colleagues, who have investigated hormone-behavior relationships in a wide range of avian species in their natural environments (Wingfield 2005); Wingfield and Silverin 2002).
Beach and colleagues began studying the behavioral endocrinology of Japanese quail in the 1960s (Beach and Inman 1965; Sachs 1967, 1969), and in the 1970s Adkins-Regan established basic facts about the hormonal control of male reproductive behavior in quail (Adkins 1975), 1978; Adkins and Adler 1972). She demonstrated not only the dependence of the expression of male-typical reproductive behaviors on the action of both estrogenic and androgenic metabolites of testosterone (Adkins 1977); Adkins and Nock 1976; Adkins et al. 1980) but also the role of estrogens in the sexual differentiation of the neural substrate mediating male-typical sexual behavior (Adkins 1975), 1978). As a result, one of us (JB) who had been studying behavioral neuroendocrine processes in ducks and ring doves became interested in the role of testosterone metabolism as a key rate-limiting factor for the activation of reproductive behavior and began using quail for these types of investigations (Balthazart et al. 1979; Balthazart and Schumacher 1983).
Concurrent with this work on sexual behavior, Follett established a laboratory in the United Kingdom to study the photoperiodic control of seasonal reproduction in birds and he too came to focus on quail in the 1970s (Follett 1976); Follett and Maung 1978; Gledhill and Follett 1976). His group showed that long photoperiods are necessary to induce full gonadal development in quail and that photoperiodic experience can render the birds nonresponsive to photoperiods that previously stimulated reproductive development (Robinson and Follett 1982).
Thus the groundwork was established over 30 years ago for more mechanistic studies of the neuroendocrine control of reproduction and reproductive behavior in Japanese quail.
Japanese Quail: Taxonomic Status and History of Domestication
The Japanese quail is closely related to the common quail (Coturnix coturnix) and in fact was for many years considered a subspecies (del Hoyo et al. 1994). C. coturnix ranges from Western Europe across Eurasia to Mongolia, and C. japonica ranges from Mongolia eastward to Japan. Their ranges overlap in Mongolia where apparently little interbreeding occurs, suggesting that they are separate species albeit closely related. However, Japanese and common quail do interbreed in captivity, which has led some authors to maintain that they should be considered a subspecies of a single Paleoartic species (Derégnaucourt et al. 2002). Others refer to the C. coturnix (del Hoyo et al. 1994) and C. japonica populations as a “superspecies.” In this review we consider Japanese quail a separate species from the common (or European) quail.
Japanese quail were apparently domesticated in Asia by the 12th century (Mills et al. 1997), bred initially for the enjoyment of their vocalizations and later for eggs and meat. They can be easily bred in captivity with the help of an incubator. They readily exhibit high rates of sexual behavior under standardized captive conditions in which sexually receptive females are present, making them excellent subjects for studies of sexual behavior and of factors that influence it, such as social interactions and social learning.
Effects of Social Experience on Mate Choice and Sexual Behavior in Quail
Because Japanese quail mate so readily in captivity one can reliably assess the effects of social milieu on mate choice in a way that is not possible in other species (Galef and White 2000; Mills et al. 1997).
Basic studies on sexual imprinting demonstrated that exposure to a specific color morph of quail during the first 15 days after hatching (and most effectively during the first 5 days) induces a long-lasting preference for partners of the same color (Gallagher 1977), 1978). More recent studies have identified more complex effects of the early social environment. For example, Galef and White (2000) found that a female’s observation of a nonpreferred mate copulating with another female increased her preference for that male. This change in preference is not dependent on the fact that the male has had additional copulatory experience that might in turn change his behavior in such a way as to make him more attractive to the female. In contrast, males exhibit less of a preference for a female they have seen mate with another male (Galef and White 2000). Other work by Galef and colleagues has found that females allowed to choose males have a higher percentage of fertilized eggs than those forced to mate with a particular male (Persaud and Galef 2005a) and, conversely, that females forced to mate with a male who harassed them have a very low probability of laying fertile eggs (Persaud and Galef 2005b).
Quail have also been used to establish the effects of early experience on mating preference; for example, males prefer slightly unfamiliar females to females with which they were raised (Bateson 1978). These findings were interpreted as an example of “optimal outbreeding,” meaning that based on early experience individuals choose a mate somewhat—but not too—different from those with which they were raised (Bateson 1978). Further studies of optimal outbreeding revealed that male and female quail raised with full siblings preferred first cousins of the opposite sex (Bateson 1982).
Studies of Learning and Memory in a Reproductive Context
The ease and reliability with which quail display sexual behavior in captive conditions have made them important subjects in studies of sexual learning, and these studies have not only provided a fruitful line of inquiry about ecologically relevant learned associations but also challenged views of how certain fundamental learning processes are controlled (Domjan et al. 2004).
Studies of quail have been particularly important in establishing what may be called the “functional approach” to Pavlovian conditioning—the study of such conditioning in a natural context that leads to outcomes with clear biological significance (Domjan 2005). Conditioned responses are relatively easy to achieve in quail through Pavlovian conditioning procedures using sexual reinforcements (Domjan et al. 2004). A male bird’s visual exposure to a female can serve as an effective unconditioned stimulus, but actual access to and the opportunity to copulate with a female are more effective. Studies have paired copulatory opportunity with diverse stimuli such as tones, colored lights, foam or wood blocks, and pairs of terrycloth cylinders attached at a 90° angle (thus roughly shaped like a squatting female quail) (see Mills et al. 1997 for review). These conditioned stimuli (CS1) were then used, when presented alone, to modify aspects of male sexual behavior; for example, in one study such a CS elicited the strutting display that males normally exhibit toward females (Farris 1967). Arbitrary CS can also elicit approach reactions (Mills et al. 1997), but there are important differences between ecologically relevant and arbitrary CS2: the former elicit a wider range of sexual responses and are more resistant to blocking and extinction (Domjan et al. 2004).
The basic argument of those advocating the functional approach to Pavlovian conditioning is that natural stimuli are those that would ordinarily predict a functional outcome (e.g., the presence of a female predicts a successful bout of copulation) (Domjan 2005). However, natural stimuli are not arbitrary given that individuals have experience with them already, whereas most CS in traditional Pavlovian studies are selected because they are arbitrary and therefore neutral for the animal (Domjan 2005). If one wants to study how learning facilitates natural rewards one should not expect the most important stimuli to be neutral.
The fact that natural stimuli facilitate functional outcomes in quail is evident from greater reproductive success after sexual learning. One study showed that male quail are more responsive to minimal cues from females if these are presented in a context previously paired with access to a female (Hilliard et al. 1997). In conditions that predict the arrival of the female, males also released a greater volume of sperm and greater numbers of spermatozoa than control subjects mating with the female in a new environment (Domjan et al. 1998). Probably as a consequence, quail whose access to the sexual partner was paired during conditioning trials with a small light (the CS) displayed a higher copulatory efficiency and produced a larger percentage of fertilized eggs when they mated immediately after exposure to the CS compared to birds that mated without such exposure. This effect was observed only if both males and females had been conditioned but not if only one member of the pair associated the CS with sexual interactions (Mahometa and Domjan 2005). Adkins-Regan and MacKillop (2003) further demonstrated the functional significance of sexual conditioning by showing that it increases the number of fertilized eggs. In their experiment, conditioning of the male alone was sufficient to produce a significant effect on egg fertility, but this discrepancy is probably due to differences in experimental procedures.
We recently demonstrated that the association of an arbitrary CS with access to copulation enhances activation of brain areas that control sexual behavior. The expression of Fos, the protein that results from activation of the immediate early gene c-fos, was more intense (as indicated by a larger number of labeled cells) in the medial preoptic area and the bed nucleus of the stria terminalis3 of male birds exposed to the CS before pairing with the female than in those exposed to either the CS or the female alone (Taziaux et al. 2008a). This differential brain activation could represent at least a component of the mechanism that mediates improved copulatory efficiency associated with the presentation of a CS before sexual interactions.
Learned Social Proximity Response
Sexual associative learning has proven to be useful for the study of appetitive sexual behavior in male quail. Male sexual behavior has arousal and satiety components, comparable to the appetitive and consummatory aspects of behavior articulated by ethologists such as Niko Tinbergen, Konrad Lorenz, and Gerard Baerends (and even earlier) based on the pioneering work of Wallace Craig, among others (see Balthazart and Ball 1998b). Arousal involves the pursuit of females and/or behavioral and physiological responses to cues provided by distal females, and satiety results from the act of copulation itself (Beach 1956). Appetitive behaviors are an expression of sexual motivation, and the functional outcome of stereotyped consummatory behavior is a reduction in motivation (Timberlake and Silva 1995). Although the value of this distinction has its critics (Sachs 2007), it is useful for the mechanistic analysis of male sexual behavior and other rewarding activities (Ball and Balthazart 2008).
The identification of reliable measures of appetitive behavior has been problematic in quail. Although crowing (a mating call that attracts females; Goodson and Adkins-Regan 1997) might provide an easily discernible and sensitive measure, its frequency is highly variable among males and influenced by a variety of external stimuli that are not clearly identified, so its reliability as an indicator of motivation is fairly weak. In the 1980s Domjan and Hall identified a form of associative learning that they named learned social proximity response. In their study, males allowed to copulate with females in an arena learned to stand in front of a narrow window that provided a view of a female. The study showed a remarkable change in the behavior of the males: they initially paid no attention to the female behind the window but after a single copulation started spending most of their time (80–90%, up to 14 hours a day) in front of the window (Domjan and Hall 1986). This response shares features normally associated with both classical and instrumental conditioning and has thus been labeled a form of associative learning in males that have been allowed to copulate with females (see Domjan et al. 1992 for discussion).
This learned response is relatively easy to achieve under laboratory conditions and provides a sensitive measure of both male appetitive sexual behavior and its sexual motivation. We developed a faster version of this test in which the proximity response is quantified during a 5-minute period of presentation of the female after the male has been habituated to the test chamber (Figure 1). The entire test can be completed in 25 minutes (as opposed to the procedure used by Domjan and collaborators in which birds were tested throughout the day) and thus makes it possible to quantify appetitive sexual behavior in a large number of subjects each day (see Balthazart et al. 1995 for full description). A suite of experiments using this procedure has enabled characterization of the endocrine and neural controls of sexual motivation in male quail (see section below).
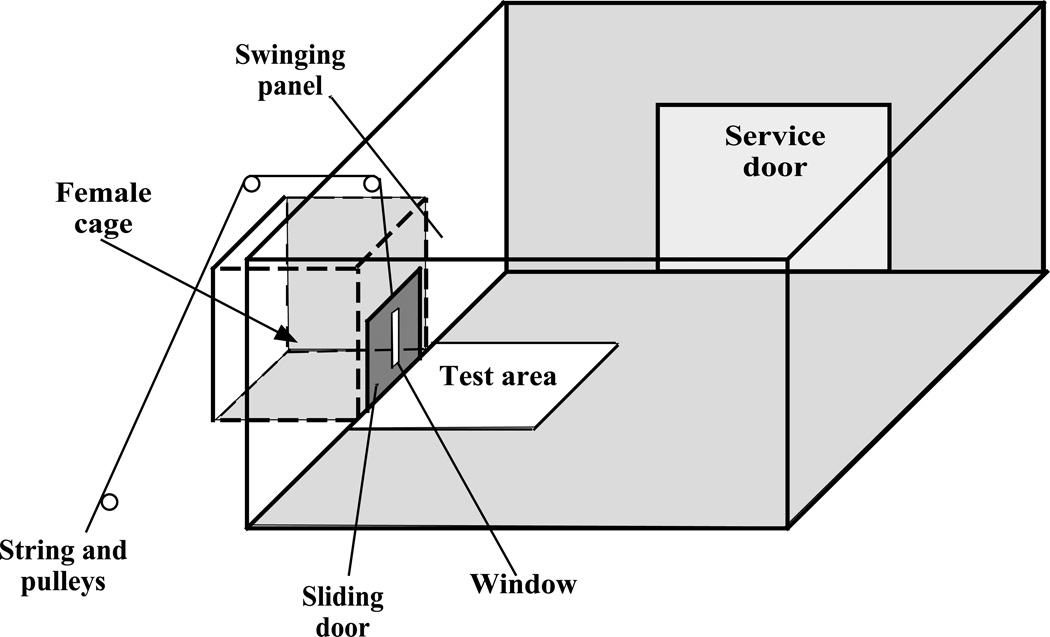
Figure 1.
Schematic representation of the two-compartment cage used to observe and measure the learned social proximity response, a form of appetitive sexual behavior, in male Japanese quail (Coturnix japonica). The experimental male is introduced into the larger compartment (90 × 90 cm) that is separated by a sliding door from an adjacent smaller cage (20 × 26 cm) containing the stimulus female. A small vertical “window” (1 × 15 cm) that can be closed by an opaque swinging panel (not shown) is located in the middle of the sliding door and provides the male with a limited visual access to the female. When the window is open, the male can see the female through the window only if he stands in the test area in front of the window. Modified from Balthazart et al. (2004).
One potential problem with the use of the social proximity response as a measure of appetitive sexual behavior is that the response is learned after reinforcement through copulation. This linkage prevents the assessment of appetitive male sexual behavior in subjects that fail to copulate. It is thus impossible to discern whether experimental manipulations that inhibit copulation also affect the appetitive sexual behavior per se or only the ability to learn a new response.
Rhythmic Contractions of the Cloacal Gland
Another behavioral procedure that allows measurement of appetitive sexual behavior in quail is based on studies conducted by Seiwert, Thompson, and Adkins-Regan and their colleagues. They showed that rhythmic contractions of the cloacal sphincter muscles (RCSM1) and cloacal gland are much more likely to occur in males, including sexually naïve males, when the stimulus animal is a female as compared to when it is a male (Seiwert et al. 1998; Thompson et al. 1998). The contractions produce a meringue-like foam that transfers to females during copulation and enhances the probability that the sperm will fertilize the egg (Seiwert et al. 1998). They are comparable to noncontact erections in mammalian species (Sachs 1995) in that they involve muscle movements that control effector organs associated with consummatory aspects of male sexual behavior in response to sensory cues emitted by a female. Although naïve males produce this response, the frequency of the contractions is modulated by the birds’ past experience. We observed, for example, that RCSM frequency decreases progressively during successive tests in which the male never attains access to the female, presumably due to a lack of sexual reinforcement (Cornil et al. 2006b). Frequency also differs significantly depending on males’ previous visual exposure to either a male or female (Cornil and Ball 2010).
It is notable that the production of RCSM can also be conditioned to an arbitrary stimulus. Males repeatedly exposed to a neutral CS (a terrycloth-covered foam block with an 8 × 2 cm pillar at one long end, vaguely evocative of a squatting female) paired with copulatory access to a female progressively learn to express RCSM at the simple view of the CS. This response is also steroid dependent, like the nonconditioned RCSM (Cornil et al. 2004; Holloway et al. 2005).
Studies of the Neuroendocrine Basis for Hormonal Activation of Reproduction
The ease with which quail exhibit sexual and social behaviors and lay eggs in the laboratory has made them an ideal species for studies of the neuroendocrine control of reproduction and sexual behavior (Balthazart and Ball 1998b). Such studies range across levels of analysis from the cellular/molecular to the behavioral. In this section we discuss specific features of Japanese quail that make them useful subjects in this research and we review significant findings from studies of the neuroendocrine control of reproduction. We begin with a look at studies of the sexual differentiation of male- and female-typical phenotypes.
External Markers of Endocrine Condition
Japanese quail display a number of morphological features that constitute a major practical advantage for research in behavioral (neuro)endocrinology and provide useful dependent measures of steroid hormone action. For example, the plumage of adult Japanese quail is sexually dimorphic: the chest feathers of males are uniformly dark brown while those of females are spotted in a mix of dark and light brown, and black and white contrasting marks on the male face are absent or much less contrasting on females. These distinctions provide a convenient way for sexing birds as soon as they are approximately 3 weeks old. Before that age, the sex of the gonads is relatively easily to determine by laparotomy, which yields an accurate result starting at day 9 of incubation (Schumacher et al. 1988). Before embryonic day 9, molecular sexing is a reliable method (Takada et al. 2006).
Quail also have a gland at the posterior end of the cloaca that produces a white foam important for egg fertilization (as mentioned above); it is present but underdeveloped in females as well as in juvenile males, castrated males, and males subjected to short days (a treatment that prevents testicular development, as discussed in the next section). The size of the gland, which is closely and positively correlated with both the size of the testes and testosterone plasma levels (Delville et al. 1984a; Ottinger and Brinkley 1978, 1979; Sachs 1967), is strictly androgen dependent and so provides an external marker of the circulating testosterone concentration (Sachs 1967): it grows markedly (from ±35 mm2 to 250–300 mm2) when subjects are exposed to high levels of plasma androgens (e.g., after exposure to long days or treatment of castrated males with exogenous testosterone).
Female-typical plumage is under the control of estrogens, so it is relatively easy to determine whether a female has been successfully ovariectomized without having to resort to additional surgery (i.e., exploratory laparotomy) or radioimmunoassays for the measurement of plasma steroids. After molting, a fully ovariectomized female grows male-typical plumage whereas a bird with a partial ovariectomy still displays the spotted chest plumage characteristic of females (Balthazart and Schumacher 1984; Gibson et al. 1975). It is also possible to visually ascertain circulating concentrations of estrogens in females on a shorter time scale: these steroids cause a significant enlargement of the cloacal diameter (from about 8–10 to 15 mm) and a change in the color of the surrounding skin from pink to black (Delville and Balthazart 1987).
Thus it is possible to assess the endocrine status (both androgen and estrogen concentrations) of male and female quail within seconds by a simple morphological inspection that does not require invasive procedures or complicated and time-consuming assays of plasma hormones. These morphological features also provide an easy indicator of the steroid concentration in a bird over a longer time period than is possible through radioimmunoassay analysis of a single plasma sample.
Photoperiod-Induced Changes in Reproductive Physiology and Behavior
In general, reproduction in Japanese quail is tightly controlled by the photoperiod (Robinson and Follett 1982). Adult birds remain sexually active throughout the year as long as they are exposed to a photoperiod simulating the long days of spring and summer (i.e., with at least 12–12½ hours of light per day, up to 16L:8D). Under these conditions, males’ testes remain large and fully active, producing sperm and secreting high concentrations of testosterone that activate male copulatory behaviors. Females also maintain a fully active hypothalamopituitary gonadal (HPG) axis and lay an egg (almost) every day throughout the year.
In contrast, juvenile quail raised under short days either do not develop sexually or show a very delayed maturation (Delville et al. 1984b), but a change from short to long days rapidly induces HPG activity as well as increases in both male testis size and female oviduct weight within 2 weeks from a few milligrams to several grams (Follett 1976). Conversely, if sexually active birds are transferred to short days (less than 12 hours of light per day; e.g., 8L:16D), HPG activity rapidly decreases in both sexes—females stop laying eggs and males no longer engage in copulatory interactions as their testes regress.
Although all quail are photoperiodic to some degree, some strains do not regress when transferred to short days or regress only transiently (Delville et al. 1985; Follett and Nicholls 1984; Robinson and Follett 1982); one study, for example, showed that cold temperatures in combination with short days were necessary to fully regress the HPG axis (Wada 1993). It has been suggested that the lack of response to short days is associated with small variations in plumage pattern (Oishi and Konishi 1983). In light of these reports, researchers should be aware that “photic castration” (functional castration by exposure to short days), which has been proposed as an easy way to obtain male and female quail that are reproductively quiescent and in which effects of exogenous sex steroid can be easily tested (Adkins 1973), might not work in some strains of quail; instead, surgical castration or ovariectomy may be necessary to obtain quiescent birds to test the action of steroids.
As with HPG levels, circulating concentrations of testosterone in male and estradiol in females are almost 10 times larger in long days than in short days (Delville et al. 1985; Laugier et al. 1978) and the behavioral effects of these massive endocrine changes are quite dramatic, especially in males. Copulatory behavior disappears within a few days after transfer to short days and is activated as rapidly after transfer to long days (Sachs 1969). Because these behavioral changes occur in almost all (90–95%) individuals in a given population, extensive work has been done on photoperiodic regulation of the termination of reproduction in quail (e.g., Follett and Pearce-Kelly 1990) and on the role of neuroendocrine systems in this regulation (e.g., the gonadotropin-releasing hormone neuronal system, Creighton and Follett 1987; the melatonin system, Kumar et al. 1993; and the more recently discovered gonadotropin-inhibiting hormone, Chowdhury et al. 2010; Tsutsui et al. 2000).
Activation of Male-Typical Sexual Behaviors by Gonadal Steroid Hormones
Even in relatively impoverished laboratory conditions, quail readily display a variety of reproductive behaviors that are quite sensitive to the activating effects of sex steroids. In the absence of a female, sexually motivated male quail produce a loud, broadband, two- to three-syllable vocalization, referred to as the “crow,” that is attractive to female quail (Goodson et al. 1997). When the male comes in contact with a female, he very reliably engages in a stereotyped sequence of copulatory behaviors that includes a pre- (and also often post-) copulatory display called strutting as well as the copulatory sequence sensu stricto during which he grabs the female’s neck feathers (neck grab; NG), attempts to mount her (mount attempts; MAs), eventually succeeds in mounting (mount; M), and finally apposes his cloaca to the female’s cloaca so that sperm transfer can occur (Adkins and Adler 1972; Hutchison 1978). Because it is often difficult, if not impossible, to ensure that successful copulation has taken place, researchers usually count only the cloacal contact movement (CCM) as a measure of attempts to achieve cloacal apposition.
All these behaviors are exquisitely sensitive to testosterone: they rapidly disappear after castration and return within 2 to 5 days after treatment with exogenous testosterone (Beach and Inman 1965; Sachs 1969). There is thus a very tight relationship between all aspects of male sexual behavior in quail and the presence of high circulating levels of sex steroids such as testosterone in the plasma—indeed, for behaviors such as CCM the presence of androgen almost seems to determine their occurrence. This connection distinguishes quail from most vertebrates, in which steroid hormones are thought to have probabilistic effects on the activation of behavior in the presence of a particular stimulus: they modify sensitivity of perception so that either the likelihood or the intensity of the animal’s behavioral response increases. In most cases, though, so-called hormone-dependent behaviors occur at some level of intensity and at some probability in the absence of the hormone (Nelson 2005). In contrast, castrated quail never exhibit most of the sexual behaviors described above, whereas they occur almost invariably in subjects exposed to high levels of steroids in the presence of a female. Such a high reliability of the behavioral effects of testosterone has been observed in only a few other cases (e.g., the lordosis reflex in female rats and the bow-coo display in male ring doves) and makes the quail an unusually useful model system to analyze the neuroendocrine mechanisms underlying sexual behaviors.
Some hormone-dependent behaviors, such as strutting and crowing, are directly activated by androgens (either testosterone or its androgenic metabolite 5α-dihydrotestosterone) that interact with androgen receptors at the cellular level, whereas activation of the copulatory sequence (NG-MA-M-CCM) depends mostly on estrogens produced by transformation in the brain of androgens into estrogens (e.g., 17β-estradiol), a process known as aromatization (Adkins et al. 1980; Balthazart et al. 2004; Schumacher and Balthazart 1983). There is, however, a synergistic action of androgens and estrogens on the copulatory sequence: low doses of 17β-estradiol or of 5α-dihydrotestosterone that are almost behaviorally inactive by themselves induce a substantial level of behavioral activity if given concomitantly to castrated males.
The differential activation of certain reproductive behaviors by androgens or estrogens means that behavioral indices of endocrine specificity are readily apparent in quail, making them useful in studies of the correlation between steroids and behavior.
Endocrine Specificity for the Activation of Appetitive and Consummatory Sexual Behaviors
Several studies have analyzed the steroid specificity of the activation of two sexually motivated responses that are used to assess appetitive sexual behavior: the learned social proximity response and RCSM. The former does not naturally develop in castrated quail but can be acquired through systemic treatment with both testosterone and an estrogen (e.g., 17β-estradiol or the synthetic estrogen diethylstilbestrol [DES]; Balthazart et al. 1995). This finding suggested that testosterone may act through its estrogenic metabolites to activate appetitive sexual behavior, a notion supported by the observation that daily injections of the antiestrogen tamoxifen could block expression of the learned proximity response (Balthazart et al. 1995). Similarly, injections of the aromatase inhibitor R76713 (racemic vorozole) progressively inhibit expression of the social proximity response, which declines to the low levels characteristic of castrates after about 2 weeks of treatment (Balthazart et al. 1997) (Figure 2).
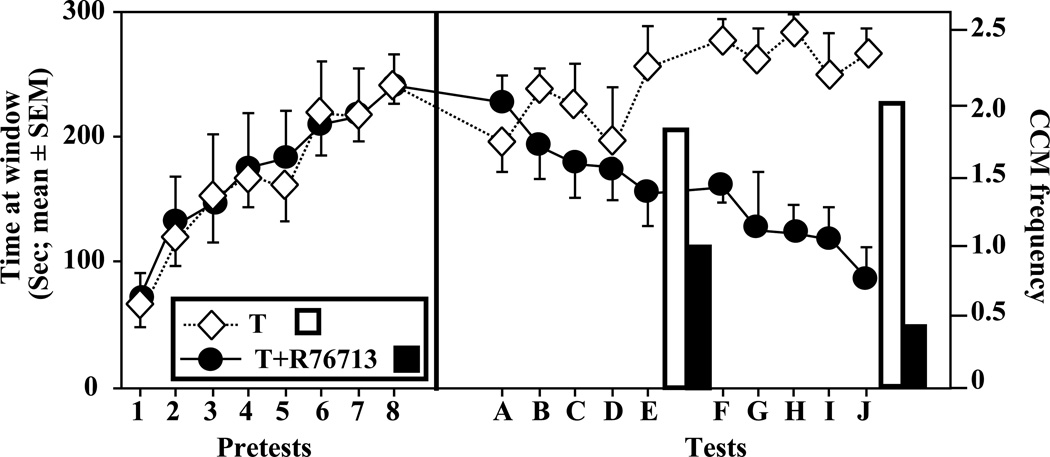
Figure 2.
Acquisition and expression of the learned social proximity response in castrated male Japanese quail (Coturnix japonica) that were treated either with testosterone in silastic capsules (T) or testosterone in association with the aromatase inhibitor R76713 (T + R76713). In the first phase of the experiment (left panel), birds underwent eight training tests during which they were treated with T and learned the social proximity response indicative of appetitive sexual behavior (i.e., they spent increasing amounts of time in front of the window). During the second phase of the experiment (right panel), access to the female was provided to males only on the 5th and 10th test (E and J) and therefore cloacal contact movement (CCM) could be recorded only during these tests (results represented by bar graphs). The 8 pretests and 10 tests were performed over a period of 10 days each. The aromatase inhibitor, injected only during the second phase of the experiments, produced a significant decrease in both appetitive and consummatory aspects of male sexual behavior, supporting the claim that estrogenic metabolites of T are necessary to activate and maintain both aspects of male-typical sexual behavior (redrawn from data in Balthazart et al. 1997). SEM, standard error of the mean
Importantly, during the period when these injections were administered, most of the tests of appetitive sexual behavior took place under conditions of extinction (i.e., the door providing physical access to the female remained closed); the subjects had free access to the female (the door was open) only in every fifth test (the 10 tests were performed daily over a 10 day period). In this way we were able to quantify the effects of the inhibition of aromatase without exposing the birds to the possible negative effects of complete inability to copulate with the female. This study clearly indicated that the expression of the learned social proximity response is activated by estrogenic metabolites of testosterone, quite independently of the activation of copulatory behavior.
The endocrine specificity of the RCSM seems to be similar. In response to the view of a female the RCSM frequency increases nearly 20-fold in castrated males treated with testosterone as compared to castrates that received no testosterone (Balthazart et al. 1998). However, the administration of both testosterone treatment and daily injections of R76713 leads to a progressive decline in female-elicited RCSM frequency (Taziaux et al. 2004).
These experiments demonstrate that appetitive and consummatory aspects of male sexual behavior in quail are activated by testosterone acting through its estrogenic metabolites. The endocrine specificity is therefore similar, if not identical, for the sexual differentiation and activation of both aspects of sexual behavior. This similarity might have been expected from an evolutionary point of view: it makes sense that a group of behaviors that must occur in sequence in order to culminate in successful reproduction have evolved to be controlled by the same endocrine mechanisms.
Neural Control of Appetitive and Consummatory Sexual Behaviors
The neural sites of action of testosterone on quail sexual behavior are well characterized. The preoptic area (POA1) contains a dense cluster of neurons called the medial preoptic nucleus (POM1). The volume of this nucleus is significantly larger in males than in females (Viglietti-Panzica et al. 1986) and reflects circulating testosterone concentrations—it decreases after castration and is restored by treatments with exogenous testosterone (Panzica et al. 1987). This structure may thus be implicated in the testosterone-induced activation of male copulatory behavior, a notion confirmed by the experiments described below.
Electrolytic lesions of the POM, but not of the surrounding POA, completely suppress copulatory behavior activated in castrated males by silastic implants containing testosterone (Balthazart and Surlemont 1990). Conversely, stereotaxic implants filled with testosterone reliably activate all aspects of copulatory behavior in castrated males if their tip is located within the cytoarchitectonic boundaries of the POM but not if it is located in the adjacent POA (Balthazart and Surlemont 1990; Balthazart et al. 1992). Testosterone action in the POM is thus sufficient to activate copulatory behavior in adult male quail (provided adequate stimuli are present) even if these data do not rule out an action of this steroid at additional sites in the central nervous system (see Balthazart and Ball 2007 for detail). Similar experiments suggest that the two aspects of appetitive sexual behavior are, like copulation, controlled by testosterone action in the POA.
Lesions of the POM that abolished consummatory sexual behavior also markedly decreased both the expression of RCSM in males in response to the view of a sexually mature female and measures of the learned social proximity response (Balthazart et al. 1998). But the two components of male sexual behavior were differentially affected by lesions of different subregions of the POM. Lesions affecting primarily the caudal POM approximately at the level of the anterior commissure resulted in the complete inhibition of consummatory sexual behavior (with little or no decrease in the social proximity response), whereas damage to the rostral part of the POM selectively inhibited appetitive sexual behavior.
This anatomical specialization in the POM in the control of appetitive and consummatory sexual behavior was confirmed independently during a study of the induction of the immediate early gene fos resulting from the expression of either copulatory behavior or RCSM (Taziaux et al. 2006). Males that performed these behaviors and were then allowed to copulate freely with a female exhibited an increase in the FOS protein expression throughout the rostrocaudal area of the POM. In contrast, males that had only visual access to the female and in response expressed a large number of RCSM displayed increased FOS expression in the rostral POM only.
Together these data strongly support the idea that the preoptic region is important in the regulation of motivational as well as performance aspects of male sexual behavior but that there is at least a partial anatomical dissociation in the POM between structures involved in the control of both aspects of the behavior (Balthazart et al. 1998; Balthazart and Ball 2007).
Although testosterone action is necessary and sufficient to activate male sexual behavior in quail, this activation obviously also involves multiple other brain areas directly or indirectly connected to the POM (Figure 3; Ball and Balthazart 2004 for review), a key integrative area in all vertebrates that is part of a neural circuit still being described in birds (Ball and Balthazart 2004). Sensory inputs that convey information about the female must reach this nucleus. Quail primarily rely on visual and auditory information for the activation of male-typical sexual behavior, but inputs to the POM from the telencephalic areas that decode this information have not been definitively identified. A projection from the dorsal thalamus to the POM could relay some visual information but probably does not affect control of sexual behavior since at the thalamic level visual information is likely in too early a stage of processing to be used to effectively interpret female behavior. But the POM and another aromatase-rich area, the medial part of the stria terminalis, are bidirectionally connected and receive inputs from the nucleus taeniae of the amygdala, a recipient of olfactory inputs. Recent work has suggested that olfactory signals may contribute to the regulation of copulatory behavior in quail (Taziaux et al. 2008b); further research is warranted to analyze the specific role of these connections.
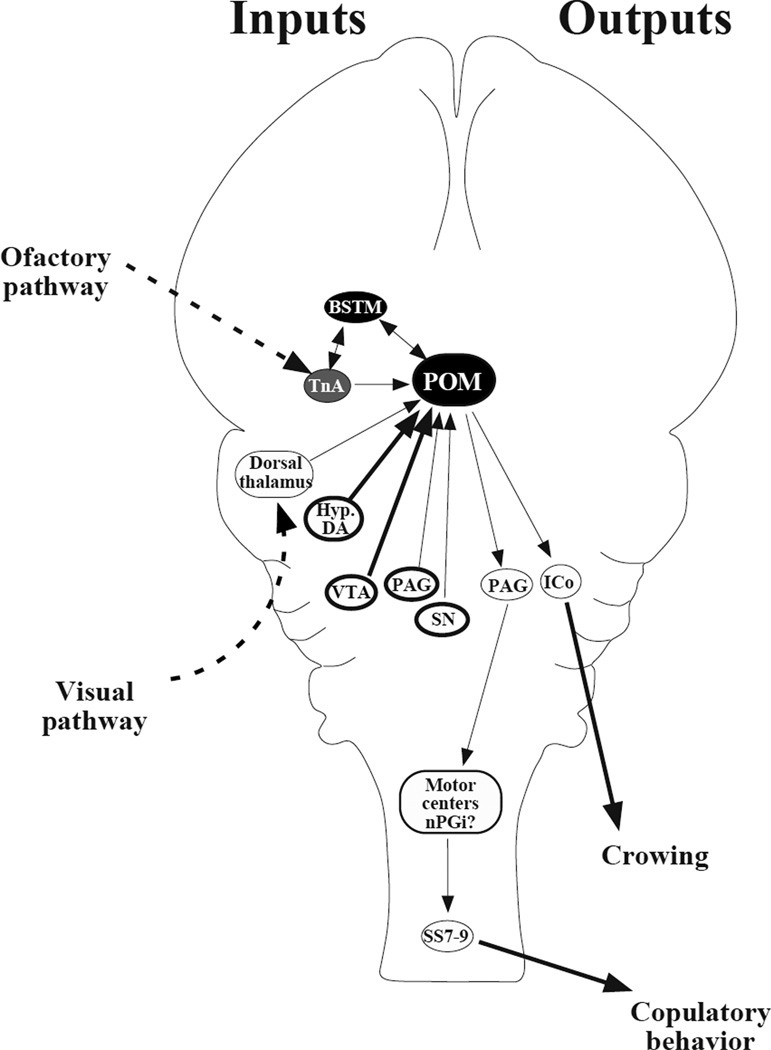
Figure 3.
Schematic view of the neural circuit controlling male sexual behavior in Japanese quail (Coturnix japonica). The aromatase-rich (black filling) medial preoptic nucleus (POM) and medial part of the bed nucleus of the stria terminalis (BSTM) are bidirectionally connected and critical to the activation of sexual behavior. Both receive inputs from the nucleus taeniae of the amygdala (TnA) that contains a small number of aromatase-expressing neurons (gray filling) and may relay olfactory information. We do not know how visual and auditory inputs that reach the telencephalon influence the POM but some visual information may reach the POM from the dorsal thalamus. The POM also receives dopaminergic inputs (thick lines) from dopaminergic cells (nuclei surrounded by a thick line) in the ventral tegmental area (VTA), the hypothalamus (Hyp.DA), and, to a lesser extent, the periaqueductal gray (PAG) and substantia nigra (SN). Outputs from POM that may contribute to the control of reproductive behaviors include a projection to the mesencephalic nucleus intercollicularis (ICo) and a projection specifically from the aromatase-positive neurons that reaches the PAG. In mammals the PAG projects to the nucleus paragigantocellularis (nPGi), which in turn projects to spinal motoneurons; this projection has not been identified in quail but motoneurons located in synsacral segments 7 to 9 (SS7-9) project directly to the muscles of the cloacal gland (Seiwert and Adkins-Regan 1998). Based on data from Absil et al. 2001a; Balthazart et al. 1994; Balthazart and Absil 1997; Carere et al. 2007; for more detail, Ball and Balthazart 2004.
The POM also receives complex modulatory inputs from various neurotransmitter systems (for detailed reviews, Absil et al. 2001a; Panzica et al. 1996b, 1997). Prominent dopaminergic inputs to this nucleus originate mostly from the ventral tegmental area and from dopaminergic cell groups in the hypothalamus, with a minor contribution from the periaqueductal gray and the substantia nigra (Balthazart and Absil 1997). Dopaminergic activity (as assessed by in vivo microdialysis) changes in the POM during sexual interactions in male quail (Kleitz-Nelson et al. 2010), and pharmacological experiments have demonstrated that, as in mammals, this activity plays a key role in the activation of sexual behavior (for review, Absil et al. 2001a; Balthazart et al. 2002).
Finally, to modulate behavior, outputs from the POM must connect to motor and premotor centers. Light projections to the mesencephalic nucleus intercollicularis have been identified by tract tracing studies (Balthazart et al. 1994; Balthazart and Absil 1997) and may activate courtship vocalizations. A robust and sexually differentiated (males > females) projection from the POM to the periaqueductal gray is probably important in the regulation of copulatory behavior specifically (Absil et al. 2001b; Carere et al. 2007).
A lot of work remains to be done to yield an integrated view of the neural circuits that control sexual behavior, and quail are an excellent animal model for such studies. Their behavior is readily expressed in laboratory conditions, it is highly sensitive to activation by steroids, and stereotaxic surgery can easily be performed in this species using a rat or mouse stereotaxic frame with only minor adaptations (e.g., to the bar used to block the upper mandible).
Correlation between Extensive Neural Plasticity and Changes in Endocrine State
Endocrine and behavioral modifications that result from photoperiod-induced changes in the steroid milieu are associated with a prominent degree of neural plasticity. As noted above, the POM is a key site of testosterone action for the activation of male copulatory behavior (in vertebrates in general, Hull et al. 2005; in quail: Balthazart et al. 1998; Balthazart and Ball 2007). In addition, the overall volume of the POM shows a remarkable plasticity in response to steroids: it is 25–30% larger in birds exposed to high concentrations of testosterone (males exposed to long days, castrates treated with exogenous testosterone) than in subjects exposed to low concentrations (birds in short days, castrates). It is also larger in males than in females (see Panzica et al. 1996b for review). Furthermore, recent experiments in our lab showed that these volumetric changes can occur very rapidly in response to steroid treatment (Charlier et al. 2005, 2008). In particular, the treatment of castrated males with testosterone induced within 24 hours a significant increase of the POM volume as identified by the dense population of aromatase-immunoreactive cells (Charlier et al. 2008). No adult neurogenesis has been detected in the avian hypothalamus-preoptic area; detailed histological studies demonstrated that the volume increase was related to increased cell spacing and cell size (Panzica et al. 1996a,b). In the short term, changes in the ratio of extra- vs. intracellular water content may also be involved, as indicated by recent in vivo magnetic resonance imaging studies (Van Der Linden et al. 2006). Additional research may shed light on the cellular mechanisms mediating the unusually rapid neuronal plasticity in the quail POA.
Testosterone Aromatization: Its Control and Function
Given the critical importance of testosterone aromatization for the control of appetitive and consummatory sexual behavior in quail (Balthazart et al. 2004), a large number of studies have focused on analysis of both the neuroanatomical distribution of the enzyme and the controls of its activity.
Quail, like many other birds, display a much higher brain aromatase activity (AA1) than rats or mice. AA is undetectable by standard assays in untreated adult mice (Bakker et al. 2004), whereas it is relatively easy to measure in microdissected quail brain regions (Schumacher and Hutchison 1986) and even in specific nuclei dissected using the Palkovits punch technique (Balthazart et al. 1990c; Schumacher and Balthazart 1987). These methods have enabled localization of the highest levels of AA in the medial POA and in the periventricular hypothalamus from the ventromedial nucleus to the infundibular level (Schumacher and Balthazart 1987). Studies have also shown that AA in the POA is induced by testosterone: in castrated males after 2 days of treatment with testosterone, it increases from a basal level to levels typical of adult sexually mature males (Balthazart et al. 1990b; Schumacher and Hutchison 1986).
This high enzymatic activity is quite probably related to a higher concentration of aromatase protein in the quail as compared to the rodent brain and is presumably why it has been reasonably easy to design antibodies and immunohistochemical procedures that permit visualization of the enzyme in the quail brain. We showed in 1990 that antibodies to human placental aromatase enabled the identification of aromatase-immunoreactive (ARO-ir) neurons in the quail brain (Balthazart et al. 1990a). A few years later, after the cloning of the corresponding messenger RNA (mRNA), the development of antibodies to a specific quail aromatase recombinant facilitated an even more accurate neuroanatomical localization of the enzyme in quail (Foidart et al. 1995). With these tools it was possible to analyze very precisely the neuroanatomical distribution of the enzyme and its control by steroids, and to define (by double-labeling studies) the neurochemical phenotype of ARO-ir cells and the nature of their afferent inputs (for detailed reviews, Absil et al. 2001a; Balthazart and Ball 1998a; Balthazart et al. 2004).
The sequencing of the quail aromatase mRNA (Balthazart et al. 2003; Harada et al. 1992) enabled the design and use of specific primers to quantify this mRNA by either reverse transcription polymerase chain reaction (RT-PCR) or in situ hybridization. These methods in turn made it possible to study aromatase in the quail brain at three different levels: the concentration of its mRNA, the concentration of the enzymatic protein assessed semiquantitatively by counting in standardized conditions the numbers of ARO-ir cells in specific brain areas, and the level of enzymatic activity quantified in in vitro assays, either the product formation assay (Schumacher and Hutchison 1986) or the tritiated water assay (Baillien and Balthazart 1997). These three approaches demonstrated that testosterone, the substrate of aromatase, increases AA mostly by increasing the concentration of the enzyme and its mRNA, suggesting that the steroid directly regulates transcription of the aromatase gene (Figure 4) (for review, Absil et al. 2001a; Balthazart and Ball 1998a).
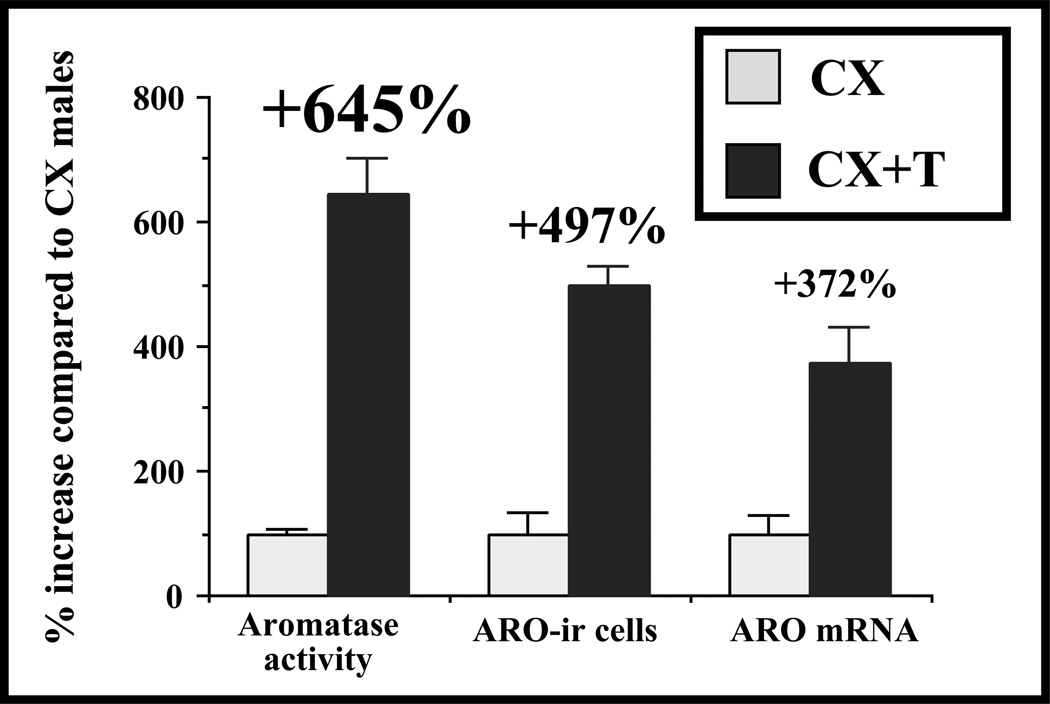
Figure 4.
Testosterone (T) increases the aromatase messenger RNA (ARO mRNA) concentrations, the number of aromatase-immunoreactive (ARO-ir) cells, and the aromatase activity in the preoptic area of castrate (CX) male Japanese quail (Coturnix japonica). These effects of T have approximately the same magnitude for the three dependent variables even if the effect increases slightly from the transcriptional level (mRNA; +372%) to the protein (ARO-ir cells; +497%) and to the enzymatic activity (+645%). One implication of these data is that aromatase activity may be regulated by mechanisms acting at the post-transcriptional level in addition to the major effect of the steroid on the transcription of the corresponding gene. Redrawn from data in Balthazart and Foidart (1993).
The steroid specificity of these effects can also be assessed thanks to the high level of aromatase expression in the quail brain. Effects of testosterone at the mRNA, protein, and enzymatic levels are almost completely reproducible through estrogen treatment, whereas nonaromatizable androgens such as 5α-dihydrotestosterone had almost no effect. As observed in the activation of copulatory behavior, there is also a clear synergism between androgens and estrogens in the activation of aromatase: inactive doses of 5α-dihydrotestosterone markedly increase the effects of estrogens on levels of aromatase mRNA, protein, and activity (Absil et al. 2001a).
Rapid Variation of Brain Aromatase Activity and Effects of Locally Produced Estrogens
Studies described in the previous section demonstrated that testosterone-induced changes in preoptic AA are paralleled by changes in enzyme concentration that are largely mediated by the transcriptional action of the steroid. However, the apparent magnitude of this effect increases with the progression from DNA transcription to enzyme activity, suggesting that testosterone may also regulate enzymatic activity by other means—for example, by affecting the translation of the mRNA or directly modulating the activity of enzymatic molecules.
In vitro studies have shown that AA measured in preoptic-hypothalamic homogenates is markedly inhibited within 10 to 15 minutes after the exposure of homogenates to elevated but physiological concentrations of adenosine triphosphate, magnesium, and calcium (Ca2+). This inhibition is prevented either by compounds that chelate divalent ions or by kinase inhibitors, indicating that it is caused by calcium-dependent phosphorylation processes (Balthazart et al. 2001, 2003); protein phosphorylation is widely used to modulate enzymatic activity or receptor affinity (e.g., Nestler and Greengard 1999). Thus well-characterized methods are available to analyze these regulations of enzymatic activity and eventually manipulate them both in vitro and in vivo.
Rapid (within 5 min) AA inhibition was detected in quail preoptic-hypothalamic explants in which the cellular integrity of the neurons and a large part of their connectivity are maintained after exposure to either conditions that increase intracellular Ca2+ concentration (e.g., a potassium-induced depolarization) or thapsigargin, a drug that mobilizes intracellular pools of Ca2+ (Balthazart et al. 2001). Exposure to glutamatergic agonists (the amino acid AMPA and kainate combined, and, to a lesser extent, N-methyl D-aspartate), but not γ-aminobutyric acid, similarly resulted in rapid inhibition of AA (Balthazart et al. 2006) (Figure 5).
Figure 5.
Rapid changes in aromatase activity (AA) in preoptic-hypothalamic explants of Japanese quail (Coturnix japonica) in which the cumulative enzymatic activity is measured every 5 minutes based on the release of tritiated water from tritiated androstenedione. Enzymatic activity was measured in paired hypothalamic explants incubated in vitro in which one explant was exposed for 10 minutes (between 20 [up arrow] and 30 [down arrow] min) (A) to thapsigargin, a lactone that mobilizes intracellular calcium ion (Ca2+) stores, (B) to kainate, or (C) to kainate associated or not with a preincubation with the non-NMDA glutamate antagonist NBQX. All data are expressed in percentage of basal release, defined as the activity during the period preceding the experimental manipulation (15–20 min). All data are means ± standard error of the mean (SEM). Redrawn from data in Balthazart et al. (2001, 2006).
These rapid changes in AA presumably also modulate the local estrogen brain concentration and recent work suggests that they have a significant impact on the expression of male sexual behavior; indeed, an acute injection of estradiol facilitates within 5 to 15 minutes the expression of both appetitive and consummatory aspects of male sexual behavior (Cornil et al. 2006a). Conversely, a single systemic injection of a large dose of Vorozole, a nonsteroidal aromatase inhibitor, quickly (15–30 min) reduces most aspects of sexual behavior in sexually active male quail (Cornil et al. 2006b).
These studies were greatly facilitated by the fact that male sexual behavior in quail is remarkably dependent on the presence of steroid hormones and that AA is present at very high levels in the quail brain. It is therefore relatively easy to monitor AA both with assays of brain homogenates and in hypothalamic explants maintained in vitro.
Studies of the Sexual Differentiation of Brain and Behavior
The expression of copulatory behavior is highly differentiated between male and female quail (Adkins 1975), 1978; Adkins-Regan et al. 1982). The behavior is readily observed in males tested in laboratory conditions but is never exhibited by females even after treatment with amounts of testosterone that are behaviorally effective in males. In fact, plasma testosterone levels, although significantly higher in male than in female quail, overlap extensively in the two sexes (Balthazart et al. 1987) and should be sufficient to activate male-typical copulatory behavior in at least those females that are at the high end of the natural range of concentrations (Balthazart et al. 1996).4 Together these data indicate that male and female quail differ markedly in the way their brain responds to testosterone. We focus here on the sexual differentiation of male behavior.
Because quail readily breed in the lab, it has been relatively easy to analyze the mechanisms that control the sexual differentiation of behavior during ontogeny. Recent work has clearly demonstrated that the sex difference in response to testosterone develops after females’ exposure to ovarian estrogens during a period of development that ends on day 12 of incubation (Adkins 1975); Adkins-Regan et al. 1982; Balthazart and Adkins-Regan 2002). Injections of estradiol benzoate into male embryos demasculinize adult copulatory behavior, whereas injections of an aromatase inhibitor in female embryos block demasculinization and lead to adult females that show the full range of male sexual behavior if exposed to exogenous testosterone (Balthazart et al. 2002, 2009). The critical role of estrogen in female demasculinization is also supported by the observation that the concentration of estradiol is much higher in female than in male quail embryos during the period when estrogens are thought to exert their organizing action (between the 9th day of incubation and hatching), according to experiments in which exogenous steroids were administered to males (Ottinger et al. 2001; Schumacher et al. 1988).
Taken together, these studies indicate that the influence of estrogens during embryonic life blocks the subsequent ability of female quail to perform masculine sexual behavior and that estrogens are presumably the only hormonal stimulus responsible for this demasculinization (since the blockade of their synthesis results in adult females with a behavioral phenotype indistinguishable from that of normal males). This represents a pattern of differentiation that appears to be the opposite of the mammalian pattern, in which steroids secreted by the male induce differentiation; male rodents, for example, are masculinized and defeminized by testes-produced testosterone, which exerts most of its effects on the brain after being aromatized into an estrogen (Goy and McEwen 1980). Bearing in mind, however, that the heterogametic sex is the male in mammals (XY) whereas it is the female in birds (ZW), it is possible to formulate a single rule that is applicable to both birds (or at least quail) and mammals: differentiation results from steroid action in the heterogametic sex.
Other aspects of sexual behavior, however, such as the RCSM in males exposed to females, do not appear to be sexually differentiated, in the organizational sense, in quail, as they can be elicited in adult females after treatment with testosterone (Adkins-Regan 2009; Adkins-Regan and Leung 2006).
The magnitude of the sex difference relating to testosterone action on male-typical copulatory behavior (essentially an all or none phenomenon) and the associated ability to fully sex-reverse the behavioral phenotype of males and females (by treating them in ovo with estradiol or an aromatase inhibitor, respectively) provide an outstanding experimental system to analyze the brain mechanisms that mediate this sexually differentiated expression of behavior in adult quail. A large number of neuroanatomical and neurochemical sex differences have been identified in the quail brain but most appear to relate to a differential activation by testosterone in males and females—when both sexes are exposed to the same endocrine conditions, these differences disappear (see Balthazart et al. 1996, 2009).
The vasotocinergic system seemed to be an exception to this rule. The density of vasotocin-immunoreactive (VT-ir) fibers is higher in males than in females in several brain areas involved in the regulation of reproduction. This difference is partly controlled by circulating concentrations of testosterone in the adult: castration decreases and testosterone treatment restores the density of the relevant VT-ir fibers. Interestingly, however, ovariectomized females treated with the same amount of testosterone do not develop the dense VT-ir fiber network typical of males (for review, Panzica et al. 1996b, 1997, 2001). This finding suggested that the sex difference in vasotocinergic system responsiveness to testosterone is organized during ontogeny, and this was indeed shown to be the case: treatment of male embryos with estrogens demasculinized the response of vasotocin to testosterone in adulthood (i.e., the adult estradiol-treated males did not develop a dense VT-ir network in response to testosterone). Conversely, blocking estrogen secretion in female embryos during incubation by injection of an aromatase inhibitor produced male-like females that in adulthood developed a dense network of VT-ir fibers in response to testosterone treatment (Panzica et al. 1998).
But this peptidergic sex difference—organized, like copulatory behavior, by the prenatal action of estrogens—does not seem by itself to explain sex differences in behavior. Indeed, treatment with vasotocin inhibits while treatment with a vasotocin antagonist stimulates copulatory behavior in adult castrated males (Castagna et al. 1998). It is beyond the scope of this review to discuss the possible explanations of this mismatch between sex difference or control of VT-ir fibers and behavioral effects of VT or its antagonist (for more detail, Castagna et al. 1998); we simply acknowledge here that available data on neurochemical sex differences in quail do not explain the prominent sex differences in behavior. More recent work points to differences in connectivity between brain nuclei as a possible basis for the organized sex differences in the ability of testosterone to activate male-typical behavior (in rats: De Vries and Simerly 2002; Simerly 2002; in quail: Carere et al. 2007).
Practical Issues Concerning Quail Husbandry
The breeding and maintenance of Japanese quail in the laboratory are quite easy. Under long-day conditions quail reach sexual maturity by 7 to 8 weeks of age, start reproducing, and produce fertile eggs (Huss et al. 2008). They are also smaller than chickens and so are easier to keep in laboratory settings. In addition, quail are very resistant to infections and colonies can be maintained for years without any outbreak of contagious disease (Huss et al. 2008; authors’ observations). However, based on personal experience, we recommend careful assessment of new birds that arrive in a facility to ensure that they are healthy and not a danger to the birds already present. Some infections can be quite serious; for example, the arrival of birds infected with Dermanyssus gallinae, a parasite that causes severe anemia, could have severe health consequences for the existing birds (JB, personal observation).
Several useful publications provide practical advice for maintaining quail in scientific laboratories (e.g., Huss et al. 2008; Padgett and Ivey 1959; Woodard et al. 1973), and new recommendations from the European Union on the housing of quail in laboratory conditions should be consulted especially by scientists working in Europe.5 These guidelines include suggestions about group housing strategies that facilitate healthy social development and minimize both intermale and male-female aggression.
Conclusion
An important trend in the life sciences that has contributed to development of the field of systems biology is recognition of the importance of interrelationships among levels of analysis (Bateson 2005); for example, cellular/molecular studies can contribute to knowledge of organ systems and thus advance understanding of complex organism–level phenomena such as behavior and cognition. The ability to make connections among these levels requires appropriate experimental subjects. For Japanese quail and other avian species there is a wealth of information about embryology, physiology, and behavior. Quail in particular have been the subject of extensive behavioral studies as well as mechanistic studies of both the regulation of these behaviors and biological development. This wealth of information provides an excellent platform for future studies that can incorporate sophisticated genomic and proteomic approaches.
One obvious limitation to studies in quail was until recently the relative lack of genetic information for this species. The recent sequencing of the chicken genome, a species closely related to quail, will be of great help (Consortium IGS 2004; Ellegren 2005; Replogle et al. 2008).
Thus quail have a bright future as a species that will contribute to the next generation of fundamental discoveries in the biomedical and behavioral sciences, especially in the field of sexual behavior, sexual learning, sexual differentiation, and neural plasticity. As illustrated elsewhere in this issue, other avian species also provide outstanding model systems for the analysis of related topics such as vocal learning, brain plasticity (e.g., generation and learning of complex sequential behaviors;), and in general brain-behavior relationships.
Acknowledgments
The research described in this paper was supported by a grant from the National Institute of Mental Health (NIMH; R01 MH50388) to GFB and JB and by grants from the Belgian National Fund of Scientific Research (FRFC; N° 2.4537.9) and the University of Liège (Crédits spéciaux) to JB. We thank Dr. P.V. Drion (University of Liège) for useful comments on an earlier version of this manuscript.
Footnotes
Abbreviations used in this article: AA, aromatase activity; CS, conditioned stimuli; POA, preoptic area; POM, medial preoptic nucleus; RCSM, rhythmic contractions of the cloacal sphincter muscles
An arbitrary CS has no connection to the stimulus that elicits the unconditional response (e.g., a bell substituting for food that would normally elicit a salivary response); an ecologically relevant CS would normally be associated with the unconditional stimulus in the real world (e.g., visual exposure to a female that normally precedes the opportunity to copulate with her).
We describe these brain areas and their role in the control of male sexual behavior under Neural Control of Appetitive and Consummatory Sexual Behaviors.
In contrast, it is possible, with an appropriate estrogen treatment, to elicit in male quail the receptive sexual behavior (squatting) that is characteristic of females (Adkins 1975; Adkins and Adler 1972).
Contributor Information
Gregory F. Ball, Department of Psychological and Brain Sciences at Johns Hopkins University in Baltimore, Maryland..
Jacques Balthazart, Chargé de Cours in Behavioral Neuroendocrinology at the University of Liège, GIGA Neurosciences in Belgium..
References
- Absil P, Baillien M, Ball GF, Panzica G, Balthazart J. The control of preoptic aromatase activity by afferent inputs in Japanese quail. Brain Res Rev. 2001a;37:38–58. doi: 10.1016/s0165-0173(01)00122-9. [DOI] [PubMed] [Google Scholar]
- Absil P, Riters LV, Balthazart J. Preoptic aromatase cells project to the mesencephalic central gray in the male Japanese quail (Coturnix japonica) Horm Behav. 2001b;40:369–383. doi: 10.1006/hbeh.2001.1702. [DOI] [PubMed] [Google Scholar]
- Adkins EK. Functional castration of the female Japanese quail. Physiol Behav. 1973;10:619–621. doi: 10.1016/0031-9384(73)90232-1. [DOI] [PubMed] [Google Scholar]
- Adkins EK. Hormonal basis of sexual differentiation in the Japanese quail. J Comp Physiol Psychol. 1975;89:61–71. doi: 10.1037/h0076406. [DOI] [PubMed] [Google Scholar]
- Adkins EK. Effects of diverse androgens on the sexual behavior and morphology of castrated male quail. Horm Behav. 1977;8:201–207. doi: 10.1016/0018-506x(77)90037-x. [DOI] [PubMed] [Google Scholar]
- Adkins EK. Sex steroids and the differentiation of avian reproductive behavior. Amer Zool. 1978;18:501–509. [Google Scholar]
- Adkins EK, Adler NT. Hormonal control of behavior in the Japanese quail. J Comp Physiol Psychol. 1972;81:27–36. doi: 10.1037/h0033315. [DOI] [PubMed] [Google Scholar]
- Adkins EK, Nock BL. Behavioral responses to sex steroids of gonadectomized and sexually regressed quail. J Endocrinol. 1976;68:49–55. doi: 10.1677/joe.0.0680049. [DOI] [PubMed] [Google Scholar]
- Adkins EK, Boop JJ, Koutnik DL, Morris JB, Pniewski EE. Further evidence that androgen aromatization is essential for the activation of copulation in male quail. Physiol Behav. 1980;24:441–446. doi: 10.1016/0031-9384(80)90233-4. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E. Is the snark still a boojum? The comparative approach to reproductive behavior. Neurosci Biobehav Rev. 1990;14:243–252. doi: 10.1016/s0149-7634(05)80224-6. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E. Hormones and sexual differentiation of avian social behavior. Dev Neurosci. 2009;31:342–350. doi: 10.1159/000216545. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E, Leung CH. Hormonal and social modulation of cloacal muscle activity in female Japanese quail. Physiol Behav. 2006;87:82–87. doi: 10.1016/j.physbeh.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E, MacKillop EA. Japanese quail (Coturnix japonica) inseminations are more likely to fertilize eggs in a context predicting mating opportunities. Proc Biol Sci. 2003;270:1685–1689. doi: 10.1098/rspb.2003.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins-Regan EK, Pickett P, Koutnik D. Sexual differentiation in quail: Conversion of androgen to estrogen mediates testosterone-induced demasculinization of copulation but not other male characteristics. Horm Behav. 1982;16:259–278. doi: 10.1016/0018-506x(82)90026-5. [DOI] [PubMed] [Google Scholar]
- Baillien M, Balthazart J. A direct dopaminergic control of aromatase activity in the quail preoptic area. J Steroid Biochem Mol Biol. 1997;63:99–113. doi: 10.1016/s0960-0760(97)00080-0. [DOI] [PubMed] [Google Scholar]
- Bakker J, Baillien M, Honda S, Harada N, Balthazart J. Relationships between aromatase activity in the brain and gonads and behavioural deficits in homozygous and heterozygous aromatase knockout mice. J Neuroendocrinol. 2004;16:483–490. doi: 10.1111/j.1365-2826.2004.01191.x. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol Behav. 2004;83:329–346. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. How useful is the appetitive and consummatory distinction for our understanding of the neuroendocrine control of sexual behavior? Horm Behav. 2008;53:307–311. doi: 10.1016/j.yhbeh.2007.09.023. author reply 315-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Absil P. Identification of catecholaminergic inputs to and outputs from aromatase-containing brain areas of the Japanese quail by tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Comp Neurol. 1997;382:401–428. [PubMed] [Google Scholar]
- Balthazart J, Adkins-Regan E. Sexual differentiation of brain and behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. vol 4. San Diego: Academic Press; 2002. pp. 223–301. [Google Scholar]
- Balthazart J, Ball GF. New insights into the regulation and function of brain estrogen synthase (aromatase) TINS. 1998a;21:243–249. doi: 10.1016/s0166-2236(97)01221-6. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. The Japanese quail as a model system for the investigation of steroid-catecholamine interactions mediating appetitive and consummatory aspects of male sexual behavior. Ann Rev Sex Res. 1998b;9:96–176. [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Topography in the preoptic region: Differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Foidart A. Brain aromatase and the control of male sexual behavior. J Steroid Biochem Mol Biol. 1993;44:521–540. doi: 10.1016/0960-0760(93)90256-v. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Schumacher M. Testosterone metabolism and sexual differentiation in quail. In: Balthazart J, Pröve E, Gilles R, editors. Hormones and behaviour in higher vertebrates. Berlin: Springer-Verlag; 1983. pp. 237–260. [Google Scholar]
- Balthazart J, Schumacher M. Estradiol contributes to the postnatal demasculinization of female Japanese quail (Coturnix coturnix japonica) Horm Behav. 1984;18:287–297. doi: 10.1016/0018-506x(84)90017-5. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Surlemont C. Copulatory behavior is controlled by the sexually dimorphic nucleus of the quail POA. Brain Res Bull. 1990;25:7–14. doi: 10.1016/0361-9230(90)90246-v. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Massa R, Negri-Cesi P. Photoperiodic control of testosterone metabolism, plasma gonadotropins, cloacal gland growth, and reproductive behavior in the Japanese quail. Gen Comp Endocrinol. 1979;39:222–235. doi: 10.1016/0016-6480(79)90227-2. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Delville Y, Sulon Y, Hendrick JC. Plasma levels of luteinizing hormone and of five steroids in photostimulated, castrated and testosterone-treated male and female Japanese quail (Coturnix coturnix japonica) Gen Endocrinol (Life SciAdv) 1987;5:31–36. [Google Scholar]
- Balthazart J, Foidart A, Harada N. Immunocytochemical localization of aromatase in the brain. Brain Res. 1990a;514:327–333. doi: 10.1016/0006-8993(90)91428-j. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Hendrick JC. The induction by testosterone of aromatase activity in the preoptic area and activation of copulatory behavior. Physiol Behav. 1990b;47:83–94. doi: 10.1016/0031-9384(90)90045-6. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Schumacher M, Evrard L. Sex differences and steroid control of testosterone-metabolizing enzyme activity in the quail brain. J Neuroendocrinol. 1990c;2:675–683. doi: 10.1111/j.1365-2826.1990.tb00464.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Surlemont C, Harada N. Aromatase as a cellular marker of testosterone action in the preoptic area. Physiol Behav. 1992;51:395–409. doi: 10.1016/0031-9384(92)90158-x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Dupiereux V, Aste N, Viglietti-Panzica C, Barrese M, Panzica GC. Afferent and efferent connections of the sexually dimorphic medial preoptic nucleus of the male quail revealed by in vitro transport of DiI. Cell Tissue Res. 1994;276:455–475. doi: 10.1007/BF00343944. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Reid J, Absil P, Foidart A, Ball GF. Appetitive as well as consummatory aspects of male sexual behavior in quail are activated by androgens and estrogens. Behav Neurosci. 1995;109:485–501. [PubMed] [Google Scholar]
- Balthazart J, Tlemçani O, Ball GF. Do sex differences in the brain explain sex differences in the hormonal induction of reproductive behavior? What 25 years of research on the Japanese quail tells us. Horm Behav. 1996;30:627–661. doi: 10.1006/hbeh.1996.0066. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Castagna C, Ball GF. Aromatase inhibition blocks the activation and sexual differentiation of appetitive male sexual behavior in Japanese quail. Behav Neurosci. 1997;111:381–397. [PubMed] [Google Scholar]
- Balthazart J, Absil P, Gérard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J Neurosci. 1998a;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol. 2001;13:61–71. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Interactions between aromatase (estrogen synthase) and dopamine in the control of male sexual behavior in quail. Comp Biochem Physiol [B] 2002;132:37–55. doi: 10.1016/s1096-4959(01)00531-0. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur J Neurosci. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: Slow and fast mechanisms of action. Physiol Behav. 2004;83:247–270. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Arnold AP, Adkins-Regan E. Sexual differentiation of brain and behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. vol 3. San Diego: Academic Press; 2009. pp. 1745–1787. [Google Scholar]
- Bateson P. Sexual imprinting and optimal outbreeding. Nature. 1978;273:659–660. doi: 10.1038/273659a0. [DOI] [PubMed] [Google Scholar]
- Bateson P. Preferences for cousins in Japanese quail. Nature. 1982;295:236–237. [Google Scholar]
- Bateson P. The return of the whole organism. J Biosci. 2005;30:31–39. doi: 10.1007/BF02705148. [DOI] [PubMed] [Google Scholar]
- Beach FA. Hormones and Behavior. New York: Paul B. Hoeber; 1948. [Google Scholar]
- Beach FA. Characteristics of masculine "sex drive". Nebraska Symposium on Motivation. 1956;4:1–32. [Google Scholar]
- Beach FA. Behavioral endocrinology: An emerging discipline. Am Sci. 1975;63:178–187. [PubMed] [Google Scholar]
- Beach FA. Historical origins of modern research on hormones and behavior. Horm Behav. 1981;15:325–376. doi: 10.1016/0018-506x(81)90001-5. [DOI] [PubMed] [Google Scholar]
- Beach FA, Inman NG. Effects of castration and androgen replacement on mating in male quail. Proc Natl Acad Sci U S A. 1965;54:1426–1431. doi: 10.1073/pnas.54.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carere C, Ball GF, Balthazart J. Sex differences in projections from preoptic area aromatase cells to the periaqueductal gray in Japanese quail. J Comp Neurol. 2007;500:894–907. doi: 10.1002/cne.21210. [DOI] [PubMed] [Google Scholar]
- Castagna C, Absil P, Foidart A, Balthazart J. Systemic and intracerebroventricular injections of vasotocin inhibit appetitive and consummatory components of male sexual behavior in Japanese quail. Behav Neurosci. 1998;112:233–250. doi: 10.1037//0735-7044.112.1.233. [DOI] [PubMed] [Google Scholar]
- Carere C, Ball GF, Balthazart J. Sex differences in projections from preoptic area aromatase cells to the periaqueductal gray in Japanese quail. J Comp Neurol. 2007;500:894–907. doi: 10.1002/cne.21210. [DOI] [PubMed] [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Inhibition of steroid receptor coactivator-1 blocks estrogen and androgen action on male sex behavior and associated brain plasticity. J Neurosci. 2005;25:906–913. doi: 10.1523/JNEUROSCI.3533-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Rapid action on neuroplasticity precedes behavioral activation by testosterone. Horm Behav. 2008;54:488–495. doi: 10.1016/j.yhbeh.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury VS, Yamamoto K, Ubuka T, Bentley GE, Hattori A, Tsutsui K. Melatonin stimulates the release of gonadotropin-inhibitory hormone by the avian hypothalamus. Endocrinology. 2010;151:271–280. doi: 10.1210/en.2009-0908. [DOI] [PubMed] [Google Scholar]
- Consortium IGS. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Ball GF. Effects of social experience on subsequent sexual performance in naïve male Japanese quail (Coturnix japonica) Horm Behav. 2010;57:515–522. doi: 10.1016/j.yhbeh.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Holloway KS, Taziaux W, Balthazart J. The effects of aromatase inhibition on testosterone-dependent conditioned rhythmic cloacal sphincter movements in male Japanese quail. Physiol Behav. 2004;83:99–105. doi: 10.1016/j.physbeh.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res. 2006a;166:110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm Behav. 2006b;49:45–67. doi: 10.1016/j.yhbeh.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton JA, Follett BK. Changes in gonadotrophin-releasing hormone and LH in Japanese quail during the first few days of photostimulation. J Endocrinol. 1987;113:419–422. doi: 10.1677/joe.0.1130419. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Simerly RB. Anatomy, development, and function of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. vol 4. San Diego: Academic Press; 2002. pp. 137–191. [Google Scholar]
- del Hoyo J, Elliott A, Sargatal J. Handbook of the Birds of the World. vol 2. Barcelona: Lynx Edition; 1994. [Google Scholar]
- Delville Y, Balthazart J. Hormonal control of female sexual behavior in the Japanese quail. Horm Behav. 1987;21:288–309. doi: 10.1016/0018-506x(87)90016-x. [DOI] [PubMed] [Google Scholar]
- Delville Y, Hendrick JC, Sulon J, Balthazart J. Testosterone metabolism and testosterone-dependent characteristics in Japanese quail. Physiol Behav. 1984a;33:817–823. doi: 10.1016/0031-9384(84)90053-2. [DOI] [PubMed] [Google Scholar]
- Delville Y, Sulon J, Balthazart J. Hormonal correlates of gonadal regression and spontaneous recovery in Japanese quail exposed to short day-lengths. Arch Int Physiol Bioch. 1985;93:123–133. doi: 10.3109/13813458509079598. [DOI] [PubMed] [Google Scholar]
- Delville Y, Sulon J, Hendrick JC, Balthazart J. Effect of the presence of females on the pituitary-testicular activity in male Japanese quail (Coturnix coturnix japonica) Gen Comp Endocrinol. 1984b;55:295–305. doi: 10.1016/0016-6480(84)90115-1. [DOI] [PubMed] [Google Scholar]
- Derégnaucourt S, Guyomarc'h J-C, Aebischer NJ. Hybridization between European quail Coturnix coturnix and Japanese quail Coturnix japonica. Ardea. 2002;90:15–21. [Google Scholar]
- Domjan M. Pavlovian conditioning: A functional perspective. Annu Rev Psychol. 2005;56:179–206. doi: 10.1146/annurev.psych.55.090902.141409. [DOI] [PubMed] [Google Scholar]
- Domjan M, Hall S. Determinants of social proximity in Japanese quail (Coturnix coturnix japonica): Male behavior. J Comp Psychol. 1986;100:59–67. [PubMed] [Google Scholar]
- Domjan M, Akins C, Vandergriff DH. Increased responding to female stimuli as a result of sexual experience: Tests of mechanisms of learning. Q J Exp Psychol. 1992;45B:139–157. doi: 10.1080/14640749208401014. [DOI] [PubMed] [Google Scholar]
- Domjan M, Blesbois E, Williams J. The adaptive significance of sexual conditioning: Pavlovian control of sperm release. Psychol Sci. 1998;9:411–415. [Google Scholar]
- Domjan M, Cusato B, Krause M. Learning with arbitrary versus ecological conditioned stimuli: Evidence from sexual conditioning. Psychon Bull Rev. 2004;11:232–246. doi: 10.3758/bf03196565. [DOI] [PubMed] [Google Scholar]
- Ellegren H. The avian genome uncovered. Trends Ecol Evol. 2005;20:180–186. doi: 10.1016/j.tree.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Farris HE. Classical conditioning of courting behavior in the Japanese quail, Coturnix coturnix japonica. J Exp Anal Behav. 1967;10:213–217. doi: 10.1901/jeab.1967.10-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foidart A, Reid J, Absil P, Yoshimura N, Harada N, Balthazart J. Critical re-examination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J Chem Neuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- Follett BK. Plasma follicle-stimulating hormone during photoperiodically induced sexual maturation in male Japanese quail. J Endocrinol. 1976;69:117–126. doi: 10.1677/joe.0.0690117. [DOI] [PubMed] [Google Scholar]
- Follett BK, Maung SL. Rate of testicular maturation, in relation to gonadotrophin and testosterone levels, in quail exposed to various artificial potoperiods and to natural daylengths. J Endocrinol. 1978;78:267–280. doi: 10.1677/joe.0.0780267. [DOI] [PubMed] [Google Scholar]
- Follett BK, Nicholls TJ. Photorefractoriness in Japanese quail: Possible involvement of the thyroid gland. J Exp Zool. 1984;232:573–580. doi: 10.1002/jez.1402320325. [DOI] [PubMed] [Google Scholar]
- Follett BK, Pearce-Kelly A. Photoperiodic control of the termination of reproduction in Japanese quail (Coturnix coturnix japonica) Proc R Soc Lond [Biol] 1990;242:225–230. doi: 10.1098/rspb.1990.0128. [DOI] [PubMed] [Google Scholar]
- Galef BG, White DJ. Evidence of social effects on mate choice in vertebrates. Behav Processes. 2000;51:167–175. doi: 10.1016/s0376-6357(00)00126-1. [DOI] [PubMed] [Google Scholar]
- Gallagher JE. Sexual imprinting: A sensitive period in Japanese quail (Coturnix coturnix japonica) J Comp Physiol Psychol. 1977;91:72–78. [Google Scholar]
- Gallagher JE. Sexual imprinting: Variables influencing the development of mate preference in Coturnix coturnix japonica. Behav Biol. 1978;24:484–491. [Google Scholar]
- Gibson WR, Follett BK, Gledhill B. Plasma levels of luteinizing hormone in gonadectomized Japanese quail exposed to short or to long daylengths. J Endocrinol. 1975;64:87–101. doi: 10.1677/joe.0.0640087. [DOI] [PubMed] [Google Scholar]
- Gledhill B, Follett BK. Diurnal variation and the episodic release of plasma gonadotrophins in Japanese quail during a photoperiodically induced gonadal cycle. J Endocrinol. 1976;71:245–257. doi: 10.1677/joe.0.0710245. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Adkins-Regan E. Playback of crows of male Japanese quail elicits female phonotaxis. Condor. 1997;99:990–993. [Google Scholar]
- Goy RW, McEwen BS. Sexual differentiation of the brain. Cambridge MA: MIT Press; 1980. [Google Scholar]
- Harada N, Yamada K, Foidart A, Balthazart J. Regulation of aromatase cytochrome p-450 (estrogen synthetase) transcripts in the quail brain by testosterone. Mol Brain Res. 1992;15:19–26. doi: 10.1016/0169-328x(92)90146-3. [DOI] [PubMed] [Google Scholar]
- Hilliard S, Nguyen M, Domjan M. One-trial appetitive conditionning in the sexual system. Psychonomic Bull Rev. 1997;4:237–241. doi: 10.3758/BF03209399. [DOI] [PubMed] [Google Scholar]
- Hinde RA. Interaction of internal and external factors in integration of canary reproduction. In: Beach FA, editor. Sex and Behavior. New York: John Wiley and Sons; 1965. pp. 381–415. [Google Scholar]
- Holloway KS, Balthazart J, Cornil CA. Androgen mediation of conditioned rhythmic cloacal sphincter movements in Japanese quail (Coturnix japonica) J Comp Psychol. 2005;119:49–57. doi: 10.1037/0735-7036.119.1.49. [DOI] [PubMed] [Google Scholar]
- Hull EM, Wood RI, McKenna KE. The neurobiology of male sexual behavior. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd ed. San Diego, New York: Academic Press; 2005. pp. 1729–1824. [Google Scholar]
- Huss D, Poynter G, Lansford R. Japanese quail (Coturnix japonica) as a laboratory animal model. Lab Anim (NY) 2008;37:513–519. doi: 10.1038/laban1108-513. [DOI] [PubMed] [Google Scholar]
- Hutchison RE. Hormonal differentiation of sexual behavior in Japanese quail. Horm Behav. 1978;11:363–387. doi: 10.1016/0018-506x(78)90038-7. [DOI] [PubMed] [Google Scholar]
- Kleitz-Nelson HK, Dominguez JM, Cornil CA, Ball GF. Is sexual motivational state linked to dopamine release in the medial preoptic area? Behav Neurosci. 2010;124:300–304. doi: 10.1037/a0018767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Emlen ST, Ricklefs RE, Wingfield JC. Contributions of bird studies to biology. Science. 1989;246:465–472. doi: 10.1126/science.2683069. [DOI] [PubMed] [Google Scholar]
- Kumar V, Follett BK. The circadian nature of melatonin secretion in Japanese quail (Coturnix coturnix japonica) J Pineal Res. 1993;14:192–200. doi: 10.1111/j.1600-079x.1993.tb00502.x. [DOI] [PubMed] [Google Scholar]
- Laugier C, Clastrat B, Brard E. Plasma estradiol levels in quail during sexual development and after different estrogenic treatments. C R Acad Sci Hebd Seances Acad Sci D. 1978;287:293–296. [PubMed] [Google Scholar]
- Lehrman DS. Ethology and psychology. Rec Adv Biol Psychiat. 1961;4:86–94. doi: 10.1007/978-1-4684-8306-2_11. [DOI] [PubMed] [Google Scholar]
- Lehrman DS. Interaction between internal and external environments in the regulation of the reproductive cycle of the ring dove. In: Beach FA, editor. Sex and Behavior. New York: John Wiley and Sons; 1965. pp. 355–380. [Google Scholar]
- Mahometa M, Domjan M. Classical conditioningincreases reproductive success in Japanese quail Coturnix japonica. Anim Behav. 2005;69:983–989. [Google Scholar]
- Marler P. Ethology and the origins of behavioral endocrinology. Horm Behav. 2005;47:493–502. doi: 10.1016/j.yhbeh.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Mills AD, Crawford LL, Domjan M, Faure JM. The behavior of the Japanese or domestic quail Coturnix japonica. Neurosci Biobehav Rev. 1997;21:261–281. doi: 10.1016/s0149-7634(96)00028-0. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. In: An Introduction to Behavioral Endocrinology. 3rd ed. Nelson RJ, editor. Sunderland MA: Sinauer Associates; 2005. [Google Scholar]
- Nestler EJ, Greengard P. Serine and threonine phosphorylation. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Philadelphia: Lippincott-Raven; 1999. pp. 471–495. [Google Scholar]
- Oishi T, Konishi T. Variations in the photoperiodic cloacal response of Japanese quail; association with testes weight and feather color. Gen Comp Endocrinol. 1983;50:1–10. doi: 10.1016/0016-6480(83)90236-8. [DOI] [PubMed] [Google Scholar]
- Ottinger MA, Brinkley HJ. Testosterone and sex-related behavior and morphology: Relationship during maturation and in the adult Japanese quail. Horm Behav. 1978;11:175–182. doi: 10.1016/0018-506x(78)90046-6. [DOI] [PubMed] [Google Scholar]
- Ottinger MA, Brinkley HJ. Testosterone and sex related physical characteristics during the maturation of the male Japanese quail (Coturnix coturnix japonica) Biol Reprod. 1979;20:905–909. doi: 10.1095/biolreprod20.4.905. [DOI] [PubMed] [Google Scholar]
- Ottinger MA, Pitts S, Abdelnabi MA. Steroid hormones during embryonic development in Japanese quail: Plasma, gonadal, and adrenal levels. Poult Sci. 2001;80:795–799. doi: 10.1093/ps/80.6.795. [DOI] [PubMed] [Google Scholar]
- Padgett CA, Ivey WD. Coturnix quail as a laboratory research animal. Science. 1959;129:267–268. doi: 10.1126/science.129.3344.267. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Viglietti-Panzica C, Calcagni M, Anselmetti GC, Schumacher M, Balthazart J. Sexual differentiation and hormonal control of the sexually dimorphic preoptic medial nucleus in quail. Brain Res. 1987;416:59–68. doi: 10.1016/0006-8993(87)91496-x. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Castagna C, Aste N, Viglietti-Panzica C, Balthazart J. Testosterone effects on the neuronal ultrastructure in the medial preoptic nucleus of male Japanese quail. Brain Res Bull. 1996a;39:281–292. doi: 10.1016/0361-9230(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: A key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol. 1996b;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Aste N, Castagna C, Balthazart J, Viglietti-Panzica C. Sexual dimorphism, steroid-induced plasticity and behavioral significance of the vasotocinergic innervation of the avian brain. In: Korf HW, Usadel KH, editors. Neuroendocrinology: Retrospect and Prospect. Heidelberg, Berlin: Springer; 1997. pp. 127–150. [Google Scholar]
- Panzica GC, Castagna C, Viglietti-Panzica C, Russo C, Tlemçani O, Balthazart J. Organizational effects of estrogens on brain vasotocin and sexual behavior in quail. J Neurobiol. 1998;37:684–699. doi: 10.1002/(sici)1097-4695(199812)37:4<684::aid-neu15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Panzica G, Viglietti-Panzica C, Balthazart J. Sexual dimorphism in the neuronal circuits of the quail preoptic and limbic regions. Microsc Res Tech. 2001;54:364–374. doi: 10.1002/jemt.1149. [DOI] [PubMed] [Google Scholar]
- Persaud KN, Galef BG., Jr Eggs of a female Japanese quail are more likely to be fertilized by a male that she prefers. J Comp Psychol. 2005a;119:251–256. doi: 10.1037/0735-7036.119.3.251. [DOI] [PubMed] [Google Scholar]
- Persaud KN, Galef BG., Jr Female Japanese quail (Coturnix japonica) mated with males that harassed them are unlikely to lay fertilized eggs. J Comp Psychol. 2005b;119:440–446. doi: 10.1037/0735-7036.119.4.440. [DOI] [PubMed] [Google Scholar]
- Replogle K, Arnold AP, Ball GF, Band M, Bensch S, Brenowitz EA, Dong S, Drnevich J, Ferris M, George JM, Gong G, Hasselquist D, Hernandez AG, Kim R, Lewin HA, Liu L, Lovell PV, Mello CV, Naurin S, Rodriguez-Zas S, Thimmapuram J, Wade J, Clayton DF. The songbird neurogenomics (song) initiative: Community-based tools and strategies for study of brain gene function and evolution. BMC Genomics. 2008;9:131. doi: 10.1186/1471-2164-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JE, Follett BK. Photoperiodism in Japanese quail: The termination of seasonal breeding by photorefractoriness. Proc R Soc Lond B Biol Sci. 1982;215:95–116. doi: 10.1098/rspb.1982.0030. [DOI] [PubMed] [Google Scholar]
- Sachs BD. Photoperiodic control of the cloacal gland of the Japanese quail. Science. 1967;157:201–203. doi: 10.1126/science.157.3785.201. [DOI] [PubMed] [Google Scholar]
- Sachs BD. Photoperiodic control of reproductive behavior and physiology of the male Japanese quail (Coturnix coturnix japonica) Horm Behav. 1969;1:7–24. [Google Scholar]
- Sachs BD. Bancroft J. The Pharmacology of Sexual Function and Dysfunction. Amsterdam: Elsevier; 1995. Context-sensitive variation in the regulation of erection; pp. 97–108. [Google Scholar]
- Sachs BD. A contextual definition of male sexual arousal. Horm Behav. 2007;51:569–578. doi: 10.1016/j.yhbeh.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Balthazart J. The effects of testosterone and its metabolites on sexual behavior and morphology in male and female Japanese quail. Physiol Behav. 1983;30:335–339. doi: 10.1016/0031-9384(83)90135-x. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Balthazart J. Neuroanatomical distribution of testosterone metabolizing enzymes in the Japanese quail. Brain Res. 1987;422:137–148. doi: 10.1016/0006-8993(87)90548-8. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Hutchison JB. Testosterone induces hypothalamic aromatase during early development in quail. Brain Res. 1986;377:63–72. doi: 10.1016/0006-8993(86)91191-1. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Sulon J, Balthazart J. Changes in serum concentrations of steroids during embryonic and post-hatching development of male and female Japanese quail (Coturnix coturnix japonica) J Endocrinol. 1988;118:127–134. doi: 10.1677/joe.0.1180127. [DOI] [PubMed] [Google Scholar]
- Seiwert CM, Adkins-Regan E. The foam production system of the male Japanese quail: Characterization of structure and function. Brain Behav Evol. 1998;52:61–80. doi: 10.1159/000006553. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: Organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Takada S, Ota J, Kansaku N, Yamashita H, Izumi T, Ishikawa M, Wada T, Kaneda R, Choi YL, Koinuma K, Fujiwara S, Aoki H, Kisanuki H, Yamashita Y, Mano H. Nucleotide sequence and embryonic expression of quail and duck sox9 genes. Gen Comp Endocrinol. 2006;145:208–213. doi: 10.1016/j.ygcen.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Taziaux M, Cornil CA, Balthazart J. Aromatase inhibition blocks the expression of sexually motivated cloacal gland movements in male quail. Behav Proc. 2004;67:461–469. doi: 10.1016/j.beproc.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Taziaux M, Cornil CA, Dejace C, Arckens L, Ball GF, Balthazart J. Neuroanatomical specificity in the expression of the immediate early gene c-fos following expression of appetitive and consummatory male sexual behaviour in Japanese quail. Eur J Neurosci. 2006;23:1869–1887. doi: 10.1111/j.1460-9568.2006.04719.x. [DOI] [PubMed] [Google Scholar]
- Taziaux M, Kahn A, Moore J, 3rd, Balthazart J, Holloway KS. Enhanced neural activation in brain regions mediating sexual responses following exposure to a conditioned stimulus that predicts copulation. Neuroscience. 2008a;151:644–658. doi: 10.1016/j.neuroscience.2007.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taziaux M, Keller M, Ball GF, Balthazart J. Site-specific effects of anosmia and cloacal gland anesthesia on fos expression induced in male quail brain by sexual behavior. Behav Brain Res. 2008b;194:52–65. doi: 10.1016/j.bbr.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RR, Goodson JL, Ruscio MG, Adkins-Regan E. Role of the archistriatal nucleus taeniae in the sexual behavior of male Japanese quail (Coturnix japonica): A comparison of function with the medial nucleus of the amygdala in mammals. Brain Behav Evol. 1998;51:215–229. doi: 10.1159/000006539. [DOI] [PubMed] [Google Scholar]
- Timberlake W, Silva KM. Appetitive behavior in ethology, psychology, and behavior systems. In: Thompson NS, editor. Perspectives in Ethology, vol 11: Behavioral Design. New York: Plenum Press; 1995. pp. 211–253. [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp JP. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- Van Der Linden A, De Groof G, Charlier TD, Balthazart J. Abst Soc Neurosci Abst Program No.660.8. 2006 Neuroscience Meeting Planner. Atlanta: Society for Neuroscience; 2006. Rapid testosterone-induced apparent diffusion coefficient (adc) changes in the sexually dimorphic medial preoptic nucleus of male Japanese quail. 2006. CD-ROM:A. [Google Scholar]
- Viglietti-Panzica C, Panzica GC, Fiori MG, Calcagni M, Anselmetti GC, Balthazart J. A sexually dimorphic nucleus in the quail preoptic area. Neurosci Ltrs. 1986;64:129–134. doi: 10.1016/0304-3940(86)90087-x. [DOI] [PubMed] [Google Scholar]
- Wada M. Low temperature and short days together induce thyroid activation and suppression of lh release in Japanese quail. Gen Comp Endocrinol. 1993;90:355–363. doi: 10.1006/gcen.1993.1091. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. Historical contributions of research on birds to behavioral neuroendocrinology. Horm Behav. 2005;48:395–402. doi: 10.1016/j.yhbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Silverin B. Ecophysiological studies of hormone-behavior relations in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. vol 2. San Diego: Academic Press; 2002. pp. 587–647. [Google Scholar]
- Woodard AE, Abplanalp H, Wilson WO, Vohra P. Japanese quail husbandry in the laboratory. Davis: University of California; 1973. Available online ( http://animalscience.ucdavis.edu/Avian/Coturnix.pdf) [Google Scholar]